Abstract
Neurons synthesizing thyrotropin-releasing hormone, substance P and serotonin in the medullary caudal raphe nuclei project to the dorsal vagal complex and play a role in the central vagal regulation of gastric function. Neurons in the parapyramidal region in the ventral medulla share similar biochemical coding and projections as those in the caudal raphe nuclei. The role of the parapyramidal region in the autonomic regulation of gastric acid secretion was investigated in urethane-anesthetized rats. Unilateral microinjection of kainate into the parapyramidal region at 10, 15 and 20 ng induced a dose-related stimulation of gastric acid secretion (net increases: 22.2 ± 11.2, 40.5 ± 8.5 and 89.8 ± 19.4 μmol/60 min, respectively), while injection of vehicle had no effect (net change: −0.1 ± 1.4 μmol/60 min). Time-course studies showed a nine-fold peak increase over basal at 30 min after parapyramidal injection of kainate (20 ng) and acid secretion returned to basal level at 70 min. Microinjections of kainate (15–20 ng) outside the parapyramidal region or into the parapyramidal region in vagotomized rats had no effect. Exposure to cold (4°C) for 2 h, which is known to induce vagally mediated gastric secretory and motor responses through medullary thyrotropin-releasing hormone pathways, increased the number of Fos-positive cells in the caudal, middle and rostral parts of the parapyramidal region to 4.3 ± 0.4, 9.4 ± 0.9 and 18.4 ± 1.6/section, respectively, compared with 0.1 ± 0.1, 0.1 ± 0.0 and 0.7 ± 0.6/section, respectively, in rats maintained at room temperature. Most of the Fos-labeled cells co-expressed pro-thyrotropin-releasing hormone messenger RNA signal and/or were serotonin immunoreactive.
These data show that chemical activation of neurons in the parapyramidal region results in a vagal-dependent stimulation of gastric acid secretion and that acute cold exposure activates parapyramidal neurons containing pro-thyrotropin-releasing hormone and/or serotonin, suggesting a potential role of the parapyramidal region, in addition to the caudal raphe nuclei, as medullary sites involved in the vagal regulation of gastric function.
Keywords: kainate, raphe pallidus, Fos, vagus, TRH mRNA, 5-HT
Thyrotropin-releasing hormone (TRH)-, substance P-and serotonin (5-HT)-containing neurons in the raphe pallidus (Rpa) and the raphe obscurus (Rob) project to the dorsal vagal complex (DVC).16,24 Accumulated evidence shows that this caudal raphe-DVC pathway is physiologically relevant in the vagal regulation of the visceral function in rats.20,36 Chemical activation of cell bodies in the Rpa or Rob with kainate or L-glutamate stimulates gastric acid secretion,39 mucosal blood flow,17 motor functions9,14,26 and erosion formation,18 and at lower doses, increases the resistance of gastric mucosa to ethanol injury.19 These gastric responses result from the stimulation of vagal cholinergic pathways induced by the excitatory effect of TRH on the dorsal motor nucleus neurons, along with the modulation of the TRH action by co-released 5-HT and substance P in the DVC.28,39,40,43 Likewise, acute cold exposure induces Fos expression5,38 and enhances TRH gene expression in the Rpa and Rob,41 and stimulates medullary 5-HT metabolism,42 which are involved in the vagal cholinergic-dependent stimulation of gastric acid output,2,30 motility8,25 and hemorrhagic lesion formation13,31 induced by acute cold exposure.
The parapyramidal region (PPR) consists of neurons located in the medulla close to the ventral surface and lateral to the pyramidal tract.12 These neurons display similar neurochemical characteristics and efferent projections to those in the Rpa and Rob. In particular, TRH-and 5-HT-containing PPR neurons are known to project directly to the DVC and the intermediolateral cell column of the spinal cord.12,24,33,37 We reported previously that cold exposure induced Fos expression not only in the Rpa and Rob, but also in the PPR.5 These morphological features provide a neuroanatomical substrate by which PPR can be involved in the vagal regulation of gastric function at the medullary level. However, such functional evidence is still lacking. In the present study, we investigated whether chemical activation of PPR cell bodies by microinjection of kainate into the PPR influences gastric secretory function through autonomic pathways in urethane-anesthetized rats. First, we assessed the dose-related effect and time-course of kainate microinjected into the PPR on gastric acid secretion. Second, we studied the role of the vagus in mediating the effect of PPR activation. Finally, we examined whether acute cold exposure activates TRH mRNA-containing or 5-HT-immunoreactive (IR) neurons in the PPR in conscious rats using double labeling with Fos-IR as a marker of neuronal activation.21
EXPERIMENTAL PROCEDURES
Animals
Male Sprague-Dawley rats (Harlan Laboratory, San Diego, CA) weighing 280–320 g were housed in controlled temperature (22–24°C) and illumination (light on 6.00 a.m. to 6.00 p.m.). Animals received ad libitum Purina Laboratory Chow (Ralston Purina, St Louis, MO) and tap water. Rats were deprived of food for 24 h, but allowed free access to water until the beginning of treatments. Animal protocols were approved by the Veterans Administration Medical Center/West Los Angeles Research Service Animal Committee.
Measurement of gastric acid secretion
In urethane (1.5 g/kg, Sigma Chemical, St Louis, MO)-anesthetized rats, the esophagus was ligated at the cervical level and a laparotomy was performed. The pylorus was ligated and a double-lumen gastric cannula was implanted into the forestomach. After a 30-min stabilization period with flushing the stomach, the gastric acid secretion was monitored as described previously.39 The stomach was flushed through the double-lumen gastric cannula, with two 5-ml boluses of saline at room temperature and one 5-ml bolus of air at the end of each 10-min period. Acid output was determined by titration of the flushed perfusate with 0.01 N NaOH using an autotitrator (TTT80 Titrator, Radiometer, Copenhagen, Denmark). Net gastric acid output was calculated by subtracting the average acid secretion in the two 10-min periods before microinjection from each 10-min acid secretion value after microinjection.
