Summary
Background
Previous research by our team identified factors associated with in-hospital mortality in patients with a diagnosis of COVID-19 in England between March and May 2020. The aim of the current paper was to investigate the changing role of demographics and co-morbidity, with a particular focus on ethnicity, as risk factors for in-hospital mortality over an extended period.
Methods
This was a retrospective observational study using the Hospital Episode Statistics administrative dataset. All patients aged ≥ 18 years in England with a diagnosis of COVID-19 who had a hospital stay that was completed (discharged alive or died) between 1st March and 30th September 2020 were included. In-hospital mortality was the primary outcome of interest. Multilevel logistic regression was used to model the relationship between in-hospital mortality with adjustment for the covariates: age, sex, deprivation, ethnicity, date of discharge and a number of comorbidities.
Findings
Compared to patients in March-May (n = 93,379), patients in June-September (n = 24,059) were younger, more likely to be female and of Asian ethnicity, but less likely to be of Black ethnicity. In-hospital mortality rates, adjusted for covariates, declined from 33–34% in March to 11–12% in September. Compared to the March-May period, Bangladeshi, Indian and Other Asian ethnicity patients had a lower relative odds of death (compared to White ethnicity patients) during June-September. For Pakistani patients, the decline in-hospital mortality rates was more modest across the same time periods with the relative odds of death increasing slightly (odds ratio (95% confidence interval)) 1.24 (1.10 to 1.40) and 1.35 (1.08 to 1.69) respectively. From March-May to June-September the relative odds of death in patients with a diagnosis of metastatic carcinoma increased (1.90 (1.73 to 2.08) vs 3.01 (2.55 to 3.54)) but decreased for male patients (1.44 (1.39 to 1.49) vs 1.27 (1.17 to 1.38)) and patients with obesity (1.42 (1.34 to 1.52) vs 0.97 (0.83 to 1.14)) and diabetes without complications (1.14 (1.10 to 1.19) vs 0.95 (0.87 to 1.05)).
Interpretation
In-hospital mortality rates for patients with a diagnosis of COVID-19 have fallen substantially and there is evidence that the relative importance of some covariates has changed since the start of the pandemic. These patterns should continue to be tracked as new variants of the virus emerge, vaccination programmes are rolled out and hospital pressures fluctuate.
Funding
None.
Keywords: COVID-19, Coronavirus, Mortality, Ethnicity, Risk factors
Research in context.
Evidence before this study
We searched PubMed on 5th January 2021 for articles that documented risk factors for COVID-19-related in-hospital mortality using search terms “SARS-CoV-2″ OR “COVID-19″ AND “mortality” AND “hospital” in the article title or abstract. Of the 2067 papers identified, we found 132 publications identified as pertaining to "England" OR "United Kingdom". Of these, 85 were original research studies involving patient data of which 16 investigated mortality in COVID-19 patients. Of the remaining 69, five were clinical trials, seven developed or evaluated risk prediction algorithms and 45 focused on a specific disease or patient population. The only United Kingdom-based study that covered an entire hospital population nationally over an extended period was an earlier study by our team. Most studies focused on patient with specific medical conditions.
Added value of this study
We present data for 117,438 patients admitted to hospital for the first seven months of the COVID-19 pandemic in England. Adjusted in-hospital mortality rates fell from 33–34% in March to 13–14% in early June and stabilised around 11–12% during July-September. Bangladeshi, Indian and Other Asian ethnicity patients had a higher odds of death during March-May than June-September. However, for patients of Pakistani ethnicity the odds of death was higher in the later time period. The odds of death in patients with a diagnosis of metastatic carcinoma increased from March-May to June-September but decreased for male patients and patients with obesity and diabetes without complications.
Implications of all the available evidence
Our study emphasises the importance of considering trends over time in evaluating the association between various risk factors and in-hospital mortality in patients with COVID-19. The reasons why mortality rates have declined over time is likely to be multifactorial, but changing patterns of infection in those most at risk of hospitalisation as lockdown measures were implemented and relaxed, illness severity on presentation, greater community awareness of COVID-19, health seeking behaviour, hospital admissions policies, and changing practice and service organisation within hospitals are likely to be key drivers. Being aware of these changes and how they relate to the risk profile of certain groups is particularly important when designing triage systems based on risk of poor outcomes.
In comparing patients from two distinct time periods, we recognise that there may be a degree of collider bias with regard to the profile of patients on unmeasured variables, such as illness severity on admission. We emphasise that our findings should not be extrapolated to community-based cohorts, where the pattern of community transmission within high risk groups is likely to be a key determinant of the mortality rate.
Alt-text: Unlabelled box
1. Introduction
There have been a number of reports of factors which are associated with mortality in people with a diagnosis of COVID-19. Early in the pandemic, community-based data from England identified a number of demographic factors and comorbidities which were associated with an increased probability of mortality [1,2]. In a study of over 17 million people in England by Williamson et al. [2], age (hazard ratio (HR) 343.3 aged 80 years and over relative to aged 18–39 years) was by far the strongest predictor, although male sex (HR 1.6 relative to females), deprivation (HR 1.8 most deprived relative to least deprived quintile), ethnicity (HR 1.5 Black, 1.5 Asian, 1.4 Mixed relative to White ethnicity) and comorbidities including obesity, respiratory disease, chronic heart failure, diabetes, cancer, liver disease, kidney disease, dementia and stroke were also associated with higher hazards of death. Studies focussing on deaths in hospital patients in England have reported similar findings, although the HR or odds ratios (OR) reported are generally smaller, reflecting the different denominator populations [3,4].
The Getting It Right First Time (GIRFT) programme aims to investigate variation in practice and patient outcomes across the National Health Service (NHS). As part of GIRFT's interest in assessing the extent of such variation in relation to COVID-19, previous work by our team using Hospital Episodes Statistics (HES) data for the entire NHS patient population in England from March-May 2020 noted similar risk factors and identified a decline in-hospital mortality as the pandemic progressed [5]. The aim of the current study was to update this initial work and focus on the changing influences of various potential risk factors on in-hospital mortality over a seven-month period. Of particular interest to the GIRFT programme, we wanted to understand which risk factors have a consistent relationship with in-hospital mortality over time and which may be more amenable to change with improved management of COVID-19 in hospital and improved control of virus spread in the community.
2. Methods
2.1. Ethics
Consent from individuals involved in this study was not required. The analysis and presentation of data follows current NHS Digital guidance for the use of HES data for research purposes. Reported data are anonymised to the level required by ISB1523 Anonymisation Standard for Publishing Health and Social Care Data [6].
2.2. Study design and data collection
This was a retrospective analysis of HES administrative data. HES data are collected by NHS Digital for all NHS-funded patients admitted to hospitals in England. Hospitals in England are run by hospital trusts, with a trust typically running between one and four large secondary care hospitals in a geographically defined catchment area. Data are entered by trained coders in each hospital trust and data collection and reporting is mandatory.
