Abstract
Rationale: In the IMPACT (Informing the Pathway of COPD Treatment) trial, single-inhaler fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) triple therapy reduced exacerbation risk versus FF/VI and UMEC/VI and mortality risk versus UMEC/VI. However, pneumonia incidence was higher in the inhaled corticosteroid (FF)–containing arms, raising questions about the relative benefit of exacerbation reduction compared with the increased risk of pneumonia.
Objectives: Determine benefit–risk of the three treatments by evaluating time-to-first and rates of composite exacerbation or pneumonia outcomes.
Methods: We evaluated time-to-first (prespecified) and rates (post hoc) of investigator-reported pneumonia, serious pneumonia leading to hospitalization or death, and the composite endpoints of 1) moderate (required antibiotics/corticosteroids)/severe (hospitalized) exacerbation or pneumonia and 2) severe exacerbation or serious (hospitalized) pneumonia. Analyses were repeated for radiographically confirmed pneumonia (post hoc).
Results: Moderate/severe exacerbations occurred in 47%, 49%, and 50% of patients randomized to FF/UMEC/VI, FF/VI and UMEC/VI, and pneumonias in 8%, 7%, and 5%, respectively. FF/UMEC/VI reduced the risk of combined moderate/severe exacerbation or pneumonia (time-to-first) versus FF/VI (hazard ratio, 0.87 [95% confidence interval (CI), 0.82–0.92]) and UMEC/VI (0.87 [0.81–0.94]), as well as the risk of combined severe exacerbation or serious pneumonia versus UMEC/VI (0.83 [0.72–0.96]). FF/UMEC/VI reduced the rate of combined moderate/severe exacerbation or pneumonia (rate ratio, 0.78 [0.72–0.84]) and combined severe exacerbation or serious pneumonia (rate ratio, 0.76 [0.65–0.89]) versus UMEC/VI. Results were similar for radiographically confirmed pneumonia endpoints.
Conclusions: Despite higher incidence of pneumonia in FF-containing arms, these composite exacerbation/pneumonia outcomes support a favorable benefit–risk profile of FF/UMEC/VI versus FF/VI and UMEC/VI in patients with symptomatic chronic obstructive pulmonary disease and a history of exacerbations.
Keywords: chronic obstructive pulmonary disease, benefit–risk assessment, pneumonia, exacerbations, corticosteroids
Chronic obstructive pulmonary disease (COPD) is a known risk factor for community-acquired pneumonia (1), and factors further enhancing pneumonia risk in this population include older age, prior exacerbation or pneumonia, low body mass index (BMI), and severe airflow limitation (2, 3). Although inhaled corticosteroids (ICSs) reduce the risk of exacerbations of COPD, they also increase the risk of pneumonia, regardless of whether pneumonia is recorded as an investigator-reported adverse event (AE) or based on the presence of chest X-ray–confirmed infiltrates (4, 5). The signs and symptoms of exacerbations and pneumonia overlap and the treatments for the two are similar. However, observational studies suggest that the presence of infiltrates on chest X-ray increases the risk of intensive care unit admission, the need for mechanical ventilation, length of stay, and mortality in patients hospitalized for exacerbations (6, 7).
The current Global Initiative for Obstructive Lung Disease (GOLD) strategy document recommends triple therapy with an ICS, long-acting β2-agonist (LABA), and long-acting muscarinic antagonist (LAMA) for patients with COPD who remain symptomatic or continue to suffer exacerbations despite maintenance treatment with either an ICS/LABA or LABA/LAMA combination (8). The IMPACT (Informing the Pathway of COPD Treatment) trial demonstrated a reduction in the risk of moderate or severe exacerbation with fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) as compared with dual therapy with either FF/VI or UMEC/VI, as well as a lower risk of death compared with UMEC/VI (9–11). IMPACT enrolled patients with documented airflow limitation (forced expiratory volume in 1 second [FEV1]/forced vital capacity <0.70), a significant burden of chronic symptoms as defined by a COPD Assessment Test score of 10 or higher, and a history of exacerbations. The study was designed before the modification to the GOLD strategy that eliminated consideration of lung function for risk assessment; thus, the exacerbation requirement varied based on FEV1. Patients with FEV1 <50% predicted were required to have one or more moderate or severe exacerbations in the year before screening; those with FEV1 between 50% and <80% predicted were required to have two or more moderate or one or more severe exacerbations. Compatible with prior reports, the incidence of investigator-reported pneumonia was higher in patients assigned to ICS-containing arms (8% for FF/UMEC/VI, 7% for FF/VI, and 5% for UMEC/VI) (9). We aimed to determine the overall benefit of exacerbation reduction compared with the risk of pneumonia for triple therapy with FF/UMEC/VI compared with each dual therapy by examining the combined outcome of exacerbation and pneumonia. We also examined whether the benefit–risk was altered based on whether pneumonia was confirmed radiographically and based on the severity of those events.
Methods
The IMPACT trial (GlaxoSmithKline study CTT116855; NCT02164513) was a phase III, randomized, double-blind, parallel-group, multicenter study evaluating the effects of once-daily single-inhaler triple therapy, containing FF/UMEC/VI 100/62.5/25 μg, or once-daily dual therapy (FF/VI 100/25 μg or UMEC/VI 62.5/25 μg), on the rate of moderate/severe exacerbations over 52 weeks in symptomatic patients with COPD and ≥1 moderate/severe exacerbation in the previous year (9). Patients were randomized in a 2:2:1 ratio to FF/UMEC/VI, FF/VI, or UMEC/VI, respectively.
Occurrence of exacerbations during the study was evaluated based on the worsening for ≥2 consecutive days of ≥2 major symptoms (dyspnea, sputum volume, or purulence), or any one major symptom together with any one minor symptom (sore throat, colds [nasal discharge/congestion], fever, increased cough, or wheeze). Moderate exacerbations were defined as worsening of symptoms requiring treatment with antibiotics or oral/systemic corticosteroids. Severe exacerbations were defined as worsening of symptoms resulting in hospitalization or death.
Safety endpoints included the incidence of on-treatment AEs of special interest, defined as AEs that are pharmacologically related to ICSs, LAMAs, or LABAs, allowing for a comprehensive review of safety data not limited to a specific Preferred Term as coded using the Medical Dictionary for Regulatory Activities (MedDRA Version 20.0; International Federation of Pharmaceutical Manufacturers and Associations, Geneva, Switzerland).
Pneumonia AEs of special interest included all investigator-reported pneumonia-related terms and is referred to as investigator-reported pneumonia throughout. Chest radiographs were required by protocol for any investigator-reported moderate/severe exacerbation or pneumonia and were independently reviewed to determine if infiltrates compatible with pneumonia were present. This subset of investigator-reported pneumonia is reported as radiographically confirmed pneumonia throughout. Time to first (TTF) pneumonia and TTF serious pneumonia (resulting in hospitalization, prolonged hospitalization, or death) were analyzed using a Cox proportional hazards model with covariates of treatment group and geographical region. TTF pneumonia or moderate/severe exacerbation and TTF serious pneumonia or severe exacerbation composite endpoints were analyzed using a Cox proportional hazards model with covariates of treatment group, sex, exacerbation history (≤1, ≥2 moderate/severe), smoking status (Screening), geographical region, and postbronchodilator percent predicted FEV1 (Screening). These prespecified endpoints were repeated for radiographically confirmed pneumonia as post hoc analyses.
