Abstract
Background
The initial therapeutic strategy for hormone receptor-positive (HR+), HER2-negative (HER2-) breast cancer is based on the first metastatic site; however, little evidence is available regarding the influence of metastatic distribution patterns of first metastatic sites on prognosis. In this study, we aimed to identify the metastatic distribution patterns of first metastatic sites that significantly correlate with survival after recurrence.
Methods
We performed a retrospective review of records from 271 patients with recurrent metastatic HR+/HER2- breast cancer diagnosed between January 2000 and December 2015. We assessed survival after recurrence according to the metastatic distribution patterns of the first metastatic sites and identified significant prognostic factors among patients with single and multiple metastases.
Results
Prognosis was significantly better in patients with a single metastasis than in those with multiple metastases (median overall survival after recurrence: 5.86 years vs. 2.50 years, respectively, p < 0.001). No metastatic organ site with single metastasis was significantly associated with prognostic outcome, although single metastasis with diffuse lesions was an independent risk factor for worse prognosis (HR: 3.641; 95% CI: 1.856–7.141) and more easily progressing to multiple metastases (p = 0.002). Multiple metastases, including liver metastasis (HR: 3.145; 95% CI: 1.802–5.495) or brain metastasis (HR: 3.289; 95% CI: 1.355–7.937), were regarded as significant independent poor prognostic factors; however, multiple metastases not involving liver or brain metastasis were not significantly related to prognosis after recurrence.
Conclusions
Single metastases with diffuse lesions could more easily disseminate systemically and progress to multiple metastases, leading to a poor prognosis similar to multiple metastases. Our findings indicate that the reconsideration of the determinant factors of therapeutic strategies for first recurrence in HR+/HER2- breast cancer may be needed.
Keywords: Breast cancer, Recurrence, Survival, Hormone receptor-positive, HER2-negative, Metastatic pattern
Introduction
Breast cancer is the most commonly diagnosed cancer in women and a leading cause of cancer-related mortality worldwide [1, 2]. Despite recent improvements in adjuvant treatment, 20–30% of early breast cancer patients will develop distant metastasis and be generally incurable, in which cases the main treatment goal is symptom palliation [3–5]. More than 70% of patients present with hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) breast cancer [6]. According to the current guidelines and international consensus statements, the metastatic patterns of first metastatic sites are important determinants of the initial therapeutic strategy for HR+/HER2- metastatic patients [7–9]. However, the metastatic patterns of the first metastatic sites, such as multiple metastases, immediately life-threatening disease, or rapidly progressive visceral metastasis or crisis, have not been clearly evaluated. Although previous real-world studies have demonstrated several significant prognostic factors of survival after recurrence [10–24], little attention has been given to the influence of the metastatic distribution patterns of first metastatic sites on patient prognosis. We felt that the metastatic distribution patterns should be analyzed in more detail to evaluate the relationship with survival after recurrence in HR+/HER2- patients. Therefore, we used the clinical data of HR+/HER2- recurrent breast cancer patients with distant metastasis to identify the correlation between the metastatic distribution patterns of first metastatic sites and survival after recurrence. We evaluated the prognosis of each HR+/HER2- patient with single and multiple metastases separately. We also classified single metastases into diffuse lesions and non-diffuse lesions, which may help us determine more beneficial therapeutic strategies and meet the unmet needs of these patients. A deeper understanding of the metastatic distribution pattern in HR+/HER2- patients may be needed for the delivery of appropriate healthcare to poor-prognosis groups and play an important role in optimal treatment and care, thereby improving the prognosis of these patients.
Methods
Study design and patients
This study was conducted using a retrospective longitudinal cohort design with the use of hospital electronic patient records. Patients with a first diagnosis of recurrent HR+/HER2- breast cancer made between January 2000 and December 2015 were identified from Sakai City Medical Center and Kindai University Hospital. Patients treated within a clinical trial (prior to or during the study period) were excluded. The index date was that of first diagnosis of distant recurrent breast cancer. Follow-up was defined as the interval between the index date and the confirmed date of death, the censored date (if lost to follow-up) or the study end in December 2018. According to current guidelines, all patients received standard adjuvant treatment and were followed up with through a regular physical examination 1–4 times a year and annual mammography; if necessary, blood exams, ultrasonography, computed tomography (CT), bone scintigraphy, magnetic resonance imaging, or position emission tomography/CT were added for the diagnosis of recurrence. Recurrence was defined as the occurrence of distant metastasis after removal of the primary breast cancer. Patients with only locoregional recurrence and distant metastasis at initial diagnosis (de novo Stage IV metastatic disease) were excluded from this analysis. Ipsilateral breast tumor recurrence and ipsilateral axillary, infraclavicular, internal mammary, and supraclavicular lymph node metastasis were defined as locoregional recurrence. TNM staging was based on the criteria of the 8th Union for International Cancer Control. The adjuvant and metastatic treatment strategies (treatment protocol after recurrence) were all decided at the experts’ conference in the institutions based on current guidelines. This study was approved by the institutional review board of the two hospitals, and all enrolled patients provided informed consent.
