Abstract
Oxytocin (OXT) is a nonapeptide that exerts anxiolytic effects in the brain. The amygdala is an important structure involved in the modulation of fear and anxiety. A high density of OXT receptors (OXTRs) has been detected in the capsular (CeC) and lateral (CeL) nucleus of the central amygdala (CeA). Previous studies have demonstrated that activation of OXTRs induces remarkable increases in neuronal excitability in the CeL/C. However, the signaling and ionic mechanisms underlying OXTR-induced facilitation of neuronal excitability have not been determined. We found that activation of OXTRs in the CeL increased action potential firing frequency recorded from neurons in this region via inhibition of the inwardly rectifying K+ channels. The functions of phospholipase C β and protein kinase C were required for OXTR-induced augmentation of neuronal excitability. Our results provide a cellular and molecular mechanism whereby activation of OXTRs exerts anxiolytic effects.
Keywords: excitability, action potential, K+ channels, amygdala, peptide, synapse
Introduction
Oxytocin (OXT) is a nonapeptide synthesized primarily by the neurosecretory cells in the paraventricular and supraoptic nuclei of the hypothalamus. In addition to the neurosecretory projections to the posterior pituitary, OXT also travels along the axonal projections from parvocellular neurons of the hypothalamus to discrete brain regions including the amygdala, hippocampus and nucleus accumbens (Buijs, 1978; Lang et al., 1983; Hawthorn et al., 1985; Jurek & Neumann, 2018). OXT functions by interacting with OXT receptors (OXTRs) or cross-activating the V1A subtype of the vasopressin receptors (Song et al., 2014). Both OXTRs (Vrachnis et al., 2011) and V1A (Birnbaumer, 2000) are coupled to Gαq proteins resulting in activation of phospholipase Cβ (PLCβ) which hydrolyzes PIP2 to generate IP3 to increase intracellular Ca2+ release and diacylglycerol (DAG) to activate protein kinase C (PKC), although OXTRs are also coupled to Gαi proteins (Jurek & Neumann, 2018). In addition to its hormonal roles in parturition and lactation, OXT is a neuromodulator that regulates a diverse range of functions including emotional responses (Neumann, 2008; Viviani et al., 2011; Knobloch et al., 2012), social affiliation (Feldman, 2012), grooming (Amico et al., 2004), sexual behaviors (Argiolas & Gessa, 1991), social (Bielsky & Young, 2004) and spatial (Tomizawa et al., 2003) memories, and may possess therapeutic potential for neuropsychiatric disorders such as autism and schizophrenia (Zik & Roberts, 2015).
The amygdala is an important structure involved in modulating emotion (Ressler, 2010; Tye et al., 2011; Dejean et al., 2015; Janak & Tye, 2015), pain (Neugebauer et al., 2004; Veinante et al., 2013; Neugebauer, 2015), alcohol use disorders (Silberman et al., 2008; Gilpin et al., 2015) and appetite (Petrovich, 2011, 2013; Zanchi et al., 2017; Smith & Lawrence, 2018). The amygdala is a collection of nuclei comprising the lateral amygdala (LA), the basolateral amygdala (BLA), and the central amygdala (CeA). Whereas the LA and BLA mostly contain glutamatergic pyramidal neurons, the CeA is composed of distinct GABAergic neurons forming 3 subnuclei named as capsular, lateral, and medial nucleus of CeA (LeDoux, 2000) (abbreviated as CeC, CeL and CeM, respectively), although the CeL and CeC are sometimes considered together (CeL/C). The GABAergic medial intercalated cells (ITCs) (Ehrlich et al., 2009) separate the BLA from the CeA (McDonald, 1985; Nitecka & Ben-Ari, 1987). Generally, information flows from the LA and BLA, or through the ITCs, into the CeA, which sends out information through amygdala efferents (Duvarci & Pare, 2014). The LA receives multisensory information from the thalamus (LeDoux et al., 1990; Tully et al., 2007), integrated sensory information from the cortex (McDonald, 1998), and noxious stimulus information from the brainstem regions (Johansen et al., 2011). The CeA also receives noxious stimulus information from the brainstem regions (Bernard et al., 1992; Neugebauer et al., 2009). Glutamatergic neurons in the LA synapse onto glutamatergic BLA neurons and onto GABAergic medial ITCs (Ehrlich et al., 2009). The LA and BLA send dense glutamatergic projections to the CeA, with the LA projecting only to the CeL and the BLA projecting to both the CeL and the CeM (Krettek & Price, 1978; Pitkanen et al., 1995; Savander et al., 1995). Projections out of the BLA also synapse onto GABAergic ITCs, which synapse on CeA neurons (Royer et al., 1999). The CeL and CeM receive GABAergic afferents from other structures (Le Gal LaSalle et al., 1978) and contain local GABA interneurons and GABAergic projection neurons (McDonald & Augustine, 1993; Pare & Smith, 1993) that may inhibit each other via axon collaterals (Pape & Pare, 2010). The CeL projects to the CeM, with no reciprocal projection from the CeM to the CeL (Pitkänen, 2000). The CeM is the major output nucleus of the amygdala and projects to regions that produce behavioral and physiologic responses to emotionally relevant events (Hopkins & Holstege, 1978; Pitkänen, 2000; Pape & Pare, 2010), although more recent data suggest that the CeL also sends GABAergic projections to behavioral and physiologic effector regions (Penzo et al., 2014).
The OXTRs are more selectively expressed in the CeL/C and activation of OXTRs increases neuronal excitability in the CeL/C, resulting in inhibition of the projection neurons in the CeM (Huber et al., 2005). Photoexcitation of OXT-releasing fibers from the hypothalamus to the CeL suppresses fear expression (Knobloch et al., 2012), likely by exciting CeL GABAergic neurons resulting in inhibition of the CeM GABAergic neurons projecting to the periaqueductal gray (Viviani et al., 2011). This may serve as the cellular mechanisms whereby OXT exerts anxiolytic effects. However, the ionic and signaling mechanisms underlying OXTR-mediated excitation of CeL neurons have not been determined. In the present study, we aimed at determining the ionic and signaling mechanisms whereby activation of OXTRs excites CeL neurons. Our results demonstrate that activation of OXTRs increased the neuronal excitability in the CeL by PLCβ and PKC-mediated inhibition of the inwardly rectifying K+ (Kir) channels, providing a cellular and molecular mechanism explaining the anxiolytic effects of OXT.
Materials and Methods
Ethical approval
All procedures and experiments presented in this study were approved by the Institutional Animal Use and Care Committee (IACUC) of the University of North Dakota and performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health, USA. All the experiments of the present study also comply with the policy and regulations on animal experimentation of The Journal of Physiology (Grundy, 2015).
Preparation of amygdala slices
Coronal brain slices (300 μm) were prepared from virgin male and female Sprague-Dawley rats (21–35 days old). After being deeply anesthetized with isoflurane, animals were decapitated and their brains were dissected out. After the trimming of the cerebellum, the caudal pole of the brain was glue to the plate of a vibrotome (Leica VT1200S) and then bathed in the solution that contained (in mM) 130 N-methyl-D-glucamine (NMDG)-Cl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 8.0 MgCl2, and 10 glucose, saturated with 95% O2 and 5% CO2 (pH 7.4, adjusted with HCl). Cuttings were made from the rostral pole of the brain and slices were collected from both hemispheres when the structure of amygdala appeared. Slices were incubated in the above solution except NMDG-Cl was replaced with NaCl at 35°C for 1 hr for recovery and then kept at room temperature until use. All animal procedures conformed to the guidelines approved by the University of North Dakota Animal Care and Use Committee.
Recordings of action potentials, resting membrane potentials and holding currents from amygdala neurons
Whole-cell patch-clamp recordings using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA) in current- or voltage-clamp mode were made at room temperature from the neurons in the CeL visually identified with infrared video microscopy (Olympus BX51WI) and differential interference contrast optics. The recording electrodes were filled with (in mM) 100 K+-gluconate, 8 KCl, 2 MgCl2, 40 HEPES, 0.6 EGTA, 2 ATPNa2, 0.4 GTPNa, and 7 phosphocreatine (pH 7.3), unless stated otherwise. The extracellular solution comprised (in mM) 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 2.5 CaCl2, 1.5 MgCl2 and 10 glucose, saturated with 95% O2 and 5% CO2 (pH 7.4). Data were filtered at 2 kHz, digitized at 10 kHz, acquired on-line and analyzed after-line using pCLAMP 10.4 software (Molecular Devices, Sunnyvale, CA). For the recordings of action potentials (APs), the above extracellular solution was supplemented with kynurenic acid (1 mM) to block glutamatergic transmission and picrotoxin (100 μM) to block GABAergic transmission. The specific OXTR agonist, [Thr4,Gly7]-oxytocin (TGOT, 0.3 μM), was dissolved in the extracellular solution and bath applied to the slices. The rationale that we used TGOT instead of OXT for experiments was to selectively activate OXTRs avoiding OXT-mediated co-activation of V1A receptors (Huber et al., 2005). To prevent potential desensitization induced by repeated applications of the agonist, one slice was limited to only one application of TGOT. Frequency of APs was calculated by Mini Analysis 6.0.1 (Synaptosoft Inc., Decatur, GA), as described previously (Li et al., 2019). Resting membrane potentials (RMPs) and holding currents (HCs) at −60 mV were recorded in the extracellular solution supplemented with tetrodotoxin (TTX, 0.5 μM), kynurenic acid (1 mM) and picrotoxin (100 μM). I-V curves were obtained by using a ramp protocol from −120 mV to −50 mV at a speed of 0.045 mV/ms. We compared the I-V curves recorded before and after the application of TGOT at its maximal effect.
Recordings of Kir currents from CeL neurons
The above-mentioned K+-gluconate-containing intracellular solution was used to record Kir currents from CeL neurons. CaCl2 in the extracellular solution was replaced by the same concentration of MgCl2 and TTX (0.5 μM) was supplemented in the extracellular solution to annul the contaminations of voltage-gated Ca2+ and Na+ channel currents, respectively. Cells were held at −60 mV and stepped from −140 mV to −40 mV for 400 ms at a voltage interval of 10 mV every 10 s. Steady-state currents were measured within 5 ms before the end of the step voltage protocols.
Data analysis
Data are presented as the means ± SD. Mann-Whitney test or Wilcoxon matched-pairs signed rank test (abbreviated as Wilcoxon test in the text) or Two-way ANOVA was used for statistical analysis as appropriate; P values are reported throughout the text and significance was set as P < 0.05.
