Abstract
Pavlov’s pioneering work established that sham-feeding induced by sight or smell of food or feeding in dogs with permanent esophagostomy stimulates gastric acid secretion through vagal pathways. Brain circuitries and transmitters involved in the central vagal regulation of gastric function have recently been unraveled. Neurons in the dorsal vagal complex including the dorsal motor nucleus of the vagus (DMN) express thyrotropin-releasing hormone (TRH) receptor and are innervated by TRH fibers originating from TRH synthesizing neurons in the raphe pallidus, raphe obscurus and the parapyramidal regions. TRH injected into the DMN or cisterna magna increases the firing of DMN neurons and gastric vagal efferent discharge, activates cholinergic neurons in gastric submucosal and myenteric plexuses and induces a vagal-dependent, atropine-sensitive stimulation of gastric secretory (acid, pepsin) and motor functions. TRH antibody or TRH-R1 receptor oligodeoxynucleotide antisense pretreatment in the cisterna magna or DMN abolished vagal-dependent gastric secretory and motor responses to sham-feeding, 2-deoxy-D-glucose, cold exposure and chemical activation of cell bodies in medullary raphe nuclei. TRH excitatory action in the DMN is potentiated by co-released prepro-TRH-(160–169) flanking peptide, Ps4 and 5-HT, and inhibited by a number of peptides involved in the stress/immune response and inhibition of food-intake. These neuroanatomical, electrophysiological and neuropharmacological data are consistent with a physiological role of brainstem TRH in the central vagal stimulation of gastric myenteric cholinergic neurons in response to several vagal dependent stimuli including sham-feeding.
Keywords: TRH, Vagus, pChAT, Myenteric neurons, Gastric acid secretion, Dorsal motor nucleus
1. Introduction
At the beginning of last century, Ivan Pavlov’s pioneer discovery of the “psychic phase” of gastric acid secretion provided one of the first experimental evidence of brain–gut axis. He established a model of sham-feeding in dogs with permanent esophagostomy, and demonstrated that the anticipation of eating and the sight and smell of food were powerful stimulants of both gastric acid and pepsin secretion, and the gastric response required the vagal innervation (Pavlov, 1910). In humans, Cushing reported in 1932 that patients with intracranial lesions had gastric hypersecretion and developed ulcers (Cushing, 1932). Thereafter, a number of experiments using electrical stimulations and lesions of various brain areas defined specific nuclei responsible for influencing gastric acid secretion through vagal pathways (reviewed in Taché, 1987). Despite this auspicious beginning establishing that gastric acid secretion is stimulated by central vagal pathways, it has only been in the past few decades that brain circuitries and chemical messengers involved in vagal regulation of gut function have been unraveled (Taché, 1987; Hornby et al., 1991).
The present review will focus on convergent sets of evidence illustrating that brain medullary thyrotropin-releasing hormone (TRH) is involved in the neuronal cascade mediating the vagal cholinergic-dependent stimulation of gastric function in response to various stimuli including sham-feeding.
2. Brain medullary TRH is a physiological vagal stimulant of gastric secretion: neuroanatomical and electrophysiological evidence
TRH was originally isolated from mammalian hypothalami and named after its property to stimulate the release of thyroid-stimulating hormone (TSH) from the pituitary (review in Guillemin, 2005). However, the widespread distribution of TRH in the brain (Hokfelt et al., 1975; Yarbrough, 1979; Jackson and Lechan, 1983; Horita et al., 1986; Lechan and Segerson, 1989) and its neuropharmacological effects (Taché et al., 1977;Yarbrough, 1979) suggested that TRH may exert biological functions that expand far beyond its pivotal hypophysiotrophic role (Metcalf and Dettmar, 1981). In particular, we initially showed that TRH injected intracisternally (i.c.) acts in the brain to induce a vagally mediated and atropine-sensitive stimulation of gastric acid secretion (Taché et al., 1980). This report provided the first evidence that a peptide increased gastric acid secretion through central vagal pathways (Taché et al., 1980). Further studies uncovered neuroanatomical, electrophysiological and neuropharmacological evidence implicating the brain medullary TRH pathway in the central vagal regulation of gastric secretory and motor function to various stimuli (Taché et al., 1993).
2.1. TRH and TRH receptor localization in the brainstem
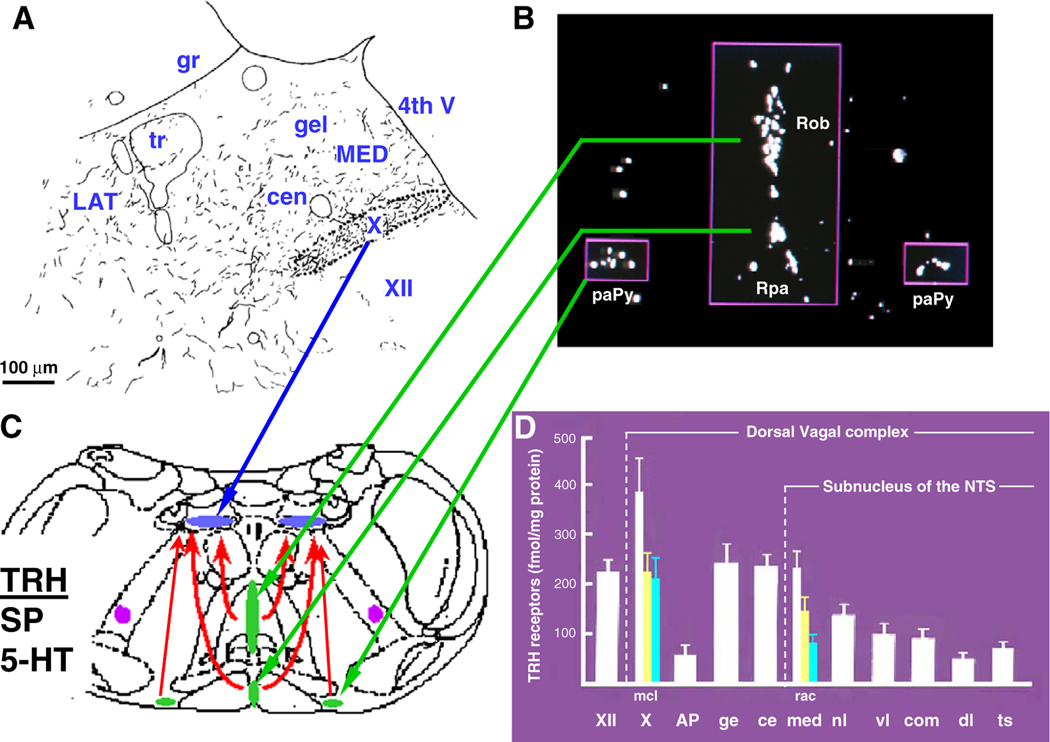
The dorsal vagal complex (DVC) encompasses the dorsal motor nucleus of the vagus (DMN) and the nucleus tractus solitarius (NTS). Tracing studies by Powley et al. (1991) established that the DMN is the source of vagal efferent fibers innervating the gut, and more prominently, the stomach. In particular, the medial parts of the right and left DMN contain neurons that project ipsilaterally to form the anterior and posterior branches of the gastric vagus, respectively (Fox and Powley, 1985). There is also evidence that dendrites of DMN neurons reached the overlying NTS more densely in the subnucleus gelatinosus of the dorsal medial NTS just rostral to the obex where gastric vagal afferent fibers project (Rinaman and Miselis, 1990). TRH-immunoreactive (IR) fibers are densely present in the DVC (Fig. 1A) and TRH-containing nerve terminals make synaptic contacts with dendrites of DMN neurons projecting to the stomach in rodents (Rinaman et al., 1989; Rinaman and Miselis, 1990). In humans, TRH-IR fibers innervating the DMN also constitute the most prominent network compared with that of twelve other neuropeptides (Fodor et al., 1994). TRH-IR fibers in the DVC do not originate from TRH-expressing neurons in the paraventricular nucleus of the hypothalamus but from direct projections from TRH synthetizing neurons located exclusively in brainstem nuclei, namely the raphe pallidus, raphe obscurus and parapyramidal regions, as delineated by knife cut and tracing methods (Palkovits et al., 1986; Segerson et al., 1987; Lynn et al., 1991; Bayliss et al., 1994) (Fig. 1B, C).
Fig. 1.
