Abstract
Hydrogen-rich water is conventionally prepared by direct current-electrolysis, but has been not or scarcely prepared by alternating current (AC)-electrolysis. The AC preparations from tap water for 20–30 minutes exhibit a dissolved hydrogen concentration of 1.55 mg/L, which was close to the theoretical maximum value of 1.6 mg/L. These preparations also displayed an oxidation-reduction potential of –270 mV (tap water: +576 mV) and pH of 7.7–7.8, being closer to physiological values of body fluids than general types of direct current-electrolytic hydrogen-rich water. We examined whether AC-electrolytic hydrogen-water is retained for hydrogen-abundance after boiling or for antioxidant abilities, and whether the oral administration of this water is clinically effective for diabetes and prevention against systemic DNA-oxidative injuries. 5,5-Dimethyl-1-pyrroline-N-oxide spin trapping and electron spin resonance revealed that the hydrogen-rich water generated by AC-electrolysis exhibited hydroxyl-radical-scavenging activities. Laser nanoparticle tracking method revealed that nanoparticle suspensions as abundant as 5.4 × 107/mL were efficiently retained (up to 3.5 × 107/mL) even after boiling for 10 minutes, being thermodynamically contrary to Henry's law. Oral intake of hydrogen-rich water, 1500 mL per day, lasted for 8 weeks in nine people with the diabetes-related serum markers beyond the normal ranges. The subjects exhibited significant tendencies for the decreased fasting blood glucose and fructosamine, and for the increased 1,5-anhydro-D-glucitol, concomitantly with significant decreases in urinary 8-hydroxy-2-deoxyguanosine contents and its rate of generation. Hydrogen-rich water prepared by AC-electrolysis may be effective in improving diverse diabetes-related markers and systemic DNA oxidative injuries through the formation of abundant heat-resistant nanobubbles and the increased hydrogen concentrations. The study protocol was officially approved by the Medical Ethics Committee of the Japanese Center for Anti-Aging Medical Sciences (approval No. 01S02) on September 15, 2009.
Keywords: alternating current-electrolysis, antioxidant activity, diabetes, DNA-oxidative injuries, hydrogen-rich water, reactive oxygen species
INTRODUCTION
Recently reported health benefits of hydrogen-rich water include skin beauty and prevention of metabolic syndrome.1,2,3,4 Many lifestyle-related chronic diseases are primarily caused or exacerbated by reactive oxygen species (ROS), which can be efficiently scavenged by hydrogen-rich water with the ability to permeate deeply into human tissues.5,6,7 Hydrogen-rich water is expected to have preventive or therapeutic effects on oxidative stress-related diseases, such as diabetes, cancer, arteriosclerosis, hypertension, apoplexy, and aging.8,9,10,11,12,13 Over the past few years, several studies in Japan have reported on hydrogen-water-manufacturing equipment.14,15,16
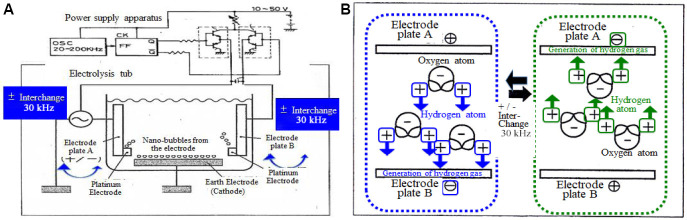
Hydrogen-rich water can be prepared using diverse methods such as electrolysis by alternating current (AC) or direct current (DC), treatment with a magnetic field, bubbling of hydrogen gas in water, and treatment with certain minerals or rocks.17,18 The present study examined the use of AC electrolysis delivered by the “Hayakawa Method,” a patented method approved by the Japan Patent Office (patient No. 2,615,308 and 2,623,204) and United States Patent Office (patient No. 5,435,894) (Figure 1A). The apparatus is composed of three electrodes: a grounding electrode and a pair of conventional electrodes, which generates the reduced hydrogen bubble rich water in the vicinity (Figure 1B). The first and second high-frequency switches are connected to DC voltage sources through a variable resistor. The electrolysis apparatus delivers high-frequency AC-electrolysis at an interchange cycle of 30 kHz. The level of the signal output from the high-frequency oscillator to the high-frequency switching commander circuit should preferably be within a range of 20 to 50 kHz and 10 to 50 V, from the viewpoint of safety. A specified frequency and voltage were selected within these ranges, in response to the quality of water to be treated. The process and apparatus are suitable for improving the taste of drinking water such as tap water, well water, and commercially available water. The success of the method runs counter to the notion that water cannot be electrolyzed by AC. The purpose of this study is to explore whether AC-electrolytic hydrogen-rich water is retained for hydrogen-abundance after boiling or for antioxidant abilities, and whether the oral administration of this water is clinically effective for diabetes and prevention against systemic DNA-oxidative injuries.
Figure 1.
The principle of alternating current (AC) electrolysis for preparation of hydrogen-rich water.
Note: (A) Generation of nanobubbles by AC electrolysis. The method used the Hayakawa Method (Patent No. for the Japan Patent Office: 5,435,894 2,615,308, 2,623,204). The high frequency switching circuit was selected within a range of 20–50 kHz and 10–50 V. (B) Polarization of water molecules and migration of protons towards the cathodal electrode interchanged at a cycle of 30 kHz in an AC-electrolysis tub.