Immunohistochemical and serological assay
Positivity for estrogen receptor (ER) or progesterone receptor (PR) was defined as a score ≥ 3 using the Allread scoring system [25]. HR positivity was defined as ER and/or PR positivity. HER2 negativity was defined as an immunohistochemistry score of 0, 1+, or 2+ and negative fluorescence in situ hybridization (ratio < 2.0). The concentrations of serum carcinoembryonic antigen (CEA) and cancer antigen 15–3 (CA15–3) were measured at the first distant recurrence using an electrochemiluminescent immunoenzymometric assay (Roche Diagnostics, Tokyo, Japan). The upper limits of normal for CEA and CA15–3 were 5 ng/ml and 25 U/ml, respectively.
Metastatic distribution patterns of first metastatic sites
The first metastatic sites were classified into single metastases or multiple metastases. Single metastases were further classified into diffuse or non-diffuse lesions. Non-diffuse lesions were defined as localized or focal lesions in a single metastatic organ or site regardless of size, whereas diffuse lesions were defined as multiple lesions widely spreading in a single metastatic organ or site. Non-diffuse lesions included a solitary metastatic lesion in one single organ (e.g., a solitary lung metastasis), the involvement of a single lymphatic site (e.g., an ipsilateral hilar lymph node metastasis), or a solitary or isolated metastatic bone lesion. On the other hand, diffuse lesions included multiple lesions in one single organ (e.g., multiple lung metastases), the involvement of two or more lymphatic sites (e.g., bilateral hilar lymph node metastases), multiple metastatic bone lesions, or pleural or peritoneal dissemination.
Survival outcomes
Overall survival (OS) was defined as the time from the date of the first distant recurrence to the time of death or last follow-up. The disease-free interval (DFI) was defined as the interval between the diagnosis of primary nonmetastatic breast cancer and the date of the first distant recurrence. Time to multiple metastases (TTM) was defined as the time from the date of the first distant recurrence at a single metastatic site to the date of the progression of disease at multiple metastatic sites.
Statistical analysis
OS plots were created using the Kaplan-Meier method, and the distributions of the survival curves were compared using log-rank tests. The Cox proportional hazard regression model was used to examine the prognostic evaluation between groups using several prognostic indicators, including patient and disease-related clinicopathological factors and metastatic organ sites and distribution patterns. A 95% confidence interval (CI) was calculated for all hazard ratios (HRs) in the Cox regression analysis. We evaluated the results of the univariate and multivariate Cox proportional hazards models with hazards ratios > 1.0 indicating an increased risk of death. All tests were two-tailed, and p-values < 0.05 were considered significant. Statistical analyses were performed using the statistical software package SPSS (v.17.0; Chicago, IL, USA).
Results
Patient characteristics
Our analysis included 271 patients with recurrent metastatic HR+/HER2- breast cancer during the study period. The median follow-up for our sample was 8.57 years (range, 1.05–19.67). The patient characteristics for the study cohort are summarized in Table 1. The median age at recurrence was 62 years (range, 29–92). The majority of the sample consisted of patients with single metastasis (n = 169, 62%), and bone was the most common metastatic organ site (n = 148, 55%) of all patients.
Table 1.
Patient characteristics
| Characteristics | All | Single metastasis | Multiple metastases | ||
|---|---|---|---|---|---|
| Number | 271 | 169 (62%) | 102 (38%) | ||
| Stage at diagnosis | |||||
| I | 37 (14%) | 24 (14%) | 13 (13%) | ||
| IIA + IIB | 84 + 89 (64%) | 58 + 53 (66%) | 62 (61%) | ||
| IIIA+IIIB+IIIC | 32 + 22 + 7 (22%) | 18 + 12 + 4 (20%) | 27 (26%) | ||
| Adjuvant chemotherapy | |||||
| Yes | 184 (68%) | 115 (68%) | 69 (68%) | ||
| No | 87 (32%) | 54 (32%) | 33 (32%) | ||
| Median age at recurrence (range, years) | 62 (29–92) | 62 (29–90) | 63 (33–92) | ||
| Median OS after recurrence (range, years) | 4.58 (0.02–13.53) | 5.86 (0.04–13.53) | 2.50 (0.02–12.21) | ||
| DFI (median, years) | 4.01 (0.35–16.85) | 3.98 (0.58–12.02) | 4.04 (0.35–16.85) | ||
| < 2 years | 56 (21%) | 35 (21%) | 21 (21%) | ||
| ≥2 years | 215 (79%) | 134 (79%) | 81 (79%) | ||
| CEA/CA15–3 serum level | |||||
| Normal | 106 (47%) | 81 (57%) | 25 (30%) | ||
| High | 120 (53%) | 62 (43%) | 58 (70%) | ||
| Initial therapy for reccurence | |||||
| Endocrine therapy | 173 (64%) | 119 (70%) | 54 (53%) | ||
| Chemotherapy | 83 (31%) | 42 (25%) | 41 (40%) | ||
| unknown | 15 (5%) | 8 (5%) | 7 (7%) | ||
| Metastatic organ site | All | Diffuse lesions | Non-diffuse lesions | ||
| All | 271 | 169 | 90 (53%) | 79 (47%) | 102 |
| Bone | 148 (55%) | 85 (50%) | 46 (54%) | 39 (46%) | 63 (62%) |
| Lymph node | 95 (35%) | 31 (18%) | 13 (42%) | 18 (58%) | 64 (63%) |
| Lung | 74 (27%) | 24 (14%) | 17 (71%) | 7 (29%) | 50 (49%) |
| Liver | 54 (20%) | 14 (8%) | 7 (50%) | 7 (50%) | 40 (39%) |
| Pleural | 41 (15%) | 12 (7%) | 6 (50%) | 6 (50%) | 29 (28%) |
| Brain | 8 (3%) | 1 (1%) | 1 (100%) | 0 (0%) | 7 (7%) |
| Others | 2 (1%) | 2 (1%) | 0 (0%) | 2 (100%) | 0 (0%) |
Survival outcomes
The median OS after recurrence estimated according to patient characteristics is given in Table 2. The median OS after recurrence for patients with single metastasis and multiple metastases was 5.86 and 2.50 years, respectively (p < 0.001; Fig. 1). Of the patients with a single metastasis, those with high serum levels of CEA/CA15–3 (p = 0.014; Fig. 2a), shorter DFI (< 2 years, p < 0.001; Fig. 2b), or diffuse lesions had significantly worse prognosis (p < 0.001; Fig. 2c), whereas any metastatic organ site with a single metastasis was not significantly associated with prognostic outcomes. Of the patients with multiple metastases, patients with shorter DFI, liver metastasis, or brain metastasis had significantly worse prognosis. However, multiple metastases not involving liver or brain metastasis had no significant relationship with prognostic outcomes.
