Abstract
Purpose:
We hypothesized that autophagy inhibition would increase response to chemotherapy in the preoperative setting for patients with pancreatic adenocarcinoma. We performed a randomized controlled trial to assess the autophagy inhibitor hydroxychloroquine in combination with gemcitabine and nab-paclitaxel.
Experimental Design:
Participants with potentially resectable tumors were randomized to two cycles of nab-paclitaxel and gemcitabine (PG) alone or with hydroxychloroquine (PGH), followed by resection. The primary endpoint was histopathologic response in the resected specimen. Secondary clinical endpoints included CA 19-9 serum biomarker response and margin negative R0 resection. Exploratory endpoints included markers of autophagy, immune infiltrate, and serum cytokines.
Results:
Thirty-four patients in the PGH arm and 30 in the PG arm were evaluable for the primary endpoint. The PGH arm demonstrated statistically improved Evans grade histopathologic responses (P = 0.00016), compared to control. In patients with elevated CA 19-9, a return to normal was associated with improved overall and recurrence-free survival (P < 0.0001). There were no differences in serious adverse events between arms and chemotherapy dose number was equivalent. The PGH arm had greater evidence of autophagy inhibition in their resected specimens (increased SQSTM1, P = 0.027, as well as increased immune cell tumor infiltration, P = 0.033). OS (P = 0.59) and RFS (P = 0.55) did not differ between the two arms.
Conclusions:
The addition of hydroxychloroquine to preoperative gemcitabine and nab-paclitaxel chemotherapy in patients with resectable pancreatic adenocarcinoma resulted in greater pathological tumor response, improved serum biomarker response, and evidence of autophagy inhibition and immune activity.
Introduction
Autophagy is an intracellular process by which damaged organelles and protein aggregates are sequestered in autophagic vesicles and the contents degraded and recycled following fusion with lysosomes.1 Autophagy promotes cell survival when the cell is stressed or damaged, such as in cancer cells. Autophagy is elevated in pancreatic ductal adenocarcinomas (PDAs) and pancreatic tumors, with higher levels of autophagic flux associated with worse overall survival (OS).2-5 Preclinical and early clinical studies support the notion that targeting autophagy may be a useful strategy in treating PDA.2,6-8,9,10 There is emerging evidence that autophagy inhibition can also enhance antitumor immunity, but to date, no clinical data support an associated increase in inflammatory cells within the tumor microenvironment.
Chloroquine and its derivatives, including hydroxychloroquine (HCQ), are synthetic 4-aminoquinolines initially developed for malaria prophylaxis. They are inexpensive, orally available drugs with a wide therapeutic index. Chloroquine blocks the acidification of lysosomes, thus inhibiting the last step in autophagy. Chloroquine or combinations of chloroquine and chemotherapy demonstrate antitumor properties in orthotopic transplantable and genetically engineered models of pancreatic cancer.1 Human tumor xenografts respond to combinations of HCQ and chemotherapy.11,12 We previously conducted a phase I/II trial examining preoperative gemcitabine in combination with oral HCQ for the treatment of patients with high-risk PDA (UPCI 09-122/ NCT01128296).8 Clinical outcomes were improved compared to those from a previously established cohort of high-risk patients at our institution.13
Based on these promising results, we launched a randomized phase II trial of neoadjuvant gemcitabine/nab-paclitaxel with HCQ (and designated the treatment as PGH) or without HCQ (designated as PG; trial identifier: UPCI 13-074/NCT01978184) to better assess the contribution of the inhibition of autophagy by HCQ to gemcitabine/nab-paclitaxel chemotherapy.
Patients and Methods
Study design and participants
This was an open-label, randomized controlled trial performed at a single large academic medical center in the midwestern United States. The trial was approved by the protocol review committee and IRB (IRB 13-074) at the Hillman Cancer Center at the University of Pittsburgh Medical Center and was registered with clinicaltrials.gov (NCT01978184). The full trial protocol is available on request. The trial was performed in full accordance with guidelines for good clinical practice and the Declaration of Helsinki. Written informed consent was obtained from all patients prior to any protocol treatment.
Patients were considered eligible for the trial if they met the National Comprehensive Cancer Network (NCCN) criteria for resectable or borderline resectable operation based on a contrast-enhanced helical chest, abdomen, and pelvis CT scan done using a pancreas mass protocol. An endoscopic ultrasound (EUS)-guided biopsy and histologic confirmation of malignancy were required. The full list of eligibility criteria can be found in the protocol.
Randomization
The trial was designed to adaptively randomize participants to either PG or PGH using the method published by Thall and Wathen.14 After 20 participants were randomized 1:1, the probability of randomization was to adapted to the probability of Evans Grade 4 histologic responses in each arm. The trial was powered to recruit 60 participants evaluable for response for a power of at least 80% for an odds ratio of 3.5 or greater on the primary endpoint. No participant in either arm experienced a grade 4 histologic response, so the randomization adaptation was not significant.
Trial procedures
Sixty-four patients were randomized to receive two cycles of gemcitabine and nab-paclitaxel (1000 mg/m2 and 125 mg/m2, respectively, on days 1, 8, and 15 of each monthly cycle) with or without the highest FDA allowable dose of HCQ (1200 mg, 600 mg twice daily) from day 1 through the evening before planned surgical extirpation. In an expansion cohort, 7 patients were separately treated with PGH alone and are reported separately in the appendix. Patients were restaged with a helical CT scan 14 days after the last dose of chemotherapy and prior to surgery. Surgical exploration and pancreatectomy were then performed if technically feasible, there was no evidence of metastatic disease, and all toxicities had resolved. Pathologic specimens were preserved, and the entire tumor was submitted for evaluation. Six to 10 weeks following the completion of successful surgical removal of their tumor, patients were free to pursue standard-of-care adjuvant therapy options at the discretion of their treating physician.
