Abstract
Grape seed procyanidin extract (GSE) has been reported to exert antineoplastic properties via the inhibition of cyclooxygenase-2 (COX-2)/prostaglandin E2 (PGE2) eicosanoid pathways. In addition, ample data link carcinogenesis to inflammatory events involving other major eicosanoid metabolic pathways, including prostacyclin (PGI2) and 15-Hydroxyeicosatetraenoic acid (15-HETE). We therefore evaluated the effects of GSE on prostacyclin synthase (PTGIS)/PGI2 and 15-lipoxigenase-2 (15-LOX-2)/15-HETE productions by human lung premalignant and malignant cells and correlated the findings with anti-proliferative or pro-apoptotic effects of GSE. The effects of GSE on PGI2 and 15-HETE productions by human bronchoalveolar lavage (BAL) cells ex vivo were also determined. We further evaluated the bioactivity of oral administration of leucoselect phytosome (a standardized GSE) in the lungs of subjects participating in a lung cancer chemoprevention trial, by comparing the anti-proliferative effects of co-culturing matched pre- vs. post-treatment BAL fluids with lung premalignant and malignant cells. GSE significantly increased PGI2 (as measured by 6-keto PGF1α) and 15-HETE productions by these cells. Transfections of PTGIS or 15-LOX-2 specific siRNA partially abrogated the anti-proliferative or proapoptotic effects of GSE in lung premalignant and malignant cells, respectively. GSE also increased PTGIS and inhibition of caspase 3 and transfection of 15-LOX-2 siRNA abrogated the GSE-induced apoptosis in A549 cells. In addition, culture supernatants from ex vivo GSE-treated baseline BAL cells, as well as BAL fluids from subjects treated with leucoselect phytosome, significantly decreased proliferations of lung premalignant and malignant cells. Our findings support the continued investigation of GSE as an anti-neoplastic and chemopreventive agent against lung cancer.
Keywords: prostacyclin synthase, 15-LOX-2, Caspase 3, SCLC, chemoprevention
INTRODUCTION
Derived from seeds of grapes (Vitis vinifera), grape seed procyanidin extract (GSE) have been shown to have antioxidant capabilities 20 and 50 times that of vitamin C and E, respectively (1). Widely available as a health food supplement, GSE is used to improve cardiovascular health, such as hypertension, hyperlipidemia, atherosclerosis, and chronic venous insufficiency. Small randomized trials have found beneficial effects of GSE for diabetic retinopathy and for vascular fragility (2). Recently, preclinical studies have shown that GSE inhibited human nonsmall cell lung cancer (NSCLC) xenografts growth in mice, in part, through the inhibition and down-regulation of cyclooxygenase-2 (COX-2) and prostaglandin (PG) E2 eicosanoid pathways (3).
Ample data link carcinogenesis to inflammatory events involving various eicosanoid metabolic pathways. Arachidonic acid (AA), released from membrane phospholipids upon cell stimulation, is converted to prostanoids by cyclooxygenases (COX), to leukotrienes by 5-lipoxygenase (5-LOX), and to 15(S)-hydroxy-eicosatetraenoic acid (15-HETE) by 15-lipoxygenases-2 (15-LOX-2)(4). Over-expression of COX-2 and subsequent overproduction of prostaglandin E2 (PGE2) are associated with a variety of well-established lung cancer risk factors (5–9). In two independent phase IIb lung cancer chemoprevention trials involving heavy former and/or active smokers, COX-2 inhibition with celecoxib significantly reduced bronchial Ki-67 expressions – a marker of cell proliferation and the primary end point for the trials (10, 11). Unfortunately, the increase in cardiovascular risks associated with selective, pharmaceutical COX-2 inhibitors prevented further evaluation of celecoxib in phase III trials. Such an increase in cardiovascular risk by COX-2 selective inhibitors is thought to be, in part, due to the reduction of PGI2, leading to an imbalance with thromboxane and is prothrombogenic.
PGI2 is an eicosanoid derived from PGH2 by prostacyclin synthase (PGIS); it is a potent vasodilator and inhibitor of platelet activation and aggregation (12). Majority of NSCLC have decreased PGIS expression (13). The potential of oral iloprost, a synthetic analogue of PGI2 as a chemopreventive agent for lung cancer has been evaluated in a multi-center phase IIb study. In that study, iloprost has been shown to improve endobronchial dysplasia in former smokers (14). However, the short half-life, side effects and costs of the agent have dampened enthusiasm for moving forward into phase III trials.
Whereas 15(S)-hydroxy-eicosatetraenoic acid (15-HETE) is primarily derived from AA by 15-lipoxygenase-2 (15-LOX-2); 15-HETE has been shown to bind to PPAR-γ nuclear receptors and induce apoptosis in A549 cells (15, 16), and reductions in 15-HETE has been associated with lung carcinogenesis (17). We therefore evaluated the effects of GSE on PTGIS/PGI2 and 15-LOX-2/15-HETE pathways in lung neoplastic cells and found that GSE also concomitantly and favorably modulated these important eicosanoids signaling pathways known to be involved in lung tumorigenesis.
