Abstract
Introduction: The SARS-CoV-2 infection is associated with significant morbidity and mortality rates. The impact of thrombotic complications has been increasingly recognized as an important component of this disease.
Case reports: We describe four cases of spontaneous acute aortic thrombosis in patients with SARS-CoV-2 infection observed from March to December 2020 at Fondazione Policlinico Universitario Gemelli IRCCS in Rome, Italy.
The SARS-CoV-2 infection is associated with significant morbidity and mortality rates. The impact of thrombotic complications has been increasingly recognized as an important component of this disease. Emerging evidence suggests that severe form of coronavirus disease 2019 (COVID-19) is mediated by a hypercoagulable state, occurring in approximately 4% of critically ill patients.1
We describe 4 cases of spontaneous acute aortic thrombosis in patients with SARS-CoV-2 infection. Written informed consent was obtained from the patients for publication of these case reports and accompanying images.
Case reports
In the period March 2020 to December 2020, 4 (0.2%) out of 2006 patients with COVID-19 (0.2%), hospitalized at Fondazione Policlinico Universitario Gemelli IRCCS in Rome, Italy, presented symptomatic spontaneous aortic thrombosis confirmed at computed tomography angiography (CTA).
During the same period, three patients suffered from acute limb ischemia COVID-related without aortic thrombus.
In all 4 patients the diagnosis of SARS-CoV-2 was confirmed by reverse transcriptase-polymerase chain reaction analysis. The Table summarizes the main laboratory results at the admission. In all cases therapeutic anticoagulation with subcutaneous enoxaparin 100 UI/Kg twice a day was started after the diagnosis of arterial thrombosis for at least 4 to 6 weeks. During the intervention, intravenous heparin was administered (50 UI/kg). Calcified plaques on the aortoiliac axis were observed in three patients. Table I .
Table I.
Main laboratory results at the admission for each patient
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Leukocytes, x 109/L | 17.31 | 20.2 | 25.21 | 5.48 |
| Lymphocytes, x 109/L | 2.65 | 1.75 | 2.52 | 0.53 |
| Red blood cells, x 1012/L | 3.98 | 3.89 | 4.62 | 5.61 |
| Hemoglobin, g/dl | 12.11 | 11.32 | 13.24 | 14.71 |
| Platelets, x 109/L | 460 | 156 | 470 | 375 |
| D-dimer, ng/mL | 674 | 1901 | 900 | 1030 |
| Fibrinogen, mg/dL | 667 | 505 | 830 | 995 |
| C-reactive protein, ng/mL | 86.92 | 180.21 | 226.32 | 154.61 |
| Creatine kinase, UI/L | 11884 | 1714 | 178 | 59 |
| Lactate dehydrogenase, UI/L | 496 | 795 | 1405 | 524 |
Patient 1
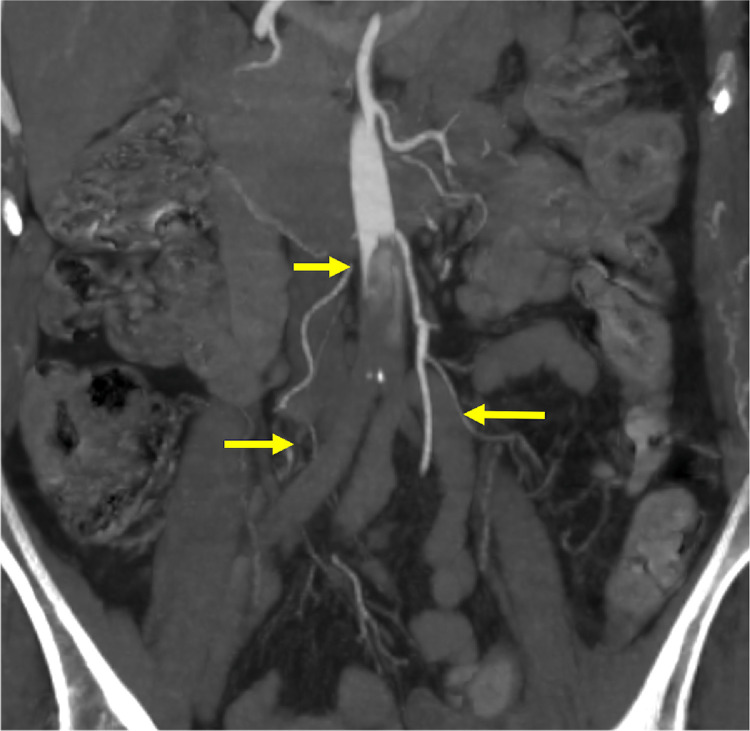
A 74-year-old woman and recent SARS-CoV-2 infection presented with bilateral acute lower limb ischemia. CTA showed complete thrombotic occlusion of the infrarenal aorta and both iliac-femoral axis. (Fig. 1 ).
Fig. 1.
Complete thrombotic occlusion of the infrarenal aorta and both iliac-femoral axis.