Participants were deemed evaluable for response if they had received at least one cycle of chemotherapy, took at least 80% of the expected HCQ doses, and underwent successful surgical extirpation of their disease.
Endpoints
The primary endpoint for this trial was the Evans grade histologic response,15 which was scored by a single expert pancreatic pathologist blinded to patients’ treatment arm (the grading system is reproduced in Supplemental Table 1). Secondary endpoints were the change in serum CA 19-9, lymph node involvement, and R0 resection. Exploratory endpoints included evidence of autophagy inhibition, immune infiltrate, serum cytokines and chemokines, pre/posttreatment SII,16 and OS and recurrence-free survival (RFS).
Statistical analysis
The effect of treatment on Evans grade (the primary endpoint) was assessed by Fisher’s exact test and a cumulative logit model, an extension of logistic regression appropriate for ordinal multinomial endpoints. In secondary analyses, the effect of baseline clinical, demographic, and biomarker variables on the relationship between treatment and Evans grade was assessed by adding those variables to the primary endpoint model. OS and RFS were characterized by the product-limit (Kaplan-Meier) estimator and compared between arms by log-rank tests. Wilcoxon tests were employed when the assumptions for the validity of the t test were violated. All point estimates are accompanied by appropriate confidence intervals. The median follow-up time for the study patients is 39.7 months.
Serum biomarkers
A Thermo Fisher (Grand Island, NY) multiplexed ProcartaPlex Immunoassay measured 64 cytokines and chemokines as well as 14 soluble checkpoint inhibitors on pre- and posttreatment sera from participants. Results were tabulated based on changes from pre-therapy (prior to chemotherapy) to post-therapy, broken down by whether patients had been randomized to receive or not receive HCQ.
Immunohistochemistry markers of autophagy in resected specimens
FFPE tissue section slides were deparaffinized in two changes of xylene for 15 minutes each, followed by rehydration in an ethanol gradient (100% ethanol, 95% ethanol, 70% ethanol, water). Antigen retrieval was used as appropriate for the individual antigen. Additional details regarding the immunohistochemical methods employed are in the supplemental materials Methods for calculating the immune infiltration score can be found in the supplemental methods.
Results
Between March 2014 and November 2018, a total of 98 participants were randomly assigned to treatment (Figure 1). Of study participants, the 92 who received at least one dose of study drug were evaluable for safety. In all, 18 patients (35%) in the PGH arm and 16 (35%) in the PG arm did not complete the protocol requirements to be considered for the primary endpoint. Reasons for attrition were 1) disease progression (4 PGH patients [8%] vs. 4 PG [9%]), 2) patient preference (7 PGH [13%] vs. 5 PG [11%]), and 3) regimen-limiting toxicity or decline in performance status (5 PGH [10%] vs. 2 PG [6%]). Four patients in the PG arm had surgery out of the preplanned window. Two patients in the PGH arm were found to have a non-PDA periampullary cancer at surgery, and 1 patient who received PG elected to pursue a different therapy.
Figure 1. Consort diagram.
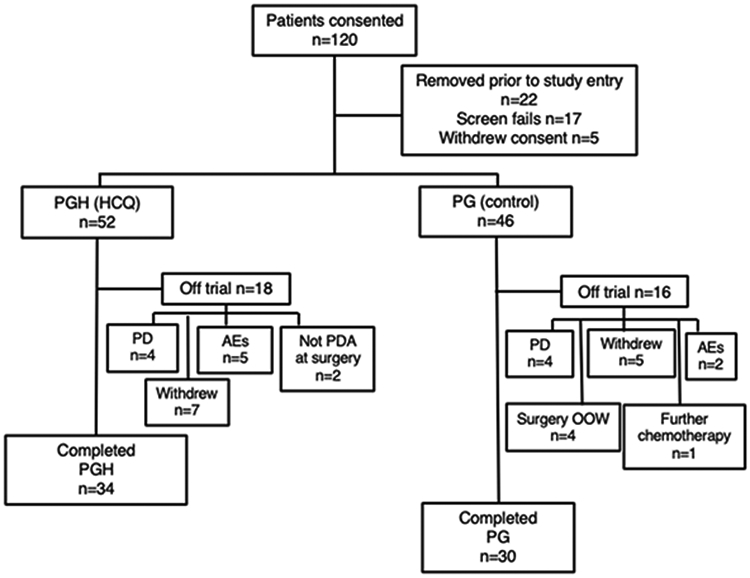
Patients were randomized to receive nab-paclitaxel and gemcitabine with hydroxychloroquine (PGH; 52 patients) or without hydroxychloroquine (PG; 46 patients). A total of 34 PGH and 30 PG patients successfully completed the regimen and were taken to operation for resection of pancreatic cancer. Abbreviations: AE, adverse event; HCQ, hydroxychloroquine; OOW, out of window; PD, progressed disease; PDA, pancreatic ductal adenocarcinoma
A total of 64 randomized participants completed the study treatment, went to surgery, had pathologically confirmed PDA, and were evaluable for the primary and secondary efficacy endpoints. Thirty-four patients completed the PGH arm and 30 completed the PG arm. The randomization process did not result in any significant imbalances in prognostic factors such as endoscopic ultrasound results for size of tumor, tumor location, vascular involvement (borderline vs. resectable), or robotic procedure. (Table 1) There was no difference in the number of cycles of chemotherapy completed by patients in either arm (data not shown).
