Abstract
The health benefits and risks of menopausal hormone therapy among women aged 50–59 years are examined in the Women’s Health Initiative randomized, placebo-controlled trials using long-term follow-up data and a parsimonious statistical model that leverages data from older participants to increase precision. These trials enrolled 27,347 healthy postmenopausal women aged 50–79 years at 40 US clinical centers during 1993–1998, including 10,739 post-hysterectomy participants in a trial of conjugated equine estrogens and 16,608 participants with a uterus in the trial of these estrogens plus medroxyprogesterone acetate. Over a (median) 18-year follow-up period (1993–2016), risk for a global index (defined as the earliest of coronary heart disease, invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, endometrial cancer, hip fracture, and all-cause mortality) was reduced with conjugated equine estrogens with a hazard ratio of 0.82 (95% confidence interval: 0.71, 0.95), and with nominally significant reductions for coronary heart disease, breast cancer, hip fracture, and all-cause mortality. Corresponding global index hazard ratio estimates of 1.06 (95% confidence interval: 0.95, 1.19) were nonsignificant for combined estrogens plus progestin, but increased breast cancer risk and reduced endometrial cancer risk were observed. These results, among women 50–59 years of age, substantially agree with the worldwide observational literature, with the exception of breast cancer for estrogens alone.
Keywords: benefits versus risks, estrogens, global index, hazard ratio, menopausal hormone therapy, multivariate failure times, progestin
Abbreviations
- CEE
conjugated equine estrogens
- CHD
coronary heart disease
- CI
confidence interval
- HR
hazard ratio
- MPA
medroxyprogesterone acetate
- WHI
Women’s Health Initiative
Following the early stoppage in 2002 (1) of the intervention in the Women’s Health Initiative (WHI) trial of 0.625 mg/day conjugated equine estrogens (CEE) plus 2.5 mg/day medroxyprogesterone acetate (MPA), among 16,608 healthy postmenopausal women with a uterus, there was a substantial reduction in use of menopausal hormone therapy in the United States (2, 3) and worldwide. The early intervention stoppage was motivated by an observed elevation in breast cancer risk, the trial’s primary safety outcome, and by a lack of evidence of risk reduction for coronary heart disease (CHD), the primary efficacy outcome, or for cardiovascular diseases more generally. The 0.625 mg/day CEE trial, among 10,739 post-hysterectomy participants, was also stopped early in 2004 (4), based in part on a stroke elevation of similar magnitude to that seen in the CEE + MPA trial.
There was a strong interest at the WHI trial-design stage to include older postmenopausal women, to allow an examination of whether the hypothesized hormone therapy benefit for CHD would extend to older ages. Hence, women aged 50–79 years were eligible for enrollment, and special efforts to recruit older women led to 32.3%, 45.2%, and 22.5% of enrollees in the 50–59, 60–69, and 70–79 age groups, respectively. The question subsequently arose as to whether trial results differed from those hypothesized, at least in part, because trial enrollees were older and further from menopause onset compared with women in the community making hormone therapy decisions, frequently motivated by vasomotor symptom control. The topic is particularly germane for breast cancer where, over cumulative follow-up of 13 years, an anticipated significant increase was observed with CEE + MPA while an unexpected significant decrease was observed with CEE alone (5). In contrast, a recent summary of worldwide observational data reported breast cancer risk increases with both estrogen-alone and estrogen + progestin regimens, with larger hazard ratios for the latter. In this report, the randomized trial evidence, mostly from the WHI trials, was described as “largely for hormone use starting after age 60 years” (6).
Here we report additional data, and additional data analyses, from the WHI hormone therapy trials, with a focus on participants who were 50–59 years of age, like many women considering hormone therapy in the community. Most trial participants continued postintervention follow-up beyond median intervention periods of 5.6 years in the CEE + MPA trial and 7.2 years in the CEE trial. Disease incidence (5) and mortality results (7), including periodic National Death Index matching for all trial participants, have been reported through September 30, 2010, and December 31, 2014, respectively. Here we report results from follow-up through December 31, 2016.
Even with such substantial follow-up, the numbers of women experiencing some important clinical outcomes remains small when restricted to women of ages 50–59 years at enrollment, leading to an unclear balance of risks and benefits in this subset (8). Consequently, to enhance precision, we present novel analyses using a “parsimonious” hazard ratio model that focuses on the age group 50–59 years while leveraging data from all randomized enrollees (see Methods). The clinical outcomes considered are the components of the protocol-defined global index, namely: CHD, (invasive) breast cancer, stroke, pulmonary embolism, colorectal cancer, endometrial cancer, hip fracture, and all-cause mortality. Additionally, we considered a multivariate global index that utilizes all the multiple global index outcomes, rather than just a participant’s earliest postrandomization outcome, in an effort to enhance precision of global benefit versus risk assessments (9, 10).
METHODS
Study design and conduct
The design of the WHI hormone therapy trials, including adherence assessment and outcome ascertainment methods, has been described (11–13). Institutional review board approval was obtained from participating clinical centers and the clinical coordinating center (clinicaltrials.gov identifier: NCT00000611). All participants provided written informed consent for their clinical trial participation. The CEE + MPA trial was stopped early on July 7, 2002, when the external Data and Safety Monitoring Board judged that health risks exceeded benefits (1), while the CEE trial was stopped early on February 29, 2004, primarily due to an increased stroke risk (4). Participants were re-consented to additional years of nonintervention follow-up that continues. Postintervention follow-up was available through December 31, 2016, for these analyses, providing a median 18 years of cumulative follow-up for most outcomes in both trials. Participants were informed of their randomized assignment following intervention stoppage. Subsequently, fewer than 4% reported postintervention use of systemic estrogens.