Chemicals
The following chemicals were products of R&D Systems: [Thr4,Gly7]-oxytocin (TGOT), kynurenic acid, picrotoxin, GDP-β-S, U73122, U73343, heparin, thapsigargin, BAPTA, chelerythrine, bisindolylmaleimide II (Bis II), phorbol 12-myristate 13-acetate (PMA), tetrodotoxin (TTX), ML 133, ML 297, tertiapin-Q and tertiapin-LQ. Drugs were initially prepared in stock solution, aliquoted and stored at −20°C. For those chemicals which are only soluble in dimethyl sulfoxide (DMSO), the concentration of DMSO was less than 0.1%. This concentration of DMSO either in the recording pipettes or in the bath had no significant effects on neuronal activity.
Results
Activation of OXTRs increases neuronal excitability in the CeL
Whereas it has been shown that activation of OXTRs facilitates neuronal excitability in the CeL/C (Huber et al., 2005), the underlying ionic and signaling mechanisms are unknown. We therefore aimed at determining the ionic and signaling mechanisms whereby activation of OXTRs augments neuronal excitability in the CeL. Because the expression of OXTRs is restricted to CeL/C region (Huber et al., 2005), we limited our recordings in the CeL region (Fig. 1A). Previous work suggests that the neurons in CeL region in rats are electrophysiologically divided into three types: regular spiking (RS, ~54%), low-threshold bursting (LTB, ~34%) and late firing (LF, 12%) (Amano et al., 2012). We thus examined the effects of OXTR activation on the excitabilities of these neurons. Because the neurons in the CeL did not demonstrate spontaneous AP firing at their RMPs, we injected a persistent positive current to elevate the membrane potential just above the firing threshold to induce sparse AP firing. We examined 18 neurons among which 11 were RS, 5 were LTB and 2 were LF. Consistent with previous results (Huber et al., 2005), activation of OXTRs by bath application of the selective OXTR agonist, TGOT (0.3 μM), enhanced the firing frequency of APs recorded from each type of neurons (Fig. 1B–D). We therefore pooled these data together. On average, TGOT increased the AP firing frequency to 407 ± 239% of control (Control: 0.68 ± 0.48 Hz, TGOT: 2.22 ± 1.29 Hz, n = 18, P < 0.0001, Wilcoxon test, Fig. 1E), demonstrating that activation of OXTRs excites CeL neurons. While the TGOT-mediated increases in AP firing frequency were reversible in 3 out of the 18 cells recorded, the effect of TGOT averaged from the 18 cells after wash for 30 min was irreversible (Control: 0.68 ± 0.48 Hz, wash 30 min: 1.97 ± 1.49 Hz, n = 18, P = 0.001, Wilcoxon test, Fig. 1E). Because TGOT usually increased the AP firing frequency in folds, we set a criterion that cells showing an increase of AP firing frequency by 50% as responsive cells. By this criterion, 15 out of the 18 cells (~83%) were responsive to bath application of TGOT. We used 0.3 μM TGOT for the remaining experiments because this is a saturating concentration (Huber et al., 2005). TGOT-induced increases in neuronal excitability were not sex-dependent because it exerted equal effects (P = 0.27, Mann-Whitney test) on male (344 ± 173% of control, n = 10 cells) and female (470 ± 272% of control, n = 8 cells) virgin rats. We thus used both male and female virgin rats for the remaining experiments.
Figure 1. Activation of OXTRs increases AP firing frequency in CeL neurons.
A, Photo taken under a microscope from a coronal slice to show different components of amygdala. EC: external capsule; LA: lateral nucleus; BLA: basolateral nucleus; CeL: lateral central amygdala; CeM: medial central amygdala. B, Bath application of TGOT (0.3 μM) increased the firing frequency of APs recorded from a regular spiking (RS) neuron in the CeL region. Voltage responses (Up panel) were evoked by injection of currents at an interval of 10 pA from −30 pA to +70 pA (Middle panel). The firing frequency of APs was persistently increased by brief (5 min) application of TGOT (Low panel). C, Bath application of TGOT (0.3 μM) increased the firing frequency of APs recorded from a low-threshold bursting (LTB) neuron in the CeL region. Voltage responses (Up panel) were evoked by injection of currents at an interval of 10 pA from −30 pA to +10 pA (Middle panel). The firing frequency of APs was persistently increased by brief (5 min) application of TGOT (Low panel). D, Bath application of TGOT (0.3 μM) increased the firing frequency of APs recorded from a late firing (LF) neuron in the CeL region. Voltage responses (Up panel) were evoked by injection of currents at an interval of 10 pA from −30 pA to +40 pA (Middle panel). The firing frequency of APs was persistently increased by brief (5 min) application of TGOT (Low panel). E, Summarized time course of TGOT-induced enhancement of AP firing frequency recorded from 18 CeL neurons.
G proteins, PLCβ and PKC are required for OXTR-mediated increases in neuronal excitability in the CeL
We examined the signaling mechanisms whereby activation of OXTRs facilitates neuronal excitability in the CeL. The OXTRs are coupled to Gαq proteins resulting in activation of PLCβ which hydrolyzes PIP2 to generate IP3 to increase intracellular Ca2+ release and DAG to activate PKC (Vrachnis et al., 2011). We thus examined the roles of this pathway in OXTR-mediated facilitation of neuronal excitability in the CeL. Intracellular perfusion of the selective G protein inactivator, GDP-β-S (0.5 mM), blocked TGOT-induced augmentation of AP-firing frequency (Control: 0.58 ± 0.46 Hz, TGOT: 0.55 ± 0.54 Hz, 105 ± 91% of control, n = 14, P = 0.81, Wilcoxon test; F(1,30) = 16.98, P = 0.0003 vs. TGOT alone, Fig. 2A). This result demonstrates that the function of G proteins is required for OXTR-mediated facilitation of AP firing frequency.
Fig. 2. G proteins, PLCβ and PKC are involved in OXTR-mediated increases in neuronal excitability.
A, Intracellular dialysis of the G-protein inactivator, GDP-β-S (0.5 mM), via the recording pipettes, blocked TGOT-induced enhancement of AP firing frequency (n = 14). B, Pretreatment of slices with U73122 (5 μM) blocked TGOT-induced augmentation of AP firing (n = 15), whereas pretreatment of slices with U73343 (5 μM) failed to alter TGOT-induced augmentation of AP firing (n = 15) significantly. C, Intracellular application of the IP3 receptor blocker, heparin (2 mg/ml, n = 14), or the sarco-endoplasmic reticulum Ca2+-ATPases inhibitor, thapsigargin (10 μM, n = 14), failed to change significantly TGOT-induced facilitation of AP firing frequency. D, Intracellular application of ryanodine (100 μM) to inhibit ryanodine receptors, did not significantly alter TGOT-induced enhancement of AP firing frequency (n = 14), whereas dialysis of BAPTA (10 mM) via the recording pipettes blocked TGOT-elicited augmentation of AP firing frequency (n = 14). E, Substitution of extracellular Ca2+ with the same concentration of Mg2+ did not significantly alter TGOT-induced enhancement of AP firing frequency (n = 15). F, Pretreatment of slices with and continuous bath application of the selective PKC inhibitor, chelerythrine (10 μM), blocked TGOT-mediated increases in AP firing frequency (n = 15). G, Pretreatment of slices with and continuous bath application of Bis II (1 μM) blocked TGOT-induced increases in AP firing frequency (n = 15). H, Bath application of PMA (1 μM) significantly increased AP firing frequency (n = 11), whereas pretreatment of slices with and continuous bath application of Bis II (1 μM, n=12) or chelerythrine (10 μM, n = 10) blocked PMA-induced enhancement of AP firing frequency.
We determined whether PLCβ is involved in TGOT-induced augmentation of neuronal excitability. Slices were pretreated with the selective PLC inhibitor, U73122 (5 μM), for >2h. As a control, separate slices were similarly pretreated with the inactive analog, U73343 (5 μM). Under these circumstances, application of TGOT failed to significantly enhance AP firing frequency in slices pretreated with U73122 (Control: 0.63 ± 0.53 Hz, TGOT: 0.60 ± 0.80 Hz, 71 ± 77% of control, n = 15, P = 0.28, Wilcoxon test, Fig. 2B), but still significantly increased the AP firing frequency in slices pretreated with U73343 (Control: 0.48 ± 0.42 Hz, TGOT: 1.85 ± 1.13 Hz, 469 ± 245% of control, n = 15, P = 0.0001, Wilcoxon test, F(1,28) = 30.3, P < 0.0001 vs. U73122, Fig. 2B). These results demonstrate that the activity of PLCβ is required for OXTR-mediated excitation of neuronal excitability in the CeL.
We tested the roles of intracellular Ca2+ release in TGOT-mediated facilitation of neuronal excitability. Application of the IP3 receptor inhibitor, heparin (2 mg/ml), via the recording pipettes, failed to significantly alter TGOT-induced enhancement of AP firing frequency (Control: 0.50 ± 0.31 Hz, TGOT: 2.24 ± 1.43 Hz, 499 ± 190% of control, n = 14, P = 0.0001, Wilcoxon test; F(1,30) = 2.59, P = 0.12 vs. TGOT alone, Fig. 2C), demonstrating that IP3 receptors are not required for TGOT-mediated facilitation of neuronal excitability. Likewise, intracellular application of the sarco-endoplasmic reticulum Ca2+-ATPase inhibitor, thapsigargin (10 μM), via the recording pipettes did not significantly change TGOT-mediated facilitation of AP firing frequency (Control: 0.68 ± 0.49 Hz, TGOT: 1.81 ± 0.77 Hz, 393 ± 306% of control, n = 14, P = 0.0006, Wilcoxon test; F(1,30) = 0.37, P = 0.55 vs. TGOT alone, Fig. 2C). We also probed a potential role of Ca2+ released from ryanodine-store by intracellular dialysis of ryanodine at 100 μM to inhibit the ryanodine receptors. Application of ryanodine did not significantly alter TGOT-elicited augmentation of AP firing frequency (Control: 0.37 ± 0.26 Hz, TGOT: 1.88 ± 1.47 Hz, 519 ± 308% of control, n = 14, P = 0.0004, Wilcoxon test; F(1,30) = 0.56, P = 0.46 vs. TGOT alone, Fig. 2D). These results demonstrate that intracellular Ca2+ release is not required for TGOT-induced augmentation of AP firing frequency. However, dialysis of BAPTA (10 mM) via the recording pipettes to chelate intracellular Ca2+ blocked TGOT-elicited augmentation of AP firing frequency (Control: 0.67 ± 0.58 Hz, TGOT: 0.70 ± 0.53 Hz, 127 ± 63% of control, n = 14, P = 0.46, Wilcoxon test; F(1,30) = 14.96, P = 0.0005 vs. TGOT alone, Fig. 2D). One explanation for these results is that TGOT-induced excitation of CeL neurons is not triggered by Ca2+ released from individual Ca2+ stores. However, BAPTA lowered the basal intracellular Ca2+ level, which may be required for the functions of Ca2+-dependent signaling molecules such as PKC (see below). We further examined the roles of extracellular Ca2+ influx by replacing the extracellular Ca2+ with the same concentration of Mg2+. In this situation, application of TGOT still induced a comparable increase of AP firing frequency (Control: 0.64 ± 0.51 Hz, TGOT: 2.30 ± 1.19 Hz, 464 ± 250% of control, n = 15, P < 0.0001, Wilcoxon test; F(1,31) = 1.01, P = 0.32 vs. TGOT alone, Fig. 2E), excluding a role of extracellular Ca2+ influx in TGOT-mediated enhancement of AP firing frequency.
