Abstract
Background
Whether infection with SARS-CoV-2 leads to excess risk of requiring hospitalization or intensive care in persons with diabetes has not been reported, nor have risk factors in diabetes associated with increased risk for these outcomes.
Methods
We included 44,639 and 411,976 adult patients with type 1 and type 2 diabetes alive on Jan 1, 2020, and compared them to controls matched for age, sex, and county of residence (n=204,919 and 1,948,900). Age- and sex-standardized rates for COVID-19 related hospitalizations, admissions to intensive care and death, were estimated and hazard ratios were calculated using Cox regression analyses.
Findings
There were 10,486 hospitalizations and 1,416 admissions into intensive care. A total of 1,175 patients with diabetes and 1,820 matched controls died from COVID-19, of these 53•2% had been hospitalized and 10•7% had been in intensive care. Patients with type 2 diabetes, compared to controls, displayed an age- and sex-adjusted hazard ratio (HR) of 2•22, 95%CI 2•13-2•32) of being hospitalized for COVID-19, which decreased to HR 1•40, 95%CI 1•34-1•47) after further adjustment for sociodemographic factors, pharmacological treatment and comorbidities, had higher risk for admission to ICU due to COVID-19 (age- and sex-adjusted HR 2•49, 95%CI 2•22-2•79, decreasing to 1•42, 95%CI 1•25-1•62 after adjustment, and increased risk for death due to COVID-19 (age- and sex-adjusted HR 2•19, 95%CI 2•03-2•36, complete adjustment 1•50, 95%CI 1•39-1•63). Age- and sex-adjusted HR for COVID-19 hospitalization for type 1 diabetes was 2•10, 95%CI 1•72-2•57), decreasing to 1•25, 95%CI 0•3097-1•62) after adjustment• Patients with diabetes type 1 were twice as likely to require intensive care for COVID-19, however, not after adjustment (HR 1•49, 95%CI 0•75-2•92), and more likely to die (HR 2•90, 95% CI 1•6554-5•47) from COVID-19, but not independently of other factors (HR 1•38, 95% CI 0•64-2•99). Among patients with diabetes, elevated glycated hemoglobin levels were associated with higher risk for most outcomes.
Interpretation
In this nationwide study, type 2 diabetes was independently associated with increased risk of hospitalization, admission to intensive care and death for COVID-19. There were few admissions into intensive care and deaths in type 1 diabetes, and although hazards were significantly raised for all three outcomes, there was no independent risk persisting after adjustment for confounding factors.
Research in context.
Evidence before this study
The majority of all persons, with and without diabetes, infected with SARS-CoV-2, survive and do not require hospitalization or intensive care. Findings from multiple studies have shown that people with both type 1 and type 2 diabetes have an increased risk of in-hospital death, of death or a composite of mortality and need of intensive care from COVID-19. Among persons with diabetes of either type COVID-19-related mortality is associated with older age, male sex, socioeconomic deprivation, non-white ethnicity, established cardiovascular disease, and impaired renal function, glycemic control and with both obesity and underweight. However, from a public health point of view COVID-19 presents a much more complex problem in that hospitals are frequently overwhelmed, both with respect to total beds but also to intensive care with an excessive toll on human and other resources. To which extent deaths, hospitalizations, and intensive care overlap will obviously depend on organization of health care and care of the elderly, as well as reporting on deaths, but with these limitations in mind, no studies have attempted to report findings for people with and without diabetes separately for these outcomes. We searched the PubMed and medRxiv using the search terms “COVID-19”, “SARS-CoV-2”, “diabetes” (in the publication title) and (hospitalization OR intensive care OR critical care OR mortality) for English-language publications. We were unable to identify any study that established risk of hospitalization, being in need of intensive care, or death, separately for each outcome, compared to population controls without diabetes.
Added value of this study
This is, to our knowledge, the first study to address to which extent persons with diabetes type 1 and 2 have an excess risk of being hospitalized, requiring intensive care, or die (in- or outside hospital) compared to persons without diabetes from the general population. We used data from a nationwide Swedish diabetes registry with 90% coverage, analyzing a total of 44,478 patients with type 1 diabetes and 385,021 persons with type 2 diabetes; comparing them with over 1.8 million controls without diabetes, matched for age, sex, and county. Altogether, 3,587 people with diabetes and 6,899 controls were hospitalized, 469 with diabetes and 947 controls were admitted to intensive care, with 1,175 with diabetes and 1,820 controls succumbing to COVID-19. Of all deaths, only 53% had been hospitalized and 11% had been in intensive care. Compared to controls, individuals with type 2 diabetes were twice as likely to be hospitalized, to require intensive care, and to die. After adjustment for comorbidities and pharmacological treatment, the excess risks were reduced but remained significant. Persons with type 1 diabetes had excess risk of being hospitalized, needing intensive care and a 3-fold risk to die compared to controls, but after adjustment for covariates there was no significant excess risk for either outcome; there were however only 21 cases requiring intensive care, and 21 deaths among those with diabetes type 1; the latter at an average age of 71 years. Among people with diabetes, poor metabolic control and obesity were associated with worse outcomes.
Implications of all the available evidence
Patients with diabetes type 2, compared to population controls, were 2 times more likely to require hospitalization, to be admitted to intensive care and to die. This was partly, but not entirely, due to comorbid conditions, differences in medications, and socioeconomic factors. Patients with type 1 diabetes had 3 times higher death rates than expected; but this was concentrated among those older than 55 years. Good metabolic control and maintaining a healthy weight are measures that may prevent persons with diabetes from falling seriously ill or die from COVID-19.
Alt-text: Unlabelled box
1. Introduction
From the start of the COVID-19 pandemic in the first months of 2020 it has been apparent that the infection may represent a real danger not only for the elderly but also for middle-aged adults [1], [2], [3], [4], where hospitalization rates and admissions to intensive care have soared in many countries. Although the course of the disease is relatively mild in most infected people, initial reports from China, Italy and USA indicated that some factors were strongly associated with serious outcomes in those infected with the virus, not only older age, but also cardiovascular disease, diabetes, hypertension and obesity [5], [6], [7], [8], [9]. A common denominator for several of these disorders are related to obesity, mainly diabetes, where increasing evidence shows that hyperglycaemia is associated with higher risk of complications and mortality from COVID-19 [5, 10, 11]. Even so, immunological and pathophysiological effects of hyperglycaemia on COVID-19 infections remain elusive, and studies are not unanimous that elevated blood glucose contributes to worse prognosis [12, 13]. Understanding the interplay between underlying cardiovascular conditions, clinical characteristics, sociodemographic factors, pharmacological treatment and cardiometabolic risk factors is essential for risk mitigation strategies and generation of population-specific models for predicting risk of severe COVID-19 cases.
Despite the lack of a formal lockdown, something which is currently not permitted by the Swedish constitution, and relying on voluntary recommendations only, the course of the pandemic in Sweden in the spring and summer of 2020 was similar to many other European countries that did apply strict lockdowns, with death rates comparable to or lower than for example France, the UK, Italy, Spain, and Belgium. Similar to other countries most deaths occurred among the very old, many of them living in long-term care facilities.