2.3. Timing, case ascertainment, inclusion and exclusion criteria
We reviewed HES data for all completed episodes of hospital care in England with a discharge date from 1st March to 30th September 2020 that involved a diagnosis of COVID-19. We only considered completed episodes of care, where the patient had been discharged and their outcome was known (either discharged alive or having died during their stay). Patients aged < 18 years were excluded. Cases of COVID-19 were identified using the International Statistical Classification of Disease and Related Health Problems 10th edition (ICD-10) codes U07.1 and U07.2. U07.1 is assigned where the presence of COVID-19 has been confirmed by laboratory testing. U07.2 is assigned to a clinical or epidemiological diagnosis of COVID-19 where laboratory confirmation is inconclusive or not available.
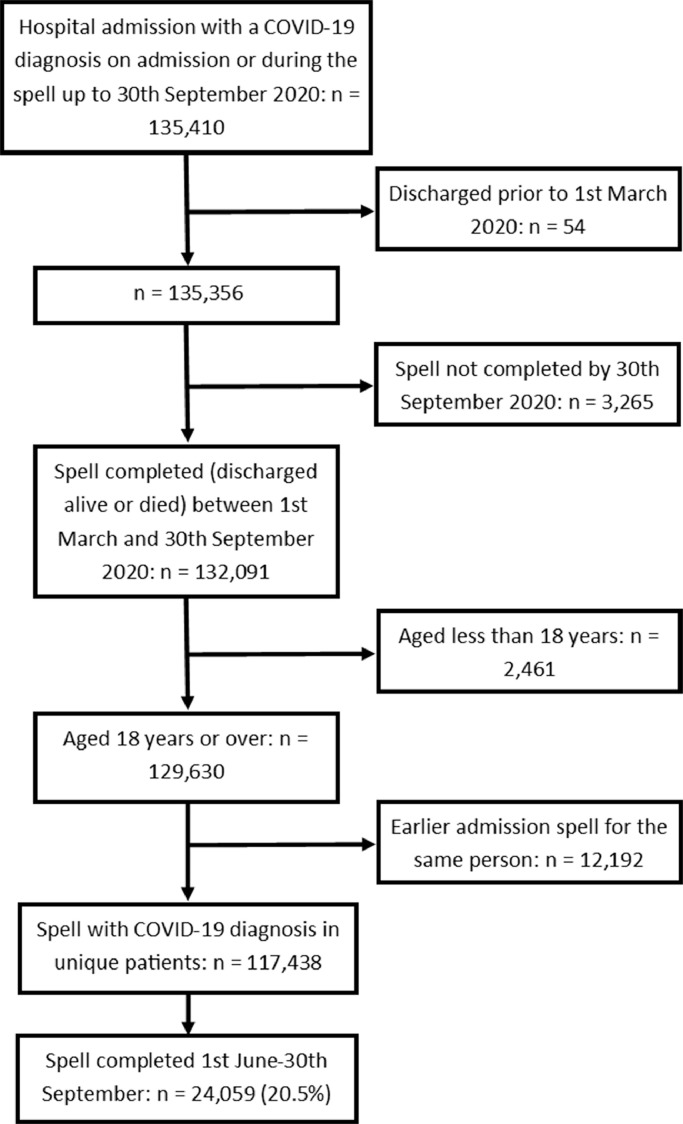
Where a patient had multiple admissions during the study period, only the chronologically last admission was retained. This ensured that all admissions were independent of one another at a patient level and avoided biasing the data by including cases where the outcome was predefined (alive at discharge) by virtue of a subsequent admission. The data extraction process is summarised in Fig. 1.
Fig. 1.
Data extraction process.
2.4. Outcome
The primary outcome was in-hospital mortality as recorded by the Office for National Statistics (ONS). An in-hospital death was recorded if the date of death was the same as or +/- one day of the date of hospital discharge recorded in HES. In previous work we have reported on the agreement between deaths recorded by ONS, directly in HES and by NHS England's COVID–19 Patient Notification System (CPNS) [7].
2.5. Covariates
Age: Categorised as 18–39, 40–49, 50–59, 60–69, 70–79 years and ≥ 80 years for exploratory analysis and treated as continuous in the final multivariate model. The categorisation was chosen to reflect that used by the OpenSAFELY study group and is felt to be clinically meaningful whilst avoiding over stratification [2].
Sex: Male or female.
Ethnicity: Coded in categories used by NHS Digital (White, Bangladeshi, Indian, Pakistani, Other Asian, Black Africa, Black Caribbean, Other Black, Mixed, Other, not stated).
Deprivation: Recorded using the Index of Multiple Deprivation (IMD) for the Lower Super Output Area (LSOA) of the patients' home address, with scores categorised into quintiles based on national averages. The IMD categorises all households in England into percentiles of relative deprivation based on their LSOA of residence. It includes items measuring income, employment, health and disability, education and skills training, crime, barriers to housing and services and living environment.
Comorbidities: These were the 14 comorbidities used to construct the Charlson Comorbidity Index (peripheral vascular disease, congestive heart failure, acute myocardial infarction, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease/rheumatic disease, peptic ulcer, liver disease (mild and moderate/severe), diabetes (with and without chronic complications), paraplegia/hemiplegia, renal disease, cancer (primary and metastatic), HIV/AIDS) [8]. The comorbidity was deemed present if it was recorded in HES as a secondary diagnosis in the index admission or as a primary or secondary diagnosis in any admission during the previous year, in accordance with the recommendations of Quan et al. [9].
Obesity: Recorded as present if the ICD-10 code E66 was used as a diagnostic code during the admission.
Temporal trends: categorised into day or month of discharge (starting from Sunday 1st March) depending on the analysis undertaken. Monthly data were used for descriptive statistics. Daily data were used for the final multivariable model.
2.6. Data management and statistical analyses
Data were extracted onto a secure encrypted server controlled by NHS England and NHS Improvement. Analysis within this secure environment took place using standard statistical software: Microsoft Excel (Microsoft Corp, Redmond, WA, USA), Stata (StataCorp LLC, College Station, TX, USA) and Alteryx (Alteryx Inc, Irvine, CA, USA). Length of stay data were non-normally distributed and summarised using the median and inter-quartile range (IQR). All other data are described by frequency and percentage.
Building on our initial work [5], mixed-effects logistic regression models for the periods March-May and June-September were constructed using the 'melogit' command in Stata. All variables were categorised for this analysis. Two-level intercept only models were constructed, allowing adjustment for clustering of patients within trusts.
