Abstract
Background
Chronotherapy is an innovative approach to improving survival through timed delivery of anti-cancer treatments according to patient daily rhythms. Temozolomide (TMZ) is a standard-of-care chemotherapeutic agent for glioblastoma (GBM). Whether timing of TMZ administration affects GBM patient outcome has not previously been studied. We sought to evaluate maintenance TMZ chronotherapy on GBM patient survival.
Methods
This retrospective study reviewed patients with newly diagnosed GBM from January 1, 2010 to December 31, 2018 at Washington University School of Medicine who had surgery, chemoradiation, and were prescribed TMZ to be taken in the morning or evening. The Kaplan–Meier method and Cox regression model were used for overall survival (OS) analyses. The propensity score method accounted for potential observational study biases. The restricted mean survival time (RMST) method was performed where the proportional hazard assumption was violated.
Results
We analyzed 166 eligible GBM patients with a median follow-up of 5.07 years. Patients taking morning TMZ exhibited longer OS compared to evening (median OS, 95% confidence interval [CI] = 1.43, 1.12–1.92 vs 1.13, 0.84–1.58 years) with a significant year 1 RMST difference (−0.09, 95% CI: −0.16 to −0.018). Among MGMT-methylated patients, median OS was 6 months longer for AM patients with significant RMST differences at years 1 (−0.13, 95% CI = −0.24 to −0.019) to 2.5 (−0.43, 95% CI = −0.84 to −0.028). Superiority of morning TMZ at years 1, 2, and 5 (all P < .05) among all patients was supported by RMST difference regression after adjusting for confounders.
Conclusions
Our study presents preliminary evidence for the benefit of TMZ chronotherapy to GBM patient survival. This impact is more pronounced in MGMT-methylated patients.
Keywords: circadian, chronotherapy, glioblastoma, MGMT, temozolomide
Key Points.
Morning temozolomide administration may improve survival for patients with glioblastoma.
Morning TMZ demonstrates greater benefit in MGMT-methylated patients.
Importance of the Study.
Glioblastoma is a dismal disease for which there have been no new drugs approved in over a decade. Chronotherapy using the existing front-line chemotherapy drug, temozolomide, enhances therapeutic efficacy and prolongs survival. This dosing modification can be quickly adopted in clinical practice. This study lays the foundation for larger scale chronomedicine trials for brain cancer.
Glioblastoma (GBM), the most common adult primary brain tumor,1 has a dismal prognosis. Standard of care treatment of GBM patients involves maximal safe surgical resection, followed by concurrent radiotherapy with temozolomide (TMZ), and maintenance treatment with TMZ thereafter. After several decades of clinical trials involving multiple chemotherapeutics, the addition of TMZ to surgery and radiation therapy was demonstrated to extend survival by 2.5 months.2,3 TMZ received FDA approval for GBM based on this modest survival improvement in 2005. In 2011, tumor-treating fields (TTF) were approved for GBM based on evidence for progression-free survival improvement by 2.7 months and overall survival by 4.9 months, when added to TMZ.4 Unfortunately, the 5-year survival rate for adults with GBM remains low at 5%–14%3–6 and further improvements in front-line treatments are necessary.
Circadian medicine considers daily rhythms in drug metabolism and cancer cell treatment response.7–9 Daily rhythms in physiology and behavior depend upon cellular circadian clocks in the brain and body. A master circadian pacemaker in the hypothalamic suprachiasmatic nucleus regulates peripheral functions through neural, endocrine, metabolic, and behavioral outputs including sleep–wake and feeding–fasting.10 These signals act on nearly all cells to synchronize their intrinsic daily rhythms. The core clock mechanism, discovery of which was awarded the Nobel Prize in 2017, drives daily rhythms in up to 50% of a cell’s transcriptome depending on the cell type11,12 and entrains to environmental timing cues like the local light cycle. Daily rhythms in metabolism,9 cell cycle regulation,13 and DNA repair14,15 likely modulate the efficacy of cancer treatments.16 Two recent studies highlighted potential benefits of treatment with drugs that target the circadian mechanism in glioblastoma. They found that chronic agonists of REV-ERB and CRY could perform as well as TMZ in GBM models implanted in mice.17,18 As an alternative approach, chronotherapy has been studied for its potential to improve treatment outcomes through optimizing the timed delivery of medication according to the patients’ circadian rhythms. Recent studies have demonstrated circadian regulation of the p38 mitogen-activated protein kinase (MAPK) signal transduction pathway in Neurospora19 and in glioma cells’ response to a p38 MAPK inhibitor.20 We previously published that patient-derived and murine-model GBM cells exhibited circadian transcription of the clock genes, Bmal1 and Period2 (Per2). Critically, murine GBM cells showed more than 3-fold greater DNA damage, activation of the apoptotic pathway and cell death following TMZ treatment at the peak of Bmal1 expression compared to at its daily minimum of expression.16 Furthermore, the expression of the protein responsible for repair of DNA double-strand breaks induced by TMZ, O-6-Methylguanine-DNA Methyltransferase (MGMT), oscillates with time of day.21–23 Sensitivity of cell cycle checkpoint mediated apoptosis has also been shown to change based on time of day via an interaction with 2 clock genes, Per1 and Per3.14,24 It is not yet known if clock gene expression delimits an optimal therapeutic time window for TMZ treatment.
A recent meta-analysis found that, of the 50 most prescribed drugs, only 4 have a recommended time of administration, over 56% of drugs target proteins that exhibit circadian variation in expression, and over 75% of 106 clinical trials involving 70 drugs found results varied with time of day.7 Chronotherapy, treatment at the optimal time of day, can increase tumor cell death and reduce side effects,14 allowing for longer or elevated dosing. Despite the success of chronotherapy in pediatric acute lymphoblastic leukemia,25,26 colorectal cancer,27–29 ovarian cancer, and some gynecological and genitourinary cancers,30 timing of drug administration is rarely accounted for in clinical trials and has not been investigated in the context of brain cancer.
TMZ readily crosses the blood brain barrier and has a short half-life.31 This makes TMZ an ideal and novel chronotherapeutic drug. The effect of TMZ chronotherapy in GBM has not previously been investigated. Based on findings in preclinical studies and the results of chronotherapy studies in other cancer types, we initiated a retrospective analysis of glioblastoma patients to compare the efficacy of maintenance TMZ treatment in the morning versus in the evening.
