Abstract
The Asian Pacific Society of Cardiology convened a consensus statement panel for optimising cardiovascular (CV) outcomes in type 2 diabetes, and reviewed the current literature. Relevant articles were appraised using the Grading of Recommendations, Assessment, Development and Evaluation system, and consensus statements were developed in two meetings and were confirmed through online voting. The consensus statements indicated that lifestyle interventions must be emphasised for patients with prediabetes, and optimal glucose control should be encouraged when possible. Sodium–glucose cotransporter 2 inhibitors (SGLT2i) are recommended for patients with chronic kidney disease with adequate renal function, and for patients with heart failure with reduced ejection fraction. In addition to SGLT2i, glucagon-like peptide-1 receptor agonists are recommended for patients at high risk of CV events. A blood pressure target below 140/90 mmHg is generally recommended for patients with type 2 diabetes. Antiplatelet therapy is recommended for secondary prevention in patients with atherosclerotic CV disease.
Keywords: Type 2 diabetes, cardiovascular, sodium-glucose cotransporter 2 inhibitor, glucagon-like protein 1 receptor agonist, consensus, Asia Pacific, prediabetes
In recent decades, there has been a significant increase in the number of people with type 2 diabetes (T2D), particularly in developing countries. Sixty per cent of the world’s diabetes cases are in Asia, largely due to its large population, posing significant social and economic burdens to most nations in the region.[1] Systematic reviews have shown that the epidemiological data on the cardiovascular (CV) complications of T2D are substantially scarcer in the Asia Pacific region compared with western countries.[2] However, the limited data suggest that the prevalence of CV disease (CVD) among T2D patients in the Asia Pacific (33.6–44.5%) is slightly higher compared with the West (27.5–46%). Furthermore, studies have also confirmed that T2D is associated with a twofold increased risk of CVD compared with non-T2D patients in the Asia-Pacific region.[3] Poor glycaemic control and high variability of plasma glucose levels are the leading causes of CV mortality in patients with T2D. While glycaemic management is essential, specific organ protection is also important in the management of patients with T2D.
These consensus statements aim to provide guidance on specific organ protection in patients with T2D to optimise CV outcomes. Despite focusing on pharmacotherapy, these consensus statements cannot emphasise enough the central role of lifestyle modification on the comprehensive management of T2D. For example, several recent studies have confirmed the benefit of intensive dietary interventions (mostly low carbohydrate diets) in the control and overall course of disease among T2D patients.[4–6] The panel endorses a tailored, multidisciplinary approach in prescribing dietary interventions for T2D patients, in accordance with clinical guidelines.[7] Similarly, recent evidence also supports the inclusion of exercise in the management of T2D patients, preferably under the supervision of qualified professionals.[8–10] These fundamental concepts should be kept in mind when applying these consensus statements in clinical practice.
Methods
The Asian Pacific Society of Cardiology (APSC) convened a 28-member multidisciplinary expert panel of cardiologists, endocrinologists and nephrologists with clinical and research expertise in the diagnosis and treatment of T2D and CVD to develop consensus statements on optimising CV outcomes in patients with T2D. The aim of these consensus statements was to guide clinicians on specific organ protection in patients with T2D in the Asia Pacific setting. These experts represented 15 territories and countries in the Asia Pacific region.
For these consensus statements, we adopted the criteria of the American Diabetes Association (ADA).[2] Prediabetes was diagnosed by fasting plasma glucose (FPG) of 5.6–6.9 mmol/l, indicating impaired fasting glucose (IFG); or a 2-hour plasma glucose (PG) during a 75-g oral glucose tolerance test (OGTT) of 7.8–11 mmol/l, indicating impaired glucose tolerance (IGT); or HbA1c of 5.7–6.4% (39–47 mmol/mol). Diabetes was diagnosed by FPG ≥7 mmol/l, or 2-hour PG ≥11.1 mmol/l during OGTT, or a HbA1c level ≥6.5% (48 mmol/mol), or classic symptoms of hyperglycaemia and a random PG of ≥11.1 mmol/l.[11] However, it should be kept in mind that countries in the Asia-Pacific region may use other cut-offs than the ADA criteria. For example, Singapore uses HbA1c ≥7% as the criterion for diabetes.[12] In contrast, guidelines from India and the United Arab Emirates use the following: HbA1c ≥6.5%, FPG ≥7 mmol/l, or 2-hour PG ≥11.1 mmol/l during OGTT.[13,14]
After a comprehensive literature search, selected articles were reviewed and analysed using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system, as follows:
High (authors have high confidence that the true effect is similar to the estimated effect).
Moderate (authors believe that the true effect is probably close to the estimated effect).
Low (true effect might be markedly different from the estimated effect).
Very low (true effect is probably markedly different from the estimated effect).[15]
The available evidence was then discussed during two consensus meetings. Consensus statements were developed during the meetings, which were then put to an online vote. Each statement was voted on by each panel member using a three-point scale (agree, neutral or disagree). Consensus was reached when 80% of votes for a statement were agree or neutral. In cases of non-consensus, the statements were further discussed using email communication, and then revised accordingly until the criterion for consensus was reached.