Table 1.
Demographics and baseline assessment of efficacy-evaluable participants. Means are accompanied by 95% confidence intervals. CCI=Charlson Comorbidity Index, EBL=estimated blood loss, EUS=endoscopic ultrasound, PG=gemcitabine+nab-paclitaxel, PGH=hydroxychloroquine+gemcitabine+nab-paclitaxel
| PG (n=30) |
PGH (n-34) |
P | ||
|---|---|---|---|---|
| Age at Diagnosis | 64 (37-83) | 67 (45-80) | 0.41 | |
| Gender | F | 14 (48%) | 15 (44%) | 1.00 |
| M | 16 (46%) | 19 (56%) | ||
| Race | Asian | 1 (3.3%) | 0 (0%) | 0.70 |
| Black | 0 (0%) | 1 (2.9%) | ||
| White | 27 (90%) | 33 (97.1%) | ||
| CCI (adjusted) | 2 | 1 (3.3%) | 1 (2.9%) | 0.75 |
| 3 | 8 (27%) | 7 | ||
| 4 | 5 (17%) | 3 (8.8%) | ||
| 5 | 8 (27%) | 15 (44%) | ||
| 6 | 5 (17%) | 5 (15%) | ||
| 7 | 3 (10%) | 2 (5.8%) | ||
| 9 | 0 (0%) | 1 (2.9%) | ||
| EUS Size (cm) | 2.6 (1.2-5.1) | 2.7 (1.2-5.5) | 0.79 | |
| Tumor Location | Head | 25 (83%) | 31 (91%) | 0.62 |
| Body | 3 (10%) | 2 (5.8%) | ||
| Tail | 2 (6.7%) | 1 (2.9%) | ||
| CT vasc. involvement | 17 (57%) | 15 (44%) | 0.45 | |
| Robotic procedure | 22 (73%) | 24 (71%) | 1.00 | |
| Vein resection | 16 (53%) | 13 (38%) | 0.32 | |
| EBL | 162 (20-2000) | 150 (20-1000) | 0.49 | |
| TNM | T1N0 | 0 (0%) | 1 (2.9%) | 0.22 |
| T1N1 | 3 (10%) | 0 (0%) | ||
| T2N0 | 4 (13%) | 2 (5.8%) | ||
| T2N1 | 3 (10%) | 7 (21%) | ||
| T3N0 | 6 (20%) | 10 (29%) | ||
| T3N1 | 14 (47%) | 13 (38%) | ||
| TxN0 | 0 (0%) | 1 (2.9%) | ||
| Stage | IA | 0 (0%) | 2 (5.8%) | 0.12 |
| IB | 5 (17%) | 1 (2.9%) | ||
| IIA | 6 (20%) | 11 (32%) | ||
| IIB | 19 (63%) | 20 (59%) | ||
Data reported as n(%) or median (range).
Supplemental Table 2 demonstrates that adverse events were not different between arms (P = 0.44 from a cumulative logit model). Supplemental Table 3 lists all grade 3-5 adverse events for all treated patients, with no significant differences demonstrated. HCQ did not add to the toxicity of chemotherapy. Patients in the PGH arm were more likely, but not statistically signficantly, to receive nab-paclitaxel as postoperative adjuvant therapy (Supplemental Table 4).
The primary endpoint for this trial was histopathologic response as described by Evans et al.15,17,18 There were 19 (55.9%) patients in the PGH arm who had an Evans IIB grade (>50% destruction of tumor cells) or greater response, versus 3 (10%) participants in the PG arm. No patients in the PG arm had an Evans grade III response (>90% destruction) versus 7 (20.6%) patients in the PGH arm. Overall, Evans grade histopathological response was statistically different between the arms, favoring PGH (Figure 2; Fisher’s exact test, P = 0.004). A cumulative logit model, which considers the ordering of the grades, confirmed the superiority of the PGH arm (P = 0.00016).
Figure 2. Evans grade response in resected patients.
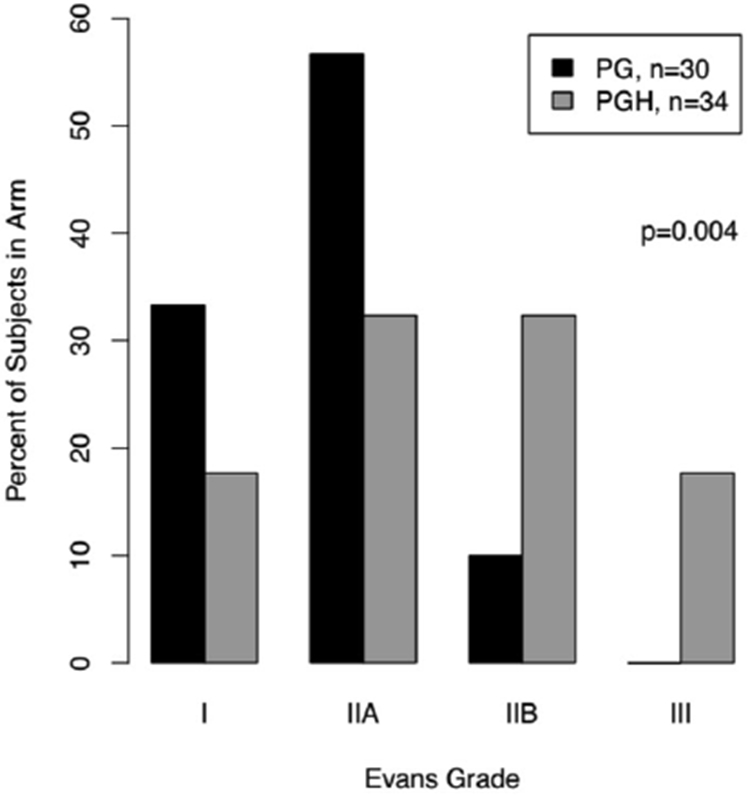
The primary endpoint for this trial was the evaluation of the Evans grade pathologic response by a blinded expert pancreatic pathologist. Patients receiving chemotherapy and hydroxychloroquine (PGH) demonstrated a statistically significant better pathologic response than those receiving chemotherapy alone (PG). The P value is from Fisher’s exact test.