Kaplan-Meier curves for pneumonia and moderate/severe exacerbations were also produced and repeated for serious pneumonia and severe exacerbations. Post hoc analyses were also performed for rate of pneumonia, pneumonia or moderate/severe exacerbation, and serious pneumonia or severe exacerbation using a generalized linear model assuming a negative binomial distribution with covariates of treatment group and geographical region with the addition of sex, exacerbation history (≤1, ≥2 moderate/severe exacerbations), smoking status (Screening), and postbronchodilator percent predicted FEV1 (Screening) for the composite endpoints. If a patient experienced both a pneumonia and an exacerbation with overlapping duration, then both events were reported with the exception of the composite endpoint, where these were counted as a single event. These analyses were repeated for radiographically confirmed pneumonia and by use of ICS within 3 days before and including the screening date (post hoc). Note that the rate of serious pneumonia or radiographically confirmed serious pneumonia were not performed owing to insufficient number of events.
Results
The overall intent-to-treat (ITT) population comprised 10,355 patients (FF/UMEC/VI, n = 4,151; FF/VI, n = 4,134; UMEC/VI, n = 2,070). Table 1 displays the demographic and clinical characteristics by randomized treatment of the ITT population as well as the characteristics of the patients who had an investigator-reported pneumonia and radiographically confirmed by chest X-ray. Compared with the overall ITT population, patients with an investigator-reported pneumonia were more likely to be older than 65 years of age, be male, have BMI ≤21 kg/m2, have a history of prior pneumonia, have had severe exacerbation in the year before enrollment, have GOLD III/IV airflow limitation, and be enrolled in Asia. There was no clear association of investigator-reported pneumonia with baseline blood eosinophils as has been reported (12). Radiographic confirmation of investigator-reported pneumonia was more common in the ICS-containing arms (FF/UMEC/VI 154/317 [49%], FF/VI 147/292 [50%], UMEC/VI 40/97 [41%]), but the pattern of risk factors was similar to those for investigator-reported pneumonia.
Table 1.
Baseline demographics and clinical characteristics of ITT population and patients with investigator-reported and radiographically confirmed pneumonia
| ITT (N = 10,355) | Patients with Investigator-reported Pneumonia |
Patients with Radiographically Confirmed Pneumonia |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| All Treatment (N = 706) | FF/UMEC/VI (N = 317) | FF/VI (N = 292) | UMEC/VI (N = 97) | All Treatment (N = 341) | FF/UMEC/VI (N = 154) | FF/VI (N = 147) | UMEC/VI (N = 40) | ||
| Age, yr, n (%) | n = 10,355 | n = 706 | n = 317 | n = 292 | n = 97 | n = 341 | n = 154 | n = 147 | n = 40 |
| <65 | 4,742 (46) | 225 (32) | 102 (32) | 87 (30) | 36 (37) | 103 (30) | 44 (29) | 45 (31) | 14 (35) |
| ≥65 | 5,631 (54) | 481 (68) | 215 (68) | 205 (70) | 61 (63) | 238 (70) | 110 (71) | 102 (69) | 26 (65) |
| Sex, F, n (%) | n = 10,355 | n = 706 | n = 317 | n = 292 | n = 97 | n = 341 | n = 154 | n = 147 | n = 40 |
| 3,485 (34) | 183 (26) | 83 (26) | 75 (26) | 25 (26) | 72 (21) | 37 (24) | 29 (20) | 6 (15) | |
| BMI, kg/m2, n (%) | n = 10,352 | n = 706 | n = 317 | n = 292 | n = 97 | n = 341 | n = 154 | n = 147 | n = 40 |
| ≤21 | 1,776 (17) | 179 (25) | 73 (23) | 83 (28) | 23 (24) | 98 (29) | 36 (23) | 51 (35) | 11 (28) |
| >21 | 8,576 (83) | 527 (75) | 244 (77) | 209 (72) | 74 (76) | 243 (71) | 118 (77) | 96 (65) | 29 (73) |
| Current smoker, n (%) | n = 10,355 | n = 706 | n = 317 | n = 292 | n = 97 | n = 341 | n = 154 | n = 147 | n = 40 |
| 3,587 (35) | 206 (29) | 90 (28) | 82 (28) | 34 (35) | 92 (27) | 38 (25) | 38 (26) | 16 (40) | |
| History of pneumonia, n (%) | n = 10,342 | n = 706 | n = 317 | n = 292 | n = 97 | n = 341 | n = 154 | n = 147 | n = 40 |
| 2,343 (23) | 265 (38) | 110 (35) | 118 (40) | 37 (38) | 127 (37) | 57 (37) | 54 (37) | 16 (40) | |
| GOLD stage predicted, n (%) | n = 10,347 | n = 705 | n = 316 | n = 292 | n = 97 | n = 341 | n = 154 | n = 147 | n = 40 |
| I | 22 (<1) | 3 (<1) | 3 (<1) | 0 (0) | 0 (0) | 3 (<1) | 3 (2) | 0 (0) | 0 (0) |
| II | 3,719 (36) | 205 (29) | 96 (30) | 90 (31) | 19 (20) | 97 (28) | 46 (30) | 46 (31) | 5 (13) |
| III | 4,982 (48) | 359 (51) | 157 (50) | 147 (50) | 55 (57) | 168 (49) | 77 (50) | 67 (46) | 24 (60) |
| IV | 1,624 (16) | 138 (20) | 60 (19) | 55 (19) | 23 (24) | 73 (21) | 28 (18) | 34 (23) | 11 (28) |
| Number of moderate exacerbations in year prior, n (%) | n = 10,355 | n = 706 | n = 317 | n = 292 | n = 97 | n = 341 | n = 154 | n = 147 | n = 40 |
| 1 | 3,542 (34) | 244 (35) | 107 (34) | 104 (36) | 33 (34) | 107 (31) | 52 (34) | 44 (30) | 11 (28) |
| ≥2 | 4,877 (47) | 269 (38) | 125 (39) | 107 (37) | 37 (38) | 123 (36) | 56 (36) | 48 (33) | 19 (48) |
| Number of severe exacerbations in year prior, n (%) | n = 10,355 | n = 706 | n = 317 | n = 292 | n = 97 | n = 341 | n = 154 | n = 147 | n = 40 |
| 1 | 2,300 (22) | 220 (31) | 96 (30) | 93 (32) | 31 (32) | 125 (37) | 53 (34) | 60 (41) | 12 (30) |
| ≥2 | 371 (4) | 51 (7) | 28 (9) | 18 (6) | 5 (5) | 22 (6) | 13 (8) | 8 (5) | 1 (3) |
| Baseline blood eosinophils, cells/μl, n (%) | n = 10,333 | n = 705 | n = 317 | n = 292 | n = 96 | n = 341 | n = 154 | n = 147 | n = 40 |
| <150 | 4,482 (43) | 316 (45) | 143 (45) | 133 (46) | 40 (42) | 151 (44) | 66 (43) | 67 (46) | 18 (45) |
| ≥150 | 5,851 (57) | 389 (55) | 174 (55) | 159 (54) | 56 (58) | 190 (56) | 88 (57) | 80 (54) | 22 (55) |
| Geographic region, n (%) | n = 10,355 | n = 706 | n = 317 | n = 292 | n = 97 | n = 341 | n = 154 | n = 147 | n = 40 |
| Western Europe | 3,164 (31) | 154 (22) | 70 (22) | 54 (18) | 30 (31) | 70 (21) | 30 (19) | 29 (20) | 11 (28) |
| Eastern Europe | 685 (7) | 40 (6) | 18 (6) | 15 (5) | 7 (7) | 28 (8) | 13 (8) | 11 (7) | 4 (10) |
| Asia | 1,644 (16) | 210 (30) | 91 (29) | 98 (34) | 21 (22) | 117 (34) | 47 (31) | 59 (40) | 11 (28) |
| North America | 2,639 (25) | 205 (29) | 99 (31) | 86 (29) | 20 (21) | 80 (23) | 45 (29) | 29 (20) | 6 (15) |
| South America | 1,708 (16) | 73 (10) | 29 (9) | 30 (10) | 14 (14) | 29 (9) | 12 (8) | 12 (8) | 5 (13) |
| Other | 515 (5) | 24 (3) | 10 (3) | 9 (3) | 5 (5) | 17 (5) | 7 (5) | 7 (5) | 3 (8) |
Definition of abbreviations: BMI = body mass index; FF = fluticasone furoate; GOLD = Global Initiative for Chronic Obstructive Lung Disease; ITT = intent-to-treat; n = number of patients with available data; UMEC = umeclidinium; VI = vilanterol.
