Abstract
Background: Eastern equine encephalitis virus (EEEV) is a mosquito-borne virus that is primarily found in North America and the Caribbean. Over the past decade there has been an increase in virus activity, including large outbreaks in human and horse populations. Predicted climate change is expected to affect the range of mosquitoes including vectors of EEEV, which may alter disease risk posing a public health concern.
Methods: A scoping review (ScR) was conducted to identify and characterize the global evidence on EEEV. A thorough search was conducted in relevant bibliographic databases and government websites. Two reviewers screened titles and abstracts for relevance and the characteristics of relevant articles were extracted using a uniformly implemented data collection form. The study protocol was developed a priori and described the methods and tools used and this article follows the PRISMA-ScR guidelines for reporting ScRs.
Results: The ScR included 718 relevant research articles. The majority of the articles originated from North America (97%) between 1933 and 2019. EEEV has been identified in 35 species of mosquitoes, over 200 species of birds, various domestic animals, wild mammals, reptiles, and amphibians. Articles identified in this ScR primarily covered three topic areas: epidemiology of hosts and vectors (344 articles) including surveillance results (138), pathogenesis of EEEV in hosts (193), and in vitro studies characterizing EEEV (111). Fewer articles evaluated the accuracy of diagnostic tests (63), the efficacy of mitigation strategies (62), transmission dynamics (56), treatment of EEEV in hosts (10), societal knowledge, attitudes, and perceptions (4), and economic burden (2).
Conclusion: With the projected impact of climate change on mosquito populations, it is expected that the risk of EEEV could change resulting in higher disease burden or spread into previously unaffected areas. Future research efforts should focus on closing some of the important knowledge gaps identified in this ScR.
Keywords: eastern equine encephalitis virus, EEEV, scoping review, knowledge synthesis, vector-borne disease, mosquito-borne disease
Introduction
Eastern equine encephalitis virus (EEEV) is a mosquito-borne virus belonging to the Alphavirus genus, Togaviridae family and is closely related to Western equine encephalitis virus and Venezuelan equine encephalitis virus. EEEV was first recorded in North American horses in 1831. In 1938, the first human case was identified in Massachusetts, United States, during an Eastern equine encephalitis (EEE) outbreak involving 38 human and 248 horse cases. EEEV is considered an endemic virus in North America (mainly Atlantic/Gulf Coast States and Great Lake Regions), and has been reported on islands in the Caribbean. Historically there have been four EEEV lineages: Group I is responsible for most human cases and includes EEEV strains from North America and the Caribbean, whereas Groups II, III, and IV primarily cause illness in horses and include EEEV strains from Central and South America (Brault et al. 1999). Due to genetic divergence and differences in ecology and pathogenesis, strains from lineages II–IV were reclassified in 2010 as a distinct virus now known as Madariaga virus (MADV) (Arrigo et al. 2010). Although EEEV and MADV are distinct species, recent phylogenetic analyses show that there is overlap in their geographic regions (Hoyos et al. 2015, Burgueno et al. 2018).
EEEV is transmitted through the bite of an infected mosquito and most spillover transmission occurs in low-lying areas with hardwood trees and swamps conducive of mosquito larvae development. Culiseta melanura is the primary vector transmitting EEEV, which feeds mainly on birds (Molaei et al. 2015). Thus, C. melanura is considered to circulate EEEV among wild birds in an enzootic cycle. Bridge vectors transmit the virus from viremic birds to other susceptible hosts outside the enzootic cycle (Armstrong and Andreadis 2010). Susceptible hosts are usually dead-end hosts, such as equine species (horses, zebras, donkeys, etc.), humans, other birds (emus, ostriches, owls, etc.), or other livestock species (e.g., swine and cattle).
The neuroinvasive disease associated with severe cases of EEEV makes it one of North America's most severe arboviral encephalitidies. Over the past decade there has been an increase in virus activity, including a large outbreak in the summer of 2019 (ProMED 2019). Environmental changes associated with climate change may alter disease risk by changing the abundance and distribution of the primary mosquito vector, by lengthening the virus-transmission season, and by extending the transmission range to areas previously unsuitable (Ng et al. 2017, Khan et al. 2020).
Vector-borne diseases likely to emerge or expand in range due to climate change are a public health concern. A scoping review (ScR) was conducted to collect and characterize the existing global evidence on EEEV and identify knowledge gaps that may assist in decision-making on this public health issue.
Methods
Review topic and scope
An ScR uses reproducible and rigorous methodology to identify and map the scope and volume of literature available on a topic (Peters et al. 2015, 2017, Tricco et al. 2016, Munn et al. 2018). In addition to synthesizing the knowledge on a particular topic, an ScR can also identify areas of research saturation or gaps to ultimately guide future research questions and decision-making (Peters et al. 2015, 2017, Tricco et al. 2016, Munn et al. 2018). This ScR adheres to the Joanna Briggs Institute methodology for ScRs, which is similar to the structure and rigor of Cochrane Collaboration systematic reviews (Higgins et al. 2020, Peters et al. 2015, 2017).
The objective of this ScR was to summarize the characteristics of the global evidence on EEEV. For the purpose of this ScR, EEEV is defined as genotype I, commonly occurring in North America and the Caribbean. The other EEEV genotypes (II, III, and IV), have been reclassified as MADV and are outside the scope of the review. All articles that reported on observational studies outside of North America and the Caribbean were excluded.
Review question and inclusion criteria
The research question for this ScR was “What are the characteristics of the global evidence on EEEV?” The following inclusion criteria was applied:
-
(1)
Publication date: All
-
(2)
Country: All (Studies from any country were eligible as long as the topic was EEEV—Group I)
-
(3)
Language: English and French
-
(4)
Document type: All primary research (e.g., journal articles, government reports, predictive models using primary research, theses describing primary research). All other documents were excluded (secondary research, literature reviews, books, etc.)
-
(5)
Agent/disease: EEEV—Group I
-
(6)
Study design: All
Review protocol and team
An ScR protocol was developed a priori to ensure the methods were transparent, consistent, and reproducible (Supplementary Document S1). The protocol includes the search strategy and algorithm, inclusion and exclusion criteria for relevance screening, and the data characterization form for data extraction. A multidisciplinary team with expertise in knowledge synthesis, epidemiology, vector-borne diseases, and public health created the protocol and conducted the ScR. The reporting of this ScR follows the guidelines of PRISMA-ScR (Tricco et al. 2018) (Supplementary Document S2). A critical appraisal of the evidence was not conducted in this ScR.
Search strategy and verification
The search algorithm (“eastern equine encephalitis” OR “eastern equine encephalomyelitis”) OR (EEE and virus) was applied to five databases that were appropriate to the ScR: Scopus, PubMed/MEDLINE, Embase, Cochrane Central Register of Controlled Trials, and ProQuest. The Cochrane library was searched for any relevant trials in the trial registry and ProQuest was used to search for relevant theses and dissertations. The initial search was conducted in May 2017 and was updated in May 2019.
A complementary search for gray literature was conducted by hand searching state health department websites for published primary reports, news bulletins, and surveillance reports that reported EEEV cases that were not captured by the original electronic search. The Centers for Disease Control and Prevention (CDC) Stacks, Public Health Agency of Canada, Caribbean Public Health Agency (CARPHA), Public Health Ontario, and Institute National De santé Publique Du Québec were also searched for reports on cases of EEEV (confirmed and probable). In addition, each United States state website that had a confirmed EEEV case in any reservoir, vector, or incidental host was searched for annual communicable disease reports. The gray literature search was conducted in May 2017 and was updated in May 2019.
To confirm that all relevant primary research was found during the search, the reference lists of 12 literature reviews/key articles focused on EEEV identified by the ScR team were hand searched for additional articles (Sellers 1989, Freier 1993, Calisher 1994, Deresiewicz et al. 1997, Olsen et al. 1997, Armstrong and Andreadis 2010, 2013, Zacks and Paessler 2010, Silverman et al. 2013, Arechiga-Ceballos and Aguilar-Setien 2015, Kumar and Patil 2017, Chapman et al. 2018). At the point of saturation, when no new references were identified, the ScR team stopped evaluating reference lists. Eleven references that had been omitted by the electronic and gray literature search were added to the ScR from the search verification process.
Review management
All potentially relevant citations that were found by the search strategy were imported into the reference management software EndNote (EndNote X7, Clarivate Analytics) and duplicate citations were removed. The unique citations were then imported into the web-based systematic review management software DistillerSR (Evidence Partners, Ottawa, Canada) where additional duplicates were removed. All subsequent stages of this ScR, including relevance screening and data characterization, were performed within this software.
Relevance screening and data characterization
A relevance screening form was developed a priori to incorporate the inclusion and exclusion criteria of the ScR (Supplementary Document S1). Two reviewers independently used this form to screen citation titles, abstracts, and keywords for relevance to the ScR topic. Due to limited translation resources, citations in languages other than English and French were excluded. Full-text articles were then procured and independently reviewed by two individuals using a data characterization form developed a priori (Supplementary Document S1). The data characterization form aimed to categorize the research on EEEV to understand where there are areas of knowledge saturation and gaps. Each article was categorized into one or more research focus areas: epidemiology, surveillance, pathogenesis, virus study, diagnostic tests, mitigation, treatment, societal knowledge, and economic analysis. Additional pertinent information from each relevant article such as study design, population, and key outcomes were then extracted. Definitions for what was included in each focus area, study designs, and information extracted are outlined in detail in the protocol (Supplementary Document S1). During both stages, reviewers resolved conflicts by consensus or a third party reviewer where necessary.
Data analysis
The final dataset was exported into MS Excel (Excel 2010; Microsoft Corporation, Redmond, WA), cleaned, and tabulated for use in the descriptive analysis and summarization of results. A repository of relevant articles including bibliographic details and the full dataset from the completed ScR are available in Supplementary Dataset S3.