As a part of a pilot study to determine the feasibility of leucoselect phytosome, a standardized GSE preparation complexed with soy phospholipid to improve bioavailability, for lung cancer chemoprevention, heavy current and former smokers are being recruited and treated with a 3-month course of leucoselect phytosome. To determine the effects of leucoselect phytosome on altering surrogate end point biomarkers (SEBM) of cancerization in the lung, serial bronchoscopies with pre- and post-treatment BAL were performed. Ex vivo conditioning of baseline BAL cells with GSE significantly increased the production of PGI2 and 15-HETE, and co-culture with these GSE-treated BAL cell culture supernatants decreased lung premalignant and malignant cell proliferations. Moreover, proliferation of lung neoplastic cells was significantly reduced by co-culturing with post-leucoselect phytosome treatment BAL fluid in comparison with matched, pre-treatment BAL fluid from the same subjects.
MATERIAL AND METHODS
Leucoselect Phytosome Clinical Study Design
A single arm, dose escalation phase I lung cancer chemoprevention study of 3 months of oral leucoselect phytosome, comprised of standardized oligomeric procyanidins complexed with soy phospholipid (1:2.6 w/w; Indena, Milan, Italy), is being conducted in 20 high risk heavy active or ex-smokers 21 years of age or older with a smoking history of at least 30 pack-years. Written informed consent is obtained in accordance with the New Mexico VA Health Care System (NMVAHCS) Institutional Review Board (IRB). Participants are screened with chest X-ray and fluorescence bronchoscopy (Onco-Life, Novadaq Technologies, Inc. Ontario, Canada) to rule out the presence of lung cancer. Qualified participants meeting all entry criteria (Table 1) were enrolled and treated with 1 capsule (cap) /day for week one, 2 caps/day for week 2, 3 caps/day for week 3, then 4 cap/day for the rest of the treatment duration as tolerated. Repeat fluorescence bronchoscopy is performed at the end of treatment with BAL, bronchial brushings, bronchial biopsies to collect samples for biomarker analysis.
Table 1.
Entry criteria for the phase I lung cancer chemoprevention trial with leuoselect phytosome.
| Inclusion Criteria: |
| 1) Age over 21. |
| 2) Smoking history > 30 pack years. |
| Exclusion Criteria: |
| 1) Inability to provide informed consent (e.g. cognitive impairment, severe psychiatric disorders). |
| 2) Hypersensitivity to grapes and related products. |
| 3) Liver dysfunction (abnormal liver function tests). |
| 4) Renal dysfunction (abnormal serum creatinine). |
| 5) End stage respiratory disease (FEV1<0.8 liters, resting or exertional hypoxemia, to select patients with adequate reserve to undergo bronchoscopy and complete the study). |
| 6) Unstable angina. |
| 7) Malignancy within 5 years, excluding non-melanoma type skin cancer or stage I NSCLC post curative resection without evidence of recurrence. |
| 8) Pregnancy. |
| 9) Systemic corticoid steroid therapy. |
| 10) Coagulopathy. |
| 11) Concurrent use of grapes or grape related products. |
| 12) Unwilling to refrain from drinking more than 1 glass of wine a day. |
| 13) Patients with concurrent medical conditions that may interfere with completion of tests, therapy, or the follow up schedule. |
Bronchoalveolar Lavage and Ex Vivo Conditioning of Alveolar Macrophages
Bronchoscopies were performed as previously described (18). Briefly, subjects were prepped with a combination of topical anesthesia (4% lidocaine atomized to posterior pharynx plus 1–2% aliquots of topical lidocaine along the trachea-laryngo-bronchial tree as needed) and moderate sedation using incremental doses of midazolam and Fentanyl according to institutional guidelines. A fiberoptic videobronchoscope (BF 20D, Olympus America) was advanced into the airway and wedged into a subsegment of the right middle lobe. Four 60-mL aliquots of room temperature saline were serially lavaged and recovered by manual syringe suction. Recovered fluid was passed through a 100-μm sterile nylon filter (Becton Dickinson, San Jose, CA) to remove mucus and particulates, pooled, and centrifuged at 300 × g for 8 minutes at 4°C. The BAL cells and fluid were then harvested, processed, aliquoted, and stored at −80°C until analysis.
BAL cell pellets were first washed in Hank’s balanced salt solution (Sigma-Aldrich, St. Louis, MO) and then resuspended in X-Vivo 15 serum-free medium (BioWhittaker, Walkersville, MD) to a concentration of 0.5 × 106 cells/ml. After 2 h incubation/adherence, cells were treated with GSE overnight at 37°C for 18–24 h. Culture supernatants were harvested for eicosanoids determination.