Number of patients in the ITT population: FF/UMEC/VI, N = 4,151; FF/VI, N = 4,134; UMEC/VI, N = 2,070.
Risk of Exacerbation or Pneumonia
In the FF/UMEC/VI, FF/VI, and UMEC/VI groups, the number of patients who experienced a moderate/severe exacerbation up to Week 52 was 1,959 (47%), 2,039 (49%), and 1,036 (50%), respectively (Figure 1A), and that for severe exacerbations was 447 (11%), 461 (11%), and 272 (13%), respectively (Figure 2). The incidence of investigator-reported pneumonia up to Week 52 was higher in ICS- versus non–ICS-containing treatment arms (FF/UMEC/VI, N = 317 [8%]; FF/VI, N = 292 [7%]; UMEC/VI, N = 97 [5%]) (Table 1 and Figure 1A), as was the incidence of investigator-reported serious pneumonia (FF/UMEC/VI, N = 199 [5%]; FF/VI, N = 170 [4%]; UMEC/VI, N = 57 [3%]) (Figure 2A). The cumulative plot of moderate/severe exacerbations and investigator-reported pneumonia events, and of severe exacerbations and investigator-reported serious pneumonia events, are presented in Figures 1B and 2B, respectively.
Figure 1.
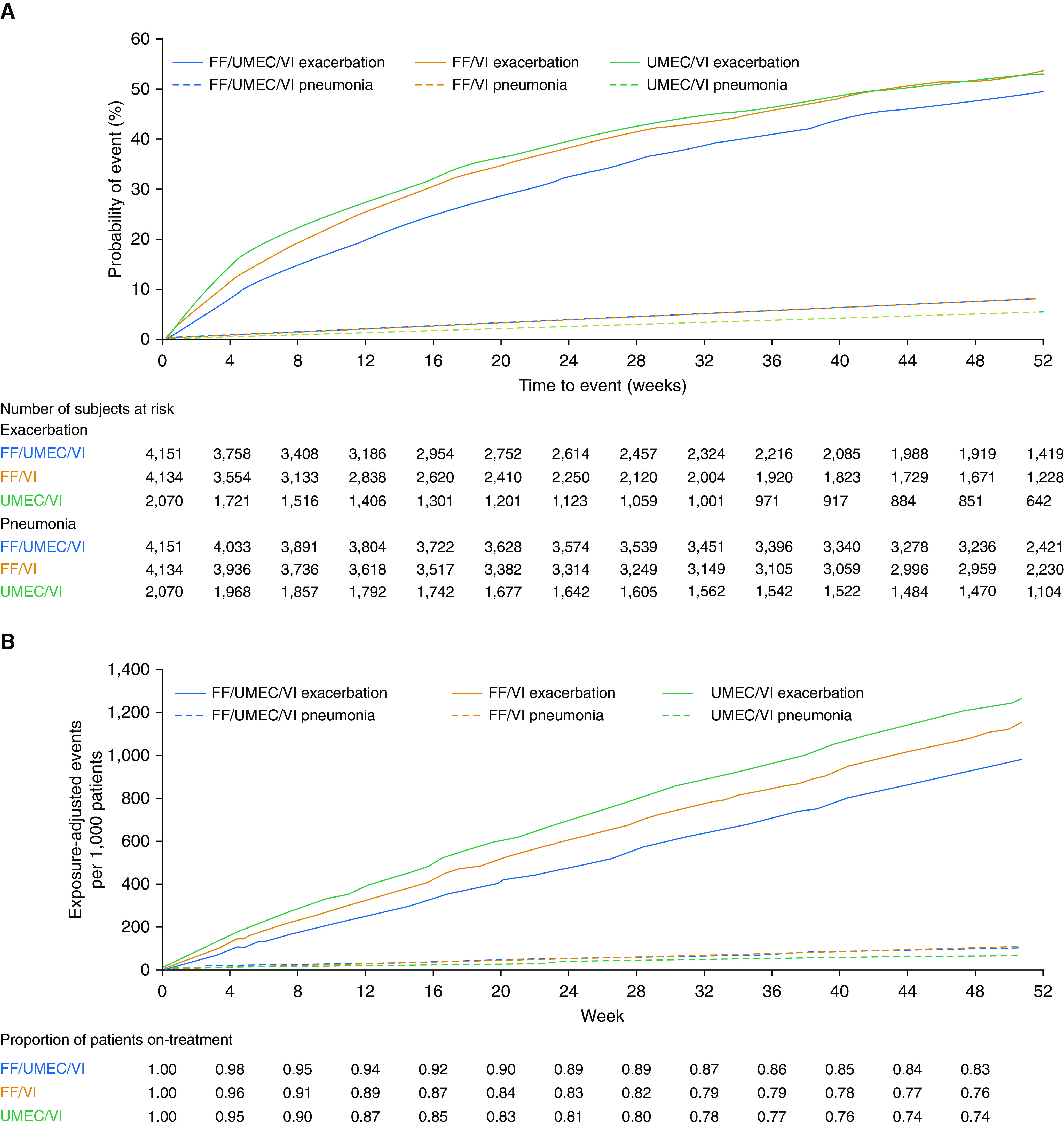
Moderate/severe exacerbation and investigator-reported pneumonia: (A) Kaplan-Meier plot of time-to-first event and (B) cumulative plot. (A) Patients experiencing a moderate/severe exacerbation up to Week 52: FF/UMEC/VI, n = 1,959 (47%); FF/VI, n = 2,039 (49%); UMEC/VI, n = 1,036 (50%). Patients with investigator-reported pneumonia up to Week 52: FF/UMEC/VI, n = 317 (8%); FF/VI, n = 292 (7%); UMEC/VI, n = 97 (5%). FF = fluticasone furoate; UMEC = umeclidinium; VI = vilanterol.
Figure 2.