Microinjections
The microinjection procedure was performed as described previously.39 Urethane-anesthetized rats with double-lumen gastric cannulae were positioned on a stereotaxic instrument (Kopf model 900) and the dorsal surface of the medulla, including the obex region and the surrounding area, was exposed by resecting the cervical musculature and removing the occipital skull plate. A glass micropipette (50–70 μm diameter) filled with either 0.1 M phosphate buffer (PB, vehicle used to dissolve kainate; Sigma) or kainate (Sigma, K0250) was positioned into the right or left PPR. Unilateral microinjection into the PPR (50 nl) was performed by pressure ejection over 1 min with a 1-μ1 Hamilton syringe attached to a glass micropipette with a PE-50 polyethylene catheter filled with water. The micropipette was left in place for another 3 min, then withdrawn and the animal was removed from the stereotaxic apparatus. The coordinates used for microinjection into the PPR were (in mm): ventral from the surface of the brainstem 3.7, anterior from the caudal tip of the area postrema 1.5, lateral from the midline 1.2. The coordinates selected for PPR microinjection were based on our unpublished observations showing that, at this level, TRH-, 5-HT-or substance P-IR neurons form a relatively larger and clearly defined cell group population. At the end of the experiment, rats were killed by decapitation, and brains were removed and fixed in a 10% formalin/20% sucrose solution for at least two days. Frozen sections were sliced at 30 μm, mounted on to glass slides and stained with Toluidine Blue. Histological sections were examined microscopically. The location of the microinjection sites was identified as the point of the termination of the micropipette track and marked on plates reproduced from the atlas of Paxinos and Watson.32
Experimental protocols
Effect of microinjection of kainate into the parapyramidal region on gastric acid secretion.
In rats anesthetized with urethane and acutely implanted with a double gastric lumen cannula, basal gastric acid secretion was measured at 10-min intervals for 20 min, then animals were positioned on the stereotaxic equipment for microinjection of vehicle (0.1 M PB, 50 nl) or kainate (10, 15 and 20 ng in 50 nl PB) into the PPR. Gastric acid secretions were monitored every10 min for a 90-min period after the microinjection. In another experiment, bilateral cervical vagotomy was performed immediately before the esophagus ligation (through the same cervical midline incision) and the gastric cannula implantation, and gastric acid secretions were monitored under the same conditions before and after kainate (20 ng) microinjected into the PPR.
Effect of cold exposure on Fos expression in the parapyramidal region in conscious rats.
Conscious rats fasted for 24 h were placed individually in flat-bottomed stainless steel cylindrical cages (16 cm × 5.5 cm × 5.5 cm) with perforations to allow ventilation. Then, semi-restrained rats were either maintained at ambient temperature (23 ± 2°C) or placed in a room at 4°C for 2 h. Previous studies showed that such semi-restraint conditions at room temperature do not induce changes in gastrointestinal function or Fos expression in the brain.5 At the end of the 2-h period of cold exposure, rats were killed and brains were processed for Fos immunohistochemistry. Brains of the cold-exposed rats were also processed for double labeling of Fos with 5-HT or pro-TRH mRNA.
Immunohistochemistry and in situ hybridization procedures
Fos immunohistochemistry and cell counting.
Fos-IR was detected as described previously. 5 Rats exposed to cold or maintained at room temperature in semi-restraint conditions for 2 h were deeply anesthetized with pentobarbital (70 mg/kg, i.p.) and transcardially perfused with 100 ml of isotonic saline followed by 500 ml of 4% paraformaldehyde in 0.1 M PB (pH 7.4). Brains were removed, postfixed for 3 h at 4°C in the same fixative and subsequently cryoprotected overnight in 20% sucrose in 0.1 M PB. Coronal frozen secretions (30 μm) of the brainstem were cut in a cryostat (Microtome, IEC, MA) at the inter-aural levels of –2.30 to –4.80 mm according to the atlas of Paxinos and Watson.32 These levels included the whole rostrocaudal length of the PPR located in the ventral medulla. Free-floating sections were incubated with the primary antibody [Fos Ab-5 rabbit polyclonal antibody, Oncogen Research Products; dilution 1:10,000 in 0.01 M phosphate-buffered saline (PBS) containing 0.3% Triton X-100 and 3% normal goat serum] at 4°C for 24 h followed by 1 h at room temperature with a biotinylated secondary antibody (goat anti-rabbit, Jackson ImmunoResearch Laboratories, West Grove, PA; dilution 1:1000). Sections were finally processed for avidin-biotin-peroxidase using diaminobenzidine as the chromogen, then mounted on slides (Superfrost/Plus, Fisher Scientific, Pittsburgh, PA), dehydrated in ethanol, cleared in xylene and coverslipped. The presence of Fos-IR was detected with bright-field microscopy as a dark brown reaction product in the cell nuclei.
Since the size of the PPR varies from caudal to rostral levels, the number of neurons at different levels also varies. To make data comparable, we divided the whole length of the PPR into three regions: caudal, from the caudal pole of the area postrema to the opening of the fourth ventricle (interaural −5.08 to −4.30 mm); middle, from the opening of the fourth ventricle to the middle level of the medial nucleus of the inferior olive (−4.30 to −3.30 mm); and rostral, from − 3.30 mm to the caudal part of the facial nucleus (−2.30 mm).32 The numbers of Fos-IR-positive cells in the PPR were counted and quantified as the average number of 20 random, although closely spaced, sections per level per rat. Cell counts from both left and right PPRs were added together and taken as one region. Double-counting errors were corrected by the following formula proposed by Abercrombie, 1 to estimate nuclear populations from microtome sections: P = A × [M/(L + M)], with P being the corrected cell count, A the total cell count, M the section thickness (μm) and L the average diameter of the nucleus (μm). To determine the average diameter of the nucleus, 10 randomly selected Fos-positive nuclei in each section of PPR neurons were measured with a microruler and in at least five sections per region.
Double staining by Fos and serotonin immunohistochemistry.
Following incubations with Fos antibody and biotinylated goat antirabbit immunoglobulin G as described above, medullary sections of rats exposed to cold for 2 h were processed for avidin–biotin–peroxidase by using diaminobenzidine enhanced with nickel ammonium sulfate as the first chromogen. Sections were then rinsed with PBS for 3 h at room temperature, and incubated with a rabbit polyclonal antibody raised against 5-HT (1:10,000; Incstar, Stillwater, MN) in 0.01 M PBS containing 0.1% Triton and 3% normal goat serum. Sections were finally processed by an avidin–biotin–peroxidase procedure using diamonobenzidine as the second chromogen. Fos-IR was detected as a dark blue reaction product in the nuclei and 5-HT appeared as a light brown reaction product in the cytoplasm.
Combination of Fos immunohistochemistry with pro-thyrotropinreleasing hormone messenger RNA in situ hybridization.
