Abstract
Acute stress affects gut functions through the activation of corticotropin-releasing factor (CRF) receptors. The impact of acute stress on pelvic viscera in the context of chronic stress is not well characterized. We investigated the colonic, urinary, and locomotor responses monitored as fecal pellet output (FPO), urine voiding, and ambulatory activity, respectively, in female and male CRF-overexpressing (CRF-OE) mice, a chronic stress model, and their wild-type littermates (WTL). Female CRF-OE mice, compared with WTL, had enhanced FPO to 2-min handling (150%) and 60-min novel environment (155%) but displayed a similar response to a 60-min partial restraint stress. Female CRF-OE mice, compared with WTL, also had a significantly increased number of urine spots (7.3 ± 1.4 vs. 1.3 ± 0.8 spots/h) and lower locomotor activity (246.8 ± 47.8 vs. 388.2 ± 31.9 entries/h) to a novel environment. Male CRF-OE mice and WTL both responded to a novel environment but failed to show differences between them in colonic and locomotor responses. Male WTL, compared with female WTL, had higher FPO (113%). In female CRF-OE mice, the CRF1/CRF2 receptor antagonist astressin B and the selective CRF2 receptor agonist mouse urocortin 2 (injected peripherally) prevented the enhanced defecation without affecting urine or locomotor responses to novel environment. RT-PCR showed that CRF1 and CRF2 receptors are expressed in the mouse colonic tissues. The data show that chronic stress, due to continuous central CRF overdrive, renders female CRF-OE mice to have enhanced pelvic and altered behavioral responses to superimposed mild stressors and that CRF1-initiated colonic response is counteracted by selective activation of CRF2 receptor.
Keywords: chronic stress, colon, urine, locomotor activity, astressin B, mouse urocortin 2
EARLY ON, CLINICAL OBSERVATIONS and experimental studies established a link between psychological stress and motor function alterations of gut (1) and bladder (62). Recent studies in humans showed the role of psychosocial factors in the onset or development of functional bowel and bladder diseases, particularly irritable bowel syndrome (IBS) (40) and interstitial cystitis (IC) (55). This led to the notion that the level of chronic life stress predicts the clinical outcome in these patients (6, 19). Numerous studies in rodents have characterized the alterations of motor and mucosal barrier functions in the gastrointestinal and urinary tracts, mainly under acute stress conditions (13, 58, 65).
Persistent elevation of central drive of the hypothalamicpituitary-adrenal (HPA) axis is one of the distinguishing characteristics of chronic stress in animals and humans (9). Corticotropin-releasing factor (CRF) is a 41-amino acid hypothalamic peptide that initiates HPA axis activation by stimulating the secretion and biosynthesis of pituitary ACTH, which, in turn, causes adrenal glucocorticoid secretion (4). In addition, activation of CRF signaling pathways induces a battery of acute behavioral, autonomic and visceral stress responses (4, 17, 65). Hypersecretion of CRF in the central nervous system has been implicated in stress-related human pathologies, including major depressive disorders (2). The actions of CRF and other related endogenous peptides, urocortin 1 (Ucn 1), Ucn 2, and Ucn 3, are mediated through the activation of two G-protein-coupled receptors, CRF1 and/or CRF2 (4, 21). Convergent reports indicate that acute stress and central injection of CRF and Ucn 1 stimulate propulsive colonic motility, primarily through the activation of brain CRF1 receptors as established by the use of selective CRF1 and CRF2 receptor antagonists in rodents (67) and monkeys (17).
Recent studies also support a role for peripheral CRF receptors in the colonic response to stress in rats (68). Peripheral injection of CRF1/CRF2 agonists such as CRF and Ucn 1 and exposure to stressors such as restraint or water avoidance stimulate motility and increase permeability of the colon. These responses are blocked in rats by peptide CRF antagonists acting peripherally (68). In contrast to CRF and Ucn 1, peripheral injection of selective CRF2 agonists, Ucn 2 or Ucn 3, had no measurable effects on basal colonic function in rats and mice (39, 43). However, emerging evidence points to a role for CRF2 receptors in counterbalancing CRF1-initiated anxiety, stimulation of the pituitary adrenal axis, and visceral hyperalgesia responses to stress, thereby contributing to the restoration of homeostasis (3, 11, 12, 50), although recent studies indicate that brain septal CRF2 plays different roles (anxiogenic or no effect) depending on the level of background stress (25). With regard to the gut, there are recent reports showing that peripheral injection of Ucn 2 inhibits the CRF1-mediated hyperalgesia induced by intestinal distension in rats (42, 44, 47).
CRF injected daily or intermittent exposure to stressors has been used to model a state of chronic stress (26, 33). An alternative strategy is to use transgenic mice that overexpress CRF (CRF-OE) (59). CRF-OE mice were generated by a chimeric CRF transgene comprising the methallothionein promoter driving the rat CRF gene, including introns (59) or the Thy-1 promoter (15, 16). These transgenic mice overexpress CRF primarily in the brain and show several features that parallel those characteristic of chronic stress (12), including the chronic stimulation of pituitary ACTH release and elevated corticosterone levels (16, 59). CRF-OE mice are also a validated model of chronic stress that bear an anxiety-like behavioral profile featuring hypoactivity when placed in a novel environment (60, 72), hyperemotionality and impaired learning (23, 70), and diminished sexual receptivity (22). CRF-OE mice display immunologic alterations (12, 46) and increased body temperature and heart rate during the inactive phase of the diurnal cycle (15). Although CRF-OE mice have been largely characterized to assess the consequences of chronic constitutive CRF overdrive on the endocrine, behavioral, cardiac, and immune systems under basal or acute stress, to date, this model has not been used to explore alterations of pelvic function well known to be susceptible to stress (28, 40, 65).
In the present study, we examined pelvic visceral functions, primarily propulsive colonic motility, and, to a lesser extent, bladder function and locomotor responses in CRF-OE mice after exposure to acute stressors that were superimposed on their life-long CRF overexpression. Studies were conducted in male and female CRF-OE mice and wild-type littermates (WTL) because studies suggest the presence of sex differences in intestinal motor responses to chronic wrap restraint in rats (76), as well as in the prevalence of IBS (24) and IC (10). A link between IBS and overactive bladder has also been suggested (45). The role of peripheral CRF signaling pathways in mediating the pelvic changes induced by exposing CRF-OE mice and WTL to a novel environment stressor was assessed by peripheral pretreatment with the long-acting CRF1/CRF2 peptide antagonist astressin B (54). We also investigated whether acute stress-induced stimulation of pelvic propulsive motor activity in CRF-OE mice is modulated by activation of CRF2 receptors using peripheral injection of Ucn 2.
MATERIALS AND METHODS
Animals.
Adult male and female CRF-OE mice and WTL (Oregon Health and Science University, Portland, OR) and male C57BL/6 mice (Harlan, Indianapolis, ID), aged 3–6 mo, were used. CRF-OE mice were generated as previously detailed (59, 60) with the use of a chimeric CRF transgene comprising the methallothionein protomer driving the rat CRF gene and backcrossed eight generations onto the C57BL/6 mouse strain. Mice were housed in standard plastic cages (outer dimension: 27 × 12 × 16 cm) in groups of three or four of the same sex, in a temperature-, humidity-, and light cycle (lights on 0600)-controlled environment. Mice were provided ad libitum access to water and a commercially available laboratory rodent diet (Prolab RMH 2500–5P14; Purina LabDiet, St. Louis, MO). All of the procedures were approved by the Animal Research Committees at UCLA and Veteran Affairs Greater Los Angeles Healthcare System (Protocols 05058–02 and 11084–03).
Peptides.
Mouse Ucn 2 and astressin B (Peptide Biology Laboratory, The Salk Institute, La Jolla, CA) were synthesized by the solid-phase approach and the Boc strategy as previously described (54) and stored in powder form at −80°C. Immediately before the experiments, astressin B was dissolved in sterile water and Ucn 2 in saline (pH 7.0). Subcutaneous and intraperitoneal injections were performed (in 0.1 ml).
Acute stressors.
Three stressors were used: brief handling, novel environment, and partial restraint. Brief handling consisted of handling for 2 min. The novel environment consisted of transferring mice from their home cage (in <20 s) to a new clean plastic cage (outer dimension: 27 × 12 × 16 cm). The cage floor was lined with filter paper instead of bedding, and each mouse stayed alone in this novel environment for 60 min without food or water. Partial restraint stress consisted of placing the mouse head first into a ventilated 50-ml falcon tube for 60 min, as previously described (38).
Monitoring colon, bladder, and behavioral parameters.