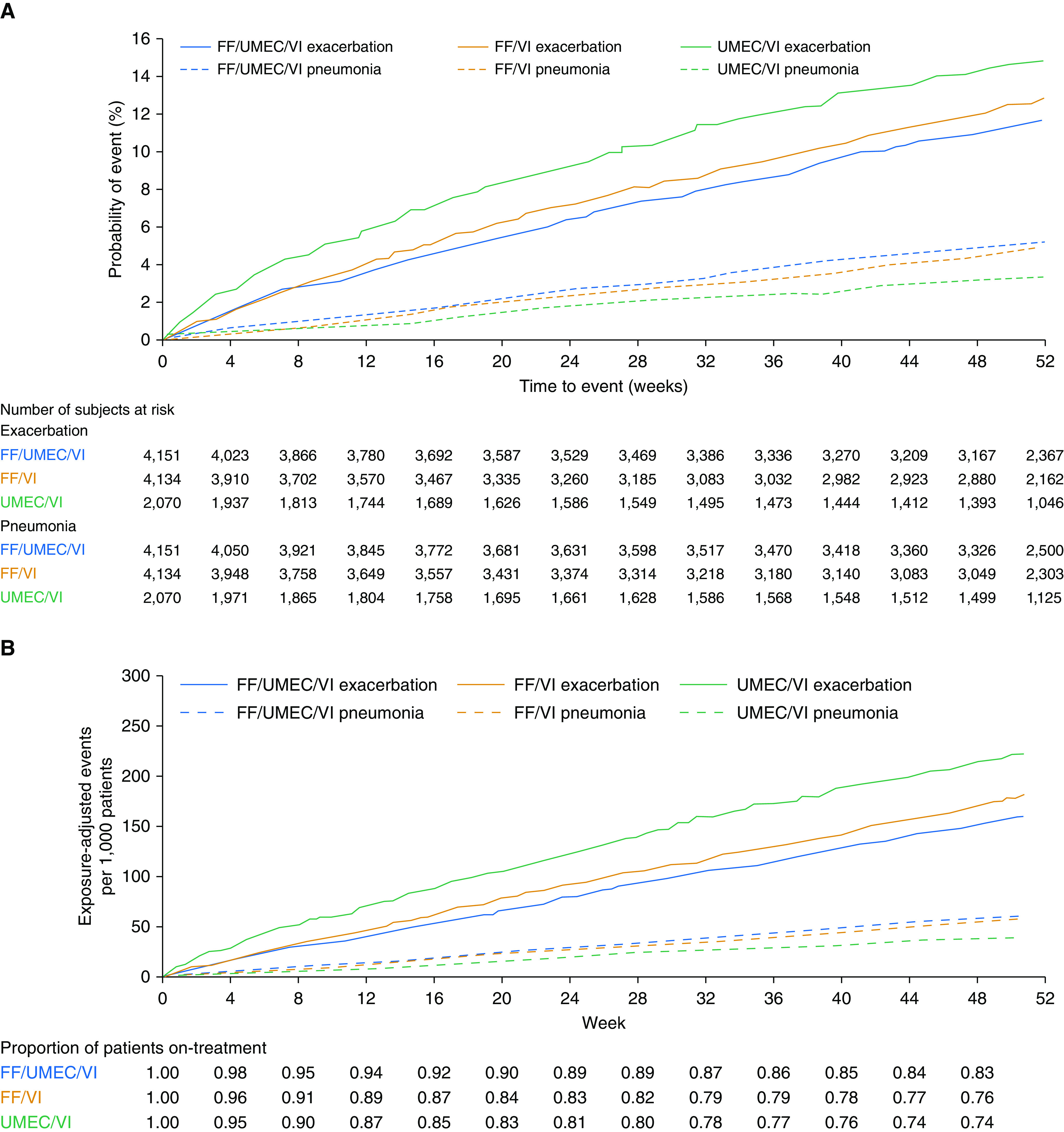
Severe exacerbation and investigator-reported pneumonia resulting in hospitalization/prolonged hospitalization or death: (A) Kaplan-Meier plot of time-to-first event and (B) cumulative plot. (A) Patients experiencing a severe exacerbation up to Week 52: FF/UMEC/VI, n = 447 (11%); FF/VI, n = 461 (11%); UMEC/VI, n = 272 (13%). Patients with investigator-reported pneumonia resulting in hospitalization/prolonged hospitalization or death up to Week 52: FF/UMEC/VI, n = 199 (5%); FF/VI, n = 170 (4%); UMEC/VI, n = 57 (3%). FF = fluticasone furoate; UMEC = umeclidinium; VI = vilanterol.
FF/UMEC/VI versus FF/VI
There was no difference in TTF pneumonia or TTF serious pneumonia between FF/UMEC/VI and FF/VI regardless of whether the pneumonia was investigator reported or radiographically confirmed (Figure 3A). Similarly, there was no difference in the rate ratios for investigator-reported pneumonia and radiographically confirmed pneumonia between FF/UMEC/VI and FF/VI (Figure 3B). By TTF analysis, FF/UMEC/VI reduced the risk of combined investigator-reported pneumonia or moderate/severe COPD exacerbation (hazard ratio [HR], 0.87; 95% confidence interval [CI], 0.82–0.92) and radiographically confirmed pneumonia or moderate/severe exacerbation (HR, 0.85; 95% CI, 0.80–0.91) compared with FF/VI (Figure 3A). Similar differences were observed for the rates of combined investigator-reported and radiographically confirmed pneumonia or moderate/severe exacerbations (rate ratio, 0.86; 95% CI, 0.81–0.91 and 0.85; 95% CI, 0.80–0.91, respectively) (Figure 3B). No differences in the rates of combined serious pneumonia or severe exacerbation were observed.
Figure 3.