Cell Cultures
As models to evaluate the anti-neoplastic effect of GSE against lung cancer, the human NSCLC cell lines A549 and H23, the small cell lung cancer (SCLC) cell line DMS114 (ATCC; Manassas, VA), and the bronchial premalignant cell line 1198 (derived from tracheal transplant of BEAS-2B cells treated with cigarette smoke condensate, generously provide by Dr. Klein-Santos, Fox Chase Cancer Center, Philadelphia, PA)(19), were studied in vitro. Experiments involving all commercial cell lines were initiated within 6 months of purchase. Cell lines were not further authenticated. ATCC uses Short Tandem Repeat profiling for cell line authentication. Cells were maintained as monolayers in an atmosphere of 5% CO2 in air at 37°C in 25-cm2 tissue culture flasks containing 5.0 ml of RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 100 units/ml of penicillin, 0.1 mg/ml of streptomycin, and 2 mM of glutamine (JRH Biosciences; Lenexa, KS) for A549; RPMI 1640 medium with 2 mM L-glutamine adjusted to contain 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10 mM HEPES, and 1.0 mM sodium pyruvate, 10% FBS, for H23 cells; and Waymouth’s MB 752/1 medium with L-Glutamine and 10% FBS for DMS114
Aliquots of 0.1 × 106 cells were incubated at 37°C for 2 h. Varying doses of GSE (6, 12, 15, 18, 30, 45, or 60 μg/ml),were added and the cells were incubated at 37°C for 18–44 h. The dose range of GSE was chosen based on considerations from prior published studies (20, 21). Primary normal human bronchial epithelial (NHBE) cells (Lonza, Walkersville, MD) and BEAS-2B cells (non-tumorigenic immortalized bronchial epithelial cells, ATCC) were used as controls and maintained according to the manufacturer’s instructions. NHBE and BEAS-2B cells were plated in wells at 0.5 × 106 cells/ml and treated with varying doses of GSE for 18–24h.
To ascertain the involvement of caspase 3 in our model systems, the caspase 3 inhibitor Z-DEVD-FMK (100 μM, R & D Systems; Minneapolis, MN), were added one hour prior to the addition of GSE. Experiments with dose titrations of the inhibitor were first carried out to determine the optimal inhibitory dose without compromising cell viability. Standardized GSE (90% procyanidins) was purchased from commercially available sources (Organic Herb Inc. China). Stock solutions of GSE were made by dissolving the extract with deionized water. Aliquots of the stock were stored at −80°C and used only once for each set of experiments.
For MTT assays, cells were plated at concentration of 8–15 × 103 cells/well in 96 well plates, treated and cultured, then subjected to the MTT assay.
All culture supernatants, cell lysates for total protein, and total RNA harvested from cell lysates were stored at −80°C until analysis, when applicable. Samples of mRNA were collected using the miRNeasy Mini Kit (Qiagen Inc, Valencia, CA), per the manufacturer’s instructions.
MTT Assay
To quantify cellular proliferation in conditioned cells, The MTT Cell Proliferation Assay (ATCC; Manassas, VA) was used to measures the reduction of a tetrazolium component (MTT) into an insoluble formazan product by the mitochondria of viable cells according to the manufacturer’s instructions.
Cell Death ELISA
For quantification of apoptosis, the cell death detection ELISA kit (Roche; Indianapolis, IN) was used. To quantify apoptosis in the conditioned cells, specific measurements of mono- and oligonucleosomes by immunochemical determination of histone-complexed DNA fragments in the cytoplasmic fraction of -cell culture lysates were performed using the cell death ELISA kit according to the manufacturer’s instructions.
Quantigene Assay
Following overnight treatment with GSE, cells were washed twice with PBS, counted, followed by addition of lysis buffer to 400 cells/μl. The gene expression level was determined using the QuantiGene Plex 2.0 Assay kit (Affymetrix, CA, USA) per the manufacturer’s instructions. The assay is based on DIRECT quantification of the RNA targets using xMAP Luminex beads and branched DNA (bDNA) signal amplification technology. For each RNA molecule of interest, an oligonucleotide probe set, containing three types of synthetic probes, capture extenders (CEs), label extenders (LEs), and blockers (BLs) that hybridize and span contiguous sequences of the target RNA, is used.
SiRNA transfection
Transfections of PTGIS- and 15-LOX-2-specific siRNA into lung neoplastic cells were achieved using specific siRNA and transfecting reagents from Qiagen Inc. (Valencia, CA). The cell numbers and the amount of siRNA mimics and transfection reagent were first optimized according to manufacturer’s instructions. Briefly, 6–8 × 103 of cells in 150 μl medium were aliquoted into 96-well plates. After 1 h of adherence, 50 μl of specific miRNA transfection complexes were gently added to the cells with gentle swirling. The cells were incubated for 42 h, then treated overnight with GSE for 24 h, followed by the MTT assay. Cell conditions were scaled up 10 fold in 12 well plates for total RNA harvest.
Quantification of 15-HETE
Measurements of 15-HETE concentrations in conditioned culture supernatants were performed using enzyme specific immunoassay (EIA) according to the manufacturer’s EIA kit protocol (Cayman Chemical; Ann Arbor, MI).
Quantification of 6-keto PGF1α
Measurements of 6-keto PGF1α concentrations in conditioned culture supernatants were performed using enzyme specific immunoassay (EIA) according to the manufacturer’s EIA kit protocol (Cayman Chemical; Ann Arbor, MI).
PTGIS ELISA
Levels of PTGIS protein were measured in GSE-treated A549 cell lysate using a PTGIS-specific ELISA per manufacturer’s instruction (Cloud-Clone Corp., Wuhan, Hubei, China).
Quantification of caspase 3 activity
For quantification of caspase 3 activity, the Caspase 3/CPP32 colormetric Assay Kits (Biovision, Mountainview, CA) were used. The assay is based on spectrophotometric detection of the chromophore p-nitroanilide (pNA) after cleavage from the labeled substrate DEVD-pNA. The pNA light emission was quantified using a microtiter plate reader at 405 nm. Freshly harvested cell extracts from conditioned cultures were assayed per manufacturer’s instructions.