Fecal pellet output (FPO) was recorded as the number of fecal pellets expelled at 2 min for the handling stressor; at 5, 15, 30, 45, and 60 min for the novel environment; and at 15, 30, 45, and 60 min for the partial restraint stressor. Urine voiding was monitored essentially as previously described (14) with some modifications. Mice were placed individually in a new cage with the floor lined with filter paper that was divided with a marker in 12 equal squares of 5.2 × 5.2-cm size. Urine spots left on the filter paper were viewed at the end of the 60-min novel environment with a UV transilluminator and photographed. The number and area of spots were determined with NIH Image software (Scion, Frederick, MD). Locomotor activity was monitored in mice placed individually in a new cage with the floor lined with filter paper that was divided with a marker in 12 equal squares of 5.2 × 5.2-cm size. Locomotor activity was assessed as the number of squares crossed during the first 5, 15, 30, 45, and 60 min, as previously described (5).
CRF receptor gene expression in the colon.
Proximal and distal colon tissue samples were harvested from naive female CRF-OE mice and female WTL to assess CRF receptor gene expression by RT-PCR. For RNA isolation and cDNA synthesis, total RNA was extracted from the proximal and distal colons of CRF-OE female mice (n = 5) and their WTL (n = 3) using RNA-Bee (TEL-TEST, Friendswood, TX), according to the manufacturer’s protocol, which included a DNase step. Briefly, the colonic tissue was homogenized in RNA-Bee with the FastPrep system (FP120A homogenizer and lysing matrix D; QBiogene, Carlsbad, CA), 200 μl of chloroform were added, and the solution was centrifuged for 15 min at 4°C. The aqueous layer was precipitated with isopropanol. The RNA pellet was suspended in diethyl pyrocarbonate water and digested for 60 min at 37°C using DNase I (Promega, Madison, WI). The quality and amount of final total RNA yield were estimated based on the ratio of absorbance at 260 to 280 nm by UV spectrophotometer (ND-1000; NanoDrop, Wilmington, DE). Total RNA (5 μg) was denatured at 65°C for 5 min and used to synthesize first-strand cDNA in a total volume of 20-μl reaction by the ThermoScript RT-PCR system (Invitrogen, Carlsbad, CA). The reverse transcription was terminated by incubation at 85°C for 5 min. RNase H was then added to the reaction mixture to remove the total RNA template.
The following specific mouse CRF1, CRF2, and ribosomal protein S16 oligonucleotide primers were used in the PCR: for mouse CRF1, 5′-GGTGTGCCTTTCCCCATCATT-3′ and 5′-CAACATGTAGGTGATGCCCAG-3′ corresponding to nucleotides 740–760 (sense) and 998–1018 (antisense) (74) and the predicted size of the band of 279 bp; for mouse CRF2, 5′-GGCAAGGAAGCTGGTGATTTG-3′ and 5′-GGCGTGGTGGTCCTGCCAGCG-3′ corresponding to nucleotides 957–977 (sense) and 1314–1334 (antisense) (61) and the predicted size of the band of 378 bp; and for ribosomal protein S16, 5′-TGCGGTGTGGAGCTCGTGCTTGT-3′ and 5′-GCTACCAGGCCTTTGAGATGGA-3′ corresponding to nucleotides 369–391 (sense) (75) and the predicted size of the band is 309 bp. PCR reactions were performed in a final volume of 30 ml using the RedTaq system (Sigma-Aldrich, St. Louis, MO). The reaction was predenatured at 95°C for 2 min and then amplified 34 times (94°C, 40 s; 59°C, 40 s; 72°C, 2 min), followed by a 5-min extension at 72°C in Thermal Cycler (PTC-200; MJ Research, San Francisco, CA). S16 was used as an internal control to ensure cDNA quality and equal loading. Negative control contained all reagents, except that 1 μl of H2O was substituted for reverse transcriptase in the RT reaction to exclude the possibility of genomic or other DNA contamination. The agarose gel image of PCR products corresponding to CRF receptors was acquired by the Kodak EDAS 290 system and semiquantified by densitometric measurement using NIH Image software (Scion). All results were normalized by the internal control.
Experimental protocols.
All experiments were performed during the light phase between 0830 and 1200 in nonfasted mice.
Effects of various acute stressors on FPO and urine voiding.
Female CRF-OE mice (22.1 ± 0.9 g) and WTL (21.2 ± 0.3 g) were handheld for 2 min (handling stress), and the FPO during the 2-min handling period was monitored. Female CRF-OE mice (22.6 ± 1.1 g) and WTL (21.7 ± 0.3 g) were restrained for 60 min (partial restraint stressor), and FPO was monitored every 15 min for 60 min. Female (24.7 ± 1.3 g) and male (32.4 ± 2.4 g) CRF-OE mice and female (21.4 ± 0.4 g) and male (31.8 ± 1.0 g) WTL were placed in the novel environment for 60 min (novel environment stressor). FPO and locomotor activity were monitored at 5, 15, 30, 45, and 60 min. Urine output during the 60-min period was determined after the end of experiment. To avoid the confounding effect of pheromone, experiments were conducted in isolated individual cages for each mouse. Male and female mice were not housed together under any circumstances, either for regular housing or during the experiment. The cages used for each study were cleaned and autoclaved at the Animal Facility where experiments were conducted. No single cage was used more than once.
Effects of CRF receptor antagonist on colonic response to acute stressors in female CRF-OE mice and WTL and male C57BL/6 mice.
Astressin B (100 μg/kg) or water was injected subcutaneously 30 min before the 60-min novel environment exposure of female CRF-OE mice (24.2 ± 2.2 g) and WTL (22.1 ± 0.8 g), and FPO, locomotor activity, and urine voiding were monitored for 60 min. A similar injection protocol was used with male C57BL/6 mice (34.3 ± 0.6 g) before the 60-min exposure to partial restraint stress, and FPO was monitored. The dose and route of CRF antagonist administration were selected based on previous experiments in mice and rats that characterized the regimen of injection for astressin B to block peripheral CRF-induced stimulation of distal colonic transit or endocrine response (39, 54).
Effects of Ucn 2 on colonic response to a novel environment in female CRF-OE mice and WTL.
Female CRF-OE mice (28.9 ± 1.7 g) and WTL (23.9 ± 0.5 g) were injected intraperitoneally with vehicle or Ucn 2 (1, 3 and 10 μg/kg) 10 min before exposure to a novel environment for 60 min. The FPO, urine voiding, and behavioral locomotor responses were monitored for 60 min. Doses and route of Ucn 2 administration were chosen on the basis of previous studies on gut functions in rodents (38, 43).
Statistical analysis.
All values are reported as means ± SE. One-way repeated-measures ANOVA followed by the Newman-Keuls multiple comparison test were used to analyze the time courses of FPO and locomotor activity. The number of urine spots and volume were analyzed by one-way ANOVA followed by Student’s t-test to compare differences at one time point. Two-way repeated-measures ANOVA was used when two-factor effects, genotype and treatment, were analyzed. A P value of <0.05 was considered statistically significant.
RESULTS
Differential FPO responses to acute stressors in female and male CRF-OE and WTL mice.
