To the Editor:
Individuals with cystic fibrosis (CF) are at markedly increased risk of pulmonary nontuberculous mycobacteria (NTM) infection (1–3), which is associated with accelerated lung function decline. Although structural lung disease likely contributes to elevated NTM risk in this population, identification of modifiable risk factors may help to reduce these morbid infections in CF. Vitamin D is important for host control of Mycobacterium tuberculosis (4, 5), but to date, few studies have explored the relationship between vitamin D deficiency (VDD) and NTM infection (6). Because of pancreatic exocrine insufficiency, individuals with CF are at high risk for VDD (7). In this analysis, we investigate our hypothesis that VDD is a risk factor for incident NTM respiratory isolation in CF.
Methods
We conducted a retrospective cohort study of adults (≥18 yr old) with CF cared for at the Johns Hopkins CF Center between January 1, 2007, and December 31, 2018 (institutional review board approval #IRB00153445). Clinical and demographic data were extracted from the CF Foundation Patient Registry (8) and chart review. Individuals with at least one serum 25-OH vitamin D value during the study period and a mycobacterial culture performed within the subsequent 2 years were eligible for inclusion; individuals could contribute multiple measurements. A 2-year follow-up period from vitamin D concentration measurement was used to account for the indolent nature of NTM infection. Exclusion criteria included history of NTM and/or lung transplantation. The primary outcome of interest was incident respiratory isolation of a potentially pathogenic NTM from sputum or bronchoalveolar lavage. VDD was the primary exposure of interest and was defined as a vitamin D concentration of <20 ng/ml (9).
Statistical analysis
Baseline characteristics were compared between individuals by NTM acquisition status during follow-up. The primary unit of analysis was individual’s vitamin D test; as such, an individual could contribute more than one vitamin D measurement to analyses, and multiple vitamin D measurements could have the same mycobacterial culture outcome, if the culture fell within the 2 year follow-up period for each measurement. This methodology was chosen to use all collected data and to allow for more precise estimation of effects while still including individual-level characteristics in a multivariable regression model. Multivariable Cox proportional hazard regression models with robust SEs to account for multiple observations within individuals were used to evaluate the association between VDD and time to incident NTM acquisition. Time at risk was defined as the time from vitamin D test to either NTM acquisition (for those who acquired NTM during follow-up) or the earliest occurrence of either death, lung transplant, administrative censoring, or 2 years (for those who did not acquire NTM). Models included adjustment for a priori–identified individual-level factors including age at vitamin D testing, sex, highest body mass index and forced expiratory volume in 1 second (FEV1) percentage predicted in year before vitamin D testing, pancreatic insufficiency (defined as pancreatic enzyme use) in previous year, and macrolide use in previous year. In a secondary analysis, vitamin D concentration was analyzed as a continuous variable. Results of regression models are presented as hazard ratios (HRs) with corresponding 95% confidence intervals (CIs). Analyses were performed using STATA version 15.1 (StataCorp).
Results
Study population
We identified 254 individuals who met the inclusion criteria (Figure 1). During the 12-year study period, 50 individuals (19.7%) had a positive NTM culture, with Mycobacterium avium as the most commonly identified NTM species (58%). Individuals who acquired NTM had significantly higher FEV1 and lower rates of macrolide use and Pseudomonas aeruginosa (PsA) infection at the time of study entry, but baseline vitamin D concentrations did not differ significantly (Table 1).
Figure 1.