Medullary sections of rats exposed 2 h to cold were processed for Fos immuno-histochemistry as described above, except that all solutions were made with sterile diethylpyrocarbonate–water and the solution diluting Fos antibody and biotinylated secondary antibody was 0.3% Triton X-100/0.25% heparin sodium salt USP (ICN Biomedicals, Aurora, OH) in 0.01 M PBS. Thereafter, sections were mounted on to FisherBiotech ProbeOn microscope slides (Fisher Scientific, Pittsburgh, PA), dried overnight and processed using in situ hybridization histochemistry. In brief, sections were fixed in 4% paraformaldehyde, digested by proteinase K and acetylated in 0.25% acetic anhydride. 35S-Labeled cRNA probe (1 × 106 c.p.m./slide) was added to a hybridization mixture (90 μ1/slide), which consisted of 50% formamide, 600 mM NaCl, 80 mM Tris–HCl (pH 7.5), 4 mM EDTA, 0.1% sodium pyrophosphate, 0.2% sodium dodecyl sulfate, 10% dextran sulfate, 100 mM dithiothreitol, 1 × Denhardt’s solution and 0.5 mg yeast transfer RNA per milliliter. Final hybridization was carried out for 20 h at 55°C. Then, slides were rinsed in saline–sodium citrate buffer, treated with ribonuclease A, desalted in 1–0.1 × saline–sodium citrate buffer and dehydrated. Labeled sections were coated with liquid emulsion (Kodak NTB-2 diluted 1:1 with water), exposed for four weeks at 4°C, developed in D-19 developer and fixed in Kodak Fixer. Fos-IR was detected using light-field microscopy as a dark brown reaction product in the nuclei and pro-TRH mRNA as silver grains in the cytoplasm.
The hybridization probe was prepared as described in our previous studies.41 All the reagents were purchased from Promega (Madison, WI). The 1322-bp EcoRI pro-TRH DNA fragment cloned in plasmid pUC12 (kindly provided by Dr R. Goodman, Vollum Institute, Portland, OR) was resubcloned into pGEM-3. The antisense cRNA probe was synthesized by T7 RNA polymerase using 1 μg SmaI-linearized plasmid DNA and with the incorporation of [α-35S]UTP (Du Pont, Boston, MA). The specificity of the pro-TRH probe has been validated in our previous studies.41
Statistical analysis
Results are expressed as mean ± S.E.M. Comparisons between two groups were performed by Student’s t-test and multiple group comparisons by ANOVA followed by Dunn’s contrast. Comparisons between pre-and post-injection levels were performed by paired t-test. A P value less than 0.05 was considered statistically significant.
RESULTS
Microinjection of kainate into the parapyramidal region stimulated gastric acid secretion in urethane-anesthetized rats
The low basal gastric acid secretions in urethane-anesthetized rats were similar before and after microinjection of vehicle (0.1 M PB) into the PPR (ranging from 2.9 ± 0.9 to 3.9 ± 1.2 μmol/10 min). Unilateral microinjection of kainate into the PPR (10, 15 and 20 ng) induced a dose-related stimulation of gastric acid secretion compared with the respective basal values and vehicle-treated group. The peak responses were reached at 30 min after the microinjection and thereafter the elevated gastric acid secretion gradually decreased to basal levels within 50–70 min post-injection (Fig. 1). The net acid response (μmol/60 min) was significantly increased to 40.5 ± 8.5 and 89.9 ± 19.4 by microinjection of kainate into the PPR at 15 and 20 ng, respectively, compared with the vehicle-microinjected group (−0.1 ± 1.4 μmol/60 min; Fig. 2). The acid response to 10 ng kainate (22.2 ± 11.2 μmol/60 min) did not reach statistical significance (Fig. 2). Bilateral cervical vagotomy completely prevented the acid stimulatory action of kainate (20 ng) microinjected into the PPR (Fig. 1). The localizations of microinjection sites are illustrated in Fig. 3.
Fig. 1.
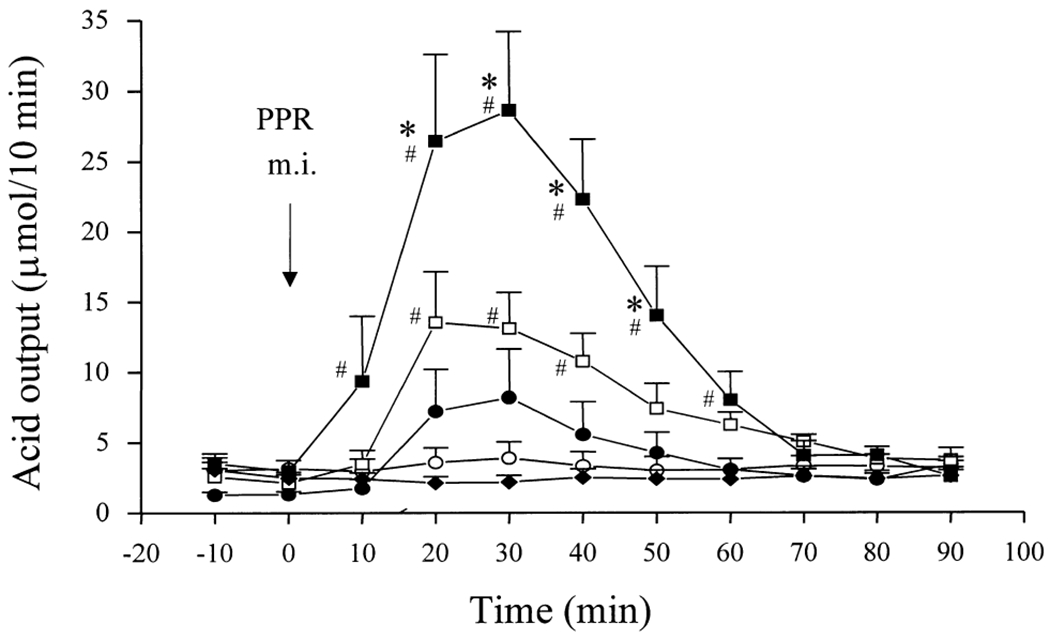
Time-course of vagal-dependent gastric acid response to kainate microinjected (m.i.) into the PPR of the ventral medulla in urethane-anesthetized rats. Each point represents the mean ± S.E.M. of the number of rats indicated in parentheses. (O) 0.1 M PB (vehicle, 50 nl, n = 5); (●) 10 ng kainate (n = 5); (□) 15 ng kainate (n = 5); (■) 20 ng kainate (n = 7); (◆) 20 ng kainate in vagotomized rats (n = 7). *P < 0.05 compared with corresponding values in the vehicle group; *P < 0.05 compared with respective pre-injection basal values.
Fig. 2.