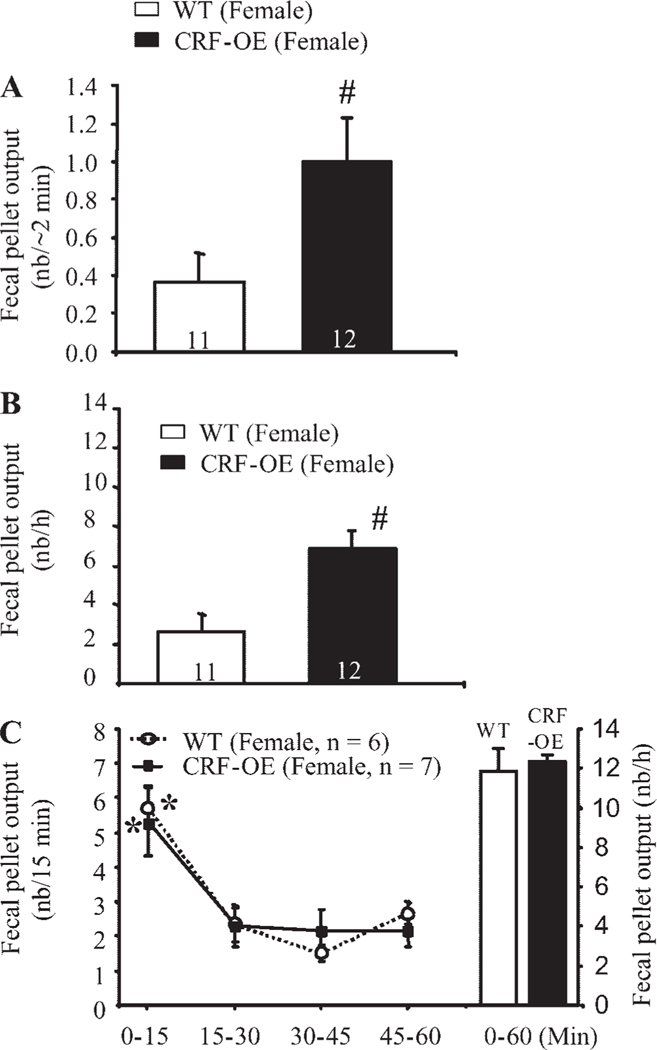
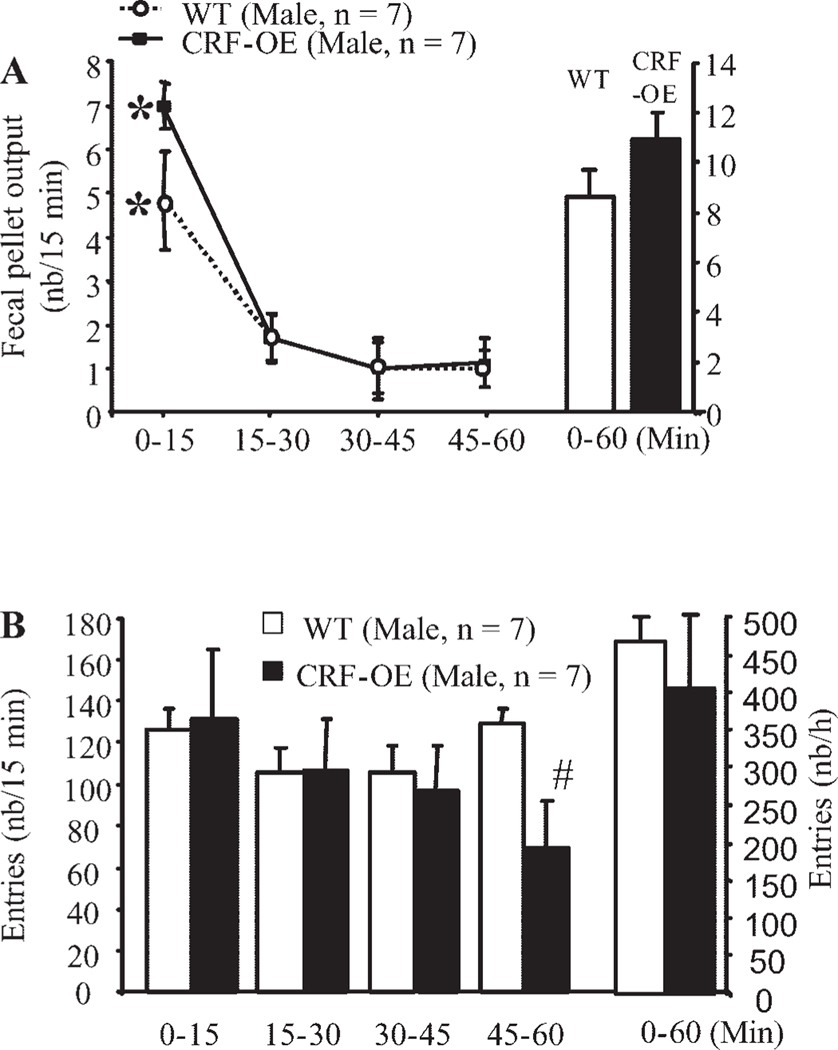
Female CRF-OE mice responded to 2-min handling with a higher FPO than female WTL (1.0 ± 0.2 vs. 0.4 ± 0.2 pellets/2 min, respectively, P < 0.05; Fig. 1A). Similarly, female CRF-OE mice exposed to a novel environment for 60 min displayed significantly higher cumulative FPO/h than did the WTL (6.9 ± 0.9 pellets/h, n = 12, vs. 2.7 ± 0.9 pellets/h, n = 11, respectively, P < 0.05; Fig. 1B). Time-course studies showed that the peak FPO in female CRF-OE mice occurred within the first 15 min of the novel environment exposure and was sevenfold higher than that of the corresponding WTL (5.4 ± 0.8 vs. 0.7 ± 1.0 pellets/15 min, respectively, P < 0.05; Fig. 2A). Thereafter, FPO/15 min in female CRF-OE mice remained low and was not significantly different from that of female WTL, with values between 0.6 and 1 pellet/15 min (Fig. 2A). In response to partial restraint, however, both female CRF-OE mice and WTL had similarly high cumulative FPO (12.3 ± 0.4 pellets/h, n = 7, vs. 11.8 ± 1.2 pellets/h, n = 6, respectively, P > 0.05) with a similar peak response at 15 min (5.7 ± 0.6 vs. 5.3 ± 1.0 pellets/min) and significantly decreased plateau response during the remaining 45 min (Fig. 1C). Because the novel environment stressor showed a significant difference between the female CRF-OE mice and WTL, we selected this stressor for all subsequent studies involving CRF-OE mice and their WTL. In male mice exposed to a novel environment, both CRF-OE mice and WTL had a significantly higher FPO score during the first 15 min (7.0 ± 0.5 vs. 4.9 ± 1.1 pellets/15 min, respectively) than that shown in the remaining 45 min, monitored every 15 min (Fig. 3A). However, neither the peak (7.0 ± 0.5 vs. 4.9 ± 1.1 pellets/15 min, P > 0.05) nor the cumulative defecation response (10.9 ± 1.2 vs. 8.6 ± 1.1 pellets/h, P > 0.05) reached significant difference between male CRF-OE mice and WTL (Fig. 3A).
Fig. 1.
Mild stress (A and B) but not strong restraint stress (C) induces enhanced fecal pellet output (FPO) in female corticotropin-releasing factor overexpressing (CRF-OE) mice compared with their wild-type (WT) littermates. Mice were handled for 2 min (A), placed in individual new cages for 60 min (B), or partially restrained for 60 min (C). Each bar or point represents the mean ± SE of the number of mice indicated at the bottom of columns or in parentheses. #P < 0.05 vs. WT littermates (t-test); *P < 0.05 vs. all other time points (repeated one-way ANOVA). nb, number.
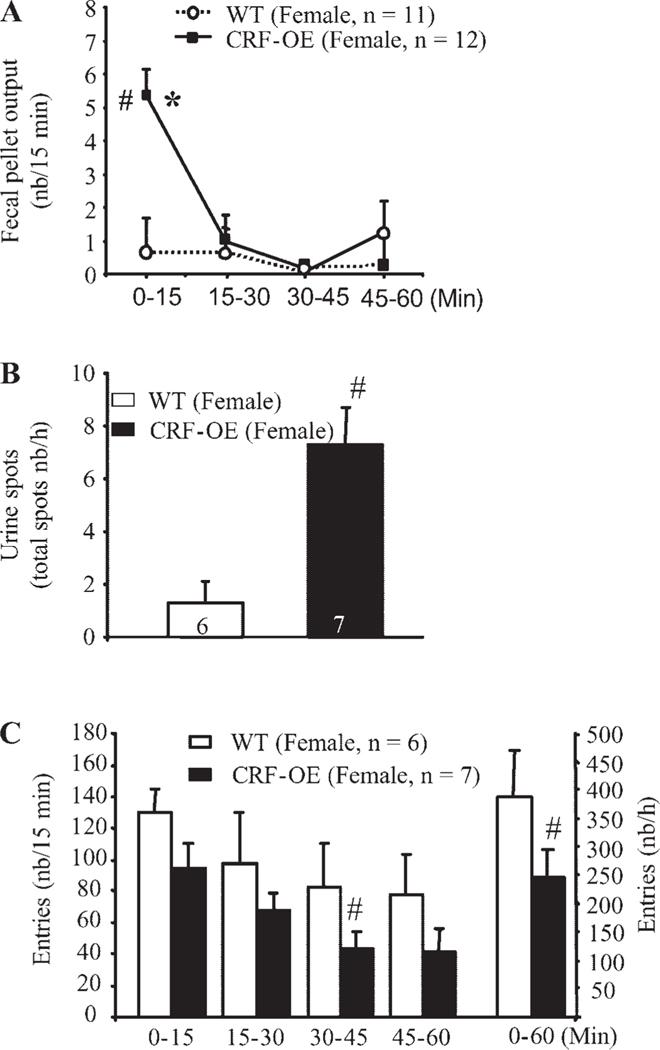
Fig. 2.
Novel environment stress for 60 min induces a rapid defecatory peak response in the first 15 min (A), increased urine spots (B), and decreased ambulatory response (C), as monitored concomitantly in female CRF-OE mice compared with their WT littermates. Each bar or point represents the mean ± SE of the number of mice indicated at the bottom of columns or in parentheses. *P < 0.05 vs. all other time points (repeated one-way ANOVA); #P < 0.05 vs. WT littermates (t-test).
Fig. 3.
Effects of novel environment on colonic (A) or locomotor (B) responses in male CRF-OE mice and WT littermates. Time course analysis shows enhanced colonic response during the first 15 min in both CRF-OE mice and WT littermates (A) and decreased locomotor response during the 45–60 min (B) of the stress session monitored concomitantly. Each bar or point represents the mean ± SE of number of mice indicated in parentheses. *P < 0.05 vs. all other time points (repeated one-way ANOVA); #P < 0.05 vs. WT littermates at 45–60 min time point (t-test).