We further tested the roles of PKC in OXTR-mediated excitation of CeL neurons. Slices were pretreated with the selective PKC inhibitor, chelerythrine (10 μM), for >2 h, and the extracellular solution continuously contained the same concentration of chelerythrine. Under these circumstances, application of TGOT did not significantly increase the AP firing frequency (Control: 0.83 ± 0.34 Hz, TGOT: 0.91 ± 0.79 Hz, 108 ± 79% of control, n = 15, P = 0.90, Wilcoxon test; F(1,31) = 17.10, P = 0.0003 vs. TGOT alone, Fig. 2F). Furthermore, pretreatment of slices with and continuous bath application of bisindolylmaleimide II (Bis II,1 μM), another PKC inhibitor, blocked TGOT-induced augmentation of AP firing frequency (Control, 0.63 ± 0.52 Hz, TGOT, 0.68 ± 0.48 Hz, 121 ± 45% of control, n = 15, P = 0.33, Wilcoxon test; F(1,31) = 17.52, P = 0.0002 vs. TGOT alone, Fig. 2G). Consistent with the requirement of PKC, bath application of the PKC activator, phorbol 12-myristate 13-acetate (PMA, 1 μM), significantly increased AP firing frequency in CeL neurons (Control: 0.61 ± 0.41 Hz, PMA: 1.30 ± 1.16 Hz, 225 ± 111% of control, n = 11, P = 0.019, Wilcoxon test, Fig. 2H). Pretreatment of slices with and continuous bath application of Bis II (1 μM) blocked PMA-mediated enhancement of AP firing frequency (Control: 0.42 ± 0.22 Hz, PMA: 0.51 ± 0.37 Hz, 122 ± 68% of control, n = 12, P = 0.27, Wilcoxon test; F(1,21) = 8.32, P = 0.009 vs. PMA alone, Fig. 2H). Furthermore, pretreatment of slices with chelerythrine (10 μM) blocked PMA-induced increase in AP firing frequency (Control: 0.64 ± 0.29 Hz, PMA: 0.89 ± 0.87 Hz, 137 ± 136% of control, n = 13, P = 0.45, Wilcoxon test; F(1,22) = 7.96, P = 0.01 vs. PMA alone, Fig. 2H), further demonstrating that the effect of PMA was mediated by activation of PKC. These results together indicate that PKC is required for OXTR-mediated enhancement of neuronal excitability in the CeL.
OXTR activation induces membrane depolarization, increases input resistance and time constants of CeL neurons
We then included TTX (0.5 μM) in the extracellular solution to block AP firing and recorded the RMPs in response to bath application of TGOT. Application of TGOT induced depolarization of CeL neurons (Control: −62.9 ± 3.5 mV, TGOT: −58.6 ± 6.2 mV, net depolarization: 4.3 ± 3.4 mV, n = 8, P = 0.008, Wilcoxon test, Fig. 3A1-A2) in current clamp. Pretreatment of slices with and continuous bath application of the selective OXTR antagonist, L-371,257 (1 μM) (Deing et al., 2013; Ali et al., 2019), blocked TGOT-mediated depolarization (L-371,257: −64.4 ± 3.7 mV, L-371,257 with TGOT: −64.1 ± 3.8 mV, net depolarization: 0.37 ± 1.15 mV, n = 14, P = 0.38, Wilcoxon test, Fig. 3A3). In voltage clamp, TGOT induced an inward current recorded at a holding potential of −60 mV (−11.9 ± 10.2 pA, n = 18, P < 0.0001, Wilcoxon test, Fig. 3B1-B2). These results suggest that activation of OXTRs facilitates neuronal excitability in the CeL by generating membrane depolarization.
Fig. 3. Activation of OXTRs induces membrane depolarization, increases the input resistance and enlarged membrane time constants.
A1-A3, Bath application of TGOT depolarized CeL neurons via activation of OXTRs. A1, RMP recorded from a CeL neuron before, during and after the application of TGOT. A2, Summary data for TGOT-induced depolarization (n=8). The empty circles represent the values from individual cells and the solid symbols are their averages. A3, Pretreatment of slices with and continuous bath application of the selective OXTR antagonist, L-371,257 (1 μM) blocked TGOT-induced depolarization (n = 14). B1-B2, Bath application of TGOT induced an inward current in voltage clamp. B1, Holding current recorded at −60 mV from a CeL neuron before, during and after the application of TGOT. B2, Summary net currents induced by TGOT recorded at −60 mV from 18 CeL neurons. Empty circles represent the values from individual cells and bar graph is their average. C1-C3, TGOT increased the input resistance (Rin). C1, Voltage responses evoked by injection of negative currents from −100 pA to −20 pA at an interval of 20 pA before (left) and during (right) the application of TGOT from the same cell. C2, Current-voltage relationship averaged from 6 cells. Rin was obtained by linear fitting of the current-voltage relationship. C3, Summary graph for Rin before and after the application of TGOT (n = 6). D1-D3, TGOT increased the time constants of CeL neurons. D1, Voltage response evoked by −100 pA before and after application of TGOT. D2, Expansion of the voltage transient shown in the box in D1 to demonstrate TGOT-induced augmentation of membrane time constants. D3, Summary graph for membrane time constants before and after the application of TGOT (n = 7).
We then measured the alterations of the input resistance (Rin) in response to TGOT application by injecting negative currents from −20 pA to −100 pA with 20 pA steps for a duration of 600 ms before and after the application of TGOT when the maximal effect was reached. We fit the current-voltage relationship with a linear function for each cell to obtain Rin, which equals the slope of the linear fitting (Fig. 3C1-C2). Application of TGOT significantly increased Rin (Control: 265 ± 92 MΩ, TGOT: 320 ± 101 MΩ, n = 6, P = 0.03, Wilcoxon test, Fig. 3C3), indicating that TGOT increases Rin. The membrane time constant obtained by fitting a single exponential function to the voltage transient (100 ms from the baseline) induced by −100 pA current step was significantly increased (Control: 26.7 ± 5.0 ms, TGOT: 35.8 ± 9.4 ms, n = 7, P = 0.016, Wilcoxon test, Fig. 3D1-D3).
OXTR-induced increases in neuronal excitability are mediated by inhibiting inwardly rectifying K+ channels
We determined the ionic mechanisms whereby OXTR activation depolarizes neurons in the CeL by measuring the reversal potential of the currents generated by TGOT. Because the effect of TGOT was independent of extracellular Ca2+ (Fig. 2E), we substituted Ca2+ with the same concentration of Mg2+ to avoid the contamination of voltage-gated Ca2+ currents and supplemented TTX (0.5 μM) in the extracellular solution to block voltage-gated Na+ currents. We used a ramp protocol (from −120 mV to −50 mV) to measure the I-V curve before and after the application of TGOT at its maximal effect. The TGOT-elicited net currents showed an inward rectification with a reversal potential of −90.8 ± 5.7 mV (n = 6, Fig. 4A1-A2), close to the calculated K+ reversal potential (–89.6 mV), suggesting that activation of OXTRs depolarizes CeL neurons by closing an inwardly rectifying K+ (Kir) channel.
Fig. 4. OXTR activation excites CeL neurons via inhibition of inwardly rectifying K+ (Kir) channels.
A1-A2, TGOT-elicited inhibition of Kir channel currents revealed by a voltage-ramp protocol. A1, I-V curve elicited by the ramp protocol before and after the application of TGOT (n = 6). A2, I-V curve of the net current induced by TGOT by subtracting the traces recorded in control condition (IControl) from those recorded after TGOT application (ITGOT) (n = 6). B1-B4, TGOT-induced inhibition of Kir channels revealed by a voltage-step protocol. B1, The voltage-step protocol used. Cells were held at −60 mV and stepped from −140 mV to −40 mV for 400 ms at a voltage interval of 10 mV in every 10 s. B2, Currents elicited by the voltage-step protocol before (left) and after (middle) bath application of TGOT and the net current obtained by subtraction (right) from a CeL neuron. B3, I-V curve averaged from 13 cells before and after application of TGOT. ** P<0.01 by Wilcoxon test. B4, I-V curve of the net current obtained by subtracting the currents in control condition from those after application of TGOT (n = 13).
Our result that TGOT-induced inward current recorded at −60 mV which is close to the RMP is small (Fig. 3B1-B2) also supports the involvement of Kir channels because the voltage dependence of the Kir channels would allow more currents to pass through the channels at negative potentials than at potentials positive to the K+ reversal potential. We therefore further characterized the effects of TGOT on the Kir channel currents recorded from the CeL neurons at different voltages. Cells were held at −60 mV and stepped from −140 mV to −40 mV for 400 ms at a voltage interval of 10 mV in every 10 s (Fig. 4B1). Steady-state currents were measured within 5 ms before the end of the step voltage protocols. Under these circumstances, the currents recorded before and after the application of TGOT showed inward rectification (n = 13, Fig. 4B2-B3), suggesting that Kir channels are expressed in the CeL neurons. Subtraction of the I-V curve in control condition from that after the application of TGOT demonstrated an inwardly rectified I-V curve (n = 13, Fig. 4B4), further supporting the notion that activation of OXTRs excites CeL neurons by inhibiting Kir channels.