Results
ScR descriptive statistics
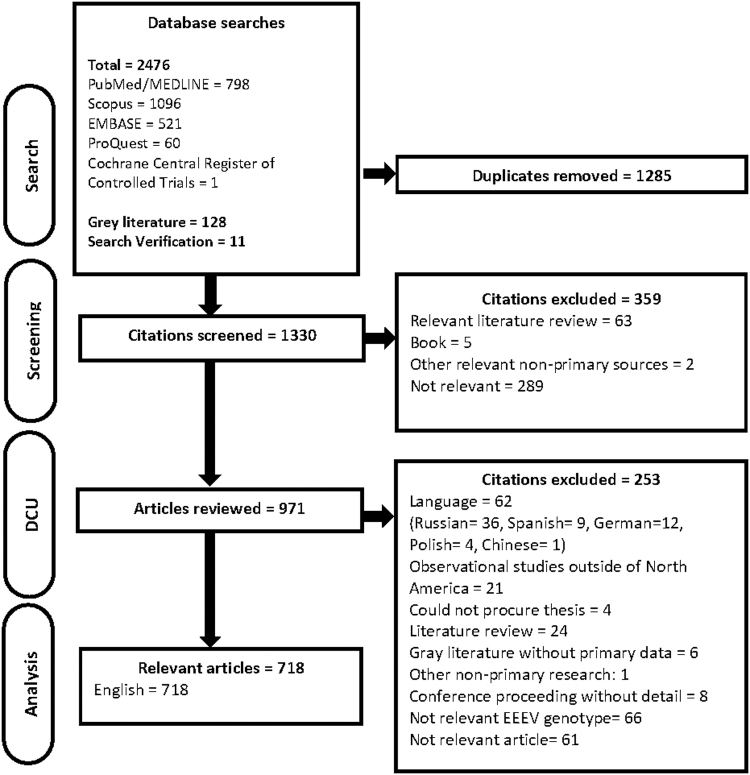
There were 1330 unique citations screened for relevance, of which 971 were potentially relevant and full articles were procured. Articles in languages other than English and French were excluded from this ScR due to a lack in translational resources. The articles excluded because of language were in Russian (n = 36), German (12), Spanish (9), Polish (4), and Chinese (1). Since EEEV typically occurs in North America and the Caribbean, we believe there is a low potential for language bias in this ScR. Data characterization was conducted for 718 relevant articles included in this ScR (Fig. 1).
FIG. 1.
PRISMA flow diagram of articles through the scoping review process. DC, data characterization.
Articles were published between 1933 and 2019 with 28.4% (204/718) published since 2011. The majority of the articles originated from North America (696/718) and were primary research (580/718) (Table 1). North America includes the United States, Canada, Mexico, and the Caribbean.
Table 1.
General Characteristics of 718 Included Primary Research Publications on Eastern Equine Encephalitis Virus
| Category | Count |
|---|---|
| Type of document | |
| Primary peer-reviewed research | 580 |
| Gray literature with primary data | 108 |
| Thesis | 22 |
| Conference proceeding abstract with data | 8 |
| Continenta | |
| North America | 696 |
| South America | 21 |
| Europe | 13 |
| Asia | 10 |
| Africa | 1 |
| Population category | |
| Nonhuman hosts | 280 |
| Humans | 206 |
| Vectorsb | 209 |
| Animal models | 170 |
| Virus only | 134 |
| Study designa | |
| Observational | 415 |
| Surveillance or monitoring program | 194 |
| Prevalence | 84 |
| Case series or report | 88 |
| Cross-sectional | 34 |
| Outbreak investigation | 32 |
| Longitudinal | 5 |
| Case–control | 2 |
| Experimental | 269 |
| Challenge trial | 191 |
| Molecular characterization | 45 |
| Controlled trial | 26 |
| Otherc | 9 |
| Quasi-experimental | 8 |
| Evaluation of diagnostic tests | 63 |
| Molecular epidemiology | 8 |
| Otherd | 2 |
| Economic model | 1 |
Total number sums to >718 as some studies were conducted on more than one continent, had more than one type of study design, or sampled more than one population
Includes arthropod and mosquito species.
Other includes: experimental methods for cultivating and purifying EEEV.
Other includes: investigating a system for deriving EEEV antigens from infected chick embryos and content analysis (mixed methods).
EEEV, eastern equine encephalitis virus.
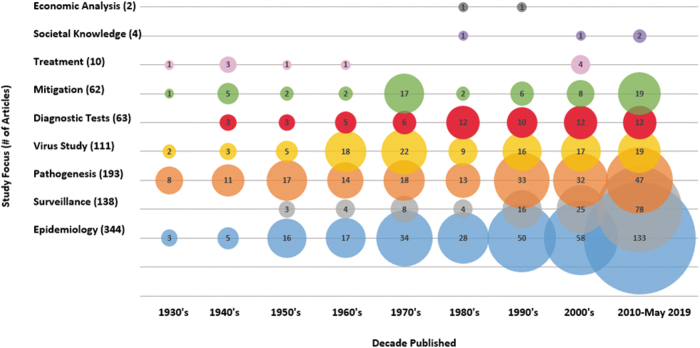
Articles primarily covered three EEEV topic areas as shown in Fig. 2: epidemiology of hosts and vectors (344/718) including surveillance (138/718), pathogenesis of EEEV in hosts (193/718), and in vitro studies characterizing EEEV (111/718) (Fig. 2). Other EEEV topics such as the accuracy of diagnostic testing (63/718), efficacy of mitigation strategies (62/718), EEEV transmission dynamics (56/718), treatment of EEEV in hosts (10/718), societal knowledge, attitudes, and perceptions (4/718), and economic burden (2/718) were captured to a lesser extent. No predictive models on the impact of climate change on EEEV were identified. Some articles did not fit into the predetermined topic areas (5/718) and included articles on studies analyzing experimental methods for cultivating and purifying EEEV and the precipitating effect of methanol on EEEV. Observational study designs were the most widely used (415/718), of which the majority were estimates of the burden of EEEV in vectors or hosts through surveillance activities (194/718), provided prevalence estimates in a defined population (84/718), or presented cases series or reports (88/718).
FIG. 2.
Bubble chart of the number of different article foci published in the years 1933–2019.
EEE virus
In vitro methods were used (134/719 studies) to characterize the virus, evaluate the impact of an intervention, or described methods for cultivating EEEV. Studies that focused specifically on the virus were reported in 111 of 134 articles. EEEV has a single-stranded, positive sense RNA genome; the virus is spherical in shape with a diameter of 60–65 nm (Aguilar et al. 2007). Many of the in vitro studies focused on the structure and function of selected part of the virus. Categorization of these articles included those that reported on the pathogenic attributes of the virus (68/111), transmission characteristics (3/111), and the molecular characterization of EEEV (51/111). Of the articles that reported molecular characterization of EEEV, there was a phylogenetic analysis in approximately half of the studies: 8 of 51 were molecular epidemiology studies, 17 of 51 reported phylogenetic trees, and 6 of 51 reported whole genome sequencing results.
Other research conducted in vitro reported on the effectiveness of antiviral treatments under experimental conditions in 5 of 134 articles (Katz et al. 1975, Amaya et al. 2015, Rico 2016, Lundberg et al. 2018, Jonsson et al. 2019). Possible mitigation options evaluated in vitro (12/134 articles) examined possible formulas for vaccination (7/12) and the use of biological and chemical agents as virucides (5/12). Four of these articles focused on laboratory methods for working with EEEV. These included articles on experimental methods for cultivating and purifying EEEV (Palmer et al. 1968), deriving EEEV antigens from chick embryos (Soret and Sanders 1954), the precipitation effect of methanol on EEEV (Pollard et al. 1949), and evaluation methods to screen EEEV for purity and contamination (Gollapudi et al. 2017).
Accuracy of diagnostic tests
The accuracy of diagnostic tests to diagnose EEEV was studied in 63 of 718 articles. Several techniques to isolate and/or identify EEEV or identify exposure to EEEV were evaluated including virus isolation (16/63), molecular tests (22/63), immunoassays (41/63), and clinical diagnosis (1/63). The performance of these tests were evaluated in different types of hosts including humans (10/63), naturally exposed animals (20/63), experimentally infected animals (14/63), mosquitoes (16/63), and well-characterized sample libraries (23/63). Information regarding sensitivity, specificity, test agreement, or raw data were provided in 26 of 63 of these articles. The citations for each of these categories can be identified in Supplementary Dataset S3.
EEEV in vectors
Research on vectors of EEEV was reported in 209 of 718 articles. While the majority of these articles focused on epidemiology (155/209), there was also research on EEEV transmission dynamics (43/209) and mitigation strategies (3/209).
Over 150 mosquito and arthropod species have been tested for naturally occurring exposure or infection with EEEV in 155 of 209 articles. Arthropod species included midges, mites, fleas, flies (sand flies, horse flies, deer flies, stable flies, louse flies, and house flies), bedbugs, mealworms, lice, and ticks. Vector populations were frequently trapped, identified, and tested for arboviruses as part of prescribed surveillance activities. Evidence of EEEV in wild mosquito and arthropod species was reported in 127 of 155 articles, mostly from 24 states in the United States (122/127) and to a lesser extent in the Caribbean (3/127) and Canada (3/127) (Table 2).
Table 2.
Mosquitoes and Arthropod Vectors Naturally Infected with Eastern Equine Encephalitis Virus Reported in 127 Articles Across North America (Including the Caribbean) Between 1947 and 2018
| Vector species | No. of articles |
|---|---|
| United States | |
| Mosquitoes | |
| Aedes albopictusa | 3 |
| Aedes atlanticus | 2 |
| Aedes atlanticus/tormentor | 1 |
| Aedes canadensisa | 19 |
| Aedes cantatora | 2 |
| Aedes cinereus | 4 |
| Aedes infirmatus | 3 |
| Aedes japonicasa | 2 |
| Aedes mitchellae | 1 |
| Aedes sollicitansa | 2 |
| Aedes taeniorhynchusa | 2 |
| Aedes triseriatusa | 2 |
| Aedes trivittatus | 2 |
| Aedes vexansa | 14 |
| Anopheles crucians | 11 |
| Anopheles punctipennisa | 5 |
| Anopheles quadrimaculatusa | 7 |
| Anopheles walkeri | 1 |
| Coquillettidia perturbansa | 27 |
| Culex erraticusa | 9 |
| Culex (melanoconion) spp. | 1 |
| Culex nigripalpus | 6 |
| Culex peccator | 1 |
| Culex pipiens | 3 |
| Culex pipiens/restuans | 9 |
| Culex restuans | 3 |
| Culex salinariusa | 10 |
| Culex spp., not specified | 6 |
| Culex territans | 2 |
| Culiseta melanoconion | 1 |
| Culiseta melanuraa | 71 |
| Culiseta minnesotae | 1 |
| Culiseta morsitans | 14 |
| Mosquito spp., not specified | 36 |
| Psorophora ferox | 3 |
| Uranotaenia sapphirina | 5 |
| Arthropods | |
| Culicoides (sp. not specified) | 1 |
| Dermanyssus gallinae | 1 |
| Menacanthus stramineus | 1 |
| Canada | |
| Mosquitoes | |
| A. vexans | 1 |
| C. perturbans | 2 |
| C. melanura | 3 |
| Caribbean | |
| Mosquitoes | |
| Culex nigripalpus | 2 |
| Culex taeniopus | 3 |
Mosquito species that have been used in transmission and/or competence studies. Other species including Aedes aegypti, Aedes atropalpus, and Culex taeniopus have been used in transmission and/or competence studies, but there have been no articles reporting natural infection of EEEV in these mosquitoes.
