Abstract
Background
The objective of this study was to explore racial/ethnic factors that may be associated with survival in patients with glioblastoma by querying the National Cancer Database (NCDB).
Methods
The NCDB was queried for patients diagnosed with glioblastoma between 2004 and 2014. Patient demographic variables included age at diagnosis, sex, race, ethnicity, Charlson–Deyo score, insurance status, and rural/urban/metropolitan location of zip code. Treatment variables included surgical treatment, extent of resection, chemotherapy, radiation therapy, type of radiation, and treatment facility type. Outcomes included 30-day readmission, 30- and 90-day mortality, and overall survival. Multivariable Cox regression analyses were performed to evaluate variables associated with race and overall survival.
Results
A total of 103 652 glioblastoma patients were identified. There was a difference in the proportion of patients for whom surgery was performed, as well as the proportion receiving radiation, when stratified by race (P < .001). Black non-Hispanics had the highest rates of unplanned readmission (7.6%) within 30 days (odds ratio [OR]: 1.39 compared to White non-Hispanics, P < .001). Asian non-Hispanics had the lowest 30- (3.2%) and 90-day mortality (9.8%) when compared to other races (OR: 0.52 compared to White non-Hispanics, P = .031). Compared to White non-Hispanics, we found Black non-Hispanics (hazard ratio [HR]: 0.88, P < .001), Asian non-Hispanics (HR: 0.72, P < .001), and Hispanics (HR: 0.69, P < .001) had longer overall survival.
Conclusions
Differences in treatment and outcomes exist between races. Further studies are needed to elucidate the etiology of these race-related disparities and to improve outcomes for all patients.
Keywords: glioblastoma, health disparities, overall survival, race, readmissions
Key Points.
Racial differences exist in mortality and readmissions for glioblastoma patients.
Black non-Hispanics had the highest rate of unplanned 30-day readmission (7.6%).
White non-Hispanics had the lowest median survival (9.03 months).
Importance of the Study.
We conducted this study to explore racial/ethnic factors that may be associated with survival in patients with glioblastoma by querying the National Cancer Database (NCDB). We found that racial differences do exist in 30- and 90-day readmissions and mortality rates, as well as overall medial survival. This study is a useful addition to the current literature in large part due to the comprehensive scope of NCDB, as it encompasses approximately 70% of all new cancer diagnoses in the United States. Our study fills a gap in current literature surrounding race/ethnicity in glioblastoma treatment and outcomes and calls for future work to be done to further understand the reasons behind disparities in glioblastoma patient outcomes.
Glioblastoma (GBM), a World Health Organization grade IV diffuse glioma of astrocytic lineage,1 is the most commonly diagnosed primary malignant brain tumor with approximately 11 833 new diagnoses per year in the United States.2 Most recent data show that treatment with a standard of care including resection, adjuvant temozolomide-based chemotherapy, and radiation yields 5-year and 10-year survival rates of 5.4% and 2.7%, respectively.3 In recent years, efforts have been refocused on identifying the epidemiologic factors that contribute to the diagnosis, implemented treatment strategies, and survivorship in cancer patients, including in GBM.2,4–11 Past studies have shown associations between GBM risk or survival and insurance type12–14 or socioeconomic status.15,16 The datasets utilized in these studies all have limitations that analysis with the US nationwide collected dataset would otherwise yield. The National Cancer Database (NCDB) is one such central registry containing compiled data from over 1500 diverse treatment centers and represents more than 70% of newly diagnosed cancer cases nationwide.17 It provides a more complete representation compared to both the population-based Surveillance, Epidemiology, and End Results (SEER) database, which represents only 28% of the US population,18 and National Inpatient Sample, which similarly represents only 20% of hospital admissions in the United States.19 Although prior work on GBM has shown differences in overall survival between races, no prior study has examined different aspects of care such as readmission rates.20 The purpose of this study is to utilize this complete dataset to better assess the role of race/ethnicity in differences in care (eg, treatment type, readmissions) and clinical outcomes for GBM patients.
Methods
The NCDB was queried for adults at least 18 years of age diagnosed with primary GBM between 2004 and 2014. International Classification of Diseases for Oncology, Third Edition (ICD-O-3)-Oncology codes included morphologic codes 9440, 9441, and 9442 and topographical codes C71.0–C71.9. Only patients with a histologically confirmed diagnosis of GBM were included. Baseline patient demographics, hospital characteristics, and treatment variables were compared and stratified by race/ethnicity. Patient demographic variables included age at diagnosis, sex, race (White, Black, Asian), ethnicity (Hispanic status [yes/no]), Charlson–Deyo score (identical to the Charlson Comorbidity), insurance status, and rural/urban/metropolitan location of zip code. Patients with reported race or ethnicities of “other” or “unknown” were excluded from this analysis. Hospital characteristics included treatment facility type: Community Cancer Program, Comprehensive Community Cancer Program, Academic/Research Program, and Integrated Network Cancer Program. Treatment and tumor characteristics included extent of surgical resection, unifocal or multifocal disease, type of radiation performed, and chemotherapy performed. Extent of surgical resection was categorized as no surgery, biopsy, subtotal resection, or gross total resection. Clinical outcomes examined included 30-day readmission, 30- and 90-day mortality, and overall survival. Exempt approval was obtained from the University Hospitals Institutional Review Board for this study.
Statistical Analyses
Descriptive statistics are presented and include means and standard deviations for continuous variables and frequency and proportions for categorical variables by race/ethnicity. Chi-squared tests were applied to test differences between racial and ethnic groups. Multivariable Cox regression analysis was performed to evaluate potential variables associated with the 4 defined outcomes by race and Hispanic status (ie, race and ethnicity). Additional covariables included in the multivariable analyses were facility type, age at diagnosis, sex, primary payer (Medicare and Medicaid are government insurances in which taxpayers represent the payer), urban/rural/metro, Charlson–Deyo score, surgical resection, unifocal/multifocal, radiation, and chemotherapy. Kaplan–Meier survival curves stratified by race and ethnicity were generated. Statistical significance was set at P value less than .05.
