Abstract
Many patients with obstructive sleep apnea (OSA) experience excessive daytime sleepiness (EDS), which can negatively affect daily functioning, cognition, mood, and other aspects of well-being. Although EDS can be reduced with primary OSA treatment, such as continuous positive airway pressure (CPAP) therapy, a significant proportion of patients continue to experience EDS despite receiving optimized therapy for OSA. This article reviews the pathophysiology and clinical evaluation and management of EDS in patients with OSA. The mechanisms underlying EDS in CPAP-treated patients remain unclear. Experimental risk factors include chronic intermittent hypoxia and sleep fragmentation, which lead to oxidative injury and changes in neurons and brain circuit connectedness involving noradrenergic and dopaminergic neurotransmission in wake-promoting regions of the brain. In addition, neuroimaging studies have shown alterations in the brain’s white matter and gray matter in patients with OSA and EDS. Clinical management of EDS begins with ruling out other potential causes of EDS and evaluating its severity. Tools to evaluate EDS include objective and self-reported assessments of sleepiness, as well as cognitive assessments. Patients who experience residual EDS despite primary OSA therapy may benefit from wake-promoting pharmacotherapy. Agents that inhibit reuptake of dopamine or of dopamine and norepinephrine (modafinil/armodafinil and solriamfetol, respectively) have demonstrated efficacy in reducing EDS and improving quality of life in patients with OSA. Additional research is needed on the effects of wake-promoting treatments on cognition in these patients and to identify individual or disorder-specific responses.
Keywords: OSA, neuronal damage, intermittent hypoxia, neurology
Obstructive sleep apnea (OSA) is a sleep-related breathing disorder resulting from repetitive collapse of the upper airway, which causes intermittent episodic hypoxia and impaired ventilation during sleep (1). OSA is estimated to affect >900 million adults (aged 30–69 yr) worldwide (2). OSA is diagnosed based on the presence of positive polysomnography (PSG) or home sleep apnea test findings of predominantly obstructive respiratory events (e.g., apneas, hypopneas, or respiratory effort–related arousals) (1, 3, 4). Diagnostic criteria specify ≥15 respiratory events/hour alone or ≥5 events/hour in combination with typical symptoms of OSA, such as snoring, fatigue, and excessive daytime sleepiness (EDS), or comorbid conditions such as hypertension, coronary artery disease, or stroke (1, 3, 4).
EDS can be a prominent symptom of OSA and occurs when sleepiness intrudes into activities of daily living (5, 6). EDS can have a negative impact on safety, functioning, productivity, mood, cognition, and quality of life (QoL) (7–10). For example, EDS in patients with OSA is associated with an increased risk of motor vehicle and occupational accidents (11, 12), and a higher prevalence of depression and anxiety (13, 14). Patients with OSA and EDS, treated or not, can demonstrate impairments in attention, memory, and higher-order executive functions (15–17).
Although EDS can be reduced with primary OSA treatment, such as continuous positive airway pressure (CPAP) therapy, a substantial proportion of patients continue to experience EDS despite receiving optimized primary OSA therapy (9, 10). This article reviews the pathophysiology, clinical evaluation, and management of EDS in patients with OSA.
Epidemiology of EDS in OSA
EDS has been reported to affect 40.5–58% of individuals with OSA (depending on severity of OSA) at initial diagnosis (18) but can persist even when the OSA is treated with continuous positive airway pressure (CPAP) therapy. Residual EDS is reported by an estimated 9–22% of CPAP-treated patients in population-based studies (9, 10). A prospective clinical practice–based study found that 34% of patients with EDS at baseline reported daytime sleepiness after 3 months of CPAP use; this percentage was lower (22%) among patients who used CPAP ≥6 hours/night (19). A large 6-month randomized controlled trial reported 22% of patients (overall) had residual EDS after 6 months of CPAP treatment; the rate was higher among patients with CPAP use ≤4 hours/night (31%) compared with those with CPAP use >4 hours/night (18%; P = 0.003) (20).
Several characteristics and conditions have been associated with a greater risk of residual EDS in CPAP-treated patients with OSA. For example, in one study, patients with pretreatment EDS, diabetes, heart disease, and lower respiratory disturbance index at baseline had greater odds of having EDS after 6 months of CPAP use (21). It is common in clinical practice for patients with idiopathic central hypersomnias to be incidentally found to have a mild degree of OSA, with sleepiness naturally persisting after PAP treatment. This may explain the association of EDS with lower respiratory disturbance indexes. A randomized controlled trial found that females and patients using CPAP >4 hours/night had significantly lower odds of experiencing EDS after 6 months of CPAP use, whereas chronic pain, depression, and baseline EDS were associated with significantly higher odds of having residual EDS (20). Such analyses are challenged by disentangling sleep restriction/deprivation and bidirectional and multidimensional relationships occurring with time, obesity, age, and activities.
Pathophysiology of EDS in OSA
Mechanisms underlying residual EDS in patients with treated OSA are likely confounded by the unknown length of time an individual has been exposed to the condition and differences in individual susceptibility to the consequences of OSA, as well as comorbid conditions such as mood disorders and neurologic conditions. Animal models of sleep apnea, exposure to hypoxia, and sleep fragmentation as well as neuroimaging studies in humans are beginning to provide evidence for plausible explanations.
Animal and Human Data on Neuronal Damage and Injury
OSA is characterized by two physiological consequences: episodic, intermittent hypoxia and sleep fragmentation (Figure 1) (22). Animal studies have demonstrated that both are associated with neuronal injury and degeneration in wake-promoting brain regions.
Figure 1.
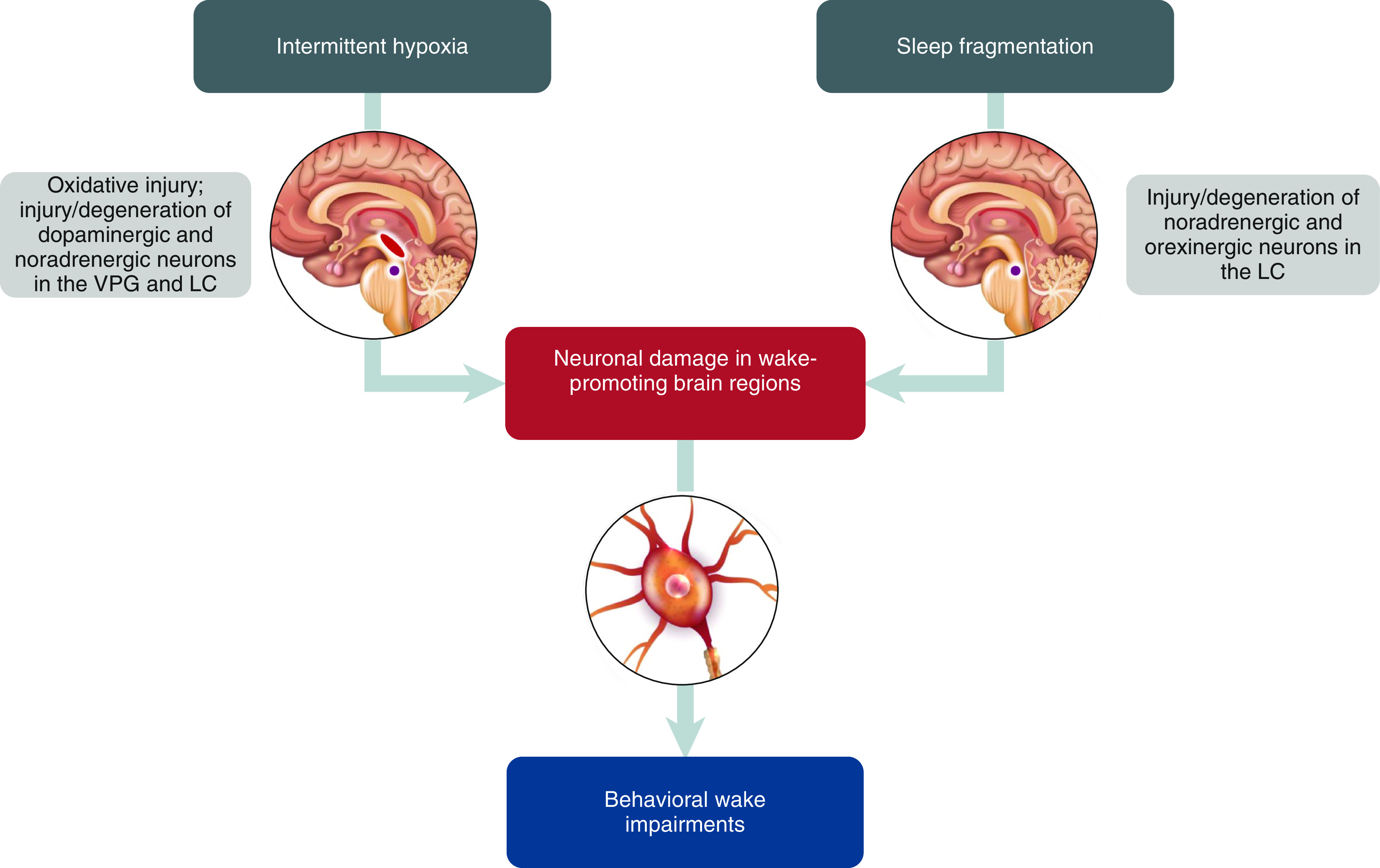
Proposed mechanisms of disease underlying residual excessive daytime sleepiness in obstructive sleep apnea. LC = locus coeruleus (purple); VPG = ventral periaqueductal gray (red).
