Abstract
Background
Geographical differences in patient characteristics, management and outcomes in heart failure (HF) trials are well recognized. The aim of this study was to assess the consistency of the treatment effect of coenzyme Q10 (CoQ10) in the European sub-population of Q-SYMBIO, a randomized double-blind multinational trial of treatment with CoQ10, in addition to standard therapy in chronic HF.
Methods
Patients with moderate to severe HF were randomized to CoQ10 300 mg daily or placebo in addition to standard therapy. At 3 months the primary short-term endpoints were changes in New York Heart Association (NYHA) functional classification, 6-min walk test, and levels of N-terminal pro–B type natriuretic peptide. At 2 years the primary long-term endpoint was major adverse cardiovascular events (MACE).
Results
There were no significant changes in short-term endpoints. The primary long-term endpoint of MACE was reached by significantly fewer patients in the CoQ10 group (n = 10, 9%) compared to the placebo group (n = 33, 27%, p = 0.001). The following secondary endpoints were significantly improved in the CoQ10 group compared with the placebo group: all-cause and cardiovascular mortality, NYHA classification and left ventricular ejection fraction (LVEF). In the European sub-population, when compared to the whole group, there was greater adherence to guideline directed therapy and similar results for short- and long-term endpoints. A new finding revealed a significant improvement in LVEF.
Conclusions
The therapeutic efficacy of CoQ10 demonstrated in the Q-SYMBIO study was confirmed in the European sub-population in terms of safely reducing MACE, all-cause mortality, cardiovascular mortality, hospitalization and improvement of symptoms.
Keywords: chronic heart failure, coenzyme CoQ10, ubiquinone, randomized controlled trial, major adverse cardiovascular events, mortality, hospitalization
Introduction
Heart failure (HF) is a progressive worsening of cardiac function, due to a variety of causes including ischemic heart disease, hypertension, cardiomyopathy and diabetes. Despite considerable advances in treatment options, HF continues to be associated with a high symptomatic burden, frequent hospitalizations and a poor long-term prognosis with 50% of HF patients dying within 5 years of diagnosis [1].
Coenzyme Q10 (CoQ10) is an essential component in the production of cellular energy (ATP) in mitochondria. In addition, CoQ10 has strong antioxidative properties that protects against cellular damage from free radicals including reactive oxygen species [2–4]. CoQ10 is primarily synthesized endogenously and in sufficient amounts during normal physiological conditions. However, endogenous production of CoQ10 declines with age and an actual deficiency is observed in a number of pathophysiological conditions including HF [5–7]. The biochemical rationale of CoQ10 supplementation in HF patients is to correct a documented deficit in heart tissue CoQ10 that may lead to failure in mitochondrial bioenergetics and a compromised cellular antioxidant capacity of the myocardium [8–11].
The Q-SYMBIO study, a multinational prospective, randomized, double-blind trial, demonstrated that treatment with CoQ10, in addition to standard therapy for patients with chronic HF, improved symptoms and reduced adverse cardiovascular events and mortality [12]. In Q-SYMBIO, patients with HF were enrolled from European and non-European (mainly Asian) centers.
Geographic differences in patient characteristics and management have the potential to affect the outcome of clinical trials. These differences have recently been analyzed and described in large HF trials [13–15]. For example, the Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure (PARADIGM) trial included patients from 5 regions including European and Asian countries, and notable regional differences were found in baseline characteristics and background HF therapy. Furthermore, differences in event-rates of HF outcomes were found, however the benefit of angiotensin–neprilysin inhibition was consistent across regions [16].
The aim of the present study was to assess the consistency of the treatment effect of CoQ10 in a European sub-population (n = 231) of the total population of Q-SYMBIO (n = 420).
Methods
The efficacy of CoQ10 in a European sub-population (n = 231) of the Q-SYMBIO trial (n = 420) by post-hoc analysis of baseline characteristics for short-term (3 months) and long-term (2 years) endpoints were investigated. Patients with moderate to severe HF were enrolled from 14 centers in 6 European countries (Poland, Denmark, Sweden, Hungary, Austria and Slovakia) and were randomized in parallel groups to either CoQ10 300 mg (Ubiquinone, Pharma Nord ApS) daily (n = 108) or placebo (n = 123) in addition to standard HF therapy.
The short-term primary endpoints were changes in New York Heart Association (NYHA) functional class, 6-min walk test (6MWT), and N-terminal pro–B-type natriuretic peptide (NT-proBNP). The secondary short-term endpoint was the scoring of symptoms (dyspnea, fatigue, and change in symptoms) by patients on visual analogue scale (VAS).