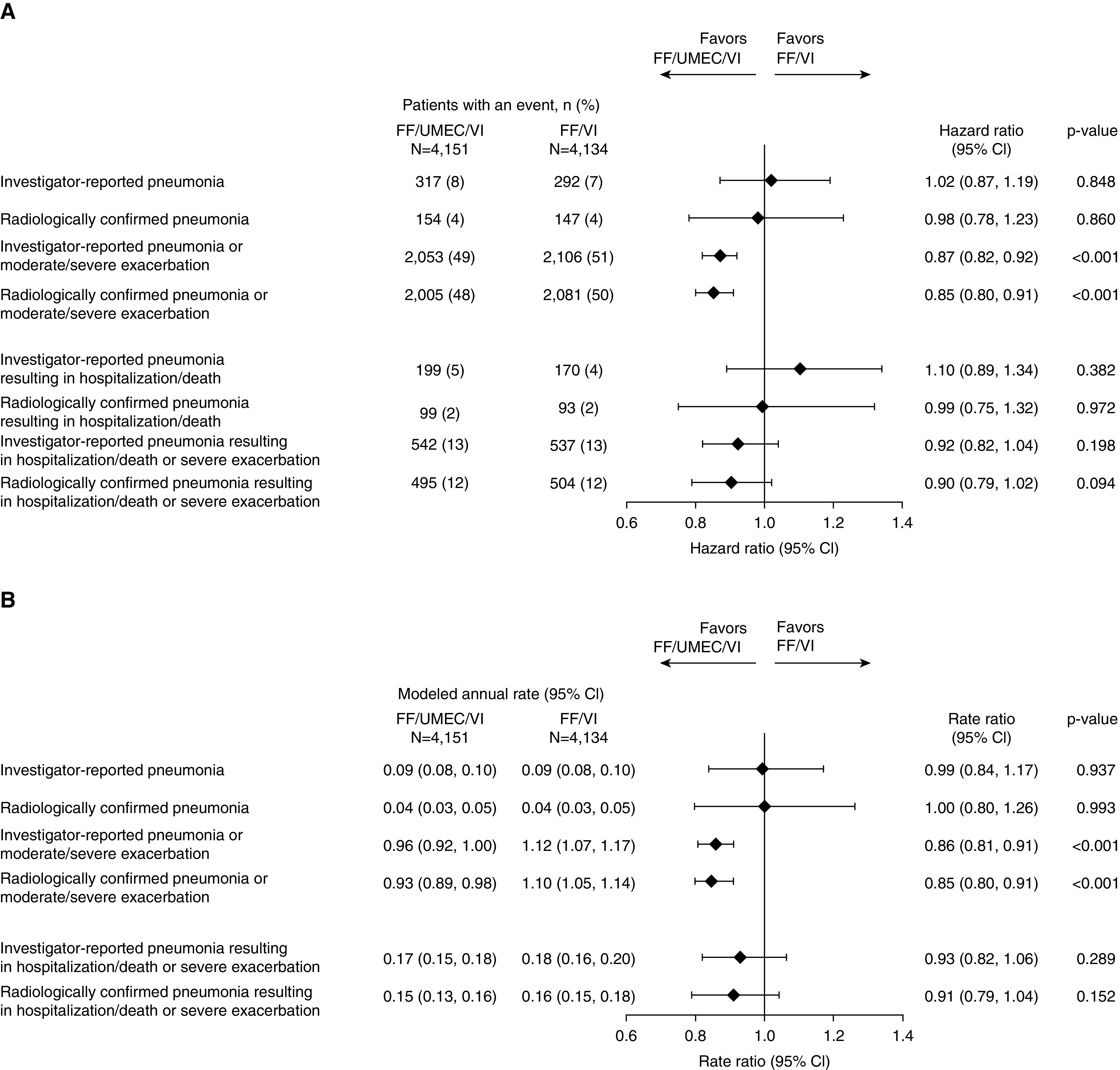
Forest plot of (A) hazard ratios for time-to-first and (B) rate ratios for pneumonia alone or combined with exacerbation: FF/UMEC/VI versus FF/VI. CI = confidence interval; FF = fluticasone furoate; UMEC = umeclidinium; VI = vilanterol.
FF/UMEC/VI versus UMEC/VI
By TTF analysis, FF/UMEC/VI increased the risk of investigator-reported pneumonia (HR, 1.53; 95% CI, 1.22–1.92) and investigator-reported serious pneumonia (HR, 1.62; 95% CI, 1.21–2.17) compared with UMEC/VI. An increase in the risk of radiographically confirmed pneumonia was also observed with FF/UMEC/VI (Figure 4A). The rates of pneumonia and radiographically confirmed pneumonia were also higher with FF/UMEC/VI than with UMEC/VI (rate ratio, 1.53; 95% CI, 1.21–1.94 and rate ratio, 1.90; 95% CI, 1.33–2.72, respectively) (Figure 4B). By both TTF and rates, FF/UMEC/VI reduced the risk of combined pneumonia or exacerbation, radiographically confirmed pneumonia or exacerbation, serious pneumonia or severe exacerbation, and serious radiographically confirmed pneumonia or severe exacerbation compared with UMEC/VI (Figures 4A and 4B).
Figure 4.
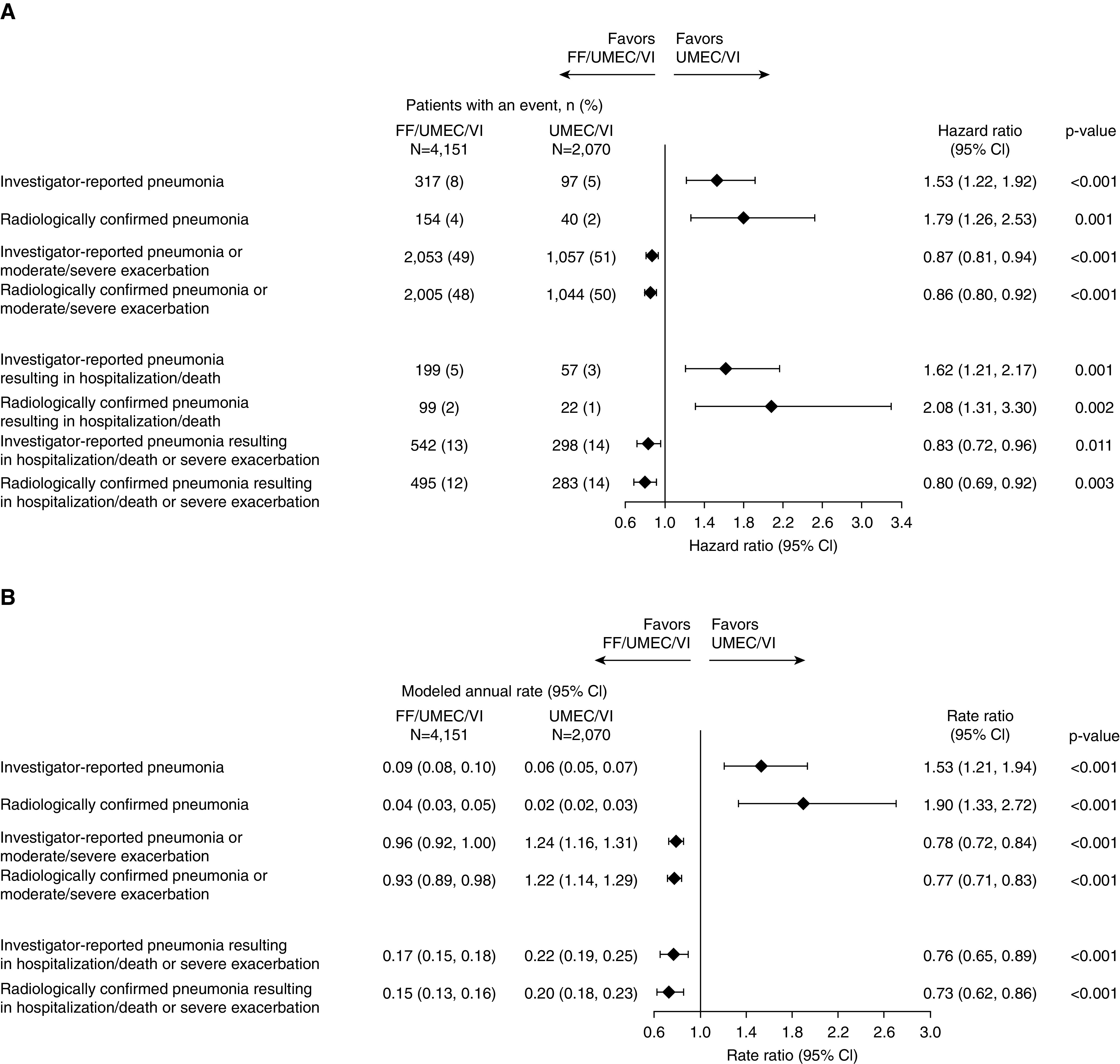
Forest plot of (A) hazard ratios for time-to-first and (B) rate ratios for pneumonia alone or combined with exacerbation: FF/UMEC/VI versus UMEC/VI. CI = confidence interval; FF = fluticasone furoate; UMEC = umeclidinium; VI = vilanterol.