An additional 13 patients were enrolled in a nonrandomized expansion cohort of the PGH arm following completion of the randomized trial. Of these, 7 receved their chemotherapy and underwent surgical resection. Their baseline demographics and outcomes were consistent with those of the patients randomized to the PGH arm (Supplemental Tables 3 and 5 and Supplementary Figures 1-3). Due to the small number of nonrandomized patients, further analyses were not undertaken.
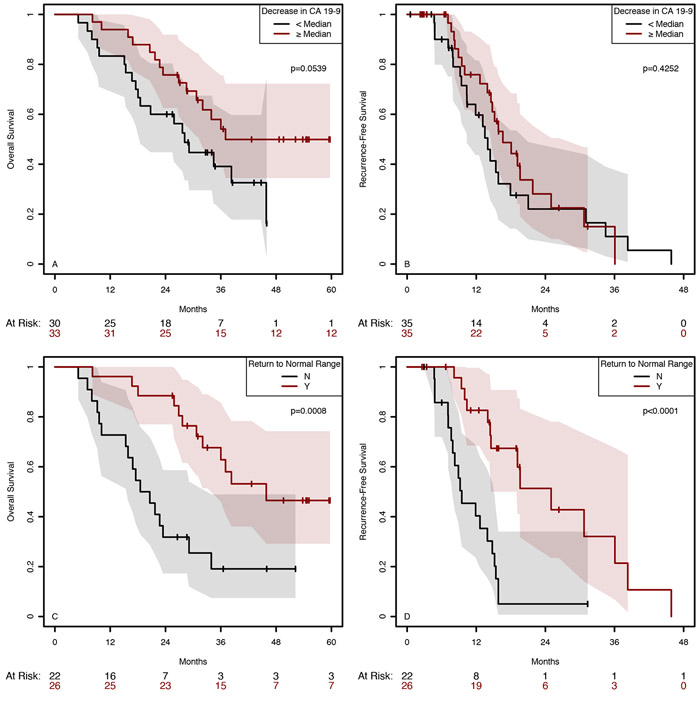
Secondary endpoints included the change in pre- and posttreatment CA19-9 as well as the rate of R0 resection. Prior to treatment, a higher CA19-9 was seen overall (a negative prognostic factor) in the PGH arm (1320 U/mL; 95% CI 260–2380) than in the PG arm (352 U/mL; 95% CI 38–665; Figure 3A; P = 0.019) but no differences were seen in CA19-9 between treatment arms following treatment (Figure 3B). After treatment, a statistically significant decrease in CA19-9, a positive prognostic factor (Figure 3C) favored the PGH arm. CA 19-9 generally decreased for participants in both treatment arms, with the exception of 5 participants in each arm. One participant in each arm experienced a greater than 100-fold increase in CA 19-9 (data not shown). The decrease in CA 19-9 was greater in the PGH arm (Wilcoxon test, P = 0.001; Figure 3C). In those patients with elevated CA 19-9 levels at baseline (>37 U/mL), a similar decrease was observed (Wilcoxon test, P = 0.017). In a cumulative logit model, the drop in CA 19-9 trended with a better Evans grade histopathologic response (Figure 3D). There was no statistically significant difference between the PGH and PG treatment arms in the proportion of participants who underwent an R0 resection (82% vs. 70%, P = 0.38; Supplemental Table 6)
Figure 3. Pre- and posttreatment CA 19-9 levels by treatment arm and Evans grade response.
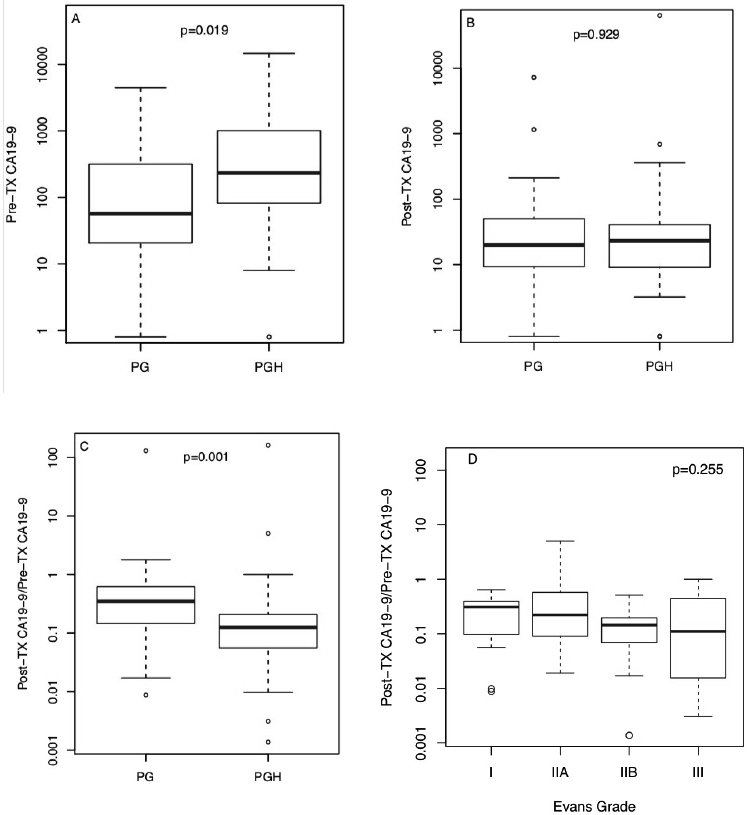
A) Pre-treatment CA 19-9 levels; B) Post-treatment CA 19-9 levels; C) Ratio between post-treatment and pre-treatment CA 19-9 levels. D) Correlation of CA19-9 response to Evan’s grade. The P values are from Wilcoxon tests.
