Abstract
Rationale: The natural history of recovery from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) remains unknown. Because fibrosis with persistent physiological deficit is a previously described feature of patients recovering from similar coronaviruses, treatment represents an early opportunity to modify the disease course, potentially preventing irreversible impairment.
Objectives: Determine the incidence of and describe the progression of persistent inflammatory interstitial lung disease (ILD) following SARS-CoV-2 when treated with prednisolone.
Methods: A structured assessment protocol screened for sequelae of SARS-CoV-2 pneumonitis. Eight hundred thirty-seven patients were assessed by telephone 4 weeks after discharge. Those with ongoing symptoms had outpatient assessment at 6 weeks. Thirty patients diagnosed with persistent interstitial lung changes at a multidisciplinary team meeting were reviewed in the interstitial lung disease service and offered treatment. These patients had persistent, nonimproving symptoms.
Results: At 4 weeks after discharge, 39% of patients reported ongoing symptoms (325/837) and were assessed. Interstitial lung disease, predominantly organizing pneumonia, with significant functional deficit was observed in 35/837 survivors (4.8%). Thirty of these patients received steroid treatment, resulting in a mean relative increase in transfer factor following treatment of 31.6% (standard deviation [SD] ± 27.6, P < 0.001), and forced vital capacity of 9.6% (SD ± 13.0, P = 0.014), with significant symptomatic and radiological improvement.
Conclusions: Following SARS-CoV-2 pneumonitis, a cohort of patients are left with both radiological inflammatory lung disease and persistent physiological and functional deficit. Early treatment with corticosteroids was well tolerated and associated with rapid and significant improvement. These preliminary data should inform further study into the natural history and potential treatment for patients with persistent inflammatory ILD following SARS-CoV-2 infection.
Keywords: COVID-19, interstitial lung disease, organizing pneumonia, fibrosis
In late 2019, a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in Wuhan, China, and has since spread globally, infecting more than 31 million people. The clinical course of infection appears to be extremely variable, from asymptomatic to severe pneumonia with multiorgan failure requiring critical care. At the time of writing, at least 1,122,036 people are known to have died following infection, but data on morbidity in survivors are scarce. Lung injury is a predominant feature of acute SARS-CoV-2 infection, and understanding the longer-term implications is critical given the number of affected patients.
The most common radiological pattern of acute infection with SARS-CoV-2 is of bilateral ground-glass opacification with or without consolidation in a subpleural distribution, and a radiological and histological pattern of organizing pneumonia pattern is described in many cases (1, 2). Radiological findings alter as the disease progresses, but persistent computed tomographic (CT) imaging abnormalities beyond Day 14 of symptoms and up to Day 37 have been reported (3, 4).
However, no data exist as to the natural history of inflammatory infiltrates during recovery from SARS-CoV-2 or the utility of any treatment in patients with persistent inflammatory interstitial lung disease (ILD) following infection with coronavirus. However, corticosteroids are the mainstay of treatment for organizing pneumonia of other causes (5), and when used acutely in the management of acute respiratory distress syndrome (ARDS) caused by SARS-CoV-2 have been associated with a reduction in mortality (6, 7).
Although interest in potential pulmonary, and specifically fibrotic, complications after SARS-CoV-2 infection grows, the long-term respiratory morbidity remains unclear (8). Given the number of infected patients, persistent functional deficits in even a relatively small proportion is likely to represent a significant disease burden and prompt therapy may avoid potentially permanent fibrosis and functional impairment. In this study, using a structured assessment protocol, we aimed to establish the incidence of persistent inflammatory ILD in patients after infection with SARS-CoV-2, and to report the progression of disease when treated with steroids.
Methods
Study Design
This was a single-center prospective observational study of patients with a diagnosis of SARS-CoV-2 pneumonitis 6 weeks after discharge from hospital. After screening, anyone with ongoing symptoms underwent structured assessment and patients identified as having ILD at a multidisciplinary team meeting (MDT) were further reviewed in the interstitial lung disease service. When clinically appropriate, they were offered treatment with corticosteroids following detailed discussion of the risks and benefits. For safety, patients were followed up weekly by telephone by an ILD specialist nurse and had access to a telephone helpline. At 3 weeks, patients had repeat lung function and repeat high-resolution CT imaging, which were reviewed by a physician. This project was internally submitted for service improvement and accepted by the quality improvement and patient safety committee (REF 11007) before commencement, and external ethical approval was not required.