Methods
Patients
A total of 498 patients who were diagnosed with glioblastoma from January 1, 2010 to December 31, 2018 at Washington University School of Medicine (WUSM) were screened for inclusion in the study. GBM patients seen at WUSM during this period received radiation therapy (RT) with concurrent TMZ in the morning. Approximately 4 weeks post-concurrent chemoradiation, maintenance TMZ was initiated as morning or evening dosing per provider preference. Following screening (see flow chart of inclusion/exclusion criteria in Supplementary Figure 1), 180 patient records were deemed evaluable for this study. For the maintenance TMZ in this cohort, 3 (GA, GL, DT) of the 4 GBM oncologists consistently prescribed TMZ to be taken on an empty stomach in the morning (AM), while 1 physician (J.C.) consistently prescribed TMZ to be taken in the evening (PM). In the event that a patient preferred to take TMZ at a time other than habitually prescribed, this was recorded in the patient record. Data on adverse events were not collected on these patients during this retrospective study. The study was approved by the institutional human research protection office (HRPO#201507048).
Statistical Analysis
The primary patient outcome is overall survival (OS), calculated as the time interval from the start date of maintenance TMZ (post-chemoradiation) to the date of death if a patient died or to the date of last contact. Patient characteristics were summarized using descriptive statistics, count, and percentages for categorical characteristics and median and interquartile range (IQR) for quantitative characteristics, overall and by TMZ timing, while the distribution difference by TMZ timing was assessed by Fisher’s exact test and Wilcoxon rank sum test for categorical and quantitative characteristics, respectively. The Kaplan–Meier (KM) method was applied to estimate empirical survival probability to report median OS estimates with 95% confidence interval (CI) and the KM curves were generated for visualization. The log-rank test was used to compare the survival difference between patient groups. The Cox proportional hazard regression model was applied to estimate unadjusted and adjusted hazard ratio (HR) without and with adjustment for other covariates, correspondingly. The proportional hazards (PH) assumption underlying both the log rank test and the Cox regression model was examined graphically and by statistical testing32 on the weighted residuals.
The resultant KM curves crossed and thus, were in violation of the proportional hazard assumptions underlying the log rank test and Cox regression model. Therefore, we resorted to the restricted mean survival time (RMST, equivalently, t-year mean survival time) method33,34 to compare the survival difference between TMZ administered in AM versus PM. The RMST method quantifies the area under the KM curve up to year t (specified by users) as a summary measure of survival. Intuitively, a greater area under the KM curve and a greater RMST estimate indicates better survival. We calculated the RMST-based difference of PM relative to AM group to quantify the survival difference. RMST does not depend on the proportional hazard assumption and serves as a more robust and widely applicable survival analysis approach. RMST modeling without and with adjustment for covariates was performed at years 1–5. In the univariate RMST analysis, a negative RMST difference indicates worse survival in the PM group (better survival in AM). In the multivariate RMST regression analysis, a negative coefficient estimate corresponds to better survival in the AM group, adjusting for influence from other covariates.
In consideration of existence of potential biases in observational data, we also evaluated the impact of TMZ timing using the propensity score (PS) method35,36 as popularly employed for observational studies. The propensity score, that is, the likelihood of GBM patients receiving TMZ in the PM versus AM, was modeled by a logistic regression with all available baseline patient characteristics, including age (continuous), sex (male vs female), extent of surgical resection (subtotal/biopsy vs gross total resection), MGMT promoter methylation status (methylated vs unmethylated), Karnofsky performance status (KPS ≥80 vs <80), baseline steroid use (Yes vs No), enrollment in DCVax-L clinical trial (Yes vs No), and enrollment in other trials (Yes vs No), and was subsequently predicted from this full logistic regression model. The Cox proportional hazard model was applied to the PS-based inverse probability of treatment (here, TMZ timing) weighting (IPTW) cohort and the PS 1:1 nearest neighbor matched cohort. For IPTW cohort, a stable weight36 was calculated for each patient as inversely proportional to the patients’ probability of receiving TMZ at their designated time.
All the computation was conducted in R37 (version 3.6.1). The R package “survRM2” 38 was used to perform the RMST analyses. The R package “MatchIt” was used for PS matching. All statistical tests were 2-sided unless otherwise noted. Statistical significance was claimed at the 5% α level.
Results
Patient Characteristics
A total of 180 patients were identified as de novo, nonrecurrent GBM diagnosed at WUSM from January 1, 2010 to December 31, 2018. All patients underwent surgical resection or biopsy followed by concurrent chemoradiation therapy with TMZ, followed by maintenance TMZ. We excluded 14 patients (Supplementary Figure 1: 11 with IDH1/2-mutant secondary GBM, 2 with 1p/19q co-deleted oligodendroglioma, and 1 with missing vital status). The remaining 166 patients were further evaluated for this study.
Patient demographic, tumor genomic, and clinical information are summarized in Table 1. The average age at maintenance TMZ start was around 60 (IQR: 52.83–65.86) years. More than 95% of the patients were Caucasian and more than 60% were male. All the patients in the PM group were seen by 1 physician (JC) while AM patients were seen by 3 other physicians. The patient characteristics were all similar between the AM and PM group, except for KPS and enrollment rate in trials. KPS was higher in the PM group (median = 90 vs 80 in AM group, P = 5.36E−10). 33 of the 89 AM patients and 5 out of the 77 PM patients had isocitrate dehydrogenase (IDH) status missing (P = 1.89E−06). Nearly 70% of the patients in the AM group and 51% in the PM group were enrolled in other clinical trials concurrent with or after TMZ treatment (P = .017).
Table 1.
