Abstract
COVID-19, caused by SARS-CoV-2, is a highly infectious disease, and clinical laboratory detection has played important roles in its diagnosis and in evaluating progression of the disease. Nucleic acid amplification testing or gene sequencing can serve as pathogenic evidence of COVID-19 diagnosing for clinically suspected cases, and dynamic monitoring of specific antibodies (IgM, IgA, and IgG) is an effective complement for false-negative detection of SARS-CoV-2 nucleic acid. Antigen tests to identify SARS-CoV-2 are recommended in the first week of infection, which is associated with high viral loads. Additionally, many clinical laboratory indicators are abnormal as the disease evolves. For example, from moderate to severe and critical cases, leukocytes, neutrophils, and the neutrophil–lymphocyte ratio increase; conversely, lymphocytes decrease progressively but are over activated. LDH, AST, ALT, CK, high-sensitivity troponin I, and urea also increase progressively, and increased D-dimer is an indicator of severe disease and an independent risk factor for death. Severe infection leads to aggravation of inflammation. Inflammatory biomarkers and cytokines, such as CRP, SAA, ferritin, IL-6, and TNF-α, increase gradually. High-risk COVID-19 patients with severe disease, such as the elderly and those with underlying diseases (cardiovascular disease, diabetes, chronic respiratory disease, hypertension, obesity, and cancer), should be monitored dynamically, which will be helpful as an early warning of serious diseases.
Keywords: SARS-CoV-2, COVID-19, Laboratory diagnosis, Biomarker
Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; MERS-CoV, middle east respiratory syndrome coronavirus; ACE, angiotensin-converting enzyme; ACE2, angiotensin-converting enzyme 2; TMPRSS2, transmembrane protease serine type 2; ARDS, acute respiratory distress syndrome; RBD, receptor binding domains; NAAT, nucleic acid amplification testing; WHO, world health organization; PCR, polymerase chain reaction; RT-PCR, reverse transcription polymerase chain reaction; qRT-PCR, real-time quantitative reverse transcription polymerase chain reaction; ORF, open-reading frames; LOD, limit of detection; POCT, point-of-care tests; RT-LAMP, reverse transcription loop-mediated isothermal amplification; BLF, bronchoalveolar lavage fluid; RdRp, RNA-dependent RNA polymerase; LRT, lower respiratory tract; Ag-RDT, Antigen-detecting rapid diagnostic test; ELISA, enzyme-linked immunosorbent assay; CLIA, chemiluminescence-immunoassay; IgM, immunoglobulin M; IgG, immunoglobulin G; IgA, immunoglobulin A; FDA, food and drug administration’s; US, United States; EUA, emergency use authorization; ICU, intensive care unit; WBC, white blood cell; NLR, neutrophil–lymphocyte ratio; PLRs, platelet-to-lymphocyte ratios; LDH, lactate dehydrogenase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CK, creatine kinase; PE, pulmonary embolism; DIC, disseminated intravascular coagulation; FDP, fibrinogen and fibrin degradation products; PT, prothrombin time; APTT, activated partial thromboplastin time; CSS, cytokine storm syndrome; IL-1β, interleukin 1β; IL-2, interleukin 2; IL-2R, interleukin 2R; IL-7, interleukin 7; IL-10, interleukin 10; IL-6, interleukin 6; G-CSF, granulocyte-colony stimulating factor; IFN-α, interferon α; IFN-γ, interferon γ; IP10, induced protein 10; MCP1, monocyte chemoattractant protein 1; MIP-1α, macrophage inflammatory protein-1α; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor; TRAIL, TNF related apoptosis inducing ligand; CRP, C-reactive protein; SAA, serum amyloid A protein; PCT, procalcitonin; RAS, renin-angiotensin system; Ang II, angiotensin II; Ang I, angiotensin I; MasR, Mas receptor; AT2R, AT2 receptor
1. Introduction
Coronavirus disease 2019 (COVID-19), which is caused by SARS-CoV-2, is a severe acute respiratory infectious disease that has led to a global pandemic and brought a major threat to human life and health. Common clinical symptoms include fever, cough, dyspnea, myalgia, and fatigue; less common symptoms are expectoration, headache, hemoptysis, diarrhea, and loss of taste and smell [1], [2], [3]. The main complications included sepsis, respiratory failure acute respiratory distress syndrome (ARDS), acute cardiac injury, acute kidney injury, arrhythmia, gut and liver function abnormalities, shock, and secondary infection [1], [2], [4], [5]. In children, special sporadic symptoms are features of severe Kawasaki-like disease [6], [7], [8], [9].
SARS-CoV-2 infection leads to many abnormal laboratory indicators. Associated biomarkers include hematologic, biochemical, coagulation-fibrinolysis system, and inflammatory indicators. Furthermore, SARS-CoV-2 nucleic acid, antigen, and antibody detection can contribute to an etiological or serological diagnosis of SARS-CoV-2 infection, so clinical laboratory detection has played important roles in the COVID-19 pandemic. This review aims to report the current knowledge regarding clinical laboratory biomarkers of COVID-19, focusing on the diagnosis, evaluation of progression, and early warning of severe disease.
2. Mechanisms of COVID-19 action
Coronaviruses are enveloped viruses containing a positive-sense, single-stranded RNA genome and characteristic crown-like spikes on their surfaces. Coronaviruses are members of the Coronavirinae subfamily of the Coronaviridae family. This subfamily consists of four genera: alphacoronavirus, betacoronavirus, gammacoronavirus, and deltacoronavirus [10]. According to whole-genome sequencing, SARS-CoV-2 belongs to a new type of betacoronavirus [11], [12]. SARS-CoV-2 shares 79.6% and 51.8% sequence identity with severe acute respiratory syndrome coronavirus (SARS-CoV) and middle east respiratory syndrome (MERS-CoV), respectively [13]. In addition, SARS-CoV-2 is 96% identical at the whole-genome level to a bat coronavirus and 80–98% identical to a Malayan pangolin coronavirus[14], [15]. The origin of SARS-CoV-2 remains to be determined. SARS-CoV, MERS-CoV, and SARS-CoV-2 are all betacoronaviruses. These three coronaviruses can cause lower respiratory tract infections and serious inflammation.
The basic mechanism of SARS-CoV-2 infection involves viral binding to the membrane-bound form of angiotensin-converting enzyme 2 (ACE2) [12], [14]. Although sharing a close relationship with SARS-CoV, the RBD of SARS-CoV-2 differs in several key amino acids and has a stronger binding affinity for ACE2, possibly explaining its greater pathogenicity [16]. ACE2, a glycoprotein and metalloprotease, exists in membrane-bound and soluble forms [17]. The membrane-bound form contains a transmembrane domain that anchors its extracellular domain to the plasma membrane; the N-terminal domain is cleaved and secreted into the blood circulation in a soluble free form.
Cell entry of SARS-CoV-2 depends on binding of the S protein to ACE2, after which the S protein is primed by a host cell protease. Transmembrane protease serine type 2 (TMPRSS2), belonging to the type II transmembrane serine protease family, can cleave the coronavirus spike (S) protein [18], [19], [20], [21]. The S protein consists of two subunits: a globular S1 domain at the N-terminal region and a membrane-proximal S2 domain. SARS-CoV-2 utilizes a receptor-binding domain in the S1 domain to bind to ACE2, triggering cleavage of S1 and S2 by TMPRSS2 and membrane fusion for viral entry [22], [23]. As receptors and mediators of virus entry, the routes and target organs of SARS-CoV-2 infection may depend on the expression and distribution of ACE2 and TMPRSS2 [24], [25]. In the airway, ACE2 and TMPRSS2 were both expressed in multiple epithelial cell types, including ciliated cells, secretory cells, and alveolar epithelial type II cells of respiratory organs. Among these cells, the highest expression has been detected in secretory cells and ciliated cells of nasal tissue [25]. In addition to its expression in the respiratory system, ACE2 and TMPRSS2 are coexpressed in the cornea, ileum, heart, kidney, colon, esophagus, brain, gallbladder, common bile duct, and testis [24], [25], [26], with high coexpression in the gallbladder, ileum, and colon [25]. This diverse expression distribution suggests that SARS-CoV-2 infection can not only cause injury to the respiratory system but also to other organs.
The pathophysiological features of severe COVID-19 are dominated by acute lung lesions with diffuse alveolar damage, microvascular thrombosis, and inflammatory infiltrates [27]. Increasing evidence suggests that tissue damage in COVID-19 is partly mediated by an excessive immune response [28], [29]. Indeed, virus-mediated destruction of lung cells induces a wave of local inflammation involving increased secretion of proinflammatory cytokines and chemokines such as IP-10, IL-6, MCP1, and IFN-γ [1], [30], and localized release of cytokines drives recruitment of macrophages, monocytes, and T lymphocytes to the site of infection, promoting a pro-inflammatory feedback loop mediated by IFN-γ [31], [32]. In particular, the massive release of cytokines in response to viral infection can result in a cytokine storm and sepsis-like symptoms that lead to multiorgan failure and even death [30].
3. Laboratory diagnosis of COVID-19
3.1. SARS-CoV-2 nucleic acid
The full-length genome of SARS-CoV-2 is approximately 30 kb long, with an open-reading frame (ORF) at the 5′ terminus. The viral genome consists of six major ORFs accounting for approximately two-thirds of the whole genome. The 3′ terminal gene encodes 4 structural proteins and RNA-dependent RNA polymerase (RdRp). The structural proteins include the E gene encoding the envelope protein, M gene encoding the membrane protein, N gene encoding the nucleocapsid protein, and S gene encoding the spike-like glycoprotein [14]. Rapid and accurate detection of COVID-19 is crucial to control outbreaks. Studies have shown that nucleic acid technologies are generally more accurate than CT scans and serological tests for definitive diagnosis of COVID-19, as they can target and identify the specific gene sequence of SARS-CoV-2. To date, there are two main methods of SARS-CoV-2 nucleic acid detection: gene sequencing and nucleic acid amplification testing (NAAT). Each can provide evidence of an etiological diagnosis [33].