Identification of Patient Cohort
All patients presenting to Guy’s and St. Thomas’ NHS Foundation Trust (a central London teaching hospital and tertiary ILD unit) with either a polymerase chain reaction (PCR) diagnosis of SARS-CoV-2 infection or a negative swab but a highly suspected clinical-radiological diagnosis were contacted by telephone 4 weeks after their discharge. They were asked if they had returned to their baseline. Patients with ongoing symptoms were invited to a structured clinical assessment and reviewed by a senior respiratory or infectious diseases physician. Patients who declined review were excluded, and any patients who reported that they had fully recovered were offered a chest radiograph at 12 weeks in line with UK national guidance (9).
The final cohort included in the study were adults aged >18 who received an in-hospital diagnosis of SARS-CoV-2 pneumonitis and who had persistent symptoms and an MDT diagnosis of resultant ILD at 6 weeks after discharge and consented to treatment with oral corticosteroids.
Structured Clinical Assessment
All patients attending the clinic had a chest radiograph on arrival. They underwent measurement of vital signs and body mass index (BMI). Current Medical Research Council (MRC) Dyspnea score was recorded, and they were asked to retrospectively report their function before their illness. Lung function, 6-minute walk tests (6MWT), echocardiography, and electrocardiogram were available to assessing clinicians as clinically indicated on a per-patient basis. Blood tests were taken for renal profile, liver function, C-reactive protein, fibrinogen, D-dimer, and ferritin. Following medical review, patients with ongoing physiological impairment as defined by desaturation of ≥4% or abnormal lung function and/or abnormal chest radiograph had chest CT imaging on the day. Relevant radiological results were discussed in a specially convened weekly post–coronavirus disease (COVID-19) MDT comprising 3 specialist chest radiologists and 15 respiratory and infectious diseases specialists. This allowed for institutional learning and refinement of our understanding of the condition and its imaging.
Treatment
Patients who were identified as having post–SARS-CoV-2 ILD at the post–COVID-19 MDT were invited to attend the interstitial lung disease service for consideration of treatment with corticosteroids. These patients were recovered from their initial infection and were assumed to have cleared the virus based on their biochemical markers and known timeframe for clearance (10). Every patient had a detailed discussion regarding their proposed treatment and understood the rationale and potential side effects.
Patients were only offered treatment in the absence of reported weekly improvement in symptoms and in the presence of a combination of MDT-confirmed ILD with physiological and functional impairment as demonstrated by 6MWT, and lung function with gas transfer.
Statistical Analysis
Values are expressed as mean ± standard deviation or median ± interquartile range. Change in forced vital capacity (FVC) and transfer factor of the lung for carbon monoxide (TlCO), MRC, 6MWT distance, and oxygen saturation as measured by pulse oximetry and biomarkers were analyzed using a paired samples t test. Adverse events and radiology are reported descriptively. Analysis was performed using SPSS version 26 (IBM) and GraphPad Prism version 8 (GraphPad Software).
Results
Between February 28 and May 29, 2020, 1,272 patients were diagnosed with SARS-CoV-2 pneumonitis in our hospital, following either an accident and emergency attendance or hospital admission (Figure 1). Of these, 1,239 had a positive PCR result, and the remainder had a clinical-radiological diagnosis made during their hospital attendance given the sensitivity of CT imaging in diagnosing SARS-CoV-2 pneumonitis. Mortality was 19.1% (245 patients). At the time of writing, 74 patients (6%) remained inpatients. Of the remaining patients, 88% were included in the study (837/953). One hundred sixteen patients (9%) did not respond to two phone calls and a letter. Electronic health record data confirm that 4 of the 116 patients whom we were not able to contact at 28 days had died.
Figure 1.

Flowchart of the study population recruited between February and May 2020. COVID = coronavirus disease; CT = computed tomography; ILD = interstitial lung disease; MDT = multidisciplinary team meeting; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
All remaining 837 patients were initially screened by telephone. Of these, 316 (38%) reported having fully recovered. Ongoing symptoms were reported by 325 patients (39%) and these were offered structured assessment. Following acceptance of initial invitation, a further 57 patients screened by telephone (9%) declined any follow-up and 139 (16%) were unable to attend the hospital but were included in the analysis.