Enrollment in Clinical Trials, IDH Status Missing Rate, and Karnofsky Performance Status Were the Only Characteristics That Differed Between Groups. Patient and tumor characteristics summary, overall and by TMZ administration time (AM/PM)
| Variable (N) | All (N = 166) | AM (N = 89) | PM (N = 77) | P |
|---|---|---|---|---|
| Age at TMZ start (N = 166) | 60.1 (52.83–65.86) | 59.28 (52.63–63.48) | 61.65 (54.22–67.17) | .1623 |
| Sex (N = 166) | .7476 | |||
| Female | 61 (36.75) | 34 (38.2) | 27 (35.06) | |
| Male | 105 (63.25) | 55 (61.8) | 50 (64.94) | |
| Race (N = 166) | 1 | |||
| Black | 6 (3.61) | 3 (3.37) | 3 (3.9) | |
| Caucasian | 160 (96.39) | 86 (96.63) | 74 (96.1) | |
| KPS (N = 166) | 80 (70–90) | 80 (70–80) | 90 (80–90) | 5.36E-10 |
| KPS (N = 166) | 9.05E-07 | |||
| KPS < 80 | 53 (31.93) | 43 (48.31) | 10 (12.99) | |
| KPS ≥ 80 | 113 (68.07) | 46 (51.69) | 67 (87.01) | |
| Bevacizumab Prior to TMZ (N = 166) | 1 | |||
| No | 158 (95.18) | 85 (95.51) | 73 (94.81) | |
| Yes | 8 (4.82) | 4 (4.49) | 4 (5.19) | |
| Bevacizumab concurrent with TMZ (N = 166) | .7258 | |||
| No | 158 (95.18) | 84 (94.38) | 74 (96.1) | |
| Yes | 8 (4.82) | 5 (5.62) | 3 (3.9) | |
| Use of TTF (N = 166) | .1784 | |||
| No | 132 (79.52) | 67 (75.28) | 65 (84.42) | |
| Yes | 34 (20.48) | 22 (24.72) | 12 (15.58) | |
| Enrolled in DCVax trial (N = 166) | .1733 | |||
| No | 151 (90.96) | 78 (87.64) | 73 (94.81) | |
| Yes | 15 (9.04) | 11 (12.36) | 4 (5.19) | |
| Other clinical trial enrollment? (N = 166) | .0166 | |||
| No | 65 (39.16) | 27 (30.34) | 38 (49.35) | |
| Yes | 101 (60.84) | 62 (69.66) | 39 (50.65) | |
| Physician (N = 166) | 2.52E-44 | |||
| GA | 21 (12.65) | 21 (23.6) | 0 (0) | |
| JC | 80 (48.19) | 3 (3.37) | 77 (100) | |
| GL | 7 (4.22) | 7 (7.87) | 0 (0) | |
| DT | 58 (34.94) | 58 (65.17) | 0 (0) | |
| IDH status* | ||||
| WT | 128 (77.1) | 56 (62.9) | 72 (93.5) | 1.89E−06 |
| Missing | 38 (22.9) | 33 (37.1) | 5 (6.5) | |
| MGMT methylation (N = 151) | 1 | |||
| No | 95 (62.91) | 50 (63.29) | 45 (62.5) | |
| Yes | 56 (37.09) | 29 (36.71) | 27 (37.5) | |
| Extent of surgical resection (N = 165) | .9524 | |||
| Biopsy | 25 (15.15) | 13 (14.77) | 12 (15.58) | |
| Gross total resection | 93 (56.36) | 49 (55.68) | 44 (57.14) | |
| Subtotal | 47 (28.48) | 26 (29.55) | 21 (27.27) | |
| Prior RT (N = 166) | .6638 | |||
| No | 161 (96.99) | 87 (97.75) | 74 (96.1) | |
| Yes | 5 (3.01) | 2 (2.25) | 3 (3.9) | |
| Prior chemo (N = 166) | .2137 | |||
| No | 164 (98.8) | 89 (100) | 75 (97.4) | |
| Yes | 2 (1.2) | 0 (0) | 2 (2.6) | |
| Baseline steroid use (N = 166) | .4364 | |||
| No | 78 (46.99) | 39 (43.82) | 39 (50.65) | |
| Yes | 88 (53.01) | 50 (56.18) | 38 (49.35) | |
| Chemo/RT (N = 166) | .2494 | |||
| Concurrent | 163 (98.19) | 86 (96.63) | 77 (100) | |
| RT only | 3 (1.81) | 3 (3.37) | 0 (0) | |
| Cycles of TMZ treatment (N = 166) | .8902 | |||
| 7.32 (3.76–13.85) | 7.64 (3.79–13.04) | 7.21 (3.75–14.36) |
RT, radiation therapy; TMZ, temozolomide; TTF, tumor-treating fields.
Bold indicates significant P-values.
*Patients with IDH mutation have been excluded (see Supplementary Figure S1).
Increased Survival With Morning TMZ Revealed by RMST Analysis
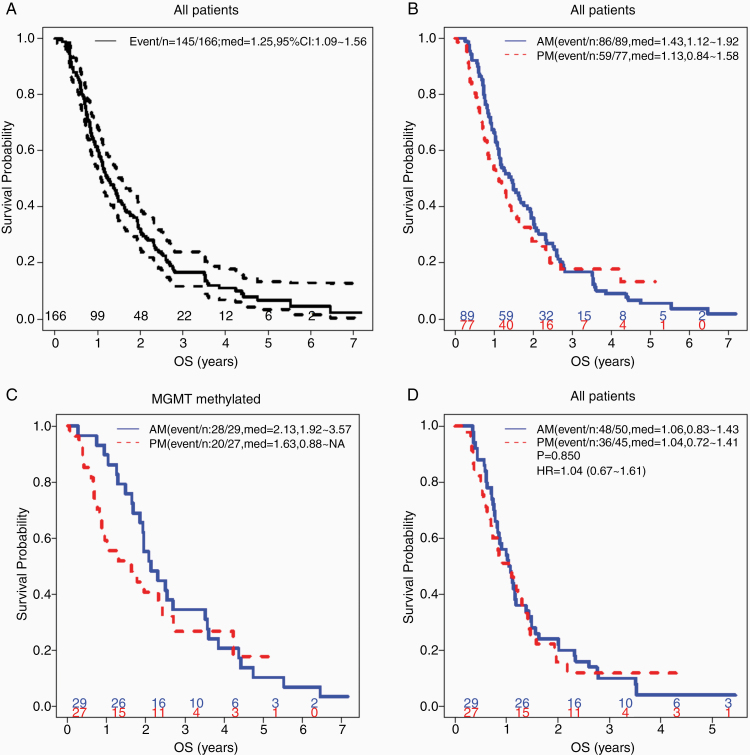
The median time to follow-up of the whole cohort of patients by the reverse KM method was 5.07 (95% CI: 4.29 to not reached [NR], range: 0.015–7.17) years. There were 145 deaths in the 166 patients during the study period. The median OS (95% CI) was estimated at 1.25 (95% CI: 1.09–1.56) years in the whole cohort (Figure 1A). The 1-year, 2-year, and 3-year OS probability was estimated at 0.6 (0.53–0.68), 0.32 (0.26–0.4), and 0.17 (0.12–0.24), respectively. AM patients trended toward longer median OS (median OS = 1.43, 95% CI: 1.12–1.92 years) than the PM patients (median OS = 1.13, 95% CI: 0.84–1.58 years), but the KM curves crossed near year 3 due to several long-surviving PM patients and their 95% CI overlapped (Figure 1B). Crossing KM curves violate the proportional hazard assumptions of the log rank test and Cox regression model, thus RMST was applied. From year 1 to year 5, the AM group consistently had a higher RMST (areas under the KM curve) compared to PM group and resulted in a negative PM-AM RMST difference, indicating better OS in AM group. When comparing all patients assigned to either morning and evening TMZ dosing, the largest RMST difference was around −0.2 observed at year 3 but statistically only reached significance in year 1 with a RMST difference of −0.09 and 95% CI that did not cross 0 (Table 2).