Prediabetes
Statement 1. Patients with prediabetes should be monitored closely and counselled regarding lifestyle interventions.
Level of evidence: High.
Level of agreement: 100% agree, 0% neutral, 0% disagree.
Large randomised, controlled trials (RCTs) have shown that, compared with controls, lifestyle intervention and weight loss in patients with IGT leads to a significant reduction in the risk of diabetes, CV and all-cause mortality.[16,17] The Diabetes Prevention Program Research Group also found that lifestyle interventions were significantly more effective than metformin in reducing the incidence of diabetes in individuals at high risk.[18,19] Consistent with the findings of these large RCTs, the panel recommends the implementation of lifestyle interventions for patients with prediabetes.
Glycaemic Target
Statement 2. Where possible, optimal glucose control should target HbA1c <7%.
Level of evidence: High.
Level of agreement: 96.4% agree, 3.6% neutral, 0% disagree.
Statement 3. Hypoglycaemia increases the risk of mortality and CV events, and should be avoided.
Level of evidence: Moderate.
Level of agreement: 100% agree, 0% neutral, 0% disagree.
Statement 4. A less stringent HbA1c target (<8%) may be appropriate in patients with advanced age, limited lifespan and comorbidities that predispose to hypoglycaemia.
Level of evidence: Low.
Level of agreement: 89.3% agree, 10.7% neutral, 0% disagree.
Statement 5. Patients with complex glycaemic management may require referral to an endocrinologist.
Level of evidence: Low.
Level of agreement: 96.4% agree, 3.6% neutral, 0% disagree.
A meta-analysis of five prospective RCTs in patients with T2D showed that, compared with standard treatment, intensive treatment that resulted in a mean HbA1c of <7% at follow-up was associated with a significantly reduced risk of non-fatal MI and coronary heart disease, but not stroke or all-cause mortality.[20] Currently, the concept of time-in-range from continuous glucose monitoring is not widely used in the Asia Pacific region; however, this may gain wider utilisation in the coming years.
The DCCT/EDIC study in patients with type 1 diabetes (T1D) also showed that targeting an HbA1c of 7%, compared with 9%, resulted in a significant reduction in non-fatal MI, stroke or death from CVD through a mean follow-up of 17 years.[21] Additionally, the UKPDS follow-up study found that patients on intensive therapy experienced a continued reduction in the risks of microvascular and emergent macrovascular complications, including MI and death from any cause, during the 10 years of post-trial follow-up.[22]
However, a review found that patients with type 1 diabetes and T2D were at increased risk of hypoglycaemia-induced CV events and mortality.[23] The DIGAMI 2 prospective study (1,253 patients with T2D with hypoglycaemic episodes) also showed that severe hypoglycaemia is a pronounced risk factor of acute MI.[24] Given these findings on the harms of hypoglycaemia, this complication should be avoided as much as possible.
Older patients with diabetes are at greater risk for hypoglycaemia compared with younger patients, even with comparable glycaemic control. The presence of comorbidities, such as chronic renal or hepatic impairment, also contributes to an increased risk of hypoglycaemia.[25] Hypoglycaemia is also associated with an increased risk of dementia and acute CV events in the elderly.[26] Therefore, a less stringent HbA1c target is recommended in certain patients with increased risk of hypoglycaemia, such as the elderly and those with other comorbidities that predispose to hypoglycaemia.
Patients at High Risk of CV Events
Statement 6. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) with proven CV benefits and sodium-glucose cotransporter-2 inhibitors (SGLT2i) are recommended in patients with T2D who have adequate renal function and are at high risk of CV events.
Level of evidence: High.
Level of agreement: 96.4% agree, 0% neutral, 3.6% disagree.
As outlined in the 2020 APSC Chronic Coronary Syndrome consensus guide, patients with T2D who have any of the risk factors categorised according to the CVD system are considered at high risk of CV events.[27]
Results of the LEADER, SUSTAIN-6 and EXSCEL RCTs in patients with high CV risk (around 75% of patients had previous CVD and around 15% had pre-existing heart failure [HF]) demonstrated that, compared with placebo, GLP-1RA led to a reduction in major adverse CV events (MACE), but not in the risk of HF admission.[28–30]
The PIONEER-6 RCT demonstrated that, compared with placebo treatment, oral semaglutide led to reduced rates of MACE (HR 0.79, p<0.001 for non-inferiority) and significantly reduced the risk for CV death and all-cause death in patients with T2D and high CV risk.[31] However, the REWIND trial found that dulaglutide was associated with a reduced rate of the primary composite outcome (non-fatal MI, non-fatal stroke or CV death; HR 0.88, p=0.026) compared with placebo, while all-cause mortality did not differ between the groups (p=0.067).[32]
A meta-analysis of several CV outcome trials (CVOTs) showed that, compared with placebo, treatment with GLP-1RA was associated with a 10% relative risk reduction (RRR) in the three-point MACE, a 13% RRR in CV mortality and a 12% RRR in all-cause mortality.[33]
Regarding SGLT2i use, a meta-analysis was conducted on four studies: CANVAS, CREDENCE, DECLARE-TIMI 58 and EMPA-REG OUTCOME. The meta-analysis showed that MACE was reduced by 12% with this drug class (HR 0.88, 95% CI[0.82–0.94]). Importantly, when used for secondary prevention, SGLT2i therapy was associated with a 14% reduction in MACE (HR 0.86, 95% CI[0.80–0.93]), a 20% reduction in CV death (HR 0.80, 95% CI[0.71–0.90]) and a 17% reduction in all-cause death (HR 0.83, 95% CI[0.75–0.91]).[34]
Finally, a recent multicentre, double-blind trial (VERTIS-CV) found that patients with T2D and atherosclerotic CVD (ASCVD) who were treated with ertugliflozin had a lower risk of first hospitalisation for HF (hHF; HR 0.70, 95% CI[0.54–0.90]).[35] While some studies, such as CANVAS, have demonstrated a small increase in the risk of amputation with canagliflozin (6.3 versus 3.4 per 1000 patient-years for placebo), SGLT2is are generally considered to have a favourable risk–benefit ratio.[34,36]
The recommended treatment options for patients with T2D at high risk of CV events are summarised in Figure 1.