Because micromolar concentration of Ba2+ (100–300 μM) has been shown to block Kir channels by at least 80% (Lacey et al., 1988; Hu et al., 2017; Li et al., 2019), we included 300 μM Ba2+ in the extracellular solution to inhibit Kir channels. Bath application of 300 μM Ba2+ indeed inhibited a current showing inward rectification (n = 14, Fig. 5A1-A3), suggesting that Ba2+ at this concentration inhibited Kir channels. In the presence of Ba2+, application of TGOT failed to elicit more currents significantly (n = 14, Fig. 5A4-A6), demonstrating that TGOT-induced excitation of CeL neurons is mediated by depression of Kir channels.
Fig. 5. TGOT-mediated inhibition of Kir channels is sensitive to micromolar concentration of Ba2+, but insensitive to ML 133.
A1-A6, Bath application of Ba2+ at 300 μM by itself inhibited Kir channel currents and blocked TGOT-elicited depression of Kir channel currents. A1, Currents evoked by the voltage-step protocol before (left) and during (middle) the application of 300 μM Ba2+ and the net current obtained by subtraction (right). A2, I-V curves of the currents elicited by the voltage-step protocol before and during the application of Ba2+ (n = 14). A3, Net currents obtained by subtracting the currents in control condition from those recorded from the same cells in the presence of Ba2+. Note that the Ba2+-sensitive currents showed inward rectification. A4, Currents recorded from a CeL cell in response to the voltage-step protocol in the presence of Ba2+ alone (left) and Ba2+ plus TGOT (middle) and the net current obtained by subtraction (right). A5, I-V curves of the currents elicited by the voltage-step protocol in the presence of Ba2+ alone and Ba2+ together with TGOT (n = 14). A6, Net currents obtained by subtracting the currents in the presence of Ba2+ alone from those recorded from the same cells in the presence of Ba2+ plus TGOT. There were no significant differences for the net currents at each voltage. B1-B6, Bath application of ML 133, a blocker for Kir2 subfamily, by itself inhibited Kir channel currents, but failed to alter TGOT-mediated inhibition of Kir currents significantly. B1, Currents recorded from a CeL cell in response to the voltage-step protocol before (left) and during (middle) the application of ML 133 (30 μM). The net currents inhibited by ML 133 are shown on the right. B2, I-V curves of the currents elicited by the voltage-step protocol before and during the application of ML 133 (n = 15). ** P<0.01, * P<0.05 by Wilcoxon test. B3, Net currents obtained by subtracting the currents in control condition from those recorded from the same cells in the presence of ML 133. Note that the ML 133-sensitive currents showed inward rectification. B4, Currents recorded from a CeL cell in response to the voltage-step protocol in the presence of ML 133 alone (left) and ML 133 plus TGOT (middle) and the net current obtained by subtraction (right). B5, I-V curves of the currents elicited by the voltage-step protocol in the presence of ML 133 alone and ML 133 together with TGOT (n = 15). ** P<0.01, * P<0.05 by Wilcoxon test. B6, Net currents obtained by subtracting the currents in the presence of ML 133 alone from those recorded from the same cells in the presence of ML 133 plus TGOT. Net currents obtained by subtracting the currents in control condition from those recorded from the same cells in the presence of TGOT were co-plotted as a comparison.
We further characterized the subtypes of the Kir channels involved in TGOT-induced facilitation of neuronal excitability. There are seven Kir channel subfamilies that can be classified into four functional groups: i) Kir2 subfamily including Kir2.1, Kir2.2, Kir2.3 and Kir2.4 form the classical Kir channels and are constitutively active; ii) Kir3 subfamily comprising Kir3.1 (GIRK1), Kir3.2 (GIRK2), Kir3.3 (GIRK3) and Kir3.4 (GIRK4) encoded by the genes KCNJ3, KCNJ6, KCNJ9, and KCNJ5, respectively, constitute the G protein-gated GIRK channels; iii) Kir6 subfamily encompassing Kir6.1 and Kir6.2 form the ATP-sensitive K+ (KATP) channels; iv) K+ transport channels include Kir1.1, Kir4.1, Kir4.2 and Kir7.1 (Hibino et al., 2010). KATP channels are unlikely to be involved because our recording solution in the pipettes contained 2 mM ATP which blocked KATP channels. We used ML 133, a specific antagonist for Kir2 subfamily (Wang et al., 2011; Kim et al., 2015; Ford & Baccei, 2016; Sonkusare et al., 2016; Huang et al., 2018) to test the roles of the Kir2 subfamily in TGOT-elicited depression of Kir channels. Bath application of ML 133 (30 μM) by itself induced a significant inhibition of Kir currents at −50 mV and −60 mV and from −100 mV to −140 mV (n = 15, Fig. 5B1-B3). In the presence of ML 133, application of TGOT still significantly depressed Kir currents at all the voltages except at −70 mV and −80 mV (n = 15, Fig. 5B4-B5). The TGOT-elicited depression of Kir currents in the presence of ML 133 was not significantly different from that induced by application of TGOT alone (n = 15, F(1,26) = 1.95, P = 0.18, Fig. 5B6). These results suggest that Kir2 subfamily are not involved in TGOT-induced excitation of CeL neurons.
We further examined the roles of GIRK channels in TGOT-induced depression of Kir channels. ML 297 is an activator of GIRK1-containing channels (Kaufmann et al., 2013) and the expression of GIRK1 mRNA has been detected more prominently in the BLA and cortical nuclei with less expression in the CeA of the amygdala (DePaoli et al., 1994). Bath application of ML297 at 10 μM (a saturating concentration) failed to affect Kir currents significantly (n = 5, Fig. 6A1-A3), suggesting that there are no functional channels of GIRK1 in the CeL neurons and TGOT-elicited excitation of CeL neurons is unlikely to be mediated by interacting with GIRK1 subtype.
Fig. 6. GIRK channels are involved in TGOT-elicited depression of Kir channel currents.
A1-A3, Application of ML 297, an activator selective for GIRK1-containing channels, did not alter Kir currents. A1, Currents recorded from a CeL cell in response to the voltage-step protocol before (left) and during (middle) the application of ML 297 (10 μM) and the net current obtained by subtraction (right). A2, I-V curves of the currents elicited by the voltage-step protocol before and during the application of ML 297 (n = 5). A3, Net currents obtained by subtracting the currents in control condition from those recorded from the same cells in the presence of ML 297. Note that application of ML 297 did not significantly alter Kir currents at any voltage. B1-B6, Bath application of tertiapin-Q (TQ) by itself inhibited Kir channel currents, and significantly decreased TGOT-mediated inhibition of Kir currents. B1, Currents recorded from a CeL cell in response to the voltage-step protocol before (left) and during (middle) the application of TQ (250 nM) and the net current obtained by subtraction (right). B2, I-V curves of the currents elicited by the voltage-step protocol before and during the application of TQ (n = 15). ** P<0.01 by Wilcoxon test. B3, Net currents obtained by subtracting the currents in control condition from those recorded from the same cells in the presence of TQ. Note that the TQ-sensitive currents showed inward rectification. B4, Currents recorded from a CeL cell in response to the voltage-step protocol in the presence of TQ alone (left) and TQ plus TGOT (middle) and the net current obtained by subtraction (right). B5, I-V curves of the currents elicited by the voltage-step protocol in the presence of TQ alone and TQ together with TGOT (n = 15). ** P<0.01, * P<0.05 by Wilcoxon test. B6, Net currents obtained by subtracting the currents in the presence of TQ alone from those recorded from the same cells in the presence of TQ plus TGOT. Net currents obtained by subtracting the currents in control condition from those recorded from the same cells in the presence of TGOT were co-plotted as a comparison. ** P<0.01, * P<0.05 by Mann-Whitney test. C1-C6, Bath application of tertiapin-LQ (T-LQ) by itself did not alter Kir currents, and failed to significantly change TGOT-mediated inhibition of Kir currents. C1, Currents recorded from a CeL cell in response to the voltage-step protocol before (left) and during (middle) the application of T-LQ (100 nM) and the net current obtained by subtraction (right). C2, I-V curves of the currents elicited by the voltage-step protocol before and during the application of T-LQ (n = 14). C3, Net currents obtained by subtracting the currents in control condition from those recorded from the same cells in the presence of T-LQ. Note that T-LQ did not alter Kir currents at each voltage. C4, Currents recorded from a CeL cell in response to the voltage-step protocol in the presence of T-LQ alone (left) and T-LQ plus TGOT (middle) and the net current obtained by subtraction (right). C5, I-V curves of the currents elicited by the voltage-step protocol in the presence of T-LQ alone and T-LQ together with TGOT (n = 14). ** P<0.01, * P<0.05 by Wilcoxon test. C6, Net currents obtained by subtracting the currents in the presence of T-LQ alone from those recorded from the same cells in the presence of T-LQ plus TGOT. Net currents obtained by subtracting the currents in control condition from those recorded from the same cells in the presence of TGOT were co-plotted as a comparison. Note that application of T-LQ failed to alter TGOT-induced depression of Kir currents significantly.
Tertiapin-Q is an antagonist of GIRK (Ki = 13.3 nM) and Kir1.1 (Ki = 1.3 nM) (Jin et al., 1999; Jin & Lu, 1999). Bath application of tertiapin-Q (250 nM) by itself inhibited Kir channel currents at the voltage range from −90 mV to −140 mV (n = 15, Fig. 6B1-B3), demonstrating the functional expression of GIRK channels in the CeL neurons. Whereas application of TGOT in the presence of tertiapin-Q still significantly depressed the Kir currents at the voltage range from −100 to −140 mV (n = 15, Fig. 6B4-B5), the TGOT-induced depression of Kir currents in the presence of tertiapin-Q was significantly smaller at −50 mV and at the voltage range from −120 mV to −140 mV (n = 15), compared with the effect of TGOT in control condition (Fig. 6B6). These results suggest that a tertiapin-Q-sensitive Kir channel subtype contributes to TGOT-induced excitation of CeL neurons.
In addition to blocking GIRK channels, tertiapin-Q also inhibits Kir1.1 (Jin et al., 1999; Jin & Lu, 1999). One possibility is that the inhibitory effect of tertiapin-Q is mediated by inhibition of Kir1.1 channels, instead of blocking GIRK channels. We therefore tested the effects of tertiapin-LQ, a selective Kir1.1 channel blocker with no effects on GIRK channels (Ramu et al., 2008), on TGOT-induced inhibition of Kir currents. Bath application of tertiapin-LQ (100 nM) by itself had no significant effects on Kir currents (Fig. 6C1-C3, n = 14) and application of TGOT in the presence of tertiapin-LQ still significantly inhibited Kir currents at all the voltages except at −80 mV and −90 mV (n = 14, Fig. 6C4-C5). The TGOT-mediated inhibition in the presence of tertiapin-LQ was not significantly different from control (F(1,25) = 0.24, P = 0.63, Fig. 6C6), excluding the involvement of Kir1.1 channels. Taken together, these results demonstrate that activation of OXTRs excites CeL neurons by inhibiting GIRK channels.