Figure 1.
Median overall survival in glioblastoma (GBM) patients tended to be longer in patients treated with temozolomide (TMZ) in the morning, especially in MGMT-methylated patient subset. (A) Overall survival (OS) Kaplan–Meier (KM) curves of all (N = 166) patients with 95% confidence band, (B) TMZ administration time (AM vs PM) among all patients, (C) TMZ administration among MGMT-methylated patients, (D) and among MGMT-unmethylated patients. Indicated in the legend are event/n: total number of death/total number of patients, med: median OS with 95% CI, P: log rank test P value; HR: Cox hazard ratio with 95% CI. Number of patients at risk from year 0 to 6 was indicated in each KM curve.
Table 2.
AM Treatment Significantly Improved Treatment Using Univariate RMST Analysis in Year 1 for All Patients and Year 1–2.5 for MGMT-methylated Patients. Univariate RMST analysis for OS was performed at year 1–5, estimating RMST for PM and AM and comparing PM and AM based on RMST difference (PM–AM), accompanied with 95% CI (nonoverlapping with 0 indicating significant difference), for all patients and MGMT-methylated patients
| Year | All Patients | MGMT-methylated Patients | ||||||
|---|---|---|---|---|---|---|---|---|
| PM RMST | AM RMST | RMST difference (PM–AM) |
P value | PM RMST | AM RMST | RMST difference (PM–AM) |
P value | |
| 1 | 0.7994 (0.7401–0.8587) | 0.8894 (0.849–0.9298) | −0.09 (−0.1617 to −0.0183) | 0.0139 | 0.8327 (0.734–0.9314) | 0.9631 (0.9113–1.0149) | −0.1304 (−0.2419 to −0.019) | 0.0218 |
| 2 | 1.1972 (1.0498–1.3447) | 1.3707 (1.248–1.4934) | −0.1735 (−0.3653 to 0.0183) | 0.0763 | 1.3386 (1.0819–1.5952) | 1.7222 (1.5554–1.889) | −0.3836 (−0.6897 to −0.0775) | 0.0140 |
| 2.5 | 1.3224 (1.1363–1.5086) | 1.5193 (1.3604–1.6782) | −0.1968 (−0.4416 to 0.0479) | 0.1149 | 1.5316 (1.1997–1.8634) | 1.9639 (1.7332–2.1947) | −0.4323 (−0.8365 to −0.0282) | 0.0360 |
| 3 | 1.416 (1.195–1.637) | 1.6192 (1.4315–1.807) | −0.2033 (−0.4932 to 0.0867) | 0.1695 | 1.6762 (1.2793–2.0732) | 2.1441 (1.8506–2.4375) | −0.4678 (−0.9615 to 0.0258) | 0.0633 |
| 4 | 1.594 (1.2934–1.8945) | 1.7553 (1.5192–1.9914) | −0.1613 (−0.5435 to 0.2209) | 0.4082 | 1.9436 (1.4019–2.4853) | 2.439 (2.0187–2.8592) | −0.4954 (−1.181 to 0.1903) | 0.1568 |
| 5 | 1.7381 (1.3623–2.1139) | 1.8289 (1.5594–2.0984) | −0.0908 (−0.5532 to 0.3717) | 0.7004 | 2.1431 (1.4736–2.8126) | 2.5958 (2.0899–3.1018) | −0.4528 (−1.2919 to 0.3864) | 0.2903 |
When restricted to the 56 MGMT methylated patients, the median OS was 6 months longer in the AM patients (median OS = 2.13, 95% CI: 1.92–3.57 years vs 1.63, 95% CI: 0.88–NR years in PM patients, Figure 1C). No significant difference was observed within the 95 unmethylated patients (median OS = 1.06 in N = 29 AM patients vs 1.04 years in N = 27 PM patients, Figure 1D). Univariate RMST difference analysis, when restricted to the MGMT-methylated patients, observed consistently negative coefficients through all the years with significant 95% CIs at year 1 (−0.1304, 95 CI: −0.2419 to −0.019) to year 2.5 (−0.4323, 95% CI: −0.8365 to −0.0282). We further performed multivariate RMST difference regression analysis at year 1–5 accounting for the relevant confounders. After the covariate adjustment, the negative coefficient estimates for TMZ timing (PM minus AM) from the RMST difference multivariate regression analyses indicated inferior OS at all the years for the PM group, both among all the patients (Table 3) and in the MGMT-methylated subset (Supplementary Table 4). Statistical significance was reached at years 1, 2, and 5 (P = .014, .039, and .048, respectively), marginally at year 3 (P = .07) but not at year 4 (P = .17) for all the patients (Table 3), while the resulting P-values range from .099 to .3, likely due to small sample size, in the MGMT-methylated subset (Supplementary Table 4). The advantage of morning TMZ was even more pronounced among the subsets of patients who were older (N = 83, age at TMZ start ≥ 60 vs <60 years: median OS = 1.28 vs 0.88 years, P = .15), enrolled in the DCVax-L trial (N = 15, median OS = 2.32 vs 1.03 years, P = .041), had bevacizumab (Avastin) concurrent with TMZ (N = 8, median OS = 1.49 vs 0.88 years, P = .046), or did not enroll in other clinical trials (N = 65, median OS = 1.09 vs 0.74 years, P = .26; Supplementary Figure 2). The multivariate Cox modeling of the cohort with incorporation of relevant covariates also indicated the trend of inferior OS in PM compared to AM (Supplementary Table 1, HR = 1.22, 95% CI: 0.83–1.78), but the proportional hazard test failed on the overall model globally (P = 5.94E−05) and on TMZ timing alone (P = .015). In all the years’ multivariate RMST regression fittings, MGMT methylation and extent of surgical resection remained highly significant while age, KPS, and enrollment in other trials also were found significant on patient survival in most results (Table 3).