Statistical methods
Hazard ratio (Cox) models were specified for the marginal hazard rates (14–17) for each of the global index outcomes. Only the first postrandomization event for each outcome type was included in analyses. Baseline rates in these models were stratified on age at enrollment (in years: 50–59, 60–69, 70–79), randomization status in the companion WHI Dietary Modification trial (intervention, comparison, not randomized), prior diagnosis of the specific disease (if applicable), race/ethnicity (White, Black, other), prior menopausal hormone therapy use (no, yes), and time-dependent study phase (intervention, postintervention). Time to response for a specific outcome was defined as days from randomization to outcome occurrence, with follow-up times for participants not experiencing the specific event type during the study phase censored at the earliest of the end of the study phase, loss to follow-up, death not due to the specific outcome, or the end of the participant’s follow-up consent period. Multivariate analyses for the set of global index outcomes used the Cox models just mentioned for marginal hazard rates, while using the marginal modeling methods (15–17) for hormone therapy hazard ratio estimation. These methods properly acknowledge dependencies among the multiple outcomes types.
Two hazard ratio models were applied, each with a focus on hormone therapy influences among women aged 50–59 years at enrollment: The first included a separate hormone therapy hazard ratio parameter for each possible combination of age at enrollment decade (in years: 50–59, 60–69, 70–79) and global index clinical outcomes. This “saturated” hormone therapy hazard ratio model included main effects by age and outcome category as well as hazard ratio interactions between age and outcome type. For participants in the 50–59 age group, standard Cox model analyses under the first model reduced to analyses using only data from the 50–59 subcohort. In comparison, analyses under the second “parsimonious” model leveraged data from all randomized participants. This parsimonious model assumed no age-by–clinical outcome interaction on the hormone therapy hazard ratio, an assumption that is readily tested for consistency with trial data, and P values for testing this assumption are presented. Alternatively stated, the hormone therapy hazard ratio’s dependence on baseline age does not differ among the outcome types under this parsimonious model. See Web Appendix 1 (available at https://academic.oup.com/aje) for an explicit explanation of hazard rate assumptions for the 2 models.
Estimated hazard ratios and 95% confidence intervals are presented for each outcome type under both the saturated and parsimonious hazard ratio models, the latter provided in the absence of evidence against parsimonious model assumptions. The parsimonious model analyses have potential to more precisely estimate treatment hazard ratios in the 50–59 age group compared with the saturated model analyses.
In addition to Cox model analyses for the global index (time to earliest of the global index outcomes), a multivariate global index hazard ratio analysis was carried out to make fuller use of multiple outcome events for individual participants using the same hazard rate models, by imposing a common hazard ratio constraint across clinical outcome categories for the saturated model (15–17) or by constructing a minimum variance weighted average of outcome-specific hazard ratio estimates for the parsimonious model (15).
Further analyses allowed hormone therapy hazard ratios to depend on additional participant characteristics. We previously presented combined analyses of WHI Clinical Trial and Observational Study data indicating more favorable hormone therapy results on breast cancer for both CEE and CEE + MPA among women having relatively long time intervals from menopause to first use of hormone therapy (18). Similar breast cancer hazard ratio patterns were seen in the Million Women’s Study cohort (19). Subsequent analyses in the WHI CEE trial showed that intervention group participants aged 50–59 years with bilateral oophorectomy in addition to hysterectomy at enrollment had a nominally significant reduction in total mortality over cumulative follow-up (20).
These subset analyses suggest that duration in a state of estrogen deprivation might be an important timing issue. Accordingly, we also calculated hormone therapy hazard ratios among women aged 50–59 years according to whether a participant’s “gap” time period of estrogen deprivation was <5 or ≥5 years, under both the saturated and parsimonious hazard ratio models. Gap time is defined as the larger of zero and the time from the earlier of bilateral oophorectomy or age 50 to the earlier of first use of menopausal hormone therapy or trial enrollment.
Two-sided P values were considered significant if 0.05, without multiple testing correction. Related to this, a cautious interpretation is needed, especially for outcomes other than CHD and breast cancer and the (univariate) global index.
Statistical analyses were conducted using the SAS (SAS Institute, Inc., Cary, North Carolina) procedure PHREG, which incorporates multivariate marginal Cox model methodology.
RESULTS
Web Figure 1 shows participant flow in the 2 hormone therapy trials through the (median) 18-year cumulative follow-up. Baseline characteristics for women 50–59 years of age at enrollment were well-balanced between randomization groups, in both the CEE and CEE + MPA trials (Table 1). Compared with the CEE + MPA trial, participants in the CEE trial had higher rates of obesity and of prior hormone therapy use.
Table 1.