MATERIALS AND METHODS
Preparation and storage of hydrogen-rich water
Hydrogen-rich water was prepared using the AQUOLIA SWM300 AC-electrolysis apparatus (Silver Seiko Co. Ltd., Tokyo, Japan). Diverse water parameters were measured as follows: dissolved hydrogen (DH) concentrations were determined using an ENH-1000 DH meter (TRUSTLEX Co. Ltd., Tokyo, Japan). Oxidation-reduction potentials (ORP) were measured using a model 9300-10D ORP meter (HORIBA Ltd., Kyoto, Japan). Dissolved oxygen (DO) concentrations were determined using a model 9520-10D DO meter (HORIBA Ltd.), and pH was measured using a model 9620-10D pH meter (HORIBA Ltd., Kyoto, Japan).
The AC-electrolytically prepared hydrogen-rich water was sealed in a screw-cap-attached aluminum-made bottle with removal of gapped internal air, and left overnight below 25°C without vibration, resulting in the retention over 99% of DH. The DH values were kept over 98% at 4°C for more than 30 days, demonstrating the long-term stability of this water.
Characterization of hydrogen-rich water
Analysis for trace-metal elements in hydrogen-rich water was performed by inductively coupled plasma-mass spectrometry. Hydrogen-rich water was prepared by a 30-minute electrolysis of tap water that had been left overnight. Trace-metal elemental analysis was done using a model ICPMS-2030 (Shimadzu Corporation, Kyoto, Japan).
Nano-particle distributions were estimated three times by nanoparticle tracking analysis with a NanoSight apparatus type-LM10V-HS (Malvern Co. Ltd., Salisburg, UK) equipped with a CMOS camera under irradiation with a violet-colored laser beam (405 nm, < 60 mW), where polystyrene latex particles at 100 nm-diameter were used as the standard particles.
The sample solution designed for examination of its ROS-scavenging ability was mixed with 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) reagent (Labotec Co., Tokyo, Japan), and was immediately poured in a flat quartz cell, which was, after 2 minutes, set in the electron spin resonance (ESR) apparatus (FR-30, JEOL Ltd., Tokyo, Japan), and then ESR measurement was started and evaluated as the relative signal intensity of DMPO adducts as compared to the internal standard Manganese(II) oxide (MnO).
Effect of oral administration of hydrogen-rich water on diabetes-related markers
Ten Japanese adults who provided the informed consent were selected based on their fasting blood glucose (FBG) levels that exceeded 6.1 mM and was below approximately 7.8 mM. FBG levels greater than 7.0 mM were considered to be beyond the regular values. The subjects were not taking any medicines that could affect FBS levels, such as hypoglycemic drugs. One person dropped out for clinical reasons, resulting in a total of nine persons finally enrolled in the study (five males and four females aged between 39 and 63 years, with an average age of 52.1 years). The subjects were orally administered 1500 mL of hydrogen-rich water per day for 8 weeks. The water was freshly prepared by AC-electrolysis of tap water left overnight. The experiment was performed as an open clinic test at the Surugadai Clinic in Tokyo (n = 4) and the Yokohama Minoru Clinic (n = 6) in Yokohama. The research was officially approved by the Medical Ethics Committee of the Japanese Center for Anti-Aging Medical Sciences (approval No. 01S02) on September 15, 2009 (Additional file 1 (1.1MB, pdf) ), a non-profit organization authenticated by the Hiroshima Prefecture Government of Japan. The approval specified that the research would be noninvasive (such as lack of hemorrhage), painless, with no physical trace/sign, and would be executed using a commercially available apparatus.
Estimation of diabetes-related clinical examination parameters
A glucose oxidase/horseradish peroxidase-colorimetric method19 for fetal bovine serum was done using a serum glucose test kit (Cosmo Bio Co. Ltd., Tokyo, Japan). 1,5-Anhydro-D-glucitol (1,5-AG) was measured using a 1,5-anhydroglucitol test kit based on adenosine diphosphate-dependent hexokinase (Determiner L; Kyowa Medix Co. Ltd., Tokyo, Japan). Fructosamine was measured using a modified-nitro blue tetrazolium colorimetric method incorporating K-assay reagent (Funakoshi Co. Ltd., Tokyo, Japan). Hemoglobin A1c (HbA1c) was measured using an International Federation of Clinical Chemistry method20 that utilized a glycohemoglobin A1c kit with an anti-human-HbA1c polyclonal antibody (Wako Pure Chemical Co. Ltd., Osaka, Japan). An enzyme-linked immunosorbent assay for urinary 8-hydroxydeoxyguanosine (8-OHdG) utilized a high-sensitivity check kit (Nikken Seil Co. Ltd., Shizuoka, Japan). A picric acid-colorimetric method for creatinine utilized a LabAssay kit (Wako Pure Chemicals, Osaka, Japan). Uric acid was measured using a 2, 4, 6-tripyridyl-s-triazine based colorimetric method21 (BioAssay System kit; Funakoshi Co. Ltd.).
Clinical examination for blood and urine
The subjects periodically underwent diverse clinical examinations before examination, and 4 and 8 weeks after intervention. Blood biochemical parameters that were measured including triglyceride, total cholesterol, high-density lipoprotein cholesterol, total phosphorus, albumin, total bilirubin, direct bilirubin, glutamic-oxaloacetic transaminase, glutamic pyruvic transaminase, gamma-glutamyltransferase, alkaline phosphatase, lactate dehydrogenase, lactic acid, pyruvic acid, Na, K, Cl, Cr, blood urea nitrogen, uric acid, FBG, fructosamine, HbA1c, 1,5-AG, total ketone body, 3-hydroxybutyric acid, and acetoacetic acid. Urine biochemical parameters that were measured included proteins, glucose, ketone body, urobilinogen, and 8-OHdG. Physical parameters that were included systolic blood pressure, diastolic blood pressure, pulse, and body weight.