Figure 1: Pharmacotherapeutic Options in Patients at High Risk of Cardiovascular Events.
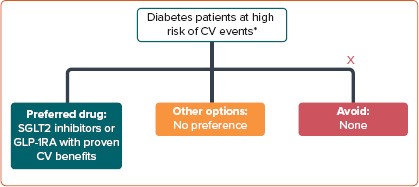
*Based on the Asian Pacific Society of Cardiology cardiovascular disease scoring.
CV = cardiovascular; GLP-1RA = glucagon-like protein 1 receptor agonist; SGLT2 = sodium–glucose cotransporter 2.
Patients with Chronic Kidney Disease
Statement 7. SGLT2i and GLP-1RA are recommended in patients with an estimated glomerular filtration rate (eGFR) >30 ml/min/1.73 m2 for their CV and renal benefits.
Level of evidence: High.
Level of agreement: 89.3% agree, 7.1% neutral, 3.6% disagree.
Statement 8. Insulin, short-acting sulfonylureas and dipeptidyl-peptidase 4 (DPP4) inhibitors are preferred in patients with end-stage renal disease (ESRD) on dialysis.
Level of evidence: Low.
Level of agreement: 92.9% agree, 7.1% neutral, 0% disagree.
Statement 9. Metformin should be avoided in patients with an eGFR <30 ml/min/1.73 m2.
Level of evidence: High.
Level of agreement: 96.4% agree, 3.6% neutral, 0% disagree.
Statement 10. Referral to a nephrologist should be considered in patients with T2D and an eGFR <30 ml/min/1.73 m2 or proteinuria >1 g/day, despite optimal blood pressure (BP) management and renin–angiotensin–aldosterone system blockade.
Level of evidence: High.
Level of agreement: 96.4% agree, 3.6% neutral, 0% disagree.
The ADA 2020 guidelines and the 2020 Kidney Disease – Improving Global Outcomes (KDIGO) Diabetes Management in CKD guidelines both recommend the use of SGLT2i for patients with T2D and diabetic kidney disease when the eGFR ≥30 ml/min/1.73 m2 to reduce the risk of chronic kidney disease (CKD) progression and CV events.[37,38]
The DECLARE-TIMI 58 trial, which compared the SGLT2i dapagliflozin with placebo, demonstrated the CV safety of dapagliflozin, but not its benefits on MACE (HR 0.93, 95% CI[0.84–1.03]). Dapagliflozin was also associated with reduced risk for the composite efficacy endpoint of CV death or hHF (HR 0.83, 95% CI [0.73–0.95]) and a 24% reduction in risk of renal endpoints.[39] A sub-analysis of the DAPA-HF study showed that the use of dapagliflozin was associated with a 28% RRR (absolute risk of 19.9% versus 26.3%, HR 0.72, 95% CI [0.59–0.86]) for the composite of CV death or worsening HF events in patients with CKD (eGFR <60 ml/min/1.73 m2 at baseline), and by a similar magnitude, in patients without CKD (13.8% versus 17.6%, HR 0.76, 95% CI [0.63–0.92]).[40]
The DAPA-CKD trial, which evaluated the efficacy of dapagliflozin compared with placebo in patients with eGFR ≥25 and ≤75 mL/min/1.73 m2 and elevated urinary albumin excretion, with and without T2D, was terminated early given the overwhelming efficacy demonstrated. The primary composite outcome (sustained decline in the eGFR of at least 50%, ESRD or death from renal or CV causes) was reduced by 39% in the dapagliflozin group (HR 0.61, 95% CI[0.51–0.72], p<0.001).[41] Due to these results, some authors proposed to use a cut-off of 25 instead of 30 ml/min/1.73 m2 for statement 7. However, for simplicity, when applying these statements in clinical practice, the current lower cut-off for CKD stage 3B (i.e. 30 ml/min/1.73 m2) is adapted.