The trial was not powered to detect differences in survival nor did the protocol prescribe adjuvant therapy post resection. The proportion of patients receiving any adjuvant chemotherapy and type of adjuvant chemotherapy was not statistically different between the arms (Supplemental Table 4). At the time of submission for publication, 19 patients (46%) in the PGH arm and 17 (57%) in the PG arm had succumbed to disease. Median OS was 32 months (95% CI 20.8–NA) in the PG arm and 36 months (95% CI 28.2–NA) in the PGH arm (P = 0.59). Similarly, the difference in RFS between the arms was not statistically significant (P = 0.55); the median RFS was 13.5 months (95% CI 9.5–34.5) in the PG arm and 16.6 months (95% CI 14.6–25) in the PGH arm (Supplemental Figure 4). On an intention to treat (ITT) basis, median OS in the PG arm is 24 months (95% CI=(17, NA)) and median OS in the HCQ arm is 31 months (95% CI=(23, NA), which were not statistically different (P<0.34). A decrease in CA19-9 greater or equal to the median CA19-9 was associated with an OS benefit (P = 0.0539) but not PFS (P = 0.43) (Figure 4A and B). However, in patients whose CA19-9 was elevated above normal at baseline, a return to normal was even further significantly associated with both improved RFS (P < 0.0001) and OS (P = 0.0008; Figure 4C and D). An Evans grade of IIB or better was associated with better OS and RFS, but the effects were not statistically significant (P = 0.29 and 0.15, respectively; Supplemental Figure 5). The proportion of patients who had any lymph node involvement was smaller in the PGH arm than in the PG arm (58.8% vs. 80%; P = 0.10) but did not reach statistical significance (Supplemental Table 7).
Figure 4. Decrease in CA 19-9 and link to overall and recurrence-free survival.
Those patients with greater than the median decrease in CA 19-9 demonstrated improved overall survival but not recurrence-free survival. Those patients who normalized their CA19-9 with treatment had both better OS and RFS.
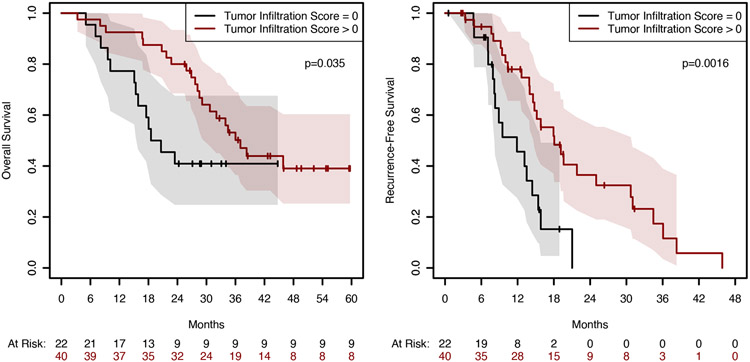
We examined a subset of the resected tumor specimens for markers of autophagy, apoptosis, and immune response (Supplemental Figure 6 and Supplemental Table 8). An increased percentage of cells positive for cytoplasmic SQSTM1 was observed in the PGH-treated patients, (43.6; 95% CI 31.7–55.6; vs. 27.5; 95% CI 16.6–38.8; P = 0.027). When examining the tumor infiltration index, HCQ increased the probability of having non-zero tumor infiltration from 0.5 (15/30) to 0.78 (25/32), with a Fisher’s exact test of P = 0.033. Moreover, a higher tumor immune infiltration score was associated with statistically improved RFS and OS (Figure 5). We also found a strong correlation between cleaved caspase 3 (CC3), HMGB1, and SQSTM1/p62 cytoplasmic staining, CD4 infiltration, and stromal immune score, and OS for all patients (Supplemental Table 9). SQSTM1/p62 in the cytoplasm correlated with worse OS (HR 1.03; 95% CI for HR 1·011–1·055; P = 0.007). The stromal infiltration score inversely correlated with survival with a HR of 4.3 (95% CI 1·59–9.10; P = 0.002). Both cytoplasmic and nuclear P62 are statistically significant, after accounting for treatment arm, in the model for Evans’ Grade, p=0.03. We also examined cytoplasmic HMGB1 because of its known role in promoting autophagy. Supplemental Figure 7 demonstrates that there was more retained nuclear HMGB1 in PGH patients.
Figure 5. Tumor immune infiltration score correlates with overall survival.
All resected tumors were evaluated for tumor immune infiltration index. A tumor infiltration index of greater than 0 was positively correlated with recurrence-free survival and overall survival.