Table 2.
Median overall survival (OS) after recurrence according to patient characteristics
| Characteristics | All | Single metastasis | Multiple metastases | |||
|---|---|---|---|---|---|---|
| Median OS (year) | p-value | Median OS (year) | p-value | Median OS (year) | p-value | |
| All patients | 4.58 | 5.86 | 2.50 | < 0.001 | ||
| Stage at diagnosis | 0.195 | 0.840 | 0.073 | |||
| I + II | 4.60 | 6.30 | 2.53 | |||
| III | 3.79 | 5.30 | 1.78 | |||
| Adjuvant chemotherapy | 0.446 | 0.149 | 0.613 | |||
| Yes | 4.44 | 5.46 | 2.50 | |||
| No | 5.55 | 7.56 | 2.35 | |||
| Age at recurrence | 0.972 | 0.259 | 0.596 | |||
| < 50y | 3.52 | 4.54 | 2.39 | |||
| ≥ 50y | 4.67 | 6.81 | 2.50 | |||
| DFI | < 0.001 | < 0.001 | < 0.001 | |||
| < 2 years | 2.17 | 3.03 | 1.35 | |||
| ≥ 2 years | 5.33 | 7.68 | 3.26 | |||
| CEA/CA15–3 serum level | < 0.001 | 0.014 | 0.390 | |||
| Normal | 8.20 | 8.20 | 3.54 | |||
| High | 3.26 | 4.83 | 2.49 | |||
| Initial therapy for reccurence | 0.077 | 0.348 | 0.524 | |||
| Endocrine therapy | 5.46 | 6.30 | 2.53 | |||
| Chemotherapy | 3.12 | 5.30 | 2.50 | |||
| Distribution pattern in single metastasis | < 0.001 | |||||
| Diffuse lesions | – | 4.60 | – | |||
| Non-diffuse lesions | – | 11.83 | – | |||
| Metastatic organ site | ||||||
| Bone | 4.51 (vs. 4.83) | 0.443 | 5.86 (vs. 6.30) | 0.869 | 1.96 (vs. 3.26) | 0.327 |
| Lymph node | 3.54 (vs. 5.46) | 0.022 | 4.54 (vs. 7.56) | 0.088 | 2.57 (vs. 2.20) | 0.279 |
| Lung | 5.72 (vs. 4.54) | 0.688 | 8.20 (vs. 5.46) | 0.062 | 2.50 (vs. 2.44) | 0.287 |
| Liver | 1.96 (vs. 5.30) | < 0.001 | 9.13 (vs. 5.72) | 0.670 | 1.88 (vs. 3.87) | < 0.001 |
| Pleura | 3.54 (vs. 4.73) | 0.204 | 2.90 (vs. 6.30) | 0.153 | 3.54 (vs. 2.39) | 0.254 |
| Brain | 0.800 (vs. 4.67) | < 0.001 | – | – | 0.800 (vs. 2.50) | 0.027 |
Fig. 1.