Safety for clinical use of hydrogen-rich water
The safety of orally administered hydrogen-rich water was assessed by clinical examination tests, such as physical, blood and urine biochemical tests. Safety was indicated by the absence of abnormalities or harmful events, with the exception of the pre-existing diabetes-related symptoms. This procedure was conducted for all nine out of ten subjects who participated. No problems were evident, except for one person who showed marked alterations in the degree of exercise and amount of food consumed. No abnormality was observed for the averages and standard deviations of the urinary 8-OHdG values, together with baseline values, for urine qualitative tests.
Statistical analyses
All the data were processed by the statistical significance analysis using the SPSS program, Ver. 11.0 (SPSS, Chicago, IL, USA) or the Wilcoxon signed-rank test, and were typically expressed as the converged average values or with probability-values, which were obtained from the experiments repeated for three to four-times. In tests for examinations of the two-sided hypothesis, the statistical significance and the statistically significant tendency were indicated at P ≤ 0.01 and 0.01 < P ≤ 0.05, respectively. Quantitative variables were expressed by estimating statistical values that included the number of subjects or experimental frequencies (number), mean, for all the clinical examinations such as urinary 8-OHdG, FBG, 1,5-AG, HbA1c and fructosamine, and standard deviation (SD), for urinary 8-OHdG, 8-OHdG/creatinine and 8-OHdG forming speed indicating the P values. The qualitative variables were described using frequency tables. The baselines for the test variables were defined as initial values observed before intake of hydrogen-rich water. As indicators for effectiveness of the estimation, descriptive statistic values including the subject number, average and SD were expressed. The changes in clinical examination parameters before and after intake of hydrogen-rich water were evaluated using the Wilcoxon signed-rank test.
RESULTS
Parameters of AC-electrolyzed hydrogen-rich water
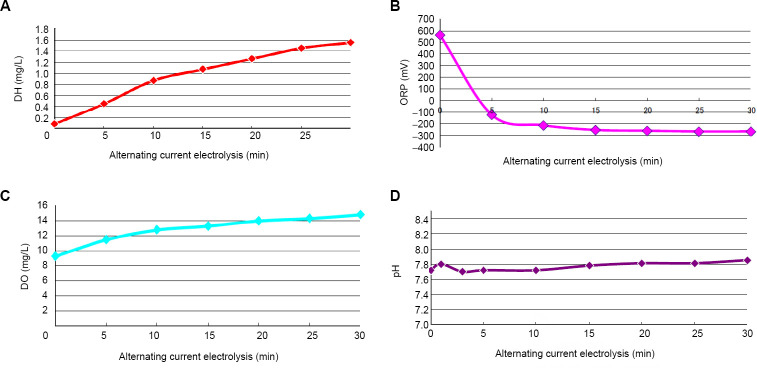
The water-characteristic parameters of hydrogen-rich water produced by AC-electrolysis of tap water according to the Hayakawa Method (Figure 1) were analyzed. DH rose to 1.55 mg/L after 30 minutes of electrolysis, representing an approximately 20-fold increase from the DH value of 0.08 mg/L prior to electrolysis (Figure 2A). The value following electrolysis was very close to the maximum DH value of 1.6 mg/mL that is attained under normal temperature and pressure. The ORP decreased from +560 mV at the start of electrolysis to –270 mV after 30 minutes of electrolysis, which indicated the intensified reducing power (Figure 2B). The DO value rose to 14.6 mg/L for 30 minutes of electrolysis, and was approximately 1.5-fold higher than the initial DO value of 9.36 mg/L (Figure 2C), indicating that aerobic water quality can promote intracellular aerobic metabolism. The pH changed within a narrow range of approximately 7.7 to 7.8 (Figure 2D), which was physiologically compatible with the human body, in contrast to pH values higher than 8 that have been observed for most of other types of DC-electrolytic hydrogen-rich water.
Figure 2.
Time courses in changes of various water-property parameters.
Note: (A–D) The time course of DH (A), ORP (B), DO (C), and pH (D) values in hydrogen-rich water generated by alternating current-electrolysis of tap water. The pH values were stable between 7.7 and 7.9. Data were expressed as the means for three measurements. DH: Dissolved hydrogen; DO: dissolved oxygen; ORP: Oxidation-reduction potential.
Trace-metal content in the AC-electrolyzed hydrogen-rich water
The trace-metal elements in the AC-electrolyzed hydrogen-rich water from tap water that had been left overnight were analyzed by inductively coupled plasma-mass spectrometry (Figure 3). The zinc (Zn) content increased to 1.37 μg/L. The level of Zn is non-toxic and is typical of beneficial trace-elements. Surplus Zn is typically secreted into the pancreatic liquid.22 Titanium (Ti) was increased to 2.20 μg/L. Ti is a biocompatible element that is sufficiently corrosion-resistant to be utilized for diverse artificial organs that are biologically safe.23 Platinum (Pt) was absent before electrolysis, but became detectable at a concentration of 0.51 μg/L, suggesting the elution of Pt ions from the electrode surface into the electrolysis fluid. Pt colloid, which is commercially available as an antioxidant oral supplement, prolonged the generation of hydrogen bubbles via the formation of a Pt-hydrogen complex. The iron (Fe) content increased to 3.66 μg/L, which was much lower than the permissible amount of 45 mg/d according to the Dietary Reference Intake indices of the American Science Academy.24
Figure 3.
