Ventilator-associated pneumonia (VAP) is the most commonly diagnosed infection among critically ill patients, with associated all-cause mortality rates of 20–50% (1–3). Prevention of VAP represents a cornerstone of infection prevention efforts and is a benchmark for hospital performance reporting (4). Although absolute mortality associated with VAP diagnosis is substantial, the excess mortality directly conferred by VAP itself remains a matter of debate (5, 6). VAP is fundamentally a complication of critical illness; thus, the substantial mortality experienced by patients with VAP is in no small part a consequence of their underlying critical illness.
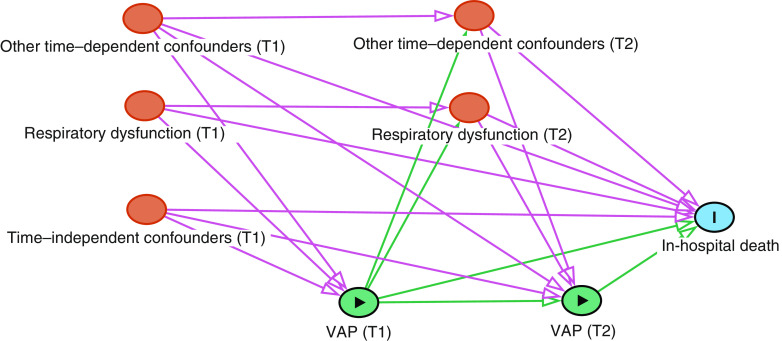
In this issue of AnnalsATS, Steen and colleagues (pp. 830–837) set out to identify rates of preventable mortality due to VAP (7). Prior studies have produced disparate estimates of VAP-attributable mortality, in part because of heterogeneity in VAP definitions and therapeutic approaches as well as potential confounding by severity of illness and other underlying differences between those who do and those who do not develop VAP. As the authors note, identifying deaths attributable to VAP is challenging for several additional reasons, impacting study design. First, the cumulative risk of VAP increases over time after intubation. This time dependence leads to biases, including immortal time bias, given that patients developing VAP must necessarily remain alive and under follow-up long enough to be diagnosed (8). Second, the outcome, death, is subject to the competing “risk” of discharge home. Failing to account for this competing risk leads to inflated estimates of VAP-associated mortality (9). Third, measuring the causal effect of perfect VAP prevention requires adjusting for factors associated with both VAP development and with death (i.e., confounders). Many such confounders (e.g., severity of respiratory dysfunction) also vary over time and, importantly, are themselves affected by VAP. This necessitates the use of analytical methods that are robust to such time-dependent confounding and exposure–confounder feedback (Figure 1) (10).
Figure 1.
Directed acyclic graph featuring time-dependent confounding, time-independent (e.g., baseline) confounding, and exposure–confounder feedback (20). T1 = Time 1; T2 = Time 2; VAP = ventilator-associated pneumonia.
Identifying the decrement in mortality that would result if all cases of VAP were successfully prevented requires comparing the observed mortality among those with VAP to the unobserved (i.e., counterfactual) rate of mortality that would have occurred had those patients not developed VAP. To accurately identify this counterfactual rate while illustrating the consequences of the sources of bias described above, the authors conduct four sequential analyses that differ in their consideration of both the time dependence of VAP onset and the temporal ordering of relevant confounders. Their ultimate approach is state of the art for addressing such complex temporal causal structures. Through its application, the authors found that fewer than 1 in 25 deaths among ventilated patients could be eliminated with perfect VAP prevention, with a majority of these occurring in the first 30 days of mechanical ventilation. Importantly, the authors also find that failure to address the time dependence both of VAP and of relevant confounders leads to potentially inflated estimates of VAP-attributable mortality.