The primary long-term endpoint was a composite of major adverse cardiovascular events (MACE) defined as unplanned hospitalization due to worsening of HF, cardiovascular death, urgent cardiac transplantation or mechanical support using time to first event analysis. Secondary long-term endpoints were mortality, changes in NYHA functional class, NT-proBNP and echocardiography (left ventricular ejection fraction [LVEF] and cavity dimensions).
Samples of serum were shipped to the core Biochemical Laboratory in Ancona, Italy and assayed for levels of CoQ10 by using high-performance liquid chromatography with ultraviolet detection [17] and NT-proBNP using the Elecsys 2010 immunoassay method (Roche Diagnostics, Mannheim, Germany) [18].
Statistical analysis
Descriptive analyses of baseline data were reported as frequencies. Percentages for categorical data and for continuous data were reported as mean ± standard deviation or mean ± standard error for normally distributed data and median and lower upper quartile for non-normal data. Baseline characteristics were compared for indepedence between patient group using the Fisher exact test for categorical data and two-tailed t-test for continous data. The significance of treatment on continuous responses was analyzed by a linear model with each investigation center treated as a random intercept effect. The treatment effects were analyzed and adjusted for pre-defined confounders. A χ2 test for independence with exact p values was calculated using the Fisher exact test for the evaluation of the treatment effect on categorical responses. Cumulative incidence curves for the risk of MACE, hospital stay for HF, total cardiovascular mortality, and all-cause mortality were constructed by the Kaplan-Meier method and were analyzed by the Cox proportional hazards regression model stratified according to the center. The rates for adverse effects were compared between treatment groups by means of a χ2 test for independence. For the short-term primary endpoints, the pre-specified objective was reached if the difference between the groups in all three endpoints had a p value < 0.05. For the primary long-term endpoint MACE, the pre-specified objective was reached if the difference between the groups had a p value < 0.05. For secondary endpoints, p values < 0.05 were used to assess statistical significance. All data were analyzed with the statistical analysis program Stata/SE 11.2 for Windows (StataCorp LP, College Station, Texas).
Results
Baseline characteristics
The two treatment groups of the European sub-population were similar regarding baseline characteristics except male gender, CoQ10 (83%) vs. placebo (71%) (p = 0.03) and systolic blood pressure, CoQ10 (127 mmHg) vs. placebo (121 mmHg) (p = 0.03; Table 1). At the beginning of the study, an average of 90% of patients were classified as NYHA class III, 6% as NYHA class II and 6% as NYHA class IV and with an LVEF of 33%. The two treatment groups were balanced for medication usage with an average of 92% patients receiving angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, 88% receiving beta-blockers, 32% digoxin, 84% diuretics, 37% anticoagulants, 55% aldosterone antagonists, 57% statin derivatives and 26% diabetic medication (Table 1).
Table 1.
Baseline characteristics of European patients.
| Baseline characteristics | Standard HF therapy + CoQ10 (47%, n = 108) | Standard HF therapy + placebo (53%, n = 123) |
|---|---|---|
| Age [years] | 65.7 ± 10 | 64.0 ± 12 |
| Male gender* | 90 (83%) | 87 (71%) |
| Weight [kg] | 83.7 ± 18 | 84.8 ± 18 |
| BMI [kg/m2] | 29 ± 5 | 29 ± 7 |
| Heart rate [bpm] | 72 ± 12 | 75 ± 12 |
| Systolic BP [mmHg]* | 127 ± 21 | 121 ± 19 |
| Diastolic BP [mmHg] | 77 ± 11 | 74 ± 11 |
| Sinus rhythm | 67 (62%) | 77 (63%) |