Neuroanatomical evidence for a physiological role of brain medullary TRH. (A) Drawing of TRH-IR fibers in rats dorsal vagal complex (DVC) rostrocaudal to the obex. Dense distribution in the dorsal motor nucleus of the vagus (DMN or X); cen: subnucleus centralis of the medial (MED) nucleus tractus solitarius (NTS); gel: subnucleus gelatinosus; adapted from Rinaman et al. (1989). (B) Dark field microscopy of cells in the raphe obscurus (Rob), raphe pallidus (Rpa) and parapyramidal area (PaPy) hydrized with the labeled antisense pro-TRH probe; adapted from Segerson et al. (1987). (C) Schematic representation of Rob, Rpa and PaPy direct projections to DMN (X) that provide TRH-IR, substance P (SP) and 5-HT innervation in the DVC. (D) Concentration of TRH receptors in the DVC; the DMN (X) is subdivided in medial (m), central (c) and lateral (l) and NTS in ge: gelatinosus, ce: central; med (medial: r, rostra; a, adjacent; and c, caudal to the area postrema); adapted from Manaker and Rizio (1989).
Consistent with a physiological role of TRH terminals in the DVC to regulate gastric function, the highest density of TRH binding sites detected by autoradiography is found in the medial column of the DMN (Manaker and Rizio, 1989) (Fig. 1D) where the majority of preganglionic motor neurons contribute to the vagal efferent innervation of the stomach (Powley et al., 1991). So far the two cloned TRH receptor subtypes, TRH-R1 and TRH-R2, are membrane proteins belonging to the family of G-protein coupled receptors (Gershengorn and Osman, 1996; Cao et al., 1998). Mapping of TRH-R1 and TRH-R2 gene expression in the rat brainstem revealed that TRH-R1 is the only subtype present in the DMN and NTS, while TRH-R2 is mainly located in the reticular formation, dorsal tegmental nucleus and spinal trigeminal nucleus which are areas processing sensory information (Heuer et al., 2000).
2.2. Activation of preganglionic vagal motor neurons and vagal efferent fibers
The expression of TRH receptors on preganglionic vagal motor neurons is indicative of a TRH modulatory action on parasympathetic outflow. Several reports showed that TRH induces an immediate and long acting excitation of individual DMN units identified electrophysiologically in urethane-anesthetized rats and in guinea pig or rat brainstem slice preparations (McCann et al., 1989; Raggenbass et al., 1990; Travagli et al., 1992; Livingston and Berger, 1993). The responsiveness to TRH observed in DMN neurons located rostral or caudal to the obex is no longer observed or decreased after repeated injections indicating that there is a sensitization or tachyphylaxis to TRH (Travagli et al., 1992; Livingston and Berger, 1993). TRH acts directly on DMN neurons since the excitatory response was still observed after synaptic blockade and was not affected by glutamate or muscarinic antagonists ruling out an action through presynaptic release of glutamate and acetylcholine (McCann et al., 1989; Raggenbass et al., 1990; Travagli et al., 1992; Livingston and Berger, 1993). The mechanisms underlying the excitatory effect involve an increase in excitatory postsynaptic currents and a reduction of fast transient A-type potassium current and calcium-dependent slow after-hyperpolarization (Travagli et al., 1992).
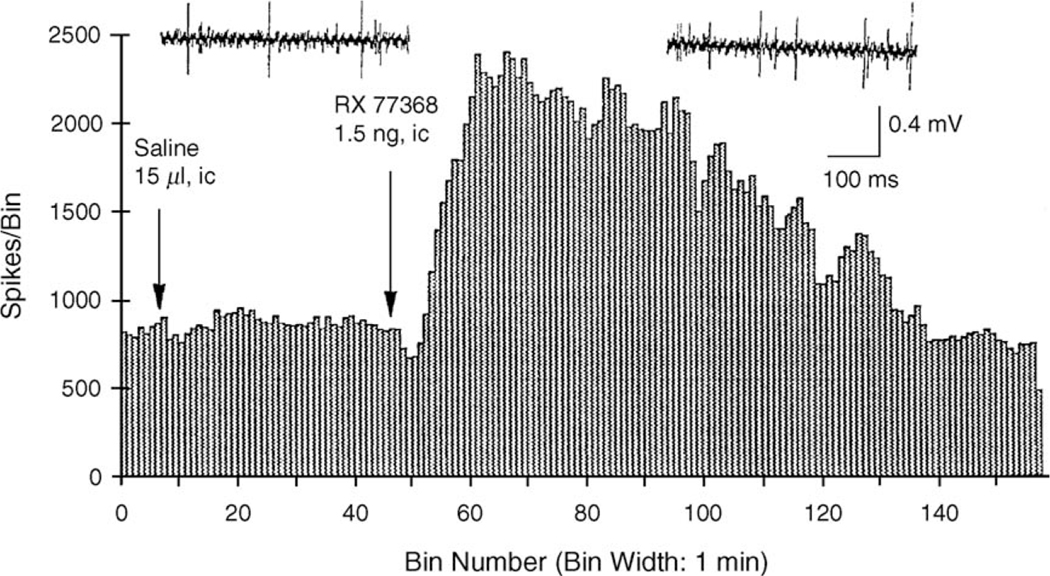
In line with the activation of preganglionic vagal motor neurons, i.c. or intracerebroventricular (i.c.v.) injection of TRH or the stable TRH analog, RX 77368 results in a doserelated sustained stimulation of efferent discharges recorded in the cervical or ventral gastric branch of the vagus in anesthetized rats (Somiya and Tonoue, 1984; Taché et al., 1985; O-Lee et al., 1997) (Fig. 2).
Fig. 2.
TRH analog, RX 77368 injected intracisternally (i.c.) stimulates gastric vagal efferent discharges (multi-unit activity) while i.c. saline had no effect in urethane anesthetized rats. Adapted from O-Lee et al. (1997).
2.3. Activation of gastric myenteric cholinergic neurons
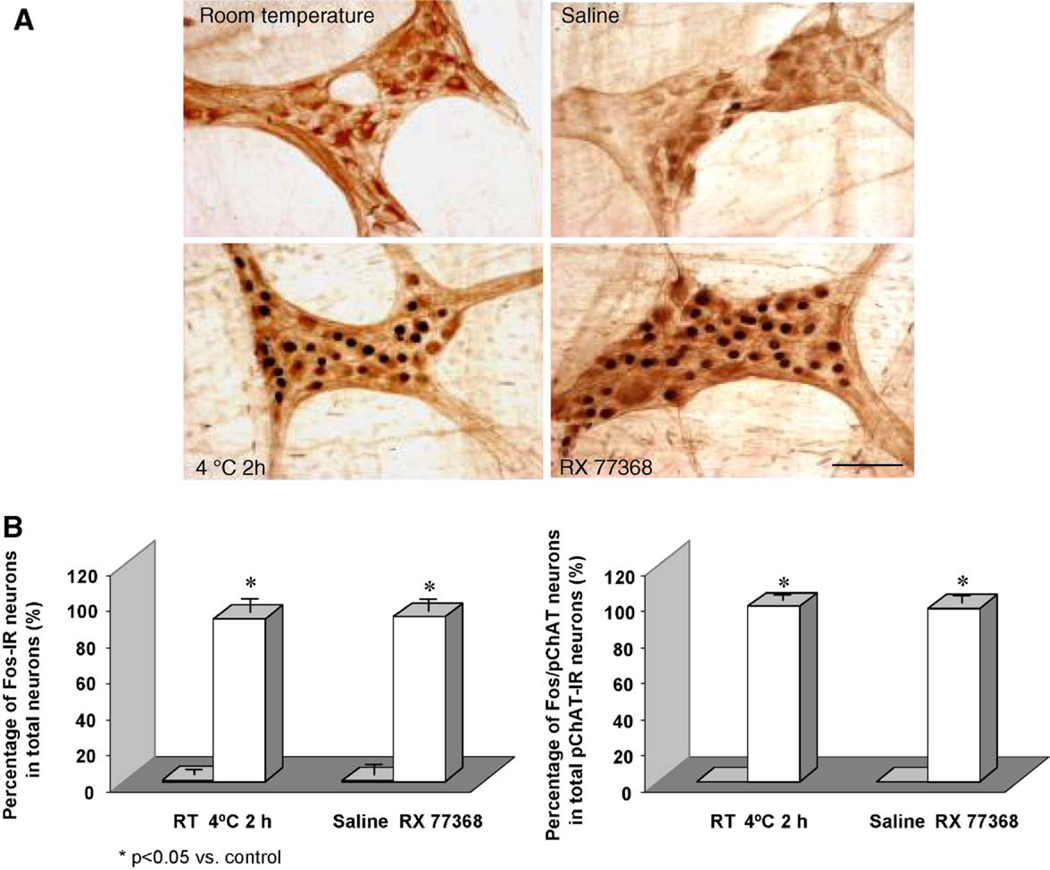
Earlier electrophysiological studies by Schemann and Grundy (1992) revealed that a high percentage of myenteric neurons in guinea pig stomach receives direct fast excitatory postsynaptic potential input from vagal efferent fibers. Anatomical support for these observations came from elegant tracing studies depicting direct input of vagal efferent fibers on gastric myenteric neurons (Berthoud, 1995; Holst et al., 1997). In the stomach, vagal efferent terminals were found to encircle or make putative contacts with all gastric myenteric and submucosal neurons (Berthoud, 1995; Holst et al., 1997). The i.c. injection of TRH provided a relevant tool to assess the activation of gastric myenteric neurons by central vagal activation in conscious rats using double labeling with Fos as a nuclear marker of neuronal synaptic activation (Krukoff, 1993) and PGP 9.5 as a neuronal marker (Krammer et al., 1993). We showed that the TRH analog, RX 77368 injected i.c., at a dose that activates gastric vagal efferent discharge (O-Lee et al., 1997), induced Fos expression in the majority (90%) of neuronal cell bodies located in the corpus and antral submucosal and myenteric ganglia (Miampamba et al., 2001; Yuan et al., 2005) (Fig. 3). Fos expression was observed in neurons densely surrounded with cholinergic fibers identified by the vesicular acetylcholine transporter (Miampamba et al., 2001). The Fos response to i.c. RX 77368 was 90% abolished by hexamethonium, but not altered by atropine (Miampamba et al., 2001). Using selective vagal denervation in the rat stomach, Fos expression in response to electrical vagal stimulation occurs in gastric myenteric neurons except in the vagally denervated area, arguing against a role of interneuronal spreading of the activation, but rather a direct cholinergic input (Zheng and Berthoud, 2000). It is well established electrophysiologically that acetylcholine acting at nicotinic acetylcholine receptors mediates most of the fast excitatory postsynaptic potentials in the enteric nervous system (Galligan, 2002). Taken together these data support that central vagal cholinergic activation-induced widespread Fos expression in gastric myenteric neurons occurs through acetylcholine acting at nicotinic acetylcholine receptors located on myenteric neurons (Kirchgessner and Liu, 1998; Galligan, 2002) although their distribution in rats myenteric neurons is still to be established.