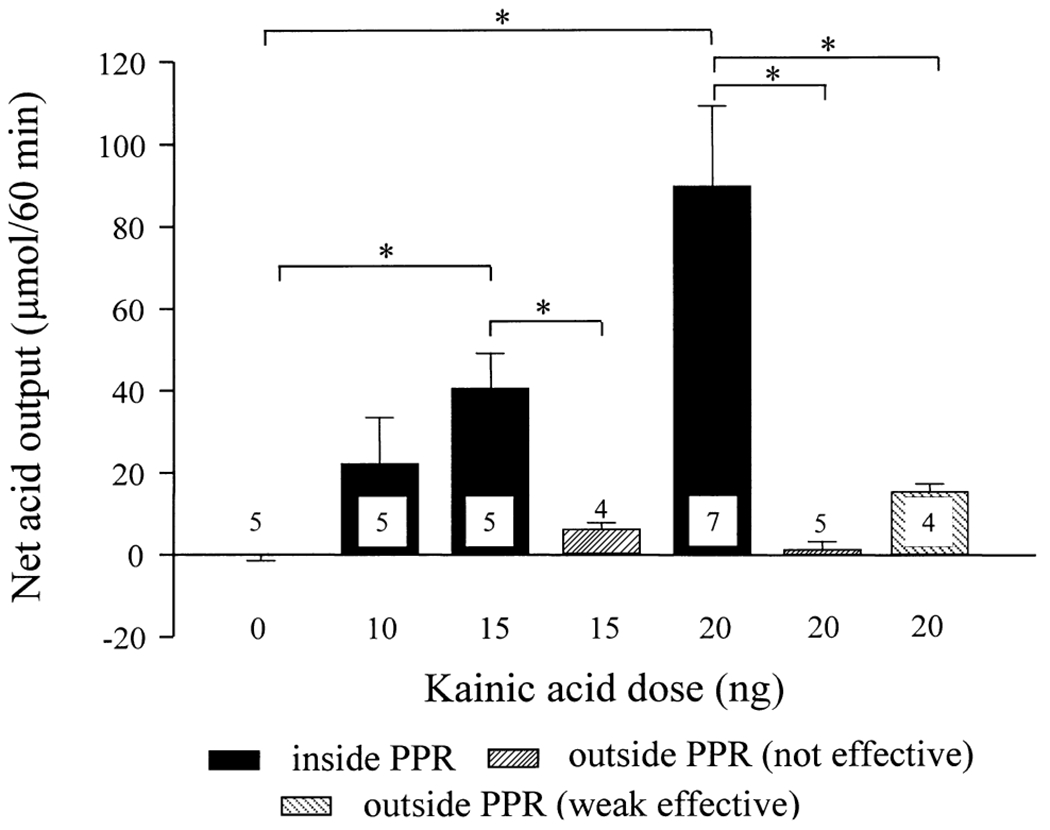
Net stimulation of gastric acid secretion induced by kainate micro-injected into the PPR or outside the PPR in the ventral medulla of urethane-anesthetized rats. Each column represents mean ± S.E.M. of the number of rats indicated in or above the column. *P<0.05 between groups as indicated.
Fig. 3.
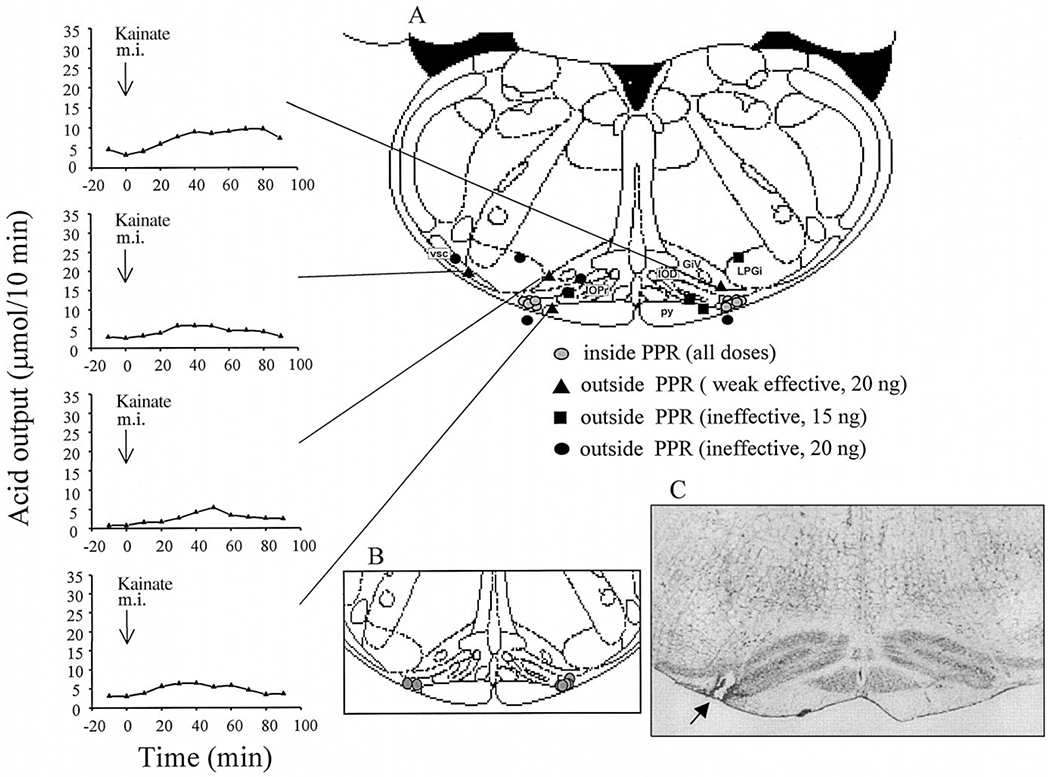
(A) Coronal brainstem section (interaural −3.30 mm) adapted from the atlas of Paxinos and Watson32 showing the microinjection sites in the PPR (including vehicle and all doses of kainate) and outside the PPR (15 and 20 ng kainate) in urethane-anesthetized rats. The weak acid responses (>10 μmol/60 min) for each microinjection site are presented individually as indicated. GiV, ventral gigantocell reticular nucleus; IOD, dorsal nucleus of the inferior olive; IOPr, principal nucleus of the inferior olive, LPGi, lateral paragigantocellular nucleus; py, pyramidal tract; vsc, ventral spinocerebellar tract. (B) Coronal brainstem section (interaural −3.30 mm) showing microinjection sites of vagotomized rats. (C) Histological sections of rat ventral medulla at interaural −3.30 mm level (× 25) showing an example of effective microinjection sites. Arrows show the location of the points of the termination of the micropipette tract.