Between 1948 and 2018, 35 specific mosquito species tested positive for EEEV (Table 2). EEEV was most commonly identified in C. melanura (74/127), Coquillettidia perturbans (29/127), Aedes canadensis (18/127), Aedes vexans (15/127), and Culiseta morsitans (14/127). The minimum infection rate was reported in 22/127 articles. In addition to mosquitoes, arthropod species (Culicoides, Dermanyssus gallinae, and Menacanthus stramineus) tested positive for EEEV in two articles from the United States between 1947 and 1959 (Table 2). A detailed list of vectors naturally infected with EEEV along with the location and dates sampled is given in Supplementary Table S1.
Risk factors that were associated with exposure to EEEV or acquiring EEEV in mosquito populations were reported in 5 of 209 articles (Crans et al. 1994, Takeda et al. 2003, Hachiya et al. 2007, Kelen et al. 2012, Skaff et al. 2017). In vectors, the method of identifying EEEV was described in 92 of 155 articles, the most common diagnostic method used was virus isolation (60/92) followed by molecular tests (37/92), or immunoassays (37/92). Many of these articles (40/92) used multiple diagnostic methods to identify EEEV in vectors.
Three articles investigated mitigation strategies to prevent EEEV infection in mosquitoes. Aerial spraying with 95% malathion was conducted in a study from Massachusetts; however, the effectiveness of this intervention was not evaluated (Grady et al. 1978). Mosquitoes were used in two studies on developing EEEV vaccine candidates. To assess the replication competence of a recombinant EEEV that was attenuated, Aedes albopictus mosquitoes were challenged with the virus (Pandya et al. 2012). This ensured the virus would only replicate in vertebrate cells, which is an important safety feature for live vaccines against mosquito-borne viruses (Pandya et al. 2012). A second study demonstrated that two chimeric vaccine candidates for EEEV were successful in reducing mosquito infectivity when tested on Aedes sollicitans and Aedes taeniorhynchus mosquitoes (Arrigo 2010).
Over 30 mosquito and arthropod species were used to study transmission dynamics and vector suitability (competence and behavior) in 44 of 209 articles. Mosquito transmission dynamics were reported in 31 of 44 studies and specific species are listed in Table 2. Transmission studies reported on vector-to-host transmission (13/31), host-to-vector transmission (24/31), and transovarial transmission (5/31). Mosquito competence, such as viral replication, dissemination, and transmission was investigated in 29 of 30 articles, whereas 2 of 30 articles studied mosquito behavior such as feeding patterns and temporal preferences. In addition, there were a few studies (6/44) that reported on EEEV transmission dynamics and competence of the following arthropod species: Haemogamasus liponyssoides, Ornithonyssus bacoti, Ixodes ricinus, Dermacentor marginatus, D. gallinae, Stomoxys calcitrans, Culicoides variipennis, and Culicoides crepuscularis. Some articles on the vector competence of arthropods have demonstrated an ability to ingest and maintain EEEV (Kissling et al. 1954, Rehacek 1958, 1960, Scanlon 1960, Clark et al. 1966), even throughout winter (Rehacek 1960). Only one article reported successful transmission of EEEV from D. gallinae to chickens under laboratory conditions (Durden et al. 1993). However, the importance of potential arthropod vectors in the EEEV transmission has not recently or extensively been investigated.
EEE and human hosts
EEEV in human populations was studied in 206 of 718 articles. Targeted observational studies were conducted in 19 of 206 articles where sample populations were evaluated for exposure to EEEV (i.e., serological antibodies) or infection with EEEV (i.e., confirmed case), of these 12 of 19 identified EEEV exposure in a proportion of the sample population between 1948 and 2013 (Table 3). Most of the human EEEV data were identified in surveillance reports (104/206) and provided data such as incidence (104/104), case fatality rates (39/104), proportion hospitalized (15/104), and prevalence of long-term sequelae (3/104). Risk factors associated with human exposure to EEEV or acquiring EEEV were reported in 2 of 206 articles (Letson et al. 1993, Hachiya et al. 2007).
Table 3.
Observational Studies That Identified Eastern Equine Encephalitis Virus in Targeted Human Populations (n = 12 Articles) Between 1948 and 2013 That Reported Positive Results for Exposure to Eastern Equine Encephalitis Virus
| Geographical location | Year(s) | Human population sampled | Outcomes reported | References |
|---|---|---|---|---|
| North America | ||||
| Caribbean | ||||
| Dominican Republic | 1948–1949 | General population in areas surrounding an outbreak | Seroprevalence | Eklund et al. (1950) |
| 1978 | General population | Seroprevalence | Calisher et al. (1979) | |
| Jamaica | 1963–1967 | General population | Seroprevalence | Belle et al. (1980) |
| Jamaica | 1975 | General population | Seroprevalence | Rowe and King (1976) |
| Mexico | ||||
| Mexico | 1974 | General population | Seroprevalence | Ruiz-Gomez and Espinosa (1981) |
| United States | ||||
| Connecticut | 1952–1953 | At risk population—pheasant farmers/handlers | Seroprevalence | Liao (1955) |
| Florida | 1970 | Patients with signs of infections of the central nervous system | Case prevalence | Centers for Disease Control and Prevention (1970) |
| Massachusetts | 1967–1976 | General population | Seroprevalence, case prevalence | Grady et al. (1978) |
| Massachusetts | 1979–2004 | General population | Case prevalence | Hachiya et al. (2007) |
| Massachusetts | 2012–2013 | Blood donors | Seroprevalence | Leiby et al. (2014) |
| Mississippi | 1989 | General population | Seroprevalence | Letson et al. (1993) |
| New York | 1966–1977 | Patients with signs of infections of the central nervous system | Seroprevalence, case-fatality rate | Deibel et al. (1979) |
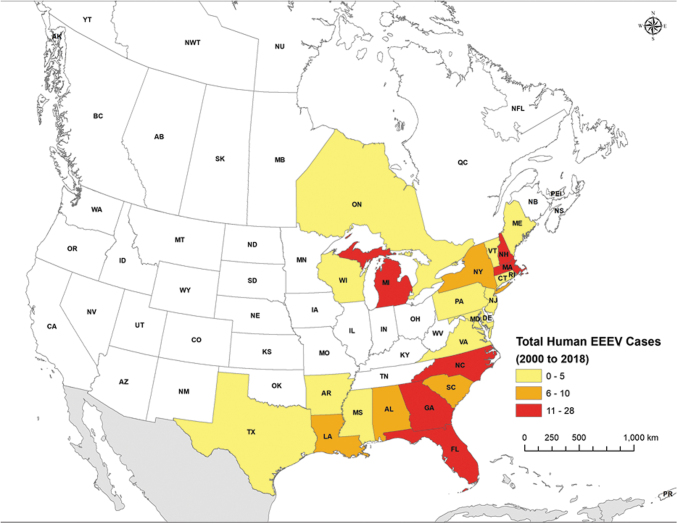
One Canadian province (Ontario) and 29 states in the United States have reported human EEEV cases through surveillance, case, or outbreak reports (Table 4, Supplementary Tables S2 and S3, and Fig. 3). The United States states include Alabama, Arkansas, Connecticut, Delaware, Florida, Georgia, Illinois, Indiana, Louisiana, Maine, Maryland, Massachusetts, Michigan, Mississippi, Missouri, Montana, New Hampshire, New Jersey, New York, North Carolina, Oklahoma, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Vermont, Virginia, and Wisconsin.
Table 4.