FF/VI versus UMEC/VI
The occurrence of investigator-reported pneumonia (TTF and rate), radiographically confirmed pneumonia (TTF and rate), serious pneumonia (TTF), and radiographically confirmed serious pneumonia (TTF) was lower with UMEC/VI than with FF/VI (Figures 5A and 5B). There was no difference between FF/VI and UMEC/VI in the TTF combined pneumonia or exacerbation endpoints, with the exception of the serious pneumonia and severe exacerbation composite endpoint and the corresponding radiographically confirmed endpoint where a numerical decrease in risk with FF/VI was observed (Figure 5A), but the rate of combined investigator-reported or radiographically confirmed pneumonia with moderate/severe exacerbation was lower with FF/VI (rate ratio, 0.91; 95% CI, 0.84–0.98 and 0.90; 95% CI, 0.83–0.97, respectively), as were the rates of combined investigator-reported or radiographically confirmed serious pneumonia with severe exacerbation (rate ratio, 0.82; 95% CI, 0.70–0.96 and 0.81; 95% CI, 0.68–0.95) (Figure 5B).
Figure 5.
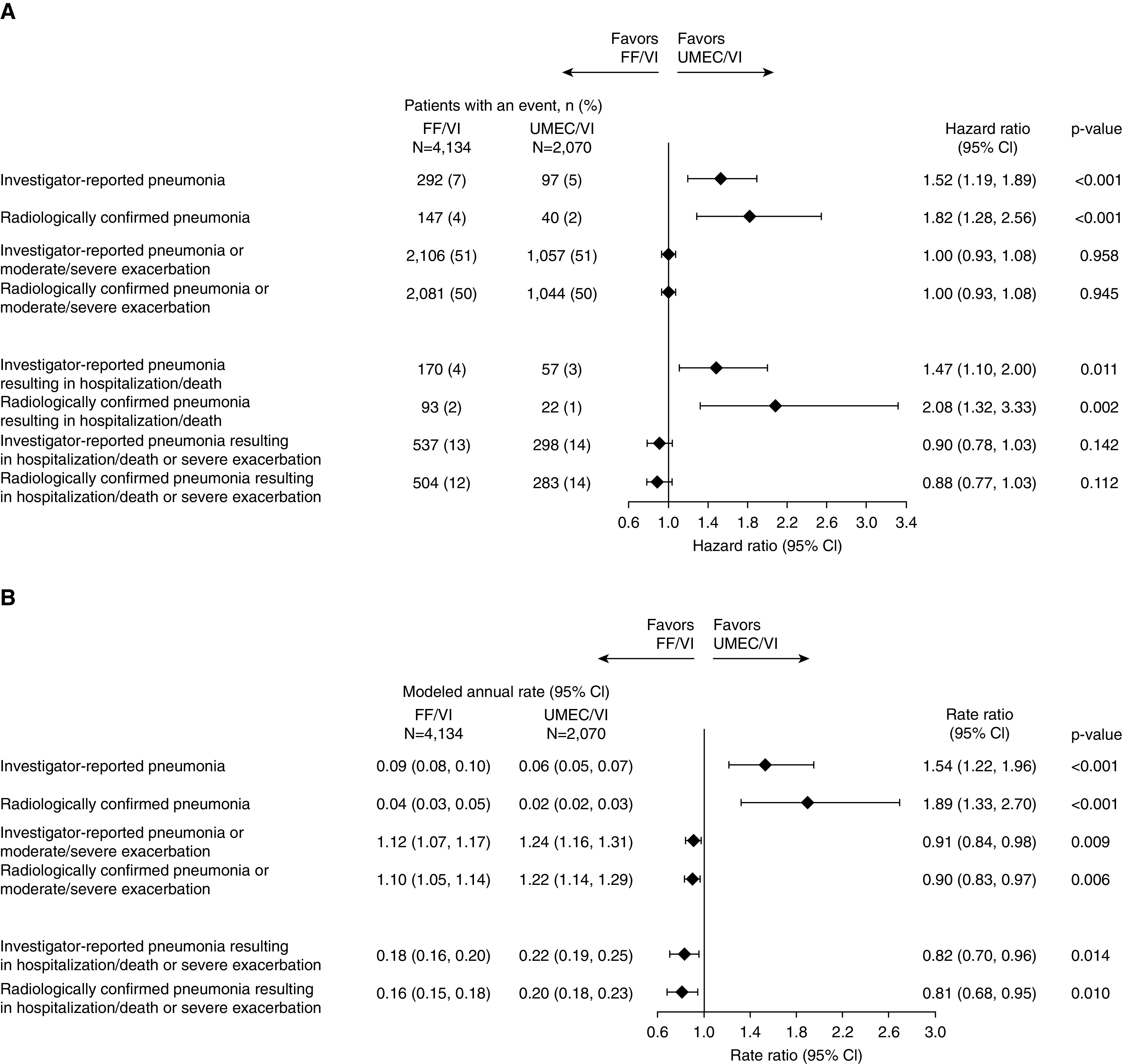
Forest plot of (A) hazard ratios for time-to-first and (B) rate ratios for pneumonia alone or combined with exacerbation: FF/VI versus UMEC/VI. CI = confidence interval; FF = fluticasone furoate; UMEC = umeclidinium; VI = vilanterol.
Analyses by Baseline ICS Use
Approximately 77% (N = 7,960/10,355) of patients enrolled in IMPACT were taking ICSs within 3 days before screening, and the overall rates of moderate/severe exacerbations or investigator-reported pneumonia after randomization were higher in ICS users compared with nonusers across study arms (Table E1 in the online supplement). The same was seen for rates of severe exacerbations and serious investigator-reported pneumonia (Table E1). Among ICS users, FF/UMEC/VI reduced the rate of moderate/severe exacerbations or pneumonia compared with FF/VI (rate ratio, 0.85; 95% CI, 0.80–0.91) and UMEC/VI (rate ratio, 0.74; 95% CI, 0.68–0.80) (Table E1 and Figure E1A). Among non-ICS users, FF/UMEC/VI reduced the rate of moderate/severe exacerbations or pneumonia compared with FF/VI (rate ratio, 0.87; 95% CI, 0.76–1.00), but there was no difference in the rate of moderate/severe exacerbation or pneumonia in the FF/UMEC/VI and UMEC/VI arms (rate ratio, 0.94; 95% CI, 0.80–1.12) (Table E1 and Figure E1B). FF/UMEC/VI reduced the risk of the combined endpoint of severe exacerbation or serious investigator-reported pneumonia compared with UMEC/VI in ICS users (rate ratio, 0.73; 95% CI, 0.61–0.87) but not in non-ICS users (rate ratio, 0.87; 95% CI, 0.60–1.24), and there was no significant difference between FF/UMEC/VI and FF/VI for this endpoint in either ICS use subgroup (Table E1 and Figures E2A and E2B).