Most population-based studies of the impact of COVID-19 have reported data on in-hospital mortality [5, 6] or on a combination of mortality and intensive care admission [14] but from a population health perspective there are several other pressing concerns, not least that hospitals are frequently overwhelmed, both with respect to total beds and to need of intensive care, with an excessive toll on human and other resources. To which extent deaths, hospitalizations, and intensive care overlap will obviously depend on organization of health care and care of the elderly, as well as reporting on deaths, but so far, no studies have reported findings for people with and without diabetes separately for these outcomes.
The National Diabetes Registry (NDR) in Sweden keeps track of over 90% of all individuals with diabetes in Sweden. The purpose of this nationwide observational study was to compare standardized incidence rates and excess risk of hospitalization, admission to intensive care and mortality as a result of COVID-19 in patients with type 1 diabetes and type 2 diabetes, compared to region-, age- and sex matched controls from the general population, during the period January 1 to August 17, 2020. In addition, we set out to assess the partial effect of cardiovascular risk factor for risk of hospitalization, admission to intensive care or death from COVID-19, in patients with diabetes.
2. Methods
Data are available from the sources stated in the paper on request to the data providers, fulfilling legal and regulatory requirements and with permission from the Swedish Ethical Review Authority.
2.1. Data sources and study cohort
The NDR [15, 16] is a nationwide tool for quality control of diabetes care in Sweden, with physicians and nurses annually reporting data from hospital outpatient clinics and primary care centres. Detailed information on clinical characteristics, risk factors, and treatments for patients with diabetes were retrieved from The National Diabetes Register. Patients aged >18 years with at least one registration in the NDR between January 1, 1998 and January 1, 2020, and who were alive at start of follow-up, i.e., January 1, 2020, were included in the study. A flow chart regarding analyses and study cohort is presented in Supplementary Figure S1.
Type 1 diabetes was defined according to epidemiologic criteria: treatment with insulin and diagnosis at 30 years of age or younger. Type 2 diabetes was also defined according to epidemiologic criteria: treatment with diet, with or without the use of oral antihyperglycemic agents, or treatment with insulin, with or without the use of oral antihyperglycemic agents; the latter category applied only to patients who were 40 years of age or older at the time of diabetes diagnosis.
Information with respect to comorbidities, medications, admission to intensive care unit, hospitalizations, and deaths was retrieved from the Swedish Inpatient Register, the Swedish Intensive Care Registry (https://www.icuregswe.org/en/) with complete coverage of all patients with COVID-19 intensive care in Sweden, the Swedish Cause of Death Register and the Swedish Prescribed Drug Register. Socioeconomic factors were retrieved from the Longitudinal integrated database for health insurance and labour market studies (LISA) registry. Data linkage is virtually complete owing to the use of unique personal identification numbers, which are assigned to all Swedes at birth or immigration.
At inclusion in the registry, each patient with diabetes was matched for age, gender and county with five controls without diabetes, randomly selected from the Swedish Total Population Registry kept by Statistics Sweden. Separate controls were selected for the cohort with type 1 diabetes and the cohort with type 2 diabetes; no person served as a control in both analyses.
2.2. Outcomes
We assessed three primary outcomes: hospitalization, intensive care unit admission and mortality due to COVID-19. Hospitalizations for COVID-19 as a principal or contributory diagnosis and comorbidities were retrieved from hospital discharge records with the use of codes in the International Classification of Diseases, 9th Revision and 10th Revision. The Swedish Inpatient Registry includes all inpatient admissions since 1987. The majority of cases that were admitted to intensive care unit (95•8%) overlapped with hospitalization for COVID-19. The specific hospital codes and anatomical therapeutic chemical classification (ATC) codes are listed in Table S1 in the supplementary appendix. Patients were followed until an event occurred or until August 17, 2020, for all outcomes except intensive-care unit admission due to COVID-19, for which registrations were available until July 12, 2020.
2.3. Variables assessed
We included the following covariates for patients with diabetes; glycated hemoglobin (HbA1c), systolic- and diastolic blood pressure, low- and high-density lipoprotein cholesterol, triglycerides, total cholesterol, body mass index, use of medications, smoking status, duration of diabetes, estimated glomerular filtration rate and physical activity level (categories: never, less than once a week, twice a week, 3–5 times per week, or daily). For each patient with diabetes, we used imputed baseline values for risk factors, i.e., imputed values at start of follow-up on January 1, 2020. Estimated glomerular filtration rate was assessed according to the Modification of Diet in Renal Disease Equation. Macroalbuminuria was defined as a urinary albumin-to-creatinine ratio >30 mg/mmol or a urinary albumin clearance of >200 μg/min or >300 mg/L. For socioeconomic variables, we categorized education into ≤9 years (pre-secondary education), > 9 to 12 years (secondary education) and ≥ 12 years (post-secondary education). Income was stratified into annual quintiles. Marital status was dichotomized into married and not-married, while country of birth was labeled as Scandinavian and other ethnicities.
Information on diabetes specific risk factors were not assessed in main analyses, they were included in regression models for ancillary analyses, i.e. optimal levels of risk factors and relative importance of risk factors. For the analyses of optimal risk factor levels, the following risk factors and cut-offs were considered: glycated haemoglobin (≥6.9% [≥53 mmol/mol]), systolic and diastolic blood pressure (either ≥130 mmHg systolic or ≥80 diastolic), estimated glomerular filtration rate (< 90 mL/min/1,73 m²), low-density lipoprotein cholesterol (>2.5 mmol/L) and body mass index (< 25 kg/m2).
2.4. Statistical analysis
For each outcome, we analysed age- and sex standardized incidence rates, the rates were standardized to the age and sex distribution in the general population of Sweden, 2019. In the incidence analyses, each person with diabetes was matched with approximately five region-, age- and gender controls, randomly selected from the general population. Numerators were the number of first events in a particular time period (i.e. month), and denominators were the number of persons at risk during the same time period. Standardized incidence rates are presented as events per 1,000 patient-years of observation with 95% confidence intervals. In addition, crude incidence rates were calculated for all outcomes and presented as events per 1,000 patient-years of observation with 95% confidence intervals. Follow-up was stratified according to eight one-month periods for all outcomes. All study participants were followed from the index date (January 1, 2020) until death or end of follow-up. Patients and controls with non-fatal outcomes continued to contribute with person-years and events for remaining outcomes. If controls died from non-COVID related causes after start of follow-up, they were right censored.
We constructed Cox proportional hazard models for all outcomes to compare excess risk between patients with diabetes and matched controls. Cox regression models for primary analyses included age, sex, socioeconomic factors, comorbidities at baseline, pharmacological treatment and a “Category” variable denoting patient with diabetes or control. In order to optimize model performance and determine accurate relative risk, age was modelled as either a continuous- or categorical variable, depending on the outcome and patient cohort. Additionally, we performed stratified Cox regression models on patients with type 1 diabetes, type 2 diabetes and their matched controls, respectively. Ethnicity was initially included in the regression models, however, due to few events in the separate ethnic groups, this covariate resulted in inflated hazard ratios and was therefore excluded from the analyses. In addition to this, we constructed Cox models that were adjusted for age and sex, in order to estimate excess risk for primary outcomes among patients with diabetes, compared to matched controls, using no further adjustment for confounders.