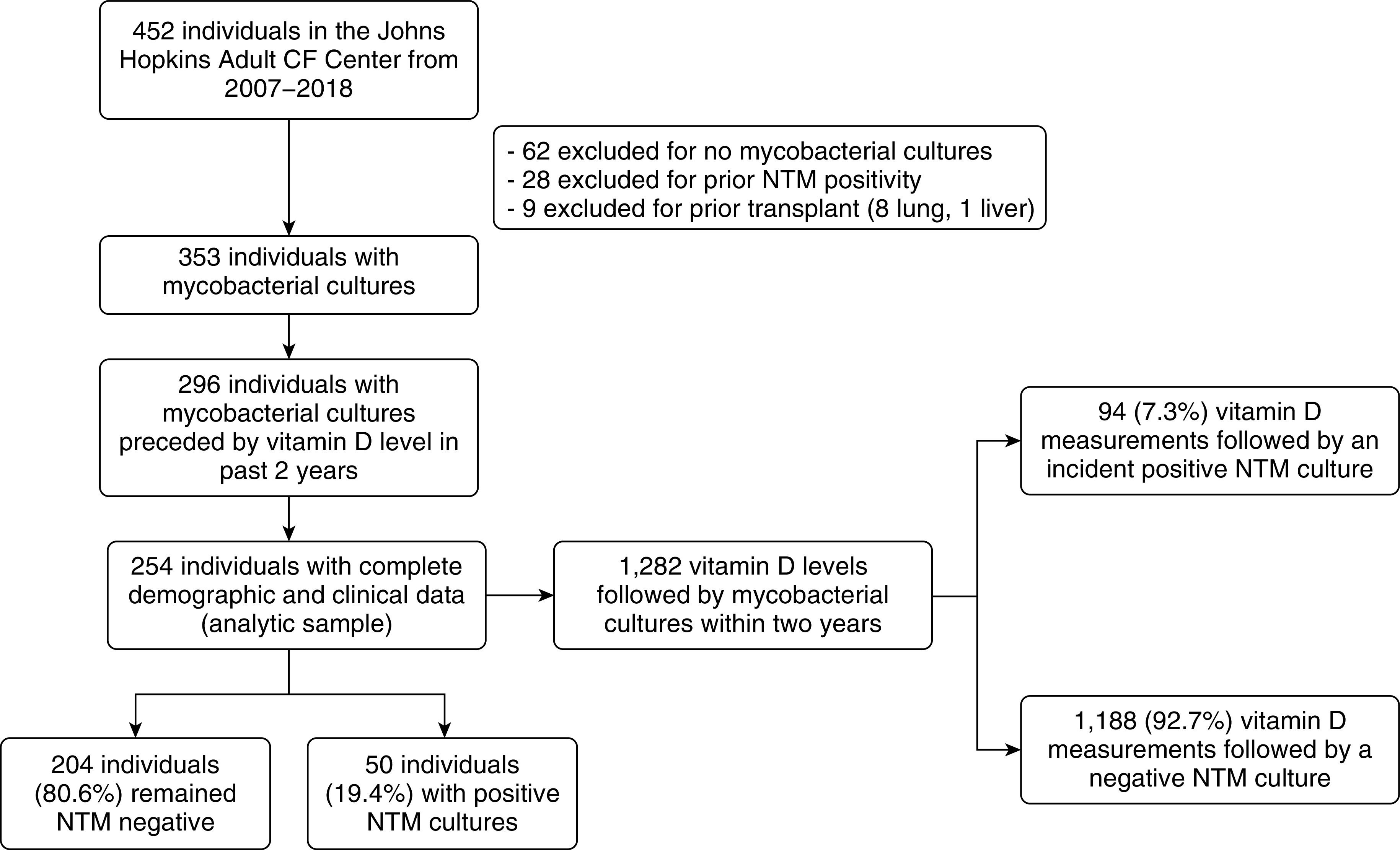
Flowchart of subjects and their vitamin D concentrations. We identified 254 individuals with mycobacterial cultures preceded by vitamin D concentrations in the prior 2 years. Our primary analysis used the vitamin D concentrations as the unit of analysis. From the 254 individuals in our cohort, 1,282 vitamin D concentrations were included in the analysis. Individuals could contribute multiple vitamin D observations, but they could not contribute any vitamin D measurements after an initial NTM positive culture. CF = cystic fibrosis; NTM = nontuberculous mycobacteria.
Table 1.
