Abstract
Introduction and Hypothesis:
Numerous analytic observational studies assess family history as a risk factor for POP and report a wide range of associations. This review aims to systematically evaluate the role of family history of POP in relation to POP risk and its recurrence.
Methods:
A review was performed of the PubMed/MEDLINE database with search criteria specifying family history, risk factors, POP, and their synonyms as title/abstract keywords, as well as MESH terms, up to March 2020. We aggregated evidence across studies with fixed effects (FE) and random effects (RE) meta-analysis.
Results:
Forty-three articles underwent full-text review. Eighteen independent studies evaluating the relationship between family history of POP and POP risk in 3,639 POP cases and 10,912 controls were eligible for meta-analysis. Four studies evaluating family history and POP recurrence in 224 recurrent cases and 400 non-recurrent cases were eligible for inclusion into another meta-analyses. A positive family history of POP is on average associated with 2.3 to 2.7-fold increased risk for POP (RE OR = 2.64; 95% CI = 2.07, 3.35), as well as a 1.4-fold increased risk for POP recurrence (FE OR = 1.44; 95% CI = 1.00, 2.08). Meta-analysis estimates of POP risk varied by study design, definition of family history and model adjustment status. We found evidence for publication bias and recall bias is a possibility.
Conclusions:
Family history of POP is a risk factor for both POP presence and recurrence.
However, reported magnitudes may be overestimates due to confounding, recall bias and publication bias.
Keywords: pelvic organ prolapse, primary prolapse, prolapse recurrence, family history, systematic review, meta-analysis
Brief Summary:
Synthesizing evidence from 24 independent studies, a positive family history of POP is associated with an increased risk of having both primary and recurrent POP.
INTRODUCTION:
Pelvic organ prolapse (POP) is defined as the descent of one or more pelvic organs into the vaginal space due to lack of support of the anterior or posterior vaginal wall, vaginal cuff, or the uterus [1,2]. Up to 50% of post-menopausal women may have POP on clinical examination, and the lifetime risk of undergoing surgery for POP is estimated at 12.6% in the US [3,4]. Prolapse can result in discomfort, obstructive defecation, increased risk of urinary tract infections, and more rarely, urinary retention. Treatment for POP can involve pelvic floor physical therapy, pessary, and surgical management. With an aging population, the economic and public health burden of POP is likely to increase [5].
Reproducible predictive models based on clearly defined risk factors and outcomes offer opportunities for accurate risk prediction and effective management strategies. Aging, vaginal birth, and obesity are well defined risk factors for POP [6,7]. Family history is an important risk factor for POP and until clinically meaningful genetic variants are discovered, family history remains the closest proxy for understanding a patient’s inherent risk for POP. Accurate estimation of the magnitude of association between clearly defined family history variables and POP can provide high predictive utility. However, family history is often broadly defined and may be inclusive of mother, sister, second-degree relatives and beyond. In 2012, a meta-analysis of eight studies reported that having a positive family history of POP is associated with 2.5-fold increased odds of having POP [8]. Multiple independent studies have since examined and reported on family history as a risk factor in relation to POP status and repeat surgery for POP. A recent study reported family history of POP as one of the strongest predictors of POP at 12 years and 20 years after delivery [9]. Collectively, the literature harbors a broad range of associations derived from studies that are heterogeneous in the populations sampled, study sample size and design, definition of family history and analytic strategy.
Here we perform a systematic review and report quantitative summaries from meta-analyses of the relationships between 1) family history of POP and POP status in women with and without POP and 2) family history of POP and POP recurrence in women who had surgical correction for POP. We hypothesize that family history is associated with a woman’s likelihood of both having primary and recurrent POP. We also evaluate study-level characteristics to identify sources of heterogeneity in effect estimates across studies. Finally, we discuss the need to clearly define family history to improve relevance, prediction and interpretation of findings.
MATERIALS AND METHODS:
We used the PubMed/MEDLINE database to perform a systematic search for articles providing adequate information to evaluate if family history of POP is associated with having POP. We used an inclusive search criterion that included a combination of search terms, family history, risk factors, POP, and their synonyms as title/abstract keywords and MESH terms to maximize article selection. Full search criteria are listed below. We implemented search criteria on March 31, 2020, and this yielded 1,019 titles and abstracts (Figure 1). We exported titles and abstracts from PubMed and uploaded into Zotero [10] for review by two independent reviewers (AG and PS). Exclusion criteria were formed a priori, and at this stage consisted of excluding articles not published in English (105 titles), not related to POP and not original research – ie. letters and reviews (609). Three hundred five articles were eligible for digital keyword review of full-text articles. Digital keyword review consisted searching for the following terms: “family history,” “mother,” “sister,” “aunt,” “grandmother,” “family,” and “history.” Full-text was not available for 13 articles and 225 articles were excluded for not including any of the keywords. A total of 43 articles were considered for full-text manual review by three reviewers (AG, PS and SJ) to determine further eligibility for systematic review and meta-analysis using a structured questionnaire implemented in a Research Electronic Data Capture (REDCap) application (SJ) hosted at Vanderbilt University Medical Center.[11,12]
Figure 1.
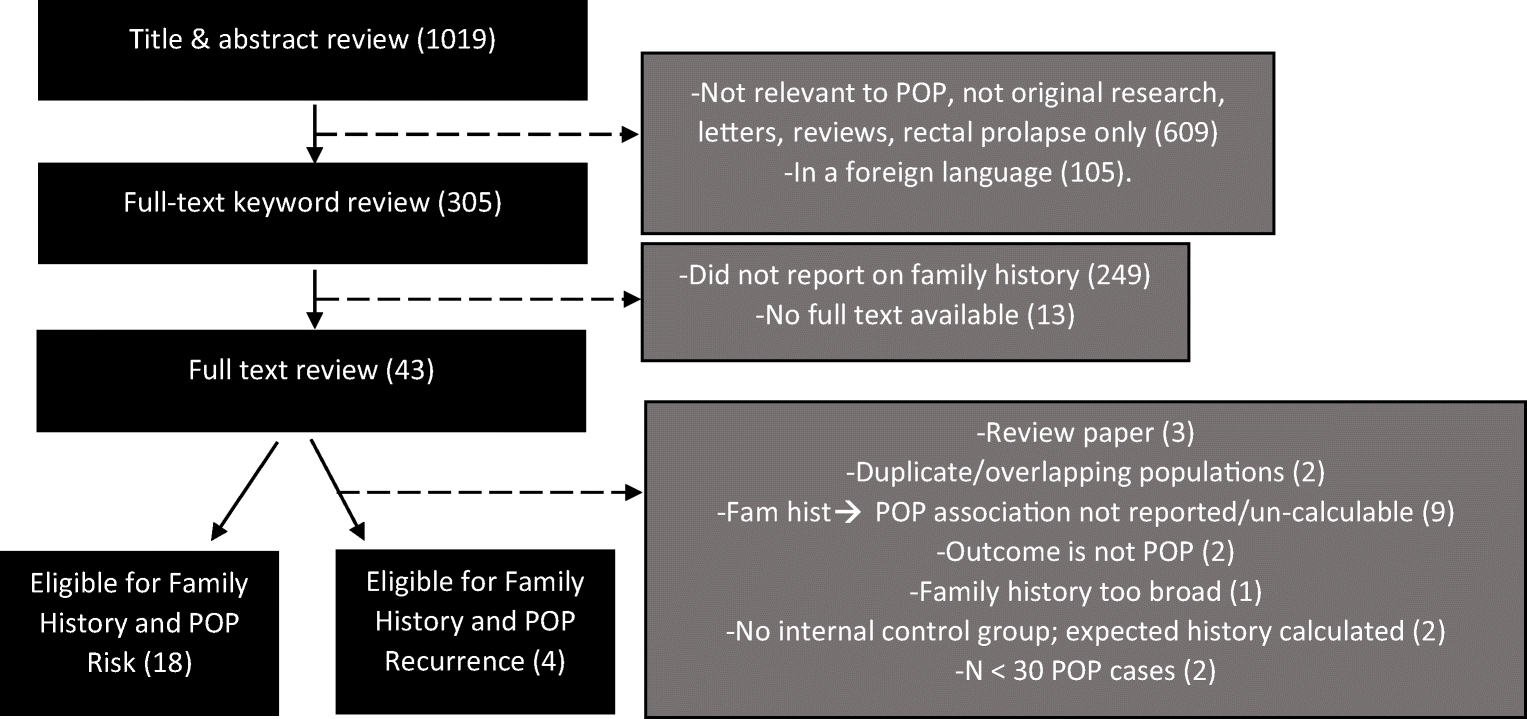
Flow chart summarizing systematic review process
Search term
((((((“Medical History Taking”[Mesh] OR “family history”[tiab] OR “family medical history”[tiab] OR “family medical histories”[tiab] OR “family health history”[tiab] OR “Risk factors”[Mesh] OR “Risk factors”[tiab]) AND (“Pelvic Organ Prolapse”[Mesh] OR “pelvic organ prolapse”[tiab])))) AND (“1800/01/01”[Date - Publication] : “2020/03/31”[Date - Publication])))
Our general strategy was to maximize the number of studies evaluating the relationship between family history of POP and participant POP status and use pre-planned sub-group analyses to explain observed estimates. All studies evaluating family history as a risk factor for POP in adult women (age 18 or older) were eligible for inclusion into meta-analysis. Two specific population types are distinguished for the purpose of this review. One consists of a population of women with and without POP at the time of assessment to allow for evaluation of the relationship between family history and POP status. The other consists of only women who underwent surgical correction for POP to allow evaluation of the relationship between family history of POP and POP recurrence. When two or more journal articles used the same or overlapping populations, we preferred the eligible study with the larger sample size. When a given article mentioned family history of POP in relation to POP status or recurrence but did not provide adequate information to compute effect estimates, the corresponding authors of the articles were contacted to request information relevant for meta-analysis.