Statistical Analysis
Data are expressed as the mean ± SD in all circumstances where mean values are compared. Data were analyzed by paired Student’s t test and/or ANOVA. Batch analyses were performed for each comparison group to eliminate interassay variability. Differences are considered significant when p <0.05.
RESULTS
GSE increased 6-keto PGF1α production by lung premalignant and malignant cells.
To explore the effects of GSE on PGI2 production by lung neoplastic cells, NSCLC cell lines A549 and H23, the SCLC cell line DMS114, and the bronchial preneoplastic cell line 1198 were treated with GSE overnight. Because of the short half-life of PGI2, levels of 6-keto PGF1α were measured in culture supernatants as a surrogate for PGI2, production in response to GSE treatment. GSE significantly increased the production of 6-keto PGF1α in A549 (Fig. 1A) and H23 cells (Fig. 1B), DMS114 (Fig. 1C) and 1198 (Fig. 1D) cells, but not in NHBE (Fig. 1E) or BEAS-2B (Fig. 1F) cells
Fig. 1.
Overnight treatment with GSE increased production of 6-keto PGF1α, a stable metabolite of PGI2, by NSCLC cell lines A549 (A) and H23 (B), SCLC cell line DMS114 (C), and the bronchial premalignant cell line 1198 (D), but not by NHBE (E) or BEAS-2B (F) cells (cell proliferation as measured by MTT also did not change significantly, data not shown). The levels of 6-keto PGF1α, were measured in culture supernatants as a surrogate for PGI2, production in response to GSE treatment. The Range of control PGI2 levels for various malignant and premaligant cell lines is 45 – 219 pg/ml. Columns, mean; Bars, SD (n = 3). *, P < 0.05; **, P < 0.01.
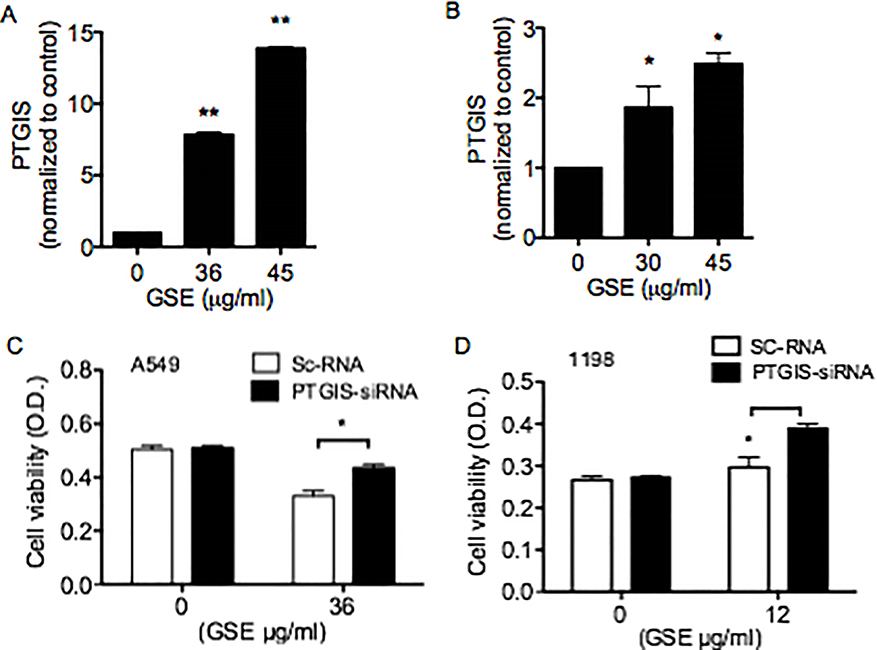
GSE increased PTGIS mRNA expression and protein production and PTGIS-siRNA transfection abrogated the anti-proliferative effects of GSE in lung premalignant and malignant cells
To further characterize the molecular mechanisms involved in GSE-mediated increase in PGI2 production, the effects of GSE treatment on PTGIS mRNA expression in A549 cells were measured following overnight treatment. GSE dose-dependently increased PTGIS mRNA expression (Fig 2A). GSE also dose-dependently increased PTGIS protein production in A549 cells (Fig 2B). Moreover, transfection of PTGIS-siRNA partially reversed the GSE-induced anti-proliferative effects on both A549 (Fig. 2C) and 1198 cells (Fig. 2D).
Fig. 2.
GSE dose-dependently increased PTGIS mRNA expression (A) and protein production (B) in A549 cells. Moreover, transfection of PTGIS-siRNA partially reversed the GSE-induced anti-proliferative effects on both A549 (C) and 1198 cells. (D). Control represents cells transfected with scrambled RNA (Sc-RNA). Columns, mean; Bars, SD (n = 3). *, P < 0.05.
GSE increased 15-HETE production and 15-LOX-siRNA transfection abrogated the apoptotic effects of GSE by lung premalignant and malignant cells.