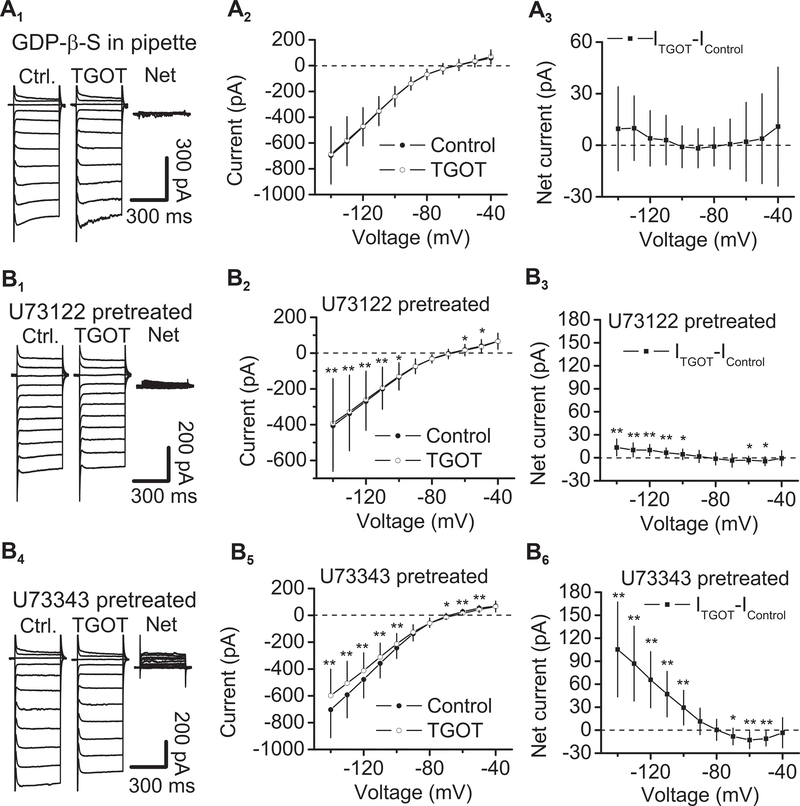
G proteins, PLCβ and PKC are required for OXTR-induced depression of Kir channels
If OXTR-elicited excitation of CeL neurons is mediated by inhibition of Kir channels, G protein, PLCβ and PKC should also be involved in TGOT-evoked depression of Kir currents. We further tested the roles of G proteins, PLCβ and PKC in OXTR-mediated inhibition of Kir currents. Intracellular perfusion of the G protein inactivator, GDP-β-S (0.5 mM), blocked TGOT-induced inhibition of Kir channels (n = 14, P > 0.05 at each voltage, Wilcoxon test, Fig. 7A1-A3). Furthermore, pretreatment of slices with U73122 (5 μM) significantly reduced TGOT-induced depression of Kir currents (n = 15, Fig. 7B1-B3), compared with the effect of TGOT in slices pretreated with the inactive analog, U73343 (5 μM, n = 14, F(1,27) = 21.71, P < 0.0001, Fig. 7B4-B6). These results demonstrate that the activity of PLC is required for OXTR-mediated depression of Kir channels in the CeL.
Fig. 7. G proteins and PLCβ are involved in OXTR-mediated depression of Kir channels.
A1-A3, Intracellular dialysis of GDP-β-S (0.5 mM) blocked TGOT-elicited depression of Kir currents. A1, Currents recorded from a CeL cell in response to the voltage-step protocol before (left) and during (middle) the application of TGOT in the presence of GDP-β-S and the net current obtained by subtraction (right). A2, I-V curves of the currents elicited by the voltage-step protocol before and during the application of TGOT (n = 14). A3, Net currents obtained by subtracting the currents in control condition from those recorded from the same cells during the application of TGOT in the intracellular solution containing GDP-β-S. Note that application of TGOT in the presence of GDP-β-S did not significantly alter Kir currents at each voltage. B1-B3, Pretreatment of slices with U73122 (5 μM), the selective PLC inhibitor, significantly reduced TGOT-elicited depression of Kir currents. B1, Currents recorded from a CeL cell in response to the voltage-step protocol before (left) and during (middle) the application of TGOT in a slice pretreated with U73122 and the net current obtained by subtraction (right). B2, I-V curves of the currents elicited by the voltage-step protocol before and during the application of TGOT (n = 15) in slices pretreated with U73122. ** P < 0.01, * P < 0.05 by Wilcoxon test. B3, Net currents obtained by subtracting the currents in control condition from those recorded from the same cells during the application of TGOT in slices pretreated with U73122. Note that pretreatment of slices with U73122 significantly reduced TGOT-elicited depression of Kir currents. B4-B6, Application of TGOT in slices pretreated with U73343 (5 μM), the inactive analog, induced a comparable depression of Kir currents. B4, Currents recorded from a CeL cell in response to the voltage-step protocol before (left) and during (middle) the application of TGOT in a slice pretreated with U73343 and the net current obtained by subtraction (right). B5, I-V curves of the currents elicited by the voltage-step protocol before and during the application of TGOT (n = 14) in slices pretreated with U73343. ** P < 0.01, * P < 0.05 by Wilcoxon test. B6, Net currents obtained by subtracting the currents in control condition from those recorded from the same cells during the application of TGOT in slices pretreated with U73343. Note that application of TGOT induced a comparable suppression in slices pretreated with U73343.
We further probed the roles of PKC in OXTR-mediated inhibition of Kir channels. Slices were pretreated with the selective PKC inhibitor, Bis II (1 μM), and the extracellular solution continuously contained the same concentration of Bis II. Bath application of TGOT in this condition did not significantly alter Kir channel currents except at −130 mV and −140 mV (n = 14, Fig. 8A1-A2). Compared with the effect of TGOT in control condition, TGOT-elicited inhibition of Kir currents in slices treated with Bis II was significantly smaller (F(1,25) = 11.55, P = 0.002 vs. TGOT alone, Fig. 8A3), demonstrating that PKC is required for TGOT-induced depression of Kir channels. Likewise, bath application of PMA (1 μM) significantly inhibited Kir currents (n = 7, Fig. 8B1-B3) and pretreatment of slices with Bis II (1 μM) blocked PMA-induced inhibition of Kir currents (n = 6, Fig. 8C1-C3), further supporting the roles of PKC in TGOT-mediated depression of Kir currents. We also tested the effect of intracellular Ca2+ level on TGOT-mediated depression of Kir currents. Intracellular perfusion of BAPTA (10 mM) significantly reduced TGOT-induced depression of Kir currents (n = 14, F(1,25) = 8.61, P = 0.007 vs. TGOT alone, Fig. 8D1-D3), suggesting that the Ca2+-dependent PKC is involved in TGOT-mediated depression of Kir channels.
Fig. 8. PKC is involved in TGOT-induced depression of Kir channels.
A1-A3, Pretreatment of slices with and continuous bath application of Bis II (1 μM), the selective PKC inhibitor, blocked TGOT-elicited depression of Kir currents at all the voltages except at −130 mV and −140 mV. A1, Currents recorded from a CeL cell in response to the voltage-step protocol before (left) and during (middle) the application of TGOT in a slice treated with Bis II and the net current obtained by subtraction (right). A2, I-V curves of the currents elicited by the voltage-step protocol before and during the application of TGOT (n = 14) in slices treated with Bis II. Note that treatment of slices with Bis II blocked TGOT-induced depression of Kir currents at all the voltages except at −130 mV and −140 mV. ** P < 0.01, * P <0.05 by Wilcoxon test. A3, Net currents obtained by subtracting the currents in control condition from those recorded from the same cells during the application of TGOT in slices treated with Bis II. Net currents obtained by subtracting the currents in control condition from those recorded from the same cells in the presence of TGOT were co-plotted as a comparison. * P < 0.05, **P < 0.01 by Mann-Whitney test. B1-B3, Bath application of a PKC activator, PMA (1 μM) inhibited Kir currents. B1, Currents recorded from a CeL cell in response to the voltage-step protocol before (left) and during (middle) the application of PMA and the net current obtained by subtraction (right). B2, I-V curves of the currents elicited by the voltage-step protocol before and during the application of PMA (n = 7). Note that bath application of PMA significantly depressed Kir currents. * P < 0.05 by Wilcoxon test. B3, Net currents obtained by subtracting the currents in control condition from those recorded from the same cells during the application of PMA. C1-C3, Pretreatment of slices with and continuous bath application of Bis II (1 μM), the selective PKC inhibitor, blocked PMA-elicited depression of Kir currents. C1, Currents recorded from a CeL cell in response to the voltage-step protocol before (left) and during (middle) the application of TGOT in a slice treated with Bis II and the net current obtained by subtraction (right). C2, I-V curves of the currents elicited by the voltage-step protocol before and during the application of PMA (n = 6) in slices treated with Bis II. C3, Net currents obtained by subtracting the currents in control condition from those recorded from the same cells during the application of PMA in slices treated with Bis II. D1-D3, Intracellular dialysis of BAPTA (10 mM) significantly reduced TGOT-induced depression of Kir currents. D1, Currents recorded from a CeL cell in response to the voltage-step protocol before (left) and during (middle) the application of TGOT in intracellular solution containing BAPTA and the net current obtained by subtraction (right). D2, I-V curves of the currents elicited by the voltage-step protocol before and during the application of TGOT (n = 14) in intracellular solution containing BAPTA. ** P < 0.01, * P < 0.05 by Wilcoxon test. D3, Net currents obtained by subtracting the currents in control condition from those recorded from the same cells during the application of TGOT in intracellular solution containing BAPTA. Net currents obtained by subtracting the currents in control condition from those recorded from the same cells in the presence of TGOT were co-plotted as a comparison. ** P < 0.01, * P < 0.05 by Mann-Whitney test.
Discussion
Our results demonstrate that activation of OXTRs excites CeL neurons by inhibition of Kir channels, likely GIRK channels. OXTR-elicited facilitation of neuronal excitability and depression of Kir channels are G-protein-dependent and mediated by activation of PLCβ and PKC. Our results provide a signaling and ionic mechanism to explain the anxiolytic effects of OXT.