These findings are not without important caveats. First, measured mortality attributable to VAP is dependent on both the incidence of VAP and on observed mortality among patients developing VAP. That VAP occurred in only 7.7% of patients, with more than 80% of these patients surviving to Day 60, is a testament to the existing quality of VAP prevention efforts and other supportive care in the study intensive care units (ICUs). Put another way, a quick way to increase the attributable mortality due to VAP would be to reverse decades of progress in ICU supportive care and VAP preventative efforts. Second, the authors leverage rich, longitudinal clinical data to account for a number of potentially important baseline and time-variant confounders. Their findings are contingent on the untestable assumption that patients developing VAP and those not developing VAP are otherwise comparable with respect to their mortality risk after accounting for these differences. Lastly, the principal determinant of VAP outcomes is timing of appropriate antimicrobial therapy (11). VAP mortality rates may therefore be contingent on local antimicrobial resistance rates and prescribing patterns, and thus generalization of a single-center experience is challenging.
Despite these limitations, this work has several important implications. The findings by White and colleagues are an important contribution to the ongoing question of the extent to which patients die with rather than of VAP. The low attributable mortality observed in this study should fuel ongoing conversation regarding the cost-effectiveness of VAP preventative strategies (12). Importantly, however, these results should not deemphasize the importance of VAP prevention efforts as a whole. For one, data from prospective randomized controlled trials of VAP prevention strategies have produced higher estimates of attributable VAP mortality than those reported in this study, ranging from 9% to 13% (13, 14). Second, although the excess mortality conferred by VAP may indeed be more modest than historically estimated, VAP remains a major source of morbidity, resource use, and healthcare expense among mechanically ventilated patients. In addition, treatment of pneumonia accounts for 50–70% of antibiotic usage within the ICU, and thus VAP prevention may curb both antimicrobial exposures and consequent rates of drug resistance (15, 16).
The results of this study also underscore the present limitations of our ability to confidently diagnose VAP. Studies using multidisciplinary expert case review or autopsy findings as reference gold standards demonstrate that a substantial portion of ICU patients treated for VAP are, in fact, misdiagnosed (17, 18). Multiple interventional studies have demonstrated significant reductions in VAP rates without any associated declines in outcomes such as duration of mechanical ventilation, hospital length of stay, or mortality (19). Prevention efforts may therefore lead to reductions in what we believe is VAP but are in fact antibiotic-nonresponsive VAP mimics, seemingly diluting the potential magnitude of attributable VAP mortality.
More broadly, investigators focused on the quality of hospital and ICU care are likely to encounter such time-dependent causal structures frequently. Decisions to start or stop a medication in response to evolving illness severity, transfer a patient to the ICU in the setting of an acute decline, or intubate and initiate mechanical ventilation in response to worsening oxygenation all present complex temporal relationships between exposures or treatments, potential confounders, and outcomes. As the authors demonstrate, observational analyses naive to these relationships risk yielding biased, incorrect results. Such results might misinform later prospective trials, or, for topics in which such trials are difficult or impossible, lead to confusion, inefficiencies, and potential harm. Rich, longitudinal data with high temporal resolution are increasingly available, enabling the use of methods attentive to these complex causal structures to arrive at more accurate conclusions.
Accurately appraising the true burden of VAP has been a central challenge to infection prevention efforts over the past few decades. Although this study does not definitively close the debate on VAP-attributable mortality, it offers a novel and nuanced contribution to our appraisal of the issue. The time for such analyses to become more commonplace—those attending to time-dependent confounding in health care–associated infections—is long overdue.