Fig. 3.
Cold exposure or intracisternal (i.c.) injection of TRH analog activates gastric myenteric cholinergic neurons in conscious rats. (A) Photomicrographs of whole mount preparations of myenteric plexus showing double staining of Fos and pChAT in the gastric corpus of conscious rats semi-restrained either at room temperature or at 4 °C for 2 h or injected i.c. with saline or RX 77368 (50 ng/rat) and euthanized 60 min later. Fos immunoreactivity was revealed as the dark blue staining in the cell nuclei and pChAT immunoreactivity appeared as brown staining in the cytoplasm. Scale bar, 100 μm. (B) Cell counts of Fos-positive and double-labeled Fos/pChAT neurons expressed in percentage of total Fos positive neurons and total pChAT neurons induced by cold exposure for 2 h or i.c. injection of RX 77368. Each column represents the mean±S.E.M. of 3 rats. *P <0.05 compared with the values of rats at room temperature or injected i.c. with saline. Adapted from Yuan et al. (2005).
The neurochemical phenotype of myenteric neurons activated by central vagal stimulation was identified to include over 90% of gastric intrinsic cholinergic neurons that represent two-thirds of total submucosal and myenteric immunoreactive neurons (Nakajima et al., 2000; Yuan et al., 2005) (Fig. 3). This was established using immunostaining with peripheral choline acetyltransferase (pChAT), a splice variant of ChAT that is expressed only in the peripheral nervous system (Tooyama and Kimura, 2000; Yuan et al., 2005). These observations provided anatomical and functional support to the vagal dependent and hexamethonium and atropine sensitive gastric acid response to i.c. injection of TRH or TRH analog in rats or medullary raphe activation in cats (Taché et al., 1980, 1984; Yanagisawa et al., 1990; White et al., 1991).
3. Involvement of medullary TRH signaling in the vagal stimulation of gastric function
The blockade of receptors by selective antagonists is a commonly used approach to assess the physiological role of endogenous transmitters and peptides. However, with regard to TRH, no specific receptor antagonists have yet been developed. Alternative strategies relied on the TRH-R1 antisense oligodeoxynucleotides to inhibit in vivo TRH-R1 expressed in the DVC (Suzuki et al., 1995; Sivarao et al., 1997) and polyclonal TRH antibody to immunoneutralize endogenous TRH localized and released in the DVC (Rinaman et al., 1989; Rinaman and Miselis, 1990).
3.1. Gastric stimulation induced by activation of TRH synthetizing neurons
Convergent reports showed that the chemical activation of TRH synthesizing cell bodies by microinjection of kainic acid into the Rpa, Rob and parapyramidal regions that project to the DVC (Lynn et al., 1991; Taché et al., 1995a) results in a vagal-dependent, atropine-sensitive stimulation of gastric acid and pepsin secretion, motility and mucosal blood flow and alterations of the resistance of the gastric mucosa to injury; these changes mimicked the gastric responses elicited by TRH or its stable analog microinjected directly into the DMN in rats and cats (Taché et al., 1984; White et al., 1991; Yang et al., 1993, 1999b, 2000a,b; Garrick et al., 1994; Kaneko et al., 1995, 1998; Kaneko and Taché, 1995). In addition, TRH antibody injected into the cisterna magna or selectively microinjected bilaterally into the DVC, or i.c. pretreatment with TRH-R1 oligodeoxynucleotide antisense prevented the stimulation of gastric acid secretion, motility and blood flow, and changes in the resistance of the gastric mucosa to injury induced by microinjection of kainic acid into the raphe obscurus or raphe pallidus (Yang et al., 1993, 2000b; Garrick et al., 1994; Kaneko et al., 1995, 1998; Kaneko and Taché, 1995; Sivarao et al., 1997). Collectively these results indicate that the excitation of TRH-synthesizing raphe medullary neurons activates endogenous TRH–TRH-R1 signaling cascade within the DMN and thereby elicits vagal cholinergic myenteric stimulation of gastric acid secretory and motor function and mucosal blood flow.
3.2. Gastric responses to acute cold exposure
Early reports established cold exposure as a model to induce vagal-dependent atropine sensitive development of gastric lesions in fasted rats (Senay and Levine, 1967). Since then, convincing data support the involvement of brain medullary TRH as part of the mechanisms underlying gastric alterations induced by cold exposure (Taché et al., 1995b). First, brain medullary proTRH mRNA expression is increased in a time-related manner by cold exposure for 1 to 3 h and in situ hybridization histochemistry revealed that this occurs exclusively in the raphe pallidus and raphe obscurus (Yang et al., 1994). Second, cold exposure activates TRH synthesizing neurons in the raphe pallidus, raphe obscurus and parapyramidal region as shown by Fos and prepro-TRH double staining (Bonaz and Taché, 1994; Wang et al., 1996; Yang et al., 2000b). Cold exposure also activates DMN neurons (Bonaz and Taché, 1994; Wang et al., 1996), vagal efferent discharge (Cho et al., 1996) and over 90% of antral and corpus submucosal and myenteric cholinergic neurons (Yuan et al., 2001, 2005) (Fig. 3). Third, acute cold exposure mimics the functional gastric responses evoked by i.c. or DMN injection of TRH or TRH analogs and induces a vagally mediated atropine-sensitive stimulation of gastric acid and pepsin secretion, motility and hemorrhagic lesion formations (Goto and Taché, 1985; Taché et al., 1988; Hernandez and Emerick, 1988; Yang et al., 1994; Okumura et al., 1994). Lastly, TRH antibody microinjected into the DVC or cisterna magna or i.c. pretreatment with the TRH-R1 oligodeoxynucleotide antisense prevents acute cold exposure-induced stimulation of gastric acid secretion, emptying, motility and lesion formation (Basso et al., 1988; Hernandez et al., 1990; Niida et al., 1991; Martinez et al., 1998).