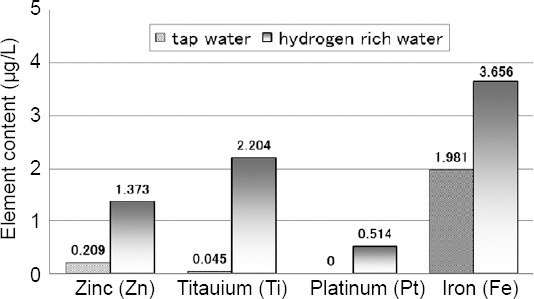
Trace-metal elements content in hydrogen-rich water generated by alternating current-electrolysis of tap water for 30 minutes, as analyzed by inductively coupled plasma-mass spectrometry method.
Note: Data are expressed as the means for three measurements.
Nanobubbles and nanoparticles in the AC-electrolyzed hydrogen-rich water
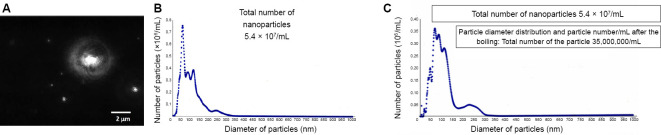
Nanobubbles generated after AC-interchange electrolysis by the Hayakawa Method were estimated by nanoparticle tracking analysis, where Brownian movement of the nanoparticles was visualized using a laser beam, and the number and diameter of nanoparticles as well as the particle size distribution were evaluated (Figure 4A). The total number of nanoparticles was determined to be 5.4 × 107/mL (Figure 4B). Even after boiling, 3.5 × 107/mL nanoparticles were retained (Figure 4C), representing a retention rate of 64.8%. This rate is unexpectedly high as normal-size bubbles, but is not applicable to nanobubble gas, since Henry's rule dictates that gas-solubility is lower at higher temperatures.25
Figure 4.
Particle-size distributions in alternating current-electrolytically prepared hydrogen-rich water.
Note: (A) Visualization of Brownian movement of nanoparticles by nanoparticle tracking analysis (NTA) using a laser beam tracing NanoSight LM20/NTA2.3 apparatus. (B) Size distribution (diameters versus numbers) of nanoparticles in hydrogen-rich water generated by alternating current-electrolysis of tap water for 30 minutes as measured by NTA. (C) Size distribution in hydrogen-rich water generated as in B and subjected to boiling and cooling. Nanoparticles after boiling were decreased to 1.9 × 107/mL, at a rate of 35.2%.
In the Hayakawa Method, electrolysis with an AC of 5 kHz is required with an oscillation (plus-minus) of 5000 times per second with hydrogen molecules gas (bubble: H2) of the nano-unit. The method with 30,000 interchanges times with the three electrodes (including the ground electrode) yielded a large quantity of hydrogen gas nanobubbles for AC-electrolysis at 30 kHz. The nanobubbles in water remained in the bulk space. They did not float up or sediment, and were retained for a long time without the fusion of mutual bubbles due to electrostatic repulsion among the negative-charges on the bubble-surface. These nanobubbles persisted for a few days.
Hydrogen molecules of nanobubbles can easily penetrate the cell membrane or skin corneum, and can reach the intracellular space and diverse organs. At these destinations, it can be converted to a harmless water molecule after removal of ROS.
Physiological benefits of AC-electrolyzed hydrogen-rich water
Hydrogen-rich water prepared by 30 minutes of AC-electrolysis was shown to appreciably scavenge superoxide anion radicals (•O2–) generated by hypoxanthine-xanthine oxidase enzymatic reaction, according to DMPO spin trapping and ESR). Hydroxyl radicals (•OH) generated by the Fenton reaction were markedly scavenged by the hydrogen-rich water, as also determined using DMPO-spin trapping/ESR (Figure 5).
Figure 5.
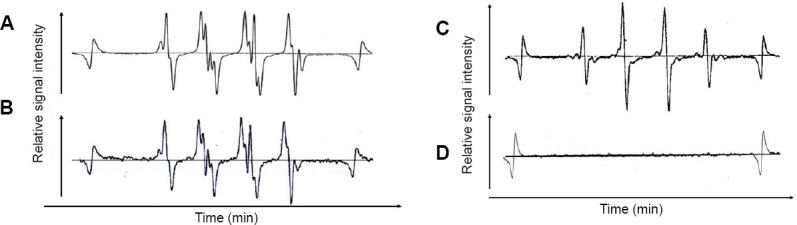
ESR spectra of superoxide anion radicals (•O2 –) and hydroxyl radicals (•OH) upon addition of alternating current-electrolytically prepared hydrogen-rich water.
Note: (A, B) An ability of hydrogen-rich water to scavenge superoxide anion radicals, as evaluated by 5,5-dimethyl-1-pyrroline-N-oxide (DMPO)-spin trapping/electron spin resonance (ESR) method. (A) superoxide anion radicals generated by hypoxanthine-xanthine oxidase enzymatic reaction. (B) superoxide anion radicals immediately after addition of hydrogen-rich water that was freshly prepared by 20-minute AC-electrolysis. (C, D) An ability of hydrogen-rich water to scavenge hydroxyl radicals, as evaluated by DMPO-spin trapping/ESR method. (C) hydroxyl radicals generated by the Fenton reaction. (D) ESR spectrum of hydroxyl radicals immediately after addition of hydrogen-rich water that was freshly prepared by 20-minute AC-electrolysis.