Sixty-Five Outbreaks Involving Humans and Animals Reported in 54 Articles Between 1933 and 2018
| Geographical location | Year(s) | No. of animal cases reported | No. of human cases reported | Reference |
|---|---|---|---|---|
| North America | ||||
| Canada | ||||
| Quebec | 2008 | Horses (19), emus (32 suspected 3 confirmed)a | Chenier et al. (2010) | |
| Caribbean | ||||
| Dominican Republic | 1948–1949 | Equine (516)a | 13a | Eklund et al. (1950) |
| 1978 | Equine (∼3600)a | Calisher et al. (1979) | ||
| Jamaica | 1962 | Equine (11)a | 12a | Belle et al. (1964); Hart et al. (1964) |
| Central | ||||
| Mexico | 1996 | Horses (113)a | Brault et al. (1999) | |
| United States | ||||
| Arkansas, Georgia, Louisiana | 1948–1949 | Horses and mules (17) | Kissling and Rubin (1951) | |
| Connecticut | 1951 | Pheasants (495)a | Luginbuhl et al. (1958) | |
| 1951 | Pheasants (610) | Luginbuhl et al. (1958) | ||
| 1951 | Pheasants (200)a | Luginbuhl et al. (1958) | ||
| 1951 | Pheasants (350)a | Luginbuhl et al. (1958) | ||
| 1953 | Pheasants (800)a, rat (1)a | Liao (1955); Luginbuhl et al. (1958) | ||
| 1955 | Pheasants (1300)a | Luginbuhl et al. (1958) | ||
| 1955 | Pheasants (7000) | Luginbuhl et al. (1958) | ||
| 1956 | Pheasants | Luginbuhl et al. (1958) | ||
| 1972 | Pheasants (27)a, turkeys (2)a, quail (1)a, dove (1)a, equine (9)a | Bryant et al. (1973) | ||
| 2003 | African penguins (14)a | Tuttle et al. (2005) | ||
| Florida | 1965 | Equine (13), sentinel chicken flock (1), chukar partridge (1) | 1 | Bigler et al. (1976) |
| 1966 | Equine (96) | Bigler et al. (1976) | ||
| 1994 | Duck (1), piglets | Centers for Disease Control and Prevention (1995) | ||
| 2018 | Emu flocks (5) | Florida Department of Health (2018) | ||
| Georgia | 1991 | Pigs (8 from 3 litters)a | Elvinger et al. (1994) | |
| 1991 | Pigs (350 from 38 litters)a, sows (10), boar (1), pigs (8) | Elvinger et al. (1994) | ||
| 1991 | Horse (1 confirmed, 4 suspected) | Centers for Disease Control and Prevention (1991) | ||
| 1991 | Commercial quail | Centers for Disease Control and Prevention (1991) | ||
| Louisiana | 1947 | Horses/mules (31), cows (2), chickens (4), geese (1), pigeons (1), dog (1) | 32 suspected, 10 confirmeda | Hauser (1948); Howitt et al. (1948); Dent (1955) |
| 1991 | Emus (2) from a flock of 24a | Tully Jr et al. (1992) | ||
| 1999 | Horses (97)a | 2 | Louisiana Department of Health (2017) | |
| Maine | 2009 | Horses (15)a, llama (1), ring-necked pheasant flocks (3), wild turkeys | Maine Center for Disease Control and Prevention (2009); Lubelczyk et al. (2013) | |
| Maryland | 1984 | Whooping cranes and Sandhill cranes (7 clinical cases, 14 seropositive)a | Centers for Disease Control and Prevention (1985); Dein et al. (1986) | |
| 1989 | Pheasant flock (1500 birds—not sure how many +)a | Centers for Disease Control and Prevention (1989) | ||
| Massachusetts | 1938 | Horses (6 confirmed 245 suspected) | 38 suspected, 8 confirmeda | Feemster (1938); Fothergill et al. (1938); Webster and Wright (1938); Farber et al. (1940); Ayres and Feemster (1949); Massachusetts Department of Health (2018) |
| 1955 | Pheasants (3)a | Faddoul and Fellows (1965) | ||
| 1955–1956 | 16a | Massachusetts Department of Health (2018) | ||
| 1956 | Pheasants (24)a | Faddoul and Fellows (1965) | ||
| 1959 | Pheasants (1)a | Faddoul and Fellows (1965) | ||
| 1973–1974 | 6a | Massachusetts Department of Health (2018) | ||
| 1982–1984 | 10a | Massachusetts Department of Health (2018) | ||
| 1990–1992 | 4a | Massachusetts Department of Health (2018) | ||
| 2004–2006 | 13a | Massachusetts Department of Health (2018) | ||
| Michigan | 1942 | Horses (102) | Ross and Kaneene (1996) | |
| 1943 | Horses (367) | Ross and Kaneene (1996) | ||
| 1973 | Horses (26) | Ross and Kaneene (1996) | ||
| 1980 | Horses (94) | Centers for Disease Control and Prevention (1980); Kappus et al. (1982); Ross and Kaneene (1996) | ||
| 1981 | Horses (56) | Ross and Kaneene (1996) | ||
| 1982 | Horses (10) | Ross and Kaneene (1996) | ||
| 1991 | Horses (14 confirmed 41 suspect)a | Ross and Kaneene (1995, 1996) | ||
| 2005 | White-tailed deer (7)a | Schmitt et al. (2007) | ||
| New Jersey | 1959 | Horses (66)a, pheasants (18 flocks) | 32a | Goldfield and Sussman (1968) |
| New York | 1971 | Equine (3)a | 1 | Morris et al. (1973) |
| 1974 | Horses (15) | Morris et al. (1975) | ||
| 1976 | Horses (37, 19 suspected) | Srihongse et al. (1978) | ||
| 1982–1983 | Equine (9)a | 1 | Howard et al. (1988) | |
| 1988 | Rufous-sided towhee (2), birds (6) | Howard et al. (2004) | ||
| 1990 | Common yellowthroat (19), rufous-sided towhee (10), song sparrow (108), birds (18), gray catbird (87), red-eyed vireo (29), veery (28), field sparrow (9), cedar waxing (19), black-capped chickadee (21), wood thrush (7), American robin (6), eastern phoebe (15) | Howard et al. (2004) | ||
| Ohio | 1991 | Horses (12 confirmed 7 probable) | Nasci et al. (1993) | |
| Rhode Island | 1956 | Pheasants (4), chukar partridge (1), wild sparrow (1)a, quail (1) | Dardiri et al. (1957) | |
| South Carolina | 1977 | Quail a | Eleazer et al. (1978) | |
| Southeastern seaboard (including FL, GA, SC) and Midwestern states (including OH, and MI) | 1991 | Horsesa | Centers for Disease Control and Prevention (1992) | |
| Tennessee | 2005 | Equine (8) | Mukherjee et al. (2012) | |
| 2008 | Equine (6)a | Mukherjee et al. (2012) | ||
| Vermont | 2011 | Emus (21)a | Saxton-Shaw et al. (2015) | |
| Virginia | 1933 | Horsesa and mulesa | Giltner and Shanan (1933) | |
| AL, FL, GA, LA, NJ, SC, MI, MS, MD, NC, RI, TN, VA, DE (human cases were in MS, MD, NC, SC) | 1989 | Equine (194–196) | 9a | Centers for Disease Control and Prevention (1990); Letson et al. (1993) |
| AL, AR, CT, FL, GA, LA, MD, MS, NC, RI, TX, VA, WA | 1996–1997 | Emus | Centers for Disease Control and Prevention (1998) | |
| East and Gulf Coast | 1982 | Pheasants, quail, horses | Centers for Disease Control and Prevention (1983) | |
Case fatalities reported.
FIG. 3.
Human cases of EEEV reported in North America between 2000 and 2018. EEEV, eastern equine encephalitis virus.
Details of 145 sporadic human EEEV cases were described in 53 of 206 surveillance and case report studies during 1955–2019, Supplementary Table S2. Of these documented sporadic cases, 47 of 53 articles reported hospitalizations and 30 of 53 reported case-fatality rates. EEEV exposure or infection was identified in both men and women between the ages of 3 months and 87 years old. However, of the 71 of 145 cases that reported age, 49.3% (35/71) were <16 years, 35.2% (25/71) were between 16 and 59 years, and 15.5% (11/71) were 60 years of age or older. In cases where gender was reported, 71.7% were male (38/53) and 28.3% (15/53) were female. Among the articles that documented sporadic cases of EEEV, 47 of 53 articles reported signs and symptoms of infection such as fever, chills, vomiting, myalgia, arthralgia, malaise, and encephalitis. In addition, these articles also reported pathology of the disease (44/53), postmortem investigations (18/53), and sequelae after infection (25/53). One article (1/206) examined the economic burden of EEEV human cases (1/206) (Villari et al. 1995).
Sixteen outbreaks involving human cases were reported in 20 articles between 1938 and 2006 (Table 4). Most outbreaks occurred in the United States (14/16), and one each in Jamaica (1/16) and Dominican Republic (1/16). Reported outbreaks ranged in size from 1 to 38 human cases and outbreaks in horses commonly preceded the identification of human cases of EEEV during the outbreak period. In the 20 articles describing outbreaks involving humans, sequelae (4/20), pathology (5/20), signs and symptoms (8/20), and postmortem (4/20) results were reported. Additional outbreaks may have occurred, however only the outbreaks that were cited in the literature as an outbreak were recorded in this table.
In articles detecting EEEV in humans through epidemiological studies, case, outbreak, and surveillance reports, 73 of 206 provided testing details; the majority used an immunoassay (72/73), virus isolation (25/73), or molecular test (8/73) for laboratory diagnosis.
The efficacy of mitigation strategies, specifically vaccination on human volunteers was assessed in 7 of 206 articles (Bartelloni et al. 1970, Darwish 1972, DeMeio et al. 1979, Strizki 1994, Strizki and Repik 1995, Pittman et al. 2009, Reisler et al. 2012). The majority of these articles (6/7) reported successful results of vaccination attempts and suggested further evaluation in human populations. Research was also conducted on societal knowledge and attitudes about EEEV (2/206) and transmission dynamics (1/206) (Liao 1955, Rodriguez 2008, Ackerson and Viswanath 2010).
EEE in nonhuman hosts and animal models
EEEV was studied in both nonhuman hosts where the animals were naturally exposed to EEEV (280/718 articles) and animal models where the animals were experimentally challenged with EEEV (170/718). Some articles (13/718) reported on both nonhuman hosts and animal models.
The majority of the articles on nonhuman hosts (280/718) focused on the epidemiology of EEEV (257/280), which included case reports, outbreak investigations, surveillance, and prevalence studies. Of these articles, 242 of 257 reported finding EEEV in nonhuman host populations including results on sentinel surveillance using hosts (e.g., chickens, quail) monitored for exposure to EEEV (42/242).
Of the articles that reported on how EEEV was identified in animal populations, the most common diagnostic tests used were immunoassays (153/167), followed by virus isolations (87/167), and molecular tests (27/167). EEEV was reported in >155 different species of passerine birds and >65 species of nonpasserine birds (Supplementary Table S4). The virus has also been documented in various domestic and farm animals such as equine, pheasants, dogs, and swine and in other mammals such as bats, white-tailed deer, and opossums. These are listed by species, date, location, and type of evidence in Supplementary Table S4. Over 15 species of reptiles and amphibians were also shown to be naturally exposed to EEEV (Supplementary Table S4). Risk factors associated with exposure to EEEV or acquiring EEEV in nonhuman host populations were reported in 15 of 280 articles (Main 1979, Emord and Morris 1984, Centers for Disease Control and Prevention 1985, Crans et al. 1994, Spalding et al. 1994, Ross and Kaneene 1995, Elvinger et al. 1996, Dunbar et al. 1998, Garvin et al. 2004, Burkett-Cadena 2010, Mutebi et al. 2011, Vander Kelen et al. 2012, Estep et al. 2013, Elias et al. 2017, Heberlein-Larson et al. 2019).