Results
Sociodemographic and treatment characteristics for the patient sample stratified by race and ethnicity (Hispanic status) are given in Table 1. A total of 103 652 patients diagnosed with GBM were identified (White non-Hispanic n = 90 709, Black non-Hispanic n = 5704, Asian n = 1843, and Hispanic n = 5396). White non-Hispanics were significantly older at the time of diagnosis when compared to other races/ethnicities. Significant differences were present in the rate of surgical resection (White non-Hispanics had the highest rate of gross total resection, 30.7%), radiation therapy (Asian non-Hispanics had the highest rate, 70.9%), and chemotherapy (White non-Hispanics had the highest rate, 65.8%).
Table 1.
Patient Sociodemographic and Treatment Characteristics, Glioblastoma, and National Cancer Database 2004–2014
| Characteristic | Overall, N = 93 477a | White Non-Hispanic, N = 81 900a | Black Non-Hispanic, N = 5124a | Asian Non-Hispanic, N = 1638a | Hispanic, N = 4815a | P valueb |
|---|---|---|---|---|---|---|
| Facility type | <.001 | |||||
| Academic/Research Program | 38 877 (44%) | 33 806 (43%) | 2422 (51%) | 774 (53%) | 1875 (43%) | |
| Community Cancer Program | 5262 (5.9%) | 4695 (6.0%) | 218 (4.6%) | 80 (5.4%) | 269 (6.2%) | |
| Comprehensive Community Cancer Program | 34 976 (39%) | 31 549 (40%) | 1416 (30%) | 497 (34%) | 1514 (35%) | |
| Integrated Network Cancer Program | 10 161 (11%) | 8723 (11%) | 660 (14%) | 117 (8.0%) | 661 (15%) | |
| Unknown | 4201 | 3127 | 408 | 170 | 496 | |
| Age | 64 (55–73) | 64 (56–73) | 60 (51–69) | 61 (50–70) | 60 (50–70) | <.001 |
| Sex | .001 | |||||
| Female | 39 748 (43%) | 34 796 (42%) | 2279 (44%) | 715 (44%) | 1958 (41%) | |
| Male | 53 729 (57%) | 47 104 (58%) | 2845 (56%) | 923 (56%) | 2857 (59%) | |
| Primary payor | <.001 | |||||
| Medicaid | 5279 (5.8%) | 3486 (4.4%) | 704 (14%) | 218 (14%) | 871 (19%) | |
| Medicare | 40 527 (44%) | 36 811 (46%) | 1810 (36%) | 480 (30%) | 1426 (31%) | |
| Not insured | 3433 (3.8%) | 2346 (2.9%) | 368 (7.4%) | 133 (8.3%) | 586 (13%) | |
| Other government | 1438 (1.6%) | 1245 (1.6%) | 130 (2.6%) | 19 (1.2%) | 44 (0.9%) | |
| Private insurance | 40 523 (44%) | 36 066 (45%) | 1989 (40%) | 756 (47%) | 1712 (37%) | |
| Unknown | 2277 | 1946 | 123 | 32 | 176 | |
| Urban/rural | <.001 | |||||
| Metro | 73 567 (82%) | 63 222 (81%) | 4424 (89%) | 1531 (98%) | 4390 (95%) | |
| Rural | 1727 (1.9%) | 1656 (2.1%) | 52 (1.1%) | 4 (0.3%) | 15 (0.3%) | |
| Urban | 14 075 (16%) | 13 354 (17%) | 471 (9.5%) | 31 (2.0%) | 219 (4.7%) | |
| Unknown | 4108 | 3668 | 177 | 72 | 191 | |
| Charlson–Deyo score | <.001 | |||||
| 0 | 67 094 (72%) | 59 159 (72%) | 3364 (66%) | 1188 (73%) | 3383 (70%) | |
| 1 | 16 055 (17%) | 13 898 (17%) | 1050 (20%) | 257 (16%) | 850 (18%) | |
| 2 | 6985 (7.5%) | 6020 (7.4%) | 453 (8.8%) | 128 (7.8%) | 384 (8.0%) | |
| 3 | 3343 (3.6%) | 2823 (3.4%) | 257 (5.0%) | 65 (4.0%) | 198 (4.1%) | |
| Surgical resection | <.001 | |||||
| Biopsy | 9306 (23%) | 8078 (23%) | 520 (22%) | 183 (21%) | 525 (23%) | |
| Gross total | 12 448 (30%) | 10 885 (31%) | 692 (29%) | 239 (28%) | 632 (28%) | |
| None | 8827 (22%) | 7653 (22%) | 529 (22%) | 201 (23%) | 444 (20%) | |
| Subtotal | 10 297 (25%) | 8790 (25%) | 621 (26%) | 235 (27%) | 651 (29%) | |
| Unknown | 52 599 | 46 494 | 2762 | 780 | 2563 | |
| Focality | .11 | |||||
| Multifocal | 7562 (19%) | 6549 (19%) | 403 (17%) | 164 (19%) | 446 (20%) | |
| Unifocal | 32 521 (81%) | 28 173 (81%) | 1906 (83%) | 690 (81%) | 1752 (80%) | |
| Unknown | 53 394 | 47 178 | 2815 | 784 | 2617 | |
| Readmission | <.001 | |||||
| Not readmitted | 85 183 (94%) | 74 778 (94%) | 4568 (92%) | 1476 (94%) | 4361 (93%) | |
| Readmitted | 5182 (5.7%) | 4376 (5.5%) | 376 (7.6%) | 101 (6.4%) | 329 (7.0%) | |
| Unknown | 3112 | 2746 | 180 | 61 | 125 | |
| Radiation | <.001 | |||||
| Not received | 27 851 (30%) | 24 214 (30%) | 1560 (31%) | 472 (29%) | 1605 (34%) | |
| Received | 65 128 (70%) | 57 266 (70%) | 3534 (69%) | 1152 (71%) | 3176 (66%) | |
| Unknown | 498 | 420 | 30 | 14 | 34 | |
| Chemotherapy | <.001 | |||||
| Not received | 31 547 (35%) | 27 247 (34%) | 1944 (40%) | 542 (35%) | 1814 (40%) | |
| Received | 59 100 (65%) | 52 338 (66%) | 2960 (60%) | 1027 (65%) | 2775 (60%) | |
| Unknown | 2830 | 2315 | 220 | 69 | 226 | |
| 30-day mortality | .017 | |||||
| Alive | 65 610 (95%) | 57 416 (95%) | 3562 (95%) | 1164 (97%) | 3468 (95%) | |
| Dead | 3587 (5.2%) | 3163 (5.2%) | 204 (5.4%) | 39 (3.2%) | 181 (5.0%) | |
| Unknown | 24 280 | 21 321 | 1358 | 435 | 1166 | |
| 90-day mortality | <.001 | |||||
| Alive | 58 095 (84%) | 50 716 (84%) | 3204 (86%) | 1070 (90%) | 3105 (86%) | |
| Dead | 10 859 (16%) | 9694 (16%) | 540 (14%) | 121 (10%) | 504 (14%) | |
| Unknown | 24 523 | 21 490 | 1380 | 447 | 1206 | |
| Survival months | 9 (3–18) | 9 (3–18) | 10 (4–20) | 12 (4–24) | 10 (4–21) | <.001 |
| Unknown | 4 | 3 | 0 | 0 | 1 | |
| Vital status | <.001 | |||||
| Alive | 9985 (11%) | 7836 (9.6%) | 751 (15%) | 331 (20%) | 1067 (22%) | |
| Dead | 83 492 (89%) | 74 064 (90%) | 4373 (85%) | 1307 (80%) | 3748 (78%) |
aStatistics presented: n (%); median (IQR).