15-HETE is one of the most abundant ecosanoid in the bronchi, has been shown to induce apoptosis in A549 cells, and is believed to be anti-carcinogenic (15–17). To determine if 15-HETE plays a role in GSE-induced apoptosis, the effects of GSE treatment on 15-HETE production by A549 (Fig. 3A), 1198 (Fig. 3B), and DMS114 (Fig. 3C) cells were evaluated. GSE significantly increased 15-HETE production by these cells. Furthermore, transfection with 15-LOX-siRNA abrogated the apoptotic effects of GSE in A549 (Fig. 3D) and 1198 (Fig. 3E) cells.
Fig. 3.
GSE significantly increased 15-HETE production by both A549 (A) and 1198 (B) and DMS 114 (C) cells, correlating with GSE-induced apoptosis. The Range of control 15-HETE levels is approximately 6 to 800, 2 to 400, and 2 to 3,000 pg/ml for A549, 1198, and DMS114 cells, respectively. Transfection with 15-LOX-siRNA partially abrogated the apoptotic effects of GSE in A549 (D) and 1198 (E) cells. Control represents cells transfected with scrambled RNA (Sc-RNA). Columns Columns, mean; bars, SD (n = 3). **, P < 0.01.
GSE increased caspase 3 activation and inhibition of caspase 3 abrogated the apoptotic effect of GSE in A549 cells
We previously reported the involvement of the 15-LOX/15-HETE pathway in caspase-3 activation (16). To determine whether the GSE-induced apoptosis is mediated by caspase 3, A549 cells were treated with GSE in the presence or absence of the caspase 3 inhibitor, Z-DEVD-FMK (DEVD). GSE significantly increased caspase 3 activation, whereas DEVD abrogated the GSE-induced apoptosis (Fig. 4). Similar findings were observed in 1198 cells (data not shown).
Fig. 4.
A549 cells were conditioned with GSE in the presence or absence of the caspase 3 inhibitor, Z-DEVD-FMK (DEVD). GSE significantly increased Caspase 3 activation, whereas DEVD abrogated the GSE-induced apoptosis. Similar findings were observed in 1198 cells (data not shown). Columns Columns, mean; bars, SD (n = 3). **, P < 0.01.
GSE dose-dependently increased 6-keto PGF1α and 15-HETE production by human BAL Cells.
BAL represents a minimally invasive bronchoscopic sampling procedure to obtain cells and molecules from the lung microenvironment. BAL cells are comprised of predominantly alveolar macrophages (AM), the major immunoeffector cells and source of eicosanoids in the lung microenvironment. Through modulations of eicosanoids, AM likely play a central role in mediating immune surveillance and antitumor immunity in the lung microenvironment. As such, we evaluated the effects of GSE on 6-keto PGF1α and 15-HETE productions by human BAL cells ex vivo. Treatment with GSE for 24 h significantly increased both 15-HETE (Fig 5A) and 6-keto PGF1α (Fig 5B) production by BAL cells. This finding suggests that GSE may favorably modulate anti-carcinogenic forces in the lung microenvironment, in part, via increasing PGI2 and 15-HETE productions from BAL cells.
Fig. 5.
GSE dose dependently increases 6-keto PGF1α (A) and 15-HETE (B) productions by human BAL cells ex vivo. 6-keto PGF1α and 15-HETE levels were measured in culture supernatants after overnight incubation with varying doses of GSE (μg/ml). The Range of control 6-keto PGF1α and 5-HETE levels are ~ 85 to 125 pg/ml and ~ 6 to 7000 pg/ml, respectively. Much less GSE is required to significantly increase 6-keto PGF1α and 15-HETE productions by BAL cells than A549 cells. mean; bars, SD (n = 3). *, P < 0.05. Proliferation of A549 (C) cells are significantly inhibited when the cells are treated with culture supernatants from GSE-pretreated BAL cells (6 μg/ml, 1:1 GSE treated BAL culture supernatant: RPMI), at a much lower dose (final concentration of GSE = 3 μg/ml) than the dose required (30 μg/ml) to induce a similar response when A549 cells are treated with GSE alone. Columns, mean; bars, SD (n = 3). *, P < 0.05.
Culture supernatants from GSE-treated BAL cell, as well as BAL fluid from human subjects treated with leucoselect phytosome, significantly inhibited growth of lung premalignant and malignant cells.
To better simulate the lung microenvironment, culture supernatants from baseline BAL cells pretreated with GSE (6 μg/ml) ex vivo was used in co-culture with A549 and significantly inhibited its cell proliferation, at 1/10th of the dose required to induce similar effects when A549 cells were treated with GSE alone (Fig. 5C). Furthermore, A549, DMS114 and 1198 cells were treated with matched BAL fluid obtained from subjects pre- and post- 3 months of oral leucoselect phytosome treatment. Post-treatment BAL fluid, despite 1:4 dilution in culture medium, significantly reduced proliferation of A549 (Fig. 6A), 1198 (Fig. 6B), and DMS114 Cells (Fig 6C).
Fig. 6.
To determine the bioactivity of oral administration of leucoselect phytosome in the lung, A549, 1198, and DMS114 cells were treated with matched BAL fluid (BAL:culture medium = 1:4) obtained from the first three subjects pre- and post- 3 months of oral leucoselect phytosome treatment. Post-treatment BAL fluid significantly reduced proliferation of A549 (A), 1198 Cells (B), and DMS114 cells (C). Columns, mean; bars, SD (n = 3). *, P < 0.05.