Sixty-five outbreaks involving nonhuman hosts were reported in 54 articles between 1933 and 2018 (Table 3). The majority of the outbreaks occurred in the United States (60/65), and to a lesser extent in Canada (1/65), Mexico (1/65), and the Caribbean (3/65). Case fatalities in nonhuman hosts were reported in 50.7% (33/65) of the outbreaks. Additional outbreaks may have occurred, however only the outbreaks that were cited in the literature as an outbreak were recorded in this table.
In nonhuman hosts, studies on pathogenesis of EEEV (47/280), efficacy of mitigation strategies (8/280), and transmission dynamics (7/280) were also captured. In addition, one article (1/280) reported on survey results about management practices of horses in Florida in addition to the economic impact of EEEV to the equine industry (Wilson et al. 1986). The pathogenesis of EEEV in nonhuman hosts was reported in 47 of 280 articles, 40 of which described the signs and symptoms of EEEV infection in nonhuman hosts including those that develop clinical disease: equine (horse, mule, emus, etc.), birds (pheasants, partridges, chickens, quail), camelids (llamas, alpacas), white-tailed deer, dogs, and swine. Results of pathology (24/47) and postmortem investigations (35/47), viremic (1/47) and incubation periods (1/47), and infection mechanism/immune response (2/47) were also reported in these articles. Mitigation strategies, specifically the efficacy of vaccinations were explored in 8 of 280 articles using nonhuman hosts such as horses, pheasants, and emus. Transmission dynamics were explored in 7 of 280 articles between nonhuman host to nonhuman host (2/7), mosquito to nonhuman host (3/7), nonhuman host to mosquito (2/7), and nonhuman host to human (1/7).
Of the 170 articles that employed animal models, 159 of 170 were experimental involving challenge trials (147/159), control trials (6/159), and quasi-experiments (4/159). Fourteen articles used animal models to evaluate diagnostic testing methods. Mice were the most common animal used in these experiments (86/170) followed by domestic birds (42/170), wild birds (17/170), guinea pigs (17/170), horses (11/170), hamsters (8/170), and to a lesser extent, other animals such as monkeys and rabbits.
The pathogenesis of EEEV in animal models was reported in 93 of 170 studies, of which 26 of 93 articles included studies on infection and immune responses in these animals. Outcomes such as signs and symptoms (46/93), pathology (35/93), and postmortem investigations (46/93) were reported in these articles. Other characteristics of EEEV infection in animal models were reported including the time between exposure and viremic period (35/93), viremic period (34/93), and intrinsic incubation period (15/93).
Transmission dynamics using animal models were reported in 37 of 170 articles. These articles looked at transmission dynamics from animal model to mosquito (23/37), mosquito to animal model (14/37), animal model to animal model (7/37), and a single article reported on differences in transmission dynamics when comparing different strains of EEEV (1/37) (Arrigo et al. 2009). The efficacy of mitigation strategies, in particular vaccination, was tested using animal models in 43 of 170 articles. The majority of these articles (38/43) reported on the effectiveness of vaccine candidates. Animal models were also used to explore EEEV treatment options such as antivirals in 6 of 170 articles.
Discussion
EEEV is a vector-borne disease of public health concern that is likely to emerge or expand in range due to climate change. To prepare for the anticipated risk of increase in EEEV cases, we summarized the characteristics of the global evidence on EEEV and identified gaps in research. Our intention is that the information synthesized and knowledge gaps identified will support decision-making and further research on this priority issue. Although we attempted to capture all relevant literature on this topic, it is possible that some research was not captured due to failure to publish or an absence of citation indexing in the bibliographic databases examined. As discussed in the results, we expect language bias will have a minimal impact on this ScR as EEEV is a North American virus.
In 2010 EEEV underwent a reclassification and three of the four lineages were classified as a distinct species due to genetic diversity and differences in the ecology and pathogenesis of the strains, lineages II–IV are now known as MADV (Arrigo et al. 2010). EEEV generally circulates in North America and the Caribbean and causes the most human disease cases. MADV, formerly known as South American EEEV circulates in Central and South America and mainly causes illness in horses and mammals. Recent phylogenetic analyses show that there is overlap in the geographic regions of the two viruses. For example, MADV was reported in a cohort of children in Haiti in 2015, and the phylogenetic analysis suggests the virus was introduced into Haiti from Panama between 2012 and 2015 (Lednicky et al. 2019). Two studies found pools of mosquitoes infected with EEEV, Culex pipiens mosquitoes in Uruguay (Burgueno et al. 2018) and mosquitoes of the Culex genera in Colombia (Hoyos et al. 2015). This raises questions about the potential spread of MADV and EEEV into new geographic areas and warrants additional epidemiological research. Because of the reclassification of EEEV in 2010 and the potential spread of MADV and EEEV into new geographic areas, it is possible that some of the studies captured before 2010 were misclassified as EEEV. This is a potential limitation to our ScR.
According to the CDC, there is an average of seven clinical EEEV cases diagnosed per year in the United States, which is estimated to represent only 4–5% of human EEEV infections that have actually occurred (Centers for Disease Control and Prevention 2019b). Historically, human cases have been relatively infrequent, as it is atypical for humans to reside in low-lying swampy areas, where the main vectors of EEEV reside. In 2019, a record number of human cases (38 confirmed) were reported to the CDC (Centers for Disease Control and Prevention 2019a). The trend over the past decade has also noted EEEV activity in new areas where its circulation was previously unknown or rare such as Montana, Arkansas, Connecticut, and Pennsylvania. Canada also reported its first human case in Southwestern Ontario in 2016 (Public Health Ontario 2017). The increased activity may represent another mosquito-borne disease that requires public health awareness and monitoring. It is also possible that the rise in cases is due to increased awareness of the disease and improved diagnostics rather than an actual increase in human exposure to EEEV. Continued surveillance and large epidemiological studies would be beneficial to address these uncertainties.
Although EEEV has a high-case fatality rate (∼33%) in humans and many patients who survive develop severe sequelae as a result of EEEV infection, there is no approved human vaccine or antiviral treatment (Centers for Disease Control and Prevention 2019a). It has been estimated that the average lifetime cost to a person who suffers sequelae as a result of EEEV is ∼$3 million USD (Villari et al. 1995). This is a very large burden and impacts on the affected case's quality of life. General public health programs about mosquito bite prevention and mosquito-borne illness are recommended in affected areas to promote protective behaviors in the general population. Horses are also particularly sensitive to EEEV; 70–90% of horses infected with EEEV die and the ones that survive have permanent brain damage (Public Health Ontario 2014). Although there is a vaccine for horses, the impact to equine and industry can be financially damaging. In 1986, it was estimated that the cost of EEEV to the state of Florida equine industry was >$1 million USD/year (Wilson et al. 1986). In conducting this ScR, outbreaks in some types of poultry such as pheasants, turkeys, ratites, and quail were identified. These birds are also susceptible to EEEV that can be financially damaging for poultry farmers income. There is a clear need for additional research on viable treatment options and updated economic analyses on burden of this disease in both human and animal populations.
Given there is no human vaccine or effective antiviral therapies, appropriate mitigation strategies should be evaluated and applied. These include mosquito control, typically in the form of insecticides to large areas by fogging. Larvacides may also be a viable option for EEEV vector control. Personal protective measures and mosquito-borne disease knowledge and awareness programs should be undertaken by public health to prevent as many cases as possible (Region of Peel 2016) There is little research on determinants of public personal protective behavior against mosquito bites and what types, intensity, and duration of program is most effective at resulting in behavioral change. This is an important area for future research to guide decision-making on preventative actions to mitigate EEEV.
EEEV has been identified in 35 species of mosquitoes, over 200 species of birds, various domestic and farm animals, wild mammals, reptiles, and amphibians. Although there are a large number of mosquitoes and animals susceptible to EEEV, there is minimal research on the transmission dynamics between species and mosquito competence. Thus, additional research on drivers of transmission and the relative importance of different species may be important in predicting and preparing for the spread of EEEV into new areas.
This ScR did not identify any predictive models examining the impact of climate change on EEEV. Environmental changes associated with climate change may alter disease risk by increasing the abundance and distribution of the primary mosquito vector, by lengthening the virus-transmission season, and by extending transmission range. Transmission of EEEV is highly seasonal, with cases occurring in late spring and early fall. Climate change could lead to warmer and later winters, producing habitats that could sustain mosquito populations and increase the window for EEEV exposure (Shah and Cherabuddi 2016). Available epidemiological and climate information could be used to create predictive models to forecast the spread of EEEV with predicted climate change.
The results of this ScR characterize the 718 global research articles on EEEV. Several knowledge gaps were highlighted in this discussion. Most troubling is the impact of EEEV on affected cases and the lack of effective treatment options. The next was an apparent knowledge gap in the dynamics EEEV spread and an explanation for the increasing number of human cases over the past decade. Whether this is due to the impacts of climate change or new competent vectors, increasing cases of EEEV is a public health concern and additional research is warranted to close some of these knowledge gaps. For public health in affected areas and areas close to those infected area, mosquito surveillance and public health mosquito bite prevention programs are important mitigation strategies in the prevention of EEEV and other mosquito-borne diseases. The summary of research on EEEV presented in this ScR provides a foundation for evidence-based policy and decision making on this important public health issue.
Supplementary Material
Acknowledgments
The authors acknowledge Nassim Haghighi, Shannon Harding, Nicole Pachal, Carl Uhland, Barb Wilhelm, Ian Young, and Kaitlin Young for assisting with relevance screening and data characterization. Also, Ian Young and Barb Wilhelm for their input into the conceptualization of this project and the Public Health Agency of Canada library for help with article procurement.
Author Disclosure Statement
All authors declare that no competing financial interests exist.
Funding Information
No funding was received for this work.