3.3. Gastric responses to 2-deoxy-D-glucose
Glucodeprivation induced by 2-deoxy-D-glucose, which impairs glucose utilization, is a well-established pharmacological tool to induce food intake and central vagal-dependent stimulation of gastric acid secretion, emptying and experimental ulcers in rats and dogs (Hirschowitz and Sachs, 1965; Smith and Epstein, 1969; Maeda-Hagiwara and Watanabe, 1983; Okumura et al., 1995b). Brain nuclei influenced by peripheral administration of 2-deoxy-D-glucose include neurons in the DMN as shown by induction of Fos expression which is indicative of activation of preganglionic vagal motor neurons (Ritter and Dinh, 1994). It is likely that brain medullary TRH plays a role in these DMN and gastric responses since i.c. injection of TRH antibody prevents the acceleration of gastric emptying and erosion formation induced by intravenous injection of 2-deoxy-D-glucose in conscious rats (Okumura et al., 1995a,b).
3.4. Gastric acid responses to sham-feeding
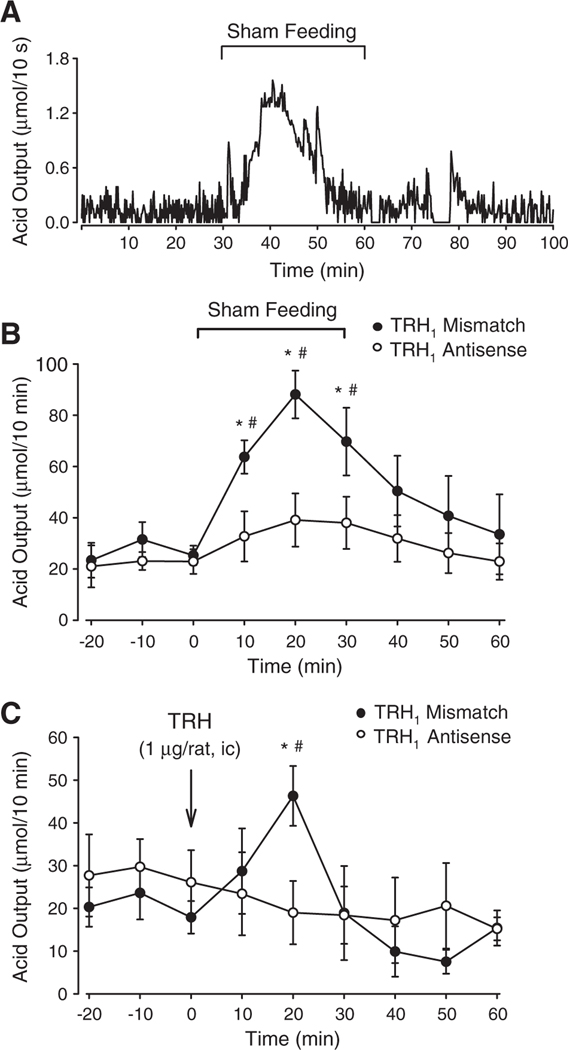
The peripheral mechanisms of the cephalic phase of gastric acid secretion has been extensively studied in dogs and in humans, however, little is known on brain circuits and transmitters involved in this vagally mediated response (Richardson et al., 1977; Feldman and Richardson, 1986; Konturek et al., 1987). In an effort to gain understanding on central mechanisms, Martinez et al. (2002) developed a rat model to study the cephalic phase of acid secretion in rats. Olfactory and visual sensory inputs of food resulted in the stimulation of gastric acid secretion in fasted rats with chronic gastric cannula and constant perfusion and titration of acid (Fig. 4A). Under these conditions, the basal acid secretion averages 22.0 ±1.6 μmol/10 min and increased within 10 min after the onset of sham-feeding to reach a 3-fold increase at 20 min, and declined to basal levels thereafter (Martinez et al., 2002) (Fig. 4B). The acid response to sham-feeding in rats is mediated by the activation of TRH–TRH-R1 signaling pathways in the brain medulla. This was established by the blockade of the acid response to sham-feeding and i.c. TRH by i.c. pretreatment with TRH-R1 antisense oligodeoxynucleotides, while TRH-R1-mismatched oligodeoxynucleotide pretreatment under similar conditions had no effect (Martinez et al., 2002) (Fig. 4B, C). Lastly, both sham-feeding and central injection of TRH, in addition to stimulate acid secretion, induce a vagal stimulation of gastroduodenal blood flow and motor function and pancreatic exocrine and endocrine secretion in experimental animals (Giduck et al., 1987; Taché et al., 1989b; Okumura et al., 1995c; Kiraly et al., 1998; Katschinski, 2000; Yang et al., 2002). Although to be further established, these data would suggest that the activation of medullary TRH and TRH-R1 in the DMN may also contribute to other digestive components of the cephalic phase of digestion that subserve optimizing the digestive (acid pepsin, propulsive motility) and metabolic processes under conditions of impending meal ingestion (Nicolaidis, 1969).
Fig. 4.
Activation of TRH–TRH-R1 signaling pathways mediates sham-feeding-induced gastric acid secretion in conscious rats. (A) Representative trace of increased gastric acid secretion induced by sham-feeding in conscious rats with chronic gastric fistula and constant perfusion with warm saline and constant recording of acid secretion while rats were maintained in a Bollman cage. Sham-feeding was induced by exposing fasted rats to the smell and sight of standard Purina chow for 30 min. (B) TRH-R1 antisense oligodeoxynucleotide pretreatment (100 μg twice, −48 and −24 h) prevents sham-feeding-stimulated gastric aid secretion, while similar pretreatment with TRH-R1 mismatch oligodeoxynucleotides did not; time course study of gastric acid output/10 min with each point representing the mean±S.E.M. of 4 rats. (C) TRH-R1 antisense oligodeoxynucleotide pretreatment (100 μg twice, −48 and −24 h) prevents i.c. TRH-induced stimulation of gastric acid secretion, while similar pretreatment with TRH-R1 mismatch oligodeoxynucleotides did not; time course study of gastric acid output/10 min with each point representing the mean±S.E.M. of 4 rats. Adapted from Martinez et al. (2002).
However, activation of medullary TRH–TRH-R1 signaling does not represent a common final pathway of vagally mediated gastric response since the i.c. injection of somatostatin analog, peptide YY (PYY) or pancreatic polypeptide that induces a vagal atropine-sensitive stimulation of gastric motor function and resistance of the mucosa to ethanol injury are not blocked by TRH-R1 antisense oligodeoxynucleotide pretreatment (Okumura et al., 1995b; Martinez et al., 1998; Yang et al., 1999a). In addition, this brain TRH pathway is not involved in the basal regulation of gastric function in the experimental models investigated since the i.c. pretreatment with the TRH antibody or TRH-R1 antisense oligodeoxynucleotides did not significantly influence basal gastric emptying or acid secretion (Okumura et al., 1995b; Martinez et al., 1998, 2002).
4. Modulation of medullary TRH action by other brain peptides
There is growing evidence that TRH excitatory action on DMN neurons does occur in concert with other potentiating influences. These modulatory effects are exerted by neuropeptides or neurotransmitters co-localized with TRH in raphe nuclei and co-released in the DVC, and through input from direct peptidergic projections to the DVC from other brain areas (Taché et al., 1995a).
4.1. Potentiation of TRH action in the dorsal vagal complex by Ps4 and 5-HT
The proteolytic cleavage of TRH prohormone generates five copies of TRH and the connecting peptides, including prepro-TRH-(160–169) (Ps4), which is co-released with TRH (Ladram et al., 1994). When Ps4 is co-injected into the DMN with TRH, the peptide potentiates the stimulation of gastric acid secretion in response to TRH, while having no effect by itself (Yang and Taché, 1994).
Serotonin (5-HT) is a neurotransmitter that is co-localized in neurons synthesizing TRH in medullary raphe nuclei and parapyramidal regions (Helke et al., 1989; Kachidian et al., 1991), and released in the DVC in response to excitation of medullary raphe neurons (Mohammed et al., 1995). Functional studies showed that 5-HT potentiates exogenous or endogenous TRH action in the DVC to stimulate gastric acid secretion and motility while 5-HT microinjected alone into the DMN did not alter basal gastric function (McCann et al., 1988; McTigue et al., 1992; Yoneda and Taché, 1995; Chi et al., 1996). Likewise, fluoxetine, a 5-HT reuptake inhibitor that enhanced extraneuronal 5-HT levels in the brain, injected i.c. potentiates i.c. RX 77368-induced increase in gastric acid secretion (Shockley et al., 1992). Pharmacologic studies using selective 5-HT receptor agonists and antagonists suggest that 5-HT action in the DVC involved interaction with 5-HT2 receptors (Yoneda and Taché, 1995; Varanasi et al., 1997).