A model for the whole seven-month period was then constructed. This model considered age, day of discharge and IMD score as continuous variables and modeled using restricted cubic splines where non-linearity was evident. Non-linearity was identified where only the first linear spline contributed to the model fit. Age was modeled using three knots at the 25th, 50th and 75th percentile of the data. For the full seven-month period, time was modeled in day of discharge using five knots at the 5th, 27.5th, 50th 72.5th and 95th percentile. For the June-September period only, three knots at the 5th, 50th and 95th percentile was found to be optimal. The optimal knot position was identified with reference to Akaike Information Criterion (AIC) and based on the data structure. Deprivation score was modeled as a linear variable. The relationship between age, time and the estimated probability of death was plotted using the 'adjustrcspline' command in Stata.
Other than for ethnicity, missing data were relatively rare (see Table 1) and no attempt was made to impute missing values. For ethnicity, a number of patients did not state their ethnicity, although an answer was recorded for all patients. Where data were missing the numbers involved are stated. The models were summarised in terms of ORs and 95% confidence intervals (CIs).
Table 1.
Patient demographic, socioeconomic and clinical profile and number of deaths for each time period.
| March-May |
June-September |
|||
|---|---|---|---|---|
| Variable | Discharges (n = 93,379) | Deaths (n = 28,344, 30.4%) | Discharges (n = 24,059) | Deaths (n = 3943, 16.4%) |
| Age band (years) | ||||
| 18–39 | 7097 | 200 (2.8%) | 2692 | 32 (1.2%) |
| 40–49 | 7275 | 506 (7.0%) | 1889 | 84 (4.4%) |
| 50–59 | 12,369 | 1725 (13.9%) | 2940 | 229 (7.8%) |
| 60–69 | 14,124 | 3574 (25.3%) | 3621 | 473 (13.1%) |
| 70–79 | 19,877 | 7329 (36.9%) | 4916 | 957 (19.5%) |
| ≥ 80 | 32,637 | 15,010 (46.0%) | 8001 | 2168 (27.1%) |
| Sex (missing = 224) | ||||
| Female | 41,570 | 11,181 (26.9%) | 11,492 | 1682 (14.6%) |
| Male | 51,622 | 17,115 (33.2%) | 12,530 | 2257 (18.0%) |
| Deprivation quintile (missing 2744) | ||||
| 1 (most deprived) | 23,412 | 6831 (29.2%) | 6773 | 1040 (15.4%) |
| 2 | 20,522 | 6158 (30.0%) | 5121 | 827 (16.1%) |
| 3 | 17,487 | 5518 (31.6%) | 4214 | 731 (17.3%) |
| 4 | 15,605 | 4973 (31.9%) | 4021 | 685 (17.0%) |
| 5 (least deprived) | 14,213 | 4408 (31.0%) | 3326 | 587 (17.6%) |
| Ethnicity (missing = 13,388) | ||||
| White | 66,215 | 21,545 (32.5%) | 17,579 | 3194 (18.2%) |
| Bangladeshi | 583 | 153 (26.2%) | 168 | 17 (10.1%) |
| Indian | 2599 | 742 (28.5%) | 731 | 91 (12.4%) |
| Pakistani | 1975 | 480 (24.3%) | 1074 | 132 (12.3%) |
| Other Asian | 2338 | 486 (20.8%) | 491 | 42 (8.6%) |
| Black African | 2184 | 380 (17.4%) | 390 | 18 (4.6%) |
| Black Caribbean | 1958 | 645 (32.9%) | 218 | 33 (15.1%) |
| Other Black | 895 | 208 (23.2%) | 142 | 7 (4.9%) |
| Mixed | 755 | 167 (22.1%) | 177 | 13 (7.3%) |
| Other | 3002 | 607 (20.2%) | 576 | 50 (8.7%) |
| Charlson Comorbidity Index items* | ||||
| Peripheral vascular disease | 4730 | 2086 (44.1%) | 1508 | 379 (25.1%) |
| Congestive heart failure | 13,185 | 6408 (48.6%) | 4092 | 1224 (29.9%) |
| Acute myocardial infarction | 8388 | 3563 (42.5%) | 2419 | 613 (25.3%) |
| Cerebrovascular disease | 8594 | 3591 (41.8%) | 2695 | 622 (23.1%) |
| Dementia | 14,437 | 6799 (47.1%) | 3223 | 848 (26.3%) |
| Chronic pulmonary disease | 24,350 | 8144 (33.4%) | 6480 | 1254 (19.4%) |
| Connective tissue disease/rheumatic disease | 2751 | 994 (36.1%) | 773 | 156 (20.2%) |
| Peptic ulcer | 597 | 213 (35.7%) | 222 | 56 (25.2%) |
| Mild liver disease | 2882 | 719 (24.9%) | 1023 | 150 (14.7%) |
| Moderate or severe liver disease | 945 | 507 (53.7%) | 371 | 155 (41.8%) |
| Diabetes without chronic complications | 23,097 | 8334 (36.1%) | 5585 | 1037 (18.6%) |
| Diabetes with chronic complications | 2746 | 1069 (38.9%) | 810 | 172 (21.2%) |
| Paraplegia and hemiplegia | 2239 | 888 (39.7%) | 668 | 139 (20.8%) |
| Renal disease | 17,061 | 7759 (45.5%) | 4420 | 1185 (26.8%) |
| Primary cancer | 5329 | 2423 (45.5%) | 1436 | 377 (26.3%) |
| Metastatic carcinoma | 2681 | 1110 (41.4%) | 940 | 310 (33.0%) |
| HIV/AIDS | 152 | 20 (13.2%) | 33 | 4 (12.1%) |
| Obesity | 8093 | 2176 (26.9%) | 2333 | 261 (11.2%) |
For the Charlson Comorbidity Index items: Only those with the disease are listed. There were no missing data. Individual patients can appear in multiple disease categories.