She underwent urgent bilateral thromboembolectomy with 3F Fogarty arterial catheter (Le Maitre, Burlington, Mass) via common femoral arteriotomies. Large thrombus material was removed, and valid pulses were obtained bilaterally. The post-operative course was uneventful, and she was discharged on post-operative day 4.
Patient 2
A 74-year-old woman presented to the emergency department with dyspnea and acute bilateral lower limb ischemia. CTA showed extensive thrombotic occlusion of the infrarenal aorta including both iliac axes.
The patient underwent surgery in urgent setting in spinal anesthesia. Thromboembolectomy with 4F Fogarty arterial catheters (Le Maitre, Burlington, Mass) via bilateral common femoral arteriotomies was performed. Abundant thrombus material was taken from both femoral arteries. A pulse was obtained in both lower extremities after the procedure.
The postoperative course was complicated by the dehiscence of the inguinal wound requiring the Vacuum-Assisted Closure therapy. The patient was discharged on post-operative day 25.
Patient 3
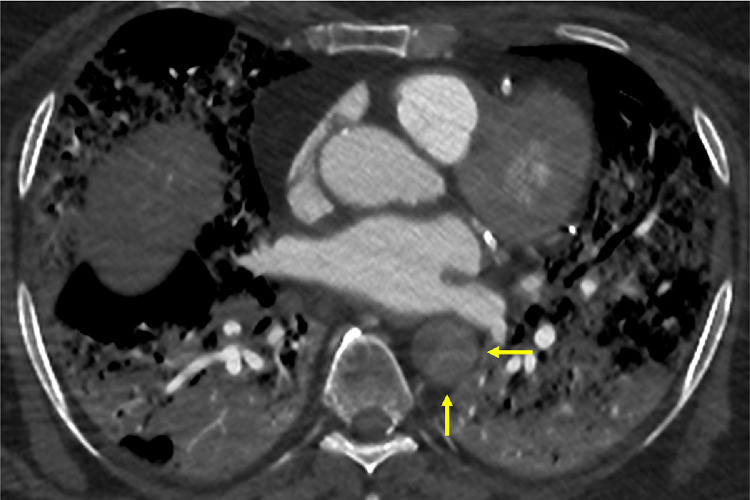
A 75-year-old man presented with cardiogenic shock. CTA reported a massive interstitial pneumonia with multifocal ground-glass opacities, typical of COVID-19 pneumopathy, were visualized in the bilateral lungs. Complete thrombosis of the descending thoracic and abdominal aorta, including visceral and renal arteries, was present. Both iliac and femoral axis were occluded. (Fig. 2 )
Fig. 2.
Complete thrombotic occlusion of the aorta, arising from the descending aorta and including all the visceral arteries.
The patient died immediately after the CT-scan.
Patient 4
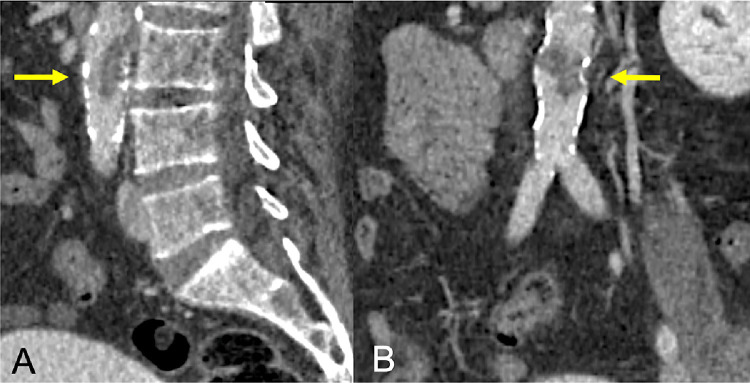
A 67-year-old SARS-COV-2 man was conducted to our emergency department for worsening of fever (<38.5°C) and dyspnea. He was hospitalized at the COVID Intensive Care Unit. The CTA showed the acute partial infrarenal aortic thrombosis and complete occlusion of the right superficial femoral artery with distal reperfusion. (Fig. 3 )
Fig. 3.
Partial infrarenal aortic thrombosis.
He underwent urgent surgical revascularization under local anesthesia: thromboembolectomy with 3F and 4F Fogarty arterial catheter (Le Maitre, Burlington, Mass) via right common femoral arteriotomy, with restoration of good inflow and outflow. The next day, because of bilateral hypothermia and absence of arterial pulses, the patient underwent another CTA scan which showed bilateral common femoral artery occlusion. He was submitted to new urgent surgical revascularization via bilateral Fogarty catheter. The post-operative course was complicated by worsening of respiratory function and massive bowel ischemia, due to acute occlusion of superior and inferior mesenteric artery, not amenable to curative treatment. The patient died on the following day from multi-organ failure.