Outcomes following Structured Outpatient Clinical Assessment
Of the 325 patients who had ongoing symptoms at telephone screening and attended structured assessment, 138 (42.9%) had no evidence of physiological impairment or persistent change on chest radiograph and were discharged with reassurance or offered formal rehabilitation. Functional or physiological impairment was observed in 110 (33.8%) in the absence of any radiological evidence of persistent lung disease or pulmonary embolism. These patients were appropriately referred to cardiology, nephrology, diabetes, psychology, or rehabilitation services via preagreed pathways. Following CT imaging, 77 patients (24%) were referred to the post–COVID-19 lung disease MDT by their clinician.
Radiological Findings
At the MDT, of the 77 patients referred, 59 (76.6%) of those imaged at 6 weeks were found to have persistent parenchymal abnormality presumed to be related to their previous infection. Diagnosis was made based on the MDT consensus of the radiological pattern, and lung biopsy was not performed. A spectrum of findings was observed including patients who had near complete resolution of chest radiographic changes but with abnormal CT. Others had persistent changes on both chest radiograph and CT imaging. In the majority, the pattern observed on initial follow-up CT imaging represented an organizing pneumonia–like pattern. Findings of bilateral subpleural ground-glass infiltrates with a mid to lower zone distribution were described as organizing pneumonia.
In a proportion of cases, this was also associated with subpleural and peribronchial linear dense consolidation. Some patients presented at first follow-up imaging with varying degrees of traction bronchiectasis that may reflect parenchymal fibrosis, consistent with a fibrotic organizing pneumonia pattern.
Of the 18 patients who did not have COVID-19–related imaging changes, 3 had normal lung parenchyma. The remainder had new findings of pulmonary embolus (one patient), lung infarct (one patient), Klebsiella pneumonia (one patient), Pneumocystis jirovecii pneumonia (one patient), and lung nodule (one patient). Some preexisting disease was identified, which included connective tissue disease–associated ILD (one patient), bronchiectasis (two patients), sickle lung disease (one patient), and airways disease (one patient).
ILD MDT Outcomes
Of the 59 patients with persistent post–COVID-19 interstitial change, the majority (59%) had organizing pneumonia. In three patients (5.0%) who had previously received steroid treatment, the pattern was felt to be fixed, with only minor ground glass (<15% of lung involvement). A further 21 patients (38.9%) had limited disease with pure ground glass with <15% lung involvement and a scattered distribution or had no functional impairment. In the remaining 35 patients (66%), given the presence of organizing pneumonia with restrictive physiology and in the absence of improving symptoms, treatment with corticosteroids was recommended. The remaining patients were followed up at 3 months with CT imaging and lung function. In 5 patients (22%) for whom therapy was recommended, it was decided by mutual agreement with the patient and clinician that steroid treatment was not appropriate, owing to either comorbid disease or limited/improving symptoms, meaning that 30 patients completed treatment and follow-up.