Supplementary Material
References
- Ackerson LK, Viswanath K. Media attention and public perceptions of cancer and eastern equine encephalitis. J Community Health 2010; 35:409–416 [DOI] [PubMed] [Google Scholar]
- Aguilar PV, Weaver SC, Basler CF. Capsid protein of eastern equine encephalitis virus inhibits host cell gene expression. J Virol 2007; 81:3866–3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya M. Keck F, Lindquist M, Voss K, et al. The ubiquitin proteasome system plays a role in Venezuelan equine encephalitis virus infection. PLoS One 2015; 10:e0124792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arechiga-Ceballos N, Aguilar-Setien A. Alphaviral equine encephalomyelitis (Eastern, Western and Venezuelan). Rev Sci Tech 2015; 34:491–501 [DOI] [PubMed] [Google Scholar]
- Armstrong PM, Andreadis TG. Eastern equine encephalitis virus in mosquitoes and their role as bridge vectors. Emerg Infect Dis 2010; 16:1869–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong PM, Andreadis TG. Eastern equine encephalitis virus—Old enemy, new threat. N Engl J Med 2013; 368:1670–1673 [DOI] [PubMed] [Google Scholar]
- Arrigo N, Watts DM, Newman PC, Weaver SC. A comparison of the infection dynamics of house sparrow and cotton rats with North and South American Eastern equine encephalitis virus. Am J Trop Med Hyg 58th Annual Meeting 2009; 81 Suppl 5:289 [Google Scholar]
- Arrigo NC. Experimental studies of the ecology and evolution of eastern equine encephalitis virus and implications for its emergence and classification. The University of Texas Medical Branch Graduate School of Biomedical Sciences, 2010. Available at http://sfx.scholarsportal.info/guelph/docview/758934282?accountid=11233
- Arrigo NC, Paige A, Weaver, SC. Evolutionary patterns of eastern equine encephalitis virus in North versus South America suggest ecological differences and taxonomic revision. J Virol 2010; 84:1014–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres JC, Feemster RF. The sequelae of eastern equine encephalomyelitis. N Engl J Med 1949; 240:960–962 [DOI] [PubMed] [Google Scholar]
- Bartelloni PJ, McKinney RW, Duffy TP, Cole FE. An inactivated eastern equine encephalomyelitis vaccine propagated in chick-embryo cell culture. II. Clinical and serologic responses in man. Am J Trop Med Hyg 1970; 19:123–126 [DOI] [PubMed] [Google Scholar]
- Belle EA, Grant LS, Thorburn MJ. An outbreak of eastern equine encephalomyelitis in Jamaica. II. Laboratory diagnosis and pathology of eastern equine encephalomyelitis in Jamaica. Am J Trop Med Hyg 1964; 13:335–341 [DOI] [PubMed] [Google Scholar]
- Belle EA, King SD, Griffiths BB, Grant LS. Epidemiological investigation for arboviruses in Jamaica, West Indies. Am J Trop Med Hyg 1980; 29:667–675 [DOI] [PubMed] [Google Scholar]
- Bigler WJ, Lassing EB, Buff EE, Prather EC, et al. . Endemic eastern equine encephalomyelitis in Florida: A twenty year analysis, 1955–1974. Am J Trop Med Hyg 1976; 25:884–890 [DOI] [PubMed] [Google Scholar]
- Brault AC, Powers AM, Chavez CL, Lopez RN, et al. . Genetic and antigenic diversity among eastern equine encephalitis viruses from North, Central, and South America. Am J Trop Med Hyg 1999; 61:579–586 [DOI] [PubMed] [Google Scholar]
- Bryant ES, Anderson CR, Van Der Heide L. An epizootic of eastern equine encephalomyelitis in Connecticut. Avian Dis 1973; 17:861–867 [PubMed] [Google Scholar]
- Burgueno A, Frabasile S, Diaz LA, Cabrera A, et al. . Genomic characterization and seroprevalence studies on alphaviruses in Uruguay. Am J Trop Med Hyg 2018; 98:1811–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett-Cadena ND. Patterns of host use in mosquitoes: Implications for virus transmission. Auburn University, 2010. Available at http://sfx.scholarsportal.info/guelph/docview/741550667?accountid=11233
- Calisher CH. Medically important arboviruses of the United States and Canada. Clin Microbiol Rev 1994; 7:89–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisher CH, Levy-Koenig E, Mitchell CJ, Cabrera FA, et al. . Eastern equine encephalitis in the Dominican Republic, 1978. Bull Pan Am Health Organ 1979; 13:380–390 [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Follow-up arbovirus surveillance—Florida, 1970. MMWR Morb Mortal Wkly Rep 1970; 19:430–436 [Google Scholar]
- Centers for Disease Control and Prevention. Mosquito-borne encephalitis—United States. MMWR Morb Mortal Wkly Rep 1980; 29(38). https://stacks.cdc.gov/view/cdc/1461 [Google Scholar]
- Centers for Disease Control and Prevention. Screening for diabetic eye disease—Mississippi. MMWR Morb Mortal Wkly Rep 1983; 32(12) [PubMed]
- Centers for Disease Control and Prevention. Arboviral infections of the central nervous system—United States, 1984. MMWR Morb Mortal Wkly Rep 1985; 34:283–286 [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Eastern equine encephalitis—United States, 1989. JAMA 1989; 262:1756. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Arboviral infections of the central nervous system—United States, 1989. MMWR Morb Mortal Wkly Rep 1990; 39:407., 413–417. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Eastern equine encephalitis—Florida, eastern United States, 1991. MMWR Morb Mortal Wkly Rep 1991; 40:533–535 [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Arboviral disease—United States, 1991. MMWR Morb Mortal Wkly Rep 1992; 41:545–548 [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Arboviral Disease—United States, 1994. MMWR Morb Mortal Wkly Rep 1995; 44:641–644 [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Arboviral infections of the central nervous system—United States, 1996–1997. MMWR Morb Mortal Wkly Rep 1998; 47:517–522 [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Eastern equine encephalitis. 2019a. Available at https://www.cdc.gov/easternequineencephalitis/index.html
- Centers for Disease Control and Prevention. Eastern equine encephalitis—Statistics & maps. 2019b. Available at https://www.cdc.gov/easternequineencephalitis/tech/epi.html
- Chapman GE, Baylis M, Archer D, Daly, JM. The challenges posed by equine arboviruses. Equine Vet J 2018; 50:436–445 [DOI] [PubMed] [Google Scholar]
- Chenier S, Cote G, Vanderstock J, Macieira S, et al. . An eastern equine encephalomyelitis (EEE) outbreak in Quebec in the fall of 2008. Can Vet J 2010; 51:1011–1015 [PMC free article] [PubMed] [Google Scholar]
- Clark GM, Lutz AE, Fadnessl. Observations on the ability of Haemogamasus liponyssoides Ewing and Ornithonyssus bacoti (Hirst) (Acarina, Gamasina) to retain eastern equine encephalitis virus: Preliminary report. Am J Trop Med Hyg 1966; 15:107–112 [DOI] [PubMed] [Google Scholar]
- Crans WJ, Caccamise DF, McNelly JR. Eastern equine encephalomyelitis virus in relation to the avian community of a coastal cedar swamp. J Med Entomol 1994; 31:711–728 [DOI] [PubMed] [Google Scholar]
- Dardiri AH, Yates VJ, Chang PW, Wheatley GH, et al. . The isolation of eastern equine encephalomyelitis virus from brains of sparrows. J Am Vet Med Assoc 1957; 130:409–410 [PubMed] [Google Scholar]
- Darwish MA. Preparation of inactivated eastern equine encephalomyelitis vaccine from chick embryo cell culture. J Egypt Public Health Assoc 1972; 47:246–255 [PubMed] [Google Scholar]
- Deibel R, Srihongse S, Woodall JP. Arboviruses in New York State. An attempt to determine the role of arboviruses in patients with viral encephalitis and meningitis. Am J Trop Med Hyg 1979; 28:577–582 [PubMed] [Google Scholar]
- Dein FJ, Carpenter JW, Clark GG, Montali RJ, et al. . Mortality of captive whooping cranes caused by eastern equine encephalitis virus. J Am Vet Med Assoc 1986; 189:1006–1010 [PubMed] [Google Scholar]
- DeMeio JL, DeSanctis AN, Thomas WJ. Persistence in humans of antibody after immunization with four alphavirus vaccines. Asian J Infect Dis 1979; 3:119–124 [PubMed] [Google Scholar]
- Dent JH. Pathological changes in the brains of children infected with the virus of eastern equine encephalomyelitis. Bull Tulane Univ Med Fac 1955; 14:85–96 [PubMed] [Google Scholar]
- Deresiewicz RL, Thaler S, Hsu L, Zamani AA. Clinical and neuroradiographic manifestations of eastern equine encephalitis. N Engl J Med 1997; 336:1867–1874 [DOI] [PubMed] [Google Scholar]
- Dunbar MR, Cunningham MW, Roof JC. Seroprevalence of selected disease agents from free-ranging black bears in Florida. J Wildl Dis 1998; 34:612–619 [DOI] [PubMed] [Google Scholar]
- Durden LA, Linthicum KJ, Monath TP. Laboratory transmission of eastern equine encephalomyelitis virus to chickens by chicken mites (Acari: Dermanyssidae). J Med Entomol 1993; 30:281–285 [DOI] [PubMed] [Google Scholar]
- Eklund CM, Brennan JM, Bell JF. Final report to the Pan American Sanitary Bureau regarding the 1948-49 outbreak of eastern equine encephalitis in the Dominican Republic. Bol Oficina Sanit Panam 1950; 29:493–516 [PubMed] [Google Scholar]
- Eleazer TH, Blalock HG, Warner JH Jr., Pearson JE. Eastern equine encephalomyelitis outbreak in coturnix quail. Avian Dis 1978; 22:522–525 [PubMed] [Google Scholar]
- Elias SP, Keenan P, Kenney JL, Morris SR, et al. . Seasonal patterns in eastern equine encephalitis virus antibody in songbirds in Southern Maine. Vector Borne Zoonotic Dis 2017; 17:325–330 [DOI] [PubMed] [Google Scholar]
- Elvinger F, Baldwin CA, Liggett AD, Tang KN, et al. . Prevalence of exposure to eastern equine encephalomyelitis virus in domestic and feral swine in Georgia. J Vet Diagn Invest 1996; 8:481–484 [DOI] [PubMed] [Google Scholar]