3.1.1. Metagenomic next-generation sequencing (mNGS)
High-throughput sequencing/metagenomic next-generation sequencing (mNGS), which is based on second-generation sequencing technology, does not require culturing, with no preference for pathogen detection. Due to the advantages of mNGS for new pathogen detection, Chinese scientists completed the identification and analysis of the SARS-CoV-2 genome in only five days, which became a key link in epidemic control [34]. Clinical specimens used for SARS-CoV-2 gene sequencing include nasopharynx swabs, sputum, and bronchoalveolar lavage fluid (BLF). The protocol of mNGS is typically divided into five steps: nucleic acid extraction, library construction, gene sequencing, biological information analysis, and interpretation. If the nucleic acid sequence is highly homologous with that of SARS-CoV-2, then the case can be confirmed. The limitations of gene sequencing include a long turn-around time, complex procedure, inadequate standardization, and high personnel qualification criteria regarding detection and data analysis [35]. Currently, DNA sequencing has been carried out only in some medical laboratories (laboratory in a hospital or independent medical laboratory).
Gene sequencing is not only used for etiological diagnosis of COVID-19, but it can also provide information such as pathogen abundance and variation [36]. According to the characteristics of the SARS-CoV-2 genetic cluster, scientists can trace the source of infection, evaluate virus variation, and carry out epidemiological monitoring [37]. For example, an important mutation of the spike protein involves a change of an aspartate (D) at position 614 to a glycine (G). Increased fatality rates correlate positively with the proportion of viruses bearing the G614 mutation on a country by country basis [38]. Recent studies suggested that new variations in Britain have occurred at N501Y of the spike protein, involving 23 separate mutations. The rate of spread of variants can be greater than 70% compared to the normal SARS-CoV-2 virus, but the variants were not found to cause more severe respiratory illness [39].
3.1.2. Manual laboratory-based nucleic acid amplification testing
Molecular NAAT methods are the standard confirmation tests for SARS-CoV-2 infections by the WHO [40]. Among NAAT methods, qRT-PCR is a common and routine approach. In fact, it can be used to detect a variety of clinical specimens, including BLF, fiber bronchoscope brush biopsies, sputum, nasal swabs, pharyngeal swabs, feces, and blood [1], [41], [42]. Kits may include one, two, or three specific primer pairs for SARS-CoV-2 nucleic acids. Specific primers and TaqMan probes are designed according to evolutionarily conserved ORF1ab, ORF1b-nsp14, RdRp1, RdRp2, spike (S), nucleocapsid (N), and envelope (E) [43], [44], [45], [46], [47], [48], [49]. If two SARS-CoV-2 targets meet or exceed the threshold, the result is positive. If only one target is detected, then the sample is recollected and tested; if a positive reaction still appears, the sample will be classified as positive. No detection of any SARS-CoV-2 target is considered a negative result. When the test results are in the “gray zone” (near the thresholds or weakly positive), using another brand kit or resampling and testing again is recommended [50]. The average period of qRT-PCR detection is 3–5 h. The qRT-PCR method can be used for a quick screen of a large number of samples from the requested population.
In addition to routine qRT-PCR for confirmation, other RT-PCR assays have been developed for the identification of SARS-CoV-2. A real-time nested RT-PCR assay has been developed for detecting low-copy-number SARS-CoV-2 with high sensitivity [51], [52]. However, nested RT-PCR can cause laboratory cross-contamination, which may lead to false-positive results [53]. Droplet digital PCR (ddPCR) has also been shown to increase the lower LOD, sensitivity, and accuracy in detecting SARS-CoV-2 [54], [55]. Overall, it is important to ensure result consistency among different molecular laboratories.
Nevertheless, “false negatives” of SARS-CoV-2 nucleic acid detection by the qRT-PCR method have been reported. In some cases, a positive result appeared after multiple sampling tests. Nasopharyngeal or throat swabs may be negative many times, with positive results eventually detected in sputum specimens or respiratory lavage fluid samples [56], [57], [58]. Several factors may affect the nucleic acid detection rate. Because the distribution of viral load varies among clinical samples, nucleic acid detection rates are usually different. For example, BLF specimens show the highest positivity rates (93%), followed by rectal swabs (87.8%), sputum (72%), nasal swabs (63%), fibrobronchoscope brush biopsy (46%), nasopharyngeal swabs (45.5%), pharyngeal swabs (32%), and feces (29%); blood has the lowest detection rate (1%) [41]. In general, lower respiratory tract specimens, such as BLF and sputum, have higher detection rates and are the recommended clinical samples. However, collection of BLF samples is somewhat invasive, and most patients have dry cough without sputum, which limits the use of BLF and sputum samples [59], [60]. Nasopharyngeal swabs, a widely used specimen, are easy to collect but have a relatively low detection rate. Furthermore, RNA fragment damage occurring during the process of SARS-CoV-2 nucleic acid extraction may cause nucleic acid loss and decrease the detection rate. Novel mutant strains of SARS-CoV-2 may also cause false negatives. For example, spike gene target failure for the qRT-PCR assay (ThermoFisher) was reported when testing viruses with H69-V70 spike deletion in France [61]. In the early stage of the COVID-19 epidemic, a large amount of PCR tests was produced, though there were some defects, such as contamination of primers/probes of SARS-CoV-2 targets [62]. Moreover, qRT-PCR technology requires professionally trained staff. Nonetheless, various measures can improve the nucleic acid detection rate, such as multiple sampling tests, adding or altering target genes according to virus variation, valid kit evaluation, external quality assessment, and laboratory staff training.
3.1.3. Rapid and point-of-care test-based nucleic acid amplification testing
Another important detection method based on NAAT is reverse transcription loop-mediated isothermal amplification (RT-LAMP) [63], [64], [65], one of the point-of-care tests (POCTs) for nucleic acid amplification. RT-LAMP was developed as a rapid, accurate, and reliable technique to amplify a target sequence at a single reaction temperature rather than the thermal cycling needed for qRT-PCR [66]. Indeed, RT-LAMP is performed in one step at 63 °C isothermal conditions, and by targeting the ORF1ab, spike (S), envelope (E), or/and N genes of SARS-CoV-2, results are obtained within 15–40 min [63], [64], [65], [67], [68]. The advantage of the LAMP method is that the amount of DNA produced is much higher than that in qRT-PCR, and a positive test result can even be seen visually without another machine. Two studies have shown evidence that RT-LAMP methods have sensitivity ranging from 89.9% to 91.4% compared to qRT-PCR when targeting the ORF1ab and nucleocapsid (N) genes [69], [70]. Additionally, the detection limit of RT-LAMP is 100–500 copies/mL, which is almost the same as that of qRT-PCR kits [64], [65]. Importantly, studies have shown that RT-LAMP analysis is more specific than qRT-PCR because it uses five or six primers to identify different regions on the target nucleic acid [64], [65]. Hence, the RT-LAMP method has wide application in SARS-CoV-2 diagnosis due to its relatively simple operation and low technical requirements for laboratory staff.
Many other rapid and point-of-care nucleic acid tests have also been used for COVID-19 diagnosis. For example, nanoparticle-based nucleic acid amplification systems have been introduced [71], [72]. However, the pretreatment steps are more complex than those of qRT-PCR. Visseaux et al. evaluated another POCT, namely, QIAstat-Dx Respiratory SARS-CoV-2 Panel, which showed sensitivity comparable to that of qRT-PCR, with an LOD of 1000 copies/mL [73]. Other kits, such as the point-of-care assays Abbott ID NOW test, Abbott m 2000 tests, Diasorin Simplex, and Xpert Xpress, reportedly have different performances [74], [75], [76], [77], [78].
3.2. SARS-CoV-2 antibody
Although nucleotide acid-based methods are recommended for assessing SARS-CoV-2 infection, they have a relatively low detection rate [79]. Therefore, many experts suggest using specific antibody detection as a supplement for nucleic acid detection. ELISA, lateral flow-based point-of-care tests, and chemiluminescence-immunoassay (CLIA) methods are commonly used for the detection of SARS-CoV-2 [80]. Many studies have reported that specific antibodies against IgG, IgM, and IgA have good sensitivity and specificity for SARS-CoV-2 detection. For example, the clinical sensitivities of serum IgM and IgG antibodies against SARS-CoV-2 E and N protein antigens are 70.24% and 96.10%, respectively, with clinical specificities of 96.20% and 92.41%, respectively, by the CLIA method. The diagnostic agreement of SARS-CoV-2 can reach 88.03% with the combined detection of antibodies and nucleic acids [81]. Another study reported sensitivities of RBD-specific IgA, IgM, and IgG of 98.6%, 96.8%, and 96.8%, with specificities of 98.1%, 92.3%, and 99.8%, respectively [82].
Overall, the seroconversion time differs with the type of antibodies; the first is total antibodies, followed by IgM and IgG. In a study based on 173 COVID-19 patients, the median seroconversion for total antibodies, IgM, and IgG directed against an RBD-specific protein occurred on day 11, day 12, and day 14, respectively. The detection rate of antibodies was <40% among patients within one week after onset and rapidly increased to 100.0% (total antibody), 94.3% (IgM), and 79.8% (IgG) after two weeks. In contrast, the detection rate of RNA decreased from 66.7% before day 7 to 45.5% during days 15–39 [83]. Therefore, the detection rate of antibodies is lower than that of nucleic acid in the first week but gradually increases; in particular, the detection rate of total antibodies was up to 100% two weeks later. In contrast, the detection rate of nucleic acids decreases gradually after one week. Therefore, combined detection of antibodies and nucleic acids has complementarity and improves the detection rate. IgG and IgM antibodies are typically present at different times, and IgG antibodies last longer. When the SARS-CoV-2 nucleic acid test becomes negative, the positivity rate of IgM decreases from 88% to 53%, whereas that for IgG changes slightly [84]. Therefore, in addition to utility in diagnosis, the combination and dynamic monitoring of IgG and IgM may reflect different stages of SARS-CoV-2 infection.
SARS-CoV-2 antibody detection has high sensitivity but is inevitably accompanied by false positives, and various factors influence antibody detection. For instance, some weak positive results near the cutoff value are usually false positives. Exogenous, including specimen hemolysis, long storage time, and incomplete coagulation, or endogenous, including rheumatoid factor, complement, heterophilic antibodies, and anti-mouse immunoglobulin antibodies induced by the use of mouse antibodies for treatment or diagnosis, interference in specimens may also cause false positives [85]. In general, serological tests alone are not used as diagnostic evidence but should be combined with epidemiological history, clinical manifestations, and basic diseases of the patient. If a case is clinically suspected COVID-19 but the nucleic acid test is negative or the patient is in the recovery period, specific antibody tests are recommended for diagnosis [33], [40].