2.7. Role of the funding source
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
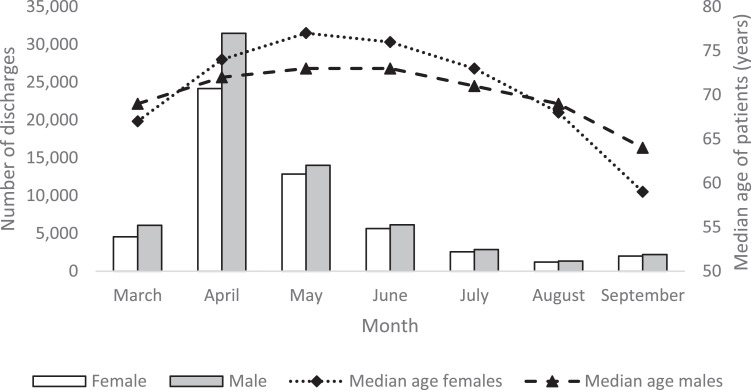
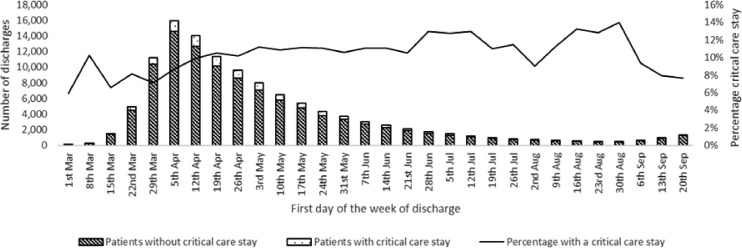
The data extraction process is summarised in Fig. 1. This gave a dataset of 117,438 unique patients who had a diagnosis of COVID-19 either on admission or during their stay. In total, 92,664 (78.8%) patients had COVID-19 confirmed by test. There were 32,287 (27.5%) in-hospital deaths. The number of discharges per month and the median age of those discharged for males and females is summarised in Fig. 2. April was the month of peak activity during the study period. In March 57.3% of discharges were males, compared to 52.1% in September. Median age peaked in May at 77 years for females and 73 years for males and declined thereafter. Median length of stay varied considerably over the seven months with a bell-shaped profile, peaking in June at 12 days (IQR 4 to 27), and lowest in March (4 days, IQR 1 to 7) and September (4 days, IQR 1 to 10). Fig. 3 shows the crude number of discharges per week and the percentage of those discharged who had a critical care stay. The proportion of patients with a critical care stay increased slightly from around 8% in March to 13% in July-August before falling back to around 8% in September.
Fig. 2.
Number of discharges and median age per month for males and females.
Fig. 3.
Number of patients and proportion with a critical care stay by month of discharge.
The demographic, socioeconomic and clinical characteristics of patients discharged in March-May and June-September are summarised in Table 1 together with the unadjusted number of in-hospital deaths. In the June-September period patients were more likely to be < 50 years of age (March-May 15.4%, June-September 19.0%), more likely to be female (March-May 44.6%, June-September 47.8%) and more likely to be of Pakistani ethnic origin (March-May 2.4%, June-September 5.0%). There was a more modest increase in the proportion of Bangladeshi and Indian ethnic origin patients and a lower proportion of Black patients (March-May 6.1%, June-September 3.5% across all Black ethnic groups). The proportion of White patients rose slightly (March-May 80.2%, June-September 81.6%).
The unadjusted in-hospital mortality rate declined substantially in all age, sex, deprivation and ethnic groups, although for ethnicity the reductions were more marked in non-White ethnic groups. The largest falls in-hospital mortality were seen in the Black African and Other Black ethnic categories. For most recorded comorbidities, deaths rates fell by approximately a half between the two time periods, with notable exceptions for moderate or severe liver disease, metastatic carcinoma, peptic ulcer, and HIV/AIDS, where more modest declines in the mortality rates were evident. In contrast, for obesity in-hospital mortality rates more than halved to 41.6% of their March-May levels.
The models using categorical variables for March-May and June-September are summarised in Table 2. There was little evidence that the association between age and in-hospital mortality changed from March-May to June-September. However, the association between male sex and in-hospital mortality reduced across the two time periods. The relationship between deprivation and in-hospital mortality was relatively weak throughout.
Table 2.
Multilevel logistic regression models of factors associated with in-hospital mortality for each period.
| Variable | March-May (odds ratio, 95% CI) | June-September (odds ratio, 95% CI) |
|---|---|---|
| Age band (years) | ||
| 18–39 | 1 (reference) | 1 (reference) |
| 40–49 | 2.05 (1.70 to 2.47) | 4.07 (2.43 to 6.84) |
| 50–59 | 4.47 (3.79 to 5.27) | 7.43 (4.60 to 11.99) |
| 60–69 | 8.47 (7.22 to 9.95) | 11.38 (7.13 to 18.19) |
| 70–79 | 14.16 (12.07 to 16.60) | 17.60 (11.05 to 28.01) |
| ≥ 80 | 20.58 (17.55 to 24.12) | 26.24 (16.51 to 41.71) |
| Sex | ||
| Female | 1 (reference) | 1 (reference) |
| Male | 1.44 (1.39 to 1.49) | 1.27 (1.17 to 1.38) |
| Deprivation quintile | ||
| 5 (least deprived) | 1 (reference) | 1 (reference) |
| 4 | 1.03 (0.97 to 1.09) | 0.96 (0.83 to 1.10) |
| 3 | 1.07 (1.01 to 1.13) | 1.05 (0.92 to 1.21) |
| 2 | 1.05 (0.99 to 1.11) | 1.01 (0.88 to 1.15) |
| 1 (most deprived) | 1.05 (1.00 to 1.12) | 1.08 (0.94 to 1.23) |
| Ethnicity | ||
| White | 1 (reference) | 1 (reference) |
| Bangladeshi | 1.47 (1.18 to 1.83) | 0.83 (0.47 to 1.48) |
| Indian | 1.26 (1.14 to 1.39) | 1.11 (0.86 to 1.44) |
| Pakistani | 1.24 (1.10 to 1.40) | 1.35 (1.08 to 1.69) |
| Other Asian | 1.13 (1.00 to 1.27) | 1.04 (0.73 to 1.47) |
| Black African | 0.99 (0.87 to 1.13) | 0.76 (0.45 to 1.29) |
| Black Caribbean | 1.08 (0.96 to 1.20) | 1.04 (0.69 to 1.57) |
| Other Black | 1.22 (1.02 to 1.46) | 0.55 (0.22 to 1.38) |
| Mixed | 1.30 (1.07 to 1.58) | 0.79 (0.43 to 1.45) |
| Other | 1.00 (0.90 to 1.11) | 1.09 (0.79 to 1.51) |
| Charlson Comorbidity Index items* | ||
| Peripheral vascular disease | 1.19 (1.12 to 1.28) | 1.25 (1.09 to 1.43) |
| Congestive heart failure | 1.54 (1.47 to 1.60) | 1.75 (1.59 to 1.92) |
| Acute myocardial infarction | 1.06 (1.00 to 1.11) | 1.20 (1.07 to 1.34) |
| Cerebrovascular disease | 1.09 (1.03 to 1.15) | 1.11 (0.98 to 1.25) |
| Dementia | 1.42 (1.36 to 1.48) | 1.36 (1.23 to 1.51) |
| Chronic pulmonary disease | 1.07 (1.03 to 1.11) | 1.12 (1.03 to 1.22) |
| Connective tissue disease/rheumatic disease | 1.21 (1.11 to 1.32) | 1.09 (0.89 to 1.33) |
| Peptic ulcer | 1.00 (0.83 to 1.21) | 1.43 (1.02 to 2.00) |
| Mild liver disease | 0.97 (0.88 to 1.08) | 1.24 (1.01 to 1.52) |
| Moderate or severe liver disease | 4.58 (3.92 to 5.35) | 6.22 (4.82 to 8.03) |
| Diabetes without chronic complications | 1.14 (1.10 to 1.19) | 0.95 (0.87 to 1.05) |
| Diabetes with chronic complications | 1.25 (1.14 to 1.37) | 1.09 (0.89 to 1.34) |
| Paraplegia and hemiplegia | 1.12 (1.06 to 1.18) | 1.10 (0.98 to 1.23) |
| Renal disease | 1.16 (1.14 to 1.19) | 1.14 (1.09 to 1.20) |
| Primary cancer | 1.55 (1.45 to 1.65) | 1.60 (1.39 to 1.84) |
| Metastatic carcinoma | 1.90 (1.73 to 2.08) | 3.01 (2.55 to 3.54) |
| Obesity | 1.42 (1.34 to 1.52) | 0.97 (0.83 to 1.14) |
The March-May models is based on data for 80,618 patients and the June-September model on data for 21,014 patients with no missing data. A stable odds ratio for the comorbidity HIV/AIDS could not be calculated due to small numbers.