Furthermore, we assessed the hazard risk functions for glycated haemoglobin, systolic blood pressure, low-density lipoprotein cholesterol, body mass index and estimated glomerular filtration rate, by modelling the association between each risk factor and the outcomes using restricted cubic splines with 4 knots for appropriate covariates. The evidence-based target level was set as reference for each risk factor. Relative importance provides an estimate of how important each risk factors is in terms of predicting the outcome. In Cox regression models for patients with diabetes, we used imputed baseline values for risk factors, i.e. measured values observed at start of the study. We used the estimated explained relative risk measure (R2 value) and proportion of explainable log-likelihood explained by each risk factor (Wald X2), to assess the partial effect of each risk factor. These methods have been described previously [17]. The R2 method is predisposed to interpret continuous variables as more important than categorical variables, whereas, the analysis of explainable log-likelihood method results in more accurate assessment of categorical variables, since all covariates are categorized in this model.
Missing data were imputed with the Multivariate Imputation by Chained Equations (MICE) algorithm. We imputed 2 complete data sets; variables used in the imputation are similar to variables used in the Cox models for patients with diabetes. Because of the exploratory nature of this study, two-sided P values of less than 0.05 were considered to indicate statistical significance. No adjustments were made for multiple comparisons, thus, the outcome of individual hypothesis tests should be interpreted with caution. All analyses were performed in RStudio (v 4.0.2).
2.5. Study design and support
The study was designed by the first and last author. All authors participated in data collection, analysis and interpretation, vouch for the accuracy and completeness of the data and analyses, and made the decision to submit the manuscript for publication. The Swedish Ethical Review Authority approved the study. Funding sources had no role in study design, data collection, data analysis, interpretation or writing of the report.
3. Results
3.1. Study population and outcomes
We identified 44,639 patients with type 1 diabetes and 204,919 matched controls, referred to as the type 1 case control cohort, and 411,976 persons with type 2 diabetes and 1,948,900 matched controls from the general population, referred to as the type 2 case control cohort. Baseline characteristics for patients with diabetes and their matched controls are presented in Table 1. Mean age among patients with type 1 diabetes was 42•6 years and 43•7% were women, whereas patients with type 2 diabetes were 66•1 years on average and 44•3% were women. Baseline characteristics by each outcome for patients and controls are presented in supplementary table S2–S4.
Table 1.
Baseline characteristics of patients with diabetes and matched controls, along with descriptive data for primary events.
| Type 1 diabetes controls | Type 1 diabetes | Type 2 diabetes controls | Type 2 diabetes | |
|---|---|---|---|---|
| Number of study participants (n) | 204919 | 44639 | 1948900 | 411976 |
| Sex = female (%) | 91150 (44.5) | 19514 (43.7) | 890839 (45.7) | 182652 (44.3) |
| Age (SD) | 40.49 (15.42) | 42.60 (16.56) | 63.37 (12.21) | 66.05 (13.24) |
| Age-categories (%) | ||||
| <45 | 124448 (60.7) | 24948 (55.9) | 142465 (7.3) | 26173 (6.4) |
| 45-54 | 38792 (18.9) | 8273 (18.5) | 297226 (15.3) | 54542 (13.2) |
| 55-64 | 25269 (12.3) | 5984 (13.4) | 520342 (26.7) | 91206 (22.1) |
| 65-74 | 13812 (6.7) | 4184 (9.4) | 647852 (33.2) | 125875 (30.6) |
| >75 | 2598 (1.3) | 1250 (2.8) | 341015 (17.5) | 114180 (27.7) |
| Sociodemographic factors | ||||
| Education (%) | ||||
| Pre-secondary education ≤ 9 years | 33508 (16.4) | 7825 (17.5) | 453334 (23.3) | 139237 (33.8) |
| Secondary education >9 to 12 years | 94605 (46.2) | 21073 (47.2) | 864551 (44.4) | 185338 (45.0) |
| Post-secondary education ≥ 12 years | 76806 (37.5) | 15741 (35.3) | 631015 (32.4) | 87401 (21.2) |
| Marital status = Not married (%) | 133885 (65.3) | 28736 (64.4) | 906198 (46.5) | 206005 (50.0) |
| Ethnicity = Not Scandinavia (%) | 18108 (8.8) | 2849 (6.4) | 235097 (12.1) | 80931 (19.6) |
| Income family interquartile range (IQR) (%) | ||||
| IQR 1 | 32486 (15.9) | 8192 (18.4) | 463023 (23.8) | 141503 (34.3) |
| IQR 2 | 48550 (23.7) | 11130 (24.9) | 477297 (24.5) | 115635 (28.1) |
| IQR 3 | 55477 (27.1) | 11739 (26.3) | 495158 (25.4) | 90225 (21.9) |
| IQR4 | 68406 (33.4) | 13578 (30.4) | 513422 (26.3) | 64613 (15.7) |
| Income interquartile range (IQR) (%) | ||||
| IQR 1 | 79426 (38.9) | 17674 (39.7) | 412802 (21.3) | 132480 (32.2) |
| IQR 2 | 47195 (23.1) | 10938 (24.6) | 478147 (24.6) | 116281 (28.3) |
| IQR 3 | 43688 (21.4) | 9092 (20.4) | 510021 (26.3) | 89811 (21.9) |
| IQR4 | 33730 (16.5) | 6808 (15.3) | 539640 (27.8) | 72427 (17.6) |
| Primary outcomes | ||||
| Covid hospitalization (%) | 281 (0.1) | 144 (0.3) | 6618 (0.3) | 3443 (0.8) |
| Admission to intensive care (%) | 41 (0.0) | 21 (0.0) | 906 (0.0) | 448 (0.1) |
| Covid death (%) | 19 (0.0) | 21 (0.0) | 1801 (0.1) | 1154 (0.3) |