Discussion
This analysis of the results of the IMPACT study confirms multiple prior studies showing that although ICSs reduce the risk of acute exacerbations, they also increase the risk of pneumonia, regardless of whether pneumonia is captured as an investigator-reported adverse event or confirmed with chest radiographs (4, 5, 13). However, as we now demonstrate, the risk of the combined pneumonia or exacerbation endpoint was lower with FF/UMEC/VI compared with both FF/VI and UMEC/VI. The benefits of triple therapy compared with UMEC/VI were most pronounced in those who were taking ICSs at baseline, reflecting their higher baseline risk of exacerbations and reinforcing the GOLD recommendations supporting ICS use in those with frequent events. These findings, along with the lower risk of death in those randomized to triple therapy (9), support a favorable benefit–risk profile of once-daily FF/UMEC/VI compared with FF/VI and UMEC/VI in symptomatic patients with COPD who are at risk for exacerbation.
The incidence of pneumonia in patients randomized to FF-containing treatments was between 1.5- and twofold the incidence in patients randomized to UMEC/VI, regardless of pneumonia severity or whether the pneumonia was radiographically confirmed. This is comparable to the increased risk reported in some (4), but not all (14), prior studies of FF/VI versus VI, as well as with fluticasone propionate compared with salmeterol (2) and indacaterol/glycopyrronium (13). There has been debate about whether this is an FF- or fluticasone propionate–specific risk, but differences in study populations, event definitions, and reporting requirements confound comparisons with other molecules, and a Cochrane Review and an Assessment Report issued by the European Medicines Agency have concluded that pneumonia is likely an ICS-related class effect (5, 15).
As has been the case in other studies (4), between 40% and 50% of investigator-reported pneumonias were confirmed on chest radiographs submitted as part of the protocol. This rate was somewhat higher in patients randomized to FF-containing treatments, perhaps suggesting differences in the clinical presentation of respiratory events in those receiving ICSs. Although the overall results were not impacted by the definition of pneumonia that was used, investigator-reported pneumonia was viewed as the most conservative endpoint as individual investigators may have had access to clinical or radiographic data, including follow-up chest X-rays or computed tomography scans, not available for independent review.
We did not identify new risk factors for pneumonia but confirmed many that have been previously reported including older age, male sex, prior pneumonia, low BMI, and more severe airflow limitation (2, 4, 16). These risks were similar regardless of whether pneumonia was recorded as investigator reported or X-ray confirmed. We did not identify current smoking as a clear risk factor, although this has been reported in prior studies in the general population (1) as well as in some COPD trials (4). It is possible that the effect of smoking was confounded by the fact that patients with more severe airflow limitation and at higher risk for pneumonia were more likely to be former smokers.
There was also no relationship between blood eosinophils and the risk of pneumonia, as has been reported (12). This contrasts with data from the Copenhagen General Population Study that demonstrated an adjusted risk for pneumonia of 2.17 in individuals with COPD, FEV1 <50%, and blood eosinophils greater than 340 cells/μL compared with those with counts less than that threshold (17). The adjusted risk was even higher (4.52) in those with elevated blood eosinophils, COPD, FEV1 <70% predicted, and recent exacerbation. It also contrasts with a pooled analysis of trials that found a higher risk of pneumonia in patients with COPD and blood eosinophils <2% (18). It is difficult to reconcile these disparate results, but the current data suggest that blood eosinophils do not affect the risk of pneumonia in patients meeting inclusion criteria for IMPACT, regardless of treatment assignment.
Although the use of triple therapy was associated with a reduced risk of combined pneumonia and exacerbation events, it could be argued that the overall benefit–risk is not favorable because pneumonic exacerbations are associated with worse outcomes than nonpneumonic events. Indeed, data from the European COPD audit found that the presence of infiltrates on admission for COPD exacerbation, which occurred in 19% of more than 14,000 cases reviewed, was associated with longer length of stay, more severe acidosis, and higher adjusted mortality than exacerbations without infiltrates (odds ratio for death, 1.36; 95% CI, 1.20–1.55) (6).
Although adjusted for in the analysis, the presence of infiltrates was also associated with other factors that might influence outcomes including older age, overall and cardiovascular comorbidity, and frequent admission in the year prior, and thus, residual confounding related to these or other characteristics could affect the estimates of risk. It is important to note that the presence of infiltrates does not definitively indicate a pneumonia as they may be caused by other processes including pulmonary edema, atelectasis, lung cancer, or bleeding that have no association with inhaled steroids but may relate to prognosis. In the European COPD audit, it is notable that the presence of infiltrates was not associated with prior use of inhaled steroids. A similar 2014 UK COPD Audit found the same 19% rate of consolidation at the time of admission for exacerbation and again the risk of mortality in that group was higher than that in those without infiltrates (6.7% vs. 3.6%) (19, 20). The presence of consolidation is also included in the Dyspnea, Eosinopenia, Consolidation, Acidemia, and Atrial Fibrillation (DECAF) prognostic scoring system for in-hospital mortality during exacerbations that has been prospectively validated (21, 22).
Prior observational studies have also shown that hospitalized pneumonic exacerbations are associated with a stronger inflammatory response than nonpneumonic events (23) as well as a greater need for intensive care unit admission and mechanical ventilation (7, 23), but at least one report suggests that the short- and long-term consequences of each are generally similar but with a higher risk of 30-day readmission in those without infiltrates on chest radiograph (23).
Less is known about differences in pathobiology and outcomes for exacerbations with and without infiltrates treated in the outpatient setting, although data from Williams and colleagues suggest a comparable rate of detected infiltrates of 20% and again a greater inflammatory response in those cases (24). That study also demonstrated no major differences in bacterial detection or lung microbiota between these groups, suggesting exacerbations and pneumonia occur along a continuum rather than as distinct entities. Despite convincing data that ICSs increase the risk of pneumonia, and that exacerbations associated with infiltrates appear associated with worse outcomes, we observed lower overall mortality in patients randomized to FF/UMEC/VI than to UMEC/VI, supporting an overall benefit to treatment despite an increased pneumonia risk (9). This is compatible with the results of the majority of observational and randomized trials showing either no difference or reduced mortality in patients with COPD taking ICSs who develop pneumonia (25).
Limitations
There are several limitations to our analysis. Despite the availability of clinical summaries for all pneumonias and exacerbations requiring hospitalization, it was not possible to directly compare the manifestations, severity, or outcomes of these events. As such, it is not possible to draw conclusions about the relative prognostic implications of these episodes, and in these analyses, the benefits of exacerbation reduction are given equal weight to the risk of pneumonia. The results of these analyses do not inform the relative exacerbation benefit and pneumonia risk of ICS-containing treatments in patients with COPD not meeting the IMPACT eligibility criteria.
Conclusions
In summary, there was an increased risk of pneumonia in the FF-containing arms in patients at risk for exacerbations enrolled in IMPACT. However, FF/UMEC/VI reduced the overall risk and rate of combined exacerbation and pneumonia events as well as overall mortality compared with UMEC/VI.
Supplementary Material
Footnotes
Supported by GlaxoSmithKline (study number CTT116855). The funders of the study had a role in the study design, data analysis, data interpretation, and writing of the report. Editorial support (in the form of assembling figures, collating author comments, and grammatical editing) was provided by Chrystelle Rasamison, at Fishawack Indicia Ltd., UK, and was funded by GlaxoSmithKline. D.S. is supported by the National Institute for Health Research Manchester Biomedical Research Centre.