We did not impose any inclusion restrictions for method of POP assessment. Studies reporting POP based on the POP-Quantification (POP-Q) system, Baden-Walker Halfway system, clinical assessment, chart review, treatment for POP, and surgical codes were all eligible, as were studies based on self-reported symptoms. Based on preliminary review of the literature, an a priori decision was made to evaluate POP as a dichotomous outcome using the definition of POP provided by the study investigators. We included all studies that reported on any type of POP regardless of anatomical location within the pelvis, including cystocele, rectocele, uterine prolapse, or vaginal wall prolapse in isolation or in combination. Studies evaluating rectal prolapse were not eligible as this may include both men and women.
Three components of family history were pre-defined: method of assessment (self-report, chart review of linked records), whose history was being collected (first, second or third degree relative), and history of which condition(s) (POP, connective tissue disorder, hernia) recorded. Information regarding these components were collected but not used as criteria for exclusion from meta-analysis to allow for downstream assessment of heterogeneity by type of family history collected.
Analytic observational studies including cohort (prospective and retrospective), case-control and cross-sectional study designs reporting on the relationship between family history of POP as a dichotomous independent variable and participant POP status were considered in the meta-analysis. Studies with less than 30 POP cases were excluded from meta-analysis because of possible unstable effect estimates. Studies needed to report appropriate effect estimate (odds ratio [OR], relative risk [RR], hazard ratio [HR]) or provide enough information for meta-analysts to calculate relevant effect estimates for either POP status or POP recurrence. Studies computing risk ratios using expected values from population rates for comparison groups were not included. Only analytic designs with appropriate internal comparison groups were eligible to be included in the primary meta-analysis sets.
After full text review and determination of eligibility for inclusion into meta-analysis, the following fields were abstracted from each article: study title, first author, publication date, study design, central measure of age (mean/median) or percentage of post-menopausal women if available, ethnicity or country of study, method of POP assessment (self-report/symptomatic, POP-Q, Baden-Walker, clinical assessment, treatment or surgical codes), components of family history (collection method, relation [mother, sister], disease history), whether analysis was adjusted for confounders (yes/no), and relevant data for effect estimates. We collected multiple types of data to maximize inclusion of each study into meta-analysis sets including multivariable adjusted effect estimates, unadjusted effect estimates, and raw numbers to compute effect estimates. For studies reporting two or more effect estimates for varying definitions of POP or family history, all relevant estimates were abstracted as separate entries and flagged as overlapping to allow for sensitivity analyses while avoiding aggregation of correlated data during analysis. The quality of evidence in each of the studies was assessed using the NewCastle-Ottawa scale [13] by two of the authors (PS and AG). As the original scale was designed to be used for case-control and cohort studies, cross-sectional studies were assessed using the case-control scale. Scores for individual studies are reported in Tables 1 and 2. These quality scores were constructed for the reader’s benefit. We did not perform any weighting of articles based on quality scores or stratification of articles by quality to avoid the potential for inducing bias in meta-analysis [14,15].
Table 1.
Description of 18 studies eligible for systematic review and meta-analysis evaluating risk of pelvic organ prolapse
| Study | Study design, sample size | Mean age (SD) and % postmenopausal | Country of study or race/ethnicity | Data collection methods | POP measurement | Covariates adjusted for | Results RR/OR/hazard ratio (95% CI) | Comments; NOS Scorea |
|---|---|---|---|---|---|---|---|---|
| Chiaffarino et al (1999), Reproductive factors, family history, occupation and risk of urogenital prolapse | Hospital-based, case-control; 208 | Mean age not provided; 62% are 57 y or greater | Italian women; ethnicity not specified | Elicited by questionnaire; Maternal: 27.6%; Sister: 30.0% | Baden-Walker classification | Age | aOR Maternal history 3.2 (1.1–7.6); OR Sister’s history 2.4 (1.0–5.6) | Controls were extracted from non-gynecologic hospital admissions; 6 |
| Altman et al (2006), Pelvic organ prolapse and urinary incontinence in women with surgically managed rectal prolapse: a population-based case-control study | Population-based, case-control; 213 | Mean age of uterine prolapse surgery: 54.7 y; percent postmenopausal not provided and could not be inferred | Sweden, 100% Caucasian | Elicited by questionnaire; maternal or sister | Respondents reported treatment for POP | Could not be specifically identified | aOR hysterectomy for uterine prolapse 2.9 (1.4–6.7) | Family history independently associated with POP surgery; patients identified from previous group that underwent surgery for rectal prolapse; 4 |
| DeLancey et al (2007), Comparison of Levator Ani Muscle Defects and Function in Women With and Without Pelvic Organ Prolapse | Population-based, case-control; 286 | Cases: 56.4 y, 55.5% postmenopausal; controls: 56.6 y, 57.4% | United States; 94% white, 3.3% African American, 2.7% other | Elicited by questionnaire; maternal, sister, or grandmother | POP-Q measurement, cases ≥ −1 | None | Not calculated; Cases: 31.1%, controls 13.3%, p <.001 | Power calculated from findings based on previous study of levator ani defects; 6 |
| McLennan et al (2008), Family history as a risk factor for pelvic organ prolapse | Hospital-based, cohort; 458 | Cases: 52.8 y; controls: 46.6 y; percent postmenopausal not provided and could not be inferred | United States; 77.5% Caucasian, 18.7% African American, 3.8% unknown/other | Elicited by questionnaire; male family members with hernia and females with hernia/prolapse | Baden-Walker classification; Grade 0 vs ≥ 1 | Vaginal deliveries, hysterectomy, urinary incontinence | RR mild prolapse 1.14 (0.70–1.87); RR severe prolapse 1.48 (1.14–1.9); overall cRR 1.8 (1.4–2.3); overall aRR 1.4 (1.2–1.8) | Identified confounders by 10% difference between aRR and cRR; 19 patients answering “do not know” were analyzed separately; 5 |
| Rodrigues et al (2008), COL1A1 Sp1-binding site polymorphism as a risk factor for genital prolapse | Hospital-based, case-control; 316 | Cases: 66.3 y; controls: 60.8 y; percent postmenopausal not provided and could not be inferred | Brazil; 60.3 % white, 39.7% non-white | Elicited by questionnaire, for any familial history | POP-Q measurement, cases Stage 3–4, control defined as Stage 0–1 | Could not be specifically identified | aOR 2.27 (1.04–4.9) | Estimates for family history were derived from questionnaire responses; 4 |
| Forsgren et al (2008), Risk factors for vaginal vault prolapse surgery in postmenopausal hysterectomized women | Population-based, case-control; 323 | Cases: 54.6 y; controls: 59.4 y; percent postmenopausal not provided and could not be inferred | Sweden; ethnicity not reported | Elicited by questionnaire, for any familial history | Codes for vaginal vault surgery used, no degree or staging information provided | Age, vaginal and cesarean delivery, severe vaginal laceration, delivery of >4000g child, hormone therapy, prolapse or incontinence surgery before hysterectomy or vaginal vault surgery | aOR 1.6 (0.6–4.0) | When adjusted for unknown factors, family history is no longer significant; 5 |
| Slieker-ten et al (2009)b, Symptomatic pelvic organ prolapse and possible risk factors in a general population | Population-based, cross-sectional study; 971 | 58.0 y; 72.2% postmenopausal | Netherlands; 96.7% white, 1.4% non-white | Elicited by questionnaire, maternal | Symptomatic POP measured through questionnaire | Age, assumed adjusted covariates: hysterectomy, incontinence surgery, current heavy physical work, POP symptoms during pregnancy, mother incontinence | aOR 1.67 (1.10–2.54) | Provided shortened questionnaire for initial non-responders; 5 |
| Miedel et al (2009), Nonobstetric risk factors for symptomatic pelvic organ prolapse | Population-based, case-control; 558 | Cases: 53.3 y, controls: 49.1 y; age at menopause collected but not reported in study | Sweden; ethnicity not reported | Elicited by questionnaire, maternal and sister | Symptomatic POP measured through questionnaire | Age, parity | aOR 3.26 (1.67–6.35) | Case participants were queried from a previous survey for POP; 7 |
| Braekken et al (2009), Pelvic floor function is independently associated with pelvic organ prolapse | Hospital-based, case-control; 98 | Cases: 47.3 y, controls: 47.0 y; 36.7% postmenopausal | Norway; ethnicity not reported | Elicited by questionnaire; maternal and grandmother | POP-Q measurement, cases stage ≥ 2, controls defined as stage 0–1 | BMI, socioeconomic status | aOR 1.9 (0.5–7.6) for unknown family history; 2.2 (0.6–8.1) for positive family history | Association between family history and POP seen without adjustments for BMI and socioeconomic status; 7 |
| Lukanovic et al (2010), Risk factors for vaginal prolapse after hysterectomy | Hospital-based, case-control; 206 | Cases: 52.2 y, controls: 53.3 y; 32% postmenopausal | Slovenia, ethnicity not reported | Elicited by questionnaire; for any familial history | Cases were chosen for undergoing post-hysterectomy POP procedure; not objectively measured | Could not be specifically identified | aOR 2.2 (1.2–4.3) | Both cases and controls had hysterectomy for benign indications (including POP); 5 |
| Levin et al (2012), Characterizing the phenotype of advanced pelvic organ prolapse | Hospital-based, case-control; 346 | Cases: 64.7 y, controls: 68.6 y; percent postmenopausal not provided and could not be inferred | United States, 90.9% white, other ethnicities not reported | Elicited by questionnaire, maternal and sister | POP-Q measurement, cases defined as stage 3–4, controls as 0–1; stage 2 not recruited | White race, BMI, constipation, vaginal parity, family history of hernias | aOR 3.74 (2.16–6.46) | Study was a secondary analysis of prospective cohort study for genetic epidemiologic study; preferentially recruited younger patients with prolapse and older patients with no prolapse; 6 |