| Comorbidities at baseline | ||||
| Coronary heart disease (%) | 4251 (2.1) | 3490 (7.8) | 178099 (9.1) | 85814 (20.8) |
| Acute myocardial infarction (%) | 2189 (1.1) | 1736 (3.9) | 85547 (4.4) | 42581 (10.3) |
| Stroke (%) | 2126 (1.0) | 1357 (3.0) | 85140 (4.4) | 35793 (8.7) |
| Heart failure (%) | 1941 (0.9) | 1474 (3.3) | 82138 (4.2) | 44215 (10.7) |
| Valvular disease (%) | 1193 (0.6) | 528 (1.2) | 43765 (2.2) | 15278 (3.7) |
| Atrial fibrillation (%) | 3667 (1.8) | 1212 (2.7) | 155352 (8.0) | 56522 (13.7) |
| Hypertension (%) | 14173 (6.9) | 15474 (34.7) | 498672 (25.6) | 228441 (55.5) |
| Peripheral arterial disease (%) | 571 (0.3) | 1518 (3.4) | 24512 (1.3) | 15904 (3.9) |
| Chronic obstructive pulmonary disease (%) | 1382 (0.7) | 489 (1.1) | 59848 (3.1) | 21210 (5.1) |
| Dementia (%) | 383 (0.2) | 243 (0.5) | 29509 (1.5) | 10834 (2.6) |
| Asthma (%) | 11178 (5.5) | 2965 (6.6) | 82731 (4.2) | 26936 (6.5) |
| Alcoholism (%) | 7950 (3.9) | 2651 (5.9) | 64353 (3.3) | 16785 (4.1) |
| Substance abuse (%) | 11317 (8.0) | 3726 (8.4) | 98096 (6.2) | 29018 (7.5) |
| Schizophrenia (%) | 2183 (1.5) | 399 (0.9) | 19910 (1.3) | 8963 (2.3) |
| Renal disease (%) | 1562 (0.8) | 5470 (12.3) | 45546 (2.3) | 35382 (8.6) |
| Cancer (%) | 10499 (5.1) | 2701 (6.1) | 339594 (17.4) | 81715 (19.8) |
| Pharmalogical treatment | ||||
| Antihypertensive medication (%) | 30414 (14.8) | 17262 (38.7) | 849737 (43.6) | 318206 (77.2) |
| Statins (%) | 13296 (6.5) | 18666 (41.8) | 438688 (22.5) | 270773 (65.7) |
| Anticoagulant medication (%) | 5280 (2.6) | 1620 (3.6) | 189632 (9.7) | 64292 (15.6) |
| Antithrombotic medication (%) | 7229 (3.5) | 6775 (15.2) | 280676 (14.4) | 126909 (30.8) |
| Variables from the National Diabetes Registry (NDR) | ||||
| Age of onset of disease (SD) | 17.65 (10.26) | 56.90 (13.25) | ||
| Duration of diabetes (SD) | 13.67 (11.87) | 3.15 (5.34) | ||
| Glycated hemoglobin (HbA1c) (SD) | 65.32 (17.31) | 55.35 (17.65) | ||
| Glycated hemoglobin categories (%) | ||||
| HbA1c < 48 mmol/mol | 5503 (13.1) | 144650 (39.1) | ||
| HbA1c >= 48 & HbA1c <= 53 mmol/mol | 4899 (11.6) | 81851 (22.1) | ||
| HbA1c >= 54 & HbA1c <= 58 mmol/mol | 5314 (12.6) | 40086 (10.8) | ||
| HbA1c >= 59 & HbA1c <= 74 mmol/mol | 16015 (38.1) | 58436 (15.8) | ||
| HbA1c >= 75 & HbA1c <= 85 mmol/mol | 5647 (13.4) | 17945 (4.8) | ||
| HbA1c >= 86 mmol/mol | 4706 (11.2) | 27452 (7.4) | ||
| Smoking = Yes (%) | 5647 (14.3) | 52696 (16.8) | ||
| Albuminuria (%) | ||||
| Macroalbuminuria (>30) | 1148 (4.0) | 9106 (4.2) | ||
| Microalbuminuria (3-30) | 2532 (8.9) | 27431 (12.6) | ||
| No albuminuria | 24662 (86.8) | 180848 (82.8) | ||
| Normalized value | 65 (0.2) | 1013 (0.5) | ||
| Retinopathy (%) | 4570 (28.5) | 18794 (17.6) | ||
| Systolic blood pressure (SD) | 123.27 (14.72) | 136.54 (16.84) | ||
| Diastolic blood pressure (SD) | 73.24 (8.91) | 79.94 (9.96) | ||
| Total-cholesterol (SD) | 4.61 (1.00) | 5.10 (1.18) | ||
| High-density lipoprotein cholesterol (SD) | 1.35 (0.31) | 1.24 (0.30) | ||
| Triglycerides (mean (SD)) | 1.15 (0.94) | 2.05 (1.62) | ||
| Physical activity (%) | ||||
| < 1/week | 2253 (14.0) | 31835 (13.5) | ||
| Daily | 3668 (22.8) | 72016 (30.6) | ||
| Never | 1152 (7.2) | 29645 (12.6) | ||
| Regularly 1-2/week | 3673 (22.8) | 48226 (20.5) | ||
| Regularly 3-5/week | 5350 (33.2) | 53616 (22.8) | ||
| Insulin treatment method = insulin pump (%) | 4583 (19.4) | 151 (0.4) | ||
| LDL-cholesterol (SD) | 2.61 (0.83) | 3.00 (1.01) | ||
| Estimated glomerular filtration rate (SD) | 101.5 (29.52) | 85.94 (24.76) | ||
| S-creatinine (SD) | 73.00 (29.22) | 74.99 (22.39) | ||
| Body mass index (SD) | 24.78 (6.28) | 30.84 (7.47) | ||
SD denotes standard deviation.
Pharmacological treatment and comorbidities are presented as number of events that occurred or individuals with ongoing medical treatment and (%).
During the study period there were 10,486 hospitalizations, 1,416 admissions into intensive care (where all but 60 cases overlapped with hospitalizations), and 2,995 deaths. Death rates for hospitalizations and admissions into intensive care were 12•2% and 22•6%, corresponding to 1,280 and 320 deaths respectively, while 1,395 of the deaths (46•6%) took place among individuals that were not hospitalized (the latter mainly occurred in long-term care facilities as reported by the Swedish National Board of Health and Welfare (https://www.socialstyrelsen.se/en/about-us/).
3.2. Hospitalization
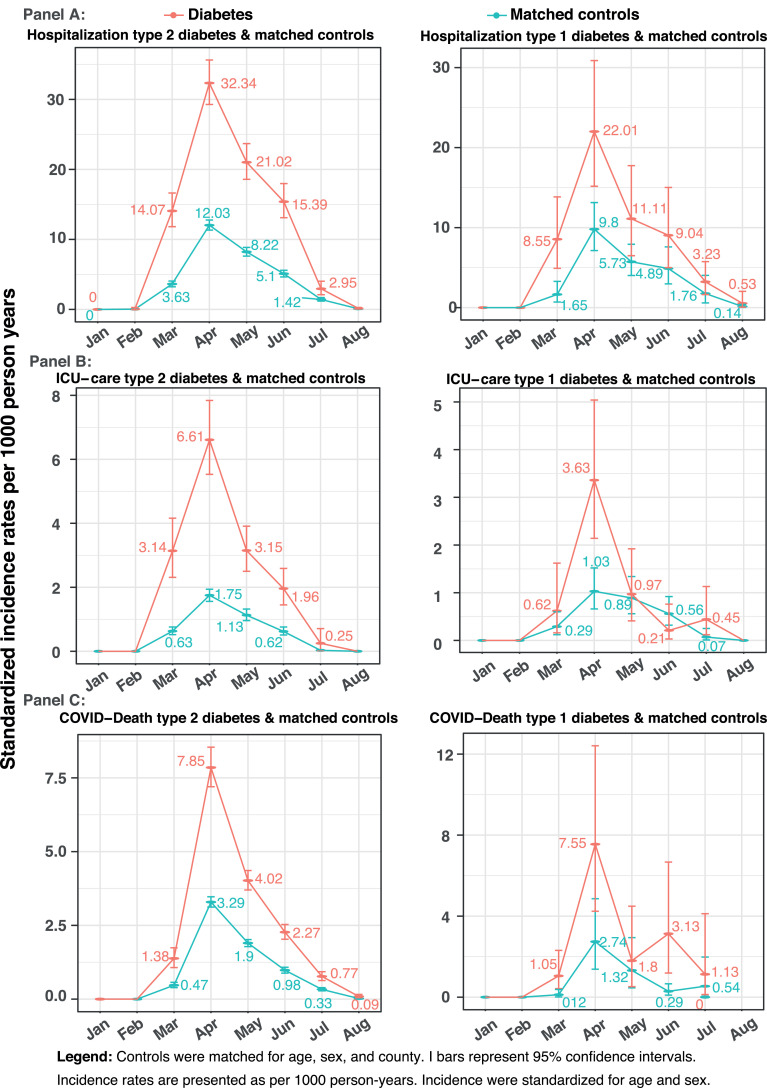
Incidence rates standardized for age and sex for hospitalizations were higher in individuals with diabetes, compared to controls (Fig. 1 Panel A), peaking in April at 22•01 and 32•34 cases per 1000 person-years in type 1 and type 2 diabetes, respectively, while the corresponding figures for controls were 9•8 and 12•03. Crude and standardised rates for hospital admissions, admissions into intensive care and deaths in type 1 and 2 diabetes are shown in supplementary Tables S5-S7. Age- and sex-adjusted hazard ratios (HRs) for being hospitalized in patients with type 2 diabetes, compared to controls, was 2•22, 95% CI 2•13-2•32, (Supplementary figure S2). The corresponding hazard for type 1 diabetes was 2•10, 95% CI 1•72-2•57.