Effect of AC-electrolyzed hydrogen-rich water on diabetes-related serum markers
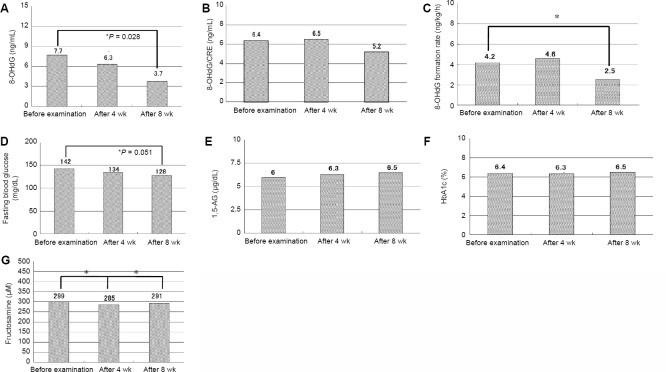
8-OHdG is an established urine marker for DNA oxidative injury.26 8-OHdG was decreased significantly after the 8-week daily consumption of hydrogen-rich water (P = 0.028), suggesting the systemic decrease of •OH by hydrogen absorbed into human body fluid (Figure 6A). The 8-OHdG/creatinine values were unchanged after 4 weeks and were decreased after 8 weeks (Figure 6B). The rate of 8-OHdG generation decreased from 4.2 ng/kg per hour before intake to 2.5 ng/kg per hour after 8 weeks (Figure 6C). The difference was not statistically significant.
Figure 6.
Effect of alternating current-electrolyzed hydrogen-rich water on diabetes-related serum markers in nine subjects with higher blood sugar levels.
Note: (A) 8-Hydroxydeoxyguanosine (8-OHdG) level in urine; (B) 8-OHdG/creatinine (Cre) in urine; (C) 8-OHdG formation per hour in urine; (D) fasting blood glucose; (E) 1,5-anhydro-D-glucitol (1,5-AG) in blood; (F) hemoglobin A1c (HbA1c); (G) fructosamine in blood. Data are expressed as mean, and analyzed by Wilcoxon signed-rank test. *P < 0.05.
FBG levels were evaluated for the nine diabetic patients who consumed the hydrogen-rich water (1500 mL per day for 8 weeks) prepared by AC-electrolysis of tap water. FBG levels decreased significantly (P = 0.051) after the 8-week regimen (Figure 6D).
1,5-AG is a marker reflecting the average concentrations of blood glucose.27 The 1,5-AG value of 9.0 μg/mL prior to intake of hydrogen-rich water was increased slightly to 9.4 μg/mL at 4 weeks and 9.5 μg/mL at 8 weeks (Figure 6E). Although the slight increases in 1,5-AG were not statistically significant, the desired blood level of 1,5-AG as an indicator for glucose reabsorption in the kidney should normally exceed 12 µg/mL, whereas 1,5-AG in blood is known to be 10 µg/mL in case of the past blood glucose of 10.3 mM, being doubted for diabetes. More than 99% of blood 1,5-AG is reabsorbed in the kidney. However, the reabsorption of 1,5-AG, a structural analogue to glucose, is competitively inhibited by secreted urinary glucose, because of the abundant blood glucose. This phenomenon is an extremely fast response to urinary glucose secretion, enabling it to estimate blood glucose concentration within several days.28
HbA1c levels tended to decline after 4 weeks of intake, but were unchanged from the baseline after 8 weeks. The findings indicate the necessity for long-term intake (more than 8 weeks) because HbA1c values reflect the average concentrations of blood glucose for the past 1 to 2 months and taking into consideration that the initial HbA1c values were up to 6.4% (Figure 6F).
ROS generation has been implicated in the development and progression of diabetes, since saccharified proteins, such as HbA1c, increase proportionately with ROS generation.12 Diabetic patients with higher FBG levels were orally administered hydrogen-rich water for 8 weeks, and examined for temporal changes in serum levels of FBG, HbA1c, fructosamine, 1,5-AG, and uric acid, and urinary levels of 8-OHdG, 8-OHdG/creatinine and 8-OHdG formation rate. The levels of fructosamine, a marker for serum glucose over a period of 1 to 2 weeks, was significantly decreased (P = 0.008) after 4 weeks of intake (Figure 6G).
Statistically significant changes were evident for platelet, total phosphorus, albumin, total bilirubin, aspartate transaminase and gamma-glutamyltransferase. Statistically significant tendency was observed for direct bilirubin, lactic acid, pyruvic acid and Na. These values were within the margin of deviation from baseline values, before and after the intake test, and did not exceed the levels indicative of abnormal changes, suggesting the safety of the 8-week regimen of 1500 mL/d of the AC-electrolytic hydrogen-rich water.
DISCUSSION
The hydrogen-rich water prepared by the Hayakawa Method displayed near-neutral pH that is compatible for human use; low ORP (–270 mV) indicative of intensified reducing power; DH of approximately 1.55 mg/L (which is very similar to the theoretical maximum DH of 1.6 mg/L under normal temperature and pressure conditions); low surface-tension and high osmotic capability indicative of easy absorption into cells; binding of hydrogen molecules to Pt nano-colloid that is of sizes for nanometers in a diameter, facilitating the dissociation to the atomic hydrogen; scavenging of ROS, particularly •OH; and an appropriate balance of positive and negative mineral ions.