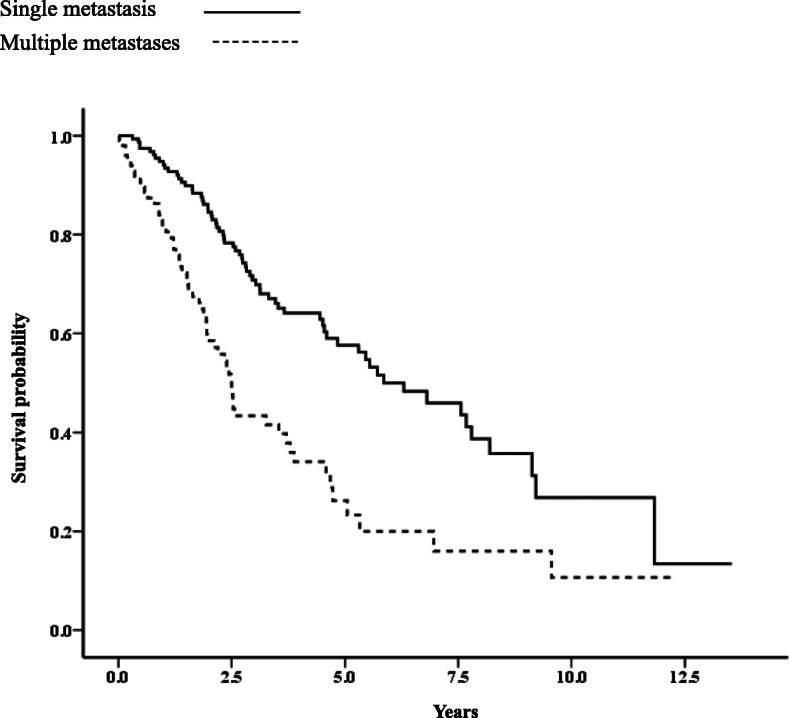
Kaplan-Meier curve for survival after recurrence between single and multiple metastases (p < 0.001)
Fig. 2.
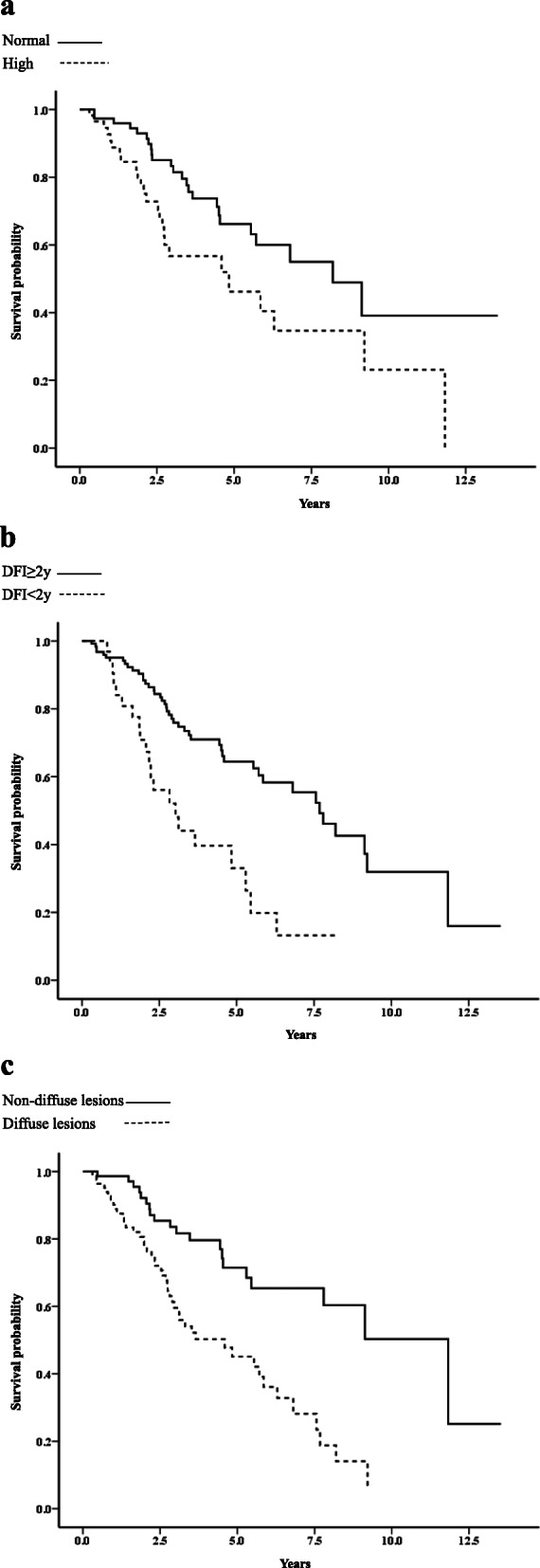
Kaplan-Meier curves for survival after recurrence according to a serum CEA/CA15–3 levels in single metastasis (p = 0.014), b DFI < 2 years and DFI ≥ 2 years in single metastasis (p < 0.001), and c diffuse lesions and non-diffuse lesions in single metastasis (p < 0.001)
Univariate and multivariate analysis of factors related to survival after recurrence
Multivariate analysis was performed according to the prognostic factors that were significant in the univariate analysis in patients with single or multiple metastases. The multivariate analysis revealed two significantly independent prognostic factors related to poor survival after recurrence in patients with single metastasis: shorter DFI and diffuse lesions (Table 3). The multivariate analysis revealed three significantly independent prognostic factors related to poor survival after recurrence in patients with multiple metastases: shorter DFI, liver metastasis, and brain metastasis (Table 4).
Table 3.