The CREDENCE trial in patients with T2D and CKD (eGFR 30–90 ml/min/1.73 m2) demonstrated that, compared with placebo, canagliflozin led to a reduced risk of renal composite endpoints, including progression to ESRD, and on CV mortality, MACE and hHF. The efficacy of canagliflozin in reducing MACE, hHF, CV mortality and renal endpoints was similar regardless of the baseline status of CVD or CKD grades 2–3.[42]
A subanalysis of patients of Asian ethnicity in the EMPA-REG OUTCOME trial demonstrated that empagliflozin reduced the risk, or the worsening, of nephropathy (HR 0.64, 95% CI[0.49–0.83]), progression to macroalbuminuria (HR 0.64, 95% CI[0.49–0.85]) and the composite of doubling of serum creatinine, initiation of renal replacement therapy or renal death (HR 0.48, 95% CI[0.25–0.92]). Furthermore, empagliflozin-treated patients showed slower eGFR decline versus placebo-treated patient, and showed rapid urine albumin-to-creatinine ratio reduction at week 12, which was maintained through to week 164, with effects most pronounced in those with baseline microalbuminuria or macroalbuminuria.[43]
A meta-analysis of the SGLT2i CVOT suggested a class effect in reducing the risk of CKD progression across high and lower CVD risk subgroups by reducing the risk of worsening eGFR, ESRD or renal death (HR 0.55, 95% CI [0.48–0.64], p<0.001).[44]
In addition to SGLT2Is, GLP-1RAs have been recommended by the 2020 KDIGO guidelines.[38] This was based on a meta-analysis of CVOTs (including patients with eGFR >15 ml/min/1.73 m2), which showed a 15% reduction in the risk of MACE (HR 0.85, 95% CI [0.76–0.95]).[45] However, data on GLP-1RA in those with more severe CKD are limited.
For patients with CKD, including those with ESRD or those on dialysis, all currently available DPP4 inhibitors may be used.[46] It has also been suggested that DPP4 inhibitors, may exert a kidney-protective effect by reducing the incidence of albuminuria.[47,48] Three studies have also reported an improvement in albuminuria with DPP4 inhibitors.[49–51] However, the panel did not find enough evidence to recommend DPP4 inhibitors as a preferred drug over other agents for patients on dialysis. For such patients, insulin or short-acting sulfonylureas are recommended, based on the extensive clinical experience with these drugs.
As metformin is excreted renally, it is contraindicated in patients with eGFR <30 ml/min/1.73 m2.
The ADA Standards of Medical Care in Diabetes––2020 state that referral to a nephrologist should take place when there is uncertainty about the aetiology of kidney disease, for difficult management issues (anaemia, secondary hyperparathyroidism, metabolic bone disease, resistant hypertension or electrolyte disturbances), or when there is advanced kidney disease (eGFR <30 ml/min/1.73 m2) requiring discussion of renal replacement therapy for ESRD.[37]
The recommended treatment options for improving CV and renal outcomes in patients with T2D and CKD are summarised in Figure 2.
Figure 2: Pharmacotherapeutic Options for Type 2 Diabetes Patients Stratified by eGFR.
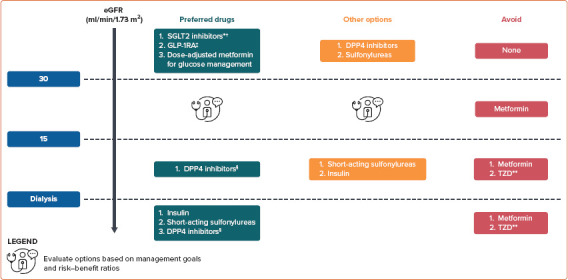
*Avoid initiating if eGFR <30 ml/min/1.73 m2. †For renal and cardiovascular protection. ‡For cardiovascular benefits. §Linagliptin is recommended as no dose adjustment is required. **Due to fluid overload. DPP4 = dipeptidyl peptidase 4; eGFR = estimated glomerular filtration rate; GLP-1RA = glucagon-like protein 1 receptor agonist; SGLT2 = sodium–glucose cotransporter 2; TZD, thiazolidinedione.
Patients with Heart Failure
Statement 11. SGLT2i are recommended in patients with HF with reduced ejection fraction (HFrEF; ≤40%) to reduce hospitalisation due to HF and CV death.
Level of evidence: High.
Level of agreement: 89.3% agree, 10.7% neutral, 0% disagree.
The DAPA-HF RCT in patients with HFrEF (≤40%) demonstrated that, compared with placebo, dapagliflozin led to a significant reduction in the risk of hospitalisation or urgent visit due to HF or CV death, as well as for HF events and total mortality due to hHF and CV death.[52] It was also demonstrated in the DECLARE-TIMI 58 trials, CANVAS Program and EMPA-REG OUTCOME that SGLT2i significantly reduced the risk CV death and hHF.[36,39,53] A subanalysis of the DECLARE-TIMI 58 trial also showed that, compared with placebo, dapagliflozin led to around a 45% reduction in the risk of CV death and all-cause mortality in patients with HFrEF. These effects were not observed in patients without HFrEF.[54] Finally, the EMPEROR-Reduced trial showed that, regardless of the presence or absence of diabetes, patients given empagliflozin had a lower risk of CV death or hospitalisation for worsening HF compared with those in the placebo group.[55]
The recommended treatment options for improving CV outcomes in patients with T2D and HFrEF are summarised in Figure 3.