No significant difference was observed between the net increase of gastric acid secretion induced by kainate (20 ng) microinjected into the left (93.1 ± 38.9 μmol/60 min, n = 3) or right PPR (87.2 ± 23.4 μmol/60 min, n = 4). Microinjections of kainate (20 ng) into sites located outside the PPR, but in nearby areas, namely into the pyramidal tract (n = 1), the ventral gigantocellular reticular nucleus (n = 2) or the ventral spinocerebellar tract (n = 1), induced a weak acid response (15.6 ± 1.8, n = 4) that was significantly lower than the responses to 20 ng kainate microinjected into the PPR (Figs 2, 3). Kainate (15 ng) microinjected into the pyramidal tract (n = 1), the principal interior olive nuclei (n = 2) or the lateral paragigantocellular nucleus (n = 1), or 20 ng into the ventral spinocerebellar tract (n = 1), the dorsal interior olive nuclei (n = 1), the lateral paragigantocellular nucleus (n = 1) or outside the surface of the ventral medulla (n = 2), failed to induce an increase in gastric acid secretion greater than 10 μmol/60 min (Figs 2, 3).
Cold exposure induced Fos expression in thyrotropin-releasing hormone messenger RNA- and serotonin-containing neurons in the parapyramidal region
Rats maintained in semi-restraint cylindrical cages at ambient temperature for a 2-h period showed no or a few Fos-IR-positive cells in the caudal, middle and rostral PPR (0.13 ± 0.05, 0.08 ± 0.03 and 0.67 ± 0.59/section, respectively; Fig. 4B). Cold exposure (4°C) for 2 h induced Fos expression on both sides of the PPR, reaching 4.29 ± 0.44, 9.35 ± 0.87 and 18.43 ± 1.58 cells/section in the caudal, middle and rostral levels, respectively (Fig. 4B). Representative Fos-positive cells in the middle to rostral PPR (interaural −3.30 mm) induced by cold exposure compared with the lack of Fos-IR in rats maintained at ambient temperature are illustrated in Fig. 4A.
Fig. 4.
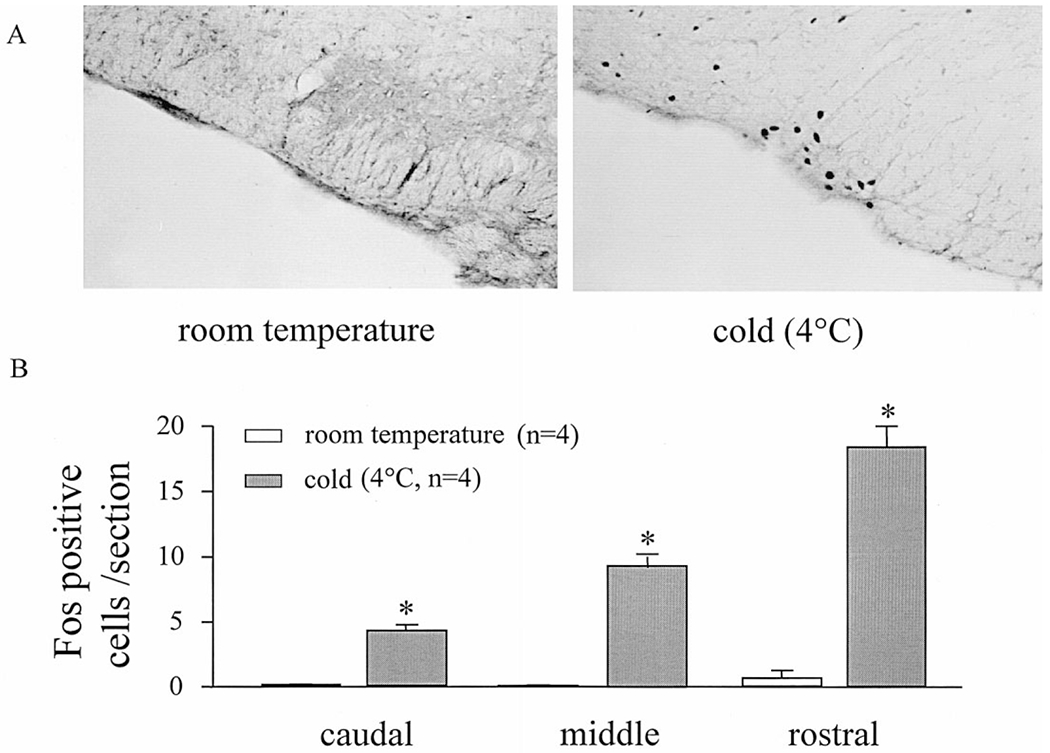
(A) Representative ventral medulla section (interaural −3.30 mm, × 120) showing Fos-IR in the parapyramidal region in semi-restrained conscious rats exposed for 2 h either at room temperature or in the cold (4°C). (B) Increase in Fos-positive cells in the PPR induced by 2-h cold exposure. Each column represents the mean ± S.E.M. of Fos-positive cells/section (left plus right PPR) of four rats in each group. *P < 0.05 compared with Fos levels in rats semi-restrained at room temperature.
Combined in situ hybridization histochemistry for pro-TRH mRNA and Fos immunohistochemistry demonstrates the presence of hybridized cells in the PPR of 2-h cold-exposed rats. Grains of pro-TRH mRNA signals accumulated over the majority of neurons that showed Fos-positive nuclei (Fig. 5A). Likewise, in cold-exposed rats many PPR neurons were 5-HT-IR and double labeled with Fos (Fig. 5B).
Fig. 5.