Univariate and multivariate analyses in patients with single metastatasis
| Characteristics | Single metastasis | |||
|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||
| HRs (95%CI) | p-value | HRs (95%CI) | p-value | |
| Stage at diagnosis | ||||
| I + II | 1.00 | 0.840 | ||
| III | 1.068 (0.564–2.024) | |||
| Adjuvant chemotherapy | ||||
| Yes | 1.00 | 0.152 | ||
| No | 0.661 (0.375–1.165) | |||
| Age at recurrence | ||||
| < 50y | 1.00 | |||
| ≥ 50y | 0.726 (0.415–1.270) | 0.261 | ||
| CEA/CA15–3 serum level | ||||
| Normal | 1.00 | |||
| High | 2.001 (1.138–3.517) | 0.016 | 1.543 (0.866–2.750) | 0.141 |
| DFI | ||||
| < 2 years | 2.808 (1.643–4.797) | < 0.001 | 3.527 (1.891–6.576) | < 0.001 |
| ≥ 2 years | 1.00 | |||
| Initial therapy for reccurence | ||||
| Endocrine therapy | 1.00 | 0.349 | ||
| Chemotherapy | 1.285 (0.761–2.170) | |||
| Distribution pattern | ||||
| Diffuse lesions | 2.922 (1.699–5.025) | < 0.001 | 3.641 (1.856–7.141) | < 0.001 |
| Non-diffuse lesions | 1.00 | |||
| Metastatic organ site | ||||
| Bone | 0.960 (0.589–1.565) | 0.869 | ||
| Lymph node | 1.678 (0.919–3.067) | 0.092 | ||
| Lung | 0.497 (0.235–1.050) | 0.067 | ||
| Liver | 1.221 (0.488–3.055) | 0.670 | ||
| Pleura | 1.927 (0.770–4.831) | 0.161 | ||
| Brain | – | – | ||
Table 4.
Univariate and multivariate analyses in patients with multiple metastases
| Characteristics | Multiple metastases | |||
|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||
| HRs (95%CI) | p-value | HRs (95%CI) | p-value | |
| Stage at diagnosis | ||||
| I + II | 1.00 | 0.076 | ||
| III | 1.695 (0.946–3.030) | |||
| Adjuvant chemotherapy | ||||
| Yes | 1.00 | 0.613 | ||
| No | 1.151 (0.667–1.989) | |||
| Age at recurrence | ||||
| < 50y | 1.00 | |||
| ≥ 50y | 0.807 (0.366–1.782) | 0.596 | ||
| CEA/CA15–3 serum level | ||||
| Normal | 1.00 | |||
| High | 1.337 (0.688–2.595) | 0.391 | ||
| DFI | ||||
| < 2 years | 3.229 (1.768–5.895) | < 0.001 | 3.082 (1.669–5.694) | < 0.001 |
| ≥ 2 years | 1.00 | |||
| Initial therapy for reccurence | ||||
| Endocrine therapy | 1.00 | 0.524 | ||
| Chemotherapy | 1.187 (0.700–2.013) | |||
| Metastatic organ site | ||||
| Bone | 0.769 (0.455–1.301) | 0.328 | ||
| Lymph node | 1.334 (0.790–2.255) | 0.281 | ||
| Lung | 0.757 (0.452–1.266) | 0.289 | ||
| Liver | 2.915 (1.689–5.025) | < 0.001 | 3.145 (1.802–5.495) | < 0.001 |
| Pleura | 1.406 (0.781–2.530) | 0.256 | ||
| Brain | 2.532 (1.078–5.952) | 0.033 | 3.289 (1.355–7.937) | 0.008 |
TTM for diffuse lesions and non-diffuse lesions
Survival plots showed that TTM was significantly shorter for patients with diffuse lesions among those with single metastasis than for those with non-diffuse lesions (median TTM: 24.2 months vs. 52.0 months, p = 0.002; Fig. 3).
Fig. 3.
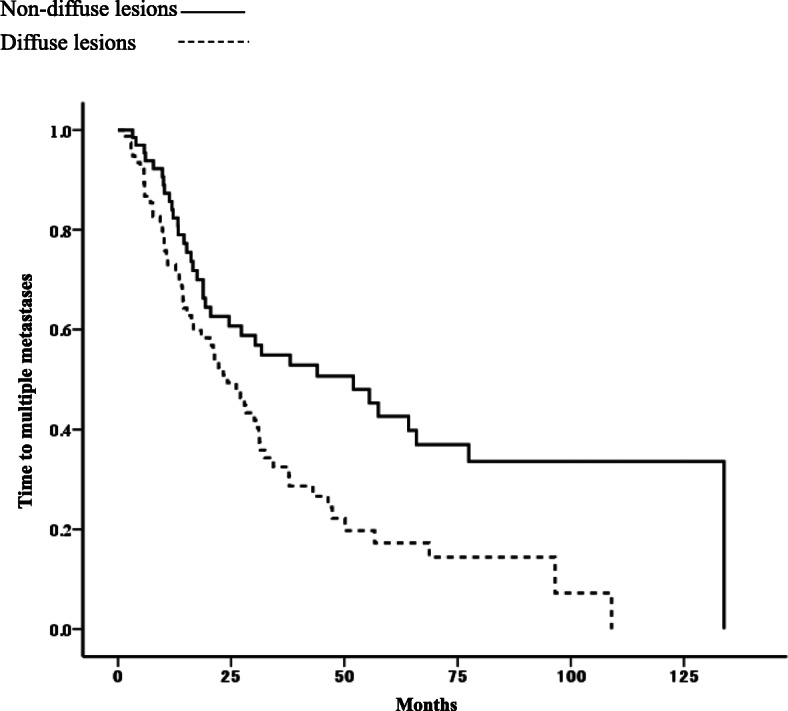
Time to multiple metastases in patients with diffuse lesions and non-diffuse lesions in single metastasis (p = 0.002)
Discussion
Metastatic breast cancer represents a biologically heterogeneous population with diverse metastatic patterns exhibiting highly unpredictable clinical behaviors [26–28]. Prognosis varies significantly among patient subtypes [29, 30], and several concepts regarding the prognosis of HR+/HER2- metastatic patients have been considered important in evaluating survival after recurrence [11]. Previous studies have shown that first metastatic sites (single, multiple, liver, or other visceral metastases) are significantly related to survival after recurrence in HR+/HER2- patients [10–13]; however, there has been clinical diversity in the metastatic distribution patterns of both single metastasis and multiple metastases [26–28]. Therefore, we studied the prognosis of HR+/HER2- patients with single and multiple metastases separately, with emphasis on the metastatic distribution patterns.