4.2. Inhibition of TRH action in the dorsal vagal complex by peptides
Several peptides innervating the DVC exert an inhibitory influence on TRH-induced stimulation of gastric function. Substance P (SP) is expressed in TRH-containing neurons in the raphe pallidus, raphe obscurus and parapyramidal regions projecting to the DVC (Kachidian et al., 1991; Taché et al., 1995a). Retrograde labeling studies showed that DMN neurons projecting to the stomach are in contact with SP terminals and express neurokinin-1 receptors (NK1) (Ladic and Buchan, 1996). The activation of NK1 receptors in the DMN reduces the gastric secretory and motor stimulatory responses to exogenous TRH microinjected into the DVC or endogenously released by stimulation of Rpa or Rob (Yang and Taché, 1997; Krowicki and Hornby, 2000). Therefore, SP co-released with TRH in the DVC dampens the excitatory action of TRH.
A number of other brain peptides co-injected with TRH into the DMN or cerebrospinal fluid inhibit the vagal-dependent stimulation of gastric secretory and motor function induced by TRH. Among them are those that are involved in the stress/immune response, including corticotrophin-releasing factor (CRF), urocortin 1, opioid peptides, interleukin-1 and tumor necrosis factor-α (Morley et al., 1981; Taché et al., 1983; Garrick et al., 1988; Saperas et al., 1990; Heymann-Mönnikes et al., 1991; Taché and Saperas, 1992; Hermann and Rogers, 1995; Hermann et al., 1999; Yang et al., 2000a; Chen et al., 2002), as well as peptides or conditions inhibiting food intake such as gastrin-releasing peptide/bombesin, PYY, Y2 agonist, calcitonin gene-related peptide, adrenomedullin and i.v. glucose (Hughes et al., 1984; Chen et al., 1997; Martinez and Taché, 2000; Yuan and Yang, 2002; Doong and Yang, 2003).
These pharmacological observations may have relevance in the context of known inhibition of vagally mediated digestive function including acid, pepsin or upper gastrointestinal motility under stress conditions (Taché et al., 1989a). However, additional studies are still needed to establish the physiological relevance and mechanisms of actions that modulate TRH–TRH-R1-induced activation of DMN preganglionic neurons.
Acknowledgements
The authors’ work was supported by the NIHDDK grant R01 30110 (YT) and DK 33061 (YT), the Center grant DK 41301 (Animal Core, YT), VA Senior Scientist Award (YT), DK 50255 (HY) and VA Merit Award (HY). The authors thank Miss Teresa Olivas for her help in the preparation of the manuscript.
References
- Basso N, Bagarani M, Pekary E, Genco A, Materia A, 1988. Role of thyrotropin-releasing hormone in stress ulcer formation in the rat. Dig. Dis. Sci 33, 819–823. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Viana F, Kanter RK, Szymeczek-Seay CL, Berger AJ, Millhorn DE, 1994. Early postnatal development of thyrotropin-releasing hormone (TRH) expression, TRH receptor binding, and TRH responses in neurons of rat brainstem. J. Neurosci 14, 821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, 1995. Anatomical demonstration of vagal input to nicotinamide acetamide dinucleotide phosphate diaphorase-positive (nitrergic) neurons in rat fundic stomach. J. Comp. Neurol 358, 428–439. [DOI] [PubMed] [Google Scholar]
- Bonaz B, Taché Y, 1994. Induction of Fos immunoreactivity in the rat brain after cold-restraint induced gastric lesions and fecal excretion. Brain Res. 652, 56–64. [DOI] [PubMed] [Google Scholar]
- Cao J, O’Donnell D, Vu H, Payza K, Pou C, Godbout C, Jakob A, Pelletier M, Lembo P, Ahmad S, Walker P, 1998. Cloning and characterization of a cDNA encoding a novel subtype of rat thyrotropin-releasing hormone receptor. J. Biol. Chem 273, 32281–32287. [DOI] [PubMed] [Google Scholar]
- Chen CH, Stephens RLJ, Rogers RC, 1997. PYYand NPY: control of gastric motility via action on Y1 and Y2 receptors in the DVC. Neurogastroenterol. Motil 9, 109–116. [DOI] [PubMed] [Google Scholar]
- Chen CY, Million M, Adelson DW, Martinez V, Rivier J, Taché Y, 2002. Intracisternal urocortin inhibits vagally stimulated gastric motility in rats: role of CRF(2). Br. J. Pharmacol 136, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi J, Kemerer J, Stephens RL, 1996. 5-HT in DVC: disparate effects on TRH analogues-stimulated gastric acid secretion, motility and cytoprotection. Am. J. Physiol 271, R368–R372. [DOI] [PubMed] [Google Scholar]
- Cho CH, Qui BS, Bruce IC, 1996. Vagal hyperactivity in stress induced gastric ulceration in rats. J. Gastroenterol. Hepatol 11, 125–128. [DOI] [PubMed] [Google Scholar]
- Cushing H, 1932. Peptic ulcers and the interbrain. Surg. Gynecol. Obstet 55, 1–34. [Google Scholar]
- Doong ML, Yang H, 2003. Intravenous glucose infusion decreases intracisternal thyrotropin-releasing hormone induced vagal stimulation of gastric acid secretion in anesthetized rats. Neurosci. Lett 340, 49–52. [DOI] [PubMed] [Google Scholar]
- Feldman M, Richardson CT, 1986. Role of thought, sight, smell and taste of food in the cephalic phase of gastric acid secretion in humans. Gastroenterology 90, 426–433. [DOI] [PubMed] [Google Scholar]
- Fodor M, Pammer C, Gorcs T, Palkovits M, 1994. Neuropeptides in the human dorsal vagal complex: an immunohistochemical study. J. Chem. Neuroanat 7, 141–157. [DOI] [PubMed] [Google Scholar]
- Fox EA, Powley TL, 1985. Longitudinal columnar organization within the dorsal motor nucleus represents separate branches of the abdominal vagus. Brain Res. 341, 269–282. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, 2002. Pharmacology of synaptic transmission in the enteric nervous system. Curr. Opin. Pharmacol 2, 623–629. [DOI] [PubMed] [Google Scholar]
- Garrick T, Veiseh A, Sierra A, Weiner H, Taché Y, 1988. Corticotropin-releasing factor acts centrally to suppress stimulated gastric contractility in the rat. Regul. Pept 21, 173–181. [DOI] [PubMed] [Google Scholar]
- Garrick T, Prince M, Yang H, Ohning G, Taché Y, 1994. Raphe pallidus stimulation increases gastric contractility via TRH projections to the dorsal vagal complex in rats. Brain Res. 636, 343–347. [DOI] [PubMed] [Google Scholar]
- Gershengorn MC, Osman R, 1996. Molecular and cellular biology of thyrotropin-releasing hormone receptors. Physiol. Rev 76, 175–191. [DOI] [PubMed] [Google Scholar]
- Giduck SA, Threatte RM, Kare MR, 1987. Cephalic reflexes: their role in digestion and possible roles in absorption and metabolism. J. Nutr 117, 1191–1196. [DOI] [PubMed] [Google Scholar]
- Goto Y, Taché Y, 1985. Gastric erosions induced by intracisternal thyrotropin-releasing hormone (TRH) in rats. Peptides 6, 153–156. [DOI] [PubMed] [Google Scholar]
- Guillemin R, 2005. Hypothalamic hormones a.k.a. hypothalamic releasing factors. J. Endocrinol 184, 11–28. [DOI] [PubMed] [Google Scholar]
- Helke CJ, Thor KB, Sasek CA, 1989. Chemical neuroanatomy of the parapyramidal region of the ventral medulla in the rat. Prog. Brain Res 81, 17–28. [DOI] [PubMed] [Google Scholar]
- Hermann G, Rogers RC, 1995. Tumor necrosis factor-alpha in the dorsal vagal complex suppresses gastric motility. Neuroimmunomodulation 2, 274–281. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Tovar CA, Rogers RC, 1999. Induction of endogenous tumor necrosis factor-alpha: suppression of centrally stimulated gastric motility. Am. J. Physiol 276, R59–R68. [DOI] [PubMed] [Google Scholar]