| Rodriguez-Mias et al (2015), Pelvic organ prolapse and stress urinary incontinence, do they share the same risk factors? | Hospital-based, cross-sectional; 502 | 59.6 y; 69.4 postmenopausal | Spain, ethnicity not reported | Elicited by questionnaire, for any familial history | Defined POP as “vaginal bulge” | Could not be specifically identified | aOR 3.91 (2.20–6.96) for POP compared to SUI | POP patients compared with UI and mixed pathology, no controls; 4 |
| Mothes et al (2016), Risk index for pelvic organ prolapse based on established individual risk factors | Hospital-based, case-control; 736 | Mean ages not reported, percent postmenopausal not provided and could not be inferred, however 55.3% ≥ 60 y | Germany, 100% Caucasian | Controls: elicited by questionnaire, for maternal, sister, or grandmother; cases: medical records reviewed | Baden-Walker classification; cases defined as grade 3–4; controls had pre-operative exam to exclude POP | Years from menopause, difficult OB history | aOR 7.28 (4.65–11.40) | Multivariable regression analysis excluded hernia, age, and heavy lifting due to their correlation to similar to other variables; 4 |
| Wang et al (2015), Association of matrix metalloproteinase-10 polymorphisms with susceptibility to pelvic organ prolapse | Hospital-based, case-control; 263 | Cases: 58.7 y, controls: 56.1 y; 46.4% postmenopausal | China, ethnicity not reported | Method of family history collection not specified | POP-Q measurement, cases defined as stage ≥ 2, controls as stage ≤ 1 | None | 10.99 vs 0.58%, p < 0.001 | Degree of family history and method of collection unknown; only raw numbers provided by authors, thus meta-analysis computed crude OR; 3 |
| He et al (2016), MicroRNA-92 expression may be associated with reduced estrogen receptor b1mRNA levels in cervical portion of uterosacral ligaments in women with pelvic organ prolapse | Hospital-based, case-control; 104 | Cases: 58.4, controls: 56.3; percent postmenopausal not provided and could not be inferred | China, ethnicity not reported | Elicited by questionnaire, for any familial history | POP-Q measurement, cases defined as ≥ 2, controls as < 1 | None | 10.71 vs 2.08%, p < 0.083 | Sub-analysis performed for stages I and II, with no significant difference in family history; only raw numbers provided by authors, thus meta-analysis computed crude OR; 4 |
| Asresie et al (2016), Determinants of pelvic organ prolapse among gynecologic patients in Bahir Dar, North West Ethiopia: a case–control study | Hospital-based, case-control; 349 | Cases: 46 y, controls: 39 y; percent postmenopausal not provided and could not be inferred | Ethiopia, ethnicity not reported | Elicited by questionnaire, for any familial history | POP-Q measurement, cases defined as stage 3–4, controls as stage 0; stage 1–2 were excluded | Could not be specifically identified | aOR 4.9 (1.94–12.63) | Majority of cases were delivered by non-health professionals at home compared to controls; 4 |
| Jelovsek et al (2018), Predicting risk of pelvic floor disorders 12 and 20 years after delivery | Hospital-based, cohort; ProLong: 2095, SwePOP: 2585 | Unable to infer from study | ProLong: United Kingdom and New Zealand, 96% non-Asian, 4% Asian; SwePOP: Sweden, ethnicity no reported | Elicited by questionnaire; ProLong: for any familial history; SwePOP for maternal history | ProLong: POP-Q measurement; SwePOP: POP via symptom questionnaire | ProLong: Adjusted for 11 covariates, refer to Supplementary Table A SwePOP: Adjusted for 9 covariates, refer to Supplementary Table B |
ProLong: OR 1.53 (1.17–1.99); SwePOP: 2.56 (1.92–3.40) | Population was from 2 longitudinal cohort studies: ProLong with patients 12 y after birth and SwePOP with patients 20 y after birth, only primiparous women Author was contacted for study specific odds ratios and confidence intervals; 6 |
| Forner et al (2019), Symptoms of pelvic organ prolapse in women who lift heavy weights for exercise: a cross-sectional study | Population-based, cross-sectional; 3934 | 40.3 y; 17.4% postmenopausal; Cases: 45.9 y; 32.2% postmenopausal | Australia, ethnicity not reported | Elicited by questionnaire, for any familial history | Symptomatic POP measured through questionnaire | Age, parity, forceps delivery, cesarean delivery, menopausal status, hysterectomy, constipation/hemorrhoids, activity level (inactive, light, moderate, and heavy lifting) | aOR 2.21 (1.72–2.84) | Effect of family history not calculated by weight (lb) lifting subset; 5 |
NewCastle-Ottawa Quality Assessment Scale
Two studies by Slieker-ten et al refer to the same study population and are referenced within the manuscript
Table 2.
Description of study characteristics of 6 studies evaluating risk of pelvic organ prolapse recurrence and genealogical linkage
| Studies evaluating risk of pelvic organ prolapse recurrence | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Study design, sample size | Mean age (SD) and % postmenopausal | Country of study or race/ethnicity | Family history | POP measurement | Covariates adjusted for | Results RR/OR/hazard ratio (95% CI) | Comments; NOS Scorea |
| Diez-Itza et al (2007), Risk factors for the recurrence of pelvic organ prolapse after vaginal surgery: a review at 5 years after surgery | Hospital-based, case-control; 134 | Cases: 60.5 y, controls: 64.9; percent postmenopausal not provided and could not be inferred | Spain, ethnicity not reported | Method of family history collection not specified | POP-Q measurement, cases defined as stage ≥ 2 or with symptomatic recurrence | None | 27.4 vs 30 %, p = 1 | Univariate analysis performed unless finding borderline significance; 4 |
| Jeon et al (2008), Risk factors for the recurrence of pelvic organ prolapse | Hospital-based, cohort; 212 | 59.3 y; 86.8% postmenopausal | Korea, ethnicity not reported | Elicited by questionnaire, maternal and sister | POP-Q measurement, cases defined as stage ≥ 2 | None | HR 2.0 (1.0–4.1) | Family history was a significant risk factor with univariate analysis, however significance not found with multivariable analysis; 6 |
| Weemhoff et al (2012), Avulsion of puborectalis muscle and other risk factors for cystocele recurrence: a 2-year follow-up study | Hospital-based, cohort; 156 | Average age and percent postmenopausal could not be inferred | Netherlands, ethnicity not reported | Elicited by questionnaire, maternal and sister | POP-Q measurement, cases defined as stage ≥ 2 or with symptomatic recurrence | Could not be specifically identified | aOR 2.4 (1.2–4.9) | Family history collected as part of a follow-up survey; participants contacted from a previous study pool; see Vergeldt et al; 6 |
| Vergeldt et al (2016), Anatomical cystocele recurrence: development and internal validation of a prediction model | Notten subgroup: Case-control, 122 Weemhoff subgroup, 148 | Average age and percent postmenopausal could not be inferred | Netherlands, ethnicity not reported | Elicited by questionnaire, maternal and sister | POP-Q measurement, cases defined as stage ≥ 2 or with symptomatic recurrence | Could not be specifically identified | OR 0.8 (0.4–1.7) | Prediction model based on secondary analysis of two studies; Author was contacted for estimates from Notten and Weemhoff sub-groups; 4 |
| Studies evaluating genealogical linkage to prolapse (proband studies) | ||||||||
| Hamer et al (2013), Familial predisposition to pelvic floor dysfunction: prolapse and incontinence surgery among family members and its relationship with age or parity in a Swedish population | Population-based, cohort; 61,323 | Unable to infer from study | Sweden, ethnicity not reported | Elicited from linking two Swedish registers: The Hospital Discharge Register (inpatient discharges) and Multi-Generation Register (familial information) | Cases defined from surgical codes for prolapse; controls extrapolated from population risk | Stratified analysis by parity, and age were performed for pelvic surgery. Analysis for POP surgery was not adjusted. | RR 2.56 (2.41–2.73) with affected mothers; RR 6.58 (6.32–6.86) with affected sisters | Expected risk calculated from specific age and parity; RR for any pelvic surgery (urinary incontinence and POP) increased by increasing age and parity at time of surgery |
| Allen-Brady et al (2020), Risk of pelvic organ prolapse treatment based on extended family history | Population-based, cohort; 453,522 | Unable to infer from study | United States, ethnicity not reported | Elicited from Utah Population Database, consisting of both genealogic and medical data | Cases defined by ICD codes for both prolapse diagnosis and treatment; controls extrapolated from population risk | No adjustment | RR ≥1 FDR 2.36 (2.15–2.58); RR ≥1 SDR 1.23 (1.10–1.38); RR ≥1 TDR 1.08 (1.00–1.16) | RR calculated by first, second, and third-degree relative subsets; authors included probands only, defined as women with data from at least 12 of 14 relatives |
NewCastle-Ottawa Quality Assessment Scale
Two main categories of meta-analyses were undertaken: one evaluating association between family history and POP status (yes/no), another evaluating association between family history and POP recurrence (yes/no) among women who had corrective surgery for POP. A priori, effect-estimates from non-overlapping studies were aggregated together using random-effects models if there were ten or more studies in a given meta-analysis set and inverse variance-weighted fixed-effects models otherwise. For completeness and transparency, meta-analysis estimates from fixed-effects and random-effects models were computed and reported. We used OR as the primary measure of association for both categories of meta-analyses as this measure of association was most commonly reported. With the exception of one study that reported a relative risk and another that reported a hazard ratio, all other studies reported odds ratios. When possible, ORs were computed, otherwise the given effect estimate was treated as an OR and meta-analyzed together. Meta-analysis effect estimates will be referred to as meta-analysis ORs hereafter.