DISCUSSION
In this study, we demonstrate, for the first time, the roles of PTGIS/PGI2 and 15-LOX-2/15-HETE in mediating the anti-neoplastic and chemopreventive properties of GSE against lung cancer. GSE increases PGI2 (as measured by 6-keto PGF1α) productions in human lung premalignant, malignant, and BAL cells, suggesting that GSE may function both as a natural COX-2 inhibitor and an inducer of PGI2. Furthermore, GSE significantly increased 15-HETE production by lung premalignant, malignant and BAL cells, and inhibition of caspase 3 abrogated the GSE-induced apoptosis in A549 cells. In addition, culture supernatants from GSE-treated BAL cell, as well as BAL fluid from human subjects treated with leucoselect phytosome, significantly inhibited growth of lung premalignant and malignant cells. The fact that GSE did not significantly alter PGI2 production in NHBE and BEAS-2B cells suggests that GSE acts on later stages of carcinogenesis. Our findings reveal novel anti-neoplastic mechanisms involving major ecosanoid signaling pathways by GSE, and support the continue investigation of GSE as an anti-neoplastic and chemopreventive agent for lung cancer.
Whereas the anti-cancer mechanism of GSE is still incompletely understood, preclinical studies have shown that the anti-cancer effects of GSE is mediated, at least in part, through the inhibition and down-regulation of COX-2/PGE2 pathways (3). The unexpected increase in cardiovascular risk associated with pharmaceutical COX-2 inhibitors has dampened enthusiasm for their use in chemoprevention. Exploring the potential utility of a natural COX-2 inhibitor with favorable side effect profiles that may also be cardio-protective, such as GSE, for treatment and prevention of lung cancer is clearly justified.
To translate the preclinical findings into a lung cancer chemoprevention trial, we first performed a literature search to identify and select a GSE agent. Because the absorption of GSE is affected by molecular weight (22), variable compositions of GSE polyphenol in different products on the market further contribute to low and erratic bioavailability (22, 23). An inexpensive GSE standardized to smaller size oligomeric procyanidins (OPC) and complexed with soy phospholipids into phytosomes to improve bioavailability (Leucoselect), is available over the counter. Leucoselect had been shown to improve the total antioxidant capacity of plasma and reduce LDL susceptibility to oxidative stress in heavy smokers (24). As such, we evaluated its efficacy in our preclinical study, using an A549 lung cancer xenograft nude mouse model, and demonstrated significant efficacy in tumor growth inhibition (25). These findings set the stage for our lung cancer chemoprevention trial with leucoselect phytosome. Whereas leucoselect phytosome has been used as a health food supplement to promote cardiovascular health for decades, the mechanism by which leucoselect may exert benefits on cardiovascular health is poorly understood. Our findings suggest that the increase in PGI2 production may contribute to its cardiovascular benefits.
In view of the fact that the ability of GSE to inhibit COX-2 may lead to shunting of AA toward 15-LOX pathways, we evaluated the effects of GSE on 15-HETE productions in lung premalignant and malignant cells and human BAL cells (ex vivo). GSE dose-dependently increased 15-HETE productions by these cells, but GSE did not increase expression of 15-LOX (1 or 2) in A549 cells (data not shown), suggesting that the increase of 15-HETE by GSE is also a result of shunting, as was previously found in a phase IIb clinical trial with Celecoxib (11). Taken together, these findings reflect the additional influence of increased 15-HETE in the setting of COX-2 inhibition on dampening the driving force of cancerization in the lungs.
During the sequence of events associated with apoptosis, caspase 3, an intracellular cysteine protease that exists as a proenzyme, becomes activated. Caspase-3 activation represents an important mechanism of apoptosis induced by a wide variety of apoptotic signals (26). In our study we find that GSE-induced apoptosis in NSCLC cells involves activation of caspase-3, and inhibition of caspase-3 significantly abrogates the GSE-induced apoptosis in A549 cells. Our group previously described the involvement of 15-LOX pathway in caspase-3 activation (16). Accordingly, the increase of 15-HETE production by GSE in lung premalignant and malignant cells likely plays a role in activation of caspase-3 and induction of apoptosis. GSE-mediated apoptotic cell death in NSCLC cell lines has been previously shown to involve the activation of caspase 9 (27), and 15-HETE has also been reported to activate caspase 9 (28). Caspase 9 is an initiator caspase that cleaves/activates procaspase 3. As such, GSE-induced 15-HETE production likely activates caspase 3 via caspase 9. Whether or not GSE activates caspase 9 through other mechanisms independent of 15-HETE is unclear and remains to be elucidated.
Another interesting observation from the present study is that the SCLC cell line DMS114 is far more sensitive to the antiproliferative effects of GSE with an in vitro IC50 of approximately 3 fold less than that of the NSCLC cell lines (data not shown). Approximately 10–15% of lung cancer cases are SCLC (29). Although SCLC is very responsive to platinum-based chemotherapy and radiation (30), resistance to therapy quickly develops. The overall prognosis of SCLC is dismal (31, 32). People with advanced stage SCLC usually survive less than 1 year. Even with limited-stage disease, the median survival time is less than 2 years. Furthermore, people with small cell lung cancer have the highest rate of developing a second primary cancer, usually in 5 to 10% of cases, but as high as 30%. Almost 4 decades since the introduction of the platinum-etoposide doublet chemotherapy, therapeutic options have remained virtually unchanged (33). To our knowledge, this is the first report on anti-neoplastic effects of GSE against SCLC cancer. Conceivably, GSE may also present important opportunities for SCLC chemoprevention and treatment.