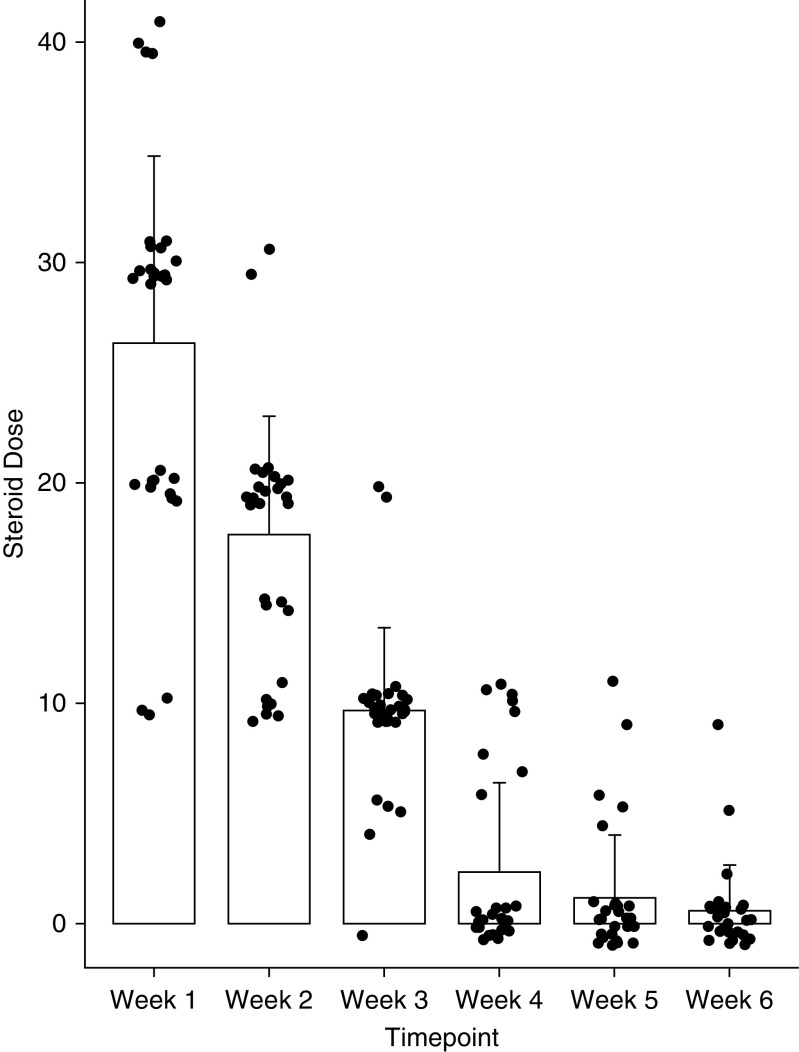
Patients were treated at Day 61 (±19) after discharge. A maximum initial dose of 0.5 mg/kg prednisolone was proposed based on the local standard protocol for managing organizing pneumonia. The average starting dose was 26.6 mg and a rapid wean over 3 weeks was felt to be appropriate given the presumed lack of an ongoing driver of inflammation. In some cases, the dose was reduced by the treating clinician in view of comorbid disease (Figure 2).
Figure 2.
Steroid dosing by week. Data are presented as median and interquartile range.
Characteristics of Patients with Persistent Inflammatory ILD
Patients with post–COVID-19 ILD were predominantly male (71.5%) and overweight with a mean BMI of 28.3 ± 4.0, although only 26% were obese (Table 1). Most had at least one comorbidity, with the commonest being diabetes and asthma (22.9%). The mean admission was 16.9 ± 12.5 days. Oxygen therapy was required by 82.9%, with half (55%) requiring intensive care unit (ICU) admission and 46% requiring invasive mechanical ventilation (Table 2).
Table 1.
Baseline characteristics of patients with interstitial lung disease following infection with SARS-CoV-2 (n = 35)
| Characteristic | Value |
|---|---|
| Age, yr | 60.5 ± 10.7 |
| Sex | |
| Male | 25 (71.4) |
| Female | 10 (28.6) |
| BMI | 28.3 ± 4.0 |
| Smoking history | |
| Ever-smoker | 21 (34.2) |
| Never-smoker | 14 (65.7) |
| Comorbidities | |
| Obesity | 9 (25.7) |
| Hypertension | 11 (31.4) |
| Diabetes | 8 (22.9) |
| CKD | 2 (5.8) |
| HIV | 1 (2.9) |
| Sickle cell | 1 (2.9) |
| Asthma | 8 (22.9) |
| COPD | 2 (5.8) |
| Preexisting ILD | 0 |
Definition of abbreviations: BMI = body mass index; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; HIV = human immunodeficiency virus; ILD = interstitial lung disease; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Data are presented as n (%) or mean ± standard deviation as appropriate.
Table 2.
Admission data from patients with interstitial lung disease following infection with SARS-CoV-2
| Admission Data | Value |
|---|---|
| Length of stay | 16.9 ± 12.5 |
| O2 therapy (>24 h) | 29 (82.9) |
| Max O2 requirement, %, median ± IQR | 38.0 ± 48 |
| Days O2 therapy, median ± IQR | 13.9 ± 12.1 |
| Inpatient steroid treatment, n (%) | 6 (17.1) |
| Critical care admission | 19 (54.5) |
| Invasive mechanical ventilation | 16 (45.7) |
| SpO2 on discharge | 95.1 ± 2 |
Definition of abbreviations: IQR = interquartile range; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SpO2 = oxygen saturation as measured by pulse oximetry.
Data are presented as n (%) or mean ± standard deviation unless otherwise stated.
Biochemical markers indicated improving systemic inflammation on follow-up, with C-reactive protein falling from a mean of 230.2 ± 162.6 mg/L at peak illness to 30.9 ± 37.5 mg/L at discharge and 6.1 ± 9.79 mg/L in clinic. Likewise, ferritin was high at the peak with a mean of 1,592.4 ± 1,274.6 μg/L, falling to 807.6 ± 450.0 μg/L at discharge and 179.0 ± 141.8 μg/L on structured assessment. Patients had a high d-dimer during their admission 17.2 ± 8.1 mg/L, and although this had fallen by 6 weeks, it was persistently elevated at 2.35 ± 3.7 mg/L (Table 3).