Multiple metastases are considered a poor prognostic factor [10–18]. The prognosis of patients with multiple metastases may vary with the metastatic organ sites due to the heterogeneity [26–28], but there has been no report investigating prognosis by each relevant metastatic organ site involved in multiple metastases. In the current study, multiple metastases including the liver or brain were a strong independent prognostic factor for worse outcomes in HR+/HER2- patients, whereas multiple metastases not involving the liver or brain had no significant relationship with prognosis after recurrence. This finding supports the assumption that multiple metastases involving liver or brain metastasis are indicative of extensive spreading, dissemination of cancer cells, or lethal organ dysfunction, leading to poor survival outcomes [11, 15, 24]. In real-world practice, patients with multiple metastases are more likely to receive cytotoxic therapy because their vital organs are potentially damaged or in “visceral crisis” [19–21]. However, due to their own heterogeneity, not all multiple metastases may lead to poor outcomes [19]; therefore, we should determine the most reliable and decisive prognostic factors for HR+/HER2- patients with multiple metastases.
Single metastasis has been regarded as a better prognostic factor than multiple metastases due to the probability of less tumor burden [10–18]. However, most patients with a single metastasis will eventually develop multiple metastases, eventually leading to poor outcomes. In this study, we analyzed the prognosis of patients with single metastases by classifying single metastases into diffuse and non-diffuse lesions. To the best of our knowledge, this is the first study to investigate the prognosis of patients with single metastases based on the metastatic distribution patterns between diffuse and non-diffuse lesions, and to evaluate the time to dissemination of single metastases to multiple metastases. We found that diffuse lesions in a single metastasis were independently related to worse prognosis and easier systemic dissemination into multiple metastases. Diffuse lesions in single metastases may likely behave as multiple metastases due to the dissemination potential. According to traditional guidelines [7–9], noncytotoxic therapy is indicated as a first-line treatment for HR+/HER2- patients with a single metastasis. Thus, our study suggests reconsidering the therapeutic guidelines, and additional treatment strategies should be sought for patients with diffuse lesions in single metastases. Our proposal may meet the unmet need for more efficacious treatments for HR+/HER2- patients with diffuse lesions in a single metastasis. The initial use of more advantageous treatments, including novel targeted agents [31], could provide more beneficial effects and better prognoses for these patients.
Our study had some limitations. First, our study was performed as a retrospective chart review without validation, and sampling biases may have been unavoidable. Second, the sample size of our study was small, and our results should be interpreted with caution. However, the selection of patients with HR+/HER2- recurrent breast cancer and exclusion of HER2+, triple-negative, and de novo breast cancer patients may have allowed the recruitment of a patient population with relative homogeneity. Future studies with a larger cohort of patients may yield more conclusive evidence of the prognostic findings in this study. Despite these limitations, the current study with a uniform population provides important insights into the real-world clinical outcomes for patients with recurrent metastatic HR+/HER2- breast cancer.
Conclusion
Our study presents new evidence of metastatic patterns and real-world clinical outcomes for HR+/HER2- recurrent metastatic breast cancer. We confirmed that multiple metastases not involving liver or brain metastasis had no significant relationship with prognosis after recurrence. We also confirmed that single metastasis with diffuse lesions was an independent factor for worse prognosis, with easier systemic dissemination and progression to multiple metastases than non-diffuse lesions. These findings may require reconsideration of the determinants of initial therapy for HR+/HER2- recurrent metastatic breast cancer and provide frontline physicians with new important clinical clues to achieve optimal treatment, leading to effective therapeutic strategies to improve the prognosis of this metastatic disease.
Acknowledgements
Not applicable.