When two or more estimates were provided or could be computed from a given study, an effect-estimate was preferentially chosen in the following order: multivariable adjusted OR, unadjusted OR given by the authors, and unadjusted OR computed by meta-analyst based on raw numbers that were provided. When two or more effect estimates were provided by a given study either due to different definitions of POP or family history, two sets of were analyses were completed: one considering the smallest of the two or more effect estimates analyzed in one meta-analysis set (referred to hereafter as the minimum set) and another considering the largest of the two or more effect estimates analyzed in another meta-analysis set (referred to hereafter as maximum set). In all analyses no positive family history of POP was considered as the referent group. Heterogeneity for each meta-analysis was reported using the Q-statistic and the I2 statistic. Evidence for small-study bias/publication bias was evaluated through visual inspection of funnel plots and the Egger test.
Finally, we investigated whether the relationship between family history of POP and POP status evaluated in the primary analyses varied by key study characteristics. These included POP assessment type (clinical assessment or self-report/symptomatic), study design (case-control, cohort, cross-sectional), multivariable adjustment (yes or no), family history of disorder(s) (POP only, connective tissue disorders, or unspecified family history), and relation (first/second degree relatives or unspecified). Sub-group analyses were performed using inverse variance weighted fixed effects and random effects meta-analytic approaches for completeness. All meta-analysis estimates from primary analyses are presented as ORs and corresponding 95% confidence intervals (95% CI). A secondary meta-analysis was conducted with two registry-based genealogical studies not eligible for primary meta-analysis reporting risk ratios estimated from POP cases and population-based expectations for controls [16,17]. All analyses were performed with STATA/MP (College Station, TX) [18]. This review was performed in adherence to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines, and results are reported in adherence to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) criteria.
RESULTS:
Full text review of 43 articles found 18 independent studies [19–37] were eligible for inclusion into meta-analyses evaluating the relationship between family history of POP and participant POP risk (Table 1). These studies reported a total of 21 effect estimates. One study reported separate estimates for mother’s history and sister’s history of POP [19]. Another reported three estimates by severity of POP, overall, and by strata of severity (mild and moderate severity) (McLennan et al.) [22]. Jelovsek et al. [36] reported as graphs the relationship between family history and POP-status evaluated in two independent populations [38,39]. Dr. Jelovsek provided adjusted ORs, confidence intervals and raw numbers for both of the independent studies upon request. These were included as separate entries the meta-analysis [36]. Fifteen effect estimates were presented in articles as multivariable adjusted. Thirteen were reported as case-control studies, three as cohort and two as cross-sectional. Of the 18 studies evaluating primary risk of POP, the NewCastle-Ottawa Quality Assessment Scale (NOS) scores ranged from 3–7, with a median score of 5. The largest meta-analysis set included a total of 19 independent effect estimates from a total of 3,639 POP cases and 10,912 controls.
Two genealogical studies [16,17] linked to electronic health records reported risk ratios for family history of POP. These studies computed unadjusted risk ratios based on observed proband POP cases and expected controls estimated from population rates. Due to lack of an internal comparison group, these studies did not meet a priori criteria and were ineligible for primary meta-analysis. A separate meta-analysis set was constructed for this class of studies.
Four studies provided adequate information to evaluate the relationship between family history of POP and POP recurrence (Table 2) [40–42]. The median NOS score of the four studies was 5. Vergeldt et. al evaluated risk factors for POP recurrence using data from two independent studies originally described by Weemhoff et al. [42] and Notten et al. [43]. Dr. Vergeldt was contacted for study-specific effect estimates relating to family history of POP and POP recurrence for the Weemhoff and Notten databases. Four effect estimates were available for this meta-analysis in a total of 224 recurrent cases and 400 controls (no recurrence). Three of these effect estimates were from cohort studies [40–42].
Meta-analysis: Family history and risk of having POP
Women reporting a positive family history of POP were more likely to have POP themselves compared to women who did not report a positive family history of POP, with a random-effects OR of 2.64 (95% CI = 2.07, 3.35) for the minimum analysis set, and 2.68 (95% CI = 2.12, 3.39) in the maximum analysis set (Figure 2 and Supplementary Table 1). Estimates from fixed effects models were slightly attenuated in magnitude (Supplementary Table 1). Both the minimum and maximum models showed evidence of heterogeneity across studies owing to factors other than chance (I2 estimates: 71% – 73%) (Supplementary Table 1). Visual inspection of funnel plots showed asymmetry contributed by a few studies suggesting some evidence of small study bias in minimum and maximum scenarios (Figure 3a-b). The Egger test also showed evidence for small study bias particularly for the maximum analysis set (Bias coefficient = 1.80; P = 0.031). In sensitivity analyses, removing Wang et. al [33] from analyses attenuated the bias (Bias coefficient = 1.68; P = 0.064).
Figures 2a-b.
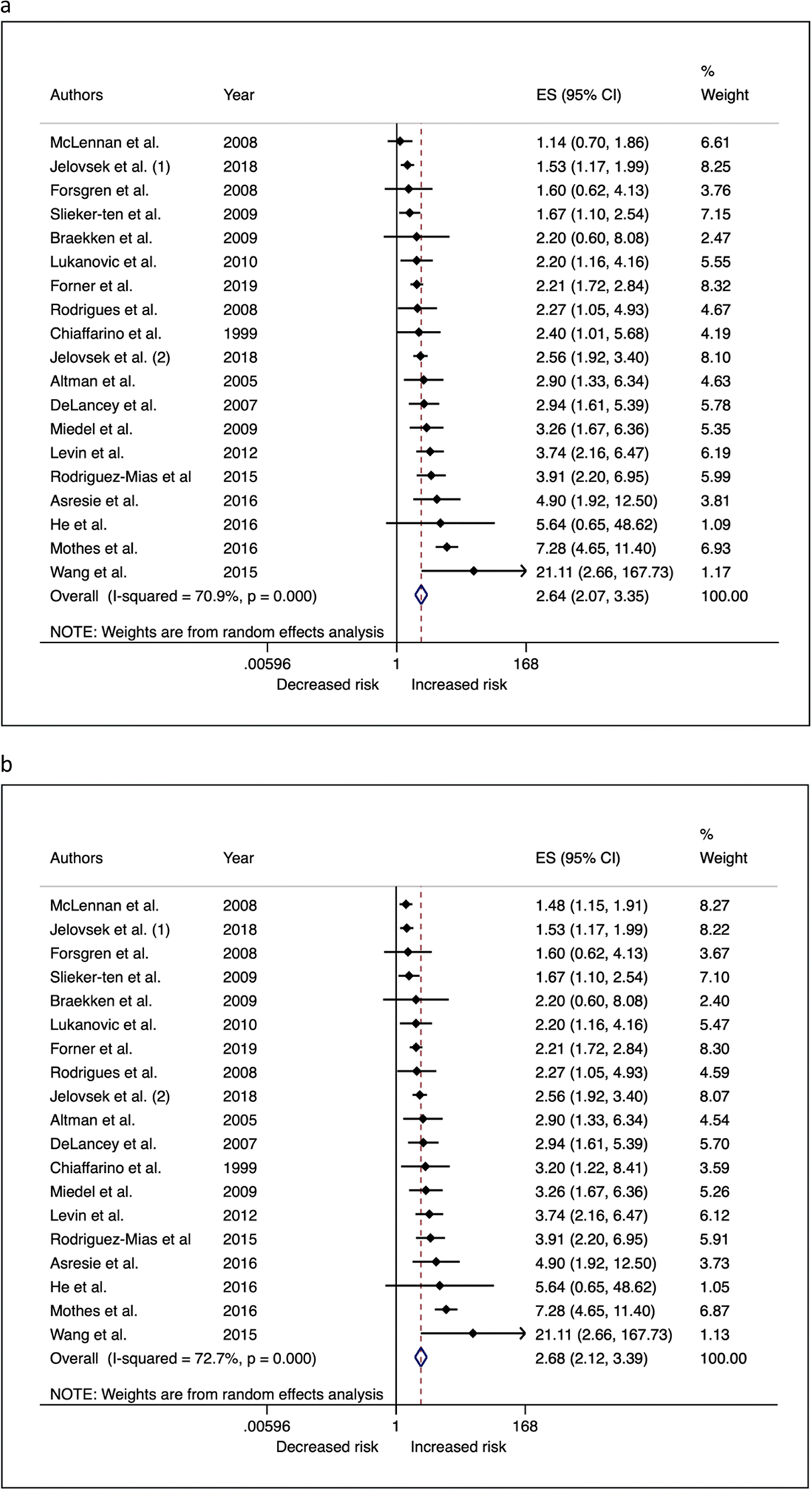
Forest plots showing (a) minimum and (b) maximum meta-analysis odds ratios for the association between family history of POP and POP in participants.