Table 3.
Patients demonstrated improvement in markers of systemic inflammation at 6 weeks after discharge
| Marker | Peak | Discharge | Clinic |
|---|---|---|---|
| CRP (0–4 mg/L) | 230.2 ± 162.6 | 30.9 ± 37.5 | 6.1 ± 9.79 |
| Ferritin (30–400 μg/L) | 1592.4 ± 1274.6 | 807.6 ± 450.0 | 179.0 ± 141.8 |
| Fibrinogen (1.7–3.9 g/L) | 12.3 ± 1.1 | 5.4 ± 1.6 | 4.2 ± 2.6 |
| D-dimer (0.00–0.55 mg/L) | 17.2 ± 8.1 | 10.2 ± 6.7 | 2.35 ± 3.7 |
| Creatinine (59–104 μmol/L) | 150.2 ± 30.3 | 87.6 ± 89.0 | 62.5 ± 33.2 |
| Nadir | Discharge | Clinic | |
|---|---|---|---|
| Lymphocyte (1.2–3.5 × 109) | 0.7 ± 0.2 | 1.6 ± 0.5 | 2.7 ± 1.4 |
Definition of abbreviation: CRP = C-reactive protein.
Normal values are presented in parentheses for each marker. Data are presented as mean ± standard deviation.
Following structured assessment, we demonstrated that patients with persistent ILD had both functional and physiological impairment. Patients reported their MRC at their 6-week structured assessment as 3 (±2) and reported their pre–COVID-19 MRC as 1 (±2). Pulmonary function testing at follow-up demonstrated a mean FVC of 91.9% predicted (±15.9) and TlCO of 60.6% predicted (±24.9) (Table 4). These were slightly lower in the treated cohort, with a mean FVC of 86.4% (±16.3) and TlCO of 56% (±19.6).
Table 4.
Results after structured assessment of patients with interstitial lung disease after infection with SARS-CoV-2
| Structured Assessment | Value |
|---|---|
| Resting SpO2, % | 95.5 ± 3 |
| MRC dyspnea score, median (IQR) | |
| Before COVID-19 | 1.0 (0–3) |
| After COVID-19 | 3.00 (1–5) |
| 6MWT distance, m | 291.2 ± 153.2 |
| 6MWT, % predicted | 54.9 ± 25.0 |
| 6MWT min SpO2 | 90.0 ± 6 |
| Lung function | |
| FEV1, L | 2.4 ± 0.7 |
| FEV1, % | 86.0 ± 13.7 |
| FVC, L | 3.2 ± 1.0 |
| FVC, % | 91.9 ± 16.0 |
| FEV1/FVC, %, median (IQR) | 77.8 (73.2–82.4) |
| TlCO, SI | 5.6 ± 2.2 |
| TlCO, % | 60.6 ± 24.9 |
| KCO, TlCO/L | 1.3 ± 0.3 |
| KCO, % | 88.0 ± 87.6–88.15 |
Definition of abbreviations: 6MWT = 6-minute walk test; COVID-19 = coronavirus disease; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; IQR = interquartile range; KCO = transfer coefficient; MRC = Medical Research Council; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SI = International System of Units; SpO2 = oxygen saturation as measured by pulse oximetry; TlCO = transfer factor of the lung for carbon monoxide.
Data are presented as mean ± standard deviation unless otherwise stated.