Intermittent hypoxia has been shown to be associated with oxidative injury that in turn is associated with wake impairments. For example, in mice, exposure (8 wk) to long-term intermittent hypoxia (LTIH) resulted in oxidative injury, specifically nitration and carbonylation, in the basal forebrain and brainstem (23). These injuries were associated with behavioral wake impairments, demonstrated by significantly shorter mean sleep latencies on a murine equivalent of the Multiple Sleep Latency Test (MSLT) (LTIH, 8.9 min; sham LTIH, 12.7 min; P < 0.01). Even after a 2-week recovery period, LTIH mice had significantly more sleep in a 24-hour period than controls (156 min; P < 0.001). In addition, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase has been implicated as a mediator of LTIH-related oxidative injury in the basal forebrain, dorsal raphe nucleus, and the locus coeruleus (LC) and as a factor in associated wake impairments (24).
LTIH can also lead to neuronal injury and cell loss. A mouse model (25) demonstrated that exposure (8–24 wk) to LTIH resulted in injury and loss of catecholaminergic wake neurons, including neurons in the dopaminergic ventral periaqueductal gray and noradrenergic LC (25); specifically, neuronal c-fos responses during wakefulness were suppressed by 70–90% and cell counts were reduced by 35–50%. The observed neuronal injury and loss was associated with behavioral wake impairments that persisted over a 6-month recovery period, including less wake time per 24 hours (120 min; P < 0.01) and shorter mean sleep latencies on the murine MSLT versus controls (10.2 min vs. 14.3 min; P < 0.001). Other wake-active neuronal populations, including orexinergic and histaminergic neurons, showed similar wake responses in LTIH and sham LTIH mice, suggesting that these neurons were not disturbed.
Chronic sleep fragmentation can result in wake impairments associated with neuronal degeneration and oxidative injury (26). Four weeks of sleep fragmentation in mice resulted in impaired excitability of wake-promoting neurons in the LC and reduced axonal projections from neurons in the LC to the frontal cortex (26). A subsequent study (27) demonstrated that chronic (14 wk) sleep fragmentation also results in degeneration of LC and orexinergic neurons, with cell counts reduced by 50% and 25%, respectively, relative to controls (P < 0.001 and P < 0.05, respectively), even after a 4-week recovery period (27). The same study (27) found that lipofuscin, a marker of oxidative stress, increased in wake-active LC and orexinergic neurons following sleep fragmentation.
Thus, several lines of evidence suggest that chronic intermittent hypoxia and sleep fragmentation, the pathophysiological hallmarks of OSA, can produce overlapping and potentially complementary modalities of injuries, including oxidative injury, neuronal damage, and cell loss in wake-promoting brain regions. These neuronal disturbances have been associated with wake impairments, demonstrated by changes in behavioral sleep/wake patterns (e.g., significantly shorter mean sleep latencies). Even after recovery periods of 2 weeks to 6 months, neuronal and wake deficits persisted, suggesting that the intermittent episodic hypoxia and sleep fragmentation associated with OSA may result in irreversible brain damage and impaired wakefulness. The advantage of animal models is that they permit a consistent, often severe, level of risk exposure and complete mitigation, whereas human expression of EDS in OSA is present in some but not all of those with mild to severe AHI levels. Therefore, these are clues rather than absolute, as factors such as genetic diversity (as a surrogate for individual susceptibility) are not examined in this literature.
Unlike animal studies, the duration and severity of exposure to OSA may vary or is difficult to quantify in human studies. Nonetheless, emerging functional evidence from patients with OSA suggests that residual EDS may be related to alterations in brain structure consistent with exposure to chronic intermittent hypoxia and/or sleep fragmentation. Patients with OSA who are adherent to CPAP therapy and have residual EDS often experience fatigue, have periodic limb movements, spend less time in stage N3 sleep, and have shorter daytime sleep latencies and more daytime sleep (28). Although animal models were developed to test assumptions of causality, the onus now is on experimental design and how findings in nonhuman models inform the next generation of human studies.
Focusing on LTIH-induced dopaminergic injury, EDS in patients with OSA was associated with overnight changes in concentrations of dopamine metabolites homovanillic acid and 3,4-dihydroxyphenylacetic acid (DOPAC); levels of both metabolites were significantly correlated with subjective EDS severity, even after adjusting for potential confounding factors (29). Furthermore, patients with OSA and EDS were 3.5 times more likely to have a nocturnal increase in urine DOPAC than patients without EDS (29). These findings suggest that EDS in OSA may be related to disruptions in the dopaminergic system, consistent with animal models. In addition, some data demonstrate normal cerebrospinal fluid levels of orexin and histamine in patients with OSA (30, 31), a finding that is consistent with data from animal models of LTIH-induced neuronal injury that suggested these neurons were not affected, as described above (25).
Human Imaging Studies
Neuroimaging methodologies have enabled evaluation of brain structural and functional changes in patients with OSA. Although informative, neuroimaging technologies are unable to distinguish causes from effects of OSA. Differences in imaging and data processing techniques, as well as in patient characteristics in individual studies, complicate comparisons across studies and the generalizability of findings.
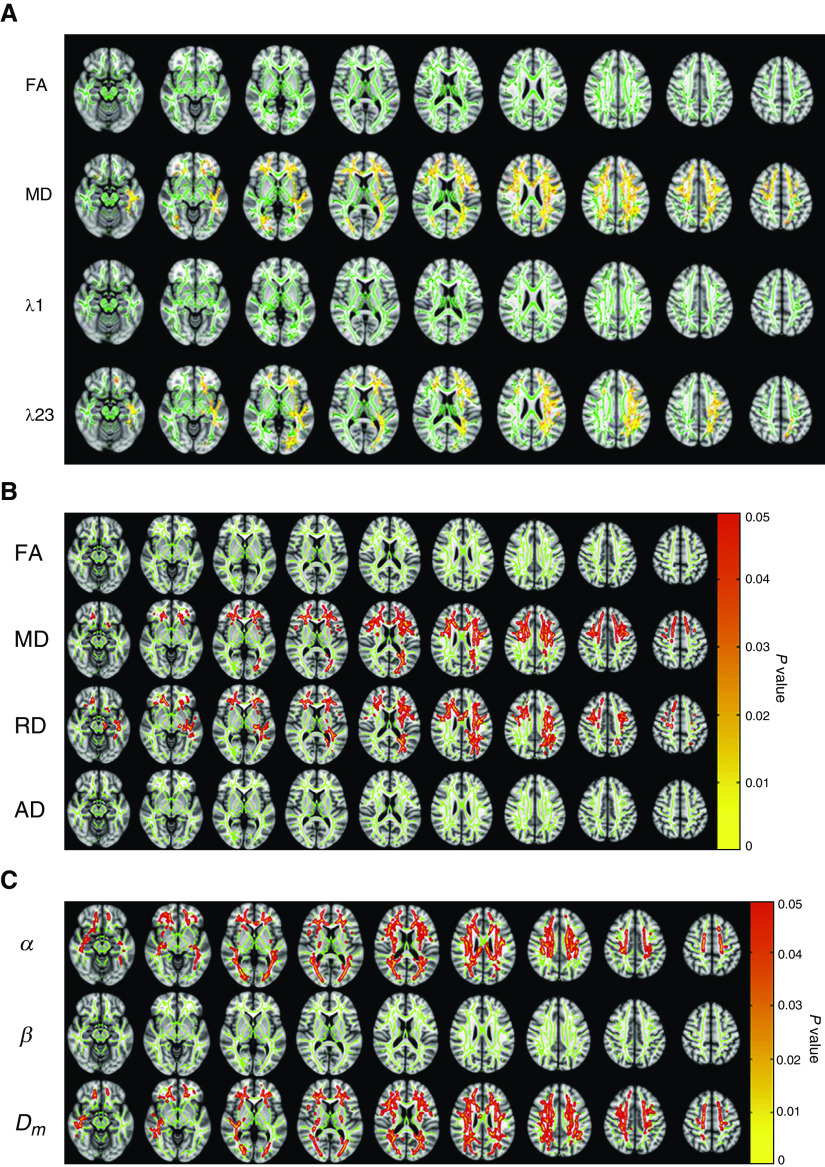
Neuroimaging studies of structure, however, have resulted in a consensus that white matter (WM) alterations are present in patients with OSA and EDS. Diffusion tensor imaging (DTI) studies showed that among patients with OSA treated with CPAP, higher whole-brain mean diffusivity (MD) was observed in patients with EDS compared with those without EDS, and that the increase was primarily caused by higher radial diffusivity (Figures 2A and 2B). These changes suggest possible myelin and/or axonal damage. In contrast, whole-brain fractional anisotropy (FA) and axial diffusivity in patients with OSA did not differ between patients with and without EDS (32, 33). In the same studies, differences in regional DTI parameters were reported, with higher regional MD (primarily related to increased radial diffusivity) and lower regional FA in patients with EDS compared with those without EDS. These differences are suggestive of decreased axonal and myelin integrity (32, 33). Specifically, these regional differences were observed in fibers of the corpus callosum, internal and external capsule, corona radiata, and sagittal stratum, with most changes seen in the left hemisphere (32).