- Elvinger F, Liggett AD, Tang KN, Harrison LR, et al. . Eastern equine encephalomyelitis virus infection in swine. J Am Vet Med Assoc 1994; 205:1014–1016 [PubMed] [Google Scholar]
- Emord DE, Morris CD. Epizootiology of eastern equine encephalomyelitis virus in upstate New York, USA. VI. Antibody prevalence in wild birds during an interepizootic period. J Med Entomol 1984; 21:395–404 [DOI] [PubMed] [Google Scholar]
- Estep LK, McClure CJW, Vander Kelen P, Burkett-Cadena ND, et al. . Risk of exposure to eastern equine encephalomyelitis virus increases with the density of northern cardinals. PLoS One 2013; 8:e57879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faddoul GP, Fellows GW. Clinical manifestations of eastern equine encephalomyelitis in pheasants. Avian Dis 1965; 9:530–535 [PubMed] [Google Scholar]
- Farber S, Hill A, Connerly ML, Dingle JH. Encephalitis in infacnts and children: Caused by the virus of the eastern variety of equine encephalitis. JAMA 1940; 114:1725–1731 [Google Scholar]
- Feemster RF. Outbreak of encephalitis in man due to the eastern virus of equine encephalomyelitis. Am J Public Health 1938; 28:1403–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florida Department of Health. Florida arbovirus surveillance week 52: December 23–29, 2018. Available at http://www.floridahealth.gov/%5C/diseases-and-conditions/mosquito-borne-diseases/_documents/2018/2018-week-52-arbovirus-surveillance-report.pdf
- Fothergill LD, Dingle JH, Farber S, Connerley ML. Human encephalitis caused by the virus of the eastern variety of equine encephalomyelitis. N Engl J Med 1938; 219:411–411 [Google Scholar]
- Freier JE. Eastern equine encephalomyelitis. Lancet 1993; 342:1281–1282 [DOI] [PubMed] [Google Scholar]
- Garvin MC, Tarvin KA, Stark LM, Woolfenden GE, et al. . Arboviral infection in two species of wild jays (Aves: Corvidae): Evidence for population impacts. J Med Entomol 2004; 41:215–225 [DOI] [PubMed] [Google Scholar]
- Goldfield M, Sussman O. The 1959 Outbreak of eastern encephalitis encephalitis in New Jersey: I. Introduction and description of outbreak. Am J Epidemiol 1968; 87:1–10 [DOI] [PubMed] [Google Scholar]
- Giltner LS, Shahan MS. The 1933 outbreak of infectious equine encephalomyelitis in the eastern states. North Am Vet 1933; 14:25 [Google Scholar]
- Gollapudi DAO, Wycuff DL, Schwartz RM, Cooper JW, et al. . Development of high-throughput and high sensitivity capillary gel electrophoresis platform method for Western, Eastern, and Venezuelan equine encephalitis (WEVEE) virus like particles (VLPs) purity determination and characterization. Electrophoresis 2017; 38:2610–2621 [DOI] [PubMed] [Google Scholar]
- Grady GF, Maxfield HK, Hildreth SW, Timperi RJ, et al. . Eastern equine encephalitis in Massachusetts, 1957–1976: A prospective study centered upon analyses of mosquitoes. Am J Epidemiol 1978; 107:170–178 [DOI] [PubMed] [Google Scholar]
- Hachiya M, Osborne M, Stinson C, Werner BG. Human eastern equine encephalitis in Massachusetts: Predictive indicators from mosquitoes collected at 10 long-term trap sites, 1979–2004. Am J Trop Med Hyg 2007; 76:285–292 [PubMed] [Google Scholar]
- Hart KL, Keen D, Belle EA. An outbreak of eastern equine encephalomyelitis in Jamaica, West Indies. I. Description of human cases. Am J Trop Med Hyg 1964; 13:331–334 [DOI] [PubMed] [Google Scholar]
- Hauser G. Human equine encephalomyelitis, eastern type, in Louisiana. New Orleans Med Surg J 1948; 100:551–558 [PubMed] [Google Scholar]
- Heberlein-Larson LA, Tan Y, Stark LM, Cannons AC, et al. . Complex epidemiological dynamics of eastern equine encephalitis virus in Florida. Am J Trop Med Hyg 2019; 100:1266–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available at www.training.cochrane.org/handbook
- Howard JJ, Morris CD, Emord DE, Grayson MA. Epizootiology of eastern equine encephalitis virus in upstate New York, USA. VII. Virus surveillance 1978-85, description of 1983 outbreak, and series conclusions. J Med Entomol 1988; 25:501–514 [DOI] [PubMed] [Google Scholar]
- Howard JJ, Oliver J, Grayson MA. Antibody response of wild birds to natural infection with Alphaviruses. J Med Entomol 2004; 41:1090–1103 [DOI] [PubMed] [Google Scholar]
- Howitt BF, Bishop LK, Gorrie RH, Kissling RE, et al. . An outbreak of equine encephalomyelitis, eastern type, in Southwestern Louisiana. Proc Soc Exp Biol Med 1948; 68:70–72 [DOI] [PubMed] [Google Scholar]
- Hoyos RL, Suaza JV, Tenorio A, Uribe S, et al. . Molecular detection of Eastern Equine Encephalitis virus in mosquitoes from La Pintada (Antioquia). Revista MVZ Cordoba 2015; 20:4800–4806 [Google Scholar]
- Jonsson CB, Cao X, Lee J, Gabbard JD, et al. . Efficacy of a ML336 derivative against Venezuelan and eastern equine encephalitis viruses. Antivir Res 2019; 167:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappus KD, Sather GE, Kaplan JE, Schonberger LB, et al. . Human arboviral infections in the united states in 1980. J Infect Dis 1982; 145:283–286 [DOI] [PubMed] [Google Scholar]
- Katz E, Margalith E, Winer B. Inhibition of the growth of hemadsorption 2 virus by three acyl derivatives of amino acids. Antimicrob Agents Chemother 1975; 7:717–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelen PTV, Downs JA, Burkett-Cadena ND, Ottendorfer CL, et al. . Habitat associations of eastern equine encephalitis transmission in Walton County Florida. J Med Entomol 2012; 49:746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SU, Ogden NH, Fazil AA, Gachon, PH, et al. . Current and Projected Distributions of Aedes aegypti and Ae. albopictus in Canada and the U.S. Environ Health Perspect 2020; 128:057007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissling RE, Chamberlain RW, Eidson ME, Sikes, RK, et al. . Studies on the North American arthropod-borne encephalitides. II. Eastern equine encephalitis in horses. Am J Hyg 1954; 60:237–250 [DOI] [PubMed] [Google Scholar]
- Kissling RE, Rubin H. Pathology of eastern equine encephalomyelitis. Am J Vet Res 1951; 12:100–105 [PubMed] [Google Scholar]
- Kumar R, Patil RD. Cryptic etiopathological conditions of equine nervous system with special emphasis on viral diseases. Vet World 2017; 10:1427–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lednicky JA, White SK, Mavian CN, El Badry MA, et al. . Emergence of Madariaga virus as a cause of acute febrile illness in children, Haiti, 2015–2016. PLoS Neglect Trop D 2019; 13:e0006972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiby DA, Hapip CA, Kenney JL, Mutebi J, et al. . Preliminary investigations of donor exposure to eastern equine encephalitis virus and implications for risk. Transfusion 2014; 54:214A [Google Scholar]
- Letson GW, Bailey RE, Pearson J, Tsai TF. Eastern equine encephalitis (EEE): A description of the 1989 outbreak, recent epidemiologic trends, and the association of rainfall with EEE occurrence. Am J Trop Med Hyg 1993; 49:677–685 [DOI] [PubMed] [Google Scholar]
- Liao SJ. Eastern equine encephalitis in Connecticut: A serological survey of pheasant farmers. Yale J Biol Med 1955; 27:287–296 [PMC free article] [PubMed] [Google Scholar]
- Louisiana Department of Health. Encephalitis, EEE & LAC Annual Report. 2017. Available at http://ldh.la.gov/assets/oph/Center-PHCH/Center-CH/infectious-epi/Annuals/EncephalitisEEELAC_LaIDAnnual.pdf
- Lubelczyk C, Mutebi JP, Robinson S, Elias SP, et al. . An epizootic of eastern equine encephalitis virus, Maine, USA in 2009: Outbreak description and entomological studies. Am J Trop Med Hyg 2013; 88:95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luginbuhl RE, Satriano SF, Helmboldt CF, Lamson AL, et al. . Investigation of eastern equine encephalomyelitis. II. Outbreaks in Connecticut pheasants. Am J Hyg 1958; 67:4–9 [DOI] [PubMed] [Google Scholar]
- Lundberg L, Brahms A, Hooper I, Carey B, et al. . Repurposed FDA-Approved drug sorafenib reduces replication of Venezuelan equine encephalitis virus and other alphaviruses. Antiv Res 2018; 157:57–67 [DOI] [PubMed] [Google Scholar]
- Main AJ. Virologic and serologic survey for eastern equine encephalomyelitis and certain other viruses in colonial bats of New England. J Wildl Dis 1979; 15:455–466 [DOI] [PubMed] [Google Scholar]
- Maine Center for Disease Control and Prevention. Reportable infectious diseases in Maine—2009 Summary. 2009. Available at https://www.maine.gov/dhhs/mecdc/infectious-disease/epi/publications/2009annualreport.pdf
- Massachusetts Department of Public Health. Massachusetts arbovirus surveillance and response plan. 2018. Available at https://www.mass.gov/lists/arbovirus-surveillance-plan-and-historical-data
- Molaei, G, Armstrong, PM, Graham, AC, Kramer, AD, et al. . Insights into the recent emergence and expansion of eastern equine encephalitis virus in a new focus in the Northern New England USA. Parasites Vectors 2015; 8:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CD, Caines AR, Woodall JP, Bast TF. Eastern equine encephalomyelitis in upstate New York, 1972–1974. Am J Trop Med Hyg 1975; 24:986–991 [DOI] [PubMed] [Google Scholar]
- Morris CD, Whitney E, Bast TF, Deibel R. An outbreak of Eastern equine encephalomyelitis in Upstate New York during 1971. Am J Trop Med Hyg 1973; 22:561–566 [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Moody EE, Lewokzco K, Huddleston DB, et al. . Eastern equine encephalitis in Tennessee: 2002–2008. J Med Entomol 2012; 49:731–738 [DOI] [PubMed] [Google Scholar]