Response to Therapy
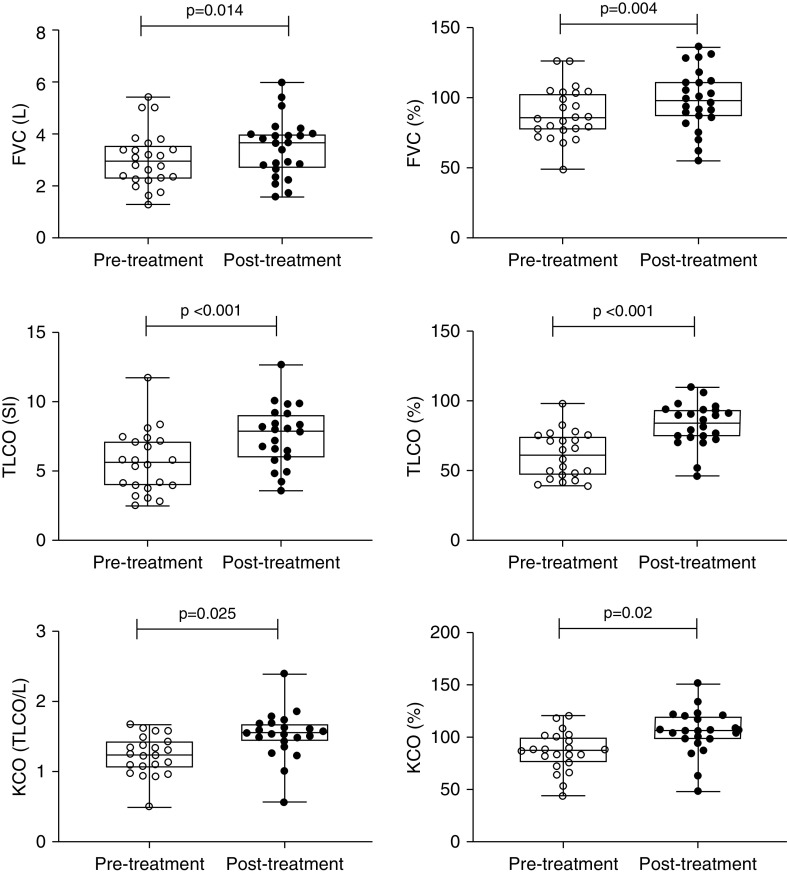
Thirty patients completed treatment and follow-up with clinical review, CT scan, and pulmonary function tests. All 30 patients report that their breathlessness and function had significantly improved following treatment with prednisolone, and median MRC had improved from 3 (±2) to 2 (±1) (P = 0.002). This was associated with a mean relative increase in FVC of 9.6% (±13.6) at 3 weeks, and the mean increase in TlCO was 31.49% (±27.7), which reached statistical significance (Table 5, Figure 3). One patient could not perform lung function, and 6MWT was used to assess response instead. At structured assessment they were able to walk 130 m (33.0% predicted) and desaturated to 89%. Following treatment, they completed 343 m (86.6% predicted), and minimum oxygen saturation as measured by pulse oximetry was 92%. No major complications of steroid treatment were observed.
Table 5.
Follow-up data from patients with interstitial lung disease after infection with SARS-CoV-2 (n = 30)
| Lung Function | Before Treatment | After Treatment | Mean Difference (95% CI) | P Value |
|---|---|---|---|---|
| FVC, L | 3.07 ± 1.12 | 3.36 ± 1.11 | 0.42 (0.28–0.56) | 0.014 |
| FVC, % | 86.8 ± 18.5 | 99.2 ± 19.1 | 9.63 (4.49–14.7) | 0.004 |
| TlCO, SI | 5.56 ± 2.56 | 7.05 ± 2.42 | 1.72 (1.18–2.25 | <0.001 |
| TlCO, % | 59.7 ± 21.1 | 82.6 ± 15.7 | 22.3 (14.1–32.5) | <0.001 |
| KCO, TlCO/L | 1.25 ± 0.34 | 1.83 ± 0.36 | 0.27 (0.16–0.37) | 0.025 |
| KCO, % | 82.9 ± 28.8 | 104.3 ± 24.0 | 19.9 (9.72–30.1) | 0.002 |
Definition of abbreviations: CI = confidence interval; FVC = forced vital capacity; KCO = transfer coefficient; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SI = International System of Units; TlCO = transfer factor of the lung for carbon monoxide.
Data are presented as mean ± standard deviation unless otherwise stated.
Figure 3.
Change in lung function after treatment with oral prednisolone in patients with interstitial lung disease after infection with SARS-CoV-2. FVC = forced vital capacity; KCO = transfer coefficient; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SI = International System of Units; transfer; TlCO = transfer factor of the lung for carbon monoxide.
All post-steroid CT imaging was rediscussed at the MDT. In all cases, the consensus was that the CT features had improved. Repeat imaging demonstrated resolution of the more solid components, leaving a subtler ground-glass pattern in the same distribution. Where the pattern was initially pure ground glass, the density of these changes decreased uniformly after treatment. At 3 weeks’ follow-up imaging, we did not observe the progression of inflammatory change to any fibrosis. A typical response is demonstrated in Figure 4, and with further examples in Figures E2 and E3 in the online supplement. However, the significance of any residual changes remains unknown and will require further longitudinal imaging.