Discussion
Although the etiopathogenesis is not fully clear, it is accepted that severe COVID‐19 is commonly complicated with coagulopathy. Cantador et al. analyzed that out of 1419 COVID-19 patients, 14 (1%) patients developed systemic arterial thrombotic events, including acute lower limb ischemia, acute coronary syndrome, and acute ischemic stroke with a mortality rate of 28.6% in these patients.2 Bellosta et al. demonstrated an increase in the incidence of acute limb ischemia in patients with COVID-19 from January to March 2020 compared to the same period of 2019 (16.3% vs. 1.8%, respectively; P < 0.001).3
Similiarly, another observational study showed 31% incidence of thrombotic complications in intensive care unit patients with COVID-19, with 3.7% arterial.4
Various mechanisms have been proposed to describe the pathophysiology of coagulopathy associated with COVID-19. Severe COVID-19 associated pneumonia patients present the characteristics of systemic hyper-inflammation, described as macrophage activation syndrome or cytokine storm, also known as secondary hemophagocytic lymphohistocytosis.5
Excessive inflammation triggered by the cytokine storm, the massive macrophages and platelet activation and endothelial dysfunction should be associated with the development of coagulopathy.6
Markers such as D-dimer, lactate dehydrogenase, ferritin, and CRP have been used to stratify patients for risk of thrombosis. Nevertheless, the treatment is controversial. Some studies suggest that only the patients meeting sepsis‐induced coagulopathy criteria or with markedly elevated D‐dimer may benefit from anticoagulant therapy mainly with low molecular weight heparin.7 , 8
In addition, potential benefit of low molecular weight heparin are also non-anticoagulant mechanisms including inhibition of heparinase activity, responsible for endothelial leakage, neutralization of chemokines, and cytokines, interference with leukocyte trafficking, reducing viral cellular entry, and neutralization of extracellular cytotoxic histones.9 In fact, anticoagulant therapy in the active infection period and during the early discharge period is necessary.
The treatment with Fogarty thromboembolectomy should always be considered in case of arterial occlusion. In our experience it was successful in 2 out of the 3 cases treated, since one patient had another thrombosis after surgery and died following the second treatment of thromboembolectomy.
In one case no treatment was possible given that a patient with a complete aortic thrombosis arising from the descending thoracic aorta died immediately after the CT-scan.
In addition, one surgery case was complicated by the wound dehiscence requiring the Vacuum-Assisted Closure therapy. This complication highlights that the sudden outbreak of COVID-19 makes the management of wounds even more complicated than usual, for both the patients and their caregivers.10 , 11
Conclusion
Patients with SARS-CoV-2 are at risk for acute arterial thromboembolic complications. In these cases, treatment dose anticoagulation and arterial embolectomy are the first treatment strategies.
Further studies are needed to investigate the cause of COVID-19 thrombotic complications.
Footnotes
Conflict of Interest: None.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions: Giovanni Tinelli: Conceptualization, Methodology, Writing, Supervion Simona Sica: Conceptualization, Methodology, Writing, Supervion. Francesca Montanari: Writing, Conceptualization. Francesco Franceschi: Conceptualization, Supervision. Marcello Covino: Conceptualization, Supervision. Domenico Dionisio: Conceptualization, Supervision. Loris Flora: Conceptualization, Supervision. Yamume Tshomba: Conceptualization, Supervision, Merthodology.
References
- 1.Cheruiyot I, Kipkorir V, Ngure B, et al. Arterial thrombosis in coronavirus disease 2019 patients: a rapid systematic review. Ann Vasc Surg. 2021;70:273–281. doi: 10.1016/j.avsg.2020.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cantador E, Núñez A, Sobrino P, et al. Incidence and consequences of systemic arterial thrombotic events in COVID-19 patients. J Thromb Thrombolysis. 2020;50:543–547. doi: 10.1007/s11239-020-02176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellosta R, Luzzani L, Natalini G, et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72:1864–1872. doi: 10.1016/j.jvs.2020.04.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGonagle D, Sharif K, O'Regan A, et al. The role of cytokines including interleukin-6 in covid-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tinelli G, Minelli F, Sica S, et al. Complete aortic thrombosis in SARS-CoV-2 infection. Eur Heart J. 2021;26 doi: 10.1093/eurheartj/ehab011. ehab011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iba T, Levy JH, Warkentin TE, et al. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost. 2019;17:1989–1994. doi: 10.1111/jth.14578. [DOI] [PubMed] [Google Scholar]
- 8.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buijsers B, Yanginlar C, Maciej-Hulme ML, et al. Beneficial non-anticoagulant mechanisms underlying heparin treatment of COVID-19 patients. EBioMedicine. 2020;59 doi: 10.1016/j.ebiom.2020.102969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabolli S, Tinelli G, Guarnera G, et al. Measuring the health status of patients with vascular leg ulcers and the burden for their caregivers. Eur J Vasc Endovasc Surg. 2007;34 doi: 10.1016/j.ejvs.2007.05.025. 613e8. [DOI] [PubMed] [Google Scholar]
- 11.Tinelli G, Sica S, Guarnera G, et al. Wound Care during COVID-19 Pandemic. Ann Vasc Surg. 2020;68:93–94. doi: 10.1016/j.avsg.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]