Figure 3: Pharmacotherapeutic Options for Type 2 Diabetes Patients with HFrEF.
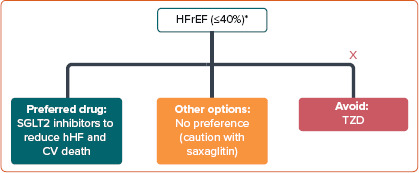
*Based on evidence from DAPA-HF[40] and EMPEROR-Reduced.[55] CV = cardiovascular; HFrEF = heart failure with reduced ejection fraction; hHF = hospitalisation for heart failure; SGLT2 = sodium–glucose cotransporter 2; TZD = thiazolidinedione.
Blood Pressure Target
Statement 12. A BP target <140/90 mmHg is generally recommended in patients with T2D. Patients with T2D and hypertension at higher CV risk, or 10-year ASCVD ≥15% or other organ involvement should aim for a target BP <130/80 mmHg.
Level of evidence: Moderate.
Level of agreement: 96.4% agree, 3.6% neutral, 0% disagree.
The UKPDS trial in 1,148 patients with T2D and hypertension demonstrated that systolic BP (SBP) control to a mean of 144 mmHg, compared with 154 mmHg, led to a significant reduction in the risks of diabetic endpoints, diabetes-related deaths, strokes and microvascular endpoints.[56] Five years post-trial monitoring of 884 patients who were not made to maintain their previously assigned therapies showed that the benefits of previously improved BP control were not sustained when between-group differences in BP were lost.[57]
The ACCORD-BP RCT showed that in approximately 4,700 participants with diabetes, targeting an SBP of <120 mmHg, compared with a target of <140 mmHg, did not reduce the rate of composite outcome of fatal and non-fatal major CV events. Compared with the standard antihypertensive therapy group, targeting SBP <120 mmHg was associated with a 41% reduction in stroke rate, but significantly increased the rate of serious adverse events.[58]
A post-hoc analysis of the ACCORD-BP trial of patients who had additional CV risk factors (n=2592) showed that intensive BP control to 120 mmHg reduced the composite of CV death, non-fatal MI, non-fatal stroke, any revascularisation and HF.[59] The SPRINT demonstrated CV benefits in patients with hypertension and without T2D randomised to SBP <120 versus <140 mmHg. Reasons proposed as to why this benefit was originally not observed in ACCORD-BP include statistical power and not because of differences in CV profile for patients with diabetes.[60] Additionally, a subgroup analysis showed that the risk of CV events was reduced in those on intensive BP treatment than in the standard BP treatment among those receiving standard glycaemic control (p=0.005), indicating a role of glycaemic control in determining net CV benefits.[61]
The American College of Cardiology/American Heart Association 2017 BP guidelines advocate a BP target of <130/80 mmHg for patients with T2D, on the basis that patients with diabetes are at a higher risk of CV events and improved CV; microvascular risk reduction is maintained at SBP <130 mmHg. Many joint guidelines advocate treating hypertension in people with diabetes to a blood pressure goal of <130/80 mmHg.[62]
While RCTs on BP targets directly relating to patients with diabetes are limited, there is an unequivocal call to treat hypertension in diabetes.[63] The panel recommends a BP target of <140/90 mmHg for patients with T2D, and a target BP of <130/80 mmHg, but not beyond <120/70 mmHg, in the presence of other organ involvement, such as CKD or CVD.
These recommendations align with western guidelines. The 2012 KDIGO guidelines state that adults with diabetes and non-dialysis-dependent CKD, with urine albumin excretion <30 mg/24 hours with consistent SBP of >140 mmHg or diastolic BP (DBP) of >90 mmHg, be treated with BP-lowering drugs to maintain a consistent SBP of ≤140 mmHg and DBP of ≤90 mmHg.[64] However, adults with diabetes and non-dialysis-dependent CKD, with urine albumin excretion >30 mg/24 hours with consistent SBP of >130 mmHg or DBP of >80 mmHg, should be treated with BP-lowering drugs to maintain a consistent SBP of ≤130 mmHg and DBP of ≤80 mmHg.
The ADA Standards of Medical Care in Diabetes – 2019 state that, for patients with diabetes and hypertension at higher CV risk (existing atherosclerotic CVD or 10-year atherosclerotic CVD risk >15%), a BP target of <130/80 mmHg may be appropriate if it can be safely attained.[65]
Antiplatelet Therapy
Statement 13. Antiplatelet therapy should be used for secondary prevention in patients with established CVD.
Level of evidence: High.
Level of agreement: 100% agree, 0% neutral, 0% disagree.
A meta-analysis of secondary prevention trials showed that aspirin leads to a greater absolute reduction in serious vascular events, with a non-significant increase in haemorrhagic stroke, but 20% reduction in total stroke and coronary events.[66] Consistent with previous recommendations, the panel recommends that antiplatelet therapy be used for secondary prevention in patients with T2D and established CVD.[67] However, despite being able to reduce ischaemic risk, the use of more potent antiplatelet therapies and prolonged intensified therapy may also be associated with an increase in bleeding complications, which continues to be a major concern.[68] Therefore, the use of P2Y12 inhibitors in patients with T2D needs to be individualised according to the overall ischaemic and bleeding risks of each patient.