Comparison of the total 60-min FPO to novel environment by sex showed that male WTL mice had significantly higher FPO response (8.6 ± 1.1, n = 7) than did the female WTL (2.7 ± 0.9 pellets/h, n = 11, P < 0.05) (Figs. 1B and 3B). Likewise, male CRF-OE mice tended to have a higher increased FPO to a novel environment than did female CRF-OE mice (10.9 ± 1.2 pellets/h, n = 7, vs. 6.9 ± 0.9 pellets/h, n = 12, P > 0.05) (Figs. 1B and 3B), although the difference did not reach significance. Because the body weight was higher in male than in female mice, FPO per 10 g body wt was computed. Analysis of FPO normalized to body weight yielded similar results. In response to a novel environment, there was no significant difference between male CRF-OE mice and WTL (3.5 ± 0.5 vs. 2.8 ± 0.4 pellets·10 g−1h−1, P > 0.05), whereas female CRF-OE mice had a significantly higher response than their WTL (2.9 ± 0.5 vs. 1.2 ± 0.4 pellets·10 g−1h−1, P > 0.05). Male WTL also had a significantly higher response to a novel environment than did female WTL (2.8 ± 0.4 vs. 1.2 ± 0.4 pellets·10 g−1h−1, P < 0.05). These data argue against body weight as a significant contribution to the observed sex difference in FPO in response to a novel environment in both WTL and CRF-OE mice.
Differential urine output and locomotor activity responses to novel environment between female CRF-OE and WTL mice.
Female CRF-OE mice exposed to the novel environment had a higher number of urine spots than did the WTL (7.3 ± 1.4 vs. 1.3 ± 0.8 spots/h, P < 0.05; Fig. 2B). No significant difference was identified in total area under the curve of the urine spots between female CRF-OE mice (32.2 ± 9.6 cm2, n = 7) and WTL (21.4 ± 9.9 cm2, n = 6, P > 0.05). The urine output response in male mice was not studied.
The ambulatory activity in response to a novel environment, assessed as the total number of squares crossed during the 60-min period, was significantly lower in the female CRF-OE mice than in the WTL (246.8 ± 47.8 vs. 388.2 ± 31.9/h, P < 0.05; Fig. 2C). The time course study showed that the activity of CRF-OE female mice was reduced starting in the first 15 min and continued to be lower than that of the WTL throughout the 60-min period with a significant effect at 30–45 min (Fig. 2C). In male CRF-OE mice, the total number of squares crossed during the 60 min of novel exposure was not different from that of WTL (404.7 ± 99.1 vs. 468.0 ± 27.3/h, respectively, P > 0.05; Fig. 3B). Inspection of the time course of the response showed similar values in both male CRF-OE and WTL mice during the first 45-min period, with a significant reduction in activity during the last 15 min in the CRF-OE mice (Fig. 3B). The ambulatory activity in male CRF-OE mice and WTL tended to be higher than that of female CRF-OE mice and WTL (Figs. 2C and 3B). Because female CRF-OE mice were more responsive to the novel environment than male CRF-OE mice compared with their WTL, further studies were mainly performed in female mice.
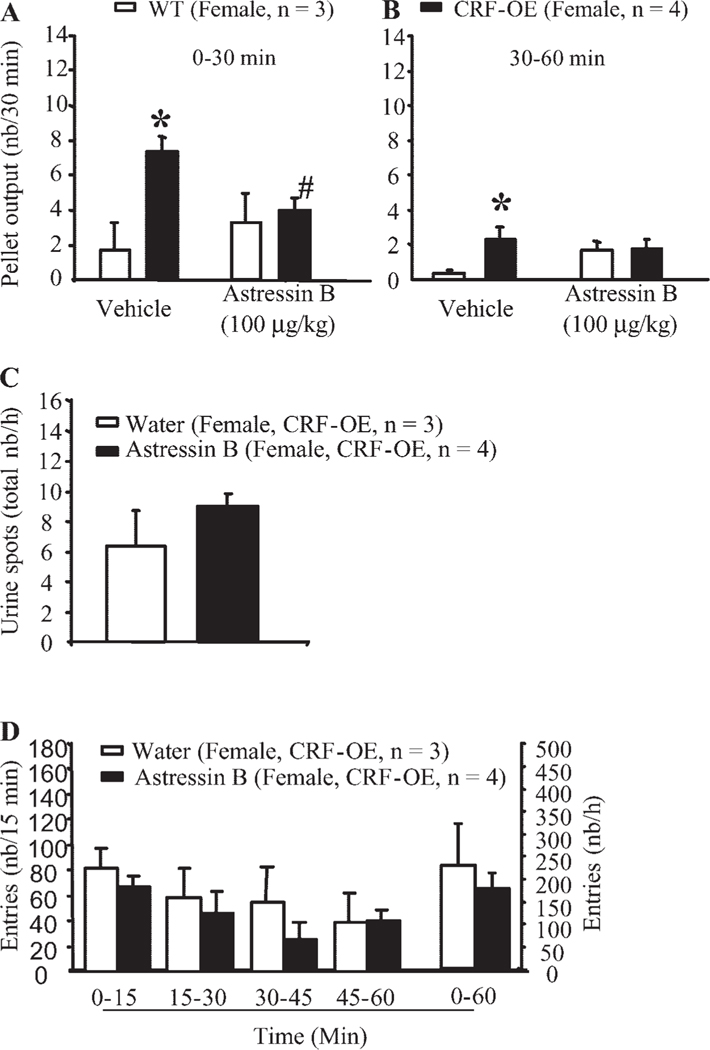
Effects of astressin B on acute stress-induced stimulation of pelvic function in CRF-OE and WTL mice.
Female CRF-OE mice injected subcutaneously with vehicle and exposed to a novel environment had a significantly higher FPO than vehicle injected subcutaneously in female WTL (0–30 min: 7.3 ± 0.9 vs. 1.7 ± 1.7 pellets/30 min, P < 0.05; 30–60 min: 2.3 ± 0.6 vs. 0.3 ± 0.2 pellets/30 min, P < 0.05; Fig. 4, A and B) consistent with previous observations in nonpretreated animals. Astressin B (100 μg/kg sc) injected 30 min before exposure to a novel environment did not significantly modify the low defecation score in female WTL but prevented the significant increase in FPO in CRF-OE mice, and values were similar to those observed in astressin B-pretreated female WTL (Fig. 4A). Likewise, astressin B (100 μg/kg sc) injection in male C57BL/6 mice as a 30-min pretreatment significantly inhibited the FPO rise induced by partial restraint stress compared with subcutaneous injection of vehicle (vehicle + restraint: 8.7 ± 0.8 pellets/h, n = 13; astressin B + restraint: 3.8 ± 0.2 pellets/h, n = 5/group, P < 0.05). Astressin B did not significantly influence FPO compared with vehicle-injected control male C57BL/6 mice (2.4 ± 1. 3 pellets/h, n = 7, vs. 1.7 ± 0.9 pellets/h, n = 7; P > 0.05). By contrast, astressin B pretreatment under the same conditions affected neither the number of urine spots (vehicle: 6.3 ± 2.4 spots/h vs. astressin B: 9.0 ± 0.8 spots/h; Fig. 4C) nor the locomotor response (vehicle: 232.0 ± 89.8 entries/h vs. astressin B: 178.0 ± 34.8 entries/h; Fig. 4D) induced by a novel environment in female CRF-OE mice.
Fig. 4.
Subcutaneous injection of a CRF1 and CRF2 receptor antagonist, astressin B, prevents novel environment stress-induced colonic response in female CRF-OE mice. The subcutaneous injection of vehicle or astressin B in female CRF-OE mice was performed 30 min before the 60-min exposure to a novel environment (A). Urinary voiding (B) and locomotor (C) responses were monitored concomitantly. Each bar represents the mean ± SE of number of mice indicated in parentheses. *P < 0.05 vs. vehicle-treated WT littermates (repeated one-way ANOVA); #P < 0.05 vs. vehicle-treated CRF-OE mice (t-test).