Table 3.
AM Treatment Significantly Improved Treatment in Years 1, 2, and 5 Using Multivariate RMST Analysis Among All the Patients. Multivariate OS RMST regression for RMST difference (PM–AM) was performed at year 1–5, adjusting for covariates (as listed in the table) among all the patients. Regression coefficients with 95% CIs and P values were reported. A negative coefficient indicates worse survival of PM versus AM
| Terms | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Regression coefficient (95% CI) | P value | Regression coefficient (95% CI) | P value | Regression coefficient (95% CI) | P value | Regression coefficient (95% CI) | P value | Regression coefficient (95% CI) | P value | |
| intercept | 0.751 (0.546,0.957) | 8.16E−13 | 1.093(0.586,1.599) | 2.36E−05 | 1.322(0.47,2.174) | .0024 | 1.517(0.42,2.614) | .007 | 1.942(0.625,3.259) | .004 |
| TMZ timing (PM vs AM) | −0.086 (−0.155,−0.017) | .014 | −0.196 (−0.382,−0.01) | .039 | −0.263 (−0.547,0.021) | .0692 | −0.27 (−0.653,0.112) | .166 | −0.422 (−0.841,−0.004) | .048 |
| Sex (Male vs Female) | 0.02(−0.046,0.086) | .556 | −0.022 (−0.202,0.159) | .813 | −0.005 (−0.275,0.266) | .9735 | 0.093(−0.293,0.478) | .637 | −0.285 (−0.781,0.211) | .260 |
| age at TMZ start (continuous) | −0.002 (−0.005,0) | .090 | −0.008 (−0.015,−0.001) | .031 | −0.015 (−0.027,−0.003) | .0147 | −0.019(−0.035,−0.004) | .015 | −0.026 (−0.047,−0.005) | .015 |
| MGMT methylation (Yes vs No) | 0.156 (0.089,0.222) | 4.53E−06 | 0.626 (0.442,0.811) | 2.71E−11 | 0.912 (0.613,1.211) | 2.2E−09 | 1.274 (0.821,1.727) | 3.59E−08 | 1.603 (1.036,2.17) | 2.99E−08 |
| KPS (≥80 vs <80) | 0.059 (−0.017,0.134) | .129 | 0.176 (−0.042,0.393) | .114 | 0.269 (−0.083,0.622) | .1341 | 0.274 (−0.296,0.845) | .346 | 0.542 (0.086,0.998) | .020 |
| Extent of Surgical Resection (gross total resection vs subtotal/biopsy) | 0.126(0.058,0.194) | .000 | 0.269 (0.102,0.435) | .002 | 0.494(0.229,0.759) | .0003 | 0.652 (0.243,1.06) | .002 | 0.696 (0.207,1.186) | .005 |
| Baseline steroid Use (Yes vs No) | −0.052 (−0.121,0.016) | .135 | −0.059 (−0.243,0.125) | .530 | 0.019 (−0.297,0.335) | .9045 | 0.014 (−0.513,0.54) | .960 | 0.174 (−0.29,0.638) | .462 |
| Novocure Optune Use (Yes vs No) | −0.023 (−0.107,0.061) | .588 | 0.002 (−0.228,0.231) | .989 | −0.12 (−0.441,0.201) | .4643 | −0.214(−0.607,0.178) | .285 | −0.181 (−0.596,0.233) | .391 |
| DCVax Trial enrollment (Yes vs No) | 0.039(−0.053,0.131) | .405 | 0.24 (−0.011,0.491) | .060 | 0.309 (−0.146,0.764) | .1836 | 0.34(−0.289,0.97) | .289 | 0.416(−0.337,1.169) | .279 |
| other Trial enrollment (Yes. vs No) | 0.174(0.1,0.247) | 3.36E−06 | 0.39(0.217,0.564) | 1.03E−05 | 0.548(0.254,0.842) | .0003 | 0.448(−0.049,0.946) | .077 | 0.31(−0.142,0.763) | .179 |
Bold indicates significant P-values.
Morning TMZ Remains Favorable After Adjusting for Potential Study Bias
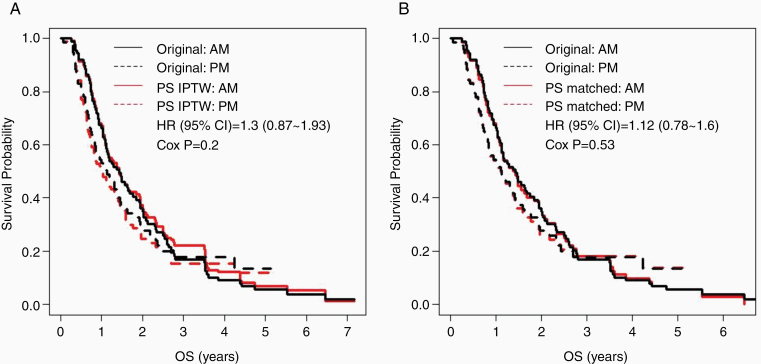
Considering potential biases in the observational study, we modeled the likelihood of patients receiving TMZ in PM versus AM using univariate (without covariates) and multivariate logistic regression model adjusting for other covariates. KPS, other trial enrollment, and DCVax-L trial enrollment were found to potentially affect the likelihood (Supplementary Table 2). Propensity score (PS) was calculated using the multivariate logistic regression to construct the IPTW weighting cohort and the PS matched (72 each in AM and PM) cohort. We subsequently performed KM analyses and multivariate Cox regression analyses of each cohort. The KM curves were like those of the original cohort, showing trends of worse OS in PM (Figure 2), with KM curves still crossing around 3.5 years after diagnosis. The multivariate Cox modeling of both cohorts yielded similar results to the multivariate Cox modeling of the original cohort, but the proportional hazard assumption still failed (Supplementary Table 3). Thus, after adjusting for differences in the likelihood of AM- and PM-treated patients starting TMZ with different initial health scores or being enrolled in other clinical trials, we still observed the trend of morning TMZ being associated with greater OS.