Figure 3a-b.
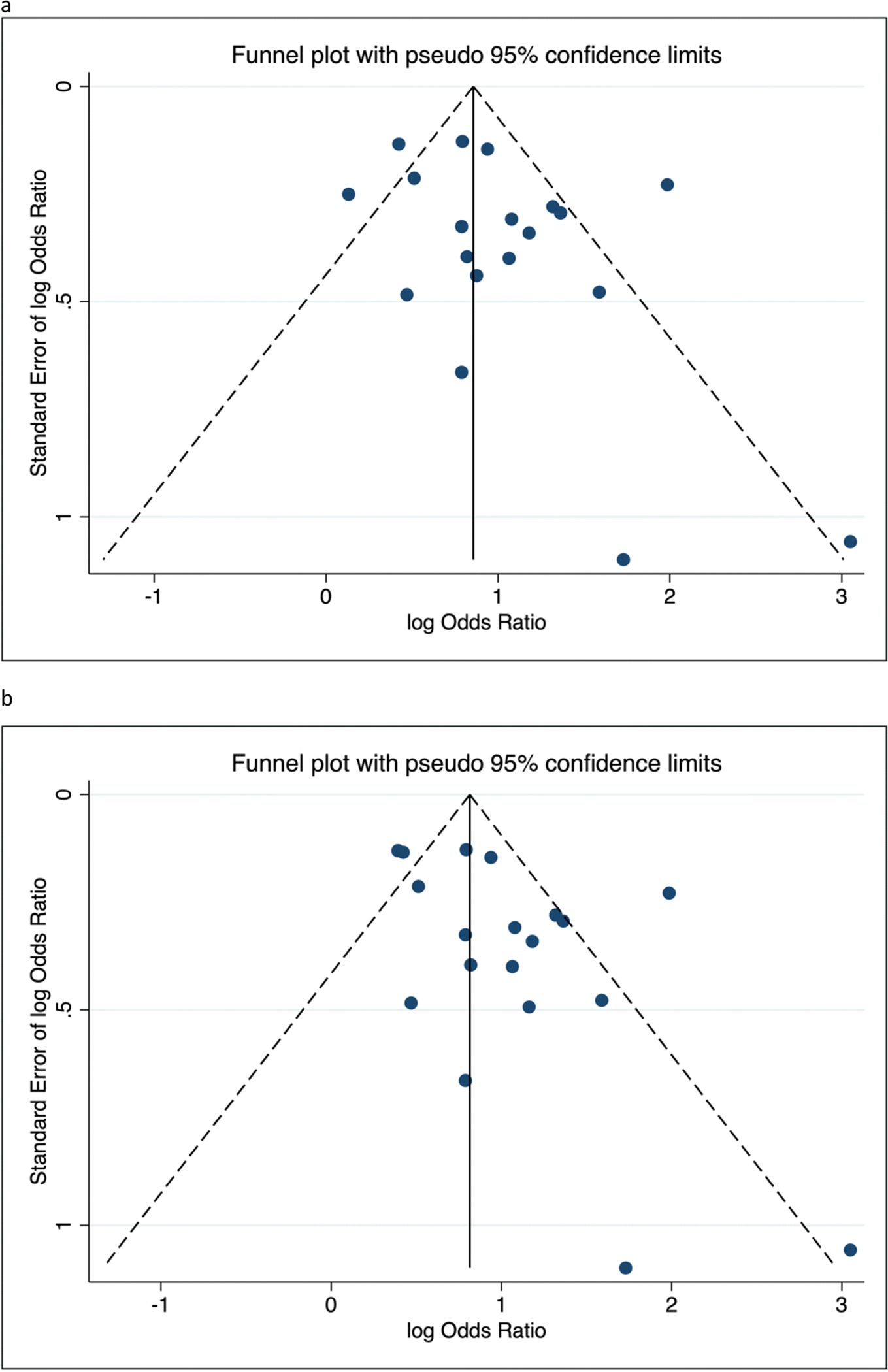
Funnel plots of studies included in the (a) minimum and (b) maximum meta-analysis sets for the association between family history of POP and POP in participants*
*Funnel plots suggest potential for small study bias as demonstrated by asymmetry in the plots. Statistical evidence for small study bias was also detected for the maximum analysis sets.
We further investigated sources of heterogeneity by performing sub-group analyses based on key study characteristics that could alter effect estimates, chosen a priori (Table 3 – fixed effects; Supplementary Table 2 – random effects; Figures 4 and 5). Studies evaluating family history in first and/or second-degree relatives on average reported stronger effect estimates (OR = 2.37; 95% CI = 2.04, 2.75) than studies that did not clearly specify family history (OR = 1.97; 95% CI = 1.72, 2.25). Studies evaluating history of connective tissue disorders (including POP, hernia or connective tissue disorders) on average had smaller effect estimates (OR = 1.86; 95% C: 1.55, 2.32) than studies that specifically reported evaluating POP (OR = 2.23; 95% CI = 1.97, 2.52) or those studies that did not clearly define family history (OR = 3.55; 95% CI = 1.93, 6.52). Case-control studies were more likely to report stronger estimates (OR = 3.55; 95% CI = 2.89, 4.36) than cohort/cross-sectional studies (OR = 1.82; 95% CI = 1.65, 2.05). Studies that did not adjust for confounding and risk factors had higher effect estimates on average (OR = 3.55; 95% CI = 2.02, 6.22) than studies that performed multivariable-adjusted regression (OR = 2.10, 95% CI = 1.90, 2.33). Meta-analysis of risk ratios from two genealogical studies showed mother’s history of POP was associated with a 2.5 fold increased risk of POP (FE RR = 2.50; 95% CI = 2.36, 2.65), and sisters history of POP was associated with 5.8 fold increased risk of POP (FE RR = 5.88; 95% CI = 5.66, 6.12).
Table 3.
Sensitivity analysis of associations between family history of POP and participant POP status by study design, adjustment of confounders and method of outcome assessment
| Sensitivity Analysis | N-estimates* | OR | 95% CI | P-value | Q-statistic | I2 |
|---|---|---|---|---|---|---|
| POP assessment type: | ||||||
| Clinical assessment | 13 | 2.23 | (1.93, 2.58) | 2.77×10−27 | 63.39 | 81.1% |
| Symptomatic/self-reported | 6 | 2.05 | (1.79, 2.36) | 2.44×10−24 | 10.99 | 54.5% |
| History in whom: | ||||||
| 1st/2nd Degree Relatives | 8 | 2.37 | (2.04, 2.75) | 2.32×10−29 | 40.75 | 82.8% |
| Family History | 11 | 1.97 | (1.72, 2.25) | 7.57×10−23 | 31.04 | 67.8% |
| History of what: | ||||||
| POP only | 12 | 2.23 | (1.97, 2.52) | 1.11×10−37 | 20.28 | 45.8% |
| Connective tissue disorder | 3 | 1.86 | (1.55, 2.32) | 2.93×10−11 | 43.26 | 95.4% |
| History not specified | 4 | 3.55 | (1.93, 6.52) | 4.50×10−5 | 6.19 | 51.5% |
| Study Design: | ||||||
| Case-control | 13 | 3.55 | (2.89, 4.36) | 1.35×10−33 | 21.54 | 44.3% |
| Cohort or cross-sectional | 6 | 1.82 | (1.65, 2.05) | 1.31×10−24 | 22.81 | 78.1% |
| Multivariable adjusted: | ||||||
| Yes | 16 | 2.10 | (1.90, 2.33) | 3.37×10−46 | 68.41 | 78.1% |
| No | 3 | 3.55 | (2.02, 6.22) | 9.42×10−6 | 3.39 | 41.0% |
Represents independent effect estimates from studies; OR = Odds Ratio 95% CI = 95% Confidence interval; I2 = Heterogeneity statistic; ORs are based on inverse variance fixed effects analyses
Figure 4a-c.