- Hernandez DE, Emerick SG, 1988. Thyrotropin-releasing hormone: medullary site of action to induce gastric ulcers and stimulate acid secretion. Brain Res. 459, 148–152. [DOI] [PubMed] [Google Scholar]
- Hernandez DE, Arredondo ME, Xue BG, Jennes L, 1990. Evidence for a role of brain thyrotropin-releasing hormone (TRH) on stress gastric lesion formation in rats. Brain Res. Bull 24, 693–695. [DOI] [PubMed] [Google Scholar]
- Heuer H, Schafer MK, O’Donnell D, Walker P, Bauer K, 2000. Expression of thyrotropin-releasing hormone receptor 2 (TRH-R2) in the central nervous system of rats. J. Comp. Neurol 428, 319–336. [PubMed] [Google Scholar]
- Heymann-Mönnikes I, Taché Y, Trauner M, Weiner H, Garrick T, 1991. CRF microinjected into the dorsal vagal complex inhibits TRH analog- and kainic acid-stimulated gastric contractility in rats. Brain Res. 554, 139–144. [DOI] [PubMed] [Google Scholar]
- Hirschowitz BI, Sachs G, 1965. Vagal gastric secretory stimulation by 2-deoxy-D-glucose. Am. J. Physiol 209, 452–460. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Fuxe K, Johansson O, Jeffcoate S, White N, 1975. Thyrotropin releasing hormone (TRH)-containing nerve terminals in certain brain stem nuclei and the spinal cord. Neurosci. Lett 1, 133–139. [DOI] [PubMed] [Google Scholar]
- Holst MC, Kelly JB, Powley TL, 1997. Vagal preganglionic projections to the enteric nervous system characterized with Phaseolus vulgaris-leucoagglutinin. J. Comp. Neurol 381, 81–100. [DOI] [PubMed] [Google Scholar]
- Horita A, Carino MA, Lai H, 1986. Pharmacology of thyrotropin-releasing hormone. Annu. Rev. Pharmacol. Toxicol 26, 311–332. [DOI] [PubMed] [Google Scholar]
- Hornby PJ, Rossiter CD, Kuhn DH, White RL Jr., Gillis RA, 1991. Neuroactive substances in medullary nuclei that control parasympathetic outflow to the stomach. In: Taché Y, Wingate D. (Eds.), Brain–Gut Interactions. CRC Press, Boca Saton, pp. 23–43. [Google Scholar]
- Hughes JJ, Levine AS, Morley JE, Gosnell BA, Silvis SE, 1984. Intraventricular calcitonin gene-related peptide inhibits gastric acid secretion. Peptides 5, 665–667. [DOI] [PubMed] [Google Scholar]
- Jackson IMD, Lechan RM, 1983. Thyrotropin releasing hormone. In: Krieger DT, Brownstein MJ, Martin JB (Eds.), Brain Peptides. Wiley-Interscience Publication, New York, pp. 661–685. [Google Scholar]
- Kachidian P, Poulat P, Marlier L, Privat A, 1991. Immunohistochemical evidence for the coexistence of substance P, thyrotropin-releasing hormone, GABA, methionine–enkephalin, and leucine-enkephalin in the serotonergic neurons of the caudal raphe nuclei: a dual labeling in the rat. J. Neurosci. Res 30, 521–530. [DOI] [PubMed] [Google Scholar]
- Kaneko H, Taché Y, 1995. TRH in the dorsal motor nucleus of the vagus is involved in gastric erosion induced by excitation of raphe pallidus in rats. Brain Res. 699, 97–102. [DOI] [PubMed] [Google Scholar]
- Kaneko H, Yang H, Ohning G, Taché Y, 1995. Medullary TRH is involved in gastric protection induced by low dose of kainic acid into the raphe pallidus. Am. J. Physiol 268, G548–G552. [DOI] [PubMed] [Google Scholar]
- Kaneko H, Kaunitz J, Taché Y, 1998. Vagal mechanisms underlying gastric protection induced by chemical activation of the raphe pallidus in rats. Am. J. Physiol 275, G1056–G1062. [DOI] [PubMed] [Google Scholar]
- Katschinski M, 2000. Nutritional implications of cephalic phase gastrointestinal responses. Appetite 34, 189–196. [DOI] [PubMed] [Google Scholar]
- Kiraly A, Suto G, Guth PH, Taché Y, 1998. Peripheral mediators involved in gastric hyperemia to vagal activation by central TRH analog in rats. Am. J. Physiol 274, G170–G177. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Liu MT, 1998. Immunohistochemical localization of nicotinic acetylcholine receptors in the guinea pig bowel and pancreas. J. Comp. Neurol 390, 497–514. [PubMed] [Google Scholar]
- Konturek SJ, Popiela T, Slowiaczek M, Bielanski W, 1987. Gastric acid and pancreatic polypeptide responses to modified sham feeding. Effects of truncal and parietal cell vagotomy. Gut 28, 280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer HJ, Karahan ST, Rumpel E, Klinger M, Kuhnel W, 1993. Immunohistochemical visualization of the enteric nervous system using antibodies against protein gene product (PGP) 9.5. Anat. Anz 175, 321–325. [DOI] [PubMed] [Google Scholar]
- Krowicki ZK, Hornby PJ, 2000. Substance P in the dorsal motor nucleus of the vagus evokes gastric motor inhibition via neurokinin 1 receptor in rat. J. Pharmacol. Exp. Ther 293, 214–221. [PubMed] [Google Scholar]
- Krukoff TL, 1993. Expression of c-fos in studies of central autonomic and sensory systems. Mol. Neurobiol 7, 247–263. [DOI] [PubMed] [Google Scholar]
- Ladic LA, Buchan AM, 1996. Association of substance P and its receptor with efferent neurons projecting to the greater curvature of the rat stomach. J. Auton. Nerv. Syst 58, 25–34. [DOI] [PubMed] [Google Scholar]
- Ladram A, Bulant M, Delfour A, Montagne JJ, Vaudry H, Nicolas P, 1994. Modulation of the biological activity of thyrotropin-releasing hormone by alternate processing of pro-TRH. Biochimie 76, 320–328. [DOI] [PubMed] [Google Scholar]
- Lechan RM, Segerson TP, 1989. Pro-TRH gene expression and precursor peptides in rat brain: observations by hybridization analysis and immunocytochemistry. Ann. N.Y. Acad. Sci 553, 29–59. [DOI] [PubMed] [Google Scholar]
- Livingston CA, Berger AJ, 1993. Response of neurons in the dorsal motor nucleus of the vagus to thyrotropin-releasing hormone. Brain Res. 621, 97–105. [DOI] [PubMed] [Google Scholar]
- Lynn RB, Kreider MS, Miselis RR, 1991. Thyrotropin-releasing hormone-immunoreactive projections to the dorsal motor nucleus and the nucleus of the solitary tract of the rat. J. Comp. Neurol 311, 271–288. [DOI] [PubMed] [Google Scholar]
- Maeda-Hagiwara M, Watanabe K, 1983. Gastric antral ulcers produced by the combined administration of indomethacin with 2-deoxy-D-glucose in the rat. Eur. J. Pharmacol 89, 243–250. [DOI] [PubMed] [Google Scholar]
- Manaker S, Rizio G, 1989. Autoradiographic localization of thyrotropin-releasing hormone and substance P receptors in the rat dorsal vagal complex. J. Comp. Neurol 290, 516–526. [DOI] [PubMed] [Google Scholar]
- Martinez V, Taché Y, 2000. Bombesin and the brain–gut axis. Peptides 21, 1617–1625. [DOI] [PubMed] [Google Scholar]
- Martinez V, Wu SV, Taché Y, 1998. Intracisternal antisense oligodeoxynucleotides to the thyrotropin-releasing hormone receptor blocked vagal-dependent stimulation of gastric emptying induced by acute cold in rats. Endocrinology 139, 3730–3735. [DOI] [PubMed] [Google Scholar]
- Martinez V, Barrachina MD, Ohning G, Taché Y, 2002. Cephalic phase of acid secretion involves activation of medullary TRH receptor subtype 1 in rats. Am. J. Physiol.: Gastrointest. Liver Physiol 283, G1310–G1319. [DOI] [PubMed] [Google Scholar]
- McCann MJ, Hermann GE, Rogers RC, 1988. Dorsal medullary serotonin and gastric motility: enhancement of effects by thyrotropin-releasing hormone. J. Auton. Nerv. Syst 25, 35–40. [DOI] [PubMed] [Google Scholar]