Lung cancer is the leading cause of cancer death in the world (32). Despite advancements in anti-cancer treatments, the 5-year survival for lung cancer remains dismal (32). The lack of effective therapy provides the impetus to search for alternative, safe and efficacious agents for lung cancer chemoprevention, to impede the driving force of cancerization, and prevent lung cancer development in at-risk individuals (34). The encouraging findings from phase IIb lung cancer chemoprevention trials with celecoxib and iloprost have engendered discussions on developing a study using a combination of the two agents, so that iloprost may negate the increased cardiovascular risk associated with pharmaceutical COX-2 inhibitors, while exerting synergistic anti-neoplastic effects. In the end, the various issues associated with both agents have prevented further investigation, yet opening doors to explore the potential utility of natural COX-2 inhibitors that may simultaneously increase PGI2 and promote cardiovascular health, such as GSE. In this study, we demonstrate novel GSE mediated anti-neoplastic mechanisms; modulation of these mechanisms may also be useful as SEBM in clinical trials. Our findings provide important rationales to continue the clinical investigation of the potential of GSE for lung cancer chemoprevention.
Acknowledgement
We wish to thank S. Kye, P Neis, E. Martinez, and A. Vargas for their excellent technical assistance. This work is supported by grants from VA Merit Review (BX002258, J. T. Mao) and National Cancer Institute (R21CA173211, J.T. Mao).
Footnotes
Conflict of Interests: The authors declare that there are no conflict of interests.
REFERENCES
- 1.Shi J, Yu J, Pohorly JE, Kakuda Y. Polyphenolics in grape seeds-biochemistry and functionality. J Med Food 2003;6:291–9. [DOI] [PubMed] [Google Scholar]
- 2.Grape seed extract, herb at a glance. NCCAM 2012. [Google Scholar]
- 3.Sharma S, Meeran S, Katiyar S. Proanthocyanidins inhibit in vitro and in vivo growth of human non-small cell lung cancer cells by inhibiting the prostaglandin E(2) and prostaglandin E(2) receptors. Molecular Cancer Therapeutics 2010;9:569–80. [DOI] [PubMed] [Google Scholar]
- 4.Ara G, Teicher BA. Cyclooxygenase and lipoxygenase inhibitors in cancer therapy. Prostaglandins Leukot Essent Fatty Acids 1996;54:3–16. [DOI] [PubMed] [Google Scholar]
- 5.Menter DG, Schilsky RL, DuBois RN. Cyclooxygenase-2 and cancer treatment: Understanding the risk should be worth the reward. Clin Cancer Res 2010;16:1384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao JT, Cui X, Reckamp K, Liu M, Krysan K, Dalwadi H, et al. Chemoprevention strategies with cyclooxygenase-2 inhibitors for lung cancer. Clinical lung cancer 2005;7:30–9. [DOI] [PubMed] [Google Scholar]
- 7.Masferrer JL, Koki A, Seibert K. COX-2 inhibitors: A new class of antiangiogenic agents. Ann N Y Acad Sci 1999;889:84–6. [DOI] [PubMed] [Google Scholar]
- 8.Huang M, Stolina M, Sharma S, Mao JT, Zhu L, Miller PW, et al. Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: Up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res 1998;58:1208–16. [PubMed] [Google Scholar]
- 9.Hida T, Kozaki K, Muramatsu H, Masuda A, Shimizu S, Mitsudomi T, et al. Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clin Cancer Res 2000;6:2006–11. [PubMed] [Google Scholar]
- 10.Kim ES, Hong WK, Lee JJ, Mao L, Morice RC, Liu DD, et al. Biological activity of celecoxib in the bronchial epithelium of current and former smokers. Cancer Prev Res (Phila) 2010;3:148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao JT, Roth MD, Fishbein MC, Aberle DR, Zhang ZF, Rao JY, et al. Lung cancer chemoprevention with celecoxib in former smokers. Cancer Prev Res (Phila) 2011;4:984–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dusting GJ, Moncada S, Vane JR. Prostacyclin: Its biosynthesis, actions, and clinical potential. Adv Prostaglandin Thromboxane Leukot Res 1982;10:59–106. [PubMed] [Google Scholar]
- 13.Tuder RM, Cool CD, Geraci MW, Wang J, Abman SH, Wright L, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. American journal of respiratory and critical care medicine 1999;159:1925–32. [DOI] [PubMed] [Google Scholar]
- 14.Keith RL, Blatchford PJ, Kittelson J, Minna JD, Kelly K, Massion PP, et al. Oral iloprost improves endobronchial dysplasia in former smokers. Cancer Prev Res (Phila) 2011;4:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shankaranarayanan P, Nigam S. IL-4 induces apoptosis in A549 lung adenocarcinoma cells: Evidence for the pivotal role of 15-hydroxyeicosatetraenoic acid binding to activated peroxisome proliferator-activated receptor gamma transcription factor. J Immunol 2003;170:887–94. [DOI] [PubMed] [Google Scholar]