Ionic mechanisms underlying OXTR-mediated excitation of CeL neurons
Activation of OXTRs has been shown to increase neuronal excitability by distinct ionic mechanisms depending on neuronal types. In the hippocampus, activation of OXTRs inhibits KCNQ-based M channels to depolarize CA2 pyramidal neurons (Tirko et al., 2018), but up-modulates L-type Ca2+ channels to excite CA1 GABAergic interneurons (Maniezzi et al., 2019). In ventral tegmental area and interfascicular nucleus of mouse midbrain, OXT increases neuronal excitability by activating a nonselective cation channel and Na+-Ca2+ exchanger (Tang et al., 2014). TRPC-like channels are targets of OXT-elicited excitation of tuberoinfundibular dopamine neurons (Briffaud et al., 2015). OXT also depolarizes substantia gelatinosa neurons of adult rat spinal cord by inhibiting a K+ conductance and opening a cationic conductance (Jiang et al., 2014). Whereas activation of OXTRs coupled to Gq/11 results in inhibition of Kir channels in the immortalized gonadotropin-releasing hormone-positive GN11 cell line (Gravati et al., 2010), the roles of Kir channels in OXTR-elicited augmentation of neuronal excitability in the brain have not been determined. Our results demonstrate that activation of OXTRs depolarizes CeL neurons by inhibition of Kir channels based on the following findings. First, activation of OXTRs increases the input resistance of the recorded CeL neurons suggesting a reduction of ionic conductance. Second, the reversal potential of TGOT-sensitive currents is close to the K+ reversal potential. Third, the I-V curve of the TGOT-sensitive currents is inwardly rectified. Fourth, the TGOT-sensitive currents are sensitive to micromolar concentration of Ba2+. Kir channels are divided into four functional groups, i.e., Kir2 subfamily (Kir2.1, Kir2.2, Kir2.3, Kir2.4), Kir3 subfamily (GIRK channels, Kir3.1, Kir3.2, Kir3.3, Kir3.4,), Kir6 subfamily (KATP channels, Kir6.1, Kir6.2), K+ transport channels (Kir1.1, Kir4.1, Kir4.2, Kir7.1) (Hibino et al., 2010). Kir6 subfamily (KATP channels) are unlikely to be responsible for TGOT-induced excitation of CeL neurons because the intracellular solution in the recording pipettes contained 2 mM ATP which could have inhibited KATP channels. ML 133 is a specific antagonist for Kir2 subfamily (Wang et al., 2011; Kim et al., 2015; Ford & Baccei, 2016; Sonkusare et al., 2016; Huang et al., 2018). Whereas application of ML 133 at a saturating concentration (30 μM) depressed Kir currents suggesting the functional expression of Kir2 subfamily in the CeL neurons, TGOT-elicited depression of Kir currents was not significantly altered in the presence of ML 133. These results demonstrate that Kir2 subfamily are unlikely to be involved in OXTR-elicited excitation of CeL neurons. Tertiapin-Q is an antagonist of GIRK and Kir1.1 channels (Jin et al., 1999; Jin & Lu, 1999). Our results demonstrate that application of tertiapin-Q significantly reduced TGOT-induced suppression of Kir currents, but application of the selective Kir1.1 channel blocker, tertiapin-LQ (Ramu et al., 2008) did not change Kir currents by itself and failed to alter TGOT-mediated inhibition of Kir currents. These results together indicate the involvement of GIRK channels.
If GIRK channels are involved in TGOT-mediated excitation of CeL neurons, GIRK channels must be constitutively active. Consistent with this prerequisite, GIRK channels are constitutively active (Chen & Johnston, 2005; Gonzalez et al., 2018). GIRK channels comprise 4 subunits including GIRK1, GIRK2, GIRK3 and GIRK4. GIRK channels are widely expressed in the brain, existing as predominantly heterotetramers of GIRK1, GIRK2 and/or GIRK3, or as homotetramers of the GIRK2 subunit (Hibino et al., 2010; Luscher & Slesinger, 2010). The expression of GIRK4 is restricted to some neuronal populations, such as Purkinje cells and neurons of the globus pallidus and the ventral pallidum (Murer et al., 1997). However, GIRK4 is not expressed in the amygdala (Murer et al., 1997). The following two lines of evidence do not support the involvement of GIRK1. First, the expression of GIRK1 mRNA has been detected more prominently in the BLA and cortical nuclei with less expression in the CeA of the amygdala (DePaoli et al., 1994). Second, application of ML 297, an activator selective for GIRK1-containing channels, failed to alter Kir currents significantly in the CeL neurons, suggesting that there is no functional GIRK1 in the CeL neurons. Therefore, the most plausible subunits underlying TGOT-induced excitation of CeL neurons are GIRK2 and GIRK3, which exist as GIRK2/GIRK3 heterotetramers or GIRK2 homotetramers (Hibino et al., 2010; Luscher & Slesinger, 2010). Consistent with this speculation, GIRK2 channels have been detected in the amygdala (Murer et al., 1997).
Signaling mechanisms whereby activation of OXTRs excites CeL neurons
Whereas activation of OXTRs has been shown to excite CeL/C neurons (Huber et al., 2005), the signaling molecules downstream of OXTRs have not been determined. Because PLC is required for OXT-induced increases in neuronal excitability (Jiang et al., 2014; Tang et al., 2014; Tirko et al., 2018), we tested the roles of PLC in OXTR-elicited excitation of CeL neurons. Our results demonstrate that PLCβ is required for OXTR-mediated suppression of Kir channels and excitation of CeL neurons. Consistent with our results, activation of OXTRs results in inhibition of Kir channels via Gq/11 and PLC pathway in the immortalized gonadotropin-releasing hormone-positive GN11 cell line (Gravati et al., 2010). Activation of PLCβ hydrolyzes PIP2 to generate IP3 which activates IP3 receptors to increase intracellular Ca2 release and DAG to activate PKC. Our results demonstrate that intracellular Ca2+ release is unnecessary, whereas the activity of PKC is necessary, for OXTR-mediated excitation of CeL neurons and inhibition of Kir channels. These results are consistent with the involvement of GIRK channels because GIRK channels are inhibited by PKC-mediated phosphorylation (Stevens et al., 1999; Mao et al., 2004; Adney et al., 2015).
PKC is classified as classical/conventional PKC (cPKC) including PKCα, PKCβ and PKCγ, novel PKC (nPKC) containing PKCδ, PKCε, PKCη and PKCθ, and atypical PKC (aPKC) comprising PKCζ and PKCλ. cPKC is both Ca2+- and DAG-dependent, whereas aPKC is neither Ca2+- nor DAG-dependent. nPKC is Ca2+-independent, but DAG-dependent. Our results that application of PMA, a DAG analog, significantly depressed Kir currents but increased AP firing frequency, support the involvement of cPKC and nPKC. Furthermore, we showed that application of BAPTA via the recording pipettes to chelate intracellular Ca2+ blocked TGOT-elicited augmentation of AP firing and significantly attenuated TGOT-induced suppression of Kir currents, supporting the involvement of cPKC. Together, these results demonstrate a role of the cPKC including PKCα, PKCβ1, PKCβ2 and PKCγ. Consistent with our results, cPKC isoforms are involved in Gαq-induced inhibition of GIRK channels (Niemeyer et al., 2019). Further experiments will identify the PKC isoform(s) involved in OXTR-mediated depression of Kir channels and excitation of CeL neurons.
By using extracellular recordings, Huber et al. reported that application of TGOT increased spontaneous spike frequencies in 21% of the CeA neurons but decreased them in more than 50% of the cells recorded (Huber et al., 2005). In the present study, we set a criterion that cells showing more than 50% increase in AP firing frequency were classified as responsive neurons. Out of the 18 CeL neurons recorded, 15 cells (83%) were responsive neurons by this criterion, demonstrating a higher response rate of TGOT in our study. There are several explanations for the discrepancy of the response rate of TGOT. First, Huber et al. calculated the response rate by counting all the cells recorded from the whole CeA which includes CeC, CeL and CeM, whereas we limited our recordings in the CeL. Because OXTRs are selectively expressed in CeL, including the numbers of cells recorded from the whole CeA likely diluted the response rate of TGOT. As demonstrated in their work, TGOT excited CeL neurons but depressed CeM neurons. Because the expression of OXTRs is limited to the CeL which exerts GABAergic inhibition on CeM, the TGOT-elicited depression of CeM neurons was an indirect effect of TGOT-induced excitation of CeL neurons. Because their results showed that more than 50% of the cells are inhibited by TGOT, it is reasonable to speculate that more than 50% of the cells used to calculate the response rate of TGOT were from CeM where OXTRs are not expressed. Thus, the actual response rate of TGOT should be much higher than that reported. Second, we included kynurenic acid and picrotoxin in the extracellular solution to block synaptic transmission and excluded any potential indirect effects of OXTR activation on CeL neuronal excitability. Nonetheless, a potential caveat of the present study is that a moderate to high response rate of TGOT in CeL neurons could still have a weakening effect on the conclusions drawn from the experiments of using the pharmacological blockers for the signaling targets of OXTRs. For example, the blocking effects of the inhibitors could be derived from the deficiency of OXTRs in the recorded cells, not the authentic actions of the inhibitors. We addressed this issue by using a relatively large sample size of cell numbers for each inhibitor. More powerful approaches such as utilization of knockout mice and gene knockdown to identify the composition of Kir channels in the CeA and especially the roles of individual members of the Kir channel family and PKC isoforms in OXTR-mediated facilitation of neuronal excitability in the CeL are still appreciated.