Figure 2.
Patient survival tended to be longer with morning temozolomide (TMZ) treatment after correcting for potential biases in patient recruitment. Kaplan–Meier (KM) curves of TMZ timing (PM: solid line; AM: dashed line) in (A) propensity score (PS)-based inverse probability of treatment weighting (IPTW) cohort and (B) PS-matched cohort (both in red), overlaid on the KM curves of the original study cohort (in black).
Discussion
In this study, we evaluated the impact of administering TMZ in the morning versus in the evening on GBM patient survival using data from our institution. We found morning TMZ dosing associated with increased overall survival in patients with MGMT methylated GBM even after accounting for potential biases in this retrospective analysis.
The median OS of 15 months and the 5-year OS probability of 7% estimated from this cohort of patients were comparable to previously reported studies such as Stupp et al.3 (median OS = ~14.6 months, 5-year OS ~10%). The patients in the AM and PM groups were quite similar in terms of age, race, sex, MGMT methylation, extent of surgical resection (which were found to be prognostic of survival as known to the field), but KPS was found significantly higher in the PM group. KPS is subjectively scored by treating physicians and varies greatly as a result. The patients in our AM cohort were seen by 3 physicians, whereas all the patients in the PM group were seen by one physician. This may have caused the KPS difference. We excluded all IDH mutated patients but included all IDH wild type or missing patients to maximize sample size. A greater percentage of AM patients were missing IDH status than PM patients (33 out of 89 vs 5 out of 77), but the impact would be minimal considering the lower IDH mutation rate ~10%.
We endeavored to additionally collect information on trial enrollments in consideration of their potential impact on survival. In our study, enrollment rate in the DCVax-L trial (NCT00045968) and the use of TTF (NCT00916409) was higher, albeit not statistically significant, in the PM group (Table 1). More patients in the AM group enrolled in other clinical trials (Table 1) after TMZ treatment. The other clinical trials in our study included NCT00884741 (radiation and TMZ with or without bevacizumab), NCT01062425 (radiation and TMZ with or without cediranib maleate), NCT01480479 (ACT IV: Adjuvant TMZ with or without rindopepimut), NCT00869401 (radiation and TMZ with or without dasatinib), NCT02179086 (TMZ and standard radiation or photon intensity-modulated radiation therapy), NCT00770471 (ABT-888: TMZ and radiation with or without veliparib), and NCT02667587 (CheckMate548: TMZ and radiation with or without nivolumab), each with fewer than 5 patients. Subset analysis observed in AM TMZ a trend toward higher (albeit statistically not significant) median OS (median OS, 95% CI = 1.09, 0.73–2.6 in AM vs 0.74, 0.6–1.95 in PM) in the subset of N = 65 patients who did not enroll in these other clinical trials, while similar survival to PM TMZ among those who enrolled in these other trials (Supplementary Figure S2). After adjusting for the known GBM prognostic factors (age, MGMT methylation, extent of surgery) and trial enrollment in DCVax-L, TTF, and others, the advantageous OS was observed in the AM group based on the multivariate RMST difference regression analyses at years 1, 2, and 5 (all P < .05).
GBM is a fatal disease and no new drugs have been approved in over a decade. Critical thinking is needed to further improve GBM patient management and survival outcome. Changing time of administration of TMZ would be cost-effective and easy to adjust to for patients. We found that TMZ administration timing impacted patient survival overall and had a greater effect in MGMT-methylated GBM patients. This is consistent with previous studies showing that MGMT silencing confers a better response to TMZ treatment.2,39–42 Based on the overall 2.5-month and MGMT-methylated 6.4-month median OS improvement by concomitant TMZ with radiation from Stupp et al. and companion translational study from Hegi et al.2,3 (NCT00006353), the concomitant treatment regimen has become standard clinical practice. In our study, maintenance TMZ administered in AM improved the median OS by 6 months in MGMT-methylated patients (with significant 95% RMST difference CIs at years 1 to 2.5) without the need of adding another therapy. On the other hand, TMZ timing had no impact on survival in MGMT-unmethylated GBM patients. These observations conform to our predictions. MGMT-unmethylated patients are generally resistant to adjuvant TMZ treatment and the mechanism of resistance is not expected to be altered by changing the timing of TMZ administration. However, in the more responsive, MGMT-methylated, GBM patients, it appears that response can be further optimized by timed treatment.
This work demonstrates the potentially powerful impact of TMZ chronotherapy in GBM. Given that TMZ rapidly absorbed, reaching peak levels in plasma within 1h after oral dosing, and is spontaneously degraded at physiological pH, with a half-life of 1.8 h, precise dose timing is possible.31,43 This is key in bringing TMZ chronotherapy to the clinic, as preclinical studies have shown maximal TMZ efficacy during a 6-h window of treatment corresponding to the peak of core clock protein BMAL1,16 which peaks just before dawn in several human tissues.44,45 Morning timing may be the most effective in humans due to daily fluctuation in absorption and excretion of the drug, as well as the sensitivity of tumor cells to DNA damage.24,46,47 TMZ chronotherapy also has potential to be customized to the patient’s unique circadian rhythm. We observed that AM TMZ may improve survival in patients over 60 years old. Older patients tend to be earlier chronotypes,48 starting their daily activity earlier, and TMZ taken in the morning better conformed to this circadian pattern and, thus, exerted a greater efficacy. Future studies should incorporate the established effects of chronotype on therapeutic responses.49 Such studies are needed to explore these potential mechanisms and to determine if peripheral clock gene expression can determine optimal dosing time for individuals.