Figure 2.
White matter changes in patients with excessive daytime sleepiness associated with obstructive sleep apnea (OSA). (A) Diffusion tensor imaging (DTI) results showing possible white matter alterations in DTI metrics including FA, MD, λ1 (AD), and λ23 (RD) between sleepy and nonsleepy patients with OSA. Green: mean FA skeleton (threshold = 0.2) without significant change. Red-yellow: fibers with increased DTI metrics in the sleepy group when compared to the nonsleepy group (P < 0.05) (32). (B) Results from DTI whole-brain analysis based on FA, MD, λ23 (RD), and λ1 (AD) showing the presence or absence of differences between sleepy and nonsleepy patients with OSA. Age was included as a covariate in the group analyses for all parameters. Green: mean fractional anisotropy (FA) skeleton (threshold = 0.2) without significant change. Red-yellow: voxels with significantly increased parameters values in the sleepy group as compared to the nonsleepy group with P < 0.05 as shown in the color bar (33). (C) Whole-brain α, β, and Dm maps showing the presence or absence of differences between sleepy and nonsleepy patients with OSA (33). (A) Reprinted with permission of John Wiley & Sons. Xiong Y, et al. Brain white matter changes in CPAP-treated obstructive sleep apnea patients with residual sleepiness. J Magn Reson Imaging. 2017;45(5):1371–1378. Copyright © 2016 International Society for Magnetic Resonance in Medicine. (B and C) Reprinted from Zhang J, et al. White matter structural differences in OSA patients experiencing residual daytime sleepiness with high CPAP use: a non-Gaussian diffusion MRI study. Sleep Med. 2019;53:51–59. doi:10.1016/j.sleep.2018.09.011, with permission from Elsevier. AD = axial diffusivity; Dm = anomalous diffusion coefficient; FA = fractional anisotropy; MD = mean diffusivity; RD = radial diffusivity.
Findings from DTI studies have also demonstrated associations with clinical outcomes. In one study described above (32), regional MD positively correlated and regional FA negatively correlated with sleepiness severity and impairment in sustained attention, suggesting that the observed WM structural differences may be one mechanism contributing to residual EDS and impaired vigilance in patients with OSA.
In a study of previously untreated patients with OSA (34), lower FA values were associated with greater sleepiness, poorer sleep quality, and delayed response time and increased errors on executive function tasks compared with controls; in contrast, higher values corresponded to better memory-based performance (34). After 3 months of CPAP treatment, pathologic WM clusters were significantly smaller and patients improved on measures of attention, executive function, and short- and long-term memory. After 12 months of treatment, more notable reversals in WM abnormalities were observed, although there was no further significant improvement in cognitive function. These findings further suggest that EDS and cognitive impairments associated with OSA may result from WM structural damage and may be at least partially reversible with CPAP treatment (34). Similarly, in older patients (≥65 yr), 3 months of CPAP treatment increased connectivity, attenuated cortical thinning, and significantly improved memory (episodic and short term) and sleep-related QoL (35).
Data based on diffusion-weighted imaging and a continuous-time random-walk model (33) also indicate WM changes in patients with OSA and EDS. Specifically, these patients had higher whole-brain Dm and whole-brain α compared with patients with OSA without EDS (Figure 2C) (33). Dm is analogous to MD, as described above, whereas α relates to temporal diffusion heterogeneity. For both parameters, higher values suggest WM damage related to shrinkage of axons and myelin. Similarly, increased regional Dm and regional α were also observed, mostly in the corpus callosum, internal and external capsule, corona radiata, superior longitudinal fasciculus, and sagittal stratum (similar to regions with differences in MD and FA (32)). Whole-brain β (which relates to spatial diffusion heterogeneity) did not differ between patients with and without EDS, although regional β was lower in the corpus callosum and longitudinal fasciculus in patients with EDS compared with those without EDS. A lower β value suggests damage to oligodendrocytes (which form myelin) and compromised myelin.
These parameters also demonstrated correlations with clinical outcomes. Specifically, regional α and Dm values were positively correlated and regional β values were negatively correlated with sleepiness severity, impairments in attention, and reaction time. Thus, these findings further support the notion that alterations in WM may underlie residual EDS in patients with OSA (33).
The authors suggest that elevated whole-brain and regional α values may be explained by OSA-related hypoxia and/or ischemia, resulting in degeneration of neurons and oligodendrocytes, and that elevated α, Dm, and MD may be attributed to shrinkage of axons and myelin sheaths in patients with EDS. Whether connectivity alterations are linked to disruptions in neurotransmitter systems involved in wakefulness, as suggested by animal studies, requires further investigation.
Changes in MD and FA values could be driven by alterations in the extracellular space, and that WM microstructure is impacted by time of day (which may vary by study), and this may in turn affect interpretation of the studies discussed above (36).
Autopsied brain tissue of patients with OSA has been studied to link OSA to hippocampal injury; specifically, OSA severity has been shown to be related to cortical thinning in the dentate gyrus, the CA1 layer of the hippocampus, and some layers of the entorhinal cortex and to decreased myelin in the deep layers of the entorhinal cortex (37). Patients who were regular CPAP users at time of death (determined by hospital records) showed no significant reductions in cortical thickness but had myelin loss in the deep layers of the entorhinal cortex, suggesting that CPAP may have a protective effect against cortical thinning but may be ineffective in preventing hippocampal myelin loss. These findings are consistent with murine models demonstrating neuronal death in the hippocampus following exposure to LTIH (38). Longitudinal studies are needed to determine the directionality of the association between OSA and hippocampal damage and to examine the impact of CPAP (39).
The specific role of these changes in EDS associated with OSA has not been determined, although evidence demonstrates an inverse correlation between hippocampal volume and severity of EDS (40). Considering the critical role of the hippocampus in memory (41), hippocampal damage associated with OSA may contribute to cognitive impairments as well as EDS.
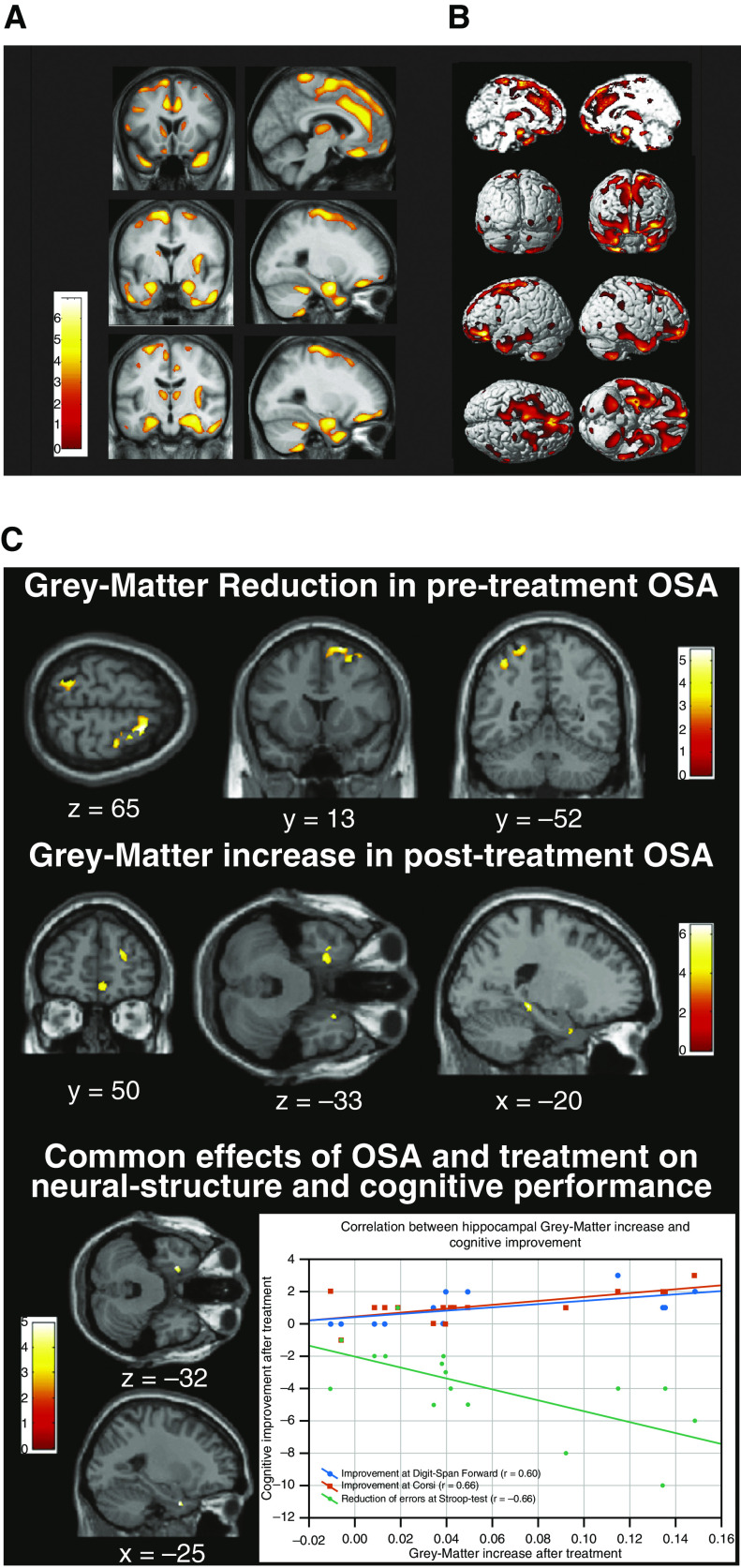
Findings regarding gray matter (GM) changes associated with OSA are mixed; the brain regions affected and types of changes are somewhat inconsistent across studies. A study in patients with severe OSA (42) found that GM concentrations were significantly reduced in cortical and subcortical regions (e.g., caudate nucleus, hippocampus, frontal cortex, anterior cingulate, thalamus, cerebellum, and temporal cortex) (Figures 3A and 3B). Another study (43) also found GM damage in the caudate and hippocampus; however, reductions in GM volume, rather than concentration, were noted. Significant reductions in GM volume in the right middle temporal gyrus have been demonstrated in patients with OSA compared with healthy controls (44). Other imaging studies have identified cortical and hippocampal GM changes (45).