Physiological significances
The CeA is actively involved in fear learning and expression. The CeL, which is composed of several classes of GABA-producing inhibitory neurons (Cassell & Gray, 1989; Cassell et al., 1999; Ehrlich et al., 2009; Haubensak et al., 2010), gates fear expression by tonically inhibiting the CeM (Ciocchi et al., 2010), the major output of amygdala (LeDoux et al., 1988). Pharmacological inactivation of CeA (Goosens & Maren, 2003; Wilensky et al., 2006) or specific inactivation of the CeL (Ciocchi et al., 2010) during conditioning blocks the formation of fear memory, whereas fear conditioning induces changes in CeL neuronal activity such that a population of cells (CeLon) becomes excited, whereas another (CeLoff) is inhibited in response to the conditioned stimulus (Ciocchi et al., 2010; Haubensak et al., 2010; Duvarci et al., 2011). Photoexcitation of OXT-releasing fibers from the hypothalamus to the CeL suppresses fear expression (Knobloch et al., 2012), likely by exciting CeL GABAergic neurons resulting in inhibition of the CeM GABAergic neurons projecting to the periaqueductal gray (Viviani et al., 2011). Whereas activation of OXTRs in the CeL/C has been shown to enhance neuronal excitability in these regions, resulting in inhibition of the projection neurons in the CeM (Huber et al., 2005), the ionic and signaling mechanisms downstream of OXTRs have not been determined. Our work filled this knowledge gap by demonstrating that activation of OXTRs augments PLCβ and possibly the Ca2+-dependent PKC activities, resulting in depression of Kir channels to facilitate neuronal excitability in the CeL. Because OXT modulates anxiety and fear responses in the amygdala, our results suggest that Kir channels, PLC and PKC could be potential targets for anxiety and fear. Consistent with our speculation, modulations of Kir channels (Pravetoni & Wickman, 2008; Wydeven et al., 2014; Victoria et al., 2016), PLC (McOmish et al., 2008; Xiao et al., 2012) and PKC (Bowers et al., 2000; Lesscher et al., 2008; Liu et al., 2014) have impact on anxiety and fear responses. Our results therefore likely provide a cellular and molecular mechanism to explain the anxiolytic effects of OXT.
Key Points Summary.
Activation of oxytocin receptors (OXTRs) facilitates neuronal excitability in rat lateral nucleus of central amygdala (CeL)
OXTR-induced excitation is mediated by inhibition of inwardly rectifying K+ (Kir) channels
PLCβ is necessary for OXTR-mediated excitation of CeL neurons and depression of Kir channels
OXTR-elicited depression of Kir channels and excitation of CeL neurons require the function of Ca2+-dependent PKC
Funding:
This work was supported by the National Institute Of General Medical Sciences (NIGMS) and National Institute Of Mental Health (NIMH) grant R01MH118258 to S.L.
Footnotes
Competing interests: The Authors declare no conflict of interest.
Data Availability Statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Adney SK, Ha J, Meng XY, Kawano T & Logothetis DE. (2015). A Critical Gating Switch at a Modulatory Site in Neuronal Kir3 Channels. J Neurosci 35, 14397–14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali II, Al-Salam S, Howarth FC & Shmygol A. (2019). Oxytocin induces intracellular Ca(2+) release in cardiac fibroblasts from neonatal rats. Cell Calcium 84, 102099. [DOI] [PubMed] [Google Scholar]
- Amano T, Amir A, Goswami S & Pare D. (2012). Morphology, PKCdelta expression, and synaptic responsiveness of different types of rat central lateral amygdala neurons. J Neurophysiol 108, 3196–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amico JA, Vollmer RR, Karam JR, Lee PR, Li X, Koenig JI & McCarthy MM. (2004). Centrally administered oxytocin elicits exaggerated grooming in oxytocin null mice. Pharmacol Biochem Behav 78, 333–339. [DOI] [PubMed] [Google Scholar]
- Argiolas A & Gessa GL. (1991). Central functions of oxytocin. Neurosci Biobehav Rev 15, 217–231. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Huang GF & Besson JM. (1992). Nucleus centralis of the amygdala and the globus pallidus ventralis: electrophysiological evidence for an involvement in pain processes. J Neurophysiol 68, 551–569. [DOI] [PubMed] [Google Scholar]
- Bielsky IF & Young LJ. (2004). Oxytocin, vasopressin, and social recognition in mammals. Peptides 25, 1565–1574. [DOI] [PubMed] [Google Scholar]
- Birnbaumer M. (2000). Vasopressin receptors. Trends Endocrinol Metab 11, 406–410. [DOI] [PubMed] [Google Scholar]
- Bowers BJ, Collins AC, Tritto T & Wehner JM. (2000). Mice lacking PKC gamma exhibit decreased anxiety. Behav Genet 30, 111–121. [DOI] [PubMed] [Google Scholar]
- Briffaud V, Williams P, Courty J & Broberger C. (2015). Excitation of tuberoinfundibular dopamine neurons by oxytocin: crosstalk in the control of lactation. J Neurosci 35, 4229–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs RM. (1978). Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Pathways to the limbic system, medulla oblongata and spinal cord. Cell Tissue Res 192, 423–435. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ & Shi C. (1999). The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci 877, 217–241. [DOI] [PubMed] [Google Scholar]
- Cassell MD & Gray TS. (1989). Morphology of peptide-immunoreactive neurons in the rat central nucleus of the amygdala. J Comp Neurol 281, 320–333. [DOI] [PubMed] [Google Scholar]
- Chen X & Johnston D. (2005). Constitutively active G-protein-gated inwardly rectifying K+ channels in dendrites of hippocampal CA1 pyramidal neurons. J Neurosci 25, 3787–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Muller C & Luthi A. (2010). Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468, 277–282. [DOI] [PubMed] [Google Scholar]
- Deing V, Roggenkamp D, Kuhnl J, Gruschka A, Stab F, Wenck H, Burkle A & Neufang G. (2013). Oxytocin modulates proliferation and stress responses of human skin cells: implications for atopic dermatitis. Exp Dermatol 22, 399–405. [DOI] [PubMed] [Google Scholar]
- Dejean C, Courtin J, Rozeske RR, Bonnet MC, Dousset V, Michelet T & Herry C. (2015). Neuronal Circuits for Fear Expression and Recovery: Recent Advances and Potential Therapeutic Strategies. Biol Psychiatry 78, 298–306. [DOI] [PubMed] [Google Scholar]
- DePaoli AM, Bell GI & Stoffel M. (1994). G protein-activated inwardly rectifying potassium channel (GIRK1/KGA) mRNA in adult rat heart and brain by in situ hybridization histochemistry. Mol Cell Neurosci 5, 515–522. [DOI] [PubMed] [Google Scholar]
- Duvarci S & Pare D. (2014). Amygdala microcircuits controlling learned fear. Neuron 82, 966–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Popa D & Pare D. (2011). Central amygdala activity during fear conditioning. J Neurosci 31, 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C & Luthi A. (2009). Amygdala inhibitory circuits and the control of fear memory. Neuron 62, 757–771. [DOI] [PubMed] [Google Scholar]
- Feldman R (2012). Oxytocin and social affiliation in humans. Horm Behav 61, 380–391. [DOI] [PubMed] [Google Scholar]
- Ford NC & Baccei ML. (2016). Inward-rectifying K(+) (Kir2) leak conductance dampens the excitability of lamina I projection neurons in the neonatal rat. Neuroscience 339, 502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA & Roberto M. (2015). The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry 77, 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JC, Epps SA, Markwardt SJ, Wadiche JI & Overstreet-Wadiche L. (2018). Constitutive and Synaptic Activation of GIRK Channels Differentiates Mature and Newborn Dentate Granule Cells. J Neurosci 38, 6513–6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA & Maren S. (2003). Pretraining NMDA receptor blockade in the basolateral complex, but not the central nucleus, of the amygdala prevents savings of conditional fear. Behav Neurosci 117, 738–750. [DOI] [PubMed] [Google Scholar]
- Gravati M, Busnelli M, Bulgheroni E, Reversi A, Spaiardi P, Parenti M, Toselli M & Chini B. (2010). Dual modulation of inward rectifier potassium currents in olfactory neuronal cells by promiscuous G protein coupling of the oxytocin receptor. J Neurochem 114, 1424–1435. [DOI] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A & Anderson DJ. (2010). Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468, 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorn J, Ang VT & Jenkins JS. (1985). Effects of lesions in the hypothalamic paraventricular, supraoptic and suprachiasmatic nuclei on vasopressin and oxytocin in rat brain and spinal cord. Brain Res 346, 51–57. [DOI] [PubMed] [Google Scholar]
- Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I & Kurachi Y. (2010). Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90, 291–366. [DOI] [PubMed] [Google Scholar]
- Hopkins DA & Holstege G. (1978). Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res 32, 529–547. [DOI] [PubMed] [Google Scholar]
- Hu B, Cilz NI & Lei S. (2017). Somatostatin depresses the excitability of subicular bursting cells: Roles of inward rectifier K(+) channels, KCNQ channels and Epac. Hippocampus 27, 971–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Lee SH, Lu H, Sanders KM & Koh SD. (2018). Molecular and functional characterization of inwardly rectifying K(+) currents in murine proximal colon. J Physiol 596, 379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D, Veinante P & Stoop R. (2005). Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 308, 245–248. [DOI] [PubMed] [Google Scholar]
- Janak PH & Tye KM. (2015). From circuits to behaviour in the amygdala. Nature 517, 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang CY, Fujita T & Kumamoto E. (2014). Synaptic modulation and inward current produced by oxytocin in substantia gelatinosa neurons of adult rat spinal cord slices. J Neurophysiol 111, 991–1007. [DOI] [PubMed] [Google Scholar]
- Jin W, Klem AM, Lewis JH & Lu Z. (1999). Mechanisms of inward-rectifier K+ channel inhibition by tertiapin-Q. Biochemistry 38, 14294–14301. [DOI] [PubMed] [Google Scholar]
- Jin W & Lu Z. (1999). Synthesis of a stable form of tertiapin: a high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry 38, 14286–14293. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE & LeDoux JE. (2011). Molecular mechanisms of fear learning and memory. Cell 147, 509–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurek B & Neumann ID. (2018). The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol Rev 98, 1805–1908. [DOI] [PubMed] [Google Scholar]
- Kaufmann K, Romaine I, Days E, Pascual C, Malik A, Yang L, Zou B, Du Y, Sliwoski G, Morrison RD, Denton J, Niswender CM, Daniels JS, Sulikowski GA, Xie XS, Lindsley CW & Weaver CD. (2013). ML297 (VU0456810), the first potent and selective activator of the GIRK potassium channel, displays antiepileptic properties in mice. ACS Chem Neurosci 4, 1278–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Jang JH, Lin H, Choi SW, Kim HR, Shin DH, Nam JH, Zhang YH & Kim SJ. (2015). Rise and Fall of Kir2.2 Current by TLR4 Signaling in Human Monocytes: PKC-Dependent Trafficking and PI3K-Mediated PIP2 Decrease. J Immunol 195, 3345–3354. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R & Grinevich V. (2012). Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73, 553–566. [DOI] [PubMed] [Google Scholar]