Low-dose aspirin might be considered for the primary prevention of ASCVD in select T2D patients with higher ASCVD risk (e.g. those with a >20% 10-year risk of CV events) who are not at increased risk of bleeding.[69] However, low-dose aspirin should not be administered on a routine basis for the primary prevention of ASCVD among adults with T2D, especially in light of recent data suggesting an increased risk of bleeding that counterbalances its CV benefit with routine use.[70–72] The decision to use aspirin for primary prevention should be done after careful consideration of the individual patient’s thrombotic and bleeding risks.
Limitations and Conclusion
These consensus statements aim to provide a comprehensive guide on the optimisation of CV outcomes among patients with T2D. However, the management of dyslipidaemia and the use of statins and other lipid-lowering drugs among patients with prediabetes and T2D were not discussed, as this falls within the scope of separate work by the APSC.
Importantly, the 13 statements presented in this paper aim to guide clinicians in providing optimum care to patients with T2D, but should not replace judicious clinical judgement. The optimisation of CV outcomes among patients with T2D should be managed on an individual basis, accounting for an individual’s baseline risk, clinical characteristics and comorbidities, as well as patient concerns and preferences.
Clinicians should also be aware of the challenges that may limit the applicability of these consensus statements, such as the availability and affordability of specific drugs, interventions and other technologies, differences in each country’s healthcare resources and currently accepted standards of care, as well as cultural factors.
Acknowledgments
Medical writing support was provided by Aizel Ebron and Ivan Olegario of MIMS Pte Ltd.
References
- 1.Guariguata L,, Whiting DR,, Hambleton I, et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–49. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Einarson TR,, Acs A,, Ludwig C,, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17:83. doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodward M,, Zhang X,, Barzi F, et al. The effects of diabetes on the risks of major cardiovascular diseases and death in the Asia-Pacific region. Diabetes Care. 2003;26:360–6. doi: 10.2337/diacare.26.2.360. [DOI] [PubMed] [Google Scholar]
- 4.Krentz AJ. DiRECT and indirect paths to reducing cardiovascular risk in diabetes: insights from Diabetes UK 2019. Cardiovasc Endocrinol Metab. 2019;8:67–8. doi: 10.1097/XCE.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unwin D,, Unwin J. Low carbohydrate diet to achieve weight loss and improve HbA1c in type 2 diabetes and pre-diabetes: experience from one general practice. Prac Diabetes. 2014;31:76–9. doi: 10.1002/pdi.1835. [DOI] [Google Scholar]
- 6.Feinman RD,, Pogozelski WK,, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. 2015;31:1–13. doi: 10.1016/j.nut.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Evert AB,, Boucher JL,, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2014;37((Suppl 1)):S120–43. doi: 10.2337/dc14-S120. [DOI] [PubMed] [Google Scholar]
- 8.Taoli W,, Yang L,, Rongzhou Z, et al. Benefit effects of aerobic exercise and resistance training on the management of type 2 diabetes. Int J Clin Exp Med. 2018;11:10433–45. [Google Scholar]
- 9.Yang Z,, Scott CA,, Mao C, et al. Resistance exercise versus aerobic exercise for type 2 diabetes: a systematic review and meta-analysis. Sports Med. 2013;44:487–99. doi: 10.1007/s40279-013-0128-8. [DOI] [PubMed] [Google Scholar]
- 10.Hordern MD,, Dunstan DW,, Prins JB, et al. Exercise prescription for patients with type 2 diabetes and pre-diabetes: a position statement from Exercise and Sport Science Australia. J Sci Med Sport. 2012/01 2012;15-::25–31. doi: 10.1016/j.jsams.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes – 2020. Diabetes Care. 2019;43((Suppl 1):):S14–31. doi: 10.2337/dc20-s002. [DOI] [PubMed] [Google Scholar]
- 12.Goh SY,, Ang SB,, Bee YM, et al. Ministry of Health Clinical Practice Guidelines: diabetes mellitus. Singapore Med J. 2014;55:334–47. doi: 10.11622/smedj.2014079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chawla R,, Madhu SV,, Makkar BM, et al. RSSDI-ESI Clinical practice recommendations for the management of type 2 diabetes mellitus 2020. Indian J Endocrinol Metab. 2020;24:1–122. doi: 10.4103/ijem.IJEM_225_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alawadi F,, Abusnana S,, Afandi B, et al. Emirates Diabetes Society consensus guidelines for the management of type 2 diabetes mellitus – 2020. Dubai Diabetes Endocrinol J. 2020;;26::1–20. doi: 10.1159/000506508. [DOI] [Google Scholar]
- 15.Balshem H,, Helfand M,, Sch–nemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Tuomilehto J,, Lindstr–m J,, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50. doi: 10.1056/nejm200105033441801. [DOI] [PubMed] [Google Scholar]
- 17.Li G,, Zhang P,, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol. 2014;2:474–80. doi: 10.1016/s2213-8587(14)70057-9. [DOI] [PubMed] [Google Scholar]