Figure 3.
Gray matter changes in patients with OSA. (A) Areas of decreased gray matter concentration in patients with severe OSA (42). (B) Areas with reduced gray matter concentrations shown in three-dimensional rendering. Reduced gray matter concentrations were observed in the bilateral superior frontal gyri, left gyrus rectus, and bilateral frontomarginal gyri, bilateral anterior cingulate gyri, right anterior insular gyrus, bilateral caudate nuclei, bilateral thalami, bilateral amygdala and hippocampi, bilateral inferior temporal gyri, and bilateral cerebellar cortices. Results were superimposed on the two-dimensional planes of averaged T1 template of all subjects. Scales in color bar are t scores. Left-hand sides of images represent the left hemisphere of the brain. Reprinted with permission of Oxford University Press. Joo EY, et al. Reduced brain gray matter concentration in patients with obstructive sleep apnea syndrome. Sleep. 2010;33(2):235–241. (C) Gray matter volume before and after continuous positive airway pressure (CPAP) treatment in patients with OSA (46). Top row: Gray matter volume is decreased in untreated patients with OSA compared with control subjects. Middle row: Gray matter volume increased after CPAP treatment compared with before treatment. Bottom row: Gray matter in left hippocampal entorhinal cortex reduced before CPAP treatment and increased after treatment. There is also a correlation between the gray matter volume increase in this region and cognitive improvement after treatment. Reprinted by permission from Reference 46. OSA = obstructive sleep apnea.
In treatment-naive patients with OSA, GM structural changes, specifically reduced GM volume in the entorhinal cortex and left posterior parietal cortex, have been associated with cognitive impairments (46). Notably, these structural changes and associated cognitive deficits appeared to be at least partially reversible with CPAP treatment (Figure 3C). After 3 months of CPAP treatment, regional and overall GM volume significantly increased and patients showed significant improvement in EDS and on measures of executive function, attention, short- and long-term memory, QoL, and mood.
Overall, neuroimaging studies provide support for WM and GM structural changes associated with OSA. Future studies controlling for data acquisition, analysis techniques, and confounding patient characteristics are needed to elucidate current findings.
Clinical Evaluation of EDS in OSA
Differential Diagnosis
When evaluating patients with OSA for residual EDS, healthcare providers should make individualized assessments of potential underlying causes of sleepiness (47). The differential diagnosis includes a review for underrecognized comorbid conditions, such as sleep deprivation, idiopathic hypersomnia, narcolepsy, hypothyroidism, circadian rhythm disorders, psychiatric illness, chronic medical conditions, concomitant medications, or illicit drug use. Work schedules (in particular shift work), use of over-the-counter and prescribed sedating medications, and lifestyle factors also should be reviewed. Furthermore, healthcare providers should ensure that the underlying airway obstruction is being adequately treated with CPAP, an oral appliance, hypoglossal nerve stimulation, or other surgical interventions (48–51).
Measures of EDS
Clinical tools to evaluate EDS include objective and self-reported assessments of sleepiness, as well as assessments of cognition or alertness (Table 1).
Table 1.
Commonly used tools for evaluating EDS
| Tool | Type | Measurement | Cutoff Value Suggestive of EDS |
|---|---|---|---|
| Maintenance of Wakefulness Test (52, 53) | Objective | Ability to stay awake (40-min session) | Sleep latency ≤19 min |
| Oxford Sleep Resistance Test (54) | Objective | Ability to stay awake (40-min session) | N/A* |
| Mean Sleep Latency Test (4, 52, 55) | Objective | Ability to fall asleep (20-min nap opportunity) | Sleep latency ≤8 min |
| Psychomotor Vigilance Task (65, 68) | Objective | Sustained attention (i.e., reaction time, lapses in attention) | N/A† |
| Epworth Sleepiness Scale (56, 60) | Subjective | Sleep propensity in daily situations‡ | Score >10 |
| Stanford Sleepiness Scale (57, 58, 63) | Subjective | Degree of sleepiness at a point in time§ | Score >3 |
| Karolinska Sleepiness Scale (59, 64) | Subjective | Degree of sleepiness at a point in time|| | Score ≥7 |
Definition of abbreviations: EDS = excessive daytime sleepiness; N/A = not applicable; OSA = obstructive sleep apnea.
No standard cutoff; however, in the original study of this test, all patients with OSA had mean sleep latencies <20 minutes whereas all healthy patients had mean sleep latencies >20 minutes (group mean sleep latency values were 10.5 min for patients with OSA and 39.8 min for healthy patients).
No standard cutoff; however, patients with EDS have been shown to have slower reaction times, greater variability in reaction times across a task, and longer and more frequent lapses (reaction time >500 ms).
Timeframe is “in recent times.” Includes eight items rated by patients on a scale from 0 to 3 (higher scores indicating greater likelihood of falling asleep); total score can range from 0 to 24.
At a specific moment in time. This is a single-item scored using a 7-point Likert-type scale (1–7), with higher scores indicating greater sleepiness.
At a specific moment in time. This is a single-item scored using a 9-point Likert-type scale (1–9), with higher scores indicating greater sleepiness.
Objective assessments for EDS include the Maintenance of Wakefulness Test (MWT), Oxford Sleep Resistance test, and MSLT (52). The MWT assesses a patient’s ability to stay awake for a defined time (a 40-min protocol is recommended) and generally includes four trials performed at 2-hour intervals, beginning 1.5–3 hours after the patient’s usual wake-up time (52). A mean sleep latency ≤19 minutes on the 40-minute MWT has been suggested as a threshold indicative of EDS (53). The Oxford Sleep Resistance test is structured similarly to the MWT but uses a computerized method for monitoring wakefulness (individuals press a switch in response to a light-emitting diode) (54). The MSLT assesses a patient’s ability to fall asleep, typically involving five nap opportunities (20 minutes each) at 2-hour intervals, beginning 1.5–3 hours after termination of nocturnal PSG (52). A mean sleep latency ≤8 minutes is considered indicative of EDS (4, 55). These tests can be used during initial evaluation of patients with EDS, although the MSLT is more typically used when narcolepsy (rather than OSA) is suspected (52). As they are time consuming and expensive, these tests may not be practical for screening or routine monitoring of treatment response unless there is some uncertainty as to the functional severity of EDS.
Self-reported measures of sleepiness include the Epworth Sleepiness Scale (ESS) (56), Stanford Sleepiness Scale (SSS) (57, 58), and Karolinska Sleepiness Scale (KSS) (59). The ESS is an eight-item self-reported measure of trait EDS in which patients rate their likelihood of dozing off or falling asleep in various scenarios (e.g., reading, watching television, riding in a car) on a Likert scale of 0–3 (total score 0–24), with higher scores indicating a greater likelihood of falling asleep (56); scores >10 are considered indicative of significant EDS (56, 60). The ESS is inexpensive and convenient; however, it may not correlate well with objective measures of EDS or PSG parameters (61, 62) such that some patients may be unaware of their sleepiness (19). The SSS and KSS are single-item self-reported assessments of sleepiness at a point in time or state sleepiness (57–59). The SSS uses a 7-point Likert-type scale (ranging from [1] feeling active, vital, alert, or wide awake to [7] no longer fighting sleep, sleep onset soon, or having dream-like thoughts) (57, 58); scores >3 are associated with sleep debt (63). The KSS uses a 9-point Likert-type scale (ranging from [1] extremely alert to [9] extremely sleepy and fighting sleep), with scores ≥7 corresponding to physiological signs of sleepiness, as determined by electroencephalography and electrooculography (59). The KSS is not typically used for clinical purposes because it is sensitive to fluctuations in prior sleep and time of day (64).