bStatistical tests performed: chi-square test of independence; Kruskal–Wallis test.
Multivariable logistic regression models for 30-day readmission by race/ethnicity are given in Table 2. Black non-Hispanics had the highest rates of unplanned readmission within 30 days, as a significant difference was seen comparing Black non-Hispanics to White non-Hispanics on multivariable modeling (odds ratio [OR] 1.39, 95% confidence interval [CI] 1.15–1.6, P < .001). Significant associations were also seen with facility type, Charlson–Deyo score, surgical resection, and focality.
Table 2.
Multivariable Logistic Regression Model for 30-Day Readmission, Glioblastoma, and National Cancer Database 2004–2014
| Characteristic | Event N | OR | 95% CI | P value |
|---|---|---|---|---|
| Race ethnicity | ||||
| White non-Hispanic | 1589 | — | — | |
| Black non-Hispanic | 139 | 1.39 | 1.15–1.66 | <.001 |
| Asian non-Hispanic | 43 | 1.24 | 0.89–1.68 | .2 |
| Hispanic | 107 | 1.11 | 0.89–1.35 | .3 |
| Facility type | ||||
| Academic/Research Program | 825 | — | — | |
| Community Cancer Program | 77 | 0.89 | 0.70–1.13 | .4 |
| Comprehensive Community Cancer Program | 687 | 1.02 | 0.92–1.13 | .7 |
| Integrated Network Cancer Program | 289 | 1.34 | 1.16–1.54 | <.001 |
| Age | 1.00 | 0.99–1.01 | .9 | |
| Sex | ||||
| Female | 777 | — | — | |
| Male | 1101 | 1.02 | 0.92–1.12 | .7 |
| Primary payer | ||||
| Medicaid | 119 | — | — | |
| Medicare | 874 | 0.96 | 0.77–1.20 | .7 |
| Not insured | 81 | 1.11 | 0.83–1.49 | .5 |
| Other government | 36 | 1.03 | 0.69–1.51 | .9 |
| Private insurance | 768 | 0.89 | 0.73–1.09 | .3 |
| Urban/rural | ||||
| Metro | 1589 | — | — | |
| Rural | 38 | 1.12 | 0.79–1.55 | .5 |
| Urban | 251 | 0.90 | 0.78–1.03 | .12 |
| Charlson–Deyo score | ||||
| 0 | 1163 | — | — | |
| 1 | 383 | 1.23 | 1.09–1.39 | <.001 |
| 2 | 227 | 1.76 | 1.51–2.04 | <.001 |
| 3 | 105 | 1.56 | 1.26–1.92 | <.001 |
| Surgical resection | ||||
| Biopsy | 467 | — | — | |
| Gross total | 698 | 1.16 | 1.03–1.32 | .015 |
| None | 74 | 0.15 | 0.11–0.19 | <.001 |
| Subtotal | 639 | 1.25 | 1.11–1.42 | <.001 |
| Focality | ||||
| Multifocal | 391 | — | — | |
| Unifocal | 1487 | 0.77 | 0.68–0.86 | <.001 |
| Radiation | ||||
| Not received | 484 | — | — | |
| Received | 1394 | 0.97 | 0.82–1.15 | .7 |
| Chemotherapy | ||||
| Not received | 553 | — | — | |
| Received | 1325 | 0.96 | 0.81–1.12 | .6 |
OR, odds ratio; CI, confidence interval.
Results of multivariable logistic regression models for 30-day and 90-day mortality by race/ethnicity are given in Tables 3 and 4, respectively. Asian non-Hispanics had the lowest odds of 30-day and 90-day mortality when compared to other races. White non-Hispanics had the highest odds of 30-day and 90-day mortality (although Black non-Hispanics had the highest unadjusted 30-day mortality rate, Table 1). A significant difference in 30-day mortality was seen on multivariable analysis when comparing Asian non-Hispanics and White non-Hispanics (OR 0.52, 95% CI 0.28–0.91, P = .031). Significant differences were seen in 90-day mortality between White non-Hispanics and Asian non-Hispanics (OR 0.64, 95% CI 0.46–0.89, P = .009) and Hispanics (OR 0.648, 95% CI 0.528–0.794, P < .001), though not Black non-Hispanics (OR 0.88, 95% CI 0.73–1.06, P = .2).
Table 3.