Baseline Characteristics of Participants Aged 50–59 Years, Postmenopausal When Enrolled in the Women’s Health Initiative Clinical Trials of Postmenopausal Hormone Therapy Across 40 Clinical Centers, United States, 1993–1998
| Trial of CEE Alone | Trial of CEE + MPA | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | CEE (n = 1,639) | Placebo (n = 1,674) | CEE + MPA (n = 2,837) | Placebo (n = 2,683) | ||||
| No. | % | No. | % | No. | % | No. | % | |
| Age at screening, yearsa | 54.9 (2.8) | 54.9 (2.9) | 55.2 (2.6) | 55.3 (2.6) | ||||
| Age group at screening, years | ||||||||
| 50–54 | 687 | 41.9 | 709 | 42.4 | 1,041 | 36.7 | 983 | 36.6 |
| 55–59 | 952 | 58.1 | 965 | 57.6 | 1796 | 63.3 | 1700 | 63.4 |
| Race/ethnicity | ||||||||
| White | 1,085 | 66.2 | 1,098 | 65.6 | 2,192 | 77.3 | 2061 | 76.8 |
| Black | 317 | 19.3 | 349 | 20.8 | 255 | 9.0 | 279 | 10.4 |
| Hispanic | 164 | 10.0 | 172 | 10.3 | 265 | 9.3 | 226 | 8.4 |
| American Indian | 18 | 1.1 | 10 | 0.6 | 11 | 0.4 | 16 | 0.6 |
| Asian/Pacific Islander | 29 | 1.8 | 23 | 1.4 | 68 | 2.4 | 63 | 2.3 |
| Unknown | 26 | 1.6 | 22 | 1.3 | 46 | 1.6 | 38 | 1.4 |
| Beyond high school/GED | 1,130 | 69.8 | 1,182 | 71.7 | 2,184 | 77.6 | 2015 | 75.9 |
| Family income at least $50,000 | 509 | 32.6 | 516 | 32.7 | 1,099 | 40.8 | 1,045 | 40.9 |
| No. of years since menopause | ||||||||
| <5 | 281 | 20.4 | 288 | 20.3 | 1,196 | 47.5 | 1,137 | 46.2 |
| 5–9 | 331 | 24.0 | 338 | 23.9 | 871 | 34.6 | 886 | 36.0 |
| ≥10 | 767 | 55.6 | 790 | 55.8 | 452 | 17.9 | 436 | 17.7 |
| HT use status | ||||||||
| Never used | 841 | 51.3 | 831 | 49.6 | 1983 | 69.9 | 1951 | 72.7 |
| Past user | 513 | 31.3 | 531 | 31.7 | 553 | 19.5 | 482 | 18.0 |
| Current userb | 285 | 17.4 | 312 | 18.6 | 301 | 10.6 | 250 | 9.3 |
| Baseline vasomotor symptoms | ||||||||
| None | 609 | 37.5 | 636 | 38.4 | 1,195 | 42.6 | 1,100 | 41.4 |
| Mild | 560 | 34.4 | 573 | 34.6 | 972 | 34.6 | 975 | 36.7 |
| Moderate/severe | 457 | 28.1 | 448 | 27.0 | 641 | 22.8 | 584 | 22.0 |
| BMIc,d | 30.2 (8.6) | 30.1 (8.7) | 27.7 (7.9) | 27.8 (8.2) | ||||
| BMIc | ||||||||
| <25 | 290 | 17.8 | 269 | 16.1 | 840 | 29.8 | 806 | 30.2 |
| 25–29 | 511 | 31.4 | 544 | 32.7 | 962 | 34.1 | 862 | 32.3 |
| ≥30 | 827 | 50.8 | 853 | 51.2 | 1,017 | 36.1 | 999 | 37.5 |
| Systolic BP, mm Hga | 124.8 (15.8) | 125.1 (16.2) | 121.7 (15.7) | 122.3 (16.1) | ||||
| Diastolic BP, mm Hga | 78.0 (9.0) | 77.9 (9.1) | 76.4 (9.0) | 76.6 (8.9) | ||||
| Smoking status | ||||||||
| Never | 789 | 48.6 | 769 | 46.2 | 1,300 | 46.2 | 1,254 | 47.3 |
| Past | 597 | 36.8 | 646 | 38.8 | 1,104 | 39.2 | 978 | 36.9 |
| Current | 237 | 14.6 | 249 | 15.0 | 411 | 14.6 | 420 | 15.8 |
| Bilateral oophorectomy | 530 | 34.1 | 599 | 37.8 | 6 | 0.2 | 5 | 0.2 |
| Treated diabetes (pills or shots) | 114 | 7.0 | 107 | 6.4 | 103 | 3.6 | 109 | 4.1 |
| Hypertensive (self-report or high BP) | 625 | 42.1 | 657 | 43.2 | 797 | 31.5 | 772 | 31.0 |
| High cholesterol | 134 | 8.2 | 158 | 9.4 | 163 | 5.7 | 167 | 6.2 |
| Statin use | 54 | 3.3 | 66 | 3.9 | 59 | 2.1 | 66 | 2.5 |
| Aspirin use of ≥80 mg for ≥30 days | 204 | 12.4 | 197 | 11.8 | 346 | 12.2 | 350 | 13.0 |
| MI ever | 22 | 1.3 | 21 | 1.3 | 18 | 0.6 | 16 | 0.6 |
| History of angina | 75 | 4.6 | 64 | 3.8 | 59 | 2.1 | 54 | 2.0 |
| History of CABG/PCI | 17 | 1.0 | 15 | 0.9 | 6 | 0.2 | 11 | 0.4 |
| Stroke ever | 9 | 0.5 | 17 | 1.0 | 12 | 0.4 | 10 | 0.4 |
| History of DVT/PE | 33 | 2.0 | 28 | 1.7 | 24 | 0.8 | 17 | 0.6 |
| History of fracture, age ≥55 | 40 | 5.1 | 42 | 5.3 | 69 | 4.8 | 62 | 4.3 |
| Family history of female relative w/breast cancer | 285 | 18.5 | 261 | 16.4 | 403 | 14.9 | 371 | 14.6 |
Abbreviations: BMI, body mass index; BP, blood pressure; CABG, coronary artery bypass graft; CEE, conjugated equine estrogens; DVT, deep vein thrombosis; GED, General Educational Development certificate; HT, menopausal hormone therapy; MI, myocardial infarction; MPA, medroxyprogesterone acetate; PCI, percutaneous coronary intervention; PE, pulmonary embolism.
a Values are expressed as mean (standard deviation).
b Required a 3-month washout prior to randomization.
c Weight (kg)/height (m)2.
d Values are expressed as median (interquartile range).
Table 2 shows outcome counts and disease rates for the global index, its multivariate extensions, and each of its components in the CEE trial. Corresponding analyses under the saturated hazard ratio model are shown on the left side of Figure 1. Neither the global index, its multivariate extension, nor any of its 7 components differed significantly between CEE and placebo over the trial intervention phase in this analysis. However, under the parsimonious model (right side of Figure 1), there was an estimated reduced risk for breast cancer and hip fracture, with CEE hazard ratios of 0.63 (95% confidence interval (CI): 0.45, 0.88) and 0.48 (0.30, 0.76), respectively. The latter estimate requires a very cautious interpretation because of the paucity of hip fractures in the 50–59 age group. A test for the no age-by–outcome type hazard ratio interaction yielded P = 0.12, so that evidence against the parsimonious model is nonsignificant. The multivariate global index was not significantly reduced for CEE during the intervention phase under either model, with a hazard ratio of 0.80 (95% CI: 0.62, 1.03) under the saturated model, and 0.81 (95% CI: 0.63, 1.04) under the parsimonious model.