Fig. 1.
Standardized incidence rates in patients with diabetes and matched controls for covid-19 related hospitalization, intensive-care and mortality
Legend: Controls were matched for age, sex, and county. I bars represent 95% confidence intervals. Incidence rates are presented as per 1000 person-years.
In the type 2 case control cohort patients with type 2 diabetes displayed 40% higher risk (HR 1•40, 95% CI 1•34-1•47) for hospitalization with COVID-19, compared to controls (Fig. 2), after adjustment for education, income, treatment and comorbidities, while type 1 diabetes was not independently associated with this outcome (adjusted HR 1•25, 95% CI 0•97 – 1•62) (Fig. 3). The risk associated with age in the type 2 case control cohort displayed a non-linear relationship where only younger age categories (i.e. 45-54 and 55-64) were more likely to be hospitalized. Hypertension (HR 2•95, 95% CI 2•77 – 3•13), dementia (HR 2•23, 95% CI 2•06 – 2•42) and renal disease (HR 2•82, 95% CI 2•67 – 2•97) displayed the highest risk for hospitalization in the type 2 case control cohort. Antihypertensive medication (HR 0.70, 95% CI 0.66 – 0.74) and statins (HR 0.87, 95% CI 0.83 – 0.92), were associated with lower risk, whereas treatment with antithrombotics or anticoagulants was associated with higher risk.
Fig. 2.
Hospitalization, mortality and intensive-care due to covid-19 in patients with type 2 diabetes and matched controls
Legend: Cox regression models for patients with type 2 diabetes and their matched controls.
Fig. 3.
Hospitalization, mortality and intensive-care due to covid-19 in patients with type 1 diabetes and matched controls
Legend: Cox regression models for patients with type 1 diabetes and their matched controls. For statistical reasons, age was modeled as categories for hospitalization for COVID-19, whereas age was modeled as a continuous variable in the regression models for admission to ICU and death, and all regression models does not include similar covariates.
In supplementary Table S8, adjusted Cox regression results of hospitalization for COVID-19 are presented separately for patients with type 1 and type 2 diabetes, and their matched controls. In type 1 diabetes, compared to individuals aged <45 years, those aged 45 to 54 years were twice as likely to require hospital care, while persons 65 and older were three times more likely to be hospitalized. Women, compared to men, with type 1 diabetes did not have a significantly lower risk for hospitalization, HR 0•90 (95% CI 0•64-1•28) while female sex did, HR 0•72 (95% CI 0•56-0•93). Heart failure (HR 2•04, 95% CI 1•20 – 3•46), hypertension (2•05, 95% CI 1•18 – 3•55), asthma (HR 1•96, 95% CI 1•19 – 3•23), renal disease (HR 1•99, 95% CI 1•34 – 2•95), and using antithrombotics (HR 1•57, 95% CI 1•00 – 2•46) or anticoagulants (HR 2•76, 95% CI 1•55 – 4•92) were all associated with increased likelihood of hospitalization in diabetes type 1. In patients with type 2 diabetes, all age groups above the age of 65 were less likely to be hospitalized than those younger than 45, while, among controls, most age categories older than 45 were more likely.
3.3. Admission to intensive care
In patients with diabetes, admissions to intensive care per 1,000 person-years increased during March and peaked during April for type 2 diabetes at 6•61 and 3•63 for type 1 diabetes (Fig. 1 Panel B), with corresponding rates for their respective controls 1•75 and 1•03 per 1,000 person-years. The age- and sex-adjusted hazard ratio for being admitted to intensive care in patients with type 2 diabetes and type 1 diabetes, compared to controls was 2•49, 95% CI 2•22-2•79 and 2•08, 95% CI 1•22-3•50, respectively (Supplementary figure S2).
In the type 2 case control cohort type 2 diabetes was associated with increased likelihood to be admitted to intensive care (HR 1•36, 95% CI 1•19 – 1•55), compared to controls, after full adjustment (Fig. 2 Panel B). Higher income and female sex were protective, whereas being married, atrial fibrillation, hypertension, chronic obstructive pulmonary disease, asthma and renal disease were associated with increased likelihood. Treatment with antihypertensives and antithrombotics and anticoagulants were associated with lower risk.
In the type 1 case control cohort, female sex, history of coronary heart disease, treatment with antihypertensives and statins were associated with lower risk for intensive care, whereas hypertension, renal disease and anticoagulants were associated with increased risk (Fig. 3 Panel B). In patients with type 1 diabetes, the risk to be admitted to intensive care was 5•00 (95% CI 1•10 – 22•70) in patients with heart failure, while renal disease was associated with the highest risk (HR 12•1, 95% CI 4•71 – 31•30) in controls (Supplementary Table S9). Hypertension, asthma, and renal disease were associated with increased risk among patients with type 2 diabetes and in their matched controls.
3.4. Mortality
COVID-19 mortality rates were higher among patients with diabetes throughout the entire follow-up period peaking during April with death rates per 1000 person years of 7•85 and 7•55 in type 2 and 1 diabetes, respectively, with corresponding rates among controls 3•29 and 2•74 (Fig. 1). In age- and sex-adjusted analyses both type 1 and type 2 patients, compared to controls, had an increased mortality risk (HR 2•90, 95% CI 1•54-5•47 and 2•19, 95% CI 2•03-2•36, respectively) (Supplementary figure S2). Of note, the number of deaths among individuals with type 1 diabetes was very limited, only 21 cases, with no death among patients aged <45 years.