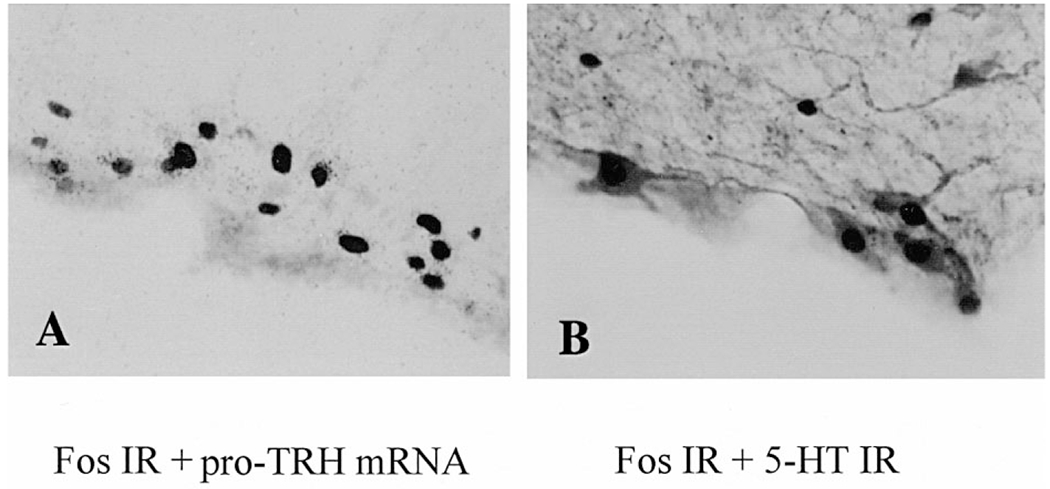
Double labeling of Fos-positive cells in TRH-and 5-HT-synthesizing neurons in the PPR in semi-restrained rats exposed to cold (4°C) for 2 h. (A) Combined Fos immunohistochemistry and pro-TRH mRNA in situ hybridization in bright field (interaural −3.30 mm, × 560). Fos-IR is shown as dark reaction product in the nuclei and pro-TRH mRNA appears as silver grains in the cytoplasm. (B) Double immunohistochemical staining for Fos-and 5-HT-IR (interaural −3.72, × 600). Fos-IR is shown as dark reaction product in the nuclei and 5-HT-IR as light/dark reaction product in the cytoplasm.
DISCUSSION
The present study shows that unilateral microinjection of kainate into the PPR stimulated gastric acid secretion in urethane-anesthetized rats. The 60-min net acid output was increased by 22-, 40-and 90-fold over basal in response to kainate microinjected into the PPR at 10, 15 and 20 ng, respectively. The effect was long lasting, as gastric acid secretion was still significantly elevated at 50 min after kainate microinjected into the PPR at 20 ng. Kainate was originally introduced as a neuron-specific and axon-sparing lesioning tool.3,6 However, doses below 0.5 μg are subthreshold for neurotoxicity, particularly under acute conditions, while being excitatory to neuron cell bodies but not to fibers of passage. 3,6 The stimulation of acid secretion induced by microinjection of kainate into the PPR at 10–20 ng, while vehicle under the same conditions had no effect, shows the chemical specificity of the response.
The microinjections were performed using coordinates at 1.5 mm rostral to the caudal tip of the area postrema. This was selected according to our unpublished immunohistochemical observations showing that pro-TRH-, 5-HT-or substance P-containing neurons at this level of the PPR form a relatively large and clearly defined cell group population, which is accessible to be targeted for microinjection. Similar acid response could be elicited when kainate was microinjected into either the right or left side of the PPR. By contrast, microinjection of kainate at 15 or 20 ng into sites nearby but outside the PPR, namely into the pyramidal tract, dorsal and principal interior olive nuclei, lateral paragigantocellular nucleus, ventral gigantocellular reticular nucleus, ventral spinocerebellar tract or outside the ventral surface of the medulla, induced significantly weaker acid response than into the PPR or had no effect. These data show the site specificity of the gastric response to activation of PPR neurons. The weak acid response to 20 ng kainate microinjected into the principal interior olive nuclei or the ventral gigantocellular reticular nucleus could be induced by diffused kainate reaching the nearby PPR neurons, or alternatively, by activating individual TRH- or 5-HT-containing neurons that are scattered in these areas (Yang, Yuan, Wang and Taché, unpublished observations).24
The neural pathway through which chemical activation of PPR neurons stimulated gastric acid secretion is vagally mediated. This is supported by the well-established stimulatory role of vagal activation in the regulation of gastric acid secretion,4 the existence of direct efferent projections from PPR neurons to the DVC24,37 and the complete inhibition of the gastric acid response to the highest dose of kainate microinjected into the PPR by bilateral cervical vagotomy (present observation). These data provide the first experimental demonstration that the PPR can influence visceral function through vagal-dependent pathways. The role of PPR in medullary autonomic regulation was previously assessed mainly in relation to its direct efferent projections to the inter-mediolateral column33 and the control of the cardiovascular system.10,11 The hypotensive response to 5-HT1A receptor agonists microinjected into the PPR was suggested to result from the modulation of the sympathetic outflow based on the presence of 5-HT1A receptors on PPR cell bodies projecting to the intermediolateral column and the inhibition of the firing rate of PPR neurons by 5-HT1A agonists microinjected into the PPR.10,11 The present study indicates that, in addition to the Rpa and Rob, which are well established to be involved in the vagal regulation of gastric function,15,26,36 the PPR in the ventral medulla is another site which is able to influence gastric secretion through vagal pathways. These functional observations are consistent with the chemical and neuroanatomical characteristics of PPR neurons, which bear a similarity with those in the Rob and Rpa, as they contain TRH-, 5-HT- and substance P-IR and project to the DVC.12,24,33 Although the biochemical coding of PPR action to stimulating gastric acid secretion needs to be characterized, it may be speculated that activation of TRH-containing neurons in the PPR projecting to the DVC24 plays an important role, as established previously for the Rpa and Rob.27,39
The assumption that PPR neurons exert a physiological role in the vagal regulation of gastric function is further supported by the demonstration that conscious semi-restrained rats exposed to cold for 2 h exhibited Fos-IR in the PPR, as observed previously in 3-h cold-exposed rats.5 The Fos protein, expressed from the proto-oncogene c-fos, belongs to the class of immediate-early gene products that are increased in specific neurons by a variety of specific stimuli.7 Fos protein forms a heterodimer with Jun B that acts as an intracellular third messenger binding to activator protein-1 transcription factor and activates genes including the TRH gene, which has an activator protein-1 site identified on its protomer. 22,23,29 The present study extends previous findings5 by quantitatively demonstrating that 2-h cold exposure significantly increased Fos-IR cells in different levels of the PPR. In addition, combined in situ hybridization histochemistry and immunohistochemistry revealed that neuronal activation in the PPR induced by cold is taking place in pro-TRH-and 5-HT-synthesizing neurons. Since neurons in the PPR may synthesize multiple peptides or peptides with 5-HT in different combinations,12,33 it is also possible that some of the cold-activated neurons contain more than one peptide or peptides in combination with 5-HT. We showed previously that acute cold exposure (2–3 h) induced Fos expression in the Rpa and Rob,5 and increased medullary TRH gene expression41 and 5-HT metabolism,42 although no double labeling was performed. The demonstration that Fos-IR was induced by cold within TRH- and 5-HT-synthesizing neurons in the PPR is consistent with the increased TRH gene expression and 5-HT metabolism in the medulla induced by cold.41,44 These findings, together with the established role of medullary TRH and 5-HT in cold-induced gastric acid secretion and erosion formation,13,30,41,42 strongly support an involvement of the PPR in vagally mediated gastric responses induced by cold exposure. Another pathophysiological situation showing that PPR neurons may play a role in autonomic regulation of visceral function is hypothyroidism. We recently found that Fos-IR was remarkably induced in the PPR, Rpa and the Rob by hypothyroidism,44 a well-known pathological situation associated with significant changes in gastrointestinal and cardiovascular functions.34,35
CONCLUSIONS
Chemical activation of PPR cell bodies by unilateral microinjection of kainate into this nucleus stimulates gastric acid secretion in urethane-anesthetized rats. The gastric acid response to kainate microinjected into the PPR is site specific and blocked by vagotomy. These data, along with the induction of Fos expression in TRH- and 5-HT-synthesizing neurons in the PPR by acute cold exposure, an environmental stimulus known to induce vagally mediated stimulation of gastric acid secretion and erosion formation through TRH-dependent mechanisms,13,30,41 provided neuroanatomical, neurochemical and functional evidence for the involvement of the PPR in the central vagal regulation of the gastrointestinal system.