The Psychomotor Vigilance Task (PVT) measures alertness and vigilance based on response to a visual stimulus (65, 66), with eight different metrics including response time and number of lapses in attention. In patients with OSA, PVT performance is impaired compared with healthy controls (67) and higher ESS scores were associated with worsened performance (66). PVT performance also was highly correlated with performance on a computer-based driving simulation task and on a highway driving test (68, 69), suggesting this test may be particularly relevant to real-world outcomes. Typically, a 10-minute test is used in research settings; a longer-duration (e.g., 20-min) test may be needed to determine milder levels of EDS. The PVT is not currently used in routine care, although a 3-minute test has been considered for this purpose (70).
Treatment of EDS in OSA
Because EDS can persist in patients with OSA despite optimal treatment and can significantly impact QoL and increase risk of motor vehicle accidents, independent and direct management of EDS may be necessary in addition to primary OSA therapy. Pharmacologic management strategies for EDS should not replace primary treatment of the underlying airway obstruction.
Pharmacotherapy of EDS in OSA
Modafinil (PROVIGIL) (71), armodafinil (NUVIGIL) (72), and solriamfetol (SUNOSI) (73) are approved in the United States for the treatment of EDS in adults with OSA; only solriamfetol is approved in the European Union for this indication (74). Modafinil and armodafinil bind to the dopamine transporter and inhibit dopamine reuptake (71, 72). Solriamfetol binds to dopamine and norepinephrine transporters and inhibits reuptake of both neurotransmitters (73, 75). Thus, these three agents have pharmacologic actions on neurotransmitters implicated in sleep–wake regulation (76).
Modafinil and armodafinil have demonstrated efficacy in improving wakefulness and reducing EDS in patients with OSA treated with CPAP in placebo-controlled short-term (4- to 12-wk) studies (Table 2) (77–80) and in open-label long-term studies (81, 82). A meta-analysis of 10 modafinil/armodafinil studies in CPAP-treated participants with OSA determined that modafinil/armodafinil reduced ESS scores by −2.2 points and increased MWT sleep latency by 3 minutes compared with placebo (83). Several of these studies also demonstrated improvements in QoL measures (Functional Outcomes of Sleep Questionnaire [FOSQ], Brief Fatigue Inventory, and/or Short Form 36 [SF-36]) (78–80, 82).
Table 2.
Efficacy data from clinical trials for FDA-approved wake-promoting agents indicated for the treatment of EDS in OSA
| Author and Year, Trial Registration (if Available) | Design | Duration | Treatment | N* | ESS Score |
MWT Sleep Latency (min) |
Summary of Efficacy Findings | ||
|---|---|---|---|---|---|---|---|---|---|
| Baseline Mean (SD) | Mean Change from Baseline† | Baseline Mean (SD) | Mean Change from Baseline† | ||||||
| Modafinil (PROVIGIL, Teva Pharmaceuticals) |
|||||||||
| Pack 2001 (77) | Randomized, double-blind, placebo-controlled | 4 wk | Placebo | 80 | 14.4 (3.2) | End of study mean, 12.4 | MSLT, 7.5 min (4.6) | MSLT end of study mean, 7.2 | Modafinil significantly improved ESS scores and MWT mean sleep latency compared with placebo |
| 200 mg (wk 1); 400 mg (wk 2–4) | 77 | 14.2 (2.9) | End of study mean, 9.6‡ | MSLT, 7.4 min (4.8) | MSLT end of study mean, 8.6‡ | ||||
| Black 2005 (78) | Randomized, double-blind, placebo-controlled | 12 wk | Placebo | 104 | N/A | −1.8 | N/A | −1.1 | Modafinil significantly improved ESS scores and MWT mean sleep latency compared with placebo; no significant difference in efficacy between doses was observed |
| 400 mg | 101 | N/A | −4.5‡ | N/A | 1.5‡ | ||||
| 200 mg | 104 | N/A | −4.5‡ | N/A | 1.6‡ | ||||
| Hirshkowitz 2007 (81) | Open-label extension | 12 mo | 200–400 mg | 266 | 14.5 (3.6) | Mean score = 10.1 (Month 12) | N/A | N/A | Modafinil maintained improvements on ESS scores over a 12-mo period |
| |
|||||||||
| Armodafinil (NUVIGIL, Teva Pharmaceuticals) |
|||||||||
| Hirshkowitz 2007 (79), NCT00079677 | Randomized, double-blind, placebo-controlled | 12 wk | Placebo | 130 | 16.0 (3.5) | N/A | 23.3 (8.2) | −1.3 (7.1) | Armodafinil significantly improved ESS scores and MWT mean sleep latency compared with placebo |
| 150 mg | 129 | 15.6 (3.5) | N/A‡ | 23.7 (8.6) | 2.3 (7.8)‡ | ||||
| Roth 2006 (80), NCT00078325 | Randomized, double-blind, placebo-controlled | 12 wk | Placebo | 131 | 15.9 (3.6) | −3.3 | 23.2 (7.7) | −1.7 | Armodafinil significantly improved ESS scores and MWT mean sleep latency compared with placebo |
| 150 mg | 133 | 15.4 (3.5) | −5.5‡§ | 21.5 (8.9) | 1.9‡§ | ||||
| 250 mg | 131 | 15.3 (3.6) | 23.3 (7.7) | ||||||
| Black 2010 (82), NCT00228553 | Open-label extension | Up to 2 yr | 100–250 mg|| | 459 | 15.8 (3.5) | −6.4 | N/A | N/A | Armodafinil improved ESS scores and remained effective over the course of the study |
| |
|||||||||
| Solriamfetol (SUNOSI, Jazz Pharmaceuticals) |
|||||||||
| Schweitzer 2019 (90), NCT02348606 | Randomized, double-blind, placebo-controlled | 12 wk | Placebo | 114 | 15.6 (3.3) | LS mean, −3.3 | 12.4 (7.2) | LS mean, 0.2 | All doses of solriamfetol improved ESS scores and MWT mean sleep latency compared with placebo |
| 37.5 mg | 56 | 15.1 (3.5) | LS mean, −5.1‡ | 13.6 (8.1) | LS mean, 4.7‡ | ||||
| 75 mg | 58 | 14.8 (3.5) | LS mean, −5.0‡ | 13.1 (7.2) | LS mean, 9.1‡ | ||||
| 150 mg | 116 | 15.1 (3.4) | LS mean, −7.7‡ | 12.5 (7.2) | LS mean, 11.0‡ | ||||
| 300 mg | 115 | 15.2 (3.1) | LS mean, −7.9‡ | 12.0 (7.3) | LS mean, 13.0‡ | ||||
| Strollo 2019 (89), NCT02348619 | Double-blind, placebo-controlled, RW | 6 wk (2-wk RW) | Placebo | 62 | 5.9¶ | LS mean, 4.5** | 29.0¶ | LS mean, −12.1** | Solriamfetol improved ESS scores and MWT sleep latency; effects were maintained in participants who continued solriamfetol vs. a loss of efficacy among those switched to placebo |
| 75, 150, or 300 mg†† | 60 | 6.4¶ | LS mean, −0.1‡** | 31.7¶ | LS mean, −1.0‡** | ||||
| Malhotra 2020 (91), NCT02348632 | Open-label extension | Up to 1 yr | 75–300 mg|| | 417 | 15.0–15.2‡‡ | End of study mean, 6.3–6.5‡‡ | N/A | N/A | Solriamfetol improved ESS scores and maintained efficacy during the 1-yr study; after ∼6 mo of treatment, participants switched to placebo worsened compared with those who remained on solriamfetol |
Definition of abbreviations: EDS = excessive daytime sleepiness; ESS = Epworth Sleepiness Scale; FDA = U.S. Food and Drug Administration; LS = least squares; MSLT = Multiple Sleep Latency Test; MWT = Maintenance of Wakefulness Test; N/A = not applicable; OSA = obstructive sleep apnea; RW = randomized withdrawal; SD = standard deviation.
All studies enrolled adults with OSA currently or previously treated with continuous positive airway pressure or another primary airway therapy.
Represents efficacy population.
Mean change from baseline to end of study, unless otherwise noted.
Significantly different than placebo (P < 0.05).
Results for combined armodafinil group.
Treatment was titrated to an efficacious and tolerable dose.
Beginning of RW phase following 4 weeks of treatment (Week 4).
Change from beginning of RW phase (Week 4) to end of RW phase (Week 6).
Based on stable dose from previous 4 weeks of treatment.
Results presented separately for two groups, differentiated based on duration between prior study completion and enrollment in the open-label extension.
In CPAP-treated patients with OSA and EDS, modafinil has demonstrated improvements in some PVT outcomes (number of lapses and inverse reaction time) (84, 85), and armodafinil has been associated with improvements in some measures of memory (79, 80, 86). Armodafinil has also been shown to improve PVT inverse reaction time and number of errors while reducing electroencephalography delta activity compared with placebo in a small study of healthy sleep-deprived adults (87). Some data suggest that modafinil and armodafinil may have pharmacologic effects in addition to those related to dopaminergic neurotransmission, including direct or indirect effects on noradrenergic, serotonergic, glutamatergic, and GABAergic neurotransmission; interactions with cholinergic and histamine neurotransmission; and other effects on brain areas involved in cognitive processes, and it has been proposed that such effects may be associated with improvements in cognitive function (88).