3.3. SARS-CoV-2 antigen
SARS-CoV-2 consists of multiple virus-encoded proteins, including S, N, E, and M, and the N or S proteins are often used as antigens [86], [87], [88], [89]. The Food and Drug Administration (FDA) of the US has granted emergency use authorization (EUA) for antigen tests that can identify SARS-CoV-2 infection [90]. Ag-RDTs, which directly detect SARS-CoV-2 antigens, produced by the replicating virus in respiratory secretions, are useful for detecting active COVID-19 infection [91]. Most Ag-RDT methods are lateral flow immunoassays (LFIs), which are simple to use and typically completed within 30 min. However, when comparing to NAATs, as there is no amplification of the target, antigen tests for SARS-CoV-2 are usually less sensitive than nucleic acid tests [91]. Most Ag-RDTs of SARS-CoV-2 require nasopharyngeal samples. Ag-RDTs will often be positive when viral loads are highest and patients most infectious, typically 1–3 days before the onset of symptoms and during the 5–7 days after onset, and then will become negative as the infection clears up and the patient recovers [91]. Therefore, Ag-RDTs perform best in the early stages (first week) of SARS-CoV-2 infection, associated with a higher viral load [90], [92].
Ag-RDTs of SARS-CoV-2 are simple and rapid, but the sensitivity and specificity vary greatly. The clinical performances of six molecular diagnostic tests and one rapid antigen immunochromatographic assay for SARS-CoV-2 have been evaluated for the diagnosis of COVID-19 using self-collected saliva. Of the 103 samples, the viral RNA detection rate was 50.5–81.6% by molecular diagnostic tests, but the antigen detection rate was only 11.7% by Ag-RDTs [63]. Scohy et al. reported that among 106 nasopharyngeal swab samples with qRT-PCR test positivity, only 32 were detected by Ag-RDTs, with an overall sensitivity of 30.2% [93]. Regardless, Lorena Porte et al. evaluated another Ag-RDT test for SARS-CoV-2 in respiratory samples. Among 82 nasopharyngeal and oropharyngeal swab samples with RT-PCR positivity, the sensitivity and specificity of Ag-RDTs were 93.9% and 100%, respectively, with a diagnostic accuracy of 96.1% and a Kappa coefficient of 0.9 [92]. In addition, Mavrikou et al. developed a portable, rapid cell-based biosensor for detecting the SARS-CoV-2 S1 protein, providing results in only 3 min, with a detection limit of 1 fg/mL [94]. Therefore, further investigations are needed to explore the best antigen targets and detection methods.
Because of the limitations of antigen detection and the large variation between different reports, it may be necessary to confirm a rapid antigen test result with a nucleic acid test, especially when the result of the antigen test is inconsistent with the clinical manifestations [90]. The minimum performance requirements of SARS-CoV-2 Ag-RDTs are ≥80% sensitivity and ≥97% specificity compared to the NAAT reference assay, and they can be used to confirm SARS-CoV-2 infection [95]. Nevertheless, the use of Ag-RDTs is not recommended in settings or populations with a low expected prevalence of COVID-19 [91].
Laboratory biomarkers related to the diagnosis of COVID-19 include nucleic acid, antibody, and antigen detection. NAAT, such as qRT-PCR, is the most commonly used and preferred method, though antibody detection is more advantageous in the recovery period. The antigen detection rate is high in the early stage. The characteristics of each biomarker are summarized in Table 1 , including main specimens, advantages, and disadvantages.
Table 1.
Laboratory biomarkers in COVID-19 diagnosis.
| Classification | Main specimens | Advantages | Disadvantages |
|---|---|---|---|
| Gene sequencing | nasopharynx swab sputum BLF |
1. Etiological diagnosis of COVID-19 2. Identification of novel pathogens 3. Simultaneous detection of multiple pathogens 4. Tracing and variation analysis of virus strains |
1. Long turn-around time(3–5 d) 2. Sophisticated instruments 3. Skillful laboratory staff |
| qRT-PCR | nasopharynx swab sputum BLF rectal swabs |
1. Manual laboratory-based NAAT, common etiological diagnosis 2. Short turn-around time(3–5h) 3. The highest detection rate was in the first week after onset and then decreased gradually |
1.False-negative |
| RT-LAMP | nasopharynx swab sputum BLF |
1. Rapid and point-of-care based NAAT, etiological diagnosis 2. Short turn-around time (1–2h) 3. Thermostatic, sensitive, and specific 4. The highest detection rate was in the first week after onset and then decreased gradually |
1. False-positive |
| antibody |
Blood Serum Plasma |
1. Serological diagnosis; More secure, low risk of infection 2. Short turn-around time (1–4h) 3. The detection rate increased gradually after onset and reached a peak (nearly 100%) two weeks later. The first seroconversion time was total antibody, followed by IgA /IgM and the last was IgG. The detection rate in the convalescence stage was higher than that in the acute stage. |
1. False-positive 2. Low positive rate in the early infection |
| Ag-RDT | Nasopharynx swab Oropharyngeal swabs |
1. Rapid and point-of-care test of antigen 2. Easy to perform and short turn-around time(<30 min) 3. The high detection rate was in the early stages of infection (1–3 days before the onset and the first week after the onset) |
1. Less sensitive 2. Suitable only in high expected prevalence setting 3. Large variation of different assays |
3.4. Laboratory diagnosis process of COVID-19
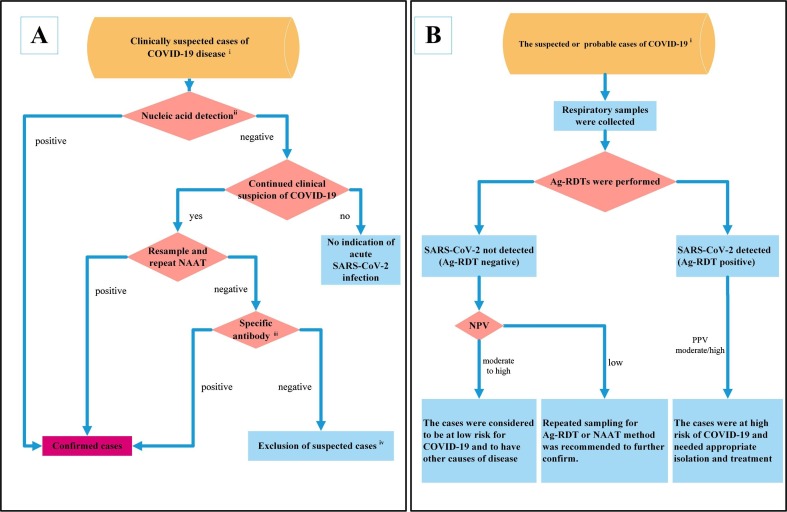
Clinical criteria for COVID-19 generally include related respiratory system symptoms, typical radiological signs, a normal or decreased WBC/lymphocyte count in the early stage, anorexia/nausea/vomiting, diarrhea, an altered mental status, and other symptoms [33], [96]. When the patients meet the clinical and epidemiological criteria of COVID-19, the cases are clinically suspected cases. According to the diagnosis and treatment guide of COVID-19 from the WHO and China [33], [40], nucleic acid testing is recommended first for a clinically suspected case to confirm the disease. The detailed laboratory diagnosis process is illustrated in Fig. 1 . A. Gene sequencing and NAAT are two methods used for nucleic acid detection. If the virus gene sequence is highly homologous with that of SARS-CoV-2 or an NAAT (such as qRT-PCR) is positive, the patient will be diagnosed with COVID-19. When nucleic acid detection is negative and the patient no longer has clinical characteristics of COVID-19, acute infection can be excluded. When the patient still has clinical characteristics of COVID-19, resampling and repeating NAAT are advised. When restesting by NAAT is positive, the case is confirmed; when restesting by NAAT is negative, specific antibody tests are advised for further diagnosis. When specific antibodies are positive, suspected cases can also be confirmed; if not, the suspected case can be ruled out.
Fig. 1.
Laboratory diagnosis flowchart of COVID-19. A: Diagnostic roles of nucleic acids and antibodies in COVID-19. i: Clinically suspected cases need to meet the epidemiological history and clinical manifestations of COVID-19. ii: Nucleic acid detection generally includes two methods: gene sequencing and NAAT. Any one of them can be used for the detection of SARS-CoV-2. iii: Specific antibody (IgM and IgG) detection is recommended in both the acute phase and convalescent phase to observe seroconversion or an increase in antibody titer. iv: Exclusion of suspected cases needs to meet criteria for SARS-CoV-2 nucleic acid and antibody negativity. B: Potential diagnostic roles of Ag-RDTs in COVID-19. With high expected prevalence, the WHO recommends Ag-RDTs to identify SARS-CoV-2 infection in the early stage when NAAT is unavailable or not sufficient. i: A case needs to meet the definition of suspected or probable COVID-19 provided by the WHO. NPV: negative predictive value; PPV: positive predictive value.
Recommendations regarding antibody testing in China are almost the same as those by the WHO [33], [40]. Both IgM and IgG antibody detection is recommended in the acute phase and in the convalescent phase and can be used to assess seroconversion or a rise in antibody titer. These two point detections can be used retrospectively to determine whether the individual has COVID-19, especially when the infection cannot be detected by NAAT. The determination of specific antibody positivity needs to meet one of the three conditions: 1. both specific IgM and IgG antibodies against SARS-CoV-2 are detectable in serum; 2. IgG antibodies become positive; 3. IgG antibodies reach a titer of at least a 4-fold increase during the convalescence phase compared with the acute phase [33]. The specific rules for the exclusion of suspected cases are as follows: SARS-CoV-2 nucleic acid is negative two consecutive times at least 24 h apart, and specific IgM and IgG of SAG-CoV-2 are still negative 7 days after onset. Thus, suspected COVID-19 can be excluded [33], [97].
In a population with a high expected prevalence of COVID-19 but where NAAT is unavailable or not sufficient, the health system may be overburdened, leading to NAAT turnaround times of >48–72 h. The WHO recommends Ag-RDTs to identify SARS-CoV-2 infection for clinically suspected or probable cases of COVID-19 [91], [95], [96]. When Ag-RDTs are positive, with a moderate or high positive predictive value, the patient is considered to be at high risk for COVID-19 and needs appropriate isolation and treatment. When Ag-RDTs are negative, with a moderate to high negative predictive value, patients are considered to be at low risk for COVID-19 and to have other causes of disease. With a low negative predictive value, repeated sampling for Ag-RDT or NAAT method is recommended for confirmation (Fig. 1. B) [91], [95].