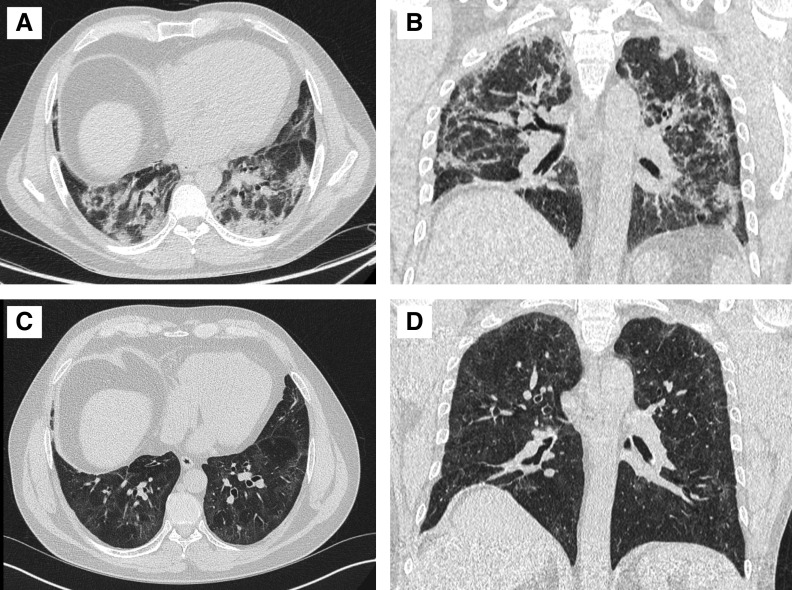
Figure 4.
Axial image and coronal reconstruction from computed tomographic (CT) imaging of the thorax acquired immediately before discharge in a previously fit and well 57-year-old man (A and B) shows a radiological pattern of organizing pneumonia disease with predominant peribronchial and perilobular dense consolidation mild traction bronchiectasis of the airways. At this stage, the patient could only walk 30 yards. Follow-up CT imaging of the thorax acquired after 3 weeks of oral prednisolone (C and D) shows resolution of consolidation with residual ground glass and fine subpleural reticulation. The airways still have a slightly nontapering appearance. The patient was now able to run for 30 minutes a day.
Follow-up has been performed for three patients who, following discussion and patient choice, did not receive steroids. Imaging was performed in two cases and revealed a degree of improvement in the ground-glass component, although of note, both follow-up scans also demonstrated traction bronchiectasis. Mean increase in FVC at 3 months was 8.9% and mean increase in TlCO was 6.9%.
Discussion
Here we describe the characteristics of a large cohort of patients recovering after hospital admission with COVID-19, including those who had residual pulmonary disease at 6 weeks after discharge. Thirty patients had persistent interstitial changes suggestive of organizing pneumonia and were treated with oral prednisolone. The objective was to prevent the development of pulmonary fibrosis with permanent functional deficit, which has been observed in the long-term follow-up of SARS-CoV-1 (11, 12).
Our telephone screening identified a cohort of persistently symptomatic patients and allowed rapid clinical triaging. Following formal assessment, a cohort of patients were identified who had both radiological inflammatory lung disease (of organizing pneumonia type) and persistent physiological and functional deficit at 6 weeks. The natural history of the disease at this time point has not previously been described, and the lack of a known recovery trajectory is a limitation of this study. It is difficult to tease out from a single time point what imaging changes are simply part of the normal recovery from lung injury. It seems likely that the patients with <15% involvement, especially given their relatively preserved lung function, may represent the “typical” recovery trajectory. However, after lung injury/ARDS due to other causes, the predominant radiological pattern is not typically that of organizing pneumonia (13, 14). Indeed, drawing parallels from other coronavirus infections, it is likely that in the absence of intervention, some patients might have been left with persistent functional deficits and permanent fibrotic change. ILD was diagnosed in 4.8% of patients who survived their original infection and 10.8% of the total patients with persistent symptoms and included those patients with relatively mild disease who did not require more than 24 hours of oxygen therapy. This may be an underestimate of the true prevalence given the proactive use of corticosteroids in the ICU at our site during the first wave in the UK. However, given the now-established benefit of dexamethasone in hospitalized patients, this study will reflect clinical practice worldwide in subsequent waves (6, 7).