Table 2.
Counts and Annualized Rates for Outcomes in the Women’s Health Initiative Estrogen-Alone Trial Among Postmenopausal Women Enrolled in 1993–1998 at Ages 50–79 Years Across 40 Clinical Centers, United States, With Cumulative Follow-up Through 2016
| Subgroup Aged 50–59 Years (n = 3,313) | Trial of CEE Alone (n = 10,739) | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | CEE (n = 1,639) | Placebo (n = 1,674) | CEE (n = 5,310) | Placebo (n = 5,429) | ||||
| No. | % | No. | % | No. | % | No. | % | |
| Intervention Phase | ||||||||
| Primary outcomes | ||||||||
| Coronary heart disease | 21 | 0.17 | 35 | 0.28 | 205 | 0.55 | 222 | 0.58 |
| Invasive breast cancer | 29 | 0.24 | 36 | 0.29 | 103 | 0.28 | 135 | 0.35 |
| Other monitored outcomes | ||||||||
| Stroke | 19 | 0.16 | 21 | 0.17 | 174 | 0.47 | 130 | 0.34 |
| Pulmonary embolism | 12 | 0.099 | 8 | 0.064 | 52 | 0.14 | 39 | 0.10 |
| Colorectal cancer | 9 | 0.074 | 13 | 0.10 | 65 | 0.17 | 58 | 0.15 |
| Hip fracture | 5 | 0.041 | 1 | 0.008 | 48 | 0.13 | 74 | 0.19 |
| All-cause mortalitya | 35 | 0.29 | 50 | 0.40 | 301 | 0.80 | 299 | 0.77 |
| Global indices | ||||||||
| Univariate | 117 | 0.98 | 142 | 1.17 | 756 | 2.09 | 754 | 2.04 |
| Multivariate | 130 | 1.07 | 164 | 1.31 | 948 | 2.51 | 957 | 2.47 |
| Cumulative Follow-up | ||||||||
| Primary outcomes | ||||||||
| Coronary heart disease | 71 | 0.27 | 91 | 0.35 | 521 | 0.69 | 550 | 0.71 |
| Invasive breast cancer | 73 | 0.28 | 92 | 0.36 | 231 | 0.30 | 291 | 0.38 |
| Other monitored outcomes | ||||||||
| Stroke | 49 | 0.19 | 58 | 0.22 | 399 | 0.53 | 392 | 0.50 |
| Pulmonary embolism | 35 | 0.13 | 34 | 0.13 | 153 | 0.20 | 150 | 0.19 |
| Colorectal cancer | 18 | 0.069 | 26 | 0.10 | 119 | 0.15 | 118 | 0.15 |
| Hip fracture | 19 | 0.073 | 20 | 0.077 | 208 | 0.27 | 229 | 0.29 |
| All-cause mortalitya | 156 | 0.59 | 184 | 0.70 | 1,258 | 1.62 | 1,277 | 1.61 |
| Global indices | ||||||||
| Univariate | 334 | 1.35 | 393 | 1.61 | 1997 | 2.84 | 2078 | 2.91 |
| Multivariate | 421 | 1.61 | 505 | 1.93 | 2,889 | 3.72 | 3,007 | 3.79 |
Abbreviation: CEE, conjugated equine estrogens
a For consistency with other monitored outcomes included in the global index, extended follow-up includes only participants who provided consent for long-term follow-up.
Figure 1.
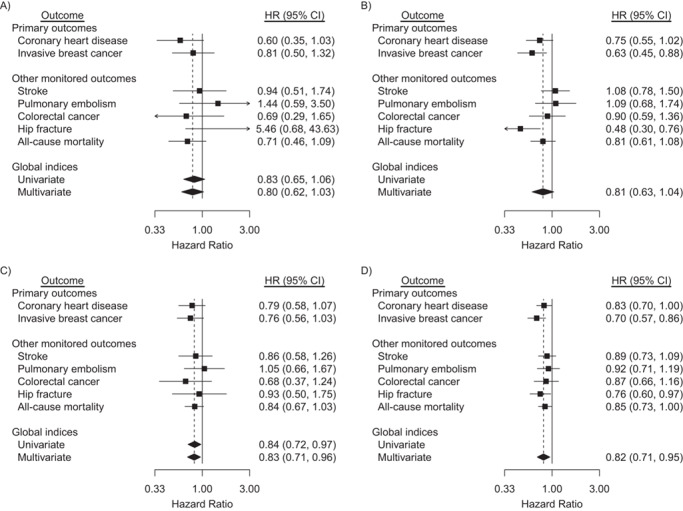
Hazard ratios (HRs) and 95% confidence intervals (CIs) for outcomes in the conjugated equine estrogens (CEE)-alone trial during the intervention phase and cumulative follow-up among participants aged 50–59 years at randomization, Women’s Health Initiative, United States, 1993–2016. Participants were postmenopausal and aged 50–79 when enrolled at 40 US clinical centers during 1993–1998. Cumulative follow-up is through 2016. HRs for primary and other monitored outcomes are from a multivariate marginal Cox regression model stratified by 10-year age group, randomization status in the Diet Modification trial, prior disease (if applicable), race/ethnicity, prior hormone therapy use, study phase (time-dependent), and outcome type. The left forest plots (saturated model, panels A and C) are derived by including regression terms for CEE and product interaction terms for CEE × age group, CEE × outcome type, and CEE × outcome type × age group. The right forest plots (parsimonious model, panels B and D) also provide estimated HRs for women aged 50–59 years but drop the 3-factor interaction, CEE × age group × outcome type, so that estimates are based on a common CEE × outcome type interaction. Dashed reference lines correspond to estimated HR for multivariate global indices. Similar summaries for women aged 60–69 and 70–79 years can be found in supplemental Web Figures 2a and 2b, respectively. Time to first event of any monitored outcome defines the univariate global index; summary statistics are from a univariate Cox regression model with stratification described above. Marginal estimate of a common HR for monitored outcomes defines the multivariate global index. Robust sandwich-estimators for variances account for within participant correlation of multivariate failure times. For consistency with other monitored outcomes included in the global index, extended follow-up includes only participants who provided consent for long-term follow-up.