4. Biomarkers associated with COVID-19 progression
COVID-19 is generally divided into mild, moderate, severe, and critical classifications based on respiratory symptoms, pulmonary imaging, and the need for mechanical ventilation assistance [33]. Statistical data released by the WHO show that approximately 82% of COVID-19 cases are mild, 15% severe and 3% critical. In total, 19.6% of all patients may develop acute respiratory distress syndrome (ARDS), though the incidence of ARDS increases to 50% in severe and critical patients. The population at high risk for severe and critical illness includes elderly individuals over 65 years old, immunodeficient patients, late-pregnancy and perinatal women, heavy smokers, and those with underlying diseases. Underlying diseases mainly include cardiovascular disease, diabetes, chronic respiratory disease, hypertension, chronic liver and kidney disease, obesity, and cancer [2], [33], [98], [99]. These high-risk factors are often associated with adverse outcomes and more mortality. As the disease evolves from moderate to severe and critical, SARS-CoV-2 infection leads to many abnormal changes in laboratory biomarkers, generally including hematologic, biochemical, coagulation-fibrinolysis system, and inflammatory biomarkers.
4.1. Hematologic biomarkers
Hematologic biomarkers include basic blood cell count and related indicators. The WBC count is normal or decreased in approximately 80% of patients, and 72.3% of the patients develop lymphocytopenia, consistent with the basic characteristics of viral infection [100]. However, the WBC count in severe cases is higher than that in moderate cases, with critical cases being highest. Compared with moderate cases, patients with severe COVID-19 have a significantly reduced lymphocyte count [101], [102]. Peripheral blood leukocytes and neutrophils are higher in patients with intensive care unit (ICU) treatment than in non-ICU patients, and lymphocytes decrease progressively in fatal cases from onset [2], [4]. An increase in neutrophils and the neutrophil-to-lymphocyte ratio (NLR) indicates the occurrence of severe or critical disease, with poor prognosis [103]. The percentages of monocytes, eosinophils, and basophils become sharply reduced in severe cases [103], [104]. As the proportion of eosinophils continues to decrease significantly before the appearance of corresponding clinical symptoms in fatal inpatients, the continued decrease in eosinophils is an indicator of a poor prognosis [105].
There are some conflicting results regarding platelets in COVID-19. More studies have found that thrombocytopenia is common in patients with severe COVID-19 than in those with nonsevere disease [2], [106]; or that the platelet count is lower in nonsurvivors than in survivors [2], [107], [108], [109]. However, some studies noted no obvious difference in median platelet count between hospitalized COVID-19 patients in the ICU versus non-ICU patients [4], [110]. For example, Wu et al. did not find a significant difference in platelet count between survivors versus nonsurvivors among COVID-19 cases complicated with ARDS [111]. On the other hand, in a retrospective analysis of 30 COVID-19 cases, patients with higher platelet counts and PLRs at platelet peak had longer average hospitalization durations [112]. Decreased platelet count may indicate platelet consumption from thrombus formation, whereas an increased platelet count suggests stimulated production of megakaryocytes and increased platelet synthesis due to inflammation [113].
Overall, obvious changes in hematologic biomarkers include continued lymphocytopenia and a decrease in monocytes, basophils, and eosinophils but a significant increase in leukocytes, neutrophils, and NLR in severe and critical COVID-19. These basic biomarkers can be helpful for early warning and identification of severe disease [114], [115].
4.2. Biochemical markers
Many biochemical markers are abnormal in COVID-19 patients because of corresponding organ damage, especially cardiac, liver, and kidney injuries. In the analysis of a large number of 1099 COVID-19 inpatients, the proportion of elevated biochemical markers gradually increased from nonsevere to severe to critical cases, and the most obvious change was for LDH, followed by AST, ALT, CK, and creatinine [106]. Chen et al. also observed markedly higher concentrations of LDH, ALT, AST, creatinine, CK, cardiac troponin I, and N-terminal pro-brain natriuretic peptide in nonsurvivors than in recovered patients [116]. Levels of LDH, hypersensitivity myocardial troponin I, urea, and creatinine increase progressively in fatal cases from onset [2], [4], and hypoalbuminemia often occurs in severe cases [1], [105]. Other biochemical markers, such as the oxygenation index and lactic acid, are reported to be associated with severe illness. For example, compared with surviving COVID-19 patients with cardiovascular disease, the oxygenation index was lower and lactic acid significantly higher in nonsurvivors [117]. Therefore, continuous elevation of these common biochemical markers, such as LDH, CK, AST, ALT, urea, and creatinine, indicates a high risk of severe illness and poor prognosis.
4.3. Biomarkers associated with coagulation and fibrinolysis system
Due to excessive inflammation and hypoxia, some COVID-19 patients present coagulation and fibrinolysis abnormalities, with increased thrombotic complications such as pulmonary embolism (PE) and disseminated intravascular coagulation (DIC) [2], [4], [106], [118]. Furthermore, coagulopathy in COVID-19 patients is associated with an increased risk of death [119]. Accordingly, abnormal coagulation and fibrinolysis biomarkers are associated with a poor prognosis in COVID-19 patients. The most typical finding is an increased D-dimer concentration. In a retrospective, clinical study, of nonsevere, severe, and critical COVID-19, the elevated proportion of D-dimer was found to gradually increase [106], and the elevated proportions of D-dimer were higher in nonsurvivors than survivors [2], [4]. Moreover, D-dimer level greater than 1 μg/mL is an independent risk factor for death among inpatients [2], [4], and increased D-dimer is predictive of coagulation-associated complications, critical illness, and death during hospitalization [120].
In addition to increases in D-dimer, other coagulation and fibrinolysis biomarkers are abnormal. For example, markedly elevated FDP was detected in nonsurvivors; prolonged APTT and PT were also observed in nonsurvivors compared to survivors on admission [119]. Furthermore, fibrinogen is higher in patients with than in those without thrombotic complications [120]. In severe COVID-19, expression of tissue factor and secretion of von Willebrand factor cause endothelial cell injury. Free thrombin activates platelets and stimulates fibrinolysis, possibly leading to high concentrations of D-dimer, FDP and fibrinogen and prolonged APTT/PT. Among these biomarkers, changes in D-dimer are the most obvious. These changes suggest coagulation activation and secondary hyperfibrinolysis conditions in severe cases[119], [121].
4.4. Biomarkers associated with the inflammatory storm
Most COVID-19 patients exhibit mild symptoms at the early stage; later, the condition of a small number of cases unexpectedly worsens and even leads to death, which is partly associated with the “inflammatory storm” or “cytokine storm syndrome (CSS) ” [122]. With a serious SARS-CoV-2 infection, the host immune system becomes overactivated and even out of control. Many cytokines are secreted to eliminate the virus; however, serious side effects cause severe damage to respiratory cells, resulting in lung injury and symptoms such as lung inflammation, edema, hyaline thrombi, and even other organ damage [105], [122]. For example, in a fatal COVID-19 case involving ARDS, CD4+ and CD8+ T cell counts in peripheral blood were substantially reduced, even though their status was hyperactivated, as manifested by high concentrations of highly proinflammatory CCR4 + CCR6 + Th17 in CD4+ cells and cytotoxic granules in CD8+ T cells, which indicated that the patient had excessive inflammatory injury [28].
Additionally, COVID-19 patients, either with or without ICU treatment, have increased levels of cytokines or chemokines compared with healthy individuals. These cytokines include IL-1β, IL-7, IL-10, G-CSF, IFN-γ, IP10, MCP1, MIP-1α, TNF-α and VEGF. Compared with patients in the non-ICU group, higher levels of IL-2, IL-7, IL-10, G-CSF, IP10, MCP1, MIP-1α and TNF-α were detected in the ICU group [1]. IL-6 and IL-2R levels are significantly higher in severe cases than in moderate cases and higher in nonsurvivors than in survivors [2], [101], [104], and concentrations of some cytokines are reported to correlate with the SARS-CoV-2 viral load. For example, IFN-α, IFN-γ, TNF-α, and TRAIL correlated positively with viral load in COVID-19 patients with both moderate and severe disease [29].
When SARS-CoV-2 infection caused an “inflammatory storm”, not only associated cytokines rise sharply, but some inflammatory biomarkers also increase, such as CRP, serum ferritin, SAA, and PCT. Compared with the moderate COVID-19 group, levels of ferritin, CRP, SAA, and PCT were significantly increased in severe and critical groups [101], [104], [123]. The specificity and sensitivity of the combined detection of CRP, SAA, and WBC can reach 78.67% and 100%, respectively, in severe and critical cases [102]. Levels of CRP, IL-6, SAA and PCT in serum were found to be in the following order: critical group > severe group > moderate group > mild group. These indicators were noted to increase significantly in the nonsurvival group compared with the survival group [124], [125]. Accordingly, increases in these inflammatory biomarkers indicate a high risk of severe illness and poor prognosis.
4.5. Biomarkers associated the renin-angiotensin system (RAS)
The RAS plays an important role in regulating electrolyte balance and blood pressure via two pathways: the ACE/Ang II/AT1R pathway and the ACE2/Ang (1–7)/Mas receptor pathway [126]. To maintain normal function, the activity of the two axes is in a dynamic equilibrium state. The main roles of ACE2 are to physiologically counterbalance angiotensin-converting enzyme (ACE) and regulate angiotensin II (Ang II). ACE2 converts Ang I to Ang-(1–9), as well as Ang II to Ang-(1–7), which is protective in cardiovascular tissue [127].
As ACE2 is also the receptor for SARS-CoV-2, SARS-CoV-2 infection may result in changes in RAS-related biomarker concentrations in plasma. There are limited data regarding levels of RAS biomarkers in COVID-19 patients. Liu et al. observed Ang II levels in the plasma of 12 COVID-19 patients to be markedly elevated and linearly associated with viral load and lung injury [128]. In contrast, Henry et al. did not observe elevated plasma Ang II in 30 COVID-19 cases, with median plasma Ang II and aldosterone concentrations being nearly equal between COVID-19 patients and healthy controls [129].
In general, higher levels of plasma ACE2 are associated with a greater risk of death, stroke, myocardial infarction, diabetes, and heart failure [130], and elevated plasma ACE2 is likely an insufficient compensatory response to overactive RAS activity rather than a cause [131], [132]. Additionally, membrane-bound ACE2 may exhibit abnormal shedding in SARS-CoV-2 infection; thus, levels of plasma ACE2 might be elevated. However, there are no data to date regarding levels of ACE2 in plasma from COVID-19 patients. Therefore, we are unable to determine a correlation between SARS-CoV-2 infection and related changes in RAS biomarker concentrations.