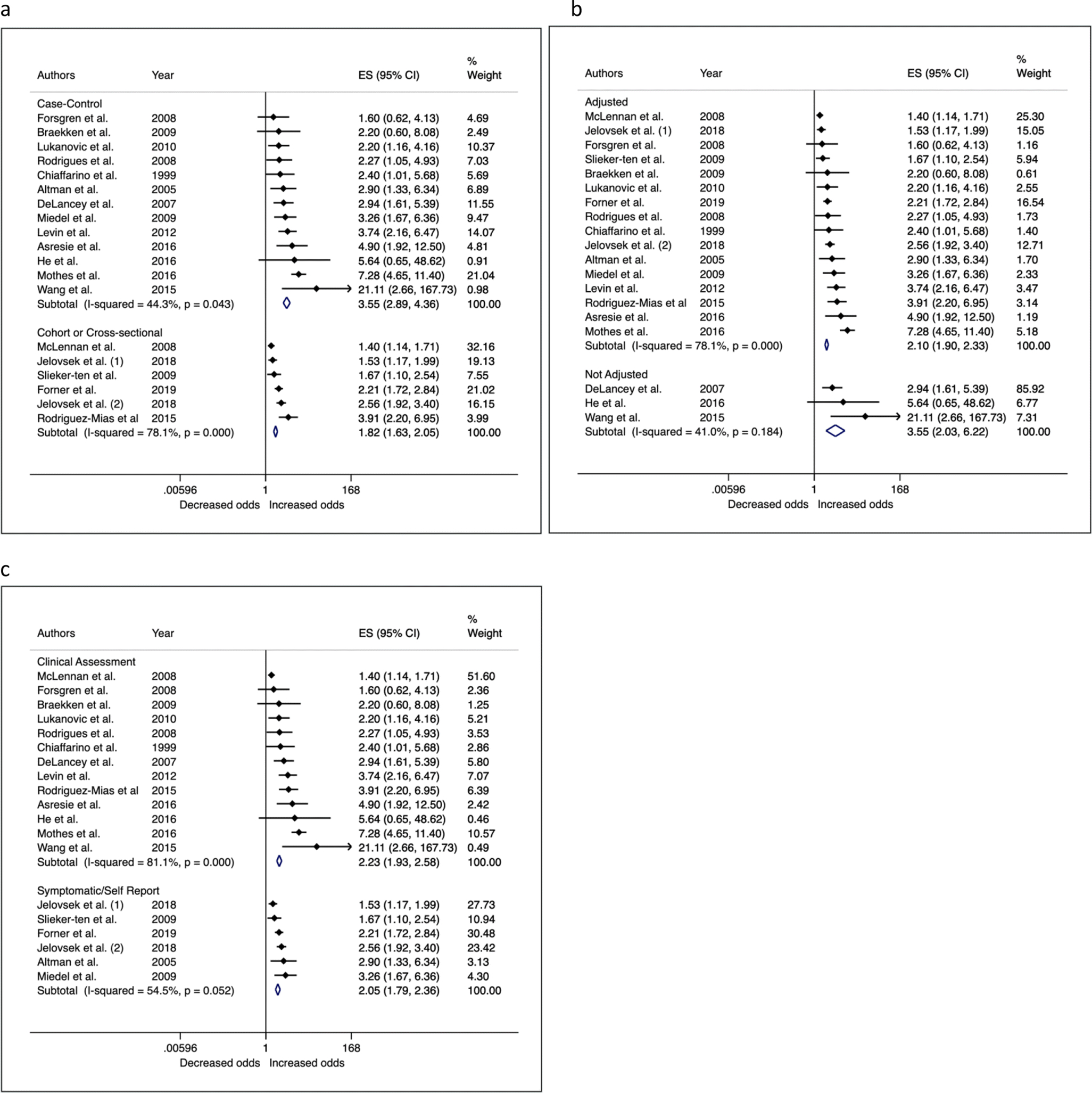
Forest plots of sensitivity analyses showing associations between family history of POP and participant POP status by study design (a), adjustment of confounders (b) and method of outcome assessment (c)
Figure 5a-b.
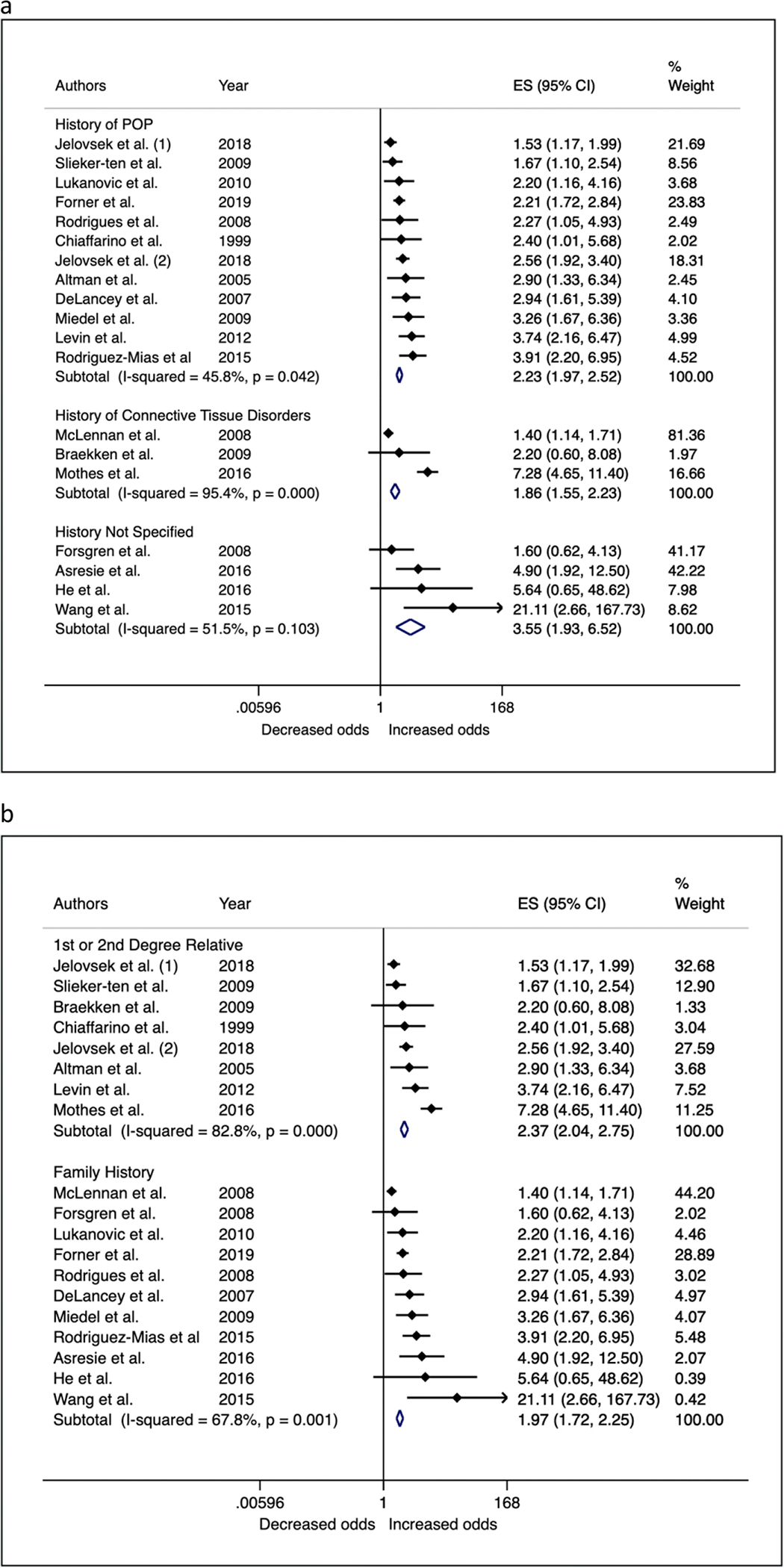
Sensitivity analysis of the association between family history of POP and participant POP status by type of family history that was assessed (a) and in whom (b)
Meta-analysis: Family history and risk of POP recurrence
Women who had surgical correction for POP and reported a positive family history of POP were on average 1.4 times as likely to have recurrent POP than women with surgical correction for POP but no family history of POP (Supplementary Table 1). Although ORs were similar between fixed (OR = 1.44; 95% CI = 1.00, 2.08) and random effects (OR = 1.43; 95% CI = 0.85, 2.39), the CI for the latter included unity. Evidence for heterogeneity between estimates was moderate (I2 = 50%) and due to the fewer number of studies, tests for small study bias, and sub-group analyses by strata of key study characteristics were not performed.
DISCUSSION:
We performed a systematic review and meta-analysis of peer-reviewed journal articles published in English to evaluate observational studies that reported associations for family history of POP in relation to POP risk and POP recurrence. Meta-analysis of eighteen estimates showed a positive family history of POP is associated with 2.3 to 2.7-fold increased risk for POP. In a smaller set of four studies consisting of women who had surgical correction for POP (224 recurrent cases and 400 POP cases without recurrence), having a positive family history of POP was associated with a 1.4-fold increased risk for POP recurrence.
In a published meta-analysis from 2012, Lince et. al reported a 2.6-fold increased odds of POP in women with a positive family history of POP compared to women without a positive family history of POP [8]. This informative study reported these estimates based on raw numbers from eight studies to perform a Mantel-Haenzel weighted meta-analysis, which is equivalent to performing a fixed-effects inverse variance weighted meta-analysis of unadjusted ORs. Our study extends findings from this previous meta-analysis and improves the quality of evidence in the following ways: 1) increases number of effect estimates from eight to 18 in the largest meta-analysis set, 2) prioritizes multivariable-adjusted effect estimates over unadjusted estimates using a method that requires only effect estimates and standard errors, 3) presents results under fixed effects and random effects assumptions to provide a range of estimates rather than a single number, 4) assesses evidence for small-study bias/publication bias, and 5) performs sub-group analyses to identify sources of heterogeneity.
Our analysis suggests that the reported effect estimates of the association between family history and POP status may be overestimated for several reasons. We found studies only reporting raw numbers or associations unadjusted for confounders tended to yield larger effect estimates (OR = 3.55) than studies that performed multivariable adjustment for confounding (OR = 2.10). Meta-analysis effect-estimates of studies identifying as case-control tended to be larger in magnitude than of studies identifying as cohort or cross-sectional. It is worth noting that studies identifying as cohort and cross-sectional had larger sample sizes than case-control studies. Recognizing that studies measure POP using variable definitions, we performed meta-analysis of studies that measured POP via clinical assessment separately from studies that measured POP through self-report. Although we did not find meaningful differences on average across these two categories of studies, we found heterogeneity was much larger in studies with clinical assessment for POP (I-squared 81.1%; Fig 4c). POP was measured using various different systems including surgical codes, the Baden-Walker system and POP-Q staging, and thresholds for what was considered POP within each system was also variable. For example, three of the examined studies excluded participants with measured Stage II prolapse and on average report large effect estimates. The varying definitions of POP within this group of studies may have in-part contributed to the breadth of associations observed. In addition to variability in POP measurement, all of the studies have two common limitations; assessment of family history of POP was based on self-report and this assessment did not precede assessment of POP status.