Over cumulative follow-up (Figure 1), the components of the global index did not significantly differ between CEE and placebo under the saturated model, although the global index was reduced, with a hazard ratio of 0.84 (95% CI: 0.72, 0.97). In comparison, under the parsimonious model, which is quite consistent with the data over cumulative follow-up (hazard ratio interaction P = 0.79), there is evidence for hazard ratio reduction in multiple global index components, with hazard ratios of 0.83 (95% CI: 0.70, 1.00) for CHD, 0.70 (95% CI: 0.57, 0.86) for breast cancer, 0.76 (95% CI: 0.60, 0.97) for hip fracture, and 0.85 (95% CI: 0.73, 1.00) for all-cause mortality. The multivariate global index has hazard ratio values of 0.83 (95% CI: 0.71, 0.96) and 0.82 (95% CI: 0.71, 0.95) under the saturated and parsimonious models, respectively. Note that outcome-specific hazard ratios are mostly similar between the 2 models, while confidence intervals are considerably narrower under the parsimonious model.
Likewise, Table 3 gives outcome counts and disease rates for CEE + MPA while Figure 2 gives corresponding CEE +MPA hazard ratio analyses. During the intervention phase, the hazard ratio does not differ significantly between CEE + MPA and placebo for the global index, its multivariate extension, or any of its 8 components. In comparison, under the parsimonious model (interaction P = 0.76), there is evidence of increased pulmonary embolism risk with CEE + MPA, as well as of reduced colorectal cancer and hip fracture risk, with respective hazard ratios of 1.91 (95% CI: 1.25, 2.90), 0.58 (95% CI: 0.38, 0.89), and 0.61 (95% CI: 0.40, 0.94). Over cumulative follow-up, neither the global index nor its multivariate extensions differ significantly between CEE + MPA and placebo. Breast cancer risk was elevated under both the saturated and the parsimonious model (interaction P = 0.74), with hazard ratios of 1.38 (95% CI: 1.11, 1.72) and 1.29 (95% CI: 1.11, 1.50) respectively, and endometrial cancer risk was reduced under the parsimonious model with hazard ratio of 0.74 (95% CI: 0.56, 0.98).
Table 3.
Counts and Annualized Rates for Outcomes in the Women’s Health Initiative Estrogen + Progestin Trial Among Postmenopausal Women Enrolled in 1993–1998 at Ages 50–79 Years Across 40 Clinical Centers, United States, With Cumulative Follow-up Through 2016
| Subgroup Aged 50–59 Years (n = 5,520) | Trial of CEE + MPA (n = 16,608) | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | CEE + MPA (n = 2,837) | Placebo (n = 2,683) | CEE + MPA (n = 8,506) | Placebo (n = 8,102) | ||||
| No. | % | No | % | No. | % | No | % | |
| Intervention Phase | ||||||||
| Primary outcomes | ||||||||
| Coronary heart disease | 38 | 0.23 | 27 | 0.17 | 196 | 0.41 | 159 | 0.35 |
| Invasive breast cancer | 55 | 0.33 | 42 | 0.27 | 205 | 0.43 | 155 | 0.35 |
| Other monitored outcomes | ||||||||
| Stroke | 26 | 0.15 | 16 | 0.10 | 159 | 0.33 | 110 | 0.24 |
| Pulmonary embolism | 18 | 0.11 | 8 | 0.051 | 87 | 0.18 | 41 | 0.091 |
| Colorectal cancer | 7 | 0.041 | 8 | 0.051 | 50 | 0.10 | 76 | 0.17 |
| Endometrial cancer | 6 | 0.035 | 5 | 0.032 | 27 | 0.056 | 30 | 0.066 |
| Hip fracture | 1 | 0.006 | 5 | 0.032 | 53 | 0.11 | 75 | 0.17 |
| All-cause mortalitya | 35 | 0.21 | 48 | 0.31 | 250 | 0.52 | 238 | 0.53 |
| Global indices | ||||||||
| Univariate | 170 | 1.03 | 141 | 0.91 | 875 | 1.88 | 737 | 1.68 |
| Multivariate | 186 | 1.10 | 159 | 1.01 | 1,027 | 2.13 | 884 | 1.96 |
| Cumulative Follow-up | ||||||||
| Primary outcomes | ||||||||
| Coronary heart disease | 129 | 0.28 | 114 | 0.26 | 710 | 0.55 | 652 | 0.53 |
| Invasive breast cancer | 198 | 0.43 | 136 | 0.31 | 574 | 0.45 | 432 | 0.36 |
| Other monitored outcomes | ||||||||
| Stroke | 87 | 0.19 | 77 | 0.17 | 579 | 0.45 | 492 | 0.40 |
| Pulmonary embolism | 55 | 0.12 | 48 | 0.11 | 235 | 0.18 | 199 | 0.16 |
| Colorectal cancer | 44 | 0.094 | 35 | 0.079 | 178 | 0.14 | 197 | 0.16 |
| Endometrial cancer | 39 | 0.083 | 47 | 0.11 | 97 | 0.74 | 127 | 0.10 |
| Hip fracture | 43 | 0.091 | 48 | 0.11 | 394 | 0.30 | 421 | 0.34 |
| All-cause mortalitya | 266 | 0.56 | 267 | 0.60 | 1870 | 1.42 | 1793 | 1.44 |
| Global indices | ||||||||
| Univariate | 673 | 1.55 | 612 | 1.47 | 3,265 | 2.78 | 3,009 | 2.67 |
| Multivariate | 861 | 1.82 | 772 | 1.74 | 4,637 | 3.52 | 4,313 | 3.45 |
Abbreviations: CEE, conjugated equine estrogens; MPA, medroxyprogesterone acetate.
a For consistency with other monitored outcomes included in the global index, extended follow-up includes only participants who provided consent for long-term follow-up.