The change trends of these biomarkers related to disease progression and prognosis in nonsevere to severe and critical cases and between survivors and nonsurvivors are summarized in Table 2 .
Table 2.
Common biomarkers associated with COVID-19 disease progression and prognosis.
| Biomarkers classification | Disease progression |
Disease prognosis |
References | ||||
|---|---|---|---|---|---|---|---|
| Non-severe patients | Severe patients | Critical patients | Survivors | Non-survivors | |||
| Hematologic | Lymphocytes | N/↓ | ↓↓ | ↓↓↓ | N/↓ | N/↓↓ | [2], [4], [106] |
| WBC | N/↓/↑ | N/↓↓/↑ | N/↓/↑↑ | N/↓/↑ | N/↓/↑/↑↑ | ||
| Neutrophil | N# | N/↑* | |||||
| Biochemical | LDH | N/↑ | N/↑/↑↑ | ↑/↑↑/↑↑↑ | N/↑ | ↑↑↑ | [2], [4], [106] |
| AST | N/↑ | N/↑ | N/↑↑ | ||||
| ALT | N/↑ | N/↑ | N/↑↑ | N/↑ | N/↑↑ | ||
| CK | N/↑ | N/↑ | N/↑↑ | N/↑ | N/↑↑ | ||
| Creatinine | N | N | N/↑ | N | N/↑ | ||
| cardiac troponin I | N# | N/↑* | N | N/↑↑ | |||
| urea | N# | N/↑* | |||||
| albumin | N/↓ | ↓↓ | |||||
| Coagulation and fibrinolysis | D-dimer | N/↑ | N/↑↑ | N/↑↑↑ | N/↑ | ↑/↑↑/↑↑↑ | [2], [106], [119] |
| FDP | N | N/↑/↑↑ | |||||
| fibrinogen | N/↑ | N/↑/↑↑ | |||||
| PT | N | N/↑ | |||||
| APTT | N | N/↑ | |||||
| Inflammation | CRP | N/↑ | N/↑/↑↑ | ↑/↑↑/↑↑↑ | ↑↑ | ↑↑↑ | [2], [104], [106], [123], [125] |
| SAA | N/↑/↑↑ | N/↑/↑↑/↑↑↑ | ↑/↑↑/↑↑↑ | ↑↑ | ↑↑↑ | ||
| PCT | N/↑ | N/↑/↑↑ | N/↑ | N/↑/↑↑/↑↑↑ | |||
| Serum ferritin | ↑/↑↑ | ↑↑/↑↑↑ | N/↑/↑↑ | ↑↑/↑↑↑ | |||
| IL-6 | N/↑ | ↑/↑↑ | N/↑ | ↑/↑↑ | |||
| IL-2R | N/↑ | N/↑/↑↑ | |||||
| TNF-α | N/↑ | N/↑/↑↑ | |||||
(N: normal; ↑: mild increase; ↑↑: moderate increase; ↑↑↑: severe increase; ↓: mild decrease; ↓↓: moderate decrease; ↓↓↓: severe decrease; #: not ICU patients; *: ICU patients).
5. Summary
Laboratory detection has played irreplaceable roles in the diagnosis and evaluation of COVID-19. SARS-CoV-2 nucleic acid and specific antibodies can be used to confirm and exclude suspected cases. A suspected case can be confirmed if etiological or serological evidence is present. If both nucleic acids and antibodies against SARS-CoV-2 are negative, SARS-CoV-2 infection can be ruled out. SARS-CoV-2 nucleic acid detection can also be used to confirm asymptomatic infections. Overall, the sensitivity of SARS-CoV-2 IgG is higher than that of IgM, but the specificity of IgM is better. If NAAT is unavailable or limited in high expected prevalence of COVID-19, Ag-RDTs are generally recommended for use in the early stages of SARS-CoV-2 infection. Regardless, nucleic acid detection, especially molecular NAAT methods such as qRT-PCR, is the preferred and standard approach for the diagnosis of COVID-19.
In addition to COVID-19 diagnosis, laboratory tests are also used for assessing the progression and prognosis of the disease. Significant changes in many biomarkers, such as decreased lymphocytes, increased D-dimer, and elevated biochemical and inflammatory markers, are found in patients with severe COVID-19. Inflammation biomarkers mainly include C-reactive protein, serum amyloid A, iron proteins, and procalcitonin. Elevated LDH, AST, CK, high-sensitivity troponin I, and urea are common biochemical markers in patients with severe illness. When these biomarkers continue to rise dynamically, they serve as early warning signals of severe disease and poor prognosis. In addition, detection of cytokines, such as IL-2, IL-6, IL-7, IL-10, IP10, and TNF-α, is recommended to assess whether there is an excessive immune response. More attention needs to be paid to COVID-19 patients with a high risk of severe illness. When warning signals of severe illness appear, appropriate treatment measures should be taken in a timely manner to reduce the occurrence of severe disease. Laboratory detection will provide better assistance for the early diagnosis of COVID-19 and for assessing disease progression.
Disclosure statement
The authors report no conflict of interest.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81703547) and Shandong Medicine and Health Science Technology Development plan (No.2016WS0479).
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2019;395(2020):497–506. doi: 10.1016/S0140-6736(20)30183-5. https://www.ncbi.nlm.nih.gov/pubmed/31986264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gautier J., Ravussin Y. A New Symptom of COVID-19: Loss of Taste and Smell. Obesity (Silver Spring) 2020;28(5):848. doi: 10.1002/oby.22809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Clinical P.Z. Characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2019 doi: 10.1001/jama.2020.1585. https://www.ncbi.nlm.nih.gov/pubmed/32031570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2019;395(2020):507–513. doi: 10.1016/S0140-6736(20)30211-7. https://www.ncbi.nlm.nih.gov/pubmed/32007143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M., Bonanomi E., D'Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viner R.M., Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395(10239):1741–1743. doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones V.G., Mills M., Suarez D., Hogan C.A., Yeh D., Segal J.B., Nguyen E.L., Barsh G.R., Maskatia S., Mathew R. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. 2020;10(6):537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 9.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. China Novel Coronavirus I Research T. A Novel Coronavirus from Patients with Pneumonia in China. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., Zhong W., Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. https://www.ncbi.nlm.nih.gov/pubmed/32007145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam T.-Y., Jia N., Zhang Y.-W., Shum M.-H., Jiang J.-F., Zhu H.-C., Tong Y.-G., Shi Y.-X., Ni X.-B., Liao Y.-S., Li W.-J., Jiang B.-G., Wei W., Yuan T.-T., Zheng K., Cui X.-M., Li J., Pei G.-Q., Qiang X., Cheung W.-M., Li L.-F., Sun F.-F., Qin S., Huang J.-C., Leung G.M., Holmes E.C., Hu Y.-L., Guan Y., Cao W.-C. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020;583(7815):282–285. doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- 16.Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J., Sheng J., Quan L., Xia Z., Tan W., Cheng G., Jiang T. Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China. Cell Host Microbe. 2020;27(3):325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R.E., Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. https://www.ncbi.nlm.nih.gov/pubmed/10969042. [DOI] [PubMed] [Google Scholar]
- 18.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwata-Yoshikawa N., Okamura T., Shimizu Y., Hasegawa H., Takeda M., Nagata N., Gallagher T. TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection. J Virol. 2019;93(6) doi: 10.1128/JVI.01815-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pohlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88(2):1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen L.W., Mao H.J., Wu Y.L., Tanaka Y., Zhang W. TMPRSS2: A potential target for treatment of influenza virus and coronavirus infections. Biochimie. 2017;142:1–10. doi: 10.1016/j.biochi.2017.07.016. https://www.ncbi.nlm.nih.gov/pubmed/28778717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens TS., Herrler G., Wu NH., Nitsche A., Muller MA., Drosten C., Pohlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. https://www.ncbi.nlm.nih.gov/pubmed/32142651 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. https://www.ncbi.nlm.nih.gov/pubmed/32155444 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong M., Zhang J., Ma X., Tan J., Chen L., Liu S., Xin Y., Zhuang L. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110678. https://www.ncbi.nlm.nih.gov/pubmed/32861070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sungnak W., Huang N., Becavin C., Berg M., Queen R., Litvinukova M., Talavera-Lopez C., Maatz H., Reichart D., Sampaziotis F., Worlock K.B., Yoshida M., Barnes J.L., Network H.C.A.L.B. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. https://www.ncbi.nlm.nih.gov/pubmed/32327758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carsana L., Sonzogni A., Nasr A., Rossi R.S., Pellegrinelli A., Zerbi P., Rech R., Colombo R., Antinori S., Corbellino M., Galli M., Catena E., Tosoni A., Gianatti A., Nebuloni M. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20(10):1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.-S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucas C., Wong P., Klein J., Castro T.B.R., Silva J., Sundaram M., Ellingson M.K., Mao T., Oh J.E., Israelow B., Takahashi T., Tokuyama M., Lu P., Venkataraman A., Park A., Mohanty S., Wang H., Wyllie A.L., Vogels C.B.F., Earnest R., Lapidus S., Ott I.M., Moore A.J., Muenker M.C., Fournier J.B., Campbell M., Odio C.D., Casanovas-Massana A., Yale I.T., Herbst R., Shaw A.C., Medzhitov R., Schulz W.L., Grubaugh N.D., Dela Cruz C., Farhadian S., Ko A.I., Omer S.B., Iwasaki A. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–469. doi: 10.1038/s41586-020-2588-y. https://www.ncbi.nlm.nih.gov/pubmed/32717743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang B., Zhou X., Qiu Y., Song Y., Feng F., Feng J., Song Q., Jia Q., Wang J., Jin X. Clinical characteristics of 82 cases of death from COVID-19. PLoS ONE. 2020;15(7):e0235458. doi: 10.1371/journal.pone.0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saksena S., Chattopadhyay P. Illuminating the immunopathology of SARS-CoV-2. Cytometry B Clin Cytom. 2021;100:33–41. doi: 10.1002/cyto.b.21988. https://www.ncbi.nlm.nih.gov/pubmed/33394568. [DOI] [PubMed] [Google Scholar]
- 33.National Health Commission of the People’s Republic of China & National Administration of Traditional Chinese Medicine. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 8). (2020). http://www.nhc.gov.cn/xcs/zhengcwj/202008/0a7bdf12bd4b46e5bd28ca7f9a7f5e5a/files/a449a3e2e2c94d9a856d5faea2ff0f94.pdf.