- Krettek JE & Price JL. (1978). A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol 178, 255–280. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB & North RA. (1988). On the potassium conductance increase activated by GABAB and dopamine D2 receptors in rat substantia nigra neurones. J Physiol 401, 437–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RE, Heil J, Ganten D, Hermann K, Rascher W & Unger T. (1983). Effects of lesions in the paraventricular nucleus of the hypothalamus on vasopressin and oxytocin contents in brainstem and spinal cord of rat. Brain Res 260, 326–329. [DOI] [PubMed] [Google Scholar]
- Le Gal LaSalle G, Paxinos G & Ben-Ari Y. (1978). Neurochemical mapping of GABAergic systems in the amygdaloid complex and bed nucleus of the stria terminalis. Brain Res 155, 397–403. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. (2000). Emotion circuits in the brain. Annu Rev Neurosci 23, 155–184. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A & Romanski LM. (1990). The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci 10, 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P & Reis DJ. (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 8, 2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher HM, McMahon T, Lasek AW, Chou WH, Connolly J, Kharazia V & Messing RO. (2008). Amygdala protein kinase C epsilon regulates corticotropin-releasing factor and anxiety-like behavior. Genes Brain Behav 7, 323–333. [DOI] [PubMed] [Google Scholar]
- Li H, Hu B, Zhang HP, Boyle CA & Lei S. (2019). Roles of K(+) and cation channels in ORL-1 receptor-mediated depression of neuronal excitability and epileptic activities in the medial entorhinal cortex. Neuropharmacology 151, 144–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Feng J & Wang JH. (2014). Protein kinase C is essential for kainate-induced anxiety-related behavior and glutamatergic synapse upregulation in prelimbic cortex. CNS Neurosci Ther 20, 982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C & Slesinger PA. (2010). Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci 11, 301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniezzi C, Talpo F, Spaiardi P, Toselli M & Biella G. (2019). Oxytocin Increases Phasic and Tonic GABAergic Transmission in CA1 Region of Mouse Hippocampus. Front Cell Neurosci 13, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Wang X, Chen F, Wang R, Rojas A, Shi Y, Piao H & Jiang C. (2004). Molecular basis for the inhibition of G protein-coupled inward rectifier K(+) channels by protein kinase C. Proc Natl Acad Sci U S A 101, 1087–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. (1985). Immunohistochemical identification of gamma-aminobutyric acid-containing neurons in the rat basolateral amygdala. Neurosci Lett 53, 203–207. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. (1998). Cortical pathways to the mammalian amygdala. Prog Neurobiol 55, 257–332. [DOI] [PubMed] [Google Scholar]
- McDonald AJ & Augustine JR. (1993). Localization of GABA-like immunoreactivity in the monkey amygdala. Neuroscience 52, 281–294. [DOI] [PubMed] [Google Scholar]
- McOmish CE, Burrows EL, Howard M & Hannan AJ. (2008). PLC-beta1 knockout mice as a model of disrupted cortical development and plasticity: behavioral endophenotypes and dysregulation of RGS4 gene expression. Hippocampus 18, 824–834. [DOI] [PubMed] [Google Scholar]
- Murer G, Adelbrecht C, Lauritzen I, Lesage F, Lazdunski M, Agid Y & Raisman-Vozari R. (1997). An immunocytochemical study on the distribution of two G-protein-gated inward rectifier potassium channels (GIRK2 and GIRK4) in the adult rat brain. Neuroscience 80, 345–357. [DOI] [PubMed] [Google Scholar]
- Neugebauer V. (2015). Amygdala pain mechanisms. Handb Exp Pharmacol 227, 261–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Galhardo V, Maione S & Mackey SC. (2009). Forebrain pain mechanisms. Brain Res Rev 60, 226–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC & Han JS. (2004). The amygdala and persistent pain. Neuroscientist 10, 221–234. [DOI] [PubMed] [Google Scholar]
- Neumann ID. (2008). Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol 20, 858–865. [DOI] [PubMed] [Google Scholar]
- Niemeyer A, Rinne A & Kienitz MC. (2019). Receptor-specific regulation of atrial GIRK channel activity by different Ca(2+)-dependent PKC isoforms. Cell Signal 64, 109418. [DOI] [PubMed] [Google Scholar]
- Nitecka L & Ben-Ari Y. (1987). Distribution of GABA-like immunoreactivity in the rat amygdaloid complex. J Comp Neurol 266, 45–55. [DOI] [PubMed] [Google Scholar]
- Pape HC & Pare D. (2010). Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90, 419–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D & Smith Y. (1993). Distribution of GABA immunoreactivity in the amygdaloid complex of the cat. Neuroscience 57, 1061–1076. [DOI] [PubMed] [Google Scholar]
- Penzo MA, Robert V & Li B. (2014). Fear conditioning potentiates synaptic transmission onto long-range projection neurons in the lateral subdivision of central amygdala. J Neurosci 34, 2432–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD. (2011). Learning and the motivation to eat: forebrain circuitry. Physiol Behav 104, 582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD. (2013). Forebrain networks and the control of feeding by environmental learned cues. Physiol Behav 121, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A (2000). Connectivity of the rat amygdaloid complex. Oxford University Press., New York. [Google Scholar]
- Pitkanen A, Stefanacci L, Farb CR, Go GG, LeDoux JE & Amaral DG. (1995). Intrinsic connections of the rat amygdaloid complex: projections originating in the lateral nucleus. J Comp Neurol 356, 288–310. [DOI] [PubMed] [Google Scholar]
- Pravetoni M & Wickman K. (2008). Behavioral characterization of mice lacking GIRK/Kir3 channel subunits. Genes Brain Behav 7, 523–531. [DOI] [PubMed] [Google Scholar]
- Ramu Y, Xu Y & Lu Z. (2008). Engineered specific and high-affinity inhibitor for a subtype of inward-rectifier K+ channels. Proc Natl Acad Sci U S A 105, 10774–10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ. (2010). Amygdala activity, fear, and anxiety: modulation by stress. Biol Psychiatry 67, 1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Martina M & Pare D. (1999). An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci 19, 10575–10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savander V, Go CG, LeDoux JE & Pitkanen A. (1995). Intrinsic connections of the rat amygdaloid complex: projections originating in the basal nucleus. J Comp Neurol 361, 345–368. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Shi L, Brunso-Bechtold JK & Weiner JL. (2008). Distinct mechanisms of ethanol potentiation of local and paracapsular GABAergic synapses in the rat basolateral amygdala. J Pharmacol Exp Ther 324, 251–260. [DOI] [PubMed] [Google Scholar]
- Smith CM & Lawrence AJ. (2018). Salt Appetite, and the Influence of Opioids. Neurochem Res 43, 12–18. [DOI] [PubMed] [Google Scholar]
- Song Z, McCann KE, McNeill JKt, Larkin TE 2nd, Huhman KL & Albers HE. (2014). Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology 50, 14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkusare SK, Dalsgaard T, Bonev AD & Nelson MT. (2016). Inward rectifier potassium (Kir2.1) channels as end-stage boosters of endothelium-dependent vasodilators. J Physiol 594, 3271–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens EB, Shah BS, Pinnock RD & Lee K. (1999). Bombesin receptors inhibit G protein-coupled inwardly rectifying K+ channels expressed in Xenopus oocytes through a protein kinase C-dependent pathway. Mol Pharmacol 55, 1020–1027. [PubMed] [Google Scholar]
- Tang Y, Chen Z, Tao H, Li C, Zhang X, Tang A & Liu Y. (2014). Oxytocin activation of neurons in ventral tegmental area and interfascicular nucleus of mouse midbrain. Neuropharmacology 77, 277–284. [DOI] [PubMed] [Google Scholar]
- Tirko NN, Eyring KW, Carcea I, Mitre M, Chao MV, Froemke RC & Tsien RW. (2018). Oxytocin Transforms Firing Mode of CA2 Hippocampal Neurons. Neuron 100, 593–608 e593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa K, Iga N, Lu YF, Moriwaki A, Matsushita M, Li ST, Miyamoto O, Itano T & Matsui H. (2003). Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci 6, 384–390. [DOI] [PubMed] [Google Scholar]
- Tully K, Li Y, Tsvetkov E & Bolshakov VY. (2007). Norepinephrine enables the induction of associative long-term potentiation at thalamo-amygdala synapses. Proc Natl Acad Sci U S A 104, 14146–14150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C & Deisseroth K. (2011). Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Yalcin I & Barrot M. (2013). The amygdala between sensation and affect: a role in pain. J Mol Psychiatry 1, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria NC, Marron Fernandez de Velasco E, Ostrovskaya O, Metzger S, Xia Z, Kotecki L, Benneyworth MA, Zink AN, Martemyanov KA & Wickman K. (2016). G Protein-Gated K(+) Channel Ablation in Forebrain Pyramidal Neurons Selectively Impairs Fear Learning. Biol Psychiatry 80, 796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, Magara F & Stoop R. (2011). Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science 333, 104–107. [DOI] [PubMed] [Google Scholar]
- Vrachnis N, Malamas FM, Sifakis S, Deligeoroglou E & Iliodromiti Z. (2011). The oxytocin-oxytocin receptor system and its antagonists as tocolytic agents. Int J Endocrinol 2011, 350546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HR, Wu M, Yu H, Long S, Stevens A, Engers DW, Sackin H, Daniels JS, Dawson ES, Hopkins CR, Lindsley CW, Li M & McManus OB. (2011). Selective inhibition of the K(ir)2 family of inward rectifier potassium channels by a small molecule probe: the discovery, SAR, and pharmacological characterization of ML133. ACS Chem Biol 6, 845–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP & LeDoux JE. (2006). Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci 26, 12387–12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wydeven N, Marron Fernandez de Velasco E, Du Y, Benneyworth MA, Hearing MC, Fischer RA, Thomas MJ, Weaver CD & Wickman K. (2014). Mechanisms underlying the activation of G-protein-gated inwardly rectifying K+ (GIRK) channels by the novel anxiolytic drug, ML297. Proc Natl Acad Sci U S A 111, 10755–10760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Jaiswal MK, Deng PY, Matsui T, Shin HS, Porter JE & Lei S. (2012). Requirement of phospholipase C and protein kinase C in cholecystokinin-mediated facilitation of NMDA channel function and anxiety-like behavior. Hippocampus 22, 1438–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanchi D, Depoorter A, Egloff L, Haller S, Mahlmann L, Lang UE, Drewe J, Beglinger C, Schmidt A & Borgwardt S. (2017). The impact of gut hormones on the neural circuit of appetite and satiety: A systematic review. Neurosci Biobehav Rev 80, 457–475. [DOI] [PubMed] [Google Scholar]
- Zik JB & Roberts DL. (2015). The many faces of oxytocin: implications for psychiatry. Psychiatry Res 226, 31–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.