In the type 2 case control cohort, diabetes was associated with 50% increased risk for death (HR 1•50, 95% CI 1•39 – 1•63) after full adjustment (Fig. 2 Panel C). Risk rose steeply with age. Increasing income and education were associated with lower risk (Fig. 2 Panel C). In patients with type 2 diabetes antihypertensive treatment and use of statins were associated with lower mortality risk. Virtually all comorbidities were associated with increased mortality risk, with dementia associated with the highest risk (HR 7•03, 95% CI 6•45 – 7•65).
After full adjustment, type 1 diabetes did not display an increased risk for death from COVID-19, compared to controls (HR 1•38, 95% CI 0•64-2•99) (Fig. 3 Panel C). In type 1 diabetes dementia and renal disease were associated with increased risk for succumbing to COVID-19 infection (Suppl Table S10). Most cardiovascular comorbidities, chronic obstructive pulmonary disease, dementia and end stage renal disease were associated with increased mortality risk in type 2 diabetes and their controls. Statins and antihypertensives were associated with reduced risk in diabetes type 2, with broadly similar reductions in type 2 controls. In type 2 diabetes, increasing age displayed an exponential risk association with mortality in the highest age-group (i•e• 75 and older) HR 24•9, 95% CI 7•94 -78•2, compared to <45 years, even more pronounced in matched controls (HR 42•1, 95% CI 17•4 – 102•0) (Suppl Table S10).
3.5. Risk factors for COVID-19 outcomes in type 1 and 2 diabetes
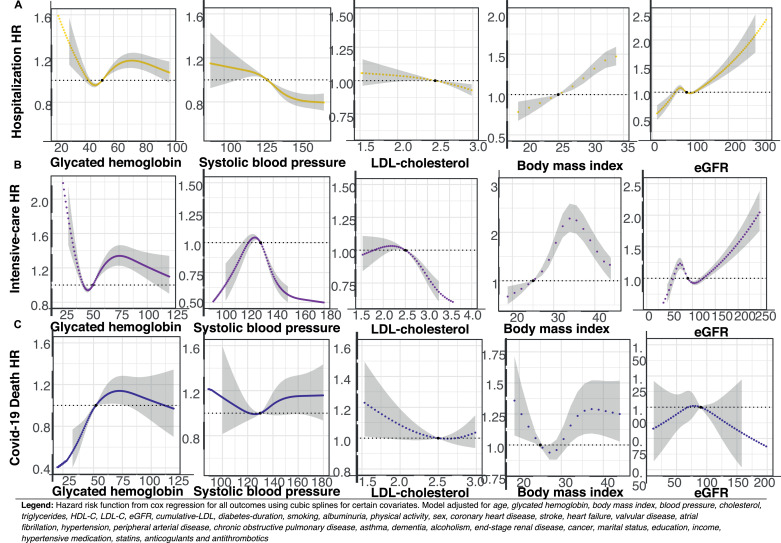
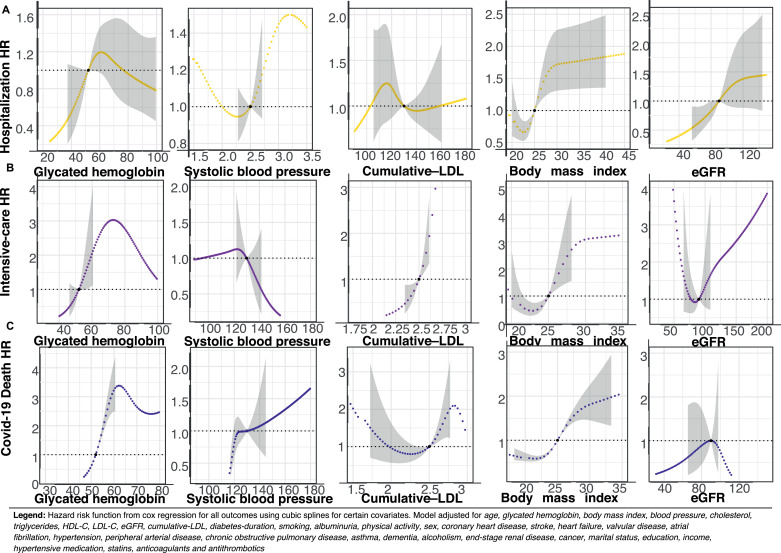
In Fig. 4–5, Panel A–C, hazard risk functions are presented for selected risk factors and hospitalization, admission to intensive care, as well as mortality. Elevated glycated hemoglobin levels were associated with increased risk for all outcomes, except for hospitalization among patients with type 1 diabetes.
Fig. 4.
Hazard function for specific risk factors and covid−19 related outcomes in patients with type 2 diabetes
Legend: Hazard risk function from cox regression for all outcomes using cubic splines for certain covariates. Model adjusted for age, glycated hemoglobin, body mass index, blood pressure, cholesterol, triglycerides, HDL-C, LDL-C, eGFR, cumulative-LDL, diabetes-duration, smoking, albuminuria, physical activity, sex, coronary heart disease, stroke, heart failure, valvular disease, atrial fibrillation, hypertension, peripheral arterial disease, chronic obstructive pulmonary disease, asthma, dementia, alcoholism, renal disease, cancer, marital status, education, income, hypertensive medication, statins, anti-coagulants and antithrombotics.
Fig. 5.
Hazard function for specific risk factors and covid−19 related outcomes in patients with type 1 diabetes
Legend: Hazard risk function from cox regression for all outcomes using cubic splines for certain covariates. Model adjusted for age, glycated hemoglobin, body mass index, blood pressure, cholesterol, triglycerides, HDL-C, LDL-C, eGFR, cumulative-LDL, diabetes-duration, smoking, albuminuria, physical activity, sex, coronary heart disease, stroke, heart failure, valvular disease, atrial fibrillation, hypertension, peripheral arterial disease, chronic obstructive pulmonary disease, asthma, dementia, alcoholism, renal disease, cancer, marital status, education, income, hypertensive medication, statins, anticoagulants and antithrombotics.
In type 2 diabetes, elevated systolic blood pressure and low-density lipoprotein cholesterol levels were associated with lower risk for hospitalization and being admitted to intensive care, but not mortality (Fig. 4). In type 1 diabetes, elevated low-density lipoprotein cholesterol was associated with increased risk for admission to intensive care, while elevated body mass index was associated with a pronounced increase in relative risk for all COVID-19 related outcomes in both cohorts with diabetes. Increasing levels of glycated hemoglobin in type 1 diabetes was associated with steeply increasing mortality risk (Fig. 5 Panel C). In type 2 diabetes, estimated glomerular filtration rate displayed a polynomial risk function where low values were associated with lower risk for hospitalization and ICU-care. For estimated glomerular filtration, marginally reduced levels below therapeutic guideline values, was associated with lower risk for hospitalization and admission to ICU in type 1 diabetes (Fig. 5 Panel A), however, renal function was not associated with mortality in any of the diabetes cohorts.