- 34.Ren L.L., Wang Y.M., Wu Z.Q., Xiang Z.C., Guo L., Xu T., Jiang Y.Z., Xiong Y., Li Y.J., Li X.W., Li H., Fan G.H., Gu X.Y., Xiao Y., Gao H., Xu J.Y., Yang F., Wang X.M., Wu C., Chen L., Liu Y.W., Liu B., Yang J., Wang X.R., Dong J., Li L., Huang C.L., Zhao J.P., Hu Y., Cheng Z.S., Liu L.L., Qian Z.H., Qin C., Jin Q., Cao B., Wang J.W. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl) 2020;133:1015–1024. doi: 10.1097/CM9.0000000000000722. https://www.ncbi.nlm.nih.gov/pubmed/32004165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tao Y., Fu Q., Mo Q. Advantages and challenges of metagenomics next-generation sequencing (mNGS) in the detection of 2019 novel coronavirus. Chin J Lab Med. 2020;43:217–220. doi: 10.3760/cma.j.issn.1009‑9158.2020.03.004. [DOI] [Google Scholar]
- 36.Chen L., Liu W., Zhang Q., Xu K., Ye G., Wu W., Sun Z., Liu F., Wu K., Zhong B., Mei Y., Zhang W., Chen Y., Li Y., Shi M., Lan K., Liu Y. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg Microbes Infect. 2020;9:313–319. doi: 10.1080/22221751.2020.1725399. https://www.ncbi.nlm.nih.gov/pubmed/32020836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang C., Liu Z., Chen Z., Huang X., Xu M., He T., Zhang Z. The establishment of reference sequence for SARS-CoV-2 and variation analysis. J Med Virol. 2020;92(6):667–674. doi: 10.1002/jmv.v92.610.1002/jmv.25762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Becerra-Flores M., Cardozo T. SARS-CoV-2 viral spike G614 mutation exhibits higher case fatality rate. Int J Clin Pract. 2020 doi: 10.1111/ijcp.13525. https://www.ncbi.nlm.nih.gov/pubmed/32374903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conti P., Caraffa A., Gallenga C.E., Kritas S.K., Frydas I., Younes A., Di Emidio P., Tete G., Pregliasco F., Ronconi G. The British variant of the new coronavirus-19 (Sars-Cov-2) should not create a vaccine problem. J Biol Regul Homeost Agents. 2021;35 doi: 10.23812/21-3-E. https://www.ncbi.nlm.nih.gov/pubmed/33377359. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. Diagnostic testing for SARS-CoV-2. Interim guidance. 11 September 2020. https://www.who.int/publications/i/item/diagnostic-testing-for-sars-cov-2, accessed 18 November 2020.
- 41.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020 doi: 10.1001/jama.2020.3786. https://www.ncbi.nlm.nih.gov/pubmed/32159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang M., Wu Q., Xu W., Qiao B., Wang J., Chen Z., Zheng H., Jiang S., Mei J., Wu Z., Deng Y., Zhou F., Wu W., Zhang Y., Lyu Z., Huang J., Guo X., Feng L., Xia Z., Li D., Liu T., Zhang P., Tong Y., Xu Z., Li Y. Analysis of 8 274 cases of novel coronavirus nucleic acid detection and co-infection in Wuhan. Chinese Journal of Laboratory Medicine. 2020;43:341–345. doi: 10.3760/cma.j.cn114452-20200222-00106. [DOI] [Google Scholar]
- 43.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brunink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. https://www.ncbi.nlm.nih.gov/pubmed/31992387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.World Health Organization. Molecular assays to diagnose COVID-19: Summary table of available protocols. (2020). https://www.who.int/publications/m/item/molecular-assays-to-diagnose-covid-19-summary-table-of-available-protocols.
- 45.China National Institute for Viral Disease Control and Prevention: Beijing. Specific Primers and Probes for Detection 2019 Novel Coronavirus. (2020). http://ivdc.chinacdc.cn/kyjz/202001/t20200121_211337.html.
- 46.Institut Pasteur P, France. Real-time RT-PCR assays for the detection of SARS-CoV-2. (2020). https://www.who.int/docs/default-source/coronaviruse/real-time-rt-pcr-assays-for-the-detection-of-sars-cov-2-institut-pasteur-paris.pdf?sfvrsn=3662fcb6_2.
- 47.Naganori N, Shirato K. Detection of Second Case of 2019-nCoV Infection in Japan. (2020). https://www.who.int/docs/default-source/coronaviruse/pcr-and-sequencing-protocols-for-2019-ncov--national-institute-of-infectious-diseases-japan.
- 48.School of Public Health, Hong Kong University. Detection of 2019 Novel Coronavirus (2019-nCoV) in Suspected Human Cases by RT-PCR. (2020). https://www.who.int/publications/i/item/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117.
- 49.Department of Medical Sciences, Ministry of Public Health, Thailand. Diagnostic Detection of Novel Coronavirus 2019 by Real Time RT-PCR. (2020). https://www.who.int/docs/default-source/coronaviruse/conventional-rt-pcr-followed-by-sequencing-for-detection-of-ncov-rirl-nat-inst-health-t.pdf.
- 50.Chinese Association of Laboratory Medicine The Expert Consensus of Novel Coronavirus 2019 nucleic acid detection. Natl Med J China. 2020;100:968–973. doi: 10.3760/cma.j.cn112137-20200206-00206. [DOI] [Google Scholar]
- 51.Shirato K., Nao N., Katano H., Takayama I., Saito S., Kato F., Katoh H., Sakata M., Nakatsu Y., Mori Y., Kageyama T., Matsuyama S., Takeda M. Development of Genetic Diagnostic Methods for Detection for Novel Coronavirus 2019(nCoV-2019) in Japan. Jpn J Infect Dis. 2020;73(4):304–307. doi: 10.7883/yoken.JJID.2020.061. [DOI] [PubMed] [Google Scholar]
- 52.Wang J., Cai K., Zhang R., He X., Shen X., Liu J., Xu J., Qiu F., Lei W., Wang J., Li X., Gao Y., Jiang Y., Xu W., Ma X. Novel One-Step Single-Tube Nested Quantitative Real-Time PCR Assay for Highly Sensitive Detection of SARS-CoV-2. Anal Chem. 2020;92(13):9399–9404. doi: 10.1021/acs.analchem.0c01884. [DOI] [PubMed] [Google Scholar]
- 53.Peiris J.S.M., Chu C.M., Cheng V.C.C., Chan K.S., Hung I.F.N., Poon L.L.M., Law K.I., Tang B.S.F., Hon TYW., Chan C.S., Chan K.H., Ng J.S.C., Zheng BJ., Ng W.L., Lai R.W.M., Guan Y., Yuen K.Y. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu X., Feng J., Zhang Q., Guo D., Zhang L., Suo T., Hu W., Guo M., Wang X., Huang Z., Xiong Y., Chen G., Chen Y., Lan K. Analytical comparisons of SARS-COV-2 detection by qRT-PCR and ddPCR with multiple primer/probe sets. Emerg Microbes Infect. 2020;9(1):1175–1179. doi: 10.1080/22221751.2020.1772679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suo T., Liu X., Feng J., Guo M., Hu W., Guo D., Ullah H., Yang Y., Zhang Q., Wang X., Sajid M., Huang Z., Deng L., Chen T., Liu F., Xu K., Liu Y., Zhang Q., Liu Y., Xiong Y., Chen G., Lan K., Chen Y. ddPCR: a more accurate tool for SARS-CoV-2 detection in low viral load specimens. Emerg Microbes Infect. 2020;9(1):1259–1268. doi: 10.1080/22221751.2020.1772678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., Sun R., Wang Y., Hu B., Chen W., Zhang Y., Wang J., Huang B., Lin Y., Yang J., Cai W., Wang X., Cheng J., Chen Z., Sun K., Pan W., Zhan Z., Chen L., Ye F. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020;92(9):1518–1524. doi: 10.1002/jmv.v92.910.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang R., Li J.M. The way to reduce the false negative results of 2019 novel coronavirus nucleic acid detection. Zhonghua Yi Xue Za Zhi. 2020;100:801–804. doi: 10.3760/cma.j.cn112137-20200215-00288. https://www.ncbi.nlm.nih.gov/pubmed/32234149. [DOI] [PubMed] [Google Scholar]
- 58.Wang C.B. Analysis of low positive rate of nucleic acid detection method used for diagnosis of novel coronavirus pneumonia. Zhonghua Yi Xue Za Zhi. 2020;100:961–964. doi: 10.3760/cma.j.cn112137-20200213-00280. https://www.ncbi.nlm.nih.gov/pubmed/32077662. [DOI] [PubMed] [Google Scholar]
- 59.Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., Guo D., Hu W., Yang J., Tang Z., Wu H., Lin Y., Zhang M., Zhang Q., Shi M., Liu Y., Zhou Y., Lan K., Chen Y. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. 2020;9(1):761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiu H., Wu J., Hong L., Luo Y., Song Q., Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. https://www.ncbi.nlm.nih.gov/pubmed/32220650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bal A., Destras G., Gaymard A., Stefic K., Marlet J., Eymieux S., Regue H., Semanas Q., d’Aubarede C., Billaud G., Laurent F., Gonzalez C., Mekki Y., Valette M., Bouscambert M., Gaudy-Graffin C., Lina B., Morfin F., Josset L., Group CO-DHS Two-step strategy for the identification of SARS-CoV-2 variant of concern 202012/01 and other variants with spike deletion H69-V70, France, August to December 2020. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.3.2100008. https://www.ncbi.nlm.nih.gov/pubmed/33478625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wernike K., Keller M., Conraths F.J., Mettenleiter T.C., Groschup M.H., Beer M. Pitfalls in SARS-CoV-2 PCR diagnostics. Transbound Emerg Dis doi:10.1111/tbed.13684. 2021;68(2):253–257. doi: 10.1111/tbed.v68.210.1111/tbed.13684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagura-Ikeda M., Imai K., Tabata S., Miyoshi K., Murahara N., Mizuno T., Horiuchi M., Kato K., Imoto Y., Iwata M., Mimura S., Ito T., Tamura K., Kato Y., Miller M.B. Clinical Evaluation of Self-Collected Saliva by Quantitative Reverse Transcription-PCR (RT-qPCR), Direct RT-qPCR, Reverse Transcription-Loop-Mediated Isothermal Amplification, and a Rapid Antigen Test To Diagnose COVID-19. J Clin Microbiol. 