Multivariable Logistic Regression Model for 30-Day Mortality, Glioblastoma, and National Cancer Database 2004–2014
| Characteristic | Event N | OR | 95% CI | P value |
|---|---|---|---|---|
| Race ethnicity | ||||
| White non-Hispanic | 1019 | — | — | |
| Black non-Hispanic | 64 | 0.88 | 0.66–1.17 | .4 |
| Asian non-Hispanic | 13 | 0.52 | 0.28–0.91 | .031 |
| Hispanic | 58 | 0.84 | 0.61–1.13 | .3 |
| Facility type | ||||
| Academic/Research Program | 455 | — | — | |
| Community Cancer Program | 53 | 1.00 | 0.72–1.37 | >.9 |
| Comprehensive Community Cancer Program | 483 | 1.10 | 0.95–1.27 | .2 |
| Integrated Network Cancer Program | 163 | 1.23 | 1.00–1.51 | .049 |
| Age | 1.02 | 1.01–1.02 | <.001 | |
| Sex | ||||
| Female | 447 | — | — | |
| Male | 707 | 1.27 | 1.11–1.45 | <.001 |
| Primary payer | ||||
| Medicaid | 56 | — | — | |
| Medicare | 718 | 1.09 | 0.79–1.53 | .6 |
| Not insured | 46 | 1.20 | 0.77–1.86 | .4 |
| Other government | 16 | 0.76 | 0.40–1.39 | .4 |
| Private insurance | 318 | 1.08 | 0.79–1.50 | .6 |
| Urban/rural | ||||
| Metro | 946 | — | — | |
| Rural | 21 | 0.87 | 0.52–1.39 | .6 |
| Urban | 187 | 0.93 | 0.77–1.11 | .4 |
| Charlson–Deyo score | ||||
| 0 | 586 | — | — | |
| 1 | 310 | 1.62 | 1.38–1.91 | <.001 |
| 2 | 142 | 1.50 | 1.21–1.85 | <.001 |
| 3 | 116 | 2.33 | 1.82–2.96 | <.001 |
| Surgical resection | ||||
| Biopsy | 417 | — | — | |
| Gross total | 327 | 0.67 | 0.57–0.80 | <.001 |
| None | 31 | 1.61 | 1.01–2.51 | .039 |
| Subtotal | 379 | 0.86 | 0.73–1.01 | .073 |
| Focality | ||||
| Multifocal | 233 | — | — | |
| Unifocal | 921 | 0.73 | 0.62–0.87 | <.001 |
| Radiation | ||||
| Not received | 1095 | — | — | |
| Received | 59 | 0.04 | 0.03–0.06 | <.001 |
| Chemotherapy | ||||
| Not received | 1074 | — | — | |
| Received | 80 | 0.21 | 0.15–0.27 | <.001 |
OR, odds ratio; CI, confidence interval.
Table 4.
Multivariable Logistic Regression Model for 90-Day Mortality, Glioblastoma, and National Cancer Database 2004–2014
| Characteristic | Event N | OR | 95% CI | P value |
|---|---|---|---|---|
| Race ethnicity | ||||
| White non-Hispanic | 3282 | — | — | |
| Black non-Hispanic | 193 | 0.88 | 0.73–1.06 | .2 |
| Asian non-Hispanic | 54 | 0.64 | 0.46–0.89 | .009 |
| Hispanic | 156 | 0.65 | 0.53–0.79 | <.001 |
| Facility type | ||||
| Academic/Research Program | 1450 | — | — | |
| Community Cancer Program | 187 | 1.20 | 0.99–1.46 | .065 |
| Comprehensive Community Cancer Program | 1594 | 1.32 | 1.20–1.45 | <.001 |
| Integrated Network Cancer Program | 454 | 1.18 | 1.03–1.35 | .017 |
| Age | 1.04 | 1.03–1.05 | <.001 | |
| Sex | ||||
| Female | 1561 | — | — | |
| Male | 2124 | 1.05 | 0.97–1.15 | .2 |
| Primary payer | ||||
| Medicaid | 180 | — | — | |
| Medicare | 2369 | 0.93 | 0.76–1.14 | .5 |
| Not insured | 134 | 1.11 | 0.84–1.46 | 0.5 |
| Other government | 55 | 0.69 | 0.47–1.00 | .056 |
| Private insurance | 947 | 0.75 | 0.61–0.91 | .004 |
| Urban/rural | ||||
| Metro | 3005 | — | — | |
| Rural | 64 | 0.75 | 0.54–1.02 | .074 |
| Urban | 616 | 1.02 | 0.91–1.15 | .7 |
| Charlson–Deyo score | ||||
| 0 | 2063 | — | — | |
| 1 | 881 | 1.44 | 1.29–1.59 | <.001 |
| 2 | 451 | 1.70 | 1.48–1.95 | <.001 |
| 3 | 290 | 2.14 | 1.79–2.55 | <.001 |
| Surgical resection | ||||
| Biopsy | 1250 | — | — | |
| Gross total | 1045 | 0.57 | 0.51–0.63 | <.001 |
| None | 75 | 1.22 | 0.88–1.69 | .2 |
| Subtotal | 1315 | 0.92 | 0.83–1.02 | .10 |
| Focality | ||||
| Multifocal | 782 | — | — | |
| Unifocal | 2903 | 0.60 | 0.54–0.67 | <.001 |
| Radiation | ||||
| Not received | 2508 | — | — | |
| Received | 1177 | 0.22 | 0.20–0.25 | <.001 |
| Chemotherapy | ||||
| Not received | 2654 | — | — | |
| Received | 1031 | 0.27 | 0.24–0.31 | <.001 |
OR, odds ratio; CI, confidence interval.