Abbreviations
- HR
Hormone receptor-positive (HR+)
- HER2-
Human epidermal growth factor receptor 2-negative
- CT
Computed tomography
- ER
Estrogen receptor
- PR
Progesterone receptor
- CEA
Carcinoembryonic antigen
- CA15–3
Cancer antigen 15–3
- OS
Overall survival
- DFI
Disease-free interval
- TTM
Time to multiple metastases
- CI
Confidence interval
- HRs
Hazard ratios
Authors’ contributions
JY and YK contributed to the concept and design of this study. JY, SK, HM, YT, WS, YH and YK provided the study materials of the patients. JY analyzed the data, performed the statistical analyses, and wrote the manuscript. All authors (JY, SK, JF, HO, HM, YT, WS, YH and YK) have read and approved the manuscript.
Authors’ information
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and analyzed during this study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the institutional review board of the two hospitals, and informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.National Cancer Institute Surveillance, Epidemiology, and End Results Program . Cancer statistics. Cancer Stat Facts: Female Breast Cancer. National Cancer Institute; 2019. [Google Scholar]
- 2.Hortobagyi GN, de la Garza SJ, Pritchard K, Amadori D, Haidinger R, Hudis CA, et al. The global breast cancer burden: variations in epidemiology and survival. Clin Breast Cancer. 2005;6(5):391–401. doi: 10.3816/CBC.2005.n.043. [DOI] [PubMed] [Google Scholar]
- 3.Giuliani J, Bonetti A. Trends in survival for patients with metastatic breast cancer: is survival improving. Tumori. 2015;101(4):347–352. doi: 10.5301/tj.5000301. [DOI] [PubMed] [Google Scholar]
- 4.Chung CT, Carlson RW. Goals and objectives in the management of metastatic breast cancer. Oncologist. 2003;8(6):514–520. doi: 10.1634/theoncologist.8-6-514. [DOI] [PubMed] [Google Scholar]
- 5.Harb WA. Management of patients with hormone receptor-positive breast cancer with visceral disease: challenges and treatment options. Cancer Manag Res. 2015;7:37–46. doi: 10.2147/CMAR.S72592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lobbezoo DJ, van Kampen RJ, Voogd AC, Dercksen MW, van den Berkmortel F, Smilde TJ, et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat. 2013;141(3):507–514. doi: 10.1007/s10549-013-2711-y. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network . NCCN clinical practice guidelines in oncology (NCCN guidelines®) with NCCN evidence blocks™. 2019. [Google Scholar]
- 8.Schnipper L, Davidson N, Wollins D, Blayney DW, Dicker AP, Ganz PA, et al. Updating the American Society of Clinical Oncology value framework: revisions and reflections in response to comments received. J Clin Oncol. 2016;34(24):2925–2934. doi: 10.1200/JCO.2016.68.2518. [DOI] [PubMed] [Google Scholar]
- 9.Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, Harbeck N, Aguilar Lopez B, Barrios CH, Bergh J, Biganzoli L, Boers-Doets CB, Cardoso MJ, Carey LA, Cortés J, Curigliano G, Diéras V, el Saghir NS, Eniu A, Fallowfield L, Francis PA, Gelmon K, Johnston SRD, Kaufman B, Koppikar S, Krop IE, Mayer M, Nakigudde G, Offersen BV, Ohno S, Pagani O, Paluch-Shimon S, Penault-Llorca F, Prat A, Rugo HS, Sledge GW, Spence D, Thomssen C, Vorobiof DA, Xu B, Norton L, Winer EP. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4) Ann Oncol. 2018;29(8):1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Saux O, Lardy-Cleaud A, Frank S, Debled M, Cottu PH, Pistilli B, et al. Assessment of the efficacy of successive endocrine therapies in hormone receptor-positive and HER2-negative metastatic breast cancer: a real-life multicentre national study. Eur J Cancer. 2019;118:131–141. doi: 10.1016/j.ejca.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Yamamura J, Kamigaki S, Tsujie M, Fujita J, Osato H, Higashi C, et al. Response to first-line recurrence treatment influences survival in hormone receptor-positive, HER2-negative breast cancer: a multicenter study. In Vivo. 2019;33(1):281–287. doi: 10.21873/invivo.11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonotto M, Gerratana L, Di Maio M, De Angelis C, Cinausero M, Moroso S, et al. Chemotherapy versus endocrine therapy as first-line treatment in patients with luminal-like HER2-negative metastatic breast cancer: a propensity score analysis. Breast. 2017;31:114–120. doi: 10.1016/j.breast.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Xie J, Hao Y, Li N, Lin PL, Ohashi E, Koo V, Wu EQ. Clinical outcomes among HR+/HER2- metastatic breast cancer patients with multiple metastatic sites: a chart review study in the US. Exp Hematol Oncol. 2015;4(1):31. doi: 10.1186/s40164-015-0023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogoz B, Houzé de l’Aulnoit A, Duhamel A, Houzé de l’Aulnoit D. Thirty-year trends of survival and time-varying effects of prognostic factors in patients with metastatic breast cancer-a single institution experience. Clin Breast Cancer. 2018;18(3):246–253. doi: 10.1016/j.clbc.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Tsuji W, Teramukai S, Ueno M, Toi M, Inamoto T. Prognostic factors for survival after first recurrence in breast cancer: a retrospective analysis of 252 recurrent cases at a single institution. Breast Cancer. 2014;21(1):86–95. doi: 10.1007/s12282-012-0358-x. [DOI] [PubMed] [Google Scholar]