Results of this study should be interpreted in light of two important sources of bias: small study/publication bias and recall bias. We assessed evidence of small study/publication bias in our meta-analysis. One of our analysis sets (maximum scenario), showed some evidence of bias suggesting that smaller studies with larger effect estimates (likely with statistically significant p-values) were more likely to publish their findings than smaller studies with statistically non-significant p-values. While the magnitude of the bias is not fully quantified, detection of this bias in one of our estimates between family history and POP status suggests an over-estimation of the true association.
POP is often viewed as a private matter and is less openly discussed with family members than conditions such as cancer or cardiovascular disease. Thus, if individuals who have POP are more likely to inquire about their family members’ history of POP than individuals without POP, recall bias is a possibility. All of the studies evaluated in the meta-analysis assessed family history through self-report at the time of POP assessment. The observed association between family history and POP status is likely overestimated due to this potential bias. The magnitude of the bias remains unknown as we have no sub-group/comparator studies that verified or validated self-reported family history through another mechanism such as health record review. However, we also found that a positive family history of POP was associated with increased risk of POP recurrence in women who had POP surgery. The mechanism of recall bias is diminished in this scenario and suggests that the observed association between family history and POP status may not be entirely due to recall bias. The smaller effect size observed for family history and recurrent POP versus that observed for POP risk is also intuitive. Since the comparator group for the recurrent POP studies includes individuals with POP – individuals who already have an increased risk of POP associated with family history – the observed association for recurrent POP could be interpreted as excess risk beyond that observed for POP risk.
Two large genealogical studies estimated relative risks based on linked electronic health records also reported increased risk of POP with mother’s history and sister’s history of POP [16,17]. Limitations of this methodology include a reliance on surgical coding for the presence of POP. Errors in coding may exist and often cannot be corrected from historical records. A notable strength of the genealogic studies is their size and analysis of a homogenous population. Although not influenced by recall bias, the magnitude of estimates from these studies could be over-estimated due to lack of control of correlated factors such as parity, obesity, and obstetric factors, as also suggested by the comparison of adjusted and unadjusted estimates in our primary meta-analysis sets. Assessments made in this review for genealogical studies, survey-based studies and those performed by Lince et al. converge towards an estimate of 2.6-fold increased risk on average. However, we cannot rule out the possibility that all three of these assessments are likely overestimates due to lack of/inadequate adjustment of confounding, recall bias, publication bias or some combination of these factors.
Only three out of the 19 eligible studies from 18 articles were conducted in predominantly diverse populations, two small studies in China [33,34] and one in Ethiopia [35]. We were not able to perform meta-analyses by strata of race/ethnic groups. The results of this study may predominantly apply to individuals of European ancestry. Race/ethnicity is potentially linked to POP, however, there is no evidence to suggest that the positive association between family history and POP would not be present in diverse populations. The three studies in non-white populations report positive associations for family history and POP, although with large standard errors due to small sample sizes.
With the exception of a twin-study that estimated a heritable component of POP at 40% [44], to our knowledge, the majority of evidence for POP heritability is inferred from family history studies. Several candidate gene studies and a few genome-wide association studies (GWAS) have investigated genetic variants in relation to POP [45–47], and with the lack of large scale GWAS on POP the search for genetic variants continues. Until we discover clinically meaningful genetic variants that reliably predict POP outcomes, family history of POP remains the closest proxy for understanding a patient’s inherent risk for POP. It is worth noting that in the absence of adjustment for social, and life-style factors shared by family structures, family history acts as a composite construct that encompasses genetic, and social and life-style similarities and has utility as a predictor for POP risk. To use family history as a meaningful predictor for POP in patients wanting to understand their individual risk, alone or in combination with other predictors, there is a need to consistently define family history with attention to two specific components: history of which condition(s) and in whom this history is being measured. Combining estimates of family history from mother, sister, and/or grandmother averages risk estimates and reduces accuracy of prediction if these estimates are different. For this average estimate to be accurate for everyone we would need to assume that the risk of POP associated with having one or more relatives with a history of POP (mother, sister and/or grandmother) alone or in combination is similar if not the same. This is an over simplified and unlikely assumption. We present evidence of this in our sub-group analysis where we find studies explicitly asking about family history of POP in first- and second-degree relatives showed stronger effect estimates on average than studies that did not clearly define family history.
Similarly, studies evaluating family history do not consistently assess the type of disease/condition measured for history. Studies evaluated in this meta-analysis included history of POP, connective tissue disorders, and hernia, and many do not clearly define the history of disease being measured. We found studies reporting a broader umbrella for history such as connective tissue disorders (inclusive of POP) tended to have estimates closer to the null than studies specifically evaluating history of POP. Studies that simply reported ‘family history’ without an explicit definition tended to yield the largest meta-analysis association. It is possible that these studies measured history of POP only. We encourage future studies evaluating family history of POP based on self-report to collect information on history of a defined condition explicitly and separately in mother, sister, grandmother, and aunt, and to report these estimates separately at the very least, and in combination if necessary. Alternatively, use of genealogic cohorts to conduct well-controlled analytic observational studies based on internally constructed controls would mitigate concerns regarding recall bias and also provide opportunity to estimate associations controlled for confounding.
Our review of the literature suggests that a positive family history of POP is associated with increased risk of having POP and POP recurrence. The accuracy of these estimates is likely affected by recall bias, publication bias and heterogeneous definitions of family history. If the primary goal is to use family history as a component in a predictive algorithm for POP risk or recurrence, future studies should focus on standardized and clear definitions of family history with external verification.
Supplementary Material
Acknowledgments
Funding: Dr. Ayush Giri was a scholar of the Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) program [2K12HD043483 (PI: Katherine Hartmann)] and a recipient of the NIDDK Research Scientist Development Award [1K01DK120631–01A1] when this work was performed.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
REFERENCES:
- [1].Weber AM, Richter HE. Pelvic organ prolapse. Obstet Gynecol 2005;106:615–34. 10.1097/01.AOG.0000175832.13266.bb. [DOI] [PubMed] [Google Scholar]
- [2].Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet 2007;369:1027–38. 10.1016/S0140-6736(07)60462-0. [DOI] [PubMed] [Google Scholar]
- [3].Barber MD, Maher C. Epidemiology and outcome assessment of pelvic organ prolapse. Int Urogynecol J 2013;24:1783–90. 10.1007/s00192-013-2169-9. [DOI] [PubMed] [Google Scholar]
- [4].Wu JM, Matthews CA, Conover MM, Pate V, Jonsson Funk M. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol 2014;123:1201–6. 10.1097/AOG.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in U.S. Women: 2010 to 2050. Obstet Gynecol 2009;114:1278–83. 10.1097/AOG.0b013e3181c2ce96. [DOI] [PubMed] [Google Scholar]
- [6].Vergeldt TFM, Weemhoff M, IntHout J, Kluivers KB. Risk factors for pelvic organ prolapse and its recurrence: a systematic review. Int Urogynecol J 2015;26:1559–73. 10.1007/s00192-015-2695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Giri A, Hartmann KE, Hellwege JN, Velez Edwards DR, Edwards TL. Obesity and pelvic organ prolapse: a systematic review and meta-analysis of observational studies. Am J Obstet Gynecol 2017;217:11–26.e3. 10.1016/j.ajog.2017.01.039. [DOI] [PubMed] [Google Scholar]