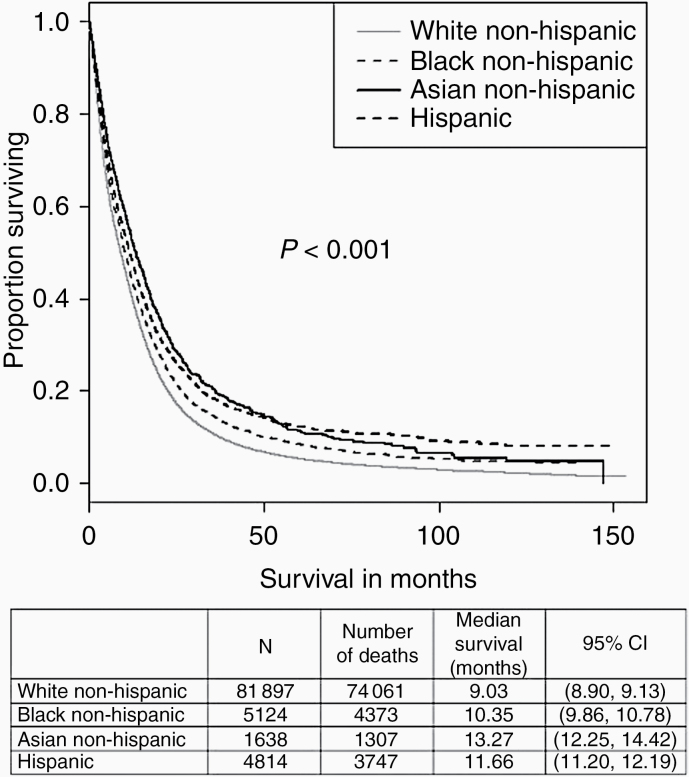
Results of multivariable Cox Proportional Hazards modeling of race/ethnicity for overall survival are given in Table 5. When compared to a reference group of White non-Hispanics, Black non-Hispanics (hazard ratio [HR]: 0.88, 95% CI 0.83–0.92, P < .001), Asian non-Hispanics (HR: 0.72, 95% CI 0.65–0.73, P < .001), and Hispanics (HR: 0.69, 95% CI 0.65–0.73, P < .001) all had significantly lower overall survival HRs. Results of multivariable Cox Proportional Hazards modeling excluding from analysis individuals who died within 90 days are given in Supplementary Table 1. White non-Hispanics still had a significantly lower overall survival (ratios were as follows: Black non-Hispanics [HR: 0.93, 95% CI 0.87–0.99, P = .030], Asian non-Hispanics [HR: 0.70, 95% CI 0.36–0.79, P < .001], and Hispanics [HR: 0.71, 95% CI 0.66–0.77, P < .001]). Kaplan–Meier survival curves by race and median survival are demonstrated in Figure 1. Race was significantly associated with adjusted overall survival (P < .001), with White non-Hispanic having the lowest median survival (9.03 months) and Asian non-Hispanic having the highest (13.27 months). Kaplan–Meier survival curves by race and median survival, excluding from analysis individuals who died within 90 days, are demonstrated in Supplementary Figure 1. Race was still significantly associated with adjusted overall survival (P < .001), with White non-Hispanic having the lowest median survival (14.2 months) and Asian non-Hispanic having the highest (18.2 months).
Table 5.
Multivariable Cox Proportional Hazards Model for Overall Survival, Glioblastoma, and National Cancer Database 2004–2014
| Characteristic | Event N | HR | 95% CI | P value |
|---|---|---|---|---|
| Race ethnicity | ||||
| White non-Hispanic | 25 525 | — | — | |
| Black non-Hispanic | 1495 | 0.88 | 0.83–0.92 | <.001 |
| Asian non-Hispanic | 507 | 0.72 | 0.65–0.78 | <.001 |
| Hispanic | 1224 | 0.69 | 0.65–0.73 | <.001 |
| Facility type | ||||
| Academic/Research Program | 12 272 | — | — | |
| Community Cancer Program | 1517 | 1.11 | 1.05–1.17 | <.001 |
| Comprehensive Community Cancer Program | 11 455 | 1.14 | 1.12–1.17 | <.001 |
| Integrated Network Cancer Program | 3507 | 1.19 | 1.14–1.23 | <.001 |
| Age | 1.03 | 1.02–1.03 | <.001 | |
| Sex | ||||
| Female | 12 105 | — | — | |
| Male | 16 646 | 1.07 | 1.05–1.10 | <.001 |
| Primary payer | ||||
| Medicaid | 1500 | — | — | |
| Medicare | 14 265 | 0.96 | 0.91–1.02 | .2 |
| Not insured | 951 | 0.96 | 0.88–1.04 | .3 |
| Other government | 477 | 0.94 | 0.85–1.04 | .2 |
| Private insurance | 11 558 | 0.85 | 0.80–0.89 | <.001 |
| Urban/rural | ||||
| Metro | 23 767 | — | — | |
| Rural | 549 | 1.06 | 0.97–1.15 | .2 |
| Urban | 4435 | 1.07 | 1.04–1.11 | <.001 |
| Charlson–Deyo score | ||||
| 0 | 19 579 | — | — | |
| 1 | 5537 | 1.20 | 1.17–1.24 | <.001 |
| 2 | 2368 | 1.25 | 1.19–1.30 | <.001 |
| 3 | 1267 | 1.43 | 1.35–1.51 | <.001 |
| Surgical resection | ||||
| Biopsy | 6623 | — | — | |
| Gross total | 8317 | 0.76 | 0.74–0.79 | <.001 |
| None | 6455 | 1.48 | 1.43–1.53 | <.001 |
| Subtotal | 7356 | 0.98 | 0.95–1.02 | .3 |
| Focality | ||||
| Multifocal | 5756 | — | — | |
| Unifocal | 22 995 | 0.75 | 0.73–0.77 | <.001 |
| Radiation | ||||
| Not received | 8197 | — | — | |
| Received | 20 554 | 0.69 | 0.67–0.72 | <.001 |
| Chemotherapy | ||||
| Not received | 9502 | — | — | |
| Received | 19 249 | 0.60 | 0.58–0.62 | <.001 |
HR, hazard ratio; CI, confidence interval.
Figure 1.
Kaplan–Meier survival analysis by race and ethnicity, glioblastoma, and National Cancer Database 2004–2014. *Median survival with 95% CI also shown by race/ethnicity.
Discussion
The purpose of this study was to examine the NCDB for an association between race and overall survival, 30-day and 90-day mortality for patients with GBM. Though the NCDB has its own limitations with regard to case coverage and reporting, its scope is quite comprehensive, covering approximately 70% of all new cancer diagnoses in the United States according to a recent comparison of national databases.17
Our study demonstrates that survival for GBM patients is in part associated with race. Such an association has also been found in various other cancers. Multiple NCDB analyses reporting on other cancer types note race as a significant factor in survival, notably in breast cancer,21 T-cell lymphoma,22 uterine cancer,23 and endometrial cancer.24 Moreover, previous analyses on GBM have similarly demonstrated that non-Hispanic Whites have the poorest survival, even account for differences in treatment. A SEER database GBM study found that Hispanics have the highest survival, followed by Blacks, and then Asian/Pacific Islanders, relative to Whites.20 White patients were also shown to have the poorest survival in other GBM datasets, including the Central Brain Tumor Registry of the United States.2 However, these studies do not address variation in short-term survival (eg, 30-day and 90-day mortality), nor do they address issues related to quality of care as reflected by 30-day readmissions.