Radiologically, the interstitial abnormalities detected after COVID-19 were dominated by an organizing pneumonia phenotype (59%). The confidence of the MDT diagnosis meant that neither bronchoalveolar lavage nor lung biopsy was undertaken on this cohort, but clearly this is an area for future research. A number of subjects had minimal residual disease and no physiological impairment (25%), and reassuringly, there was not a predominance of established fibrotic lung disease, despite the numbers of patients requiring ICU and invasive mechanical ventilation (46%). As anticipated in screening such a population with significant comorbidities, previously undiagnosed respiratory conditions were also diagnosed including IPF and connective tissue disease–associated ILD.
The radiological pattern of organizing pneumonia is concordant with the known postmortem findings of COVID-19 (3) although we demonstrate its persistence in convalescent patients. Steroids are the accepted first-line treatment of organizing pneumonia of other causes, and it was with this rationale that post–COVID-19 patients were treated. In patients with >15% lung involvement on CT scan and impairment of their respiratory function, we demonstrate a good response to immunosuppressive therapy. The treatment was well tolerated, and there was a dramatic increase in spirometry and gas transfer at 3 weeks with functional improvement in a group of patients whose clinical trajectory had plateaued before treatment. The clinical improvement was mirrored in the imaging, with radiological resolution of the inflammatory changes. This was not primarily a radiological study and the CT scans were not formally scored before/after treatment. However, each case was discussed before and after treatment at a MDT with three senior radiologists semiquantitatively in line with standard ILD MDT practice and the findings paired with the improvement in patient-reported function and physiological parameters. In addition to this, there are several other limitations to our work. First, there is some difficulty is understanding whether imaging and clinical findings represented nothing other than slow ongoing recovery. This was not a randomized control trial and treatment regimens were drawn from established best-practice protocols to treat organizing pneumonia triggered by other factors. Although we have seen good resolution following initial therapy, patients with interstitial changes will require close follow-up to observe and to understand the evolution of this condition. However, drawing parallels with organizing pneumonia secondary to other triggers, it is unlikely that once resolved this will reoccur.
The small number of patients who remain inpatients may mean we have underestimated the true prevalence the inflammatory disease. However, even at these estimates, given the continually escalating number of cases worldwide, there is likely to be a large cohort of patients at risk for these inflammatory sequelae and who would clearly benefit from early assessment and prompt therapy to prevent long-term irreversible lung damage. These preliminary data should inform further study into both the natural history and potential treatment for patients with a persistent inflammatory interstitial lung disease following SARS-CoV-2 infection.
Supplementary Material
Footnotes
Author Contributions: K.J.M., B.M., A.D., and A.G.W. contributed to the study design, clinical care, and data collection and analysis. A.M.C. contributed to the clinical care and data collection. J.L.L. contributed to data collection and analysis. G.B., S.M.M., and R.P. contributed to the image acquisition and analysis. M.T. and P.L.M. contributed to data interpretation and critical review. All authors were involved in the preparation of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol. 2020;214:1072–1077. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 2.Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series Lancet 2020396320–332.[Published erratum appears in Lancet 396:312.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan F, Ye T, Sun P, Gui S, Liang B, Li L, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Dong C, Hu Y, Li C, Ren Q, Zhang X, et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020;296:E55–E64. doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cordier JF. Organising pneumonia. Thorax. 2000;55:318–328. doi: 10.1136/thorax.55.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N Engl J Med. [online ahead of print] 17 Jul 2020. 10.1056/NEJMoa2021436. [Google Scholar]
- 7.Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, et al. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George PM, Barratt SL, Condliffe R, Desai SR, Devaraj A, Forrest I, et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020;75:1009–1016. doi: 10.1136/thoraxjnl-2020-215314. [DOI] [PubMed] [Google Scholar]
- 9.George PM, Barratt SL, Condliffe R, Desai SR, Devaraj A, Forrest I, et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020;75:1009–1016. doi: 10.1136/thoraxjnl-2020-215314. [DOI] [PubMed] [Google Scholar]
- 10.He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 11.Hui DS, Joynt GM, Wong KT, Gomersall CD, Li TS, Antonio G, et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60:401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang P, Li J, Liu H, et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study Bone Res 202088[Published erratum appears in Bone Res 8:34.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthay MA, Pittet JF, Jayr C. Just say NO to inhaled nitric oxide for the acute respiratory distress syndrome. Crit Care Med. 1998;26:1–2. doi: 10.1097/00003246-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Wilcox ME, Patsios D, Murphy G, Kudlow P, Paul N, Tansey CM, et al. Radiologic outcomes at 5 years after severe ARDS. Chest. 2013;143:920–926. doi: 10.1378/chest.12-0685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.