- [8].Lince SL, van Kempen LC, Vierhout ME, Kluivers KB. A systematic review of clinical studies on hereditary factors in pelvic organ prolapse. Int Urogynecol J 2012;23:1327–36. 10.1007/s00192-012-1704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jelovsek JE, Chagin K, Gyhagen M, Hagen S, Wilson D, Kattan MW, et al. Predicting risk of pelvic floor disorders 12 and 20 years after delivery. Am J Obstet Gynecol 2018;218:222.e1–222.e19. 10.1016/j.ajog.2017.10.014. [DOI] [PubMed] [Google Scholar]
- [10].Zotero, Corporation for Digital Scholarship. Fairfax, Virginia, USA: n.d. [Google Scholar]
- [11].Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Luchini C, Stubbs B, Solmi M, Veronese N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World Journal of Meta-Analysis 2017;5:80–4. 10.13105/wjma.v5.i4.80. [DOI] [Google Scholar]
- [14].Stone J, Gurunathan U, Glass K, Munn Z, Tugwell P, Doi SAR. Stratification by quality induced selection bias in a meta-analysis of clinical trials. J Clin Epidemiol 2019;107:51–9. 10.1016/j.jclinepi.2018.11.015. [DOI] [PubMed] [Google Scholar]
- [15].Greenland S, O’rourke K. On the bias produced by quality scores in meta‐analysis, and a hierarchical view of proposed solutions. Biostatistics 2001;2:463–71. 10.1093/biostatistics/2.4.463. [DOI] [PubMed] [Google Scholar]
- [16].Allen-Brady K, Norton PA, Hill AJ, Rowe K, Cannon-Albright LA. Risk of pelvic organ prolapse treatment based on extended family history. Am J Obstet Gynecol 2020. 10.1016/j.ajog.2019.12.271. [DOI] [PubMed] [Google Scholar]
- [17].Andrada Hamer M, Persson J. Familial predisposition to pelvic floor dysfunction: prolapse and incontinence surgery among family members and its relationship with age or parity in a Swedish population. European Journal of Obstetrics & Gynecology and Reproductive Biology 2013;170:559–62. 10.1016/j.ejogrb.2013.07.025. [DOI] [PubMed] [Google Scholar]
- [18].STATA/MP. College Station, Texas, USA: StataCorp; n.d. [Google Scholar]
- [19].Chiaffarino F, Chatenoud L, Dindelli M, Meschia M, Buonaguidi A, Amicarelli F, et al. Reproductive factors, family history, occupation and risk of urogenital prolapse. Eur J Obstet Gynecol Reprod Biol 1999;82:63–7. [DOI] [PubMed] [Google Scholar]
- [20].Altman D, Zetterstrom J, Schultz I, Nordenstam J, Hjern F, Lopez A, et al. Pelvic organ prolapse and urinary incontinence in women with surgically managed rectal prolapse: a population-based case-control study. Dis Colon Rectum 2006;49:28–35. 10.1007/s10350-005-0217-3. [DOI] [PubMed] [Google Scholar]
- [21].DeLancey JOL, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, et al. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol 2007;109:295–302. 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- [22].McLennan MT, Harris JK, Kariuki B, Meyer S. Family history as a risk factor for pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct 2008;19:1063–9. 10.1007/s00192-008-0591-1. [DOI] [PubMed] [Google Scholar]
- [23].Rodrigues AM, Girao MJBC, da Silva IDCG, Sartori MGF, de F Martins K, de A Castro R. COL1A1 Sp1-binding site polymorphism as a risk factor for genital prolapse. Int Urogynecol J Pelvic Floor Dysfunct 2008;19:1471–5. 10.1007/s00192-008-0662-3. [DOI] [PubMed] [Google Scholar]
- [24].Forsgren C, Zetterstrom J, Lopez A, Altman D. Risk factors for vaginal vault prolapse surgery in postmenopausal hysterectomized women. Menopause 2008;15:1115–9. 10.1097/gme.0b013e31817062a5. [DOI] [PubMed] [Google Scholar]
- [25].Slieker-ten Hove MCP, Pool-Goudzwaard AL, Eijkemans MJC, Steegers-Theunissen RPM, Burger CW, Vierhout ME. Symptomatic pelvic organ prolapse and possible risk factors in a general population. Am J Obstet Gynecol 2009;200:184.e1–7. 10.1016/j.ajog.2008.08.070. [DOI] [PubMed] [Google Scholar]
- [26].Slieker-ten Hove MCPh, Pool-Goudzwaard AL, Eijkemans MJC, Steegers-Theunissen RPM, Burger CW, Vierhout ME. The prevalence of pelvic organ prolapse symptoms and signs and their relation with bladder and bowel disorders in a general female population. Int Urogynecol J Pelvic Floor Dysfunct 2009;20:1037–45. 10.1007/s00192-009-0902-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Miedel A, Tegerstedt G, Maehle-Schmidt M, Nyren O, Hammarstrom M. Nonobstetric risk factors for symptomatic pelvic organ prolapse. Obstet Gynecol 2009;113:1089–97. 10.1097/AOG.0b013e3181a11a85. [DOI] [PubMed] [Google Scholar]
- [28].Braekken IH, Majida M, Ellstrom Engh M, Holme IM, Bo K. Pelvic floor function is independently associated with pelvic organ prolapse. BJOG 2009;116:1706–14. 10.1111/j.1471-0528.2009.02379.x. [DOI] [PubMed] [Google Scholar]
- [29].Lukanovic A, Drazic K. Risk factors for vaginal prolapse after hysterectomy. Int J Gynaecol Obstet 2010;110:27–30. 10.1016/j.ijgo.2010.01.025. [DOI] [PubMed] [Google Scholar]
- [30].Levin PJ, Visco AG, Shah SH, Fulton RG, Wu JM. Characterizing the phenotype of advanced pelvic organ prolapse. Female Pelvic Med Reconstr Surg 2012;18:299–302. 10.1097/SPV.0b013e31826a53de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rodriguez-Mias NL, Martinez-Franco E, Aguado J, Sanchez E, Amat-Tardiu L. Pelvic organ prolapse and stress urinary incontinence, do they share the same risk factors? Eur J Obstet Gynecol Reprod Biol 2015;190:52–7. 10.1016/j.ejogrb.2015.04.015. [DOI] [PubMed] [Google Scholar]
- [32].Mothes AR, Radosa MP, Altendorf-Hofmann A, Runnebaum IB. Risk index for pelvic organ prolapse based on established individual risk factors. Arch Gynecol Obstet 2016;293:617–24. 10.1007/s00404-015-3863-2. [DOI] [PubMed] [Google Scholar]
- [33].Wang H, Zhang Z-Q, Wang S-Z, Lu J-L, Wang X-L, Zhang Z-Y. Association of matrix metalloproteinase-10 polymorphisms with susceptibility to pelvic organ prolapse. J Obstet Gynaecol Res 2015;41:1972–81. 10.1111/jog.12809. [DOI] [PubMed] [Google Scholar]
- [34].He K, Niu G, Gao J, Liu J-X, Qu H. MicroRNA-92 expression may be associated with reduced estrogen receptor beta1 mRNA levels in cervical portion of uterosacral ligaments in women with pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol 2016;198:94–9. 10.1016/j.ejogrb.2016.01.007. [DOI] [PubMed] [Google Scholar]
- [35].Asresie A, Admassu E, Setegn T. Determinants of pelvic organ prolapse among gynecologic patients in Bahir Dar, North West Ethiopia: a case-control study. Int J Womens Health 2016;8:713–9. 10.2147/IJWH.S122459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jelovsek JE, Chagin K, Gyhagen M, Hagen S, Wilson D, Kattan MW, et al. Predicting risk of pelvic floor disorders 12 and 20 years after delivery. Am J Obstet Gynecol 2018;218:222.e1–222.e19. 10.1016/j.ajog.2017.10.014. [DOI] [PubMed] [Google Scholar]
- [37].Forner LB, Beckman EM, Smith MD. Symptoms of pelvic organ prolapse in women who lift heavy weights for exercise: a cross-sectional survey. Int Urogynecol J 2019. 10.1007/s00192-019-04163-w. [DOI] [PubMed] [Google Scholar]
- [38].Gyhagen M, Bullarbo M, Nielsen TF, Milsom I. Prevalence and risk factors for pelvic organ prolapse 20 years after childbirth: a national cohort study in singleton primiparae after vaginal or caesarean delivery. BJOG 2013;120:152–60. 10.1111/1471-0528.12020. [DOI] [PubMed] [Google Scholar]
- [39].MacArthur C, Glazener C, Lancashire R, Herbison P, Wilson D, ProLong study group. Exclusive caesarean section delivery and subsequent urinary and faecal incontinence: a 12-year longitudinal study. BJOG 2011;118:1001–7. 10.1111/j.1471-0528.2011.02964.x. [DOI] [PubMed] [Google Scholar]
- [40].Diez-Itza I, Aizpitarte I, Becerro A. Risk factors for the recurrence of pelvic organ prolapse after vaginal surgery: a review at 5 years after surgery. Int Urogynecol J Pelvic Floor Dysfunct 2007;18:1317–24. 10.1007/s00192-007-0321-0. [DOI] [PubMed] [Google Scholar]
- [41].Jeon MJ, Chung SM, Jung HJ, Kim SK, Bai SW. Risk factors for the recurrence of pelvic organ prolapse. Gynecol Obstet Invest 2008;66:268–73. 10.1159/000149851. [DOI] [PubMed] [Google Scholar]
- [42].Weemhoff M, Vergeldt TFM, Notten K, Serroyen J, Kampschoer PHNM, Roumen FJME. Avulsion of puborectalis muscle and other risk factors for cystocele recurrence: a 2-year follow-up study. Int Urogynecol J 2012;23:65–71. 10.1007/s00192-011-1524-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Notten KJB, Kluivers KB, Fütterer JJ, Schweitzer KJ, Stoker J, Mulder FE, et al. Translabial Three-Dimensional Ultrasonography Compared With Magnetic Resonance Imaging in Detecting Levator Ani Defects. Obstetrics & Gynecology 2014;124:1190–1197. 10.1097/AOG.0000000000000560. [DOI] [PubMed] [Google Scholar]
- [44].Altman D, Forsman M, Falconer C, Lichtenstein P. Genetic Influence on Stress Urinary Incontinence and Pelvic Organ Prolapse. European Urology 2008;54:918–23. 10.1016/j.eururo.2007.12.004. [DOI] [PubMed] [Google Scholar]
- [45].Ward RM, Velez Edwards DR, Edwards T, Giri A, Jerome RN, Wu JM. Genetic epidemiology of pelvic organ prolapse: a systematic review. American Journal of Obstetrics and Gynecology 2014;211:326–35. 10.1016/j.ajog.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Allen-Brady K, Cannon-Albright L, Farnham JM, Teerlink C, Vierhout ME, van Kempen LCL, et al. Identification of six loci associated with pelvic organ prolapse using genome-wide association analysis. Obstet Gynecol 2011;118:1345–53. 10.1097/AOG.0b013e318236f4b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Giri A, Wu JM, Ward RM, Hartmann KE, Park AJ, North KE, et al. Genetic Determinants of Pelvic Organ Prolapse among African American and Hispanic Women in the Women’s Health Initiative. PLoS One 2015;10. 10.1371/journal.pone.0141647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