For Charlson Comorbidity Index items the reference category is patients without the specified comorbidity. For Charlson Comorbidity Index items relating to liver disease, diabetes and cancer three mutually exclusive categories were used.
In the June-September period there was evidence that the effect of connective tissue disease, diabetes (with and without complications) and obesity had declined relative to the March-May period. For patients with metastatic carcinoma there was an crease and for patients with diabetes without complications and obesity a decrease in the relative odds of in-hospital mortality (compared to those without these conditions) across the two time periods.
For the Bangladeshi, Indian, Other Asian, Black Other and Mixed ethnic group the relative odds of death declined across the two time periods (compared to White patients). However, for the Pakistani ethnic group the relative odds of death increased from March-May to June-September.
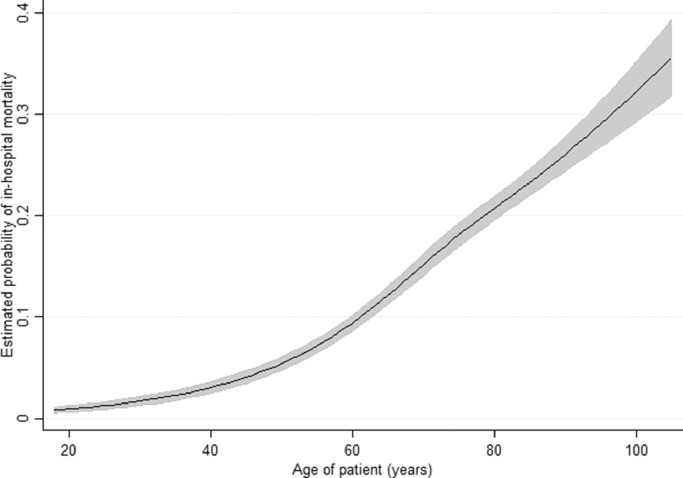
The final model for the June-September period, with age, deprivation and date of discharge treated as continuous variables is summarised in Table 3 and Fig. 4. The model is very similar to the exploratory categorical model for the same period. Age had a shallow sigmoid relationship with the probability of in-hospital mortality, rising from 1–3% in those aged under 30 years to 30–35% in those aged 90 years and over. Deprivation was not associated with in-hospital mortality. Pakistani was the only ethnic group where the odds of death was noticeably higher than for the White ethnic group.
Table 3.
Multilevel logistic regression model predicting in-hospital mortality for June-September for variables modelled as linear or categorical.
| Variable | Odds ratios (95% CIs) |
|---|---|
| Sex | |
| Female | 1 (reference) |
| Male | 1.30 (1.20 to 1.41) |
| IMD score | 1.00 (1.00 to 1.00) |
| Ethnicity | |
| White | 1 (reference) |
| Bangladeshi | 0.87 (0.49 to 1.55) |
| Indian | 1.14 (0.88 to 1.48) |
| Pakistani | 1.41 (1.13 to 1.76) |
| Other Asian | 1.07 (0.75 to 1.52) |
| Black African | 0.80 (0.47 to 1.36) |
| Black Caribbean | 1.05 (0.69 to 1.59) |
| Other Black | 0.58 (0.23 to 1.48) |
| Mixed | 0.82 (0.44 to 1.50) |
| Other | 1.12 (0.81 to 1.55) |
| Charlson Comorbidity Index items* | |
| Peripheral vascular disease | 1.25 (1.09 to 1.44) |
| Congestive heart failure | 1.73 (1.58 to 1.90) |
| Acute myocardial infarction | 1.20 (1.07 to 1.34) |
| Cerebrovascular disease | 1.09 (0.97 to 1.23) |
| Dementia | 1.33 (1.20 to 1.47) |
| Chronic pulmonary disease | 1.14 (1.05 to 1.24) |
| Connective tissue disease/rheumatic disease | 1.10 (0.90 to 1.34) |
| Peptic ulcer | 1.44 (1.03 to 2.01) |
| Mild liver disease | 1.25 (1.02 to 1.53) |
| Moderate or severe liver disease | 6.36 (4.92 to 8.21) |
| Diabetes without chronic complications | 0.97 (0.88 to 1.06) |
| Diabetes with chronic complications | 1.12 (0.91 to 1.37) |
| Paraplegia and hemiplegia | 1.11 (0.99 to 1.25) |
| Renal disease | 1.13 (1.08 to 1.18) |
| Primary cancer | 1.62 (1.40 to 1.86) |
| Metastatic carcinoma | 3.03 (2.57 to 3.57) |
| Obesity | 1.00 (0.85 to 1.17) |
Models are based on data for 21,014 patients with no missing data. Age was modeled as a continuous variable using restricted cubic splines and so the model output cannot be summarised as an odds ratio; the relationship is depicted graphically in Fig. 4. A stable odds ratio for the comorbidity HIV/AIDS could not be calculated due to small numbers. Where the 95% confidence interval does not cross 1, the model outputs are marked in bold.
For Charlson Comorbidity Index items the reference category is patients without the specified comorbidity. For Charlson Comorbidity Index items relating to liver disease, diabetes and cancer three mutually exclusive categories were used. IMD = Index of Multiple Deprivation.
Fig. 4.
Predicted probability of in-hospital mortality by age for June-September with 95% confidence intervals shown as grey shading.