We hypothesized if considerations having to do with access to care might vary by race. Our analysis demonstrates that non-Hispanic Whites are more likely than Hispanics and non-Hispanic Blacks to receive standard of care treatment. Specifically, treatment use is significantly lower in 2–3 race/ethnicity groups compared to White non-Hispanics, including surgical resection and each adjuvant therapy (radiation therapy or chemotherapy). There are several reports that have observed the survival benefits of undergoing treatment in high-volume academic centers compared to lower-volume community centers.25,26 Similar findings have been reported for patients undergoing isolated radiation therapy.27 There has been recently published data on facility volume and outcomes specifically in GBM patients, demonstrating a substantial decrease in prolonged length of stay, readmission rates, and mortality in patients who sought treatment at academic and/or high-volume centers.28 In fact, multidisciplinary “Tumor Board” conferences have shown to lead to better outcomes, including a higher likelihood of patients receiving adjuvant treatment.29 With regard to the selection of treatment facility, our analysis shows that a majority of GBM patients seek treatment at either a Comprehensive Community Cancer Program or Academic/Research Program irrespective of race, which represents high-volume facility types. This is in line with past analysis demonstrating GBM patients who travel farther to receive care at high-volume centers have superior postoperative outcomes compared to patients who receive care locally at low-volume centers.30 This may reflect the more comprehensive diagnostic capabilities of such centers, as well as the presence of oncologic care pathways that provide continuous and multidisciplinary care from the time of diagnosis through resection and adjuvant therapy. This may also reflect limitations in the ability of community programs to access services such as advanced MRI imaging or radiation therapy. Such access has previously been demonstrated as a factor in survival in other cancer types.31
Our analysis found that differences in 30-day readmissions were present; non-Hispanic Whites are less likely than Hispanics and non-Hispanic Blacks to be re-admitted, suggesting disparities in short-term outcomes. This may reflect the existence of higher rates of perioperative complications not captured by the NCDB. Racial disparities in short-term perioperative outcomes have been widely reported for other oncologic procedures,32–36 particularly when comparing Black to Caucasian populations. Additionally, readmissions may be correlated to treatment differences by race, specifically, in regard to pain management. Both oncologic and non-oncologic evidence suggest that Blacks, when compared to other races, have both higher pain scores and higher rates of inadequately treated pain.37,38 While the NCDB does not have sufficient granularity in their data to tease out these specific reasons for readmission, multiple hypotheses that have been proven in the literature include higher rates of perioperative complications, inadequately managed periprocedural pain control, racial bias in discharge planning, differences in communication of discharge expectations, and inadequate provision of socioeconomic support services.39–41 Additionally, a growing body of evidence suggests that racism, ie, systemic bias, rather than race itself may be the driving factor behind observed healthcare disparities.42,43 Cumulatively these reasons may be contributing factors to the higher rates of 30-day readmission and mortality rates for the Black non-Hispanic population.
Despite the higher rates of treatment and lower readmissions, White non-Hispanics exhibited the lowest overall survival, demonstrated by our multivariable cox proportional hazards model. Although this may be partially attributable to older ages at diagnosis in White non-Hispanics, our multivariable model did adjust for age. We found that survival was highest in Asians, followed by Hispanics and Blacks relative to Caucasians. Importantly, this finding was durable even after excluding from analysis the patient who died within the first 90 days, that is, Whites continue doing worse even beyond the 90-day window, suggesting biology may underlie these differences.
This study does have a number of limitations, as database studies are inherently limited by selection bias, missing data, and confounding factors. The overwhelming majority of GBM patients in the NCDB are White non-Hispanic. Other similar such studies utilizing the NCDB have samples that are up to 90.5% White patients.12 However, this in part reflects the known higher incidence in White patients. The Central Brain Tumor Registry of the US’s 2011–2015 primary brain tumor report shows that the incidence rate of GBM was higher in White patients (3.47) compared to Black patients (1.80) and Asian patients (1.57).2 While the NCDB captures data for approximately 70% of all new cancer diagnoses, only hospitals with Commission on Cancer accreditation are included, which only applies to about 30% of 5000 hospitals in the United States. This may present a selection bias for the study population, as racial differences may be present in the use of high-volume Commission on Cancer accredited hospitals.44 Furthermore, only patients with a histologically confirmed diagnosis were included in this dataset, which may limit inclusion of the elderly, those with a late diagnosis, and disadvantaged individuals without access to a histologically confirmed diagnosis. This could also be an indirect contributor to racial differences in survival. Exclusion of patients with undiagnosed cancers or death prior to diagnosis may also influence findings. Additionally, analyzing mortality as an outcome for a disease with as rapid a course as GBM presents the potential confounder of time of presentation influencing the duration of postoperative survival measured. With regard to missing data, most patient data did not have Karnofsky Performance Status, MGMT methylation status, extent of resection, extent of adjuvant therapy, despite all having an important relationship with survival data45,46; therefore, these had to be excluded in the multivariable analyses. As with virtually all multi-institutional registries, miscoding or erroneous entry of data variables must be acknowledged. Additional important predictors such as molecular markers were not present in this dataset, which can have significant impacts on survival as well as may have racial associations which should be investigated in future studies. Finally, race is an elusive concept, sometimes imposed by external sources (the racial NCDB may not reflect all races) or those internally defined. Healthcare outcomes can differ across communities and encompass socioeconomic factors as well as cultural and access issues that are built into the racialism that creates some and fractures many other communities.47
Conclusions
Ultimately, our findings reiterate the well-known disparities that exist in healthcare as a function of race and should alert care providers and researchers to the continued need for improvement in studying and addressing these issues. Given that GBM is the most common primary brain tumor, it is likely that such findings related to racial/ethnic differences may be true in other primary brain tumors, as our group has demonstrated similar results in gliosarcoma.48 We call for further work to be done to understand the sources behind the observed disparities in GBM patient treatment, readmissions, and outcomes, so these disparities can ultimately be overcome. Future studies should be focused on addressing such disparities, and such national databases can continue to contribute to this work in the manner in which they collect patient data.