Author Contributions: The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors, take responsibility for the integrity of the work as a whole, contributed to the writing and reviewing of the manuscript, and have given final approval for the version to be published. All authors had full access to the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. M.T.D., G.J.C., and D.M.G.H. were involved in the acquisition of data and data analysis/interpretation. C.C., N.C.D., M.K.H., C.E.J., S.K., D.L., D. A. Lomas, N.M., F.J.M., D.S., R.A.W., and P.L. were involved in data analysis/interpretation. D. A. Lipson was involved in conception/design and data analysis/interpretation.
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Almirall J, Serra-Prat M, Bolíbar I, Balasso V. Risk factors for community-acquired pneumonia in adults: a systematic review of observational studies. Respiration. 2017;94:299–311. doi: 10.1159/000479089. [DOI] [PubMed] [Google Scholar]
- 2.Crim C, Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J. 2009;34:641–647. doi: 10.1183/09031936.00193908. [DOI] [PubMed] [Google Scholar]
- 3.Müllerova H, Chigbo C, Hagan GW, Woodhead MA, Miravitlles M, Davis KJ, et al. The natural history of community-acquired pneumonia in COPD patients: a population database analysis. Respir Med. 2012;106:1124–1133. doi: 10.1016/j.rmed.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Crim C, Dransfield MT, Bourbeau J, Jones PW, Hanania NA, Mahler DA, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Ann Am Thorac Soc. 2015;12:27–34. doi: 10.1513/AnnalsATS.201409-413OC. [DOI] [PubMed] [Google Scholar]
- 5.Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;3:CD010115. doi: 10.1002/14651858.CD010115.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saleh A, López-Campos JL, Hartl S, Pozo-Rodríguez F, Roberts CM European COPD Audit team. The effect of incidental consolidation on management and outcomes in COPD exacerbations: data from the European COPD audit. PLoS One. 2015;10:e0134004. doi: 10.1371/journal.pone.0134004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieberman D, Lieberman D, Gelfer Y, Varshavsky R, Dvoskin B, Leinonen M, et al. Pneumonic vs nonpneumonic acute exacerbations of COPD. Chest. 2002;122:1264–1270. doi: 10.1378/chest.122.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53:1900164. doi: 10.1183/13993003.00164-2019. [DOI] [PubMed] [Google Scholar]
- 9.Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, et al. IMPACT Investigators. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378:1671–1680. doi: 10.1056/NEJMoa1713901. [DOI] [PubMed] [Google Scholar]
- 10.Lipson DA, Criner G, Day N, Dransfield MT, Halpin DMG, Han M, et al. Reduction in the risk of all-cause mortality with fluticasone furoate/umeclidinium/vilanterol compared to umeclidinium/vilanterol in IMPACT including previously missing or censored vital status data [abstract] Am J Respir Crit Care Med. 2020;201:A7344. [Google Scholar]
- 11.Lipson DA, Crim C, Criner GJ, Day NC, Dransfield MT, Halpin DMG, et al. Reduction in all-cause mortality with fluticasone furoate/umeclidinium/vilanterol in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;201:1508–1516. doi: 10.1164/rccm.201911-2207OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pascoe S, Barnes N, Brusselle G, Compton C, Criner GJ, Dransfield MT, et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. Lancet Respir Med. 2019;7:745–756. doi: 10.1016/S2213-2600(19)30190-0. [DOI] [PubMed] [Google Scholar]
- 13.Wedzicha JA, Banerji D, Chapman KR, Vestbo J, Roche N, Ayers RT, et al. FLAME Investigators. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374:2222–2234. doi: 10.1056/NEJMoa1516385. [DOI] [PubMed] [Google Scholar]
- 14.Vestbo J, Anderson JA, Brook RD, Calverley PM, Celli BR, Crim C, et al. SUMMIT Investigators. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387:1817–1826. doi: 10.1016/S0140-6736(16)30069-1. [DOI] [PubMed] [Google Scholar]
- 15.European Medicines Agency; Pharmacovigilance Risk Assessment Committee (PRAC) London, UK: European Medicines Agency; 2016. Inhaled corticosteroids (ICS) containing medicinal products indicated in the treatment of chronic obstructive pulmonary disease (COPD) assessment report. [accessed 2019 Nov 11]. Available from: https://www.ema.europa.eu/documents/referral/inhaled-corticosteroids-article-31-referral-ema-completes-review-inhaled-corticosteroids-chronic_en.pdf. [Google Scholar]
- 16.Agusti A, Fabbri LM, Singh D, Vestbo J, Celli B, Franssen FME, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J. 2018;52:1801219. doi: 10.1183/13993003.01219-2018. [DOI] [PubMed] [Google Scholar]
- 17.Vedel-Krogh S, Nordestgaard BG, Lange P, Vestbo J, Nielsen SF. Blood eosinophil count and risk of pneumonia hospitalisations in individuals with COPD. Eur Respir J. 2018;51:1800120. doi: 10.1183/13993003.00120-2018. [DOI] [PubMed] [Google Scholar]
- 18.Pavord ID, Lettis S, Anzueto A, Barnes N. Blood eosinophil count and pneumonia risk in patients with chronic obstructive pulmonary disease: a patient-level meta-analysis. Lancet Respir Med. 2016;4:731–741. doi: 10.1016/S2213-2600(16)30148-5. [DOI] [PubMed] [Google Scholar]
- 19.Hurst JR. Consolidation and exacerbation of COPD. Med Sci (Basel) 2018;6:E44. doi: 10.3390/medsci6020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Royal College of Physicians. London, UK: National COPD Audit Programme; 2014. COPD: who cares when it matters most? – outcomes report 2014. [accessed 2018 Jul 12]. Available from: https://www.rcplondon.ac.uk/projects/outputs/copd-who-cares-when-it-matters-most-outcomes-report-2014. [Google Scholar]
- 21.Echevarria C, Steer J, Heslop-Marshall K, Stenton SC, Hickey PM, Hughes R, et al. Validation of the DECAF score to predict hospital mortality in acute exacerbations of COPD. Thorax. 2016;71:133–140. doi: 10.1136/thoraxjnl-2015-207775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steer J, Gibson J, Bourke SC. The DECAF Score: predicting hospital mortality in exacerbations of chronic obstructive pulmonary disease. Thorax. 2012;67:970–976. doi: 10.1136/thoraxjnl-2012-202103. [DOI] [PubMed] [Google Scholar]
- 23.Huerta A, Crisafulli E, Menéndez R, Martínez R, Soler N, Guerrero M, et al. Pneumonic and nonpneumonic exacerbations of COPD: inflammatory response and clinical characteristics. Chest. 2013;144:1134–1142. doi: 10.1378/chest.13-0488. [DOI] [PubMed] [Google Scholar]
- 24.Williams NP, Ostridge K, Devaster JM, Kim V, Coombs NA, Bourne S, et al. AERIS Study Group. Impact of radiologically stratified exacerbations: insights into pneumonia aetiology in COPD. Respir Res. 2018;19:143. doi: 10.1186/s12931-018-0842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Festic E, Scanlon PD. Incident pneumonia and mortality in patients with chronic obstructive pulmonary disease: a double effect of inhaled corticosteroids? Am J Respir Crit Care Med. 2015;191:141–148. doi: 10.1164/rccm.201409-1654PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