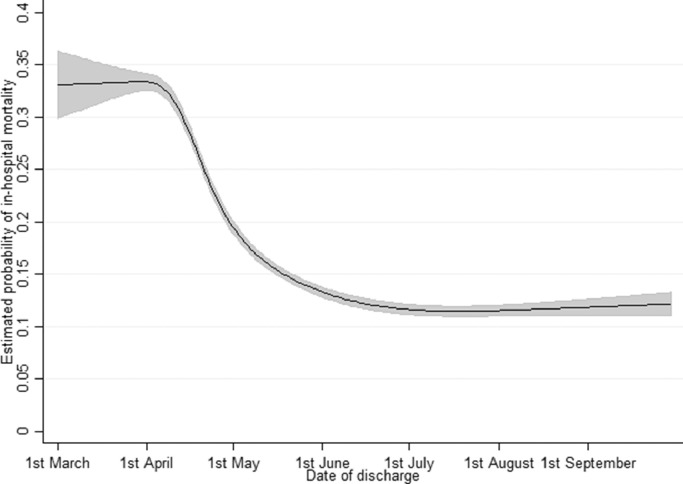
Fig. 5 shows the relationship between date of discharge and the adjusted probability of in-hospital mortality for the full seven month period, modeled using the final model. Date of discharge had a clear reverse-sigmoid relationship with in-hospital mortality, with a relatively stable adjusted in-hospital mortality rate of 33–34% in March, followed by a rapid decline in-hospital mortality to around 13–14% in early June and a consistent in-hospital mortality rate of 11–12% during July-September. Table 4 and Fig. 6 summarise the other outputs from the final model for the whole seven-month period. The model is very similar to the March-May model, with the majority of data coming from this period [5].
Fig. 5.
Predicted probability of in-hospital mortality by date of discharge for March-September with 95% confidence intervals shown as grey shading.
Table 4.
Multilevel logistic regression model predicting in-hospital mortality for the entire seven month period for variables modeled as linear or categorical covariates.
| Variable | Odds ratios (95% CIs) |
|---|---|
| Sex | |
| Female | 1 (reference) |
| Male | 1.40 (1.36 to 1.45) |
| IMD score | 1.00 (1.00 to 1.00) |
| Ethnicity | |
| White | 1 (reference) |
| Bangladeshi | 1.30 (1.06 to 1.60) |
| Indian | 1.22 (1.11 to 1.35) |
| Pakistani | 1.22 (1.09 to 1.35) |
| Other Asian | 1.13 (1.01 to 1.27) |
| Black African | 0.95 (0.84 to 1.08) |
| Black Caribbean | 1.01 (0.91 to 1.13) |
| Other Black | 1.13 (0.95 to 1.36) |
| Mixed | 1.25 (1.04 to 1.50) |
| Other | 1.01 (0.91 to 1.12) |
| Charlson Comorbidity Index items* | |
| Peripheral vascular disease | 1.24 (1.17 to 1.32) |
| Congestive heart failure | 1.60 (1.54 to 1.67) |
| Acute myocardial infarction | 1.08 (1.03 to 1.13) |
| Cerebrovascular disease | 1.14 (1.09 to 1.20) |
| Dementia | 1.44 (1.38 to 1.50) |
| Chronic pulmonary disease | 1.08 (1.05 to 1.12) |
| Connective tissue disease/rheumatic disease | 1.22 (1.12 to 1.32) |
| Peptic ulcer | 1.16 (0.98 to 1.37) |
| Mild liver disease | 1.09 (0.99 to 1.19) |
| Moderate or severe liver disease | 5.67 (4.95 to 6.49) |
| Diabetes without chronic complications | 1.12 (1.08 to 1.16) |
| Diabetes with chronic complications | 1.27 (1.16 to 1.38) |
| Paraplegia and hemiplegia | 1.15 (1.10 to 1.21) |
| Renal disease | 1.16 (1.14 to 1.18) |
| Primary cancer | 1.59 (1.50 to 1.68) |
| Metastatic carcinoma | 2.26 (2.09 to 2.45) |
| Obesity | 1.38 (1.30 to 1.47) |
Models are based on data for 101,632 patients with no missing data. A stable odds ratio for the comorbidity HIV/AIDS could not be calculated due to small numbers. Where the 95% confidence interval does not cross 1, the model outputs are marked in bold.
For Charlson Comorbidity Index items the reference category is patients without the specified comorbidity. For Charlson Comorbidity Index items relating to liver disease, diabetes and cancer three mutually exclusive categories were used. IMD = Index of Multiple Deprivation.
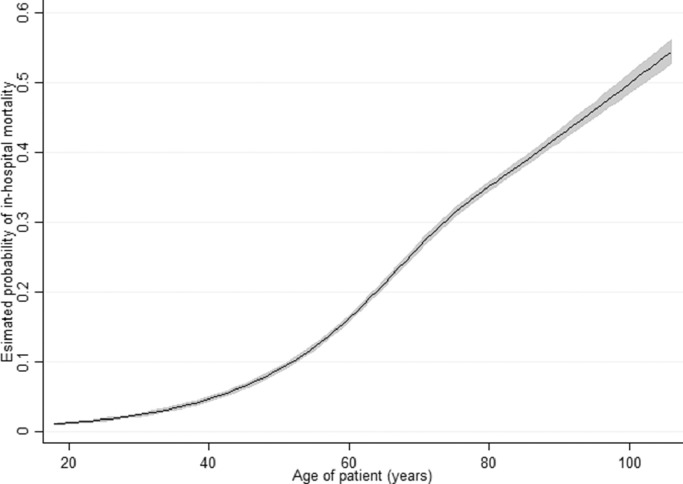
Fig. 6.
Predicted probability of in-hospital mortality by age March-September with 95% confidence intervals shown as grey shading.
4. Discussion
This study builds on previous work by our team and looks at the changing role of various patient-related factors in predicting in-hospital mortality with COVID-19 [5]. Temporal trends in COVID-19 mortality have received relatively little attention, partly due to a lack of reliable data over a sufficiently long time period. Changes in the role of ethnicity, sex and comorbidity on in-hospital mortality were evident in our study of people hospitalised with COVID-19.
We observed a substantial fall in adjusted in-hospital mortality rates over the first seven months of the pandemic in England. Adjusted in-hospital mortality rates fell dramatically during April 2020 and were relatively stable from July-September.
Large falls in critical care mortality in England have been reported for the March-June period, with high dependence unit mortality falling from 28.4% to 7.3% and intensive care unit mortality from 42.0% to 19.6% [10]. The declines in-hospital mortality reported here mirror those reported in New York City, United States by Horwitz et al. during a similar time period [11]. Across 5121 patients in three teaching hospitals adjusted mortality rates fell from 25.6% in March 2020 to 7.6% in August 2020. A much younger patient population (median age 64 years in March and 49 years in August) in this exclusively metropolitan setting is a likely explanation for much of the difference in absolute mortality rates report in their study compared to ours. Differing health system organisation, admission criteria and disease severity at admission may also play a role. However, the pattern of decline report by Horwitz et al. and in our study appears very similar, with rapid declines during spring and steadier declines during the summer months. The percentage decline of around two-thirds was very similar. In our study, the proportion of patients admitted to critical care rose modestly from March to July before declining and this alone is unlikely to explain the large falls in mortality rates over time. However, clinical management once admitted to critical care is likely to be of central importance. The authors of a study using Intensive Care National Audit & Research Centre (ICNARC) data also note declines in mortality over time and propose that rapid learning and knowledge sharing at a transnational level is likely to have avoided the continuation of potentially harmful interventions (e.g. early intubation) and supported the early dissemination of beneficial treatments (e.g. use of dexamethasone, continuous positive airways pressure and prone positioning) [12]. However, their data does not support the view that earlier presentation to hospital explains a substantial amount of the decline in mortality seen. Although our data cannot provide any information on the reason for the declines in-hospital mortality observed, we agree that these and other factors (e.g. mandatory mask wearing in enclosed public spaces reducing viral load on presentation) are likely important factors in the changes seen.