Acknowledgements—
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants DK-50255 (H.Y.), DK-30110 (Y.T.) and DK-33061 (CURE Animal Core). Paul Kirsch is acknowledged for his help in the preparation of the manuscript.
Abbreviations:
- DVC
dorsal vagal complex
- EDTA
ethylenediaminetetra-acetate
- 5-HT
serotonin
- IR
immunoreactive, immunoreactivity
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- PPR
parapyramidal region
- Rob
raphe obscurus
- Rpa
raphe pallidus
- TRH
thyrotropin-releasing hormone
REFERENCES
- 1.Abercrombie M (1946) Estimation of nuclear population from microtome sections. Anat. Rec. 94, 239–247. [DOI] [PubMed] [Google Scholar]
- 2.Arai I, Muramatsu M and Aihara H (1986) Body temperature dependency of gastric regional blood flow, acid secretion and ulcer formation in restraint and water-immersion stressed rats. Jpn. J. Pharmac. 40, 501–504. [DOI] [PubMed] [Google Scholar]
- 3.Berger ML, Lassmann H and Hornykiewicz O (1989) Limbic seizures without brain damage after injection of low doses of kainic acid into the amygdala of freely moving rats. Brain Res. 489, 261–272. [DOI] [PubMed] [Google Scholar]
- 4.Berthoud HR, Fox EA and Powley TL (1991) Abdominal pathways and central origin of rat vagal fibers that stimulate gastric acid. Gastroenterology 100, 627–637. [DOI] [PubMed] [Google Scholar]
- 5.Bonaz B and Taché Y (1994) Induction of Fos immunoreactivity in the rat brain after cold-restraint induced gastric lesions and fecal excretion. Brain Res. 652, 56–64. [DOI] [PubMed] [Google Scholar]
- 6.Coyle JT, Molliver ME and Kuhar MJ (1978) In situ injection of kainic acid: a new method for selectively lesioning neural cell bodies while sparing axons of passage. J. comp. Neurol. 180, 301–323. [DOI] [PubMed] [Google Scholar]
- 7.Dragunow M and Faull R (1989) The use of c-fos as a metabolic marker in neuronal pathway tracing. J. Neurosci. Meth. 29, 261–265. [DOI] [PubMed] [Google Scholar]
- 8.Garrick T, Buack S and Bass P (1986) Gastric motility is a major factor in cold restraint-induced lesion formation in rats. Am. J. Physiol. 250, G191–G199. [DOI] [PubMed] [Google Scholar]
- 9.Garrick T, Prince M,, Yang H, Ohning G and Taché Y (1994) Raphe pallidus stimulation increases gastric contractility via TRH projections to the dorsal vagal complex in rats. Brain Res. 636, 343–347. [DOI] [PubMed] [Google Scholar]
- 10.Helke CJ, Capuano S, Tran N and Zhuo H (1997) Immunocytochemical studies of the 5-HT(1A) receptor in ventral medullary neurons that project to the intermediolateral cell column and contain serotonin or tyrosine hydroxylase immunoreactivity. J. comp. Neurol. 379, 261–270. [PubMed] [Google Scholar]
- 11.Helke CJ, McDonald CH and Phillips ET (1993) Hypotensive effects of 5-HT1A receptor activation: ventral medullary sites and mechanisms of action in the rat. J. auton. nerv. Syst. 42, 177–188. [DOI] [PubMed] [Google Scholar]
- 12.Helke CJ, Thor KB and Sasek CA (1989) Chemical neuroanatomy of the parapyramidal region of the ventral medulla in the rat. Prog. Brain Res. 81, 17–28. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez DE, Arredondo ME, Xue BG and Jennes L (1990) Evidence for a role of brain thyrotropin-releasing hormone (TRH) on stress gastric lesion formation in rats. Brain Res. Bull. 24, 693–695. [DOI] [PubMed] [Google Scholar]
- 14.Hornby PJ, Rossiter CD, White RL, Norman WP, Kuhn DH and Gillis RA (1990) Medullary raphe: a new site for vagally mediated stimulation of gastric motility in cats. Am. J. Physiol. 258, G637–G647. [DOI] [PubMed] [Google Scholar]
- 15.Hornby PJ, Sivarao DV and Paul D (1998) Medullary raphe and regulation of gastric function. Neurogastroenterol. Motil. 10, 355. [DOI] [PubMed] [Google Scholar]
- 16.Kachidian P, Poulat P, Marlier L and Privat A (1991) Immunohistochemical evidence for the coexistence of substance P, thyrotropin-releasing hormone, GABA, methionine-enkephalin, and leucin-enkephalin in the serotonergic neurons of the caudal raphe nuclei: a dual labeling in the rat. J. Neurosci. Res. 30, 521–530. [DOI] [PubMed] [Google Scholar]
- 17.Kaneko H, Kaunitz J and Taché Y (1998) Vagal mechanisms underlying gastric protection induced by chemical activation of raphe pallidus in rats. Am. J. Physiol. 275, G1056–G1062. [DOI] [PubMed] [Google Scholar]
- 18.Kaneko H and Taché Y (1995) TRH in the dorsal motor nucleus of vagus is involved in gastric erosion induced by excitation of raphe pallidus in rats. Brain Res. 699, 97–102. [DOI] [PubMed] [Google Scholar]
- 19.Kaneko H, Yang H, Ohning G and Taché Y (1995) Medullary TRH is involved in gastric protection induced by low dose of kainic acid into the raphe pallidus. Am. J. Physiol. 268, G548–G552. [DOI] [PubMed] [Google Scholar]
- 20.Krowicki ZK (1996) Role of selected peptides in the vagal regulation of gastric motor and endocrine pancreatic function. J. Physiol. Pharmac. 47, 399–409. [PubMed] [Google Scholar]
- 21.Krukoff TL (1993) Expression of c-fos in studies of central autonomic and sensory systems. Molec. Neurobiol. 7, 247–263. [DOI] [PubMed] [Google Scholar]