2020;58(9) doi: 10.1128/JCM.01438-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang M., Pan W., Arasthfer A., Fang W., Ling L., Fang H., Daneshnia F., Yu J., Liao W., Pei H., Li X., Lass-Florl C. Development and Validation of a Rapid, Single-Step Reverse Transcriptase Loop-Mediated Isothermal Amplification (RT-LAMP) System Potentially to Be Used for Reliable and High-Throughput Screening of COVID-19. Front Cell Infect Microbiol. 2020;10:331. doi: 10.3389/fcimb.2020.00331. https://www.ncbi.nlm.nih.gov/pubmed/32626666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park G.-S., Ku K., Baek S.-H., Kim S.-J., Kim S.I., Kim B.-T., Maeng J.-S. Development of Reverse Transcription Loop-Mediated Isothermal Amplification Assays Targeting Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) J Mol Diagn. 2020;22(6):729–735. doi: 10.1016/j.jmoldx.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Notomi T., Okayama H., Masubuchi H., Yonekawa T., Watanabe K., Amino N., Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:E63. doi: 10.1093/nar/28.12.e63. https://www.ncbi.nlm.nih.gov/pubmed/10871386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang W.E., Lim B., Hsu C., Xiong D., Wu W., Yu Y., Jia H., Wang Y., Zeng Y., Ji M., Chang H., Zhang X., Wang H., Cui Z. RT-LAMP for rapid diagnosis of coronavirus SARS-CoV-2. Microb Biotechnol. 2020;13(4):950–961. doi: 10.1111/mbt2.v13.410.1111/1751-7915.13586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yan C., Cui J., Huang L., Du B., Chen L., Xue G., Li S., Zhang W., Zhao L., Sun Y., Yao H., Li N., Zhao H., Feng Y., Liu S., Zhang Q., Liu D., Yuan J. Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay. Clin Microbiol Infect. 2020;26:773–779. doi: 10.1016/j.cmi.2020.04.001. https://www.ncbi.nlm.nih.gov/pubmed/32276116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu L., Wu S., Hao X., Dong X., Mao L., Pelechano V., Chen W.H., Yin X. Rapid Detection of COVID-19 Coronavirus Using a Reverse Transcriptional Loop-Mediated Isothermal Amplification (RT-LAMP) Diagnostic Platform. Clin Chem. 2020;66:975–977. doi: 10.1093/clinchem/hvaa102. https://www.ncbi.nlm.nih.gov/pubmed/32315390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ouyang W., Yu J., Zhang J., Xie C. Alert to Potential Contagiousness: A Case of Lung Cancer With Asymptomatic Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Thorac Oncol. 2020;15:e82–e83. doi: 10.1016/j.jtho.2020.04.005. https://www.ncbi.nlm.nih.gov/pubmed/32305592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moitra P., Alafeef M., Dighe K., Frieman M.B., Pan D. Selective Naked-Eye Detection of SARS-CoV-2 Mediated by N Gene Targeted Antisense Oligonucleotide Capped Plasmonic Nanoparticles. ACS Nano. 2020;14(6):7617–7627. doi: 10.1021/acsnano.0c03822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu X., Wang X., Han L., Chen T., Wang L., Li H., Li S., He L., Fu X., Chen S., Xing M., Chen H., Wang Y. Multiplex reverse transcription loop-mediated isothermal amplification combined with nanoparticle-based lateral flow biosensor for the diagnosis of COVID-19. Biosens Bioelectron. 2020;166:112437. doi: 10.1016/j.bios.2020.112437. https://www.ncbi.nlm.nih.gov/pubmed/32692666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Visseaux B., Le Hingrat Q., Collin G., Bouzid D., Lebourgeois S., Le Pluart D., Deconinck L., Lescure F.X., Lucet J.C., Bouadma L., Timsit J.F., Descamps D., Yazdanpanah Y., Casalino E., Houhou-Fidouh N. Emergency Department Influenza Study G. Evaluation of the QIAstat-Dx Respiratory SARS-CoV-2 Panel, the First Rapid Multiplex PCR Commercial Assay for SARS-CoV-2 Detection. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00630-20. https://www.ncbi.nlm.nih.gov/pubmed/32341142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harrington A., Cox B., Snowdon J., Bakst J., Ley E., Grajales P., Maggiore J., Kahn S., McAdam A.J. Comparison of Abbott ID Now and Abbott m2000 Methods for the Detection of SARS-CoV-2 from Nasopharyngeal and Nasal Swabs from Symptomatic Patients. J Clin Microbiol. 2020;58(8) doi: 10.1128/JCM.00798-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rhoads D.D., Cherian S.S., Roman K., Stempak L.M., Schmotzer C.L., Sadri N., McAdam A.J. Comparison of Abbott ID Now, DiaSorin Simplexa, and CDC FDA Emergency Use Authorization Methods for the Detection of SARS-CoV-2 from Nasopharyngeal and Nasal Swabs from Individuals Diagnosed with COVID-19. J Clin Microbiol. 2020;58(8) doi: 10.1128/JCM.00760-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhen W., Smith E., Manji R., Schron D., Berry G.J., McAdam A.J. Clinical evaluation of three sample-to-answer platforms for detection of SARS-CoV-2. J Clin Microbiol. 2020;58(8) doi: 10.1128/JCM.00783-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wolters F., van de Bovenkamp J., van den Bosch B., van den Brink S., Broeders M., Chung N.H., Favie B., Goderski G., Kuijpers J., Overdevest I., Rahamat-Langedoen J., Wijsman L., Melchers W.J., Meijer A. Multi-center evaluation of cepheid xpert(R) xpress SARS-CoV-2 point-of-care test during the SARS-CoV-2 pandemic. J Clin Virol. 2020;128:104426. doi: 10.1016/j.jcv.2020.104426. https://www.ncbi.nlm.nih.gov/pubmed/32417674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hogan C.A., Sahoo M.K., Huang C., Garamani N., Stevens B., Zehnder J., Pinsky B.A. Five-minute point-of-care testing for SARS-CoV-2: Not there yet. J Clin Virol. 2020;128:104410. doi: 10.1016/j.jcv.2020.104410. https://www.ncbi.nlm.nih.gov/pubmed/32403009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu R., Han H., Liu F., Lv Z., Wu K., Liu Y., Feng Y., Zhu C. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin Chim Acta. 2020;505:172–175. doi: 10.1016/j.cca.2020.03.009. https://www.ncbi.nlm.nih.gov/pubmed/32156607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ejazi S.A., Ghosh S., Ali N. Antibody detection assays for COVID-19 diagnosis: an early overview. Immunol Cell Biol. 2021;99(1):21–33. doi: 10.1111/imcb.v99.110.1111/imcb.12397. [DOI] [PubMed] [Google Scholar]
- 81.Xu W., Li J., He X., Zhang C., Mei S., Li C., Li Y., Cheng S., Zhang P. The diagnostic value of joint detection of serum IgM and IgG antibodies to 2019-nCoV in 2019-nCoV infection. Chinese Journal of Laboratory Medicine. 2020;43:230–233. doi: 10.3760/cma.j.issn.1009-9158.2020.03.007. [DOI] [Google Scholar]
- 82.Ma H., Zeng W., He H., Zhao D., Jiang D., Zhou P., Cheng L., Li Y., Ma X., Jin T. Serum IgA, IgM, and IgG responses in COVID-19. Cell Mol Immunol. 2020;17(7):773–775. doi: 10.1038/s41423-020-0474-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., Wang X., Yuan J., Li T., Li J., Qian S., Hong C., Wang F., Liu Y., Wang Z., He Q., Li Z., He B., Zhang T., Fu Y., Ge S., Liu L., Zhang J., Xia N., Zhang Z. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa344. https://www.ncbi.nlm.nih.gov/pubmed/32221519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li P., Li Z., Zhao S., Li Q., Hu Y., Chen Y., Yi F., Xie Q. Preliminary study of serum 2019-nCoV IgM and IgG antibodies in the diagnosis of COVID-19. Chinese Journal of Laboratory Medicine. 2020;43:352–357. doi: 10.3760/cma.j.cn114452-20200302-00155. [DOI] [Google Scholar]
- 85.Zhang R., Li J. Reasons and solutions for “false positive results” of 2019 novel coronavirus-specific antibodies detection. Chin J Lab Med. 2020;43:507–510. doi: 10.3760/cma.j.cn114452‑20200318‑00271. [DOI] [Google Scholar]
- 86.Ward S., Lindsley A., Courter J., Assa’ad A. Clinical testing for COVID-19. J Allergy Clin Immunol. 2020;146(1):23–34. doi: 10.1016/j.jaci.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Okba N.M.A., Müller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M., Lamers M.M., Sikkema R.S., de Bruin E., Chandler F.D., Yazdanpanah Y., Le Hingrat Q., Descamps D., Houhou-Fidouh N., Reusken C.B.E.M., Bosch B.-J., Drosten C., Koopmans M.P.G., Haagmans B.L. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients. Emerg Infect Dis. 2020;26(7):1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lv H., Wu N.C., Tsang O.T., Yuan M., Perera R., Leung W.S., So R.T.Y., Chan J.M.C., Yip G.K., Chik T.S.H., Wang Y., Choi C.Y.C., Lin Y., Ng W.W., Zhao J., Poon L.L.M., Peiris J.S.M., Wilson I.A., Mok C.K.P. Cross-reactive Antibody Response between SARS-CoV-2 and SARS-CoV Infections. Cell Rep. 2020;31:107725. doi: 10.1016/j.celrep.2020.107725. https://www.ncbi.nlm.nih.gov/pubmed/32426212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu W., Liu L., Kou G., Zheng Y., Ding Y., Ni W., Wang Q., Tan L., Wu W., Tang S., Xiong Z., Zheng S., McAdam A.J. Evaluation of Nucleocapsid and Spike Protein-Based Enzyme-Linked Immunosorbent Assays for Detecting Antibodies against SARS-CoV-2. J Clin Microbiol. 2020;58(6) doi: 10.1128/JCM.00461-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Centers for Disease Control and Prevention of U.S. Interim Guidance for Rapid Antigen Testing for SARS-CoV-2. (2020). https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html.
- 91.World Health Organization. SARS-CoV-2 antigen-detecting rapid diagnostic tests: an implementation guide. 21 December 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance-publications.