Baseline demographic and clinical characteristics of subjects upon study entry by NTM acquisition status
| Subject characteristics | Overall (n = 254) | Acquired NTM Infection During Follow-Up (n = 50) | Remained NTM Negative (n = 204) |
|---|---|---|---|
| Age, mean (SD), yr | 30.1 (11.1) | 29.0 (9.1) | 30.5 (11.5) |
| Sex, F, n (%) | 132 (52.0) | 22 (44.0) | 110 (53.9) |
| BMI, mean (SD) | 23.4 (4.3) | 24.3 (3.9) | 23.1 (4.4) |
| Baseline vitamin D values, mean (SD) | 26.6 (12.0) | 23.9 (11.6) | 27.2 (11.8) |
| FEV1% predicted, mean (SD) | 69.7 (22.3) | 78.8 (18.0) | 67.5 (22.7)* |
| CFTR genotype, n (%) | |||
| F508del homozygous | 118 (46.5) | 23 (46.0) | 95 (47.0) |
| F508del heterozygous | 109 (41.7) | 23 (46.0) | 86 (42.2) |
| Other | 23 (9.1) | 4 (8.0) | 19 (9.3) |
| Unknown† | 4 (1.6) | 0 (0.0) | 4 (2.0) |
| CFRD, n (%) | 45 (17.8) | 6 (12.2) | 39 (19.0) |
| Pancreatic insufficiency, n (%) | 218 (85.8) | 45 (90.0) | 173 (84.8) |
| Chronic macrolide use, n (%) | 207 (76.9) | 39 (78.0) | 168 (82.4) |
| Pseudomonas aeruginosa, n (%) | 193 (76.9) | 32 (64.0) | 161 (80.1)* |
| Staphylococcus aureus, n (%) | 158 (63.7) | 35 (70.0) | 123 (61.2) |
| Aspergillus (any species), n (%) | 73 (29.1) | 18 (36.0) | 55 (27.4) |
| NTM species, n (%) | |||
| Mycobacterium avium complex | 29 (58.0) | — | |
| Mycobacterium abscessus/chelonae | 18 (36.0) | — | |
| Other‡ | 3 (6.0) | — | |
| NTM treatment initiated, n (%) | 23 (46.0) | — |
Definition of abbreviations: BMI = body mass index; CFRD = cystic fibrosis related diabetes; CFTR = cystic fibrosis transmembrane conductance regulator; FEV1 = forced expiratory volume in 1 s; NTM = nontuberculous mycobacteria; SD = standard deviation.
Statistically significant with a P < 0.05 using Student’s t tests with unequal variances and χ2 or Fisher exact tests for continuous and categorical variables, respectively.
Unknown includes individuals who do not have at least one allele classified. Other includes those with two known alleles, neither of which are F508del.
Vitamin D concentrations
Individuals remaining NTM negative throughout the study contributed a greater number of vitamin D values (mean ± SD, 5.4 ± 4.1) in comparison with those who became NTM positive (3.5 ± 2.7). The analytic sample was comprised of 1,282 vitamin D measurements among 254 individuals (median = 4 measurements/person; range, 1–25 measurements). The average serum vitamin D value was 28.8 ng/ml (SD = 13.7), and 23.0% (n = 295) of vitamin D values were categorized as vitamin D deficient (<20 ng/ml). A positive NTM respiratory culture within 2 years of vitamin D measurement was observed after only 94 (7.3%) vitamin D measurements, whereas the remaining 1,188 (92.7%) vitamin D measurements were followed by negative mycobacterial cultures. VDD was more common (35.1% vs. 22.1%; P < 0.01) and mean vitamin D values were lower (25.7 ng/ml [SD = 12.28] vs. 29.1 ng/ml [SD = 13.74]; P = 0.03) among vitamin D measurements before positive NTM cultures compared with those before negative NTM cultures. Persistent VDD (defined as two or more consecutive vitamin measurements that were vitamin D deficient) was more frequently observed in individuals who became NTM positive (28.0%) than those who remained NTM negative (15.7%).
Primary and secondary analysis
After adjustment for individual-level characteristics, VDD was associated with a significantly higher risk of NTM acquisition (HR, 1.74; 95% CI, 1.12–2.71). Lowering the threshold of VDD from 20 ng/ml to 12 ng/ml resulted in a similar estimate for the association between VDD and NTM infection (HR, 2.05; 95% CI, 1.07–3.91). When vitamin D concentration was evaluated as a continuous variable, it was not associated with increased risk of incident NTM isolation (HR, 0.98; 95% CI, 0.96–1.00).
Discussion
We found that VDD was associated with a higher risk of incident NTM respiratory isolation in adults with CF. Further supporting this association are findings of significantly higher rates of VDD and significantly lower average serum vitamin D concentrations before NTM isolation. Interestingly, when vitamin D was analyzed as a continuous variable, NTM isolation was not significantly associated with lower vitamin D concentrations, suggesting that a threshold may exist below which NTM susceptibility increases. The association between VDD and NTM infection has a biologically plausible explanation based on prior in vitro studies demonstrating the role of vitamin D in the immune response to other mycobacteria (5). A recent single-center retrospective study reported that adults with non-CF bronchiectasis with NTM lung disease had a higher prevalence of severe VDD compared with control subjects without NTM (6), although that study evaluated vitamin D concentrations after NTM isolation and is also limited because of its retrospective nature. To our knowledge, this is the first study that has implicated VDD as a potential risk factor in NTM acquisition in CF.