Common adverse events (AEs) associated with modafinil include headache, nausea, nervousness, rhinitis, diarrhea, back pain, anxiety, insomnia, dizziness, and dyspepsia (71). Common AEs associated with armodafinil include headache, nausea, dizziness, and insomnia (72). Rare but serious side effects (e.g., Stevens-Johnson Syndrome) can occur with modafinil/armodafinil; drug interactions with CYP3A4/5 substrates, including oral contraceptives, are also a consideration (71, 72).
Solriamfetol has demonstrated efficacy in improving wakefulness and reducing EDS in participants with OSA (who were currently using or who had previously failed primary OSA therapy) in a placebo-controlled 6-week randomized withdrawal study (89) and a placebo-controlled 12-week study (90); long-term efficacy in reducing EDS was demonstrated in a 1-year open-label extension study, which also included a 2-week randomized withdrawal period (Table 2) (91). In the 12-week study, solriamfetol (37.5, 75, 150, and 300 mg/d) dose-dependently reduced ESS scores by −1.7 to −4.7 points, and increased MWT sleep latency by 4.5 to 12.8 minutes, compared with placebo (90). Efficacy was similar in subgroups who were adherent or nonadherent to primary OSA therapy (90).
Solriamfetol treatment was also associated with improvements in QoL, functioning, and work productivity measures (SF-36v2, FOSQ short version [FOSQ-10], and Work Productivity and Activity Impairment Questionnaire: Specific Health Problem) (92). Data on cognitive outcomes with solriamfetol have not been reported, although a randomized, double-blind, placebo-controlled, crossover study to evaluate the effect of solriamfetol on on-road driving performance in participants with EDS due to OSA is currently being conducted (NCT 02806895; EudraCT 2015-003930-28).
Common AEs associated with solriamfetol are headache, nausea, decreased appetite, insomnia, and anxiety (73). Solriamfetol has been shown to improve wakefulness from 1 to 9 hours after dosing (90); therefore, solriamfetol should not be administered within 9 hours of planned bedtime as it has the potential to interfere with sleep (73).
Future Directions
Potential mechanisms of EDS in OSA have been hypothesized but are not entirely clear. Pharmacologic treatments have been shown to improve EDS and QoL in patients with OSA, but whether they have some effect on the brain changes that can be seen in patients with OSA and EDS has yet to be studied. Neuroimaging studies to evaluate brain changes in patients with OSA with EDS are not part of the routine clinical management of these patients. More specific tests to evaluate the brain changes and overall pathophysiological mechanisms involved in EDS in patients with OSA would inform the overall clinical management of these patients. This can result in a more personalized and phenotype-based approach to OSA management as we learn more about this disease state. Studies of agents with different mechanisms of action, such as the selective histamine H3 antagonist pitolisant, an agent currently approved to treat narcolepsy that also has shown efficacy in clinical trials of patients with EDS associated with OSA (93, 94), would be beneficial in this regard. Such data could provide a better understanding of potential therapeutic approaches that recruit alternate alerting pathways and the effects of OSA on these pathways.
Given that animal models show damage in several neurotransmitter systems, neuroimaging studies suggest dysfunction in both WM and GM, and the success of diverse agents, there may be functional redundancy in EDS mitigation. Future studies could investigate the action of pharmacologic agents to determine whether wake-promoting agents impact the structural or functional changes observed with neuroimaging techniques. Additionally, the impact of wake-promoting agents on cognition in patients with OSA is an area for future study. Finally, it is unknown why some individuals may respond better to one line of therapy than another.
Conclusions
An estimated 9–22% of patients with OSA continue to experience EDS despite adequate primary OSA therapy. Although neuroimaging studies demonstrate evidence of neuronal injury, the exact pathophysiologic mechanisms underlying residual EDS in OSA remain unclear. Currently marketed treatments for EDS in OSA have demonstrated improvements in various measures of EDS as well as QoL and work productivity measures. Future studies should evaluate the impact of wake-promoting agents on cognitive impairment and brain changes seen in patients with OSA with EDS.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Sherri Jones, PharmD, and Jeannette Fee of Peloton Advantage, LLC, who, under the direction of the authors, provided medical writing and editorial support for this article, which was funded by Jazz Pharmaceuticals.
Footnotes
Supported by Jazz Pharmaceuticals. Jazz Pharmaceuticals has worldwide development, manufacturing, and commercialization rights to solriamfetol, excluding certain jurisdictions in Asia. SK Biopharmaceuticals, the discoverer of the compound (also known as SKL-N05), maintains rights in 12 Asian markets, including Korea, China, and Japan.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7:687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. Obstructive sleep apnea, adult; pp. 53–62. [Google Scholar]
- 4.Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146:1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- 5.Pagel JF. Excessive daytime sleepiness. Am Fam Physician. 2009;79:391–396. [PubMed] [Google Scholar]
- 6.Dongol EM, Williams AJ. Residual excessive sleepiness in patients with obstructive sleep apnea on treatment with continuous positive airway pressure. Curr Opin Pulm Med. 2016;22:589–594. doi: 10.1097/MCP.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 7.Mulgrew AT, Ryan CF, Fleetham JA, Cheema R, Fox N, Koehoorn M, et al. The impact of obstructive sleep apnea and daytime sleepiness on work limitation. Sleep Med. 2007;9:42–53. doi: 10.1016/j.sleep.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Stepnowsky C, Sarmiento KF, Bujanover S, Villa KF, Li VW, Flores NM. Comorbidities, health-related quality of life, and work productivity among people with obstructive sleep apnea with excessive sleepiness: findings from the 2016 US National Health and Wellness Survey. J Clin Sleep Med. 2019;15:235–243. doi: 10.5664/jcsm.7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pépin JL, Viot-Blanc V, Escourrou P, Racineux JL, Sapene M, Lévy P, et al. Prevalence of residual excessive sleepiness in CPAP-treated sleep apnoea patients: the French multicentre study. Eur Respir J. 2009;33:1062–1067. doi: 10.1183/09031936.00016808. [DOI] [PubMed] [Google Scholar]
- 10.Gasa M, Tamisier R, Launois SH, Sapene M, Martin F, Stach B, et al. Scientific Council of the Sleep Registry of the French Federation of Pneumology-FFP. Residual sleepiness in sleep apnea patients treated by continuous positive airway pressure. J Sleep Res. 2013;22:389–397. doi: 10.1111/jsr.12039. [DOI] [PubMed] [Google Scholar]
- 11.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5:573–581. [PMC free article] [PubMed] [Google Scholar]
- 12.Garbarino S, Guglielmi O, Sanna A, Mancardi GL, Magnavita N. Risk of occupational accidents in workers with obstructive sleep apnea: systematic review and meta-analysis. Sleep (Basel) 2016;39:1211–1218. doi: 10.5665/sleep.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005;90:4510–4515. doi: 10.1210/jc.2005-0035. [DOI] [PubMed] [Google Scholar]
- 14.Lee SA, Han SH, Ryu HU. Anxiety and its relationship to quality of life independent of depression in patients with obstructive sleep apnea. J Psychosom Res. 2015;79:32–36. doi: 10.1016/j.jpsychores.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Lal C, Strange C, Bachman D. Neurocognitive impairment in obstructive sleep apnea. Chest. 2012;141:1601–1610. doi: 10.1378/chest.11-2214. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, Camacho M, Tang X, Kushida CA. A review of neurocognitive function and obstructive sleep apnea with or without daytime sleepiness. Sleep Med. 2016;23:99–108. doi: 10.1016/j.sleep.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Werli KS, Otuyama LJ, Bertolucci PH, Rizzi CF, Guilleminault C, Tufik S, et al. Neurocognitive function in patients with residual excessive sleepiness from obstructive sleep apnea: a prospective, controlled study. Sleep Med. 2016;26:6–11. doi: 10.1016/j.sleep.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Bjorvatn B, Lehmann S, Gulati S, Aurlien H, Pallesen S, Saxvig IW. Prevalence of excessive sleepiness is higher whereas insomnia is lower with greater severity of obstructive sleep apnea. Sleep Breath. 2015;19:1387–1393. doi: 10.1007/s11325-015-1155-5. [DOI] [PubMed] [Google Scholar]