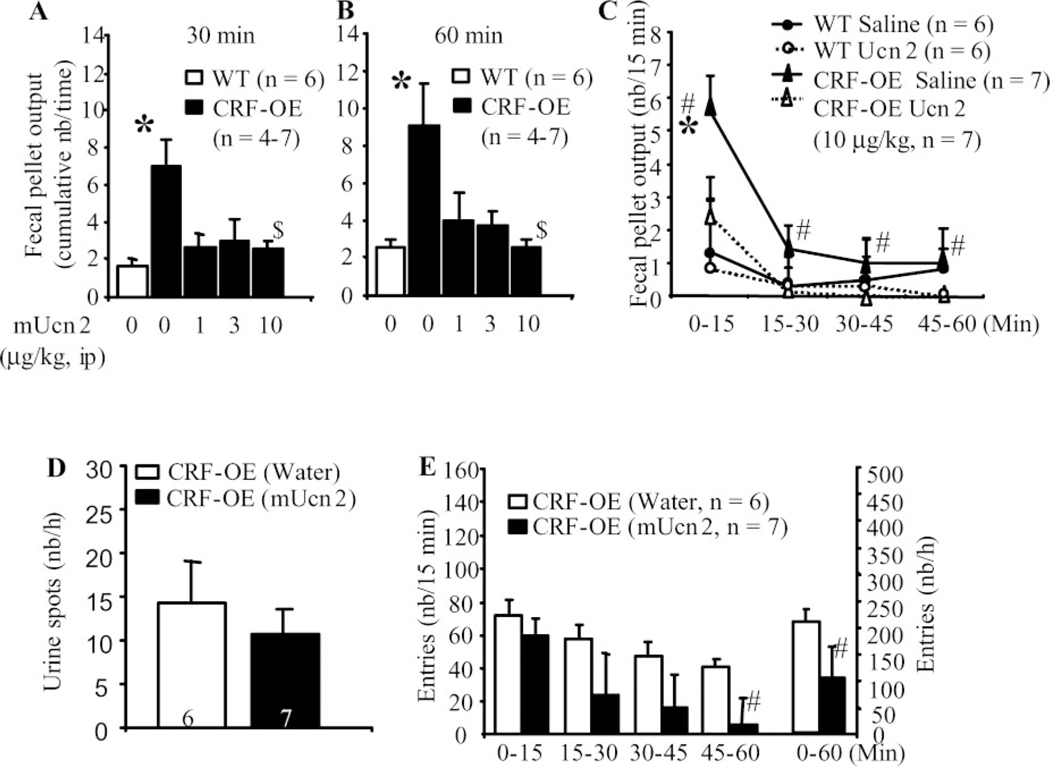
Peripheral mouse Ucn 2 blocked FPO response to novel environment in female CRF-OE mice.
CRF-OE mice injected intraperitoneally with saline had a significant 3.1- to 3.3-fold higher FPO score than did the saline-injected WTL during the first 15 or 30 min of novel environment (Fig. 5, A–C). Ucn 2 (1, 3, and 10 μg/kg ip) blocked the novel environment-induced 60-min FPO response in CRF-OE female mice (pellets/h: 4.0 ± 1.5, 3.8 ± 0.8, and 2.6 ± 0.4, respectively, vs. 9.0 ± 2.1 in vehicle, P < 0.05; Fig. 5B). By contrast, Ucn 2 (10 μg/kg ip) did not significantly affect the urine response as shown by the similar spot number in WTL and CRF-OE mice (Fig. 5D). Ucn 2 (10 μg/kg ip) treatment in female CRF-OE mice significantly reduced the novel environment-induced overall locomotor response (106.3 ± 23.5 vs. 218.4 ± 62 entries/h; Fig. 5E) with no significant effect during the first 15 min when the FPO response was maximal (Fig. 5C).
Fig. 5.
Intraperitoneal injection of selective CRF2 receptor agonist mouse urocortin 2 (mUcn 2), blocked novel environment stress-induced colonic response. Shown are dose and time courses (A–C), lack of effect on urinary voiding (D), and time course of locomotor response (D) in female CRF-OE mice compared with WT littermates. mUcn was injected intraperitoneally 10 min before exposure for 60 min to novel environment. *P < 0.05 vs. all other time points (repeated two-way ANOVA); #P < 0.05 vs. the corresponding WT littermates (one-way ANOVA); $P < 0.05 vs. vehicle CRF-OE mice (one-way ANOVA).
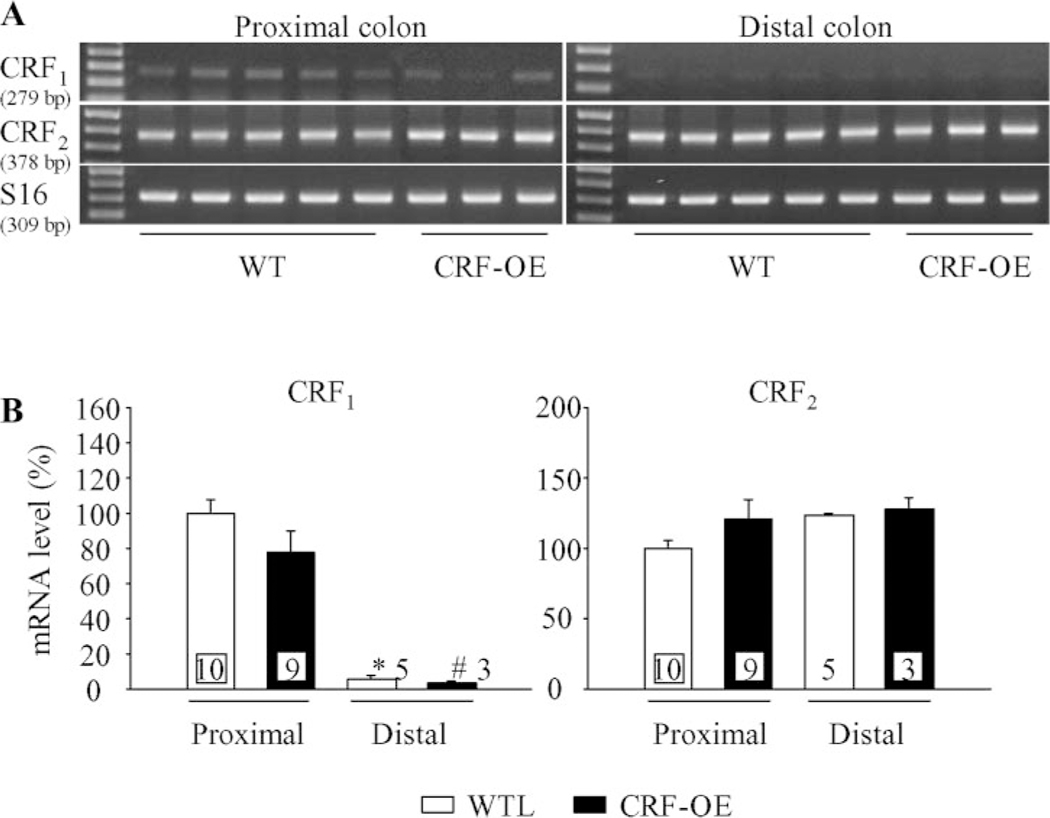
CRF1 and CRF2 gene expression in proximal and distal colons of female CRF-OE and WTL mice.
Using the primers specific for mouse CRF1 and CRF2, we found RT-PCR products for CRF1 and CRF2 with the predicted sizes in all samples of the proximal and distal mice colons that were examined (Fig. 6A). Quantitative analysis of the proximal vs. distal colon for CRF1 signal showed that CRF1 mRNA levels were significantly higher in proximal colon than in distal colon in both WTL (94.2%) and CRF-OE mice (96.1%). CRF1 mRNA levels in CRF-OE mice tended to decrease by 22.6% in the proximal colon and by 48.5% in the distal colon compared with WT mice. In contrast, similar levels of CRF2 expression was found in the proximal and distal colons with increased tendency of CRF2 mRNA level in the proximal colon of CRF-OE mice (21.4%) (Fig. 6B).
Fig. 6.
CRF1 and CRF2 gene expression in the proximal and distal colonic tissues of female CRF-OE mice and WT littermates (WTL). A: gel images of RT-PCR for mouse CRF1 and CRF2 acquired by the Kodak EDAS 290 system. The representative total RNA samples were extracted from whole thickness proximal (n = 9–10) and distal colons (n = 3–5) of WTL and CRF-OE mice. B: quantitative analysis of RT-PCR for CRF1 and CRF2 in proximal and distal colon using the NIH Image system (Scion, Frederick, MD) and standardized by taking the ratio to ribosomal protein S16 in each sample, respectively. Each column represents the mean ± SE of the number of mice indicated at the bottom of bars. P < 0.05 compared with proximal colon in WTL (*) or CRF-OE (#) mice.
DISCUSSION
Stimulation of colon and bladder motor functions (transit, defecation, urination) is a hallmark of the acute visceral response in rodents exposed to various stressors (38, 63, 65). The present study shows that female CRF-OE mice exposed to a novel environment for 60 min displayed a 6.7-fold peak increase in FPO compared with WTL. The response had a rapid onset and was short lasting. Likewise, female CRF-OE mice responded to handling for 2 min with a significant increase in FPO, compared with their WTL. By contrast, both female CRF-OE and their female WTL mice had a robust FPO response with a similar time course and magnitude during partial restraint stress. Female CRF-OE mice displayed enhanced urine voiding and decreased ambulation during exposure to the novel environment compared with their female WTL. These data are significant because they demonstrate that exposure to a novel environment triggers pelvic and behavioral manifestations of stress in female CRF-OE mice, which are under conditions of chronic central drive of CRF (12). Therefore, female CRF-OE mice provide an experimental model of enhanced pelvic responsiveness to a novel environment in the context of chronically activated stress pathways that may have relevance to stress modulation of functional disorders (19, 40, 55).