As shown in Supplemental Figures 8 and 9, an increase in serum-soluble PD-L1 of greater than 25% (pretreatment to posttreatment) was associated with worse OS (HR 3·06; 95% CI for HR 1·18–7·98; P = 0.04) and RFS (HR 6·60; 95% CI for HR 2·38–18·20; P = 0.002), while an increase in hepatocyte growth factor (HGF) of greater than 50% was associated with improved OS (HR 0·31; 95% CI for HR 0·11–0·88; P = 0.01) and RFS (HR 0·41; 95% CI for HR 0·17–0·97; P = 0.02).
Discussion
The addition of the autophagy inhibitor HCQ to preoperative gemcitabine and nab-paclitaxel improves pathological response in the tumor specimen, serum and CA 19-9 response. This confirms our previous clinical observations.8 This randomized trial provides strong evidence that autophagy is an important therapeutic target in patients with pancreatic cancer. In addition, the enhanced immune infiltrate we observed suggests a possible immunomodulatory effect of the regimen.
We utilized the pathologic response system described by Evans et al.,15 whereby complete pathologic response rates have been correlated with improved OS in retrospective series.18 We observed a remarkable improvement (P = 0.00016) in the proportion of patients demonstrating a IIB or greater response in the cohort receiving the combination of HCQ and chemotherapy. Evans grade response in this trial was statistically correlated with serum CA 19-9 response, which was associated with better OS. These findings support the use of this pathologic endpoint as an important surrogate marker of oncologic outcome in pancreatic cancer trials. Our findings suggest that the entire Evans grade scale of pathologic response has prognostic implications. No patients in this trial who received the prescribed two cycles of chemotherapy demonstrated a complete pathologic response. However, a complete pathologic response to neoadjuvant therapy in PDA is rare.
In numerous retrospective series, both the absolute level as well as the change in levels of serum glycoprotein CA 19-9 correlate with survival in pancreatic ductal adenocarcinoma. Our trial adds to the growing number of prospective trials demonstrating a correlation between changes in CA 19-9 and survival.19-21 We observed a significant correlation between decreases in CA 19-9 during treatment and OS. In addition, CA 19-9 decline had a significant correlation with Evans grade pathologic response. These observations suggest that, in the absence of radiation therapy, the response in the tumor to systemic chemotherapy mirrors the systemic response and supports the use of CA 19-9 to accelerate the completion of future trials.
There are many advantages to the evaluation of novel agents in the preoperative setting that we sought to exploit in this study. Preoperative chemotherapy has also been suggested to be the single most important factor for predicting survival in PDA patients.22 Presurgical window-of-opportunity trials, such as this one, allow earlier treatment of patients, while they have a better performance status and allow a complete evaluation of the resected tumor. In contrast, most new treatments for PDA are evaluated in the metastatic setting, by which time patients’ performance status and immunity are significantly diminished.
Overall and progression-free survival were not improved by the neoadjuvant addition of HCQ to the backbone of nab-paclitaxel and gemcitabine. In this study, we did not control for adjuvant therapy; adjuvant chemotherapy was at the discretion of the treating oncologist. Only two patients in the PGH arm, and none in the PG arm, received FOLFIRINOX which is the current standard of care as of June 2018 as accrual was nearly complete by this time. The majority of patients received nab-paclitaxel and gemcitabine as adjuvant therapy (supplementary figure 4). Recently, nab-paclitaxel added to gemcitabine demonstrated no benefit over gemcitabine alone.23 Given the limitations of the adjuvant treatment; it is not surprising that 2 months of neoadjuvant therapy did not statistically effect PFS and OS, although a non-statistical trend towards improved survival in the PGH was observed. Planned studies to examine the benefit of the addition of HCQ to FOLFIRINOX compared to PGH will include adjuvant FOLFIRINOX with HCQ continued post operatively in those patients who received it neoadjuvantly. Together these changes may facilitate the identification of an RFS and OS advantage of HCQ added to these regimens. A recent study compared PG with PGH as first line therapy in metastatic pancreatic cancer.24 The results identified a PFS but not OS survival benefit to treatment with PGH compared with PG alone. One reason for the lack of an OS benefit may have been due to not continuing HCQ during subsequent lines of therapy. Alternatively, the differences in patient performance and the inability to render a metastatic patient macroscopically disease free may have affected an OS benefit to the addition of HCQ.
There are currently over 60 trials on https://clinicaltrials.gov/ that list autophagy as a target. All the trials examine HCQ or chloroquine administration. It is well-accepted that these drugs may have multiple off-target effects that make it difficult to fully conclude that autophagy is the critical target. We therefore examined markers of autophagic flux in the resected specimens as a means to correlate autophagic changes with the trial endpoints. During autophagy, the sequestosome protein SQSTM1/p62 targets ubiquitinated proteins to the autophagosome, and its accumulation is consistent with inhibition of this step.25 We did observe a statistically significant increase in the staining of cytoplasmic SQSTM1/p62 accumulation, associated with the administration of HCQ. Since both cytoplasmic and nuclear P62 are statistically significant, after accounting for treatment arm, in the model for Evans’ grade response, these studies support a critical role for autophagy inhibition as related to Evans grade response. Furthermore, these data suggest that maximizing autophagic inhibition in future studies may positively affect response and subsequently survival. We did not observe any differences between the two cohorts in staining for the common recycled autophagy marker LC3B II/ATG8. Similarly, no differences were found in markers of apoptosis. Both of these markers are themselves rapidly cleared by either autophagy itself or ingestion and clearance of phagocytosed apoptotic cells.