- 19.Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budhiraja R, Kushida CA, Nichols DA, Walsh JK, Simon RD, Gottlieb DJ, et al. Predictors of sleepiness in obstructive sleep apnoea at baseline and after 6 months of continuous positive airway pressure therapy. Eur Respir J. 2017;50:1700348. doi: 10.1183/13993003.00348-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koutsourelakis I, Perraki E, Economou NT, Dimitrokalli P, Vagiakis E, Roussos C, et al. Predictors of residual sleepiness in adequately treated obstructive sleep apnoea patients. Eur Respir J. 2009;34:687–693. doi: 10.1183/09031936.00124708. [DOI] [PubMed] [Google Scholar]
- 22.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, et al. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27:194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- 24.Zhan G, Serrano F, Fenik P, Hsu R, Kong L, Pratico D, et al. NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am J Respir Crit Care Med. 2005;172:921–929. doi: 10.1164/rccm.200504-581OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Y, Fenik P, Zhan G, Mazza E, Kelz M, Aston-Jones G, et al. Selective loss of catecholaminergic wake active neurons in a murine sleep apnea model. J Neurosci. 2007;27:10060–10071. doi: 10.1523/JNEUROSCI.0857-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Panossian LA, Zhang J, Zhu Y, Zhan G, Chou YT, et al. Effects of chronic sleep fragmentation on wake-active neurons and the hypercapnic arousal response. Sleep (Basel) 2014;37:51–64. doi: 10.5665/sleep.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y, Fenik P, Zhan G, Xin R, Veasey SC. Degeneration in arousal neurons in chronic sleep disruption modeling sleep apnea. Front Neurol. 2015;6:109. doi: 10.3389/fneur.2015.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vernet C, Redolfi S, Attali V, Konofal E, Brion A, Frija-Orvoen E, et al. Residual sleepiness in obstructive sleep apnoea: phenotype and related symptoms. Eur Respir J. 2011;38:98–105. doi: 10.1183/09031936.00040410. [DOI] [PubMed] [Google Scholar]
- 29.Paik MJ, Kim DK, Nguyen DT, Lee G, Rhee CS, Yoon IY, et al. Correlation of daytime sleepiness with urine metabolites in patients with obstructive sleep apnea. Sleep Breath. 2014;18:517–523. doi: 10.1007/s11325-013-0913-5. [DOI] [PubMed] [Google Scholar]
- 30.Kanbayashi T, Inoue Y, Kawanishi K, Takasaki H, Aizawa R, Takahashi K, et al. CSF hypocretin measures in patients with obstructive sleep apnea. J Sleep Res. 2003;12:339–341. doi: 10.1046/j.0962-1105.2003.00373.x. [DOI] [PubMed] [Google Scholar]
- 31.Kanbayashi T, Kodama T, Kondo H, Satoh S, Inoue Y, Chiba S, et al. CSF histamine contents in narcolepsy, idiopathic hypersomnia and obstructive sleep apnea syndrome. Sleep. 2009;32:181–187. doi: 10.1093/sleep/32.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiong Y, Zhou XJ, Nisi RA, Martin KR, Karaman MM, Cai K, et al. Brain white matter changes in CPAP-treated obstructive sleep apnea patients with residual sleepiness. J Magn Reson Imaging. 2017;45:1371–1378. doi: 10.1002/jmri.25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Weaver TE, Zhong Z, Nisi RA, Martin KR, Steffen AD, et al. White matter structural differences in OSA patients experiencing residual daytime sleepiness with high CPAP use: a non-Gaussian diffusion MRI study. Sleep Med. 2019;53:51–59. doi: 10.1016/j.sleep.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castronovo V, Scifo P, Castellano A, Aloia MS, Iadanza A, Marelli S, et al. White matter integrity in obstructive sleep apnea before and after treatment. Sleep (Basel) 2014;37:1465–1475. doi: 10.5665/sleep.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalmases M, Solé-Padullés C, Torres M, Embid C, Nuñez MD, Martínez-Garcia MA, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest. 2015;148:1214–1223. doi: 10.1378/chest.15-0171. [DOI] [PubMed] [Google Scholar]
- 36.Voldsbekk I, Maximov II, Zak N, Roelfs D, Geier O, Due-Tønnessen P, et al. Evidence for wakefulness-related changes to extracellular space in human brain white matter from diffusion-weighted MRI. Neuroimage. 2020;212:116682. doi: 10.1016/j.neuroimage.2020.116682. [DOI] [PubMed] [Google Scholar]
- 37.Owen JE, BenediktsdÓttir B, Gislason T, Robinson SR. Neuropathological investigation of cell layer thickness and myelination in the hippocampus of people with obstructive sleep apnea. Sleep. 2019;42 doi: 10.1093/sleep/zsy199. 1-13. [DOI] [PubMed] [Google Scholar]
- 38.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci. 2001;21:2442–2450. doi: 10.1523/JNEUROSCI.21-07-02442.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Macey PM. Damage to the hippocampus in obstructive sleep apnea: a link no longer missing. Sleep. 2019;42:zsy266. doi: 10.1093/sleep/zsy266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dusak A, Ursavas A, Hakyemez B, Gokalp G, Taskapilioglu O, Parlak M. Correlation between hippocampal volume and excessive daytime sleepiness in obstructive sleep apnea syndrome. Eur Rev Med Pharmacol Sci. 2013;17:1198–1204. [PubMed] [Google Scholar]
- 41.Voss JL, Bridge DJ, Cohen NJ, Walker JA. A closer look at the hippocampus and memory. Trends Cogn Sci. 2017;21:577–588. doi: 10.1016/j.tics.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joo EY, Tae WS, Lee MJ, Kang JW, Park HS, Lee JY, et al. Reduced brain gray matter concentration in patients with obstructive sleep apnea syndrome. Sleep. 2010;33:235–241. doi: 10.1093/sleep/33.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torelli F, Moscufo N, Garreffa G, Placidi F, Romigi A, Zannino S, et al. Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage. 2011;54:787–793. doi: 10.1016/j.neuroimage.2010.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morrell MJ, Jackson ML, Twigg GL, Ghiassi R, McRobbie DW, Quest RA, et al. Changes in brain morphology in patients with obstructive sleep apnoea. Thorax. 2010;65:908–914. doi: 10.1136/thx.2009.126730. [DOI] [PubMed] [Google Scholar]
- 45.Celle S, Delon-Martin C, Roche F, Barthélémy JC, Pépin JL, Dojat M. Desperately seeking grey matter volume changes in sleep apnea: a methodological review of magnetic resonance brain voxel-based morphometry studies. Sleep Med Rev. 2016;25:112–120. doi: 10.1016/j.smrv.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Canessa N, Castronovo V, Cappa SF, Aloia MS, Marelli S, Falini A, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med. 2011;183:1419–1426. doi: 10.1164/rccm.201005-0693OC. [DOI] [PubMed] [Google Scholar]
- 47.Epstein LJ, Kristo D, Strollo PJ, Jr, Friedman N, Malhotra A, Patil SP, et al. Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–276. [PMC free article] [PubMed] [Google Scholar]
- 48.Strollo PJ, Jr, Malhotra A. Stimulating therapy for obstructive sleep apnoea. Thorax. 2016;71:879–880. doi: 10.1136/thoraxjnl-2016-209077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strollo PJ, Jr, Soose RJ, Maurer JT, de Vries N, Cornelius J, Froymovich O, et al. STAR Trial Group. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370:139–149. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 50.Almeida FR, Henrich N, Marra C, Lynd LD, Lowe AA, Tsuda H, et al. Patient preferences and experiences of CPAP and oral appliances for the treatment of obstructive sleep apnea: a qualitative analysis. Sleep Breath. 2013;17:659–666. doi: 10.1007/s11325-012-0739-6. [DOI] [PubMed] [Google Scholar]
- 51.Cistulli PA, Grunstein RR. Medical devices for the diagnosis and treatment of obstructive sleep apnea. Expert Rev Med Devices. 2005;2:749–763. doi: 10.1586/17434440.2.6.749. [DOI] [PubMed] [Google Scholar]
- 52.Littner MR, Kushida C, Wise M, Davila DG, Morgenthaler T, Lee-Chiong T, et al. Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–121. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 53.Doghramji K, Mitler MM, Sangal RB, Shapiro C, Taylor S, Walsleben J, et al. A normative study of the maintenance of wakefulness test (MWT) Electroencephalogr Clin Neurophysiol. 1997;103:554–562. doi: 10.1016/s0013-4694(97)00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bennett LS, Stradling JR, Davies RJ. A behavioural test to assess daytime sleepiness in obstructive sleep apnoea. J Sleep Res. 1997;6:142–145. doi: 10.1046/j.1365-2869.1997.00039.x. [DOI] [PubMed] [Google Scholar]
- 55.American Academy of Sleep Medicine Idiopathic hypersomnia International Classification of Sleep Disorders, 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. pp. 53-62 [Google Scholar]
- 56.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 57.Hoddes E, Dement W, Zarcone V. The development and use of the Stanford sleepiness scale (SSS) Psychophysiology. 1972;9:150. [Google Scholar]
- 58.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 59.Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 60.Johns M, Hocking B. Daytime sleepiness and sleep habits of Australian workers. Sleep. 1997;20:844–849. doi: 10.1093/sleep/20.10.844. [DOI] [PubMed] [Google Scholar]