Supplementary Material
Acknowledgments
This project is sponsored by the Junior Faculty Mentorship Program in the Department of Neurosurgery, UH-Cleveland Medical Center (to A.E.S.). A.E.S. is also supported by the National Institutes of Health CA217956; as well as the Peter D Cristal Chair, the Center of Excellence for Translational Neuro-Oncology, the Gerald Kaufman Fund for Glioma Research, the Kimble Family Foundation, and the Ferry Family Foundation at University Hospitals of Cleveland. J.B.S. is supported by the Research Division, University Hospitals of Cleveland.
Funding
A.E.S. is supported by the National Institutes of Health CA217956; as well as the Peter D Cristal Chair, the Center of Excellence for Translational Neuro-Oncology, the Gerald Kaufman Fund for Glioma Research, the Kimble Family Foundation, and the Ferry Family Foundation at University Hospitals of Cleveland. J.B.S. is supported by the Research Division, University Hospitals of Cleveland. T.R.H. is also supported by the UH-Cleveland Minority Faculty Career Development Grant.
Conflicts of interest statement. The authors report no conflict of interests.
Authorship Statement Conception: T.H., A.S., and J.B.S.; data collection: T.H., C.H.W., J.W., and H.G.; statistical analysis: G.C., H.G., and C.K.; drafting—original: C.M.L. and U.V.M.; drafting—revisions and editing: C.M.L., U.V.M., E.H., X.Z., and K.D.; approved final version: all authors; study supervision: T.H., A.S., and J.B.S.
References
- 1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 2. Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gittleman H, Boscia A, Ostrom QT, et al. Survivorship in adults with malignant brain and other central nervous system tumor from 2000–2014. Neuro Oncol. 2018;20(suppl 7):vii6–vii16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barnholtz-Sloan JS, Williams VL, Maldonado JL, et al. Patterns of care and outcomes among elderly individuals with primary malignant astrocytoma. J Neurosurg. 2008;108(4):642–648. [DOI] [PubMed] [Google Scholar]
- 5. Barnholtz-Sloan JS, Sloan AE, Schwartz AG. Racial differences in survival after diagnosis with primary malignant brain tumor. Cancer. 2003;98(3):603–609. [DOI] [PubMed] [Google Scholar]
- 6. Patel NP, Lyon KA, Huang JH. The effect of race on the prognosis of the glioblastoma patient: a brief review. Neurol Res. 2019;124(4):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baldi I, Huchet A, Bauchet L, Loiseau H. [Epidemiology of glioblastoma]. Neurochirurgie. 2010;56(6):433–440. [DOI] [PubMed] [Google Scholar]
- 8. Chakrabarti I, Cockburn M, Cozen W, Wang YP, Preston-Martin S. A population-based description of glioblastoma multiforme in Los Angeles County, 1974–1999. Cancer. 2005;104(12):2798–2806. [DOI] [PubMed] [Google Scholar]
- 9. Darefsky AS, King JT Jr, Dubrow R. Adult glioblastoma multiforme survival in the temozolomide era: a population-based analysis of Surveillance, Epidemiology, and End Results registries. Cancer. 2012;118(8):2163–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thakkar JP, Dolecek TA, Horbinski C, et al. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. 2014;23(10):1985–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu A, Lv T, Zhang B, Zhang W, Ou X, Huang J. Development and evaluation of an unlabeled probe high-resolution melting assay for detection of ATP7B mutations in Wilson’s disease. J Clin Lab Anal. 2017;31(4):e22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown DA, Himes BT, Kerezoudis P, et al. Insurance correlates with improved access to care and outcome among glioblastoma patients. Neuro Oncol. 2018;20(10):1374–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rong X, Yang W, Garzon-Muvdi T, et al. Influence of insurance status on survival of adults with glioblastoma multiforme: a population-based study. Cancer. 2016;122(20):3157–3165. [DOI] [PubMed] [Google Scholar]
- 14. Chandra A, Young JS, Dalle Ore C, et al. Insurance type impacts the economic burden and survival of patients with newly diagnosed glioblastoma. J Neurosurg. 2019;133(1):1–11. [DOI] [PubMed] [Google Scholar]
- 15. Pollom EL, Fujimoto DK, Han SS, Harris JP, Tharin SA, Soltys SG. Newly diagnosed glioblastoma: adverse socioeconomic factors correlate with delay in radiotherapy initiation and worse overall survival. J Radiat Res. 2018;59(suppl 1):i11–i18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Porter AB, Lachance DH, Johnson DR. Socioeconomic status and glioblastoma risk: a population-based analysis. Cancer Causes Control. 2015;26(2):179–185. [DOI] [PubMed] [Google Scholar]
- 17. Lerro CC, Robbins AS, Phillips JL, Stewart AK. Comparison of cases captured in the national cancer data base with those in population-based central cancer registries. Ann Surg Oncol. 2013;20(6):1759–1765. [DOI] [PubMed] [Google Scholar]
- 18. Janz TA, Graboyes EM, Nguyen SA, et al. A comparison of the NCDB and SEER database for research involving head and neck cancer. Otolaryngol Head Neck Surg. 2019;160(2):284–294. [DOI] [PubMed] [Google Scholar]
- 19. Batista Rodríguez G, Balla A, Fernández-Ananín S, Balagué C, Targarona EM. The era of the large databases: outcomes after gastroesophageal surgery according to NSQIP, NIS, and NCDB databases. Systematic literature review. Surg Innov. 2018;25(4):400–412. [DOI] [PubMed] [Google Scholar]
- 20. Bohn A, Braley A, Rodriguez de la Vega P, Zevallos JC, Barengo NC. The association between race and survival in glioblastoma patients in the US: a retrospective cohort study. PLoS One. 2018;13(6):e0198581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murray DCD, Bhandari S, Ngo P, et al. Race as an independent factor for survival in breast cancer patients according to analysis of the National Cancer Database (NCDB). J Clin Oncol. 2019;37(15 suppl):e18155–e18155. [Google Scholar]