The greatest decrease in the in-hospital mortality rate was seen in April, the month of peak activity. The decline in the median age of patients discharged during July-September may reflect changing admission policies, or public behaviour with regard to healthcare service use at a point when pressure on services had eased or reflect patterns of infection in the community [13]. There is evidence in England that longer time from symptom onset to hospital presentation is associated with higher mortality risk. A study of 6068 patients admitted to hospital between 8th February and 13th April 2020 found that each additional day between symptom onset and hospital admission was associated with a 1% increase in mortality risk [14]. The authors noted that late presentation was more likely in Black and Asian ethnicities, in males and in those with obesity. This may also be a reason why in-hospital mortality rates have fallen particularly in certain groups; as awareness of COVID-19 symptoms increased, people presented earlier.
Our study does not provide evidence as to why the influence of ethnicity on in-hospital mortality declined over time. However, it is notable that the changes in-hospital mortality rates mirror changes in the proportion of patients from these ethnic groups. There were large falls in the proportion of Black ethnicity patients and large increases in the proportion of patients of Pakistani ethnic background. The number of cases in regions of England will explain some of these changes, with relatively low cases numbers in London and the Midlands (where the proportion of Black ethnic group residents is relatively high) and higher cases number in the north of England during June-September. Furthermore, the proportion of admitted patients from a particular ethnic or socioeconomic group would be expected to mirror patterns of local community transmission of the virus. It may be that these transmission patterns also impact on mortality risk once infected, with viral load and physiological response to infection in certain populations possible mediators in the relationship. Improved health messaging towards hard to reach ethnic groups may also have played a role [15].
Comparing March-May to June September, the risk of in-hospital mortality declined for all patient groups, although the declines were notably greater or more modest than the general picture for patients with certain comorbidities when compared to those without the comorbidity. This is likely to partly reflect changes in management of patients with these comorbidities during the pandemic rather than management of COVID-19 itself. The increase in the relative odds of in-hospital mortality in patients with metastatic cancer with COVID-19 between the two time periods could be attributed to delays in cancer diagnosis [16] and treatment with restrictions in operating capacity [17] and reduction in initiation of chemotherapy treatment [18]. Whereas, the reduction in relative odds of in-hospital mortality in patients with diabetes may be due to early identification of glycaemic control as a prognostic factor for survival in patients with severe COVID-19 [[19], [20], [21]] and national efforts to ensure management of diabetic patients in hospital and the community was prioritised [22,23]. The relatively large odds of in-hospital mortality in patients with moderate or severe liver disease is likely to reflect the life-limiting nature of chronic liver disease.
Our findings are particularly relevant to the development of COVID-19 risk prediction algorithms. Within a short period of time, the relative importance of certain risk factors (e.g. diabetes, obesity, cerebrovascular disease) appears to be changing and these changes need to be accounted for in the models developed [[24], [25], [26]]. We emphasise the point that any risk prediction model is only valid within the population it was developed and tested in. If the characteristics of the population appear to be changing, then the model should be re-evaluated and adapted as required. Failure to do so may result in poor decision making at a patient level.
4.1. Strengths and limitations
HES data cover all NHS-funded hospital activity in England. As such they are the most complete and detailed record of hospital activity in England related to COVID-19. The seven-month study period allowed us to look at temporal trends over an extended period covering the initial surge in cases in Spring 2020 and a period of relatively low activity during summer and early autumn.
The completeness of our dataset will help to minimise collider bias when considering hospital populations [27]. However, differences in case mix across the two time periods (when compared directly) means that collider bias is likely in this analysis. As such a cautious interpretation is advised. More importantly, our findings should not be extrapolated to community-based cohorts, where the pattern of community transmission within high risk groups is likely to be a key determinant of the mortality rate. By considering only those ill enough to require hospital admission, hospital-based studies do not consider the structure of the background population or the extent to which infection levels of the SARS-Cov-2 virus are disproportionately high in certain groups. Although illness severity at presentation, health-seeking behaviour, hospital admissions policies and improved treatment are likely to be important mediators/confounders of the findings in both hospital-based and community-based studies, their relative importance in each setting will be markedly different. Nevertheless, insight can be gained when comparing the findings of hospital-based and community-based studies, provided one is aware of such biases and how the two populations relate to one another [4,5]. In addition, the associations reported here should not be interpreted as being causal.
As with any administrative dataset, there are limitations in using HES data. HES relies on individual hospital trusts compiling data accurately and in an internally and externally consistent manner. HES data are entered by trained coders based on clinical information. Only if this is recorded accurately will HES data be reliable. HES provides information on the admission diagnosis and major pre-existing diagnoses relevant to the admission. It provides no detailed information on signs and symptoms or how acutely unwell the patient was on presentation. As such we are unable to adjust for these factors. As lockdown measures were introduced and eased across England and awareness of COVID-19 increased, patterns of disease severity on admission are likely to have varied with time and contributed to the patterns of mortality reported.
We included patients who were diagnosed with COVID-19 though testing and on clinical grounds. This avoided any bias due to limited testing capacity early in the pandemic but may have identified some patients where the presence of COVID-19 was uncertain. However, the vast majority of patients in our dataset had COVID-19 confirmed by a test and this is unlikely to be a major bias in our findings. By including all recorded COVID-19-related hospitalisations our data should be as complete a record of the pandemic as is possible.
5. Conclusions
Our study provides insight into the changing nature of the COVID-19 pandemic as experienced in hospitals in England. In-hospital mortality rates fell substantially from March to June and were relatively stable from July to September. However, over the winter months COVID-19 related in-hospital mortality rates will have been influenced by the opposing forces of the emergence of SARS-CoV-2 variants with a higher transmission rate [28] coupled with the usual increased pressures on hospitals typical of the season and the mass roll out of newly introduced vaccinations to high risk groups [29,30]. The changing patterns of in-hospital mortality and the changing nature of the association between in-hospital mortality and key risk factors should be borne in mind if risk scales are used to triage patients.