The relative importance plot for patients with diabetes is presented in Supplementary Figure S3 Panel A–C, only covariates with the greatest relative predictive contribution to the model are presented. For hospitalization and admission to intensive care, higher age, renal disease, blood pressure and body mass index were the strongest predictors in both cohorts with diabetes. For death from COVID-19 in type 1 diabetes, duration of diabetes explained almost 40% of the relative predictability, considering all covariates included in the model, with age adding another 20%. In type 2 diabetes, age, dementia, ongoing lipid medication and renal disease were the strongest predictors, derived from the R2 and chi-square models.
4. Discussion
During the first wave of the COVID-19 pandemic in Sweden during the spring of 2020, these nationwide registry data show that individuals with type 2 diabetes had more than twice the likelihood for COVID-19-related hospitalization, being admitted to intensive care and death, compared to matched controls from the general population. This excess risk was reduced but persisted after adjustment for comorbidities and other factors. In age- and sex-adjusted analyses patients with diabetes type 1 had significant excess risk for hospitalization, intensive care, and death due to COVID-19, but after full adjustment for confounders and mediators, individuals with type 1 diabetes did not display any independent excess risk for COVID-19 related outcomes. This is, to our knowledge, the first study to investigate the relative and absolute risk of hospitalization, admission to ICU and death from COVID-19 by type of diabetes in an entire nation, and comparing them to a substantial proportion of the adult population without diabetes.
Virtually all comorbidities were associated with significantly higher risk for COVID-19 related outcomes among patients with type 2 diabetes and matched controls. Being treated with antihypertensive medications and statins were associated with lower risk, whilst antithrombotics and anticoagulants were associated with higher risk of outcomes. Similar patterns were observed for type 1 diabetes and their matched controls. Those with higher education, high income, and women had lower risk for outcomes, in both patients with diabetes type 2 and the general population, however, women, compared to men, with diabetes type 1 had no significant reduction in risk.
Most studies regarding prognosis in patients infected with COVID-19 so far use case series or hospital-based populations; with short-term mortality as an outcome [18]. A large nationwide study from the UK based on over 60 million people observed that both type 1 and type 2 diabetes was associated with increased risk of in-hospital death with COVID-19 but did not investigate the risk of being hospitalized or requiring intensive care [6]. A recent study of the first wave of the pandemic in Scotland found that the overall risks of fatal or critical care unit-treated COVID-19 were substantially elevated in those with type 1 and type 2 diabetes compared with the background population. However, in the Scottish study 9 out of 10 cases were fatal, at a mean age of 80 years [14]. In our study, the age distribution of the three outcomes varied substantially, with fatal cases in type 2 diabetes on average 79 years, compared to 60 years for type 2 diabetes admitted to intensive care. Corresponding figures for type 1 diabetes were 71 years for fatal cases and 54 years for intensive care. There was very little overlap between intensive care and mortality, with only 10.7% of deaths occurring in patients admitted to intensive care.
In the present study type 1 diabetes was not independently associated with excess risk for mortality indicating that the absolute risk in diabetes type 1 was mediated through the long-term complications associated with this disorder. The contrasting findings to the type 1 diabetes patients in the UK could reflect differences in dysglycemia, obesity and smoking [19], among patients with type 1 diabetes in the UK population, compared to the Swedish type 1 diabetes population. Also, there were only 21 deaths, with ensuing lack of statistical power. Of note, although the age- and sex-adjusted hazard ratios for outcomes in diabetes type 1 was at least 2-fold that of controls, absolute risks were quite low. Persons with diabetes type 1 were not specifically asked to shield, but there were still no deaths in anyone with diabetes type 1 younger than 45 years.
Using hospitalization or intensive care as outcomes is not uncomplicated because of differences between nations in organization of hospital care, and availability of beds. In the present study, higher age among patients with type 1 diabetes was associated with increased risk for hospitalization, admission to intensive care and death. The increased likelihood of hospitalization with comorbidities might be due not only to increasing risks of complications but also to that these patients might be perceived to be at higher risk and admitted on that account. In type 2 diabetes, with patients on average two decades older, increasing age reduced the likelihood for admission and intensive care, but not death from COVID-19, where older age was associated with increased risk. Fewer admissions to intensive care, given the higher mortality, in all likelihood represent selection processes where elderly patients with multiple disorders, including diabetes, will have been considered too frail to benefit from intensive care or by being hospitalized. Of note, almost half of the deaths registered in the study occurred in persons who were not hospitalized, the absolute majority of those deaths occurred in long-term care facilities, with only 4% of deaths outside either hospital or residential care.
Initially, reports were conflicting regarding the impact of diabetes or dysglycemia, where some, but not all studies found a clear association [13, 20, 21]. However, reports from China, Italy and England, are unanimous that older patients with chronic diseases, including diabetes, are at higher risk for severe complications and death from COVID-19 [6, 9, 22, 23]. Our regression models were extensively adjusted for confounding factors, nevertheless, whether diabetes itself increases susceptibility and impacts outcomes from COVID-19 infection, or whether it is the cardio-renal comorbidities frequently associated with diabetes remains controversial. Additionally, we found that ongoing treatment with antihypertensive medication and statins were associated with lower risk for most outcomes, whereas medication with anticoagulants and antithrombotics was associated with increased risk. The observed association of increasing low-density lipoprotein cholesterol levels and lower risk for COVID-19 outcomes may suggest that treatment with statins interferes positively with prognosis of severe SARS-CoV2 infection, since individuals with hyperlipidemia are more likely treated with statins. Theoretically, medications more prevalent in the diabetes population, such as ACE-inhibitors, may interfere with susceptibility and prognosis of SARS-CoV-2 [24, 25].
Increasing levels of glycated hemoglobin in patients with type 2 diabetes was associated with higher risk for outcomes, in contrast to patients with type 1 diabetes, where hyperglycemia was associated with increased risk for admission to intensive care and death. Elevated body mass index was associated with higher risk for all outcomes in both cohorts with diabetes. Relative variable importance assessment shows that age, renal disease, hypertension, chronic obstructive pulmonary disease, asthma, high body mass index, dementia and low-density lipoprotein cholesterol were the most important predictors for COVID-19 outcomes. The lower risk for hospitalization and admission to intensive care in patients with type 2 diabetes and increasing levels of low-density lipoprotein cholesterol is likely due to reverse causation.
Reports suggest that patients with diabetes and SARS-CoV-2 may develop pneumonia more frequently, which in turn is detrimental to the prognosis of COVID-19. Immune studies show that poorly controlled diabetes could inhibit lymphocyte response, and impairs the monocyte-, macrophage- and neutrophil function [26, 27]. Also, studies show that COVID-19 is associated with coagulopathic and prothrombotic conditions in particularly pulmonary microvasculature but also in extrapulmonary organs. A Cochrane review combined data from seven reports that included 5929 participants to investigate the effect of anticoagulants, with conflicting results [28, 29].