- 22.Lee SL, Stewart K and Goodman RH (1988) Structure of the gene encoding rat thyrotropin releasing hormone. J. biol. Chem. 263, 16,604–16,609. [PubMed] [Google Scholar]
- 23.Luo LG and Jackson IM (1998) Antisense oligomers of cfos and cjun block glucocorticoid stimulation of thyrotropin-releasing hormone (TRH) gene expression in cultured anterior pituitary cells. Peptides 19, 1295–1302. [DOI] [PubMed] [Google Scholar]
- 24.Lynn RB, Kreider MS and Miselis RR (1991) Thyrotropin-releasing hormone-immunoreactive projections to the dorsal motor nucleus and the nucleus of the solitary tract of the rat. J. comp. Neurol. 311, 271–288. [DOI] [PubMed] [Google Scholar]
- 25.Martinez V, Wu SV and Taché Y (1998) Intracisternal antisense oligodeoxynucleotides to the TRH receptor blocked vagal dependent stimulation of gastric emptying induced by acute cold in rats. Endocrinology 139, 3730–3735. [DOI] [PubMed] [Google Scholar]
- 26.McCann MJ, Hermann GE and Rogers RC (1989) Nucleus raphe obscurus (nRO) influences vagal control of gastric motility in rats. Brain Res. 486, 181–184. [DOI] [PubMed] [Google Scholar]
- 27.McCann MJ, Hermann GE and Rogers RC (1989) Thyrotropin-releasing hormone: effects on identified neurons of the dorsal vagal complex. J. auton. nerv. Syst. 26, 107–112. [DOI] [PubMed] [Google Scholar]
- 28.McTigue DM, Rogers RC and Stephens RLJ (1992) Thyrotropin-releasing hormone analogue and serotonin interact within the dorsal vagal complex to augment gastric acid secretion. Neurosci. Lett. 144, 61–64. [DOI] [PubMed] [Google Scholar]
- 29.Morgan JI and Curran T (1991) Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. A. Rev. Neurosci. 14, 421–451. [DOI] [PubMed] [Google Scholar]
- 30.Niida H, Takeuchi K and Okabe S (1991) Role of thyrotropin-releasing hormone in acid secretory response induced by lowering of body temperature in the rat. Eur. J. Pharmac. 198, 137–142. [DOI] [PubMed] [Google Scholar]
- 31.Niida H, Takeuchi K, Ueshima K and Okabe S (1991) Vagally mediated acid hypersecretion and lesion formation in anesthetized rat under hypothermic conditions. Dig. Dis. Sci. 36, 441–448. [DOI] [PubMed] [Google Scholar]
- 32.Paxinos G and Watson C (1997) The Rat Brain in Stereotaxic Coordinates, pp. 1–116. Academic, Orlando. [Google Scholar]
- 33.Sasek CA, Wessendorf MW and Helke CJ (1990) Evidence for co-existence of thyrotropin-releasing hormone, substance P and serotonin in ventral medullary neurons that project to the intermediolateral cell column in the rat. Neuroscience 35, 105–119. [DOI] [PubMed] [Google Scholar]
- 34.Seely EW and Williams GH (1990) Gastrointestinal manifestations of endocrine disease. In Principles and Practice of Endocrinology and Metabolism (ed. Becker KL), pp. 1503–1506. J. B. Lippincott, Philadelphia. [Google Scholar]
- 35.Seely EW and Williams GH (1990) The cardiovascular system and endocrine disease. In Principles and Practice of Endocrinology and Metabolism (ed. Becker KL), pp. 1496–1501. J. B. Lippincott, Philadelphia. [Google Scholar]
- 36.Taché Y, Yang H and Kaneko H (1995) Caudal raphe-dorsal vagal complex peptidergic projections: role in gastric vagal control. Peptides 16, 431–435. [DOI] [PubMed] [Google Scholar]
- 37.Thor KB and Helke CJ (1987) Serotonin- and substance P-containing projections to the nucleus tractus solitarii of the rat. J. comp. Neurol. 265, 275–293. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Cardin S and Martinez V (1996) Intracerebroventricular CRF inhibits cold restraint-induced c-fos expression in the dorsal motor nucleus of the vagus and gastric erosions in rats. Brain Res. 736, 44–53. [DOI] [PubMed] [Google Scholar]
- 39.Yang H, Ohning GV and Taché Y (1993) TRH in dorsal vagal complex mediates acid response to excitation of raphe pallidus neurons in rats. Am. J. Physiol. 265, G880–G886. [DOI] [PubMed] [Google Scholar]
- 40.Yang H and Taché Y (1997) Substance P in the dorsal vagal complex inhibits medullary TRH-induced gastric acid secretion in rats. Am. J. Physiol 272, G987–G993. [DOI] [PubMed] [Google Scholar]
- 41.Yang H, Wu SV, Ishikawa T and Taché Y (1994) Cold exposure elevates thyrotropin-releasing hormone gene expression in medullary raphe nuclei: relationship with vagally mediated gastric erosions. Neuroscience 61, 655–663. [DOI] [PubMed] [Google Scholar]
- 42.Yang H, Zhang XJ, Tache Y, Yoneda M and Wang CC (1995) Central serotonin influences the development of cold-restraint stress-induced gastric ulceration: role of autonomic and endocrine mechanisms. In Gastrointestinal Tract and Endocrine System (eds Singer MV, Ziegler R and Rohr G), pp. 77–82. Kluwer Academic, London. [Google Scholar]
- 43.Yoneda M and Taché Y (1995) Serotonin enhances gastric acid response to TRH analogue in dorsal vagal complex through 5-HT2 receptors in rats. Am. J. Physiol. 269, R1–R6. [DOI] [PubMed] [Google Scholar]
- 44.Yuan PQ and Yang H (1999) Hypothyroidism induces Fos-like immunoreactivity in ventral medullary neurons that synthesize TRH. Am. J. Physiol. 277, E927–E936. [DOI] [PMC free article] [PubMed] [Google Scholar]