Contributors
This study was designed and organised by AN, WKG, JD and TWRB. Data cleaning, analysis was by WKG, supported by JD. Writing of the first draft was by AN and WKG. All authors critically reviewed the manuscript and agreed to submission of the final draft.
Data availability statement
This report does not contain patient identifiable data. Consent from individuals involved in this study was not required. Requests for any underlying data cannot be granted by the authors because the data were acquired from data under licence/data sharing agreement from NHS Digital, for which conditions of use (and further use) apply. Individuals and organisations wishing to access HES data can make a request directly to NHS Digital.
Informed consent
Informed consent was not sought for the present study because it was an analysis of routine administrative data.
Ethical approval
Ethical approval was not sought for the present study because it did not directly involve human participants. This study was completed in accordance with the Helsinki Declaration as revised in 2013.
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
We acknowledge NHS Digital for permission to use their data in this report. The GIRFT programme is providing a framework for examining contemporary clinical practice in unprecedented detail and breadth. We also thank all staff within individual NHS trusts who collected and entered the data used in this study and GIRFT Clinical leads for advice: Michael Jones, Philip Dyer, Cliff Mann, Chris Moutlon, Anna Batchelor, Michael Swart, Christopher Snowden, Martin Allen, Adrian Hopper, Partha Kar, Gerry Rayman, Shona MacKinnon, Andrew Wheeler, Sue Eve-Jones and GIRFT Clinical fellows: Ini Adelaja and Pratusha Babu.
References
- 1.Atkins J.L., Masoli J.A.H., Delgado J. Preexisting comorbidities predicting COVID-19 and mortality in the UK biobank community cohort. J Gerontol A Biol Sci Med Sci. 2020 doi: 10.1093/gerona/glaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williamson E.J., Walker A.J., Bhaskaran K. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Docherty A.B., Harrison E.M., Green C.A. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zakeri R., Bendayan R., Ashworth M. A case-control and cohort study to determine the relationship between ethnic background and severe COVID-19. EClinicalMedicine. 2020;28 doi: 10.1016/j.eclinm.2020.100574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navaratnam A.V., Gray W.K., Day J., Wendon J., Briggs T.W.R. Patient factors and temporal trends associated with COVID-19 in-hospital mortality in England: an observational study using administrative data. Lancet Respir Med. 2021;9(4):397–406. doi: 10.1016/S2213-2600(20)30579-8. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Information Standard Board for Health and Social Care . NHS Digital; London, UK: 2013. Anonymisation Standard for Publishing Health and Social Care Data Specification (Process Standard) [Google Scholar]
- 7.NHS England. COVID-19 Daily Deaths. 2020. https://www.england.nhs.uk/statistics/statistical-work-areas/covid-19-daily-deaths/ (accessed 4th November 2020).
- 8.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 9.Quan H., Li B., Couris C.M. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 10.Dennis J.M., McGovern A.P., Vollmer S.J., Mateen B.A. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med. 2020 doi: 10.1097/CCM.0000000000004747. Published online 26th October. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horwitz L.I., Jones S.A., Cerfolio R.J. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2020 doi: 10.12788/jhm.3552. [DOI] [PubMed] [Google Scholar]
- 12.Doidge J.C., Gould D.W., Ferrando-Vivas P. Trends in intensive care for patients with COVID-19 in England, wales and Northern Ireland. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202008-3212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Office for National Statistics. Coronavirus (COVID-19) infection survey: characteristics of people testing positive for COVID-19 in england: october 2020. 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19infectionsinthecommunityinengland/october2020 (accessed2020).
- 14.Alaa A., Qian Z., Rashbass J., Benger J., van der Schaar M. Retrospective cohort study of admission timing and mortality following COVID-19 infection in England. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-042712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khunti K., Singh A.K., Pareek M., Hanif W. Is ethnicity linked to incidence or outcomes of covid-19? BMJ. 2020;369:m1548. doi: 10.1136/bmj.m1548. [DOI] [PubMed] [Google Scholar]
- 16.Maringe C., Spicer J., Morris M. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modeling study. Lancet Oncol. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.COVIDSurg Collaborative Elective surgery cancellations due to the COVID-19 pandemic: global predictive modeling to inform surgical recovery plans. Br J Surg. 2020;107(11):1440–1449. doi: 10.1002/bjs.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark J.J., Dwyer D., Pinwill N., Clark P., Johnson P., Hackshaw A. The effect of clinical decision making for initiation of systemic anticancer treatments in response to the COVID-19 pandemic in England: a retrospective analysis. Lancet Oncol. 2020 doi: 10.1016/S1470-2045(20)30619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barron E., Bakhai C., Kar P. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holman N., Knighton P., Kar P. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dennis J.M., Mateen B.A., Sonabend R. Type 2 diabetes and COVID-19-related mortality in the critical care setting: a national cohort study in England, March-July 2020. Diabetes Care. 2020;44(1):50–57. doi: 10.2337/dc20-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rayman G., Lumb A., Kennon B. Guidelines for the management of diabetes services and patients during the COVID-19 pandemic. Diabet Med. 2020;37(7):1087–1089. doi: 10.1111/dme.14316. [DOI] [PubMed] [Google Scholar]
- 23.Choudhary P., Wilmot E.G., Owen K. A roadmap to recovery: ABCD recommendations on risk stratification of adult patients with diabetes in the post COVID-19 era. Diabet Med. 2020:e14462. doi: 10.1111/dme.14462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clift A.K., Coupland C.A.C., Keogh R.H. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020;371:m3731. doi: 10.1136/bmj.m3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knight S.R., Ho A., Pius R. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: development and validation of the 4C mortality score. BMJ. 2020;370:m3339. doi: 10.1136/bmj.m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Razavian N., Major V.J., Sudarshan M. A validated, real-time prediction model for favorable outcomes in hospitalized COVID-19 patients. NPJ Digit Med. 2020;3:130. doi: 10.1038/s41746-020-00343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffith G.J., Morris T.T., Tudball M.J. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun. 2020;11(1):5749. doi: 10.1038/s41467-020-19478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahase E. Covid-19: what have we learnt about the new variant in the UK? BMJ. 2020;371:m4944. doi: 10.1136/bmj.m4944. [DOI] [PubMed] [Google Scholar]
- 29.Voysey M., Clemens S.A.C., Madhi S.A. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majeed A., Molokhia M. Vaccinating the UK against covid-19. BMJ. 2020;371:m4654. doi: 10.1136/bmj.m4654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This report does not contain patient identifiable data. Consent from individuals involved in this study was not required. Requests for any underlying data cannot be granted by the authors because the data were acquired from data under licence/data sharing agreement from NHS Digital, for which conditions of use (and further use) apply. Individuals and organisations wishing to access HES data can make a request directly to NHS Digital.