- McCann MJ, Hermann GE, Rogers RC, 1989. Thyrotropin-releasing hormone: effects on identified neurons of the dorsal vagal complex. J. Auton. Nerv. Syst 26, 107–112. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Rogers RC, Stephens RL, 1992. Thyrotropin releasing hormone analogue and serotonin interact within the dorsal vagal complex to augment gastric acid secretion. Neurosci. Lett 144, 61–64. [DOI] [PubMed] [Google Scholar]
- Metcalf G, Dettmar PW, 1981. Is thyrotropin releasing hormone an endogenous ergotropic substance in the brain? Lancet 1, 586–589. [DOI] [PubMed] [Google Scholar]
- Miampamba M, Yang H, Sharkey KA, Taché Y, 2001. Intracisternal TRH analog induces Fos expression in gastric myenteric neurons and glia in conscious rats. Am. J. Physiol.: Gastrointest. Liver Physiol 280, G979–G991. [DOI] [PubMed] [Google Scholar]
- Mohammed JR, Saska TA, Chi J, Stephens RL Jr., 1995. Stimulation of nucleus raphe obscurus produces marked serotonin release into the dorsal medulla of fed but not fasted rats – glutaminergic dependence. Brain Res. 695, 100–103. [DOI] [PubMed] [Google Scholar]
- Morley JE, Levine AS, Silvis SE, 1981. Endogenous opiates inhibit gastric acid secretion induced by central administration of thyrotropin-releasing hormone (TRH). Life Sci. 29, 293–297. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Tooyama I, Yasuhara O, Aimi Y, Kimura H, 2000. Immunohistochemical demonstration of choline acetyltransferase of a peripheral type (pChAT) in the enteric nervous system of rats. J. Chem. Neuroanat 18, 31–40. [DOI] [PubMed] [Google Scholar]
- Nicolaidis S, 1969. Early systemic responses to orogastric stimulation in the regulation of food and water balance: functional and electrophysiological data. Ann. N.Y. Acad. Sci 157, 1176–1203. [DOI] [PubMed] [Google Scholar]
- Niida H, Takeuchi K, Okabe S, 1991. Role of thyrotropin-releasing hormone in acid secretory response induced by lowering of body temperature in the rat. Eur. J. Pharmacol 198, 137–142. [DOI] [PubMed] [Google Scholar]
- Okumura T, Uehara A, Watanabe Y, Taniguchi Y, Kitamori S, 1994. Site-specific formation of thyrotropin-releasing hormone-induced gastric ulcers through the vagal system. Scand. J. Gastroenterol 29, 226–231. [DOI] [PubMed] [Google Scholar]
- Okumura T, Grant AP, Taylor IL, Ohning G, Taché Y, Pappas TN, 1995a. Gastric mucosal damage induced by 2-deoxy-d-glucose involves medullary TRH in the rat. Regul. Pept 55, 311–319. [DOI] [PubMed] [Google Scholar]
- Okumura T, Taylor IL, Ohning G, Taché Y, Pappas TN, 1995b. Intracisternal injection of TRH antibody blocks gastric emptying stimulated by 2-deoxy-d-glucose in rats. Brain Res. 674, 137–141. [DOI] [PubMed] [Google Scholar]
- Okumura T, Taylor IL, Pappas TN, 1995. c. Microinjection of TRH analogue into the dorsal vagal complex stimulates pancreatic secretion in rats. Am. J. Physiol 269, G328–G334. [DOI] [PubMed] [Google Scholar]
- O-Lee TJ, Wei JY, Taché Y, 1997. Intracisternal TRH and RX 77368 potently activate vagal efferent discharge in rats. Peptides 18, 213–219. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Mezey E, Eskay RL, Brownstein MJ, 1986. Innervation of the nucleus of the solitary tract and the dorsal vagal nucleus by thyrotropin-releasing hormone-containing raphe neurons. Brain Res. 373, 246–251. [DOI] [PubMed] [Google Scholar]
- Pavlov I, 1910. The work of the digestive glands. (English Translation by Thompson WH). C. Criffin & Co, London. [Google Scholar]
- Powley TL, Berthoud H-R, Prechtl JC, Fox AE, 1991. Fibers of the vagus regulating gastrointestinal function. In: Taché Y, Wingate D. (Eds.), Brain–Gut Interactions. CRC Press, Boca Raton, pp. 73–82. [Google Scholar]
- Raggenbass M, Vozzi C, Tribollet E, Dubois-Dauphin M, Dreifuss JJ, 1990. Thyrotropin-releasing hormone causes direct excitation of dorsal vagal and solitary tract neurones in rat brainstem slices. Brain Res. 530, 85–90. [DOI] [PubMed] [Google Scholar]
- Richardson CT, Walsh JH, Cooper KA, Feldman M, Fordtran JS, 1977. Studies on the role of cephalic–vagal stimulation in the acid secretory response to eating in normal human subjects. J. Clin. Invest 60, 435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, Miselis RR, 1990. Thyrotropin-releasing hormone-immunoreactive nerve terminals synapse on the dentrites of gastric vagal motoneurons in the rat. J. Comp. Neurol 294, 235–251. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Miselis RR, Kreider MS, 1989. Ultrastructural localization of thyrotropin-releasing hormone immunoreactivity in the dorsal vagal complex in rat. Neurosci. Lett 104, 7–12. [DOI] [PubMed] [Google Scholar]
- Ritter S, Dinh TT, 1994. 2-Mercaptoacetate and 2-deoxy-d-glucose induce Fos-like immunoreactivity in rat brain. Brain Res. 641, 111–120. [DOI] [PubMed] [Google Scholar]
- Saperas E, Yang H, Rivier C, Taché Y, 1990. Central action of recombinant interleukin-1 to inhibit acid secretion in rats. Gastroenterology 99, 1599–1606. [DOI] [PubMed] [Google Scholar]
- Schemann M, Grundy D, 1992. Electrophysiological identification of vagally innervated enteric neurons in guinea pig stomach. Am. J. Physiol 263, G709–G718. [DOI] [PubMed] [Google Scholar]
- Segerson TP, Hoefler H, Childers H, Wolfe HJ, Wu P, Jackson I, Lechan RM, 1987. Localization of thyrotropin-releasing hormone prohormone messenger ribonucleic acid in rat brain by in situ hybridization. Endocrinology 121, 98–107. [DOI] [PubMed] [Google Scholar]
- Senay EC, Levine RJ, 1967. Synergism between cold and restraint for rapid production of stress ulcers in rats. Proc. Soc. Exp. Biol. Med 124, 1221–1223. [DOI] [PubMed] [Google Scholar]
- Shockley RA, LePard KJ, Stephens RL Jr., 1992. Fluoxetine pretreatment potentiates intracisternal TRH analog-stimulated gastric acid secretion in rats. Regul. Pept 38, 121–128. [DOI] [PubMed] [Google Scholar]
- Sivarao DV, Krowicki ZK, Abrahams TP, Hornby PJ, 1997. Intracisternal antisense oligonucleotides to TRH receptor abolish TRH-evoked gastric motor excitation. Am. J. Physiol 272, G1372–G1381. [DOI] [PubMed] [Google Scholar]
- Smith GP, Epstein AN, 1969. Increased feeding in response to decreased glucose utilization in the rat and monkey. Am. J. Physiol 217, 1083–1087. [DOI] [PubMed] [Google Scholar]
- Somiya H, Tonoue T, 1984. Neuropeptides as central integrators of autonomic nerve activity: effects of TRH, SRIF, VIP and bombesin on gastric and adrenal nerves. Regul. Pept 9, 47–52. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Pilowsky P, Minson J, Arnolda L, Llewellyn-Smith I, Chalmers J, 1995. Antisense to thyrotropin releasing hormone receptor reduces arterial blood pressure in spontaneously hypertensive rats. Circ. Res 77, 679–683. [DOI] [PubMed] [Google Scholar]
- Taché Y, 1987. Central regulation of gastric acid secretion. In: Johnson LR, Christensen J, Jackson M, Jacobson ED, Walsh JH (Eds.), Physiology of the Gastrointestinal Tract. Raven Press, New York, pp. 911–930. [Google Scholar]
- Taché Y, Saperas E, 1992. Potent inhibition of gastric acid secretion and ulcer formation by centrally and peripherally administered interleukin-1. Ann. N.Y. Acad. Sci 659, 353–368. [DOI] [PubMed] [Google Scholar]