Harmful trace-metal elements were not generated at detectable levels by AC-electrolysis. The material of the applying electrodes was selected by taking into consideration the quality of treated water. Zn, lithium oxide, magnesium (Mg) alloys (with Zn), copper, Fe, stainless steel and Ti are candidate materials for the electrodes. Of these, Zn and Mg alloys are preferable for obtaining the drinking water. Electrodes composed of Zn and Mg gradually dissolve from the surface to enrich the water. Of the diverse types of ROS, superoxide anion radicals (•O2–) and hydrogen peroxide can be removed by intracellular enzymes, such as superoxide dismutase and catalase/glutathione-peroxidase, respectively. In contrast, •OH cannot be scavenged by any human-endogenous enzymes, resulting in injury to cellular DNA and the plasma membrane.29,30 Hydrogen-rich water prepared by AC-electrolysis, apart from the conventional DC-electrolysis, scavenges •OH and possibly protects cells against oxidative injury. In contrast, DNA cannot be directly protected from attack by •OH, resulting in elimination of some 8-OHdG fragments from the body in the urine. The relative amounts of 8-OHdG are an appropriate marker for the extent of cellular injury. Repression of urinary 8-OHdG by hydrogen-rich water that was prepared by AC-electrolysis, suggests that cells can be protected against attack from •OH through cellular conversion of the oxidative state to the reductive state, by means of the antioxidant ability of hydrogen bubbles.
The development and progression of diabetes may be correlated to ROS and peroxylipid, especially in terms of metabolic activities of glucose-taking tissues and insulin-secreting ability of the pancreatic langerhans islet β-cells. Peroxylipid values in serum are linked with the progression of diabetes, whereas blood glucose values are improved by the intake of antioxidant foods having an superoxide dismutase-like action. Furthermore, participation of ROS in diabetes may also be possible because ROS is generated from some glycoproteins, for example, HbA1c which increase as diabetes develops and progresses.31
During hyperglycemia, the glucose oxidative metabolism and the promoted metabolism of arachidonic acid may generate ROS, such as superoxide anion radicals (•O2–) and •OH, which injure endotheliocytes and lead to the accumulation of oxidative low-density lipoprotein, which in turn promotes the progression of diabetes and the diverse complications associated with the disease.32
As intrinsic improvement for diabetes, glucose transporter-4, which is located in the endoplasmic reticulum membrane, may be translocated into the cell membrane, and subsequently functions in the intracellular intake of extracellular glucose. Glucose transporter-4 location requires a higher pH of as little as slight as 0.2 pH unit in the extracellular liquid, which occurs by the activation of the Na+/H+ channel, due to the increased abundance of hydrogen bubbles in the intracellular liquid, in addition to insulin. The translocation of glucose transporter-4 from the endoplasmic reticulum to the cell membrane is disturbed even if insulin exists when the Na+/H+ channel is perturbed.33
The present study explored the effects of hydrogen-rich water intake on the FBG levels of the nine participants with relatively high blood glucose levels. The subjects drank 1500 mL of hydrogen-rich water each day for 8 weeks. HbA1c levels, fructosamine levels, 1,5-AG values and FBG levels were measured to gauge their glycemic control. The findings are presented in Figure 7. Chronologically, the glucose status was first evaluated with HbA1c reflecting blood glucose values for one to two months before the examination, then with fructosamine levels 2 weeks before, with 1,5-AG values a few days before, and finally with the FBG value at the time of examination.
Figure 7.
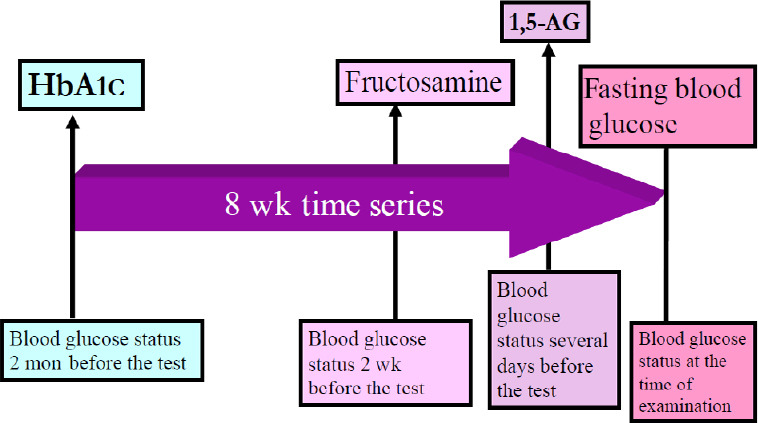
Time schedule that can predict blood glucose level before measurement by measuring markers related to diabetes.
Note: Hemoglobin A1c (HbA1c) levels, fructosamine levels, 1,5-anhydro-D-glucitol (1,5-AG) values, and fasting blood glucose levels were measured in the nine subjects who drank 1500 mL of hydrogen-rich water every day for 8 weeks, to trace their glycemic control.
In diabetes, abundant glucose in the blood has been correlated to higher glucose concentrations in erythrocytes,12 suggesting the possibility for the decreased metabolism of glucose. In contrast, active consumption of glucose might be restored, as shown presently by the decreases in FBG and fructosamine during the 8-week daily consumption of hydrogen-rich water prepared by AC-electrolysis.