Figure 2.
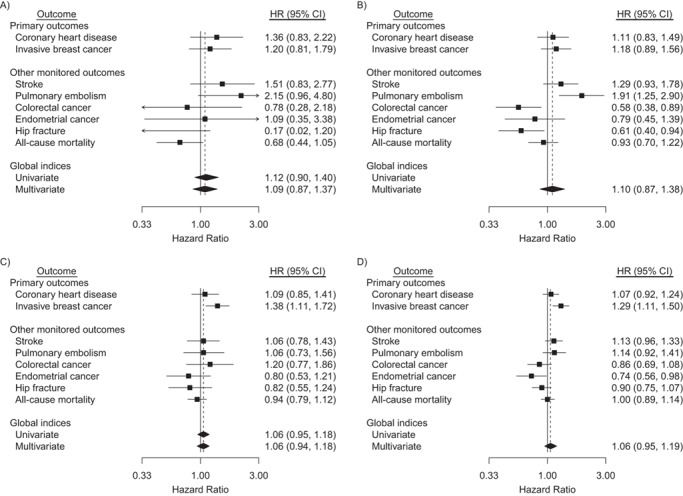
Hazard ratios (HRs) and 95% confidence intervals (CIs) for outcomes in the conjugated equine estrogens (CEE) + medroxyprogesterone acetate (MPA) trial during the intervention phase and cumulative follow-up among participants aged 50–59 years at randomization, Women’s Health Initiative, United States, 1993–2016. Participants were postmenopausal and aged 50–79 when enrolled at 40 US clinical centers during 1993–1998. Cumulative follow-up is through 2016. Panels A and B summarize the intervention phase, and panels C and D summarize cumulative follow-up. HRs for primary and other monitored outcomes are from a multivariate marginal Cox regression model stratified by 10-year age group, randomization status in the Diet Modification trial, prior disease (if applicable), race/ethnicity, prior hormone therapy use, study phase (time-dependent), and outcome type. The left forest plots (saturated model, panels A and C) are derived by including regression terms for CEE + MPA and product interaction terms for CEE + MPA × age group, CEE + MPA × outcome type, and CEE + MPA × outcome type × age group. The right forest plots (parsimonious model, panels B and D) also provide estimated HRs for women aged 50–59 years but drop the 3-factor interaction, CEE + MPA × age group × outcome type, so that estimates are based on a common CEE + MPA × outcome type interaction. Dashed reference lines correspond to estimated HR for multivariate global indices. Similar summaries for women aged 60–69 and 70–79 years can be found in supplemental Web Figures 3a and 3b, respectively. Time to first event of any monitored outcome defines the univariate global index; summary statistics are from a univariate Cox regression model with stratification described above. Marginal estimate of a common HR for monitored outcomes defines the multivariate global index. Robust sandwich-estimators for variances account within participant correlation of multivariate failure times. For consistency with other monitored outcomes included in the global index, extended follow-up includes only participants who provided consent for long-term follow-up.
Parsimonious model analyses also produce hormone therapy hazard ratios for women of ages 60–69 and 70–79 years at enrollment. These differ from those for ages 50–59 by estimated multiplicative factors that are common across outcomes. For CEE at ages 60–69 these estimated factors are 1.26 (95% CI: 0.94, 1.70) in the intervention phase, and 1.20 (95% CI: 1.01, 1.43) over cumulative follow-up. Corresponding values for ages 70–79 are 1.42 (95% CI: 1.04, 1.94) during intervention and 1.21 (95% CI: 1.01, 1.46) over cumulative follow-up. Estimated multiplicative factors for CEE + MPA at ages 60–69 years are 1.03 (95% CI: 0.78, 1.36) and 0.97 (95% CI: 0.85, 1.11) during intervention and cumulative follow-up respectively, and for ages 70–79 are 1.10 (95% CI: 0.83, 1.47) and 1.01 (95% CI: 0.87, 1.16) during intervention and cumulative follow-up respectively. Outcome-specific hormone therapy hazard ratios and confidence intervals for these older age groups are presented in Web Figures 2 and 3.
The results of analyses that allowed separate hazard ratios for participants having (estrogen deprivation) gap times <5 years and ≥5 years, while retaining all other components of the previous parsimonious model, are shown in Web Figure 4 for CEE and Web Figure 5 for CEE + MPA. From Web Figure 4 the multivariate global index provides little evidence of CEE influence, with a hazard ratio of 0.96 (95% CI: 0.69, 1.33) over the intervention period among participants with gap time <5 years. However, there is evidence of health benefits among participants having gap times ≥5 years with a multivariate global index hazard ratio of 0.64 (95% CI: 0.43, 0.96), along with evidence for breast cancer and for CHD risk reduction during the intervention period. However, a test comparing global index hazard ratios between gap time categories was not significant (P = 0.23). These hazard ratio patterns tend to continue over cumulative follow-up, but hazard ratio differences between gap time categories attenuate, with a multivariate global index hazard ratio of 0.83 (95% CI: 0.69, 1.00) for gap times <5 years and 0.78 (95% CI: 0.62, 0.99) for those with gap time ≥5 years, respectively (P = 0.85 for test of global index hazard ratio (HR) equality).
The CEE + MPA hazard ratios for breast cancer tend to be comparatively lower among participants having gap times of ≥5 years versus <5 years in both the intervention phase and over cumulative follow-up, but this pattern is not at all evident for CHD (Web Figure 5). For the intervention phase, the multivariate global index hazard ratios are 0.97 (95% CI: 0.69, 1.35) and 1.25 (95% CI: 0.91, 1.71) among participants with gap time <5 years and ≥5 years, respectively (P = 0.25 for test of global index HR equality). Over cumulative follow-up, multivariate hazard ratios for the global index are 1.05 (95% CI: 0.90, 1.24) and 1.08 (95% CI: 0.93, 1.26) for participants with gap time <5 years and ≥5 years, respectively (P = 0.76 for test of global index HR equality).