- Taché Y, Lis M, Collu R, 1977. Effects of thyrotropin-releasing hormone on behavioral and hormonal changes induced by beta-endorphin. Life Sci. 21, 841–846. [DOI] [PubMed] [Google Scholar]
- Taché Y, Vale W, Brown M, 1980. Thyrotropin-releasing hormone–CNS action to stimulate gastric acid secretion. Nature 287, 149–151. [DOI] [PubMed] [Google Scholar]
- Taché Y, Goto Y, Gunion MW, Vale W, Rivier J, Brown M, 1983. Inhibition of gastric acid secretion in rats by intracerebral injection of corticotropin-releasing factor. Science 222, 935–937. [DOI] [PubMed] [Google Scholar]
- Taché Y, Goto Y, Lauffenburger M, Lesiege D, 1984. Potent central nervous system action of p-Glu-His-(3,3-Vdimethyl)-Pro NH2, a stabilized analog of TRH, to stimulate gastric secretion in rats. Regul. Pept 8, 71–78. [DOI] [PubMed] [Google Scholar]
- Taché Y, Goto Y, Hamel D, Pekary A, Novin D, 1985. Mechanisms underlying intracisternal TRH-induced stimulation of gastric acid secretion in rats. Regul. Pept 13, 21–30. [DOI] [PubMed] [Google Scholar]
- Taché Y, Maeda-Hagiwara M, Goto Y, Garrick T, 1988. Central nervous system action of TRH to stimulate gastric function and ulceration. Peptides 9 (Suppl. 1), 9–13. [DOI] [PubMed] [Google Scholar]
- Taché Y, Gunion MM, Stephens R, 1989a. CRF: central nervous system action to influence gastrointestinal function and role in the gastrointestinal response to stress. In: De Souza EB, Nemeroff CB (Eds.), Corticotropin-Releasing Factor: Basic and Clinical Studies of a Neuropeptide. CRC Press, Boca Raton, pp. 300–307. [Google Scholar]
- Taché Y, Stephens RL, Ishikawa T, 1989b. Central nervous system action of TRH to influence gastrointestinal function and ulceration. Ann. N.Y. Acad. Sci 553, 269–285. [DOI] [PubMed] [Google Scholar]
- Taché Y, Yang H, Yoneda M, 1993. Vagal regulation of gastric function involves thyrotropin-releasing hormone in the medullary raphe nuclei and dorsal vagal complex. Digestion 54, 65–72. [DOI] [PubMed] [Google Scholar]
- Taché Y, Yang H, Kaneko H, 1995. Caudal raphe–dorsal vagal complex peptidergic projections: role in gastric vagal control. Peptides 16, 431–435. [DOI] [PubMed] [Google Scholar]
- Taché Y, Yang H, Yoneda M, Bonaz B, 1995. Role of medullary TRH in vagally mediated formation of gastric lesions. In: Szabo S, Taché Y. (Eds.), Neuroendocrinology of Gastrointestinal Ulceration. Plenum Press, New York, pp. 161–173. [Google Scholar]
- Tooyama I, Kimura H, 2000. A protein encoded by an alternative splice variant of choline acetyltransferase mRNA is localized preferentially in peripheral nerve cells and fibers. J. Chem. Neuroanat 17, 217–226. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA, Vicini S, 1992. Effects of thyrotropin-releasing hormone on neurons in the rat dorsal motor nucleus of the vagus, in vitro. Am. J. Physiol 263, G508–G517. [DOI] [PubMed] [Google Scholar]
- Varanasi S, Chi J, Stephens RL Jr., 1997. 5-CTand DOI augments TRH analog-induced gastric acid secretion at the dorsal vagal complex. Am. J. Physiol 273, R1607–R1611. [DOI] [PubMed] [Google Scholar]
- Wang L, Cardin S, Martinez V, Taché Y, 1996. Intracerebroventricular CRF inhibits cold restraint-induced c-fos expression in the dorsal motor nucleus of the vagus and gastric erosions in rats. Brain Res. 736, 44–53. [DOI] [PubMed] [Google Scholar]
- White RL Jr., Rossiter CD, Hornby PJ, Harmon JW, Kasbekar DK, Gillis RA, 1991. Excitation of neurons in the medullary raphe increases gastric acid and pepsin production in cats. Am. J. Physiol 260, G91–G96. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K, Yang H, Walsh JH, Taché Y, 1990. Role of acetylcholine, histamine and gastrin in the acid response to intracisternal injection of TRH analog, RX 77368, in the rat. Regul. Pept 27, 161–170. [DOI] [PubMed] [Google Scholar]
- Yang H, Taché Y, 1994. Prepro-TRH-(160–169) potentiates gastric acid secretion stimulated by TRH microinjected into the dorsal motor nucleus of the vagus. Neurosci. Lett 174, 43–46. [DOI] [PubMed] [Google Scholar]
- Yang H, Taché Y, 1997. Substance P in the dorsal vagal complex inhibits medullary TRH-induced gastric acid secretion in rats. Am. J. Physiol 272, G987–G993. [DOI] [PubMed] [Google Scholar]
- Yang H, Ohning G, Taché Y, 1993. TRH in dorsal vagal complex mediates acid response to excitation of raphe pallidus neurons in rats. Am. J. Physiol 265, G880–G886. [DOI] [PubMed] [Google Scholar]
- Yang H, Wu SV, Ishikawa T, Taché Y, 1994. Cold exposure elevates thyrotropin-releasing hormone gene expression in medullary raphe nuclei: relationship with vagally mediated gastric erosions. Neuroscience 61, 655–663. [DOI] [PubMed] [Google Scholar]
- Yang H, Kawakubo K, Taché Y, 1999a. Intracisternal PYY increases gastric mucosal resistance: role of cholinergic, CGRP, and NO pathways. Am. J. Physiol 277, G555–G562. [DOI] [PubMed] [Google Scholar]
- Yang H, Kawakubo K, Taché Y, 1999b. Kainic acid into the parapyramidal region protects against gastric injury by ethanol. Eur. J. Pharmacol 372, R1–R3. [DOI] [PubMed] [Google Scholar]
- Yang H, Kawakubo K, Wong H, Ohning G, Walsh J, Taché Y, 2000a. Peripheral PYY inhibits intracisternal TRH-induced gastric acid secretion by acting in the brain. Am. J. Physiol.: Gastrointest. Liver Physiol 279, G575–G581. [DOI] [PubMed] [Google Scholar]
- Yang H, Yuan PQ, Wang L, Taché Y, 2000b. Activation of the parapyramidal region in the ventral medulla stimulates gastric acid secretion through vagal pathways in rats. Neuroscience 95, 773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Taché Y, Ohning G, Go VL, 2002. Activation of raphe pallidus neurons increases insulin through medullary thyrotropin-releasing hormone (TRH)–vagal pathways. Pancreas 25, 301–307. [DOI] [PubMed] [Google Scholar]
- Yarbrough GG, 1979. On the neuropharmacology of thyrotropin releasing hormone (TRH). Prog. Neurobiol 12, 291–312. [DOI] [PubMed] [Google Scholar]
- Yoneda M, Taché Y, 1995. Serotonin enhances gastric acid response to TRH analog in dorsal vagal complex through 5-HT2 receptors in rats. Am. J. Physiol 269, R1–R6. [DOI] [PubMed] [Google Scholar]
- Yuan PQ, Yang H, 2002. Neuronal activation of brain vagal–regulatory pathways and upper gut enteric plexuses by insulin hypoglycemia. Am. J. Physiol.: Endocrinol. Metab 283, E436–E448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan PQ, Taché Y, Miampamba M, Yang H, 2001. Acute cold exposure induces vagally mediated Fos expression in gastric myenteric neurons in conscious rats. Am. J. Physiol.: Gastrointest. Liver Physiol 281, G560–G568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan PQ, Kimura H, Million M, Bellier JP, Wang L, Ohning GV, Taché Y, 2005. Central vagal stimulation activates enteric cholinergic neurons in the stomach and VIP neurons in the duodenum in conscious rats. Peptides 26, 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Berthoud HR, 2000. Functional vagal input to gastric myenteric plexus as assessed by vagal stimulation-induced Fos expression. Am. J. Physiol.: Gastrointest. Liver Physiol 279, G73–G81. [DOI] [PubMed] [Google Scholar]