As concerned with a limitation range for reliability to the present study, diverse material- or antioxidant-properties of AC-electrolytically prepared hydrogen-water were thoroughly elucidated in terms of water-property parameters such as DH, DO, ORP and pH, ROS-scavenging abilities and particle-size distributions. In contrast, from viewpoints of hydrogen-intervening clinic examination for diabetes, the diverse time-series diabetes-related indicators, such as FBS for the current time, 1,5-AG for several days before, fructosamine for 2 weeks before and HbA1c for 2 months before, were examined in the present study, indicating the data reliability that was comprehensively attributed to the systemically chronological sequence. Based on these results, hydrogen-rich water prepared by AC-electrolysis may be effective in improving diverse diabetes-related markers and systemic DNA oxidative injuries through the formation of abundant heat-resistant nanobubbles and the increased hydrogen concentrations.
Additional file 1
Additional file 1 (1.1MB, pdf) : Hospital Ethics Approval.
Footnotes
Conflicts of interest
None.
Financial support
The study was supported in part by a Grant-in-Aid #0204 for AntiAging Medical Sciences from Japanese Center for AntiAging MedSciences, Hiroshima, Japan (to RA).
Institutional review board statement
This study was approved by the Medical Ethics Committee of the Japanese Center for Anti-Aging Medical Sciences (approval No. 01S02) on September 15, 2009.
Declaration of patient consent
The authors certify that they have obtained all patient consent forms. In the form, patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published.
Biostatistics statement
The statistical methods of this study were reviewed by the epidemiologist of Osaka Prefecture University, Japan.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
Personal names of subjects or personal specific information will not be announced even upon presentation of the research results. Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:
Checked twice by iThenticate.
Peer review:
Externally peer reviewed.
Funding:
The study was supported in part by a Grant-in-Aid #0204 for AntiAging Medical Sciences from Japanese Center for AntiAging MedSciences, Hiroshima, Japan (to RA).
REFERENCES
- 1.Asada R, Saitoh Y, Miwa N. Effects of hydrogen-rich water bath on visceral fat and skin blotch, with boiling-resistant hydrogen bubbles. Med Gas Res. 2019;9:68–73. doi: 10.4103/2045-9912.260647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kato S, Saitoh Y, Iwai K, Miwa N. Hydrogen-rich electrolyzed warm water represses wrinkle formation against UVA ray together with type-I collagen production and oxidative-stress diminishment in fibroblasts and cell-injury prevention in keratinocytes. J Photochem Photobiol B. 2012;106:24–33. doi: 10.1016/j.jphotobiol.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Kato S, Saitoh Y, Miwa N. Inhibitions by hydrogen-occluding silica microcluster to melanogenesis in human pigment cells and tyrosinase reaction. J Nanosci Nanotechnol. 2013;13:52–59. doi: 10.1166/jnn.2013.6848. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka Y, Saitoh Y, Miwa N. Electrolytically generated hydrogen warm water cleanses the keratin-plug-clogged hair-pores and promotes the capillary blood-streams, more markedly than normal warm water does. Med Gas Res. 2018;8:12–18. doi: 10.4103/2045-9912.229598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao L, Miwa N. Hydrogen-rich water achieves cytoprotection from oxidative stress injury in human gingival fibroblasts in culture or 3D-tissue equivalents, and wound-healing promotion, together with ROS-scavenging and relief from glutathione diminishment. Hum Cell. 2017;30:72–87. doi: 10.1007/s13577-016-0150-x. [DOI] [PubMed] [Google Scholar]
- 6.Kato S, Hokama R, Okayasu H, Saitoh Y, Iwai K, Miwa N. Colloidal platinum in hydrogen-rich water exhibits radical-scavenging activity and improves blood fluidity. J Nanosci Nanotechnol. 2012;12:4019–4027. doi: 10.1166/jnn.2012.6163. [DOI] [PubMed] [Google Scholar]
- 7.Kato S, Matsuoka D, Miwa N. Antioxidant activities of nano-bubble hydrogen-dissolved water assessed by ESR and 2,2’-bipyridyl methods. Mater Sci Eng C Mater Biol Appl. 2015;53:7–10. doi: 10.1016/j.msec.2015.03.064. [DOI] [PubMed] [Google Scholar]
- 8.Iketani M, Sekimoto K, Igarashi T, et al. Administration of hydrogen-rich water prevents vascular aging of the aorta in LDL receptor-deficient mice. Sci Rep. 2018;8:16822. doi: 10.1038/s41598-018-35239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saitoh Y, Okayasu H, Xiao L, Harata Y, Miwa N. Neutral pH hydrogen-enriched electrolyzed water achieves tumor-preferential clonal growth inhibition over normal cells and tumor invasion inhibition concurrently with intracellular oxidant repression. Oncol Res. 2008;17:247–255. doi: 10.3727/096504008786991620. [DOI] [PubMed] [Google Scholar]
- 10.Asada R, Kageyama K, Tanaka H, et al. Antitumor effects of nano-bubble hydrogen-dissolved water are enhanced by coexistent platinum colloid and the combined hyperthermia with apoptosis-like cell death. Oncol Rep. 2010;24:1463–1470. doi: 10.3892/or_00001006. [DOI] [PubMed] [Google Scholar]