The present data also provide evidence that peripheral activation of CRF signaling pathways is part of the underlying mechanisms of acute stress-related activation of motor function in the colon. The CRF receptor antagonist, astressin B (54), prevented the significant increase in FPO of female CRF-OE mice compared with their WTL when exposed to the novel environment stressor. Similarly, in male C57BL/6 mice, the partial restraint-induced 4.1-fold increase in FPO compared with control was prevented by astressin B injected subcutaneously. Consistent with these observations, studies in female and male rats have also shown that the CRF1/CRF2 peptide antagonists, α-helical CRF9–41, or astressin (51, 53) injected intraperitoneally or intravenously prevented wrap restraint or water avoidance stress-induced acceleration of colonic transit and defecation (7, 36, 43, 77).
The blunting of the FPO response to acute stress by subcutaneous astressin B is likely to reflect a peripheral site of action (38, 68). In our study, the subcutaneous injection of astressin B did not influence the decreased locomotor activity in CRF-OE mice exposed to a novel environment. Such a decrease in exploratory activity is part of the anxiety-like behavior in CRF-OE mice, which has been well characterized in different novel environment paradigms, including open field, light-dark exploration, 16-hole board task, and elevated plus maze (35, 60, 70, 72), and shown to be mediated by brain CRF1 receptors (12, 60). CRF injected into the cerebral ventricles in as low as 0.15 nmol was previously reported to decrease locomotion in an open-field test, whereas subcutaneous injection of similar doses of CRF failed to affect locomotion (64). Thus, although there is no immunohistochemical evidence for the overexpression of CRF peptide in the peripheral tissue or elevated CRF levels in the circulation of CRF-OE mice (12, 59), an acute mild stressor superimposed on their chronic central CRF overproduction could engage the peripheral urocortin signaling (20, 31) to contribute to the colonic response.
Although astressin B binds to both CRF1 and CRF2 receptors (52), the astressin B-induced dampening of colonic motor response to a novel environment is likely to occur primarily through peripheral blockade of CRF1 receptors. This has been shown by the blockade of stress or CRF-induced colonic motor stimulation by intraperitoneal injection of astressin and CRF1 receptor antagonists (39) but not by peripheral injection of the selective CRF2 antagonist astressin B (38, 42). In addition, the CRF2 agonists Ucn 2 (6–60 μg/kg ip) and Ucn 3 (6–120 μg/kg ip) did not influence basal distal colonic transit, although they dose-dependently inhibited gastric transit monitored simultaneously in male mice (39). CRF1 receptors are localized at peripheral sites that subserve the regulation of propulsive motor activity in the colon. These include the myenteric neurons in the rat and guinea pig colon, where CRF induced a CRF1-dependent activation (8, 34, 41). We found by RT-PCR that CRF1 receptors are also expressed in CRF-OE mouse and WTL proximal colon and to a lesser extent in the distal colon, consistent with a possible site of action as established in rats. Lastly, female CRF-OE mice injected intraperitoneally with Ucn 2 in doses ranging from 1 to 10 μg/kg no longer displayed a significant increase in FPO during the 60 min of novel environment, whereas vehicle-injected CRF-OE mice did. Collectively, these findings support a role for peripheral CRF1 and not CRF2 receptors in mediating the FPO response to an acute stressor in mice, in line with previous reports in rats (36, 43).
Some of the phenotypic attributes of the CRF-OE mice, particularly those related to immunological abnormalities, derive exclusively from the CRF1-mediated chronic increase in circulating glucocorticoids (12). Other attributes, such as decreased exploratory activity and decreased sexual receptivity in females, are unrelated to the hypercorticosteronemia (12, 22, 23). Existing data do not support a glucocorticoid modulation of the CRF1-mediated colonic motor response in female CRF-OE mice. Hypophysectomy does not alter the stimulation of colonic transit by an acute stressor, and peripheral injection of ACTH or glucocorticoids does not result in colonic motor activation (66). The FPO of female CRF-OE mice was enhanced during exposure to the novel environment compared with WTL but was similar to WTL during the partial restraint stressor, indicative of differential responsiveness between the endocrine (12, 16, 60) and colonic motor changes to acute stressors (present study).
The selective CRF2 receptor agonist, Ucn 2 injected intraperitoneally, prevented the CRF1-initiated colonic motor response to a novel environment in female CRF-OE. Ucn 2 injected intravenously into mice has been reported to reach the brain parenchyma in an intact form at a moderate rate through passive diffusion (27), and a direct or indirect central action could occur. However, intraperitoneal Ucn 2 is likely to act peripherally. First, CRF2 receptors are expressed in the proximal and distal colons of both CRF-OE mice and WTL, as has been observed in rats (8). In addition, intraperitoneal Ucn 2 did not block the decrease in locomotor activity in CRF-OE mice exposed to the novel environment, a response well established to be mediated by CRF receptors in the brain (12, 60). These data support the emerging concept that activation of CRF2 receptors may counterbalance CRF1-initiated endocrine and visceral responses and thereby maintain allostasis (3, 44, 50).
Female CRF-OE mice exposed to a novel environment responded by a 4.6-fold increase in the frequency of urine voiding compared with WTL. CRF-OE mice are reported to have increased water intake (72), which may lead to increased voiding frequency. However, the fact that voiding frequency, but not the total urine output, was increased in CRF-OE mice suggests that the increased spotting in CRF-OE mice is related to an altered voiding and/or guarding reflex rather than factors affecting the overall urine volume (14). A recent review linked disorders of voiding with anxiety (29), and data in conscious rats established an important positive relationship between the state of arousal and micturition (28). The increased number of urine spots from CRF-OE mice, which also display anxiety-like behavior, is in line with the idea that their state of anxiety and/or arousal increased voiding frequency. The Barrington’s and sacral parasympathetic nuclei are synaptically linked to modulate motor function in both colon and bladder (56, 57, 71), and CRF serves as a major neurotransmitter in this pathway (56, 73). However, the precise role of CRF in the micturition reflex remains to be clarified. An inhibitory effect of central and spinal CRF on the micturition pathway in conscious and anesthetized male Sprague-Dawley rats (28, 48) and increased micturition frequency and threshold of micturition by intrathecal or intraperitoneal injection of CRF in conscious Wistar female rats have been reported (30). In the present study, astressin B or Ucn 2 injected intraperitoneally at doses that counteracted the FPO response to novel environment did not alter the voiding response in CRF-OE mice. These data suggest that peripheral CRF receptors play little role in triggering or modulating the enhanced voiding frequency under these conditions. The lack of CRF1 receptor expression in the rat bladder under noninflamed conditions (32) may explain the differential effects of peripheral astressin B on colonic vs. bladder responses, since CRF1 receptors are located on myenteric neurons in the colon, where they exert an excitatory action in response to peripheral injection of CRF agonists (8, 34, 41). Clearly, further studies will be needed to address the underlying mechanisms regulating voiding function in CRF-OE mice exposed to novel environments. Whether a reflex response from the possible cross-talk between the colon and bladder contribute to the observed effects is also not known and requires further studies.
The present data suggest that chronically stressed female mice tend to have enhanced behavioral and autonomic responses to a superimposed acute mild stress compared with male. Female CRF-OE mice, compared with their female WTL, displayed higher FPO and anxiogenic-like responses than did male CRF-OE mice, compared with their male WTL, during exposure to the novel cage stressor. Alterations of brain CRF pathways induced by restraint stress during the last week of pregnancy result in a sex difference in the offspring, whereby female offspring display a prolonged HPA axis response and increased anxiety to stressors vs. that shown in the male offspring (49). Such in utero impact of stress may have a bearing on the observed sex difference in our study. The observation that chronic stress enhances the colonic and anxiogenic responses to a superimposed acute, novel stressor, mainly in the female CRF-OE mice has relevance to sex differences observed in chronic disease conditions in humans. It is well documented that women under stress conditions develop functional diseases such as IBS (24), IC (55), and comorbid disorders (6, 78) with anxiety more prevalently than men. Whether estrogen receptors, known to influence the activity of the CRF promoter and transcriptional activity of the urocortin gene (18, 69), play a role in this sex difference warrants investigation.
In summary, the present study established that female CRF-OE mice exhibit enhanced FPO and urine voiding and reduced locomotor activity when submitted to novel environmental stress compared with female WTL. This enhanced pelvic responsiveness of female CRF-OE mice was mainly observed in the context of mild stressors and was sex specific. The present findings also point to differential peripheral mechanisms in the colon vs. bladder of female CRF-OE mice exposed to a novel environment. The FPO response to novel cage and restraint stressors involved primarily peripheral CRF1 and could be blunted by peripheral Ucn 2, strengthening the notion of a possible modulation of CRF1 stimulatory action by CRF2 receptors. By contrast, the enhanced urine spotting was largely independent of peripheral CRF1 receptors and not modulated by intraperitoneal Ucn 2, suggesting the presence of different peripheral or central mechanisms that will require further detailed investigations. The data also add to a possible link between IBS and overactive bladder, as suggested by Monga et al. (45). In view of the enhanced response to acute novel mild stressors in chronically CRF-OE female mice, this model has an important bearing on the understanding of several functional diseases in women, such as IBS and IC, which are known to be linked to chronic stress and for which growing evidence indicates that CRF signaling pathways may be of significant relevance (6, 37).