The KM curves of AM and PM crossed over and the proportional hazard assumption failed mostly. This was attributable to 6 outlier patients who were alive with long OS (>3 years) in the PM group. All 6 of the patients (3 male, 3 female) had gross total resection, none were IDH1/2 mutated, and 3 of them were MGMT methylated. PS-based survival analyses showed similar results to the original cohort analyses and violation of the proportional hazard assumption was similarly observed. Thus, we resorted to the RMST method which does not rely on the proportional hazard assumption and the multivariate RMST analyses found that TMZ in AM was superior to PM TMZ at years 1, 2, and 5 after adjusting for confounders.
As the very first paper exploring the TMZ chronotherapy effect in GBM, the study has various limitations. Due to its retrospective nature, the study may harbor potential issues (such as selection bias) which are common in observational studies. To address this, we have employed the PS-based methods (including IPTW and PS matching), which yielded results consistent with the survival analyses of the original cohort. While this is the largest patient cohort in this line of research, the sample size of the study was still moderate and thus was insufficient to detect the relatively small effect of TMZ chronotherapy in some analyses. Note that most current treatments result in modest improvement in GBM patient outcomes. GBM patient survival can be impacted by many factors. We have considered major demographical and clinical factors, and even clinical trial participation. However, confounders that were not collected/recorded could not be accounted for. The patient population was primarily Caucasian, thus findings from this study may not generalize to non-Caucasian patient populations. The AM/PM TMZ grouping was based on the treating physicians’ habitual prescription as stated in the Methods section. We assumed that patients in this cohort complied with the designated TMZ administration timing as prescribed by the treating physicians, although the compliance cannot be controlled in this retrospective study. Finally, we focused on the impact of TMZ timing on patient survival and did not collect side effect data in this study. We acknowledge that toxicity profiles may differ as a consequence of AM versus PM TMZ dosing. This should be prospectively evaluated.
Future large retrospective studies at other institutions and prospective randomized controlled trials are required to further validate our findings on the TMZ chronotherapy effect in GBM patients. A randomized 2-arm phase II trial (NCT02781792) where 30 brain tumor patients are randomized at 1:1 ratio to receive TMZ in the morning (before 10 AM) or in the evening (after 8 PM) is ongoing. This trial will examine adverse events in addition to patient survival. If our findings can be replicated in this and other studies, TMZ chronotherapy can be easily and immediately implemented without fundamentally altering the current standard of care and may improve anti-tumor efficacy in GBM patients. More broadly, chronotherapy is a growing field with potential to improve outcomes in many cancer types and diseases beyond cancer.
Supplementary Material
Acknowledgments
The authors would like to thank Qian Li, Rincon Jagarlamudi, and Ethan Roberts for their assistance in compiling data.
Funding
The Alvin J. Siteman Cancer Center Siteman Investment Program through funding from The Foundation for Barnes-Jewish Hospital and the Barnard Trust (to J.B.R., J.L.C., and E.D.H.), The Children’s Discovery Institute (to J.B.R. and E.D.H.), and the National Institutes of Health National Cancer Institute (P30CA091842 to T.E., F31CA250161 to A.R.D.).
Authorship Statement. Devised the study: J.L.C., E.D.H., and J.B.R. Reviewed charts and assembled the data: A.R.D. Analyzed the data and generated all the figures and tables: J.L. Drafted the manuscript: J.L. and A.R.D. Interpretation of results and review of the manuscript: A.R.D., J.L., R.G.N.K., G.R.T., J.B.R., E.D.H., and J.L.C.
Conflict of interest statement. The authors declare no conflict of interest to the current study.
References
- 1. Ostrom QT, Cioffi G, Gittleman H, et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21(Supplement_5):v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hegi ME, Diserens AC, Gorlia T, et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 3. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 4. Stupp R, Taillibert S, Kanner A, et al. . Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tamimi AF, Juweid M. Epidemiology and outcome of glioblastoma. In: De Vleeschouwer S, ed. Glioblastoma. Brisbane, AU: Codon Publications; 2017. Accessed August 25, 2020. http://www.ncbi.nlm.nih.gov/books/NBK470003/ [PubMed] [Google Scholar]
- 6. Darefsky AS, King JT Jr, Dubrow R. Adult glioblastoma multiforme survival in the temozolomide era: a population-based analysis of Surveillance, Epidemiology, and End Results registries. Cancer. 2012;118(8):2163–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruben MD, Smith DF, FitzGerald GA, Hogenesch JB. Dosing time matters. Science. 2019;365(6453):547–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lévi F, Okyar A, Dulong S, Innominato PF, Clairambault J. Circadian timing in cancer treatments. Annu Rev Pharmacol Toxicol. 2010;50:377–421. [DOI] [PubMed] [Google Scholar]
- 9. Masri S, Sassone-Corsi P. The emerging link between cancer, metabolism, and circadian rhythms. Nat Med. 2018;24(12):1795–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herzog ED. Neurons and networks in daily rhythms. Nat Rev Neurosci. 2007;8(10):790–802. [DOI] [PubMed] [Google Scholar]
- 11. Ruben MD, Wu G, Smith DF, et al. . A database of tissue-specific rhythmically expressed human genes has potential applications in circadian medicine. Sci Transl Med. 2018;10(458):eaat8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA. 2014;111(45):16219–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khapre RV, Samsa WE, Kondratov RV. Circadian regulation of cell cycle: molecular connections between aging and the circadian clock. Ann Med. 2010;42(6):404–415. [DOI] [PubMed] [Google Scholar]