For CEE hazard ratios among participants having gap times of 5 years or more there was evidence of breast cancer risk reduction whether participants did (HR = 0.55, 95% CI: 0.32, 0.95) or did not (HR = 0.61, 95% CI: 0.41, 0.90) have prior oophorectomy. These analyses do not identify any subsets where the hazard ratio for breast cancer was elevated with CEE, either during the intervention period or over cumulative follow-up.
DISCUSSION
Analyses presented here indicate that baseline age modifies the balance of benefits and risks with CEE, with hazard ratios 20%–40% higher for older women compared with those aged 50–59 years when starting hormone treatment. Our results are consistent with previous WHI reports (4, 5, 18), but parsimonious model analyses allow previously reported comparisons to be reexamined with considerably greater precision. Accordingly, we can now assess that (median) 7.2 years of CEE intervention yields estimated global index health benefits that exceed risks for women who were 50–59 when randomized and were followed for (median) 18 years. Specific risk reductions are estimated for breast cancer, CHD, and total mortality, without significant adverse results for any other global index outcome. Even for stroke, which played a role in early stoppage of the CEE trial, there is little evidence of risk elevation for women aged 50–59.
The parsimonious model assumption also agrees well with CEE + MPA trial data and indicates that the global index was not significantly altered by (median) 5.6 years of CEE + MPA intervention among women 50–59. However, certain global index components were evidently affected, including a breast cancer risk elevation. Again these findings are consistent with previous WHI reports, while leading to comparatively more precise results.
The strongly held belief that important CHD benefits would follow for commonly used CEE and CEE + MPA regimens was corroborated here only for CEE, and then only in a borderline significant fashion, among participants aged 50–59.
These findings continue to point to harm when MPA is added to CEE, even when focusing on participants aged 50–59. MPA might override health benefits associated with favorable sex hormone profiles (21). For breast cancer, antiinflammatory properties of MPA might interfere with apoptosis of undetected tumor cells that could otherwise be induced following a sustained period of estrogen deprivation (22, 23). Strategies to combine CEE with a selective estrogen receptor modulator, instead of MPA or other progestin, have been developed and approved by the Food and Drug Administration (24, 25).
The favorable health benefits versus risks with CEE during the intervention period, especially evident for breast cancer, might derive (Web Figure 4) primarily from participants who had some years of estrogen deprivation prior to randomization, whereas participants having the short gap times that characterize CEE use among post-hysterectomy women in the community do not show breast cancer risk reduction. Analyses cross-classified by gap time and bilateral oophorectomy status suggest that breast cancer benefits with CEE at ages 50–59 are more closely tied to a large gap time than to prior bilateral oophorectomy status (20). If the benefits of CEE in this age range are largely restricted to women having lengthy prior estrogen deprivation time periods, then the clinical relevance of these findings could be reduced.
The recent summary of the worldwide observational breast cancer literature reported a significantly elevated risk with estrogens alone (6). With focus on women aged 50–59 years, the WHI randomized trials do not suggest any such elevation overall (Figure 1), or when stratified by gap time (Web Figure 4), prior bilateral oophorectomy status, or by both gap time and oophorectomy status. In contrast, there is evidence of breast cancer risk reduction in this age group, overall and among women who begin taking estrogens following estrogen deprivation. The observational literature might include biases due to uncontrolled confounding or due to the absence of a clinical context to ensure equal mammography utilization, and to ensure equal outcome ascertainment, between hormone therapy users and nonusers. For example, in our combined analyses of WHI Clinical Trial and Observational Study data (18), we observed elevated breast cancer hazard ratios in the Observational Study component until a careful account was taken of each participant’s screening mammography history prior to and over the study follow-up period. Note, however, that WHI trial results, even under the parsimonious model, are not sufficiently precise to exclude some breast cancer risk elevation among women having gap times <5 years.
The principal strength of this report is the randomized, placebo-controlled design of the WHI hormone therapy trials of the most commonly used estrogen alone and estrogen + progestin therapy preparations in the United States at the time the trials were initiated (1993). Additional strengths include high-quality clinical outcome data over a cumulative follow-up period of (median) 18 years, providing substantial information on the long-term health effects of a median of 7.2 years (CEE) and 5.6 years (CEE + MPA) of hormone therapy intervention. Other strengths include baseline hormone therapy history obtained for all WHI participants, through personal interview, as well as the protocol-required annual mammography screening during the intervention phase of the hormone treatment trials.
A limitation is that many participants were older and had a longer period of estrogen deprivation prior to starting hormone therapy than is the case for women making hormone therapy decisions in community settings. Also, trial results are imprecise when restricted to younger women. Here we mitigate this latter limitation by leveraging the entire cohort in a manner consistent with trial data, substantially enhancing the precision of hormone therapy hazard ratio estimates. Other limitations arise from some lack of adherence to study pills (1, 4) during the trial intervention periods, from the need to re-consent participants following the intervention period (Web Figure 1), and from multiple testing issues, especially as relates to the several outcomes included in the global index for each trial.