- 16.Mao J, Nie W, Tsu I, Jin Y, Rao J, Lu Q, et al. White tea extract induces apoptosis in non-small cell lung cancer cells: The role of peroxisome proliferator-activated receptor-{gamma} and 15-lipoxygenases. Cancer Prevention Research 2010;3:1132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan H, Li MY, Ma LT, Hsin MK, Mok TS, Underwood MJ, et al. 15-lipoxygenases and its metabolites 15(S)-HETE and 13(S)-HODE in the development of non-small cell lung cancer. Thorax 2010;65:321–6. [DOI] [PubMed] [Google Scholar]
- 18.Mao JT, Roth MD, Serio KJ, Baratelli F, Zhu L, Holmes EC, et al. Celecoxib modulates the capacity for prostaglandin E2 and interleukin-10 production in alveolar macrophages from active smokers. Clin Cancer Res 2003;9:5835–41. [PubMed] [Google Scholar]
- 19.Klein-Szanto A, Iizasa T, Momiki S, Garcia-Palazzo I, Caamano J, Metcalf R, et al. A tobacco-specific N-nitrosamine or cigarette smoke condensate causes neoplastic transformation of xenotransplanted human bronchial epithelial cells. Proceedings of the National Academy of Sciences 1992;89:6693–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akhtar S, Meeran S, Katiyar N, Katiyar S. Grape seed proanthocyanidins inhibit the growth of human non-small cell lung cancer xenografts by targeting insulin-like growth factor binding protein-3, tumor cell proliferation, and angiogenic factors. Clinical Cancer Research 2009;15:821–31. [DOI] [PubMed] [Google Scholar]
- 21.Shrotriya S, Deep G, Gu M, Kaur M, Jain A, Inturi S, et al. Generation of reactive oxygen species by grape seed extract causes irreparable DNA damage leading to G2/M arrest and apoptosis selectively in head and neck squamous cell carcinoma cells. Carcinogenesis 2012;33:848–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deprez S, Mila I, Huneau J, Tome D, Scalbert A. Transport of proanthocyanidin dimer, trimer, and polymer across monolayers of human intestinal epithelial caco-2 cells. Antioxidants & Redox Signaling 2001;3:957–67. [DOI] [PubMed] [Google Scholar]
- 23.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr 2000;130:2073S–85S. [DOI] [PubMed] [Google Scholar]
- 24.Vigna GB, Costantini F, Aldini G, Carini M, Catapano A, Schena F, et al. Effect of a standardized grape seed extract on low-density lipoprotein susceptibility to oxidation in heavy smokers. Metab Clin Exp 2003;52:1250–7. [DOI] [PubMed] [Google Scholar]
- 25.Mao JT, Xue B, Smoake J, Lu QY, Park H, Henning SM, et al. MicroRNA-19a/b mediates grape seed procyanidin extract-induced anti-neoplastic effects against lung cancer. J Nutr Biochem 2016;34:118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin YH, Yoo KJ, Lee YH, Lee SK. Caspase 3-mediated cleavage of p21WAF1/CIP1 associated with the cyclin A-cyclin-dependent kinase 2 complex is a prerequisite for apoptosis in SK-HEP-1 cells. J Biol Chem 2000;275:30256–63. [DOI] [PubMed] [Google Scholar]
- 27.Tyagi A, Raina K, Gangar S, Kaur M, Agarwal R, Agarwal C. Differential effect of grape seed extract against human non-small-cell lung cancer cells: the role of reactive oxygen species and apoptosis induction. Nutr Cancer. 2013;65 Suppl 1:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li MY, Yuan HL, Ko FW, Wu B, Long X, Du J, Wu J, Ng CS, Wan IY, Mok TS, Hui DS, Underwood MJ, Chen GG. Antineoplastic effects of 15(S)-hydroxyeicosatetraenoic acid and 13-S-hydroxyoctadecadienoic acid in non-small cell lung cancer. Cancer. 2015;121 Suppl 17:3130–45. [DOI] [PubMed] [Google Scholar]
- 29.American Cancer Society. Cancer facts and figures 2016. American Cancer Society, Atlanta: 2016. [Google Scholar]
- 30.Pignon J, Arriagada R, Ihde DC, Johnson DH, Perry MC, Souhami RL, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992;327:1618–24. [DOI] [PubMed] [Google Scholar]
- 31.Stahel RA, Ginsberg R, Havemann K, Hirsch FR, Ihde DC, Jassem J, et al. Staging and prognostic factors in small cell lung cancer: A consensus report. Lung Cancer 1989;5:119–26. [Google Scholar]
- 32.Brambilla E, Travis WD. Lung cancer. in: World cancer report. World Health Organization, Lyon: 2014. [Google Scholar]
- 33.Pietanza MC, Byers LA, Minna JD, Rudin CM. Small cell lung cancer: Will recent progress lead to improved outcomes? Clin Cancer Res 2015;21:2244–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao J, Durvasula R. Lung cancer chemoprevention: Current status and future direction. Current Respiratory Care Reports 2012;1:9–20. [Google Scholar]