- 61.Erman M, Emsellem H, Black J, Mori F, Mayer G. Correlation between the Epworth Sleepiness Scale and the Maintenance of Wakefulness Test in patients with narcolepsy participating in two clinical trials of sodium oxybate. Sleep Med. 2017;38:92–95. doi: 10.1016/j.sleep.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 62.Bausmer U, Gouveris H, Selivanova O, Goepel B, Mann W. Correlation of the Epworth Sleepiness Scale with respiratory sleep parameters in patients with sleep-related breathing disorders and upper airway pathology. Eur Arch Otorhinolaryngol. 2010;267:1645–1648. doi: 10.1007/s00405-010-1250-y. [DOI] [PubMed] [Google Scholar]
- 63.Carskadon MA, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107–113. doi: 10.1111/j.1469-8986.1981.tb02921.x. [DOI] [PubMed] [Google Scholar]
- 64.Shahid A, Wilkinson K, Marcu S, Shapiro CM. Karolinska Sleepiness Scale (KSS) In: Shahid A, Wilkinson K, Marcu S, Shapiro CM, editors. STOP, THAT and One Hundred Other Sleep Scale. New York, NY: Springer; 2012. pp. 209–210. [Google Scholar]
- 65.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput. 1985;17:652–655. [Google Scholar]
- 66.Batool-Anwar S, Kales SN, Patel SR, Varvarigou V, DeYoung PN, Malhotra A. Obstructive sleep apnea and psychomotor vigilance task performance. Nat Sci Sleep. 2014;6:65–71. doi: 10.2147/NSS.S53721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.D’Rozario AL, Field CJ, Hoyos CM, Naismith SL, Dungan GC, Wong KKH, et al. Impaired neurobehavioural performance in untreated obstructive sleep apnea patients using a novel standardised test battery. Front Surg. 2018;5:35. doi: 10.3389/fsurg.2018.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thomann J, Baumann CR, Landolt HP, Werth E. Psychomotor vigilance task demonstrates impaired vigilance in disorders with excessive daytime sleepiness. J Clin Sleep Med. 2014;10:1019–1024. doi: 10.5664/jcsm.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jongen S, Perrier J, Vuurman EF, Ramaekers JG, Vermeeren A. Sensitivity and validity of psychometric tests for assessing driving impairment: effects of sleep deprivation. PLoS One. 2015;10:e0117045. doi: 10.1371/journal.pone.0117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deering S, Amdur A, Borelli J, Headapohl W, Stepnowsky CJ. A three-minute mobile version of the psychomotor vigilance task [abstract] Sleep. 2018;41:A391–A392. [Google Scholar]
- 71.Teva Pharmaceuticals; 2018. Provigil. Package insert. [Google Scholar]
- 72.Teva Pharmaceuticals; 2018. Nuvigil. Package insert. [Google Scholar]
- 73.Jazz Pharmaceuticals; 2019. Sunosi (solriamfetol) tablets. Prescribing information. [Google Scholar]
- 74.Jazz Pharmaceuticals Ireland; 2020. Sunosi (solriamfetol) tablets. Summary of product characteristics. [Google Scholar]
- 75.Baladi MG, Forster MJ, Gatch MB, Mailman RB, Hyman DL, Carter LP, et al. Characterization of the neurochemical and behavioral effects of solriamfetol (JZP-110), a selective dopamine and norepinephrine reuptake inhibitor. J Pharmacol Exp Ther. 2018;366:367–376. doi: 10.1124/jpet.118.248120. [DOI] [PubMed] [Google Scholar]
- 76.Holst SC, Landolt HP. Sleep-wake neurochemistry. Sleep Med Clin. 2018;13:137–146. doi: 10.1016/j.jsmc.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 77.Pack AI, Black JE, Schwartz JR, Matheson JK. Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea. Am J Respir Crit Care Med. 2001;164:1675–1681. doi: 10.1164/ajrccm.164.9.2103032. [DOI] [PubMed] [Google Scholar]
- 78.Black JE, Hirshkowitz M. Modafinil for treatment of residual excessive sleepiness in nasal continuous positive airway pressure-treated obstructive sleep apnea/hypopnea syndrome. Sleep. 2005;28:464–471. doi: 10.1093/sleep/28.4.464. [DOI] [PubMed] [Google Scholar]
- 79.Hirshkowitz M, Black JE, Wesnes K, Niebler G, Arora S, Roth T. Adjunct armodafinil improves wakefulness and memory in obstructive sleep apnea/hypopnea syndrome. Respir Med. 2007;101:616–627. doi: 10.1016/j.rmed.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 80.Roth T, White D, Schmidt-Nowara W, Wesnes KA, Niebler G, Arora S, et al. Effects of armodafinil in the treatment of residual excessive sleepiness associated with obstructive sleep apnea/hypopnea syndrome: a 12-week, multicenter, double-blind, randomized, placebo-controlled study in nCPAP-adherent adults. Clin Ther. 2006;28:689–706. doi: 10.1016/j.clinthera.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 81.Hirshkowitz M, Black J. Effect of adjunctive modafinil on wakefulness and quality of life in patients with excessive sleepiness-associated obstructive sleep apnoea/hypopnoea syndrome: a 12-month, open-label extension study. CNS Drugs. 2007;21:407–416. doi: 10.2165/00023210-200721050-00004. [DOI] [PubMed] [Google Scholar]
- 82.Black JE, Hull SG, Tiller J, Yang R, Harsh JR. The long-term tolerability and efficacy of armodafinil in patients with excessive sleepiness associated with treated obstructive sleep apnea, shift work disorder, or narcolepsy: an open-label extension study. J Clin Sleep Med. 2010;6:458–466. [PMC free article] [PubMed] [Google Scholar]
- 83.Chapman JL, Vakulin A, Hedner J, Yee BJ, Marshall NS. Modafinil/armodafinil in obstructive sleep apnoea: a systematic review and meta-analysis. Eur Respir J. 2016;47:1420–1428. doi: 10.1183/13993003.01509-2015. [DOI] [PubMed] [Google Scholar]
- 84.Dinges DF, Weaver TE. Effects of modafinil on sustained attention performance and quality of life in OSA patients with residual sleepiness while being treated with nCPAP. Sleep Med. 2003;4:393–402. doi: 10.1016/s1389-9457(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 85.Avellar AB, Carvalho LB, Prado GF, Prado LB. Pharmacotherapy for residual excessive sleepiness and cognition in CPAP-treated patients with obstructive sleep apnea syndrome: a systematic review and meta-analysis. Sleep Med Rev. 2016;30:97–107. doi: 10.1016/j.smrv.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 86.Roth T, Rippon GA, Arora S. Armodafinil improves wakefulness and long-term episodic memory in nCPAP-adherent patients with excessive sleepiness associated with obstructive sleep apnea. Sleep Breath. 2008;12:53–62. doi: 10.1007/s11325-007-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Conrado DJ, Bewernitz M, Ding M, Cibula J, Seubert C, Sy SK, et al. Electroencephalogram effects of armodafinil: comparison with behavioral alertness. J Clin Pharmacol. 2013;53:1058–1071. doi: 10.1002/jcph.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mereu M, Bonci A, Newman AH, Tanda G. The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders. Psychopharmacology (Berl) 2013;229:415–434. doi: 10.1007/s00213-013-3232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Strollo PJ, Jr, Hedner J, Collop N, Lorch DG, Jr, Chen D, Carter LP, et al. Tones 4 Study Investigators. Solriamfetol for the treatment of excessive sleepiness in OSA: a placebo-controlled randomized withdrawal study. Chest. 2019;155:364–374. doi: 10.1016/j.chest.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 90.Schweitzer PK, Rosenberg R, Zammit GK, Gotfried M, Chen D, Carter LP, et al. TONES 3 Study Investigators. Solriamfetol for excessive sleepiness in obstructive sleep apnea (TONES 3): a randomized controlled trial. Am J Respir Crit Care Med. 2019;199:1421–1431. doi: 10.1164/rccm.201806-1100OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Malhotra A, Shapiro C, Pepin JL, Hedner J, Ahmed M, Foldvary-Schaefer N, et al. Long-term study of the safety and maintenance of efficacy of solriamfetol (JZP-110) in the treatment of excessive sleepiness in participants with narcolepsy or obstructive sleep apnea. Sleep. 2020;43:zsz220. doi: 10.1093/sleep/zsz220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weaver T, Pepin JL, Schwab R, Shapiro C, Hedner J, Ahmed M, et al. Long-term effects of solriamfetol on quality of life in participants with excessive daytime sleepiness associated with narcolepsy or obstructive sleep apnea [abstract] Sleep. 2019;42:A239. doi: 10.5664/jcsm.9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dauvilliers Y, Verbraecken J, Partinen M, Hedner J, Saaresranta T, Georgiev O, et al. HAROSA II Study Group Collaborators Pitolisant for daytime sleepiness in patients with obstructive sleep apnea who refuse continuous positive airway pressure treatment: a randomized trial Am J Respir Crit Care Med 20202011135–1145.[Published erratum appears in Am J Respir Crit Care Med 202:154–155.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pepin JL, Dauvilliers Y, Attali V, Tamisier R, Lecomte I, Lecomte J, et al. Pitolisant evaluation in patients with OSA and treated by NCPAP but still complaining of Excessive Daytime Sleepiness (EDS) [abstract] Am J Respir Crit Care Med. 2020;201:A2718. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.