There are limitations to our study. First, although clinical guidelines recommend annual vitamin D measurement (10) and NTM respiratory cultures (11), routine ascertainment in our cohort was highly variable. Our study included only individual’s vitamin D measurements with a corresponding NTM culture within 2 years which may have introduced selection bias. At the time of first clinical encounter (separate from first vitamin D measurement), individuals from our institution who were included in this study were older, had lower FEV1% predicted and higher rates of PsA and macrolide use when compared with those not included in the study (data not shown). In addition, the exposure–outcome effect estimates may be further impacted because of unmeasured confounding; however, covariates in the final model reflect those that have been previously described to be associated with NTM acquisition. Finally, our study only evaluated incident NTM isolation in CF rather than NTM lung disease (as this was a rare event); however, NTM isolation alone is an important clinical endpoint in CF.
The potential increased risk of NTM conferred by VDD may have important clinical consequence for the management of individuals with CF. More frequent monitoring of vitamin D concentrations and targeted attempts at aggressive repletion—especially in those who are significantly deficient—may warrant investigation as to whether they would reduce the risk of NTM infection. Further prospective studies with larger populations are warranted to better define the relationship between VDD and the risk of NTM infection and disease in CF.
Supplementary Material
Footnotes
Supported by grants from the National Heart, Lung, and Blood Institute/National Institutes of Health (T32 HL007534–36 and F32HL149178–01) (W.J.R.), Cystic Fibrosis Foundation Student Traineeship Award SUN19HO (Y.S.), K08 HL139994 and Burroughs Wellcome Fund Career Award for Medical Scientists (K.A.C).
Author Contributions: Study conception and initial design: M.T.J. and K.A.C. Methodological input: K.J.P. and N.L. Data acquisition: W.J.R., Y.S., M.N.S., and J.A.N. Data analysis: Y.S. and K.J.P. First draft of the manuscript: W.J.R., Y.S., and K.J.P. Critical revision of the manuscript: A.S., N.L., M.T.J., and K.A.C. Approval of the final version of the manuscript: all authors.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Adjemian J, Olivier KN, Prevots DR. Nontuberculous mycobacteria among patients with cystic fibrosis in the United States: screening practices and environmental risk. Am J Respir Crit Care Med. 2014;190:581–586. doi: 10.1164/rccm.201405-0884OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viviani L, Harrison MJ, Zolin A, Haworth CS, Floto RA. Epidemiology of nontuberculous mycobacteria (NTM) amongst individuals with cystic fibrosis (CF) J Cyst Fibros. 2016;15:619–623. doi: 10.1016/j.jcf.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Martiniano SL, Sontag MK, Daley CL, Nick JA, Sagel SD. Clinical significance of a first positive nontuberculous mycobacteria culture in cystic fibrosis. Ann Am Thorac Soc. 2014;11:36–44. doi: 10.1513/AnnalsATS.201309-310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000;355:618–621. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 5.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 6.Jeon K, Kim S-Y, Jeong B-H, Chang B, Shin SJ, Koh W-J. Severe vitamin D deficiency is associated with non-tuberculous mycobacterial lung disease: a case-control study. Respirology. 2013;18:983–988. doi: 10.1111/resp.12109. [DOI] [PubMed] [Google Scholar]
- 7.Hall WB, Sparks AA, Aris RM. Vitamin d deficiency in cystic fibrosis. Int J Endocrinol. 2010;2010:218691. doi: 10.1155/2010/218691. 10.1155/2010/218691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, et al. The cystic fibrosis foundation patient registry: design and methods of a national observational disease registry. Ann Am Thorac Soc. 2016;13:1173–1179. doi: 10.1513/AnnalsATS.201511-781OC. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 10.Tangpricha V, Kelly A, Stephenson A, Maguiness K, Enders J, Robinson KA, et al. Cystic Fibrosis Foundation Vitamin D Evidence-Based Review Committee. An update on the screening, diagnosis, management, and treatment of vitamin D deficiency in individuals with cystic fibrosis: evidence-based recommendations from the Cystic Fibrosis Foundation. J Clin Endocrinol Metab. 2012;97:1082–1093. doi: 10.1210/jc.2011-3050. [DOI] [PubMed] [Google Scholar]
- 11.Floto A, Olivier KN, Saiman L, Daley CL, Herrmann J-L, Nick JA, et al. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax. 2016;71:i1–i22. doi: 10.1136/thoraxjnl-2015-207360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