In summary, additional analysis of WHI randomized trial data, under a parsimonious model with additional years of follow-up, are consistent with prior reports while providing stronger evidence that long-term health benefits exceed risks for CEE at ages 50–59 years in the population of post-hysterectomy women studied. In comparison, CEE + MPA among women aged 50–59 among women having an intact uterus leads to some serious health risks, precluding its use for disease-prevention purposes. These results substantially agree with the worldwide observational study literature for similar menopausal hormone therapy preparations, with the exception of CEE use and breast cancer risk.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, United States (Ross L. Prentice, Aaron K. Aragaki, Garnet L. Anderson); Los Angeles Biomedical Research Institute at Harbor–UCLA Medical Center, Torrance, California, United States (Rowan T. Chlebowski); National Heart, Lung, and Blood Institute, Bethesda, Maryland, United States (Jacques E. Rossouw); Stanford Prevention Research Center, Stanford University, Palo Alto, California, United States (Marcia L. Stefanick); Department of Epidemiology and Environmental Health, School of Public Health and Health Professions, University at Buffalo, the State University of New York, Buffalo, New York, United States (Jean Wactawski-Wende); Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania, United States (Lewis H. Kuller); Department of Epidemiology, College of Public Health, University of Iowa, Iowa City, Iowa, United States (Robert Wallace); Department of Preventive Medicine, University of Tennessee Health Science Center, Memphis, Tennessee, United States (Karen C. Johnson); Department of Family Medicine and Public Health, University of California, San Diego School of Medicine, La Jolla, California, United States (Aladdin H. Shadyab); North American Menopause Society Emeritus, Pepper Pike, Ohio, United States (Margery Gass); and Division of Preventive Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, United States (JoAnn E. Manson).
This work was supported by the National Heart, Lung, and Blood Institute through which the Women’s Health Initiative infrastructure is supported (contracts HHSN268201100046C, HSN268201600001C, HHSN268201600002C, HHSN268201600003C, HHSN268201600004C, and HHSN271201600004C) and by the National Cancer Institute (grants R01 CA119171 and R01 CA210921).
The authors acknowledge the following investigators in the Women’s Health Initiative Program: Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller. Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross L. Prentice, Andrea LaCroix, and Charles Kooperberg. Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Jennifer Robinson; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker; (University of Nevada, Reno, NV) Robert Brunner. Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Mark Espeland. For a list of all the investigators who have contributed to WHI science, please visit: https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long20List.pdf.
Women’s Health Initiative investigators and representatives of the National Heart, Lung, and Blood Institute collaborated on the design and conduct of the trial, including data interpretation, management, analysis, manuscript review and approval, and decision to submit the manuscript for publication. The views expressed are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the US Department of Health and Human Services.
R.T.C. is a consultant for Novartis, AstraZeneca, Genentech, Merck, Immunomedics, and Puma and received honoraria from Novartis and AstraZeneca. The other authors report no conflicts.
REFERENCES
- 1. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. [DOI] [PubMed] [Google Scholar]
- 2. Ettinger B, Wang SM, Scott LR, et al. Evolution of postmenopausal hormone therapy between 2002 and 2009. Menopause. 2012;19(6):610–615. [DOI] [PubMed] [Google Scholar]
- 3. Crawford SL, Crandall CJ, Derby CA, et al. Menopausal hormone therapy trends before versus after 2002: impact of the Women’s Health Initiative study results. Menopause. 2018;26(6):588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogens in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. [DOI] [PubMed] [Google Scholar]
- 5. Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collaborative Group on Hormonal Factors in Breast Cancer . Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019;394(10204):1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women's Health Initiative randomized trials. JAMA. 2017;318(10):927–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. US Preventive Services Task Force, Grossman DC, Curry SJ, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224–2233. [DOI] [PubMed] [Google Scholar]
- 9. Armstrong PW, Westerhout CM. Composite end points in clinical research: a time for reappraisal. Circulation. 2017;135(23):2299–2307. [DOI] [PubMed] [Google Scholar]
- 10. Freemantle N, Calvert M, Wood J, et al. Composite outcomes in randomized trials: greater precision but with greater uncertainty? JAMA. 2003;289(19):2554–2559. [DOI] [PubMed] [Google Scholar]
- 11. Women’s Health Initiative Study Group . Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19(1):61–109. [DOI] [PubMed] [Google Scholar]
- 12. Anderson G, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(9 suppl):S5–S17. [DOI] [PubMed] [Google Scholar]
- 13. Curb D, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13(9 suppl):S122–S128. [DOI] [PubMed] [Google Scholar]
- 14. Cox DR. Regression models and life-tables (with discussion). J Roy Stat Soc B. 1972;34(2):187–220. [Google Scholar]
- 15. Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc. 1989;84(408):1065–1073. [Google Scholar]
- 16. Spiekerman CF, Lin DY. Marginal regression models for multivariate failure time data. J Am Stat Assoc. 1998;98:1164–1175. [Google Scholar]
- 17. Lin DY. Cox regression analysis of multivariate failure time data: the marginal approach. Stat Med. 1994;13(21):2233–2247. [DOI] [PubMed] [Google Scholar]
- 18. Prentice RL, Manson JE, Langer RD, et al. Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause. Am J Epidemiol. 2009;170(1):12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beral V, Reeves G, Bull D, et al. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst. 2011;103(4):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manson JE, Aragaki AK, Bassuk SS, et al. Menopausal estrogen-alone therapy and health outcomes in women with and without bilateral oophorectomy: a randomized trial. Ann Intern Med. 2019;171(6):406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao S, Chlebowski RT, Anderson GL, et al. Sex hormone associations with breast cancer risk and the mediation of randomized trial postmenopausal hormone therapy effects. Breast Cancer Res. 2014;16(2):R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sweeney EE, Fan P, Jordan VC. Molecular modulation of estrogen-induced apoptosis by synthetic progestins in hormone replacement therapy: an insight into the Women’s Health Initiative study. Cancer Res. 2014;74(23):7060–7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abderrahman B, Jordan VC. The modulation of estrogen-induced apoptosis as an interpretation of the Women’s Health Initiative trials. Expert Rev Endocrinol Metab. 2016;11(1):81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yue W, Wang J, Atkins KA, et al. Effect of a tissue selective estrogen complex on breast cancer: role of unique properties of conjugated equine estrogen. Int J Cancer. 2018;143(5):1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pickar JH, Boucher M, Morgenstern D. Tissue selective estrogen complex (TSEC): a review. Menopause. 2018;25(9):1033–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