ACKNOWLEDGMENTS
We thank HongHui Liang for technical assistance. We also thank Teresa Olivas and Debby Doan for editing the manuscript.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK-33061 (to Y. Taché), R01 DK-57238 (to Y. Taché), R21 DK-068155 (to M. Million), P50 DK-64539 (to Y. Taché, T. Buffington, M. Million), DK-26741 (to J. Rivier), and R01 MH-65689 (to M. Stenzel-Poore).
REFERENCES
- 1.Almy TP, Kern F Jr, Tulin M. Alterations in colonic function in man under stress. II. Experimental production of sigmoid spasm in healthy persons. Gastroenterology 12: 425–436, 1949. [PubMed] [Google Scholar]
- 2.Bale TL. Sensitivity to stress: dysregulation of CRF pathways and disease development. Horm Behav 48: 1–10, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet 24: 410–414, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Bale TL, Vale WW. CRF and CRF receptor: Role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44: 525–557, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Barrachina MD, Martinez V, Wang L, Wei JY, Taché Y. Synergistic interaction between leptin and cholecystokinin to reduced short-term food intake in lean mice. Proc Natl Acad Sci USA 94: 10455–10460, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buffington CA. Comorbidity of interstitial cystitis with other unexplained clinical conditions. J Urol 172: 1242–1248, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Castagliuolo I, Lamont JT, Qiu B, Fleming SM, Bhaskar KR, Nikulasson ST, Kornetsky C, Pothoulakis C. Acute stress causes mucin release from rat colon: role of corticotropin-releasing factor and mast cells. Am J Physiol Gastrointest Liver Physiol 271: G884–G892, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Chatzaki E, Crowe PD, Wang L, Million M, Taché Y, Grigoriadis D. CRF receptor type 1 and 2 expression and anatomical distribution in the rat colon. J Neurochem 90: 309–316, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Checkley S The neuroendocrinology of depression and chronic stress. Br Med Bull 52: 597–617, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Clemens JQ, Meenan RT, O’Keeffe Rosetti MC, Brown SO, Gao SY, Calhoun EA. Prevalence of interstitial cystitis symptoms in a managed care population. J Urol 174: 576–580, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet 24: 403–409, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Coste SC, Murray SE, Stenzel-Poore MP. Animal models of CRH excess and CRH receptor deficiency display altered adaptations to stress. Peptides 22: 733–741, 2001. [DOI] [PubMed] [Google Scholar]
- 13.De Groat WC. Integrative control of the lower urinary tract: Preclinical perspective. Br J Pharmacol 147: S25–S40, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science 182: 939–941, 1973. [DOI] [PubMed] [Google Scholar]
- 15.Dirks A, Groenink L, Bouwknecht JA, Hijzen TH, Van Der GJ, Ronken E, Verbeek JS, Veening JG, Dederen PJ, Korosi A, Schoolderman LF, Roubos EW, Olivier B. Overexpression of corticotropin-releasing hormone in transgenic mice and chronic stress-like autonomic and physiological alterations. Eur J Neurosci 16: 1751–1760, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Groenink L, Dirks A, Verdouw PM, Schipholt M, Veening JG, Van Der GJ, Olivier B. HPA axis dysregulation in mice overexpressing corticotropin releasing hormone. Biol Psychiatry 51: 875–881, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Habib KE, Weld KP, Rice KC, Pushkas J, Champoux M, Listwak S, Webster EL, Atkinson AJ, Schulkin J, Contoreggi C, Chrousos GP, McCann SM, Suomi SJ, Higley JD, Gold PW. Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proc Natl Acad Sci USA 97: 6079–6084, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haeger P, Andres ME, Forray MI, Daza C, Araneda S, Gysling K. Estrogen receptors alpha and beta differentially regulate the transcriptional activity of the urocortin gene. J Neurosci 26: 4908–4916, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halpert A, Drossman D. Biopsychosocial issues in irritable bowel syndrome. J Clin Gastroenterol 39: 665–669, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Harada S, Imaki T, Naruse M, Chikada N, Nakajima K, Demura H. Urocortin mRNA is expressed in the enteric nervous system of the rat. Neurosci Lett 267: 125–128, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev 55: 21–26, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Heinrichs SC, Min H, Tamraz S, Carmouche M, Boehme SA, Vale WW. Anti-sexual and anxiogenic behavioral consequences of corticotropin-releasing factor overexpression are centrally mediated. Psychoneuroendocrinology 22: 215–224, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Heinrichs SC, Stenzel-Poore MP, Gold LH, Battenberg E, Bloom FE, Koob GF, Vale WW, Pich EM. Learning impairment in transgenic mice with central overexpression of corticotropin-releasing factor. Neuroscience 74: 303–311, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Heitkemper M, Jarrett M, Bond EF, Chang L. Impact of sex and gender on irritable bowel syndrome. Biol Res Nurs 5: 56–65, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Henry B, Vale W, Markou A. The effect of lateral septum corticotrophin-releasing factor receptor 2 activation on anxiety is modulated by stress. J Neurosci 26: 9142–9152, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hotta M, Shibasaki T, Yamauchi N, Ohno H, Benoit R, Ling N, Demura H. The effects of chronic central administration of corticotrophin-releasing factor on food intake, body weight, and hypothalamic-pituitary-adrenocortical hormones. Life Sci 48: 1483–1491, 1991. [DOI] [PubMed] [Google Scholar]
- 27.Kastin AJ, Akerstrom V. Differential interactions of urocortin/corticotropin-releasing hormone peptides with the blood-brain barrier. Neuroendocrinology 75: 367–374, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Kiddoo DA, Valentino RJ, Zderic S, Ganesh A, Leiser SC, Hale L, Grigoriadis DE. Impact of state of arousal and stress neuropeptides on urodynamic function in freely moving rats. Am J Physiol Regul Integr Comp Physiol 290: R1697–R1706, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Klausner AP, Steers WD. Corticotropin releasing factor: a mediator of emotional influences on bladder function. J Urol 172: 2570–2573, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Klausner AP, Streng T, Na YG, Raju J, Batts TW, Tuttle JB, Andersson KE, Steers WD. The role of corticotropin releasing factor and its antagonist, astressin, on micturition in the rat. Auton Neurosci 123: 26–35, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Kozicz T, Arimura A. Distribution of urocortin in the rat’s gastrointestinal tract and its colocalization with tyrosine hydroxylase. Peptides 23: 515–521, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Laberge J, Malley SE, Zvarova K, Vizzard MA. Expression of corticotropin-releasing factor (CRF) and CRF receptors in micturition pathways after cyclophosphamide (CYP)-induced cystitis. Am J Physiol Regul Integr Comp Physiol 291: R692–R703, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Linthorst AC, Flachskamm C, Hopkins SJ, Hoadley ME, Labeur MS, Holsboer F, Reul JM. Long-term intracerebroventricular infusion of corticotropin-releasing hormone alters neuroendocrine, neurochemical, autonomic, behavioral, and cytokine responses to a systemic inflammatory challenge. J Neurosci 17: 4448–4460, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu S, Gao X, Gao N, Wang X, Fang X, Hu HZ, Wang GD, Xia Y, Wood JD. Expression of type 1 corticotropin-releasing factor receptor in the guinea pig enteric nervous system. J Comp Neurol 481: 284–298, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology 146: 797–807, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Maillot C, Million M, Wei JY, Gauthier A, Taché Y. Peripheral corticotropin-releasing factor and stress-stimulated colonic motor activity involve type 1 receptor in rats. Gastroenterology 119: 1569–1579, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Martinez V, Taché Y. CRF1 receptors as a therapeutic target for irritable bowel syndrome. Curr Pharm Des 12: 1–18, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Martinez V, Wang L, Rivier J, Grigoriadis D, Taché Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J Physiol 556.1: 221–234, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez V, Wang L, Rivier JE, Vale W, Taché Y. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J Pharmacol Exp Ther 301: 611–617, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Mayer EA, Naliboff BD, Chang L, Coutinho SV. Stress and the gastrointestinal tract. V. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 280: G519–G524, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Miampamba M, Maillot C, Million M, Taché Y. Peripheral CRF activates myenteric neurons in the proximal colon through CRF1 receptor in conscious rats. Am J Physiol Gastrointest Liver Physiol 282: G857–G865, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Million M, Maillot C, Adelson DW, Nozu T, Gauthier A, Rivier C, Chrousos GP, Bayati A, Mattsson H, Taché Y. Peripheral injection of sauvagine prevents repeated colorectal distention-induced visceral pain in female rats. Peptides 26: 1188–1195, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Million M, Maillot C, Saunders PR, Rivier J, Vale W, Taché Y. Human urocortin II, a new CRF-related peptide, displays selective CRF2-mediated action on gastric transit in rats. Am J Physiol Gastrointest Liver Physiol 282: G34–G40, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Million M, Wang L, Wang Y, Adelson DW, Yuan PQ, Maillot C, Coutinho SV, McRoberts JA, Bayati A, Mattsson H, Wu VS, Wei JY, Rivier J, Vale W, Mayer EA, Taché Y. CRF2 receptor activation prevents colorectal distension-induced visceral pain and spinal ERK1/2 phosphorylation in rats. Gut 55: 172–181, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monga AK, Marrero JM, Stanton SL, Lemieux MC, Maxwell JD. Is there an irritable bladder in the irritable bowel syndrome? Br J Obstet Gynaecol 104: 1409–1412, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Murray SE, Rosenzweig HL, Johnson M, Huising MO, Sawicki K, Stenzel-Poore MP. Overproduction of corticotropin-releasing hormone blocks germinal center formation: role of corticosterone and impaired follicular dendritic cell networks. J Neuroimmunol 156: 31–41, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Nijsen M, Ongenae N, Meulemans A, Coulie B. Divergent role for CRF1 and CRF2 receptors in the modulation of visceral pain. Neurogastroenterol Motil 17: 423–432, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Pavcovich LA, Valentino RJ. Central regulation of micturition in the rat the corticotropin-releasing hormone from Barrington’s nucleus. Neurosci Lett 196: 185–188, 1995. [DOI] [PubMed] [Google Scholar]
- 49.Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL. Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology 147: 2506–2517, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Rivier CL, Grigoriadis DE, Rivier JE. Role of corticotropin-releasing factor receptors type 1 and 2 in modulating the rat adrenocorticotropin response to stressors. Endocrinology 144: 2396–2403, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Rivier J, Gulyas J, Corrigan A, Martinez V, Craig AG, Taché Y, Vale W, Rivier C. Astressin analogues (corticotropin-releasing factor antagonists) with extended duration of action in the rat. J Med Chem 41: 5012–5019, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Rivier J, Gulyas J, Kirby D, Low W, Perrin MH, Kunitake K, DiGruccio M, Vaughan J, Reubi JC, Waser B, Koerber SC, Martinez V, Wang L, Taché Y, Vale W. Potent and long-acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. J Med Chem 45: 4737–4747, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Rivier J, Rivier C, Vale W. Synthetic competitive antagonists of corticotropin-releasing factor: effect on ACTH secretion in the rat. Science 224: 889–891, 1984. [DOI] [PubMed] [Google Scholar]
- 54.Rivier JE, Kirby DA, Lahrichi SL, Corrigan A, Vale WW, Rivier CL. Constrained corticotropin releasing factor antagonists (astressin analogues) with long duration of action in the rat. J Med Chem 42: 3175–3182, 1999. [DOI] [PubMed] [Google Scholar]
- 55.Rothrock NE, Lutgendorf SK, Kreder KJ, Ratliff T, Zimmerman B. Stress and symptoms in patients with interstitial cystitis: a life stress model. Urology 57: 422–427, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Rouzade-Dominguez ML, Pernar L, Beck S, Valentino RJ. Convergent responses of Barrington’s nucleus neurons to pelvic visceral stimuli in the rat: a juxtacellular labelling study. Eur J Neurosci 18: 3325–3334, 2003. [DOI] [PubMed] [Google Scholar]
- 57.Sasaki M Role of Barrington’s nucleus in micturition. J Comp Neurol 493: 21–26, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Soderholm JD, Perdue MH II. Stress and intestinal barrier function. Am J Physiol Gastrointest Liver Physiol 280: G7–G13, 2001. [DOI] [PubMed] [Google Scholar]
- 59.Stenzel-Poore MP, Cameron VA, Vaughan J, Sawchenko PE, Vale W. Development of Cushing’s syndrome in corticotropin-releasing factor transgenic mice. Endocrinology 130: 3378–3386, 1992. [DOI] [PubMed] [Google Scholar]
- 60.Stenzel-Poore MP, Heinrichs SC, Rivest S, Koob GF, Vale WW. Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J Neurosci 14: 2579–2584, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stenzel P, Kesterson R, Yeung W, Cone RD, Rittenberg MB, Stenzel-Poore MP. Identification of a novel murine receptor for corticotrophin-releasing hormone expressed in the heart. Mol Endocrinol 9: 637–645, 1995. [DOI] [PubMed] [Google Scholar]
- 62.Straub LR, Ripley HS, Wolf S. Disturbances of bladder function associated with emotional states. JAMA 141: 1139–1143, 1949. [DOI] [PubMed] [Google Scholar]
- 63.Sudre EC, de Barros MR, Sudre GN, Schenberg LC. Thresholds of electrically induced defence reaction of the rat: short- and long-term adaptation mechanisms. Behav Brain Res 58: 141–154, 1993. [DOI] [PubMed] [Google Scholar]
- 64.Sutton RE, Koob GF, LeMoal M, Rivier J, Vale W. Corticotropin releasing factor produces behavioural activation in rats. Nature 297: 331–333, 1982. [DOI] [PubMed] [Google Scholar]
- 65.Taché Y, Martinez V, Million M, Wang L. Stress and the gastrointestinal tract. III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am J Physiol Gastrointest Liver Physiol 280: G173–G177, 2001. [DOI] [PubMed] [Google Scholar]
- 66.Taché Y, Million M. Central corticotropin-releasing factor and the hypothalamic-pituitary-adrenal axis in gastrointestinal physiology. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR and Wood J. New York: Academic, 2006, p. 791–816. [Google Scholar]
- 67.Taché Y, Martinez V, Wang L, Million M. CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br J Pharmacol 141: 1321–1330, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taché Y, Perdue MH. Role of peripheral CRF signaling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Motil 16, Suppl 1: 1–6, 2004. [DOI] [PubMed] [Google Scholar]
- 69.Torpy DJ, Papanicolaou DA, Chrousos GP. Sexual dimorphism of the human stress response may be due to estradiol-mediated stimulation of hypothalamic corticotropin-releasing hormone synthesis. J Clin Endocrinol Metab 82: 982, 1997. [DOI] [PubMed] [Google Scholar]
- 70.Tovote P, Meyer M, Ronnenberg A, Ogren SO, Spiess J, Stiedl O. Heart rate dynamics and behavioral responses during acute emotional challenge in corticotropin-releasing factor receptor 1-deficient and corticotropin-releasing factor-overexpressing mice. Neuroscience 134: 1113–1122, 2005. [DOI] [PubMed] [Google Scholar]
- 71.Valentino RJ, Miselis RR, Pavcovich LA. Pontine regulation of pelvic viscera: pharmacological target for pelvic visceral dysfunctions. Trends Pharmacol Sci 20: 253–260, 1999. [DOI] [PubMed] [Google Scholar]
- 72.Van Gaalen MM, Stenzel-Poore MP, Holsboer F, Steckler T. Effects of transgenic overproduction of CRH on anxiety-like behaviour. Eur J Neurosci 15: 2007–2015, 2002. [DOI] [PubMed] [Google Scholar]
- 73.Vincent SR, Satoh K. Corticotropin-releasing factor (CRF) immunoreactivity in the dorsolateral pontine tegmentum: further studies on the micturition reflex system. Brain Res 308: 387–391, 1984. [DOI] [PubMed] [Google Scholar]
- 74.Vita N, Laurent P, Lefort S, Chalon P, Lelias JM, Kaghad M, Le Fur G, Caput D, Ferrara P. Primary structure and functional expression of mouse pituitary and human brain corticotrophin releasing factor receptors. FEBS Lett 335: 1–5, 1993. [DOI] [PubMed] [Google Scholar]
- 75.Wagner M, Perry RP. Characterization of the multigene family encoding the mouse S16 ribosomal protein: strategy for distinguishing an expressed gene from its processed pseudogene counterparts by an analysis of total genomic DNA. Mol Cell Biol 5: 3560–3576, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Williams CL, Burks TF. Stress, opioids, and gastrointestinal transit. In: Hans Selye Symposia on Neuroendocrinology and Stress: Neuropeptides and Stress, edited by Taché Y, Morley J, and Brown MR. New York: Springer-Verlag, 1989, p. 175–187. [Google Scholar]
- 77.Williams CL, Peterson JM, Villar RG, Burks TF. Corticotropin-releasing factor directly mediates colonic responses to stress. Am J Physiol Gastrointest Liver Physiol 253: G582–G586, 1987. [DOI] [PubMed] [Google Scholar]
- 78.Wu EQ, Birnbaum H, Kang YJ, Parece A, Mallett D, Taitel H, Evans RJ. A retrospective claims database analysis to assess patterns of interstitial cystitis diagnosis. Curr Med Res Opin 22: 495–500, 2006. [DOI] [PubMed] [Google Scholar]