- 18.Knowler WC,, Barrett-Connor E,, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perreault L,, Pan Q,, Mather KJ, et al. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet. 2012;379:2243–51. doi: 10.1016/S0140-6736(12)60525-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ray KK,, Seshasai SRK,, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–72. doi: 10.1016/s0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 21.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group. Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care. 2016;39::686–93. doi: 10.2337/dc15-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holman RR,, Paul SK,, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 23.International Hypoglycaemia Study Group. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 2019;7::385–96. doi: 10.1016/S2213-8587(18)30315-2. [DOI] [PubMed] [Google Scholar]
- 24.Mellbin LG,, Malmberg K,, Waldenstrom A, et al. Prognostic implications of hypoglycaemic episodes during hospitalisation for myocardial infarction in patients with type 2 diabetes: a report from the DIGAMI 2 trial. Heart. 2009;95:721–7. doi: 10.1136/hrt.2008.152835. [DOI] [PubMed] [Google Scholar]
- 25.Bramlage P,, Gitt AK,, Binz C, et al. Oral antidiabetic treatment in type-2 diabetes in the elderly: balancing the need for glucose control and the risk of hypoglycemia. Cardiovasc Diabetol. 2012;11:122. doi: 10.1186/1475-2840-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston SS,, Conner C,, Aagren M, et al. Evidence linking hypoglycemic events to an increased risk of acute cardiovascular events in patients with type 2 diabetes. Diabetes Care. 2011;34:1164–70. doi: 10.2337/dc10-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asian Pacific Society of Cardiology Consensus Statements. Presented at: Advances in Medicine 2020, 26 September 2020
- 28.Marso SP,, Bain SC,, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44. doi: 10.1056/nejmoa1607141. [DOI] [PubMed] [Google Scholar]
- 29.Marso SP,, Daniels GH,, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holman RR,, Bethel MA,, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–39. doi: 10.1056/nejmoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Husain M,, Birkenfeld AL,, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–51. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 32.Gerstein HC,, Colhoun HM,, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–30. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 33.Bethel MA,, Patel RA,, Merrill P, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018;6:105–13. doi: 10.1016/s2213-8587(17)30412-6. [DOI] [PubMed] [Google Scholar]
- 34.Arnott C,, Li Q,, Kang A, et al. Sodium-glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9:e014908.. doi: 10.1161/JAHA.119.014908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cosentino F,, Cannon CP,, Cherney DZI, et al. Efficacy of ertugliflozin on heart failure-related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV trial. Circulation. 2020;;142::2205–15. doi: 10.1161/CIRCULATIONAHA.120.050255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neal B,, Perkovic V,, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57. doi: 10.1056/nejmoa1611925. [DOI] [PubMed] [Google Scholar]
- 37.American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes−2020. Diabetes Care. 2019;43((Suppl 1)):S135–51. doi: 10.2337/dc20-s011. [DOI] [PubMed] [Google Scholar]
- 38.de Boer IH,, Caramori ML,, Chan JCN, et al. Executive summary of the 2020 KDIGO Diabetes Management in CKD Guideline: evidence-based advances in monitoring and treatment. Kidney Int. 2020;98::839–48. doi: 10.1016/j.kint.2020.06.024. [DOI] [PubMed] [Google Scholar]
- 39.Wiviott SD,, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 40.Solomon SD,, Jhund P,, Kosiborod M. The Dapagliflozin in Heart Failure with Reduced Ejection Fraction Trial (DAPA-HF): outcomes in patients with CKD and effects on renal function. Presented at: American Society of Nephrology Kidney Week, 8 November 2019
- 41.Heerspink HJL,, Stef–nsson BV,, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383::1436–46. doi: 10.1056/nejmoa2024816. [DOI] [PubMed] [Google Scholar]
- 42.Perkovic V,, Jardine MJ,, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–306. doi: 10.1056/nejmoa1811744. [DOI] [PubMed] [Google Scholar]
- 43.Kadowaki T,, Nangaku M,, Hantel S, et al. Empagliflozin and kidney outcomes in Asian patients with type 2 diabetes and established cardiovascular disease: Results from the EMPA-REG OUTCOME® trial. J Diabetes Invest. 2019;10:760–70. doi: 10.1111/jdi.12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zelniker TA,, Wiviott SD,, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022–31. doi: 10.1161/circulationaha.118.038868. [DOI] [PubMed] [Google Scholar]
- 45.Kristensen SL,, R–rth R,, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776–85. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura Y,, Hasegawa H,, Tsuji M, et al. Diabetes therapies in hemodialysis patients: Dipeptidase-4 inhibitors. World J Diabetes. 2015;6:840–9. doi: 10.4239/wjd.v6.i6.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abe M,, Okada K. DPP-4 inhibitors in diabetic patients with chronic kidney disease and end-stage kidney disease on dialysis in clinical practice. Contrib Nephrol. 2015;185:98–115. doi: 10.1159/000380974. [DOI] [PubMed] [Google Scholar]