Supplementary Material
Footnotes
Supported by the National Heart, Lung, and Blood Institute grant F32HL149337 (A.A.). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Magill SS, O’Leary E, Janelle SJ, Thompson DL, Dumyati G, Nadle J, et al. Emerging Infections Program Hospital Prevalence Survey Team. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med. 2018;379:1732–1744. doi: 10.1056/NEJMoa1801550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muscedere JG, Day A, Heyland DK. Mortality, attributable mortality, and clinical events as end points for clinical trials of ventilator-associated pneumonia and hospital-acquired pneumonia. Clin Infect Dis. 2010;51:S120–S125. doi: 10.1086/653060. [DOI] [PubMed] [Google Scholar]
- 3.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papazian L, Klompas M, Luyt C-E. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med. 2020;46:888–906. doi: 10.1007/s00134-020-05980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muscedere J. Ventilator-associated pneumonia and mortality: the controversy continues. Crit Care Med. 2009;37:2845–2846. doi: 10.1097/CCM.0b013e3181aff7db. [DOI] [PubMed] [Google Scholar]
- 6.Carlet J. Dying from or with a nosocomial pneumonia in the intensive care unit? Crit Care Med. 2001;29:2392–2394. doi: 10.1097/00003246-200112000-00029. [DOI] [PubMed] [Google Scholar]
- 7.Steen J, Vansteelandt S, De Bus L, Depuydt P, Gadeyne B, Benoit DD, et al. Attributable mortality of ventilator-associated pneumonia: replicating findings, revisiting methods. Ann Am Thorac Soc. 2021;18:830–837. doi: 10.1513/AnnalsATS.202004-385OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor SP, Kowalkowski MA, Admon AJ. Timing is everything: the importance of alignment of time anchors for observational causal inference research. Ann Am Thorac Soc. 2021;18:769–772. doi: 10.1513/AnnalsATS.202009-1163VP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolkewitz M, Vonberg RP, Grundmann H, Beyersmann J, Gastmeier P, Bärwolff S, et al. Risk factors for the development of nosocomial pneumonia and mortality on intensive care units: application of competing risks models. Crit Care. 2008;12:R44. doi: 10.1186/cc6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniel RM, Cousens SN, De Stavola BL, Kenward MG, Sterne JA. Methods for dealing with time-dependent confounding. Stat Med. 2013;32:1584–1618. doi: 10.1002/sim.5686. [DOI] [PubMed] [Google Scholar]
- 11.Kuti EL, Patel AA, Coleman CI. Impact of inappropriate antibiotic therapy on mortality in patients with ventilator-associated pneumonia and blood stream infection: a meta-analysis. J Crit Care. 2008;23:91–100. doi: 10.1016/j.jcrc.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Kollef MH. Ventilator-associated pneumonia prevention: is it worth it? Am J Respir Crit Care Med. 2015;192:5–7. doi: 10.1164/rccm.201504-0734ED. [DOI] [PubMed] [Google Scholar]
- 13.Melsen WG, Rovers MM, Koeman M, Bonten MJ. Estimating the attributable mortality of ventilator-associated pneumonia from randomized prevention studies. Crit Care Med. 2011;39:2736–2742. doi: 10.1097/CCM.0b013e3182281f33. [DOI] [PubMed] [Google Scholar]
- 14.Melsen WG, Rovers MM, Groenwold RH, Bergmans DC, Camus C, Bauer TT, et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013;13:665–671. doi: 10.1016/S1473-3099(13)70081-1. [DOI] [PubMed] [Google Scholar]
- 15.Bergmans DC, Bonten MJ, Gaillard CA, van Tiel FH, van der Geest S, de Leeuw PW, et al. Indications for antibiotic use in ICU patients: a one-year prospective surveillance. J Antimicrob Chemother. 1997;39:527–535. doi: 10.1093/jac/39.4.527. [DOI] [PubMed] [Google Scholar]
- 16.Thomas Z, Bandali F, Sankaranarayanan J, Reardon T, Olsen KM Critical Care Pharmacotherapy Trials Network. A multicenter evaluation of prolonged empiric antibiotic therapy in adult ICUs in the United States. Crit Care Med. 2015;43:2527–2534. doi: 10.1097/CCM.0000000000001294. [DOI] [PubMed] [Google Scholar]
- 17.Nussenblatt V, Avdic E, Berenholtz S, Daugherty E, Hadhazy E, Lipsett PA, et al. Ventilator-associated pneumonia: overdiagnosis and treatment are common in medical and surgical intensive care units. Infect Control Hosp Epidemiol. 2014;35:278–284. doi: 10.1086/675279. [DOI] [PubMed] [Google Scholar]
- 18.Klompas M. Does this patient have ventilator-associated pneumonia? JAMA. 2007;297:1583–1593. doi: 10.1001/jama.297.14.1583. [DOI] [PubMed] [Google Scholar]
- 19.Klompas M. The paradox of ventilator-associated pneumonia prevention measures. Crit Care. 2009;13:315. doi: 10.1186/cc8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GTH. Robust causal inference using directed acyclic graphs: the R package “dagitty”. Int J Epidemiol. 2016;45:1887–1894. doi: 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.