In this trial, the administration of HCQ correlated with increased immune cell infiltration within the tumor. Moreover, immune cell infiltration in the resected tumor correlated with improved OS and RFS, independent of treatment arm. These findings corroborate a recent report that demonstrate that the level of immune cell infiltration within resected pancreatic tumors correlates with OS.26 The lack of response to modern immune therapies in pancreatic cancer remains incompletely understood, but may be related to a combination of T-cell exclusion and the low number of synonymous mutations found in pancreatic cancer, with which a diminished response to checkpoint therapies has been associated.27 Animal models have suggested that activated stromal cells may form a barrier to T-cell infiltration.28 Interestingly, in murine PDAC models engineered to have genetic autophagy loss, a similar change in tumor immune infiltration was identified supporting these changes are due to autophagic inhibition.29,30 Future human trials based on the design of this trial may therefore incorporate more potent autophagy inhibitors, or a combination of HCQ with other agents. These may offer a platform for incorporating and analyzing rational immune therapies in pancreatic adenocarcinoma. We are now actively investigating if HCQ affected stromal activation markers and myeloid infiltrate in this trial. Given the positive correlation of OS and RFS with immune infiltration emerging in the literature, future window-of-opportunity trials of preoperative therapy may focus on this surrogate endpoint.
Multiple cytokines/chemokines and soluble checkpoint molecules were examined prior to and after therapy. We observed that increased HGF and PDL-L1 were associated with improved OS. The concept of HGF increase as a good prognostic factor is quite interesting since HGF increases proportionally with increased apoptotic death,31 something that is a predicted outcome of successful autophagy inhibition and effective chemotherapy. HGF and its receptor MET could possibly be important targets in patients with pancreatic cancer, 32-34 and serum levels could be important for following disease progression and response to treatment, as in other diseases. PD-L1 upregulation was also associated with lower OS.
This prospective randomized trial demonstrates that the addition of the autophagy inhibitor HCQ to preoperative gemcitabine and nab-paclitaxel improves pathologic and biomarker response in potentially resectable pancreatic cancer. It further supports the use of pathologic response and CA 19-9 as surrogate markers of survival in future trials of preoperative therapy. The development of novel autophagy inhibitors and their integration into therapeutic regimens should remain a priority for the field.
Supplementary Material
Translational Relevance.
Autophagy is a critical mediator of damage-associated molecular patterns (DAMP)-induced tumor cell survival. We previously conducted a Phase I/II trial examining preoperative gemcitabine in combination with oral hydroxychloroquine (HCQ) for treatment of high-risk PDA. Gemcitabine and HCQ resulted in improved overall survival compared with the prior cohort (34.83 vs. 12.27 months, P = 0.03). Based on these promising results, we launched this NCI-supported randomized phase II trial of neoadjuvant gemcitabine/nab-paclitaxel, with and without HCQ. This study demonstrates that addition of the autophagy inhibitor HCQ to preoperative gemcitabine and nab-paclitaxel improves pathological response in the tumor specimen, serum CA 19-9 response, and lymph node involvement. It also prospectively established the utility of CA19-9 decline in the neoadjuvant treatment setting, for prediction of overall survival. In addition, the enhanced immune infiltrate we observed suggests a possible immunomodulatory effect of the regimen that can be addressed in future studies.
Acknowledgments
This work was supported by the NIH (grant no. 1R01 CA181450; to M.T. Lotze and H. Zeh) R01GM115366 (Billiar/Tang) and R01CA206012 (Facciabene/Lotze) and by pancreatic philanthropic funds to the Hillman Cancer Center at the University of Pittsburgh Medical Center. The authors thank Dave Primm of the UT Southwestern Department of Surgery for help in editing this article.
Research support for the study: 1R01 CA181450 (Zeh/Lotze) “Pancreatic Ductal Adenocarcinoma Is a Disease of Constitutive Autophagy”
Footnotes
Preliminary findings were presented at the Society of Surgical Oncology meeting, 2016.
Disclosure of Potential Conflicts of Interests
We declare no competing interests.
Data sharing
Trial data are available from the authors by request.
References
- 1.Amaravadi RK, Lippincott-Schwartz J, Yin XM, et al. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res 2011; 17(4): 654–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mukubou H, Tsujimura T, Sasaki R, Ku Y. The role of autophagy in the treatment of pancreatic cancer with gemcitabine and ionizing radiation. Int J Oncol 2010; 37(4): 821–8. [DOI] [PubMed] [Google Scholar]
- 3.Viale A, Pettazzoni P, Lyssiotis CA, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature 2014; 514(7524): 628–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perera RM, Stoykova S, Nicolay BN, et al. Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature 2015; 524(7565): 361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boone BA, Zeh HJ 3rd, Bahary N. Autophagy Inhibition in Pancreatic Adenocarcinoma. Clin Colorectal Cancer 2018; 17(1): 25–31. [DOI] [PubMed] [Google Scholar]
- 6.Pardo R, Lo Re A, Archange C, et al. Gemcitabine induces the VMP1-mediated autophagy pathway to promote apoptotic death in human pancreatic cancer cells. Pancreatology 2010; 10(1): 19–26. [DOI] [PubMed] [Google Scholar]
- 7.Yang S, Kimmelman AC. A critical role for autophagy in pancreatic cancer. Autophagy 2011; 7(8): 912–3. [DOI] [PubMed] [Google Scholar]