- 48.Bae JH,, Kim S,, Park EG, et al. Effects of dipeptidyl peptidase-4 inhibitors on renal outcomes in patients with type 2 diabetes: a systematic review and meta-analysis. Endocrinol Metab (Seoul) 2019;34:80–92. doi: 10.3803/EnM.2019.34.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan JCN,, Scott R,, Arjona Ferreira JC, et al. Safety and efficacy of sitagliptin in patients with type 2 diabetes and chronic renal insufficiency. Diabetes Obes Metab. 2008;10:545–55. doi: 10.1111/j.1463-1326.2008.00914.x. [DOI] [PubMed] [Google Scholar]
- 50.Yoon SA,, Han BG,, Kim SG, et al. Efficacy, safety and albuminuria-reducing effect of gemigliptin in Korean type 2 diabetes patients with moderate to severe renal impairment: A 12-week, double-blind randomized study (the GUARD Study). Diabetes Obes Metab. 2017;19:590–8. doi: 10.1111/dom.12863. [DOI] [PubMed] [Google Scholar]
- 51.Mosenzon O,, Leibowitz G,, Bhatt DL, et al. Effect of saxagliptin on renal outcomes in the SAVOR-TIMI 53 trial. Diabetes Care. 2016;40:69–76. doi: 10.2337/dc16-0621. [DOI] [PubMed] [Google Scholar]
- 52.McMurray JJV,, Solomon SD,, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 53.Zinman B,, Wanner C,, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. doi: 10.1056/nejmoa1504720. [DOI] [PubMed] [Google Scholar]
- 54.Kato ET,, Silverman MG,, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528–36. doi: 10.1161/CIRCULATIONAHA.119.040130. [DOI] [PubMed] [Google Scholar]
- 55.Packer M,, Anker SD,, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;;383::1413–24. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 56.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317::703–13. doi: 10.1136/bmj.317.7160.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holman RR,, Paul SK,, Bethel MA, et al. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med. 2008;359:1565–76. doi: 10.1056/nejmoa0806359. [DOI] [PubMed] [Google Scholar]
- 58.ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362::1575–85. doi: 10.1056/NEJMoa1001286. Epub 2010/03/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buckley LF,, Dixon DL,, Wohlford GF, et al. Response to comment on Buckley et al. Intensive versus standard blood pressure control in SPRINT-Eligible participants of ACCORD-BP. Diabetes Care. 2018;41::e86–7. doi: 10.2337/dci17-0066. [DOI] [PubMed] [Google Scholar]
- 60.SPRINT Research Group, Wright JT Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373::2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsujimoto T,, Kajio H. Benefits of intensive blood pressure treatment in patients with type 2 diabetes mellitus receiving standard but not intensive glycemic control. Hypertension. 2018;72:323–30. doi: 10.1161/HYPERTENSIONAHA.118.11408. [DOI] [PubMed] [Google Scholar]
- 62.Whelton PK,, Carey RM,, Aronow WS. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association. Task Force on Clinical Practice Guidelines. Kidneys. 2018;7:68–74. doi: 10.22141/2307-1257.7.1.2018.122220. [DOI] [Google Scholar]
- 63.Passarella P,, Kiseleva TA,, Valeeva FV,, Gosmanov AR. Hypertension management in diabetes: 2018 update. Diabetes Spectr. 2018;31:218–24. doi: 10.2337/ds17-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Becker GJ,, Wheeler DC,, De Zeeuw D, et al. Kidney Disease: Improving Global Outcomes (KDIGO) blood pressure work group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2012;2((Suppl)):337–414. [Google Scholar]
- 65.American Diabetes Association. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes−2019. Diabetes Care. 2019;42((Suppl 1):):S103–23. doi: 10.2337/dc19-S010. [DOI] [PubMed] [Google Scholar]
- 66.Antithrombotic Trialists Collaboration, Baigent C, Blackwell L, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373::1849–60. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2013;34::3035–87. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 68.Rivas Rios JR,, Franchi F,, Rollini F,, Angiolillo DJ. Diabetes and antiplatelet therapy: from bench to bedside. Cardiovasc Diagn Ther. 2018;8:594–609. doi: 10.21037/cdt.2018.05.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aimo A,, De Caterina R. Aspirin for primary prevention of cardiovascular disease: advice for a decisional strategy based on risk stratification. Anatol J Cardiol. 2020;;23::70–78. doi: 10.14744/AnatolJCardiol.2019.89916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin MH,, Lee CH,, Lin C, et al. Low-dose aspirin for the primary prevention of cardiovascular disease in diabetic individuals: a meta-analysis of randomized control trials and trial sequential analysis. J Clin Med. 2019;8:609. doi: 10.3390/jcm8050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McNeil JJ,, Woods RL,, Nelson MR, et al. Effect of aspirin on disability-free survival in the healthy elderly. N Engl J Med. 2018;379:1499–508. doi: 10.1056/NEJMoa1800722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bowman L,, Mafham M,, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529–39. doi: 10.1056/NEJMoa1804988. [DOI] [PubMed] [Google Scholar]