Some limitations of our study should be considered. Polymerase chain reaction (PCR) testing for the virus during the early phase of the pandemic was not always performed, particularly not in deaths occurring out-of-hospital, and diagnosis therefore rested on clinical assessment. However, any misclassification due to the lack of testing likely affected persons with and without diabetes to a similar degree. Classification of diabetes was not based on islet autoantibodies or measurement of C-peptide levels. We used the epidemiological definitions and these has been validated as accurate in 97% of the cases. Correction for multiple testing was not performed, and thus caution is needed with respect to the interpretation of significance tests. Hospitalizations and need of intensive care, although a significant part of the public health burden imposed by the pandemic, are not standard outcomes and are subject to availability of beds and resources, and to the perception of risk in a patient, and may be rationed during an epidemic. Additionally, prognosis in COVID-19 is contingent on being infected, which in turn is influenced by many factors, such as voluntary sheltering, housing, occupation, etc. where there is, so far, incomplete information, not least because the capacity for testing for the virus in the general population was limited during the first phase.
In conclusion, we report that type 2 diabetes was associated with at least twice the risk for separate outcomes of hospitalization, admission to intensive care and death due to COVID-19, compared to the Swedish general population, and that after adjustment for comorbidities and mediators there was still a significant, albeit reduced excess risk. Patients with type 1 diabetes did not display an excess risk for outcomes after adjustment; reassuringly for this group, there were very few deaths and admissions into intensive care, during the first and second wave of the COVID-19 pandemic.
5. Contributors
The study was designed by the first and last author. The first author wrote the first draft of the manuscript and performed all statistical analyses. The first and last author vouches for the data and analysis. All of the authors participated in data collection, analysis and interpretation. All authors vouch for the accuracy and completeness of the data and analyses, and made the decision to submit the manuscript for publication. All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Declaration of Competing Interests
Professor Eliasson reports personal fees (expert panels, lectures) from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Mundipharma, Navamedic, NovoNordisk, RLS Global, grants and personal fees from Sanofi, all outside the submitted work. NN declare that they have no conflict of interest.
MG reports personal fees (scientific advisory boards, lectures) from Gilead Sciences, GSK/ViiV, MSD, Biogen, Amgen, Novocure, Novo Nordic and research grants from Gilead Sciences, all outside the submitted work.
Dr. Lind reports personal fees from Astra Zeneca, personal fees from Boeheringer Ingelheim, grants and personal fees from DexCom, personal fees from Eli Lilly, grants and personal fees from Novo Nordisk, personal fees from MSD, outside the submitted work.
Dr. Sattar reports personal fees from Amgen, personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, personal fees from Eli Lilly, personal fees from Merck Sharp & Dohme, personal fees from Novartis, personal fees from Novo Nordisk, personal fees from Pfizer, personal fees from Sanofi, outside the submitted work.
Data availability
Data are available from the sources stated in the paper on request to the data providers, fulfilling legal and regulatory requirements and with permission from the Swedish Ethical Review Authority.
Funding
This work was supported by grants from: the Swedish state under an agreement concerning research and education of doctors [ALFGBG-717211]; the Swedish Heart and Lung Foundation [2018-0366]; the Swedish Research Council [2013-05187, VRREG 2019-00193, 2020-05792]
Footnotes
Funding: This work was supported by grants from: the Swedish Heart and Lung Foundation; the Swedish Research Council; the Swedish state under an agreement concerning research and education of medical practitioners.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanepe.2021.100105.
Appendix. Supplementary materials
References
- 1.Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. 2020;80(6):e14–ee8. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang SJ, Jung SI. Age-related morbidity and mortality among patients with COVID-19. Infect Chemother. 2020;52(2):154–164. doi: 10.3947/ic.2020.52.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omori R, Matsuyama R, Nakata Y. The age distribution of mortality from novel coronavirus disease (COVID-19) suggests no large difference of susceptibility by age. Sci Rep. 2020;10(1):16642. doi: 10.1038/s41598-020-73777-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levin AT, Hanage WP, Owusu-Boaitey N, Cochran KB, Walsh SP, Meyerowitz-Katz G. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur J Epidemiol. 2020 doi: 10.1007/s10654-020-00698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holman N, Knighton P, Kar P, O'Keefe J, Curley M, Weaver A. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barron E, Bakhai C, Kar P, Weaver A, Bradley D, Ismail H. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riddle MC, Buse JB, Franks PW, Knowler WC, Ratner RE, Selvin E. COVID-19 in people with diabetes: urgently needed lessons from early reports. Diabetes Care. 2020;43(7):1378–1381. doi: 10.2337/dci20-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736–EE41. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bode B, Garrett V, Messler J, McFarland R, Crowe J, Booth R. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14(4):813–821. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(6) doi: 10.1016/j.cmet.2020.04.021. 1068-77 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cariou B, Hadjadj S, Wargny M, Pichelin M, Al-Salameh A, Allix I. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Yang D, Cheng B, Chen J, Peng A, Yang C. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43(7):1399–1407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 14.McGurnaghan SJ, Weir A, Bishop J, Kennedy S, Blackbourn LAK, McAllister DA. The Lancet Diabetes & Endocrinology. 2020. Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rawshani A, Rawshani A, Franzen S, Sattar N, Eliasson B, Svensson AM. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379(7):633–644. doi: 10.1056/NEJMoa1800256. [DOI] [PubMed] [Google Scholar]
- 16.Rawshani A, Rawshani A, Gudbjornsdottir S. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;377(3):300–301. doi: 10.1056/NEJMc1706292. [DOI] [PubMed] [Google Scholar]
- 17.Heller G. A measure of explained risk in the proportional hazards model. Biostatistics. 2012;13(2):315–325. doi: 10.1093/biostatistics/kxr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8(9):782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charalampopoulos D, Hermann JM, Svensson J, Skrivarhaug T, Maahs DM, Akesson K. Exploring variation in glycemic control across and within eight high-income countries: a cross-sectional analysis of 64,666 children and adolescents with type 1 diabetes. Diabetes Care. 2018;41(6):1180–1187. doi: 10.2337/dc17-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract. 2020;162 doi: 10.1016/j.diabres.2020.108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med. 2020;58(7):1131–1134. doi: 10.1515/cclm-2020-0198. [DOI] [PubMed] [Google Scholar]
- 22.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 23.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 24.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382(25):2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.<EHJ-CVP-D-20-00546_R1.pdf>.
- 26.Moutschen MP, Scheen AJ, Lefebvre PJ. Impaired immune responses in diabetes mellitus: analysis of the factors and mechanisms involved. Relevance to the increased susceptibility of diabetic patients to specific infections. Diabete Metab. 1992;18(3):187–201. [PubMed] [Google Scholar]
- 27.Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999;26(3-4):259–265. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 28.Flumignan RLG, JDdS Tinôco, Pascoal PIF, Areias LL, Cossi MS, Fernandes MICD. Prophylactic anticoagulants for people hospitalised with COVID-19. Cochrane Database of Systematic Reviews. 2020 doi: 10.1002/14651858.CD013739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flam B, Wintzell V, Ludvigsson JF, Martensson J, Pasternak B. Direct oral anticoagulant use and risk of severe COVID-19. J Intern Med. 2020 doi: 10.1111/joim.13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the sources stated in the paper on request to the data providers, fulfilling legal and regulatory requirements and with permission from the Swedish Ethical Review Authority.