- 92.Porte L., Legarraga P., Vollrath V., Aguilera X., Munita J.M., Araos R., Pizarro G., Vial P., Iruretagoyena M., Dittrich S., Weitzel T. Evaluation of a novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int J Infect Dis. 2020;99:328–333. doi: 10.1016/j.ijid.2020.05.098. https://www.ncbi.nlm.nih.gov/pubmed/32497809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scohy A., Anantharajah A., Bodeus M., Kabamba-Mukadi B., Verroken A., Rodriguez-Villalobos H. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J Clin Virol. 2020;129:104455. doi: 10.1016/j.jcv.2020.104455. https://www.ncbi.nlm.nih.gov/pubmed/32485618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mavrikou S., Moschopoulou G., Tsekouras V., Kintzios S. Development of a Portable, Ultra-Rapid and Ultra-Sensitive Cell-Based Biosensor for the Direct Detection of the SARS-CoV-2 S1 Spike Protein Antigen. Sensors (Basel) 2020;20(11):3121. doi: 10.3390/s20113121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.World Health Organization. Antigen-detection in the diagnosis of SARS-CoV-2 infection using rapid immunoassays.Interim guidance. 11 September 2020. https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays.
- 96.World Health Organization. WHO COVID-19: Case Definitions.16 December 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance-publications?publicationtypes=df113943-c6f4-42a5-914f-0a0736769008.
- 97.National Health Commission & National Administration of Traditional Chinese Medicine. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7) Chin Med J (Engl) 2020;133:1087–1095. doi: 10.1097/CM9.0000000000000819. https://www.ncbi.nlm.nih.gov/pubmed/32358325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. https://www.ncbi.nlm.nih.gov/pubmed/32091533. [DOI] [PubMed] [Google Scholar]
- 99.Jordan R.E., Adab P., Cheng K.K. Covid-19: risk factors for severe disease and death. BMJ 368. 2020:m1198. doi: 10.1136/bmj.m1198. https://www.ncbi.nlm.nih.gov/pubmed/32217618. [DOI] [PubMed] [Google Scholar]
- 100.Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P., Xiao W., Wang Y.N., Zhong M.H., Li C.H., Li G.C., Liu H.G. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. https://www.ncbi.nlm.nih.gov/pubmed/32044814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI13724410.1172/JCI137244DS110.1172/JCI137244DS2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shi Y., Ou J., Chen X., Tan M., Li F., Liu Y. Expressions of multiple inflammation markers in the patients with COVID-19 and their clinical values. Chinese Journal of Laboratory Medicine. 2020;43:346–351. doi: 10.3760/cma.j.cn114452-20200214-00073. [DOI] [Google Scholar]
- 103.Zhang B., Zhou X., Zhu C., Song Y., Feng F., Qiu Y., Feng J., Jia Q., Song Q., Zhu B., Wang J. Immune Phenotyping Based on the Neutrophil-to-Lymphocyte Ratio and IgG Level Predicts Disease Severity and Outcome for Patients With COVID-19. Front Mol Biosci 7. 2020:157. doi: 10.3389/fmolb.2020.00157. https://www.ncbi.nlm.nih.gov/pubmed/32719810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian DS. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. https://www.ncbi.nlm.nih.gov/pubmed/32161940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C., Mou H.M., Wang L.H., Zhang H.R., Fu W.J., Luo T., Liu F., Guo Q.N., Chen C., Xiao H.L., Guo H.T., Lin S., Xiang D.F., Shi Y., Pan G.Q., Li Q.R., Huang X., Cui Y., Liu X.Z., Tang W., Pan P.F., Huang X.Q., Ding Y.Q., Bian X.W. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. https://www.ncbi.nlm.nih.gov/pubmed/32172546. [DOI] [PubMed] [Google Scholar]
- 106.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. China Medical Treatment Expert Group for C. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. https://www.ncbi.nlm.nih.gov/pubmed/32109013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu Y., Sun W., Guo Y., Chen L., Zhang L., Zhao S., Long D., Yu L. Association between platelet parameters and mortality in coronavirus disease 2019: Retrospective cohort study. Platelets. 2020;31:490–496. doi: 10.1080/09537104.2020.1754383. https://www.ncbi.nlm.nih.gov/pubmed/32297540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan. China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang X., Yang Q., Wang Y., Wu Y., Xu J., Yu Y., Shang Y. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18(6):1469–1472. doi: 10.1111/jth.v18.610.1111/jth.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fan B.E., Chong V.C.L., Chan S.S.W., Lim G.H., Lim K.G.E., Tan G.B., Mucheli S.S., Kuperan P., Ong K.H. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020;95:E131–E134. doi: 10.1002/ajh.25774. https://www.ncbi.nlm.nih.gov/pubmed/32129508. [DOI] [PubMed] [Google Scholar]
- 111.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. https://www.ncbi.nlm.nih.gov/pubmed/32167524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qu R., Ling Y., Zhang Y., Wei L., Chen X., Li X., Liu X., Liu H., Guo Z., Ren H., Wang Q. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J Med Virol. 2020;92(9):1533–1541. doi: 10.1002/jmv.v92.910.1002/jmv.25767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Amgalan A., Othman M. Hemostatic laboratory derangements in COVID-19 with a focus on platelet count. Platelets. 2020;31(6):740–745. doi: 10.1080/09537104.2020.1768523. [DOI] [PubMed] [Google Scholar]
- 114.Zheng Y., Xu H., Yang M., Zeng Y., Chen H., Liu R., Li Q., Zhang N., Wang D. Epidemiological characteristics and clinical features of 32 critical and 67 noncritical cases of COVID-19 in Chengdu. J Clin Virol. 2020;127:104366. doi: 10.1016/j.jcv.2020.104366. https://www.ncbi.nlm.nih.gov/pubmed/32302954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., LippiHematologic G. biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. https://www.ncbi.nlm.nih.gov/pubmed/32286245. [DOI] [PubMed] [Google Scholar]
- 116.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., Wang T., Guo W., Chen J., Ding C., Zhang X., Huang J., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. https://www.ncbi.nlm.nih.gov/pubmed/32217556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Peng Y.D., Meng K., Guan H.Q., Leng L., Zhu R.R., Wang B.Y., He M.A., Cheng L.X., Huang K., Zeng Q.T. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:450–455. doi: 10.3760/cma.j.cn112148-20200220-00105. https://www.ncbi.nlm.nih.gov/pubmed/32120458. [DOI] [PubMed] [Google Scholar]
- 118.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. https://www.ncbi.nlm.nih.gov/pubmed/32381264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.v18.410.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Al-Samkari H., Karp Leaf R.S., Dzik W.H., Carlson J.C.T., Fogerty A.E., Waheed A., Goodarzi K., Bendapudi P.K., Bornikova L., Gupta S., Leaf D.E., Kuter D.J., Rosovsky R.P. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. https://www.ncbi.nlm.nih.gov/pubmed/32492712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Levi M. COVID-19 coagulopathy vs disseminated intravascular coagulation. Blood Adv. 2020;4:2850. doi: 10.1182/bloodadvances.2020002197. https://www.ncbi.nlm.nih.gov/pubmed/32574369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cabler S.S., French A.R., Orvedahl A. A Cytokine Circus with a Viral Ringleader: SARS-CoV-2-Associated Cytokine Storm Syndromes. Trends Mol Med. 2020;26(12):1078–1085. doi: 10.1016/j.molmed.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li H., Xiang X., Ren H., Xu L., Zhao L., Chen X., Long H., Wang Q., Wu Q. Serum Amyloid A is a biomarker of severe Coronavirus Disease and poor prognosis. J Infect. 2020;80(6):646–655. doi: 10.1016/j.jinf.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chang Z., Yang W., Wang Q., Liao G. Clinical significance of serum hs-CRP, IL-6, and PCT in diagnosis and prognosis of patients with COVID-19. Drugs & Clinic. 2020;35:417–420. doi: 10.7501/j.issn.1674-5515.2020.03.005. [DOI] [Google Scholar]
- 125.Pieri M., Ciotti M., Nuccetelli M., Perrone M.A., Calio M.T., Lia M.S., Minieri M., Bernardini S. Serum Amyloid A Protein as a useful biomarker to predict COVID-19 patients severity and prognosis. Int Immunopharmacol. 2021;95:107512. doi: 10.1016/j.intimp.2021.107512. https://www.ncbi.nlm.nih.gov/pubmed/33735714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hampl V., Herget J., Bibova J., Banasova A., Huskova Z., Vanourkova Z., Jichova S., Kujal P., Vernerova Z., Sadowski J., Cervenka L. Intrapulmonary activation of the angiotensin-converting enzyme type 2/angiotensin 1–7/G-protein-coupled Mas receptor axis attenuates pulmonary hypertension in Ren-2 transgenic rats exposed to chronic hypoxia. Physiol Res. 2015;64:25–38. doi: 10.33549/physiolres.932861. https://www.ncbi.nlm.nih.gov/pubmed/25194138. [DOI] [PubMed] [Google Scholar]
- 127.Touyz R.M., Li H., Delles C. ACE2 the Janus-faced protein - from cardiovascular protection to severe acute respiratory syndrome-coronavirus and COVID-19. Clin Sci (Lond) 2020;134:747–750. doi: 10.1042/CS20200363. https://www.ncbi.nlm.nih.gov/pubmed/32255491. [DOI] [PubMed] [Google Scholar]
- 128.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C., Zhang Z., Wang L., Peng L., Chen L., Qin Y., Zhao D., Tan S., Yin L., Xu J., Zhou C., Jiang C., Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Henry B.M., Benoit S., Lippi G., Benoit J. Circulating plasma levels of angiotensin II and aldosterone in patients with coronavirus disease 2019 (COVID-19): A preliminary report. Prog Cardiovasc Dis. 2020 doi: 10.1016/j.pcad.2020.07.006. https://www.ncbi.nlm.nih.gov/pubmed/32659343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Narula S., Yusuf S., Chong M., Ramasundarahettige C., Rangarajan S., Bangdiwala S.I., van Eikels M., Leineweber K., Wu A., Pigeyre M., Paré G. Plasma ACE2 and risk of death or cardiometabolic diseases: a case-cohort analysis. Lancet. 2020;396(10256):968–976. doi: 10.1016/S0140-6736(20)31964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ramchand J., Patel S.K., Srivastava P.M., Farouque O., Burrell L.M., Shimosawa T. Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease. PLoS ONE. 2018;13(6):e0198144. doi: 10.1371/journal.pone.0198144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ingraham N.E., Barakat A.G., Reilkoff R., Bezdicek T., Schacker T., Chipman J.G., Tignanelli C.J., Puskarich M.A. Understanding the renin-angiotensin-aldosterone-SARS-CoV axis: a comprehensive review. Eur Respir J. 2020;56(1):2000912. doi: 10.1183/13993003.00912-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]