- 14. Kang TH, Reardon JT, Sancar A. Regulation of nucleotide excision repair activity by transcriptional and post-transcriptional control of the XPA protein. Nucleic Acids Res. 2011;39(8):3176–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang Y, Adebali O, Wu G, et al. . Cisplatin-DNA adduct repair of transcribed genes is controlled by two circadian programs in mouse tissues. Proc Natl Acad Sci USA. 2018;115(21):E4777–E4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Slat EA, Sponagel J, Marpegan L, et al. . Cell-intrinsic, Bmal1-dependent circadian regulation of temozolomide sensitivity in glioblastoma. J Biol Rhythms. 2017;32(2):121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sulli G, Rommel A, Wang X, et al. . Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature. 2018;553(7688):351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dong Z, Zhang G, Qu M, et al. . Targeting Glioblastoma stem cells through disruption of the circadian clock. Cancer Discov. 2019;9(11):1556–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lamb TM, Goldsmith CS, Bennett L, Finch KE, Bell-Pedersen D. Direct transcriptional control of a p38 MAPK pathway by the circadian clock in Neurospora crassa. PLoS One. 2011;6(11):e27149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goldsmith CS, Kim SM, Karunarathna N, et al. . Inhibition of p38 MAPK activity leads to cell type-specific effects on the molecular circadian clock and time-dependent reduction of glioma cell invasiveness. BMC Cancer. 2018;18(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martineau-Pivoteau N, Cussac-Buchdahl C, Chollet P, et al. . Circadian variation in O6-methylguanine-DNA methyltransferase activity in mouse liver. Anticancer Drugs. 1996;7(6):703–709. [DOI] [PubMed] [Google Scholar]
- 22. Pizarro A, Hayer K, Lahens NF, Hogenesch JB. CircaDB: a database of mammalian circadian gene expression profiles. Nucleic Acids Res. 2013;41(Database issue):D1009–D1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marchenay C, Cellarier E, Lévi F, et al. . Circadian variation in O6-alkylguanine-DNA alkyltransferase activity in circulating blood mononuclear cells of healthy human subjects. Int J Cancer. 2001;91(1):60–66. [DOI] [PubMed] [Google Scholar]
- 24. Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell. 2006;22(3):375–382. [DOI] [PubMed] [Google Scholar]
- 25. Rivard GE, Infante-Rivard C, Dresse MF, Leclerc JM, Champagne J. Circadian time-dependent response of childhood lymphoblastic leukemia to chemotherapy: a long-term follow-up study of survival. Chronobiol Int. 1993;10(3):201–204. [DOI] [PubMed] [Google Scholar]
- 26. Bostrom B, Erdmann G. Cellular pharmacology of 6-mercaptopurine in acute lymphoblastic leukemia. Am J Pediatr Hematol Oncol. 1993;15(1):80–86. [PubMed] [Google Scholar]
- 27. Lévi F, Zidani R, Misset JL. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet. 1997;350(9079):681–686. [DOI] [PubMed] [Google Scholar]
- 28. Innominato PF, Giacchetti S, Moreau T, et al. ; ARTBC International Chronotherapy Group . Prediction of survival by neutropenia according to delivery schedule of oxaliplatin-5-Fluorouracil-leucovorin for metastatic colorectal cancer in a randomized international trial (EORTC 05963). Chronobiol Int. 2011;28(7):586–600. [DOI] [PubMed] [Google Scholar]
- 29. Giacchetti S, Bjarnason G, Garufi C, et al. ; European Organisation for Research and Treatment of Cancer Chronotherapy Group . Phase III trial comparing 4-day chronomodulated therapy versus 2-day conventional delivery of fluorouracil, leucovorin, and oxaliplatin as first-line chemotherapy of metastatic colorectal cancer: the European Organisation for Research and Treatment of Cancer Chronotherapy Group. J Clin Oncol. 2006;24(22):3562–3569. [DOI] [PubMed] [Google Scholar]
- 30. Lin HH, Farkas ME. Altered circadian rhythms and breast cancer: from the human to the molecular level. Front Endocrinol (Lausanne). 2018;9:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ballesta A, Zhou Q, Zhang X, Lv H, Gallo JM. Multiscale design of cell-type-specific pharmacokinetic/pharmacodynamic models for personalized medicine: application to temozolomide in brain tumors. CPT Pharmacometrics Syst Pharmacol. 2014;3:e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- 33. Pak K, Uno H, Kim DH, et al. . Interpretability of cancer clinical trial results using restricted mean survival time as an alternative to the hazard ratio. JAMA Oncol. 2017;3(12):1692–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim DH, Uno H, Wei LJ. Restricted mean survival time as a measure to interpret clinical trial results. JAMA Cardiol. 2017;2(11):1179–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–2281. [DOI] [PubMed] [Google Scholar]
- 36. Austin PC, Stuart EA. Optimal full matching for survival outcomes: a method that merits more widespread use. Stat Med. 2015;34(30):3949–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. R: a language and environment for statistical computing. https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing. Accessed August 31, 2020.
- 38. survRM2-package: Comparing Restricted Mean Survival Time in survRM2: Comparing Restricted Mean Survival Time. https://rdrr.io/cran/survRM2/man/survRM2-package.html. Accessed August 31, 2020.
- 39. Kitange GJ, Carlson BL, Schroeder MA, et al. . Induction of MGMT expression is associated with temozolomide resistance in glioblastoma xenografts. Neuro Oncol. 2009;11(3):281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thon N, Kreth S, Kreth FW. Personalized treatment strategies in glioblastoma: MGMT promoter methylation status. Onco Targets Ther. 2013;6:1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee SY. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016;3(3):198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang J, Stevens MF, Bradshaw TD. Temozolomide: mechanisms of action, repair and resistance. Curr Mol Pharmacol. 2012;5(1):102–114. [DOI] [PubMed] [Google Scholar]
- 43. Baker SD, Wirth M, Statkevich P, et al. . Absorption, metabolism, and excretion of 14C-temozolomide following oral administration to patients with advanced cancer. Clin Cancer Res. 1999;5(2):309–317. [PubMed] [Google Scholar]
- 44. Cermakian N, Boivin DB. The regulation of central and peripheral circadian clocks in humans. Obes Rev. 2009;10 Suppl 2:25–36. [DOI] [PubMed] [Google Scholar]
- 45. Bjarnason GA, Jordan RC, Wood PA, et al. . Circadian expression of clock genes in human oral mucosa and skin: association with specific cell-cycle phases. Am J Pathol. 2001;158(5):1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111(1):41–50. [DOI] [PubMed] [Google Scholar]
- 47. Ballesta A, Innominato PF, Dallmann R, Rand DA, Lévi FA. Systems Chronotherapeutics. Pharmacol Rev. 2017;69(2):161–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fischer D, Lombardi DA, Marucci-Wellman H, Roenneberg T. Chronotypes in the US—Influence of age and sex. PLoS One. 2017;12(6):e0178782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9(10):764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.