- 16.Jung SY, Rosenzweig M, Sereika SM, Linkov F, Brufsky A, Weissfeld JL. Factors associated with mortality after breast cancer metastasis. Cancer Causes Control. 2012;23(1):103–112. doi: 10.1007/s10552-011-9859-8. [DOI] [PubMed] [Google Scholar]
- 17.Largillier R, Ferrero JM, Doyen J, Barriere J, Namer M, Mari V, Courdi A, Hannoun-Levi JM, Ettore F, Birtwisle-Peyrottes I, Balu-Maestro C, Marcy PY, Raoust I, Lallement M, Chamorey E. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008;19(12):2012–2019. doi: 10.1093/annonc/mdn424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andre F, Slimane K, Bachelot T, Dunant A, Namer M, Barrelier A, Kabbaj O, Spano JP, Marsiglia H, Rouzier R, Delaloge S, Spielmann M. Breast cancer with synchronous metastases: trends in survival during a 14-year period. J Clin Oncol. 2004;22(16):3302–3308. doi: 10.1200/JCO.2004.08.095. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe J, Hayashi T, Tadokoro Y, Nishimura S, Takahashi K. Clinical pattern of primary systemic therapy and outcomes of estrogen receptor-positive, HER2-negative metastatic breast cancer: a review of a single institution. Breast Cancer Res Treat. 2017;166(3):911–917. doi: 10.1007/s10549-017-4478-z. [DOI] [PubMed] [Google Scholar]
- 20.Llombart-Cussac A, Pivot X, Biganzoli L, Cortes-Funes H, Pritchard KI, Pierga JY, Smith I, Thomssen C, Srock S, Sampayo M, Cortes J. A prognostic factor index for overall survival in patients receiving first-line chemotherapy for HER2-negative advanced breast cancer: an analysis of the ATHENA trial. Breast. 2014;23(5):656–662. doi: 10.1016/j.breast.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Cazzaniga M, Pronzato P, Leto di Priolo SL, De Matteis A, Di Costanzo F, Passalacqua R, et al. Patterns of relapse and modalities of treatment of breast cancer: the ‘IRIS’ project, a multicenter observational study. Oncology. 2004;66(4):260–268. doi: 10.1159/000078325. [DOI] [PubMed] [Google Scholar]
- 22.Kwast AB, Voogd AC, Menke-Pluijmers MB, Linn SC, Sonke GS, Kiemeney LA, et al. Prognostic factors for survival in metastatic breast cancer by hormone receptor status. Breast Cancer Res Treat. 2014;145(2):503–511. doi: 10.1007/s10549-014-2964-0. [DOI] [PubMed] [Google Scholar]
- 23.Giordano SH, Buzdar AU, Smith TL, Kau SW, Yang Y, Hortobagyi GN. Is breast cancer survival improving? Cancer. 2004;100(1):44–52. doi: 10.1002/cncr.11859. [DOI] [PubMed] [Google Scholar]
- 24.Wyld L, Gutteridge E, Pinder SE, James JJ, Chan SY, Cheung KL, Robertson JFR, Evans AJ. Prognostic factors for patients with hepatic metastases from breast cancer. Br J Cancer. 2003;89(2):284–290. doi: 10.1038/sj.bjc.6601038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand—binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17(5):1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 26.Regierer AC, Wolters R, Ufen MP, Weigel A, Novopashenny I, Köhne CH, Samonigg H, Eucker J, Possinger K, Wischnewsky MB. An internally and externally validated prognostic score for metastatic breast cancer: analysis of 2269 patients. Ann Oncol. 2014;25(3):633–638. doi: 10.1093/annonc/mdt539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newton PK, Mason J, Venkatappa N, Jochelson MS, Hurt B, Nieva J, Comen E, Norton L, Kuhn P. Spatiotemporal progression of metastatic breast cancer: a Markov chain model highlighting the role of early metastatic sites. NPJ Breast Cancer. 2015;1(1):15018. doi: 10.1038/npjbcancer.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onken JS, Fekonja LS, Wehowsky R, Hubertus V, Vajkoczy P. Metastatic dissemination patterns of different primary tumors to the spine and other bones. Clin Exp Metastasis. 2019. 10.1007/s10585-019-09987-w. [DOI] [PubMed]
- 29.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 30.Kast K, Link T, Friedrich K, Petzold A, Niedostatek A, Schoffer O, Werner C, Klug SJ, Werner A, Gatzweiler A, Richter B, Baretton G, Wimberger P. Impact of breast cancer subtypes and patterns of metastasis on outcome. Breast Cancer Res Treat. 2015;150(3):621–629. doi: 10.1007/s10549-015-3341-3. [DOI] [PubMed] [Google Scholar]
- 31.Turner NC, Finn RS, Martin M, Im SA, DeMichele A, Ettl J, Diéras V, Moulder S, Lipatov O, Colleoni M, Cristofanilli M, Lu DR, Mori A, Giorgetti C, Iyer S, Bartlett CH, Gelmon KA. Clinical considerations of the role of palbociclib in the management of advanced breast cancer patients with and without visceral metastases. Ann Oncol. 2018;29(3):669–680. doi: 10.1093/annonc/mdx797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during this study are available from the corresponding author on reasonable request.