- 11.Saitoh Y, Yoshimura Y, Nakano K, Miwa N. Platinum nanocolloid-supplemented hydrogendissolved water inhibits growth of human tongue carcinoma cells preferentially over normal cells. Exp Oncol. 2009;31:156–162. [PubMed] [Google Scholar]
- 12.Kajiyama S, Hasegawa G, Asano M, et al. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr Res. 2008;28:137–143. doi: 10.1016/j.nutres.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Han L, Tian R, Yan H, et al. Hydrogen-rich water protects against ischemic brain injury in rats by regulating calcium buffering proteins. Brain Res. 2015;1615:129–138. doi: 10.1016/j.brainres.2015.04.038. [DOI] [PubMed] [Google Scholar]
- 14.Li Q, Asada R, Tanaka Y, Miwa N. Fundamental insight into the methodology of hydrogen water in biological studies. J Nanosci Nanotechnol. 2017;17:5134–5138. [Google Scholar]
- 15.Higashimura Y, Baba Y, Inoue R, et al. Effects of molecular hydrogen-dissolved alkaline electrolyzed water on intestinal environment in mice. Med Gas Res. 2018;8:6–11. doi: 10.4103/2045-9912.229597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujita R, Tanaka Y, Saihara Y, Yamakita M, Ando D, Koyama K. Effect of molecular hydrogen saturated alkaline electrolyzed water on disuse muscle atrophy in gastrocnemius muscle. J Physiol Anthropol. 2011;30:195–201. doi: 10.2114/jpa2.30.195. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto A, Yamafuji M, Tachibana T, Nakabeppu Y, Noda M, Nakaya H. Oral ‘hydrogen water’ induces neuroprotective ghrelin secretion in mice. Sci Rep. 2013;3:3273. doi: 10.1038/srep03273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noda K, Tanaka Y, Shigemura N, et al. Hydrogen-supplemented drinking water protects cardiac allografts from inflammation-associated deterioration. Transpl Int. 2012;25:1213–1222. doi: 10.1111/j.1432-2277.2012.01542.x. [DOI] [PubMed] [Google Scholar]
- 19.Abo-Haded HM, Elkablawy MA, Al-Johani Z, Al-Ahmadi O, El-Agamy DS. Hepatoprotective effect of sitagliptin against methotrexate induced liver toxicity. PLoS One. 2017;12:e0174295. doi: 10.1371/journal.pone.0174295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeppsson JO, Kobold U, Barr J, et al. Approved IFCC reference method for the measurement of HbA1c in human blood. Clin Chem Lab Med. 2002;40:78–89. doi: 10.1515/CCLM.2002.016. [DOI] [PubMed] [Google Scholar]
- 21.Kamel AH. Conventional and planar chip sensors for potentiometric assay of uric acid in biological fluids using flow injection analysis. J Pharm Biomed Anal. 2007;45:341–348. doi: 10.1016/j.jpba.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Walsh CT, Sandstead HH, Prasad AS, Newberne PM, Fraker PJ. Zinc: health effects and research priorities for the 1990s. Environ Health Perspect. 1994;102(Suppl 2):5–46. doi: 10.1289/ehp.941025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nomura N. Artificial organs: recent progress in metals and ceramics. J Artif Organs. 2010;13:10–12. doi: 10.1007/s10047-010-0487-y. [DOI] [PubMed] [Google Scholar]
- 24.Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Vol. 2001. Washington (DC): National Academies Press (US); [PubMed] [Google Scholar]
- 25.Young CL. Hydrogen and Deuterium. Commission On Solubility Data, Analytical Chemistry Division, International Union of Pure and Applied Chemistry, ed. IUPAC Solubility Data Series. 1981 [Google Scholar]
- 26.Loft S, Fischer-Nielsen A, Jeding IB, Vistisen K, Poulsen HE. 8-Hydroxydeoxyguanosine as a urinary biomarker of oxidative DNA damage. J Toxicol Environ Health. 1993;40:391–404. doi: 10.1080/15287399309531806. [DOI] [PubMed] [Google Scholar]
- 27.Yamanouchi T, Ogata N, Tagaya T, et al. Clinical usefulness of serum 1,5-anhydroglucitol in monitoring glycaemic control. Lancet. 1996;347:1514–1518. doi: 10.1016/s0140-6736(96)90672-8. [DOI] [PubMed] [Google Scholar]
- 28.Sydow K, Wiedfeld C, Musshoff F, et al. Evaluation of 1,5-anhydro-d-glucitol in clinical and forensic urine samples. Forensic Sci Int. 2018;287:88–97. doi: 10.1016/j.forsciint.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Weydert CJ, Cullen JJ. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc. 2010;5:51–66. doi: 10.1038/nprot.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Xue X, Han X, et al. Hydrogen-rich water ameliorates total body irradiation-induced hematopoietic stem cell injury by reducing hydroxyl radical. Oxid Med Cell Longev. 2017;2017:8241678. doi: 10.1155/2017/8241678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim MJ, Jung KH, Uhm YK, Leem KH, Kim HK. Preservative effect of electrolyzed reduced water on pancreatic beta-cell mass in diabetic db/db mice. Biol Pharm Bull. 2007;30:234–236. doi: 10.1248/bpb.30.234. [DOI] [PubMed] [Google Scholar]
- 32.Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10:293–302. doi: 10.1038/nrendo.2014.29. [DOI] [PubMed] [Google Scholar]
- 33.Vidotti DB, Arnoni CP, Maquigussa E, Boim MA. Effect of long-term type 1 diabetes on renal sodium and water transporters in rats. Am J Nephrol. 2008;28:107–114. doi: 10.1159/000109967. [DOI] [PubMed] [Google Scholar]