- 8.Boone BA, Bahary N, Zureikat AH, et al. Safety and Biologic Response of Pre-operative Autophagy Inhibition in Combination with Gemcitabine in Patients with Pancreatic Adenocarcinoma. Ann Surg Oncol 2015; 22(13): 4402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashimoto D, Blauer M, Hirota M, Ikonen NH, Sand J, Laukkarinen J. Autophagy is needed for the growth of pancreatic adenocarcinoma and has a cytoprotective effect against anticancer drugs. Eur J Cancer 2014; 50(7): 1382–90. [DOI] [PubMed] [Google Scholar]
- 10.Thakur PC, Miller-Ocuin JL, Nguyen K, et al. Inhibition of endoplasmic-reticulum-stress-mediated autophagy enhances the effectiveness of chemotherapeutics on pancreatic cancer. J Transl Med 2018; 16(1): 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang S, Wang X, Contino G, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev 2011; 25(7): 717–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chi KH, Wang YS, Huang YC, et al. Simultaneous activation and inhibition of autophagy sensitizes cancer cells to chemotherapy. Oncotarget 2016; 7(36): 58075–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bao P, Potter D, Eisenberg DP, et al. Validation of a prediction rule to maximize curative (R0) resection of early-stage pancreatic adenocarcinoma. HPB (Oxford) 2009; 11(7): 606–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thall PF, Wathen JK. Practical Bayesian adaptive randomisation in clinical trials. Eur J Cancer 2007; 43(5): 859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breslin TM, Hess KR, Harbison DB, et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol 2001; 8(2): 123–32. [DOI] [PubMed] [Google Scholar]
- 16.Aziz MH, Sideras K, Aziz NA, et al. The Systemic-Immune-Inflammation Index Independently Predicts Survival and Recurrence in Resectable Pancreatic Cancer and its Prognostic Value Depends on Bilirubin Levels: A Retrospective Multicenter Cohort Study. Ann Surg 2018. [DOI] [PubMed]
- 17.Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Archives of surgery (Chicago, Ill : 1960) 1992; 127(11): 1335–9. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee D, Katz MH, Rashid A, et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer 2012; 118(12): 3182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiorean EG, Von Hoff DD, Reni M, et al. CA19–9 decrease at 8 weeks as a predictor of overall survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Ann Oncol 2016; 27(4): 654–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regine WF, Winter K, Abrams RA, et al. Postresection CA19–9 and margin status as predictors of recurrence after adjuvant treatment for pancreatic carcinoma: Analysis of NRG oncology RTOG trial 9704. Adv Radiat Oncol 2018; 3(2): 154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer TM, El-Rayes BF, Li X, et al. Carbohydrate antigen 19–9 is a prognostic and predictive biomarker in patients with advanced pancreatic cancer who receive gemcitabine-containing chemotherapy: a pooled analysis of 6 prospective trials. Cancer 2013; 119(2): 285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haeno H, Gonen M, Davis MB, Herman JM, Iacobuzio-Donahue CA, Michor F. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell 2012; 148(1–2): 362–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tempero MA, Reni M, Riess H, et al. APACT: phase III, multicenter, international, open-label, randomized trial of adjuvant nab-paclitaxel plus gemcitabine (nab-P/G) vs gemcitabine (G) for surgically resected pancreatic adenocarcinoma. Journal of Clinical Oncology 2019; 37(15_suppl): 4000-. [Google Scholar]
- 24.Karasic TB, O’Hara MH, Loaiza-Bonilla A, et al. Effect of Gemcitabine and nab-Paclitaxel With or Without Hydroxychloroquine on Patients With Advanced Pancreatic Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol 2019; 5(7): 993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wurzer B, Zaffagnini G, Fracchiolla D, et al. Oligomerization of p62 allows for selection of ubiquitinated cargo and isolation membrane during selective autophagy. Elife 2015; 4: e08941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tahkola K, Mecklin JP, Wirta EV, et al. High immune cell score predicts improved survival in pancreatic cancer. Virchows Arch 2018; 472(4): 653–65. [DOI] [PubMed] [Google Scholar]
- 27.Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med 2017; 377(25): 2500–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dauer P, Zhao X, Gupta VK, et al. Inactivation of Cancer-Associated-Fibroblasts Disrupts Oncogenic Signaling in Pancreatic Cancer Cells and Promotes Its Regression. Cancer Res 2018; 78(5): 1321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang A, Herter-Sprie G, Zhang H, et al. Autophagy Sustains Pancreatic Cancer Growth through Both Cell-Autonomous and Nonautonomous Mechanisms. Cancer Discov 2018; 8(3): 276–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo JY, Karsli-Uzunbas G, Mathew R, et al. Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis. Genes Dev 2013; 27(13): 1447–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogel S, Borger V, Peters C, et al. Necrotic cell-derived high mobility group box 1 attracts antigen-presenting cells but inhibits hepatocyte growth factor-mediated tropism of mesenchymal stem cells for apoptotic cell death. Cell Death Differ 2015; 22(7): 1219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takiguchi S, Inoue K, Matsusue K, Furukawa M, Teramoto N, Iguchi H. Crizotinib, a MET inhibitor, prevents peritoneal dissemination in pancreatic cancer. Int J Oncol 2017; 51(1): 184–92. [DOI] [PubMed] [Google Scholar]
- 33.Yang XP, Liu SL, Xu JF, Cao SG, Li Y, Zhou YB. Pancreatic stellate cells increase pancreatic cancer cells invasion through the hepatocyte growth factor /c-Met/survivin regulated by P53/P21. Exp Cell Res 2017; 357(1): 79–87. [DOI] [PubMed] [Google Scholar]
- 34.Modica C, Tortarolo D, Comoglio PM, Basilico C, Vigna E. MET/HGF Co-Targeting in Pancreatic Cancer: A Tool to Provide Insight into the Tumor/Stroma Crosstalk. Int J Mol Sci 2018; 19(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.