- Munn Z, Peters MDJ, Stern C, Tufanaru C, et al. . Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol 2018; 18:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutebi JP, Lubelczyk C, Eisen R, Panella N, et al. . Using wild white-tailed deer to detect Eastern Equine Encephalitis virus activity in Maine. Vector Borne Zoonotic Dis 2011; 11:1403–1409 [DOI] [PubMed] [Google Scholar]
- Nasci RS, Berry RL, Restifo RA, Parsons MA, et al. . Eastern equine encephalitis virus in Ohio during 1991. J Med Entomol 1993; 30:217–222 [DOI] [PubMed] [Google Scholar]
- Ng V, Fazil A, Gachon P, Deuymes, G, et al. . Assessment of the probability of autochthonous transmission of chikungunya virus in Canada under recent and projected climate change. Environ Health Perspect 2017; 125:067001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GH, Turell MJ, Pagac BB. Efficacy of eastern equine encephalitis immunization in whooping cranes. J Wildl Dis 1997; 33:312–315 [DOI] [PubMed] [Google Scholar]
- Palmer DF, Bucca MA, Bird BR, Winn JF. Western equine encephalitis and eastern equine encephalitis virus antigens derived from sucrose-acetone treated chicken embryos. Proc Soc Exp Biol Med 1968; 127:514–517 [DOI] [PubMed] [Google Scholar]
- Pandya J, Gorchakov R, Wang E, Leal G, et al. . A vaccine candidate for eastern equine encephalitis virus based on IRES-mediated attenuation. Vaccine 2012; 30:1276–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters MD, Godfrey CM, Khalil H, McInerney P, et al. . Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc 2015; 13:141–146 [DOI] [PubMed] [Google Scholar]
- Peters MDJ, Godfrey C, McInerney P, Baldini Soares C, et al. Chapter 11: Scoping Reviews. In: Aromataris E, Munn Z, eds. Joanna Briggs Institute Reviewer's Manual. The Joanna Briggs Institute, 2017. Available at https://reviewersmanual.joannabriggs.org/
- Pittman PR, Liu, CT, Cannon TL, Mangiafico JA, et al. . Immune interference after sequential alphavirus vaccine vaccinations. Vaccine 2009; 27:4879–4882 [DOI] [PubMed] [Google Scholar]
- Pollard M, Connolly J, Fromm S. The precipitating effect of methanol on viruses. Proc Soc Exp Biol Med 1949; 71:290–293 [DOI] [PubMed] [Google Scholar]
- ProMED. Eastern equine encephalitis—North America (25): USA (Connecticut, Massachusetts, Michigan) human. 2019. Available at https://promedmail.org/, archive no. 2019. 0923.6688701
- Public Health Ontario. Eastern equine encephalitis virus. History and Enhanced Surveillance in Ontario. 2014. Available at https://www.publichealthontario.ca/-/media/documents/e/2014/eeev-report.pdf?la=en
- Public Health Ontario. Vector-borne diseases. 2016 Summary Report. 2017. Available at https://www.publichealthontario.ca/-/media/documents/vector-borne-diseases-2016.pdf?la=en
- Region of Peel. Vector-Borne Disease Prevention Plan 2016. 2016. Available at https://www.peelregion.ca/health/vbd/resources/pdf/2016-vbd-plan.pdf
- Rehacek J. Elimination of the virus of eastern equine encephalomyelitis (EEE) in the faeces of experimentally infected ticks Ixodes ricinus L. and Dermacentor marginatus Sulz. Acta Virol 1958; 2:158–163 [PubMed] [Google Scholar]
- Rehacek J. Experimental hibernation of the virus of the Eastern type of North American equine encephalomyelitis (EEE) in Czechoslovak species of ticks. (Ixodes ricinus L. and Dermacentor marginatus Sulz). J Hyg Epidemiol Microbiol Immunol 1960; 4:61–65 [PubMed] [Google Scholar]
- Reisler RB, Gibbs PH, Danner DK, Boudreau EF. Immune interference in the setting of same-day administration of two similar inactivated alphavirus vaccines: Eastern equine and western equine encephalitis. Vaccine 2012; 30:7271–7277 [DOI] [PubMed] [Google Scholar]
- Rico A. Development and efficacy testing of broad alphavirus vaccines and antivirals and characterization of alphavirus neuroinvasion. (1013. 7957 Ph.D.), Colorado State University, 2016. Available at http://sfx.scholarsportal.info/guelph/docview/1803939461?accountid=11233
- Rodriguez LF. Emerging arboviruses in Harris County, Texas. (3297. 432 Dr.P.H.), The University of Texas School of Public Health, 2008. Available at http://sfx.scholarsportal.info/guelph/docview/304471380?accountid=11233
- Ross WA, Kaneene JB. A case-control study of an outbreak of Eastern Equine Encephalomyelitis in Michigan (USA) equine herds in 1991. Prev Vet Med 1995; 24:157–170 [Google Scholar]
- Ross WA, Kaneene JB. Evaluation of outbreaks of disease attributable to eastern equine encephalitis virus in horses. J Am Vet Med Assoc 1996; 208:1988–1997 [PubMed] [Google Scholar]
- Rowe M, King D. Eastern equine encephalitis (EEE) antibody status in St. Thomas, Jamaica, 1975. W Indian Med J 1976; 25:146–152 [PubMed] [Google Scholar]
- Ruiz-Gomez J, Espinosa LEL. Serum epidemiology of Eastern. Western and Venezuelan equine encephalitides in Mexico. Arch Invest Med (Mex) 1981; 12:395–419 [PubMed] [Google Scholar]
- Saxton-Shaw KD, Ledermann JP, Kenney JL, Berl E, et al. . The first outbreak of eastern equine encephalitis in Vermont: Outbreak description and phylogenetic relationships of the virus isolate. PLoS One 2015; 10:e0128712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon JE. The relationship of culicoides (Diptera, Ceratopogonida) to the tranmission of the virus of eastern equine encephalitits. (6100. 428 Ph.D.), University of Maryland, College Park, 1960. Available at http://sfx.scholarsportal.info/guelph/docview/301856109?accountid=11233
- Schmitt SM, Cooley TM, Fitzgerald SD, Bolin SR, et al. . An outbreak of eastern equine encephalitis virus in free-ranging white-tailed deer in Michigan. J Wildl Dis 2007; 43:635–644 [DOI] [PubMed] [Google Scholar]
- Sellers RF. Eastern equine encephalitis in Quebec and Connecticut, 1972: Introduction by infected mosquitoes on the wind? Can J Vet Res 1989; 53:76–79 [PMC free article] [PubMed] [Google Scholar]
- Shah KJ, Cherabuddi K. Case of eastern equine encephalitis presenting in winter. BMJ Case Rep 2016; bcr2016215270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MA, Misasi J, Smole S, Feldman HA, et al. . Eastern equine encephalitis in children, Massachusetts and New Hampshire, USA, 1970–2010. Emerg Infect Dis 2013; 19:194–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaff NK, Armstrong PM, Andreadis TG, Cheruvelil KS. Wetland characteristics linked to broad-scale patterns in Culiseta melanura abundance and eastern equine encephalitis virus infection. Parasite Vector 2017; 10:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soret MG, Sanders M. In vitro method for cultivating eastern equine encephalomyelitis virus in teleost embryos. Proc Soc Exp Biol Med 1954; 87:526–529 [DOI] [PubMed] [Google Scholar]
- Spalding MG, McLean RG, Burgess JH, Kirk LJ. Arboviruses in water birds (Ciconiiformes, Pelecaniformes) from Florida. J Wildl Dis 1994; 30:216–221 [DOI] [PubMed] [Google Scholar]
- Srihongse S, Grayson MA, Morris CD, Deibel R, et al. . Eastern equine encephalomyelitis in upstate New York: Studies of a 1976 epizootic by a modified serologic technique, hemagglutination reduction, for rapid detection of virus infections. Am J Trop Med Hyg 1978; 27:1240–1245 [DOI] [PubMed] [Google Scholar]
- Strizki JM. Molecular characterization and pathogenesis of eastern equine encephalitis viruses. (9421. 865 Ph.D.), The Medical College of Pennsylvania, 1994. Available at http://sfx.scholarsportal.info/guelph/docview/304115055?accountid=11233
- Strizki JM, Repik PM. Differential reactivity of immune sera from human vaccinees with field strains of eastern equine encephalitis virus. Am J Trop Med Hyg 1995; 53:564–570 [DOI] [PubMed] [Google Scholar]
- Takeda T, Whitehouse CA, Brewer M, Gettman AD. Arbovirus surveillance in Rhode Island: Assessing potential ecologic and climatic correlates. J Am Mosq Control Assoc 2003; 19:179–189 [PubMed] [Google Scholar]
- Tricco AC, Lillie E, Zarin W, O'Brien K, et al. . A scoping review on the conduct and reporting of scoping reviews. BMC Med Res Methodol 2016; 16:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricco AC, Lillie E, Zarin W, O'Brien KK, et al. . PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med 2018; 169:467–473 [DOI] [PubMed] [Google Scholar]
- Tully TN Jr., Shane SM, Poston RP, England JJ, et al. . Eastern equine encephalitis in a flock of emus (Dromaius novaehollandiae). Avian Dis 1992; 36:808–812 [PubMed] [Google Scholar]
- Tuttle AD, Andreadis TG, Frasca S Jr., Dunn JL. Eastern equine encephalitis in a flock of African penguins maintained at an aquarium. J Am Vet Med Assoc 2005; 226:2059–2062 [DOI] [PubMed] [Google Scholar]
- Vander Kelen PT, Downs JA, Stark LM, Loraamm RW, et al. . Spatial epidemiology of eastern equine encephalitis in Florida. Int J Health Geogr 2012; 11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villari P, Spielman A, Komar N, McDowell M, et al. . The economic burden imposed by a residual case of eastern encephalitis. Am J Trop Med Hyg 1995; 52:8–13 [DOI] [PubMed] [Google Scholar]
- Webster LT, Wright FH. Recovery of Eastern equine encephalomyelitis virus from brain tissue of human cases of encephalitis in Massachusetts. Science 1938; 88:305–306 [DOI] [PubMed] [Google Scholar]
- Wilson JH, Rubin HL, Lane TJ, Gibbs EPJ. A survey of eastern equine encephalomyelitis in Florida horses: Prevalence, economic impact, and management practices, 1982–1983. Prev Vet Med 1986; 4:261–271 [Google Scholar]
- Zacks MA, Paessler S. Encephalitic alphaviruses. Vet Microbiol 2010; 140:281–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.