- 22. Allen PB, Flowers C, Lechowicz MJ, Goldstein J. Association of insurance status and race with overall survival among patients with cutaneous T-cell lymphoma: a National Cancer Database analysis. J Clin Oncol. 2018;36(15 suppl):e18624–e18624. [Google Scholar]
- 23. Fedewa SA, Lerro C, Chase D, Ward EM. Insurance status and racial differences in uterine cancer survival: a study of patients in the National Cancer Database. Gynecol Oncol. 2011;122(1):63–68. [DOI] [PubMed] [Google Scholar]
- 24. Malagon-Blackwell EM, Seagle BL, Nieves-Neira W, Shahabi S. The Hispanic Paradox in endometrial cancer: a National Cancer Database study. Gynecol Oncol. 2017;146(2):351–358. [DOI] [PubMed] [Google Scholar]
- 25. Bajaj A, Martin B, Bhasin R, et al. The impact of academic facility type and case volume on survival in patients undergoing curative radiation therapy for muscle-invasive bladder cancer. Int J Radiat Oncol Biol Phys. 2018;100(4):851–857. [DOI] [PubMed] [Google Scholar]
- 26. Urbach DR, Baxter NN. Does it matter what a hospital is “high volume” for? Specificity of hospital volume-outcome associations for surgical procedures: analysis of administrative data. BMJ. 2004;328(7442):737–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen YW, Mahal BA, Muralidhar V, et al. Association between treatment at a high-volume facility and improved survival for radiation-treated men with high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2016;94(4):683–690. [DOI] [PubMed] [Google Scholar]
- 28. Zhu P, Du XL, Zhu J-J, Esquenazi Y. Improved survival of glioblastoma patients treated at academic and high-volume facilities: a hospital-based study from the National Cancer Database. J Neurosurg. 2019;132(2):491–502. [DOI] [PubMed] [Google Scholar]
- 29. Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: a systematic review of the literature. Cancer Treat Rev. 2016;42:56–72. [DOI] [PubMed] [Google Scholar]
- 30. Lopez Ramos C, Brandel MG, Steinberg JA, et al. The impact of traveling distance and hospital volume on post-surgical outcomes for patients with glioblastoma. J Neurooncol. 2019;141(1):159–166. [DOI] [PubMed] [Google Scholar]
- 31. Coory MD. Distance to the closest radiotherapy facility and survival after a diagnosis of rectal cancer in Queensland. Med J Aust. 2011;195(11–12):661–662. [DOI] [PubMed] [Google Scholar]
- 32. Alavi K, Cervera-Servin JA, Sturrock PR, Sweeney WB, Maykel JA. Racial differences in short-term surgical outcomes following surgery for diverticulitis. J Gastrointest Surg. 2012;16(3):613–621. [DOI] [PubMed] [Google Scholar]
- 33. Sanchez D, Dubay D, Prabhakar B, Taber DJ. Evolving trends in racial disparities for peri-operative outcomes with the new Kidney Allocation System (KAS) implementation. J Racial Ethn Health Disparities. 2018;5(6):1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schneider EB, Haider AH, Hyder O, Efron JE, Lidor AO, Pawlik TM. Assessing short- and long-term outcomes among black vs white Medicare patients undergoing resection of colorectal cancer. Am J Surg. 2013;205(4):402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Payne R, Medina E, Hampton JW. Quality of life concerns in patients with breast cancer: evidence for disparity of outcomes and experiences in pain management and palliative care among African-American women. Cancer. 2003;97(1 suppl):311–317. [DOI] [PubMed] [Google Scholar]
- 36. Naik G, Akinyemiju T. Disparities in hospitalization outcomes among African-American and White prostate cancer patients. Cancer Epidemiol. 2017;46:73–79. [DOI] [PubMed] [Google Scholar]
- 37. Stein KD, Alcaraz KI, Kamson C, Fallon EA, Smith TG. Sociodemographic inequalities in barriers to cancer pain management: a report from the American Cancer Society’s Study of Cancer Survivors-II (SCS-II). Psychooncology. 2016;25(10):1212–1221. [DOI] [PubMed] [Google Scholar]
- 38. Martinez KA, Snyder CF, Malin JL, Dy SM. Is race/ethnicity related to the presence or severity of pain in colorectal and lung cancer? J Pain Symptom Manage. 2014;48(6):1050–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Muhlestein WE, Akagi DS, Chotai S, Chambless LB. The impact of race on discharge disposition and length of hospitalization after craniotomy for brain tumor. World Neurosurg. 2017;104:24–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nuño M, Mukherjee D, Elramsisy A, et al. Racial and gender disparities and the role of primary tumor type on inpatient outcomes following craniotomy for brain metastases. Ann Surg Oncol. 2012;19(8):2657–2663. [DOI] [PubMed] [Google Scholar]
- 41. Trent SA, Hasegawa K, Ramratnam SK, Bittner JC, Camargo CA Jr. Variation in asthma care at hospital discharge by race/ethnicity groups. J Asthma. 2018;55(9):939–948. [DOI] [PubMed] [Google Scholar]
- 42. Feagin J, Bennefield Z. Systemic racism and U.S. health care. Soc Sci Med. 2014;103:7–14. [DOI] [PubMed] [Google Scholar]
- 43. Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health. 2015;105(12):e60–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wasif N, Etzioni D, Habermann EB, et al. Racial and socioeconomic differences in the use of high-volume commission on cancer-accredited hospitals for cancer surgery in the United States. Ann Surg Oncol. 2018;25(5):1116–1125. [DOI] [PubMed] [Google Scholar]
- 45. Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. [DOI] [PubMed] [Google Scholar]
- 46. Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chastain DB, Osae SP, Henao-Martínez AF, Franco-Paredes C, Chastain JS, Young HN. Racial disproportionality in Covid clinical trials. N Engl J Med. 2020;383(9):e59. [DOI] [PubMed] [Google Scholar]
- 48. Wright JM, Hodges TR, Wright CH, et al. Racial/ethnic differences in survival for patients with gliosarcoma: an analysis of the National Cancer Database. J Neurooncol. 2019;143(2):349–357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.