| Atrial fibrillation | 27 (25%) | 32 (26%) |
| Rhythm, other (pace) | 14 (13%) | 14 (11%) |
| Ischemic heart disease | 68 (63%) | 84 (68%) |
| Dilated cardiomyopathy | 35 (32%) | 38 (31%) |
| Valvular heart disease | 5 (5%) | 1 (1%) |
| Duration of HF [months] | 42 ± 59 | 39 ± 41 |
| NYHA class II | 6 (6%) | 7 (6%) |
| NYHA class III | 97 (90%) | 109 (89%) |
| NYHA class IV | 5 (5%) | 7 (6%) |
| Left ventricular EF [%], [range] | 33 ± 12 [10–65] | 33 ± 12 [10–70] |
| Left ventricular EDD [mm] | 64 ± 10 | 62 ± 11 |
| Left ventricular ESD [mm] | 51 ± 12 | 50 ± 13 |
| 6MWT [m], [range] | 331 ± 91 [25–525] | 321 ± 90 [90–490] |
| Serum CoQ10 [μg/mL]§ | 0.95 ± 0.08 | 0.90 ± 0.07 |
| NT-proBNP [pg/mL]§† | 2470 ± 369, p50: 1208 | 2335 ± 398, p50: 1174 |
| Use of medications: | ||
| ACEI/ARBs | 99 (92%) | 112 (91%) |
| Beta-blockers | 94 (87%) | 110 (89%) |
| Digoxin | 35 (32%) | 39 (32%) |
| Diuretics | 90 (83%) | 104 (85%) |
| Aldosterone antagonists | 59 (55%) | 66 (54%) |
| Statins | 62 (57%) | 69 (56%) |
| Anticoagulants | 38 (35%) | 48 (39%) |
| Diabetes medication | 27 (25%) | 32 (26%) |
| Device therapy: | ||
| Cardiac resynchronization device | 2 | 5 |
| Implanted cardioverter defibrillator | 3 | 4 |
Values are mean ± standard deviation, number (percentage), mean ± standard deviation [range], mean ± standard deviation (median, p50), or number.
Values are mean ± standard error.
To convert values for NT-proBNP to picomoles per liter, divide by 8.457,
p = 0.03.
ACEI/ARB — angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; BMI — body mass index; BP — blood pressure CoQ10 — coenzyme Q10; EDD — end-diastolic diameter; ESD — end-systolic diameter; EF — ejection fraction; HF — heart failure; NT-proBNP — N-terminal pro–B-type natriuretic peptide; NYHA — New York Heart Association; 6MWT — 6-min walk test
Changes in serum CoQ10 levels
Changes in biochemical status were examined at short term (3 months) and long-term follow-up (2 years). After 3 months, serum CoQ10 significantly increased 3-fold in the CoQ10 group (p < 0.001) from 0.95 ± 0.08 μg/mL (mean ± SE) at baseline to 3.42 ± 0.21 μg/mL and was maintained during the study period with a level of 3.55 ± 0.34 μg/mL (p < 0.001) after 2 years. In the placebo group, there was a non-significant decrease in mean serum CoQ10 from 0.90 ± 0.07 μg/mL at baseline to 0.76 ± 0.04 μg/mL after 2 years (Table 2).
Table 2.
Biochemical assessments at baseline at 3 months and 2 years.
| Variable | CoQ10 (mean ± SE) 3 months: n = 80 2 years: n = 40 |
Placebo (mean ± SE) 3 months: n= 88 2 years: n = 45 |
|---|---|---|
| Serum CoQ10 [μg/mL]: | ||
| Baseline | 0.95 ± 0.08 | 0.90 ± 0.07 |
| 3 months | 3.42 ± 0.21* | 0.82 ± 0.06 |
| 2 years | 3.55 ± 0.34* | 0.76 ± 0.04 |
| Serum NT-proBNP [pg/mL]† | ||
| Baseline | 2470 ± 369 | 2335 ± 398 |
| 3 months | 2144 ± 370§ | 2343 ± 418 |
| 2 years | 1768 ± 375 | 2059 ± 390 |
p < 0.001 vs. baseline,
p = 0.052 vs. baseline,
To convert values for NT-proBNP to picomoles per liter, divide by 8.457.
CoQ10 — coenzyme Q10; NT-proBNP — N-terminal pro–B-type natriuretic peptide; SE — standard error
Effect on short-term endpoints
At 3 months there was a borderline significant reduction in serum NT-proBNP (p = 0.052) in the CoQ10 group compared to baseline but not in the placebo group (Table 2). There were no changes from baseline in the specified short-term endpoints NYHA functional class, VAS score, 6MWT or heart rate in either treatment group or between groups (Table 3).
Table 3.
Clinical and echocardiographic assessment changes from baseline.
| Variable | 3 months | 2 years | ||
|---|---|---|---|---|
|
|
|
|||
| CoQ10 (n = 98) | Placebo (n = 109) | CoQ10 (n = 81) | Placebo (n = 77) | |
| NYHA classification: | ||||
| Improvement | 27 (28%) | 26 (24%) | 39 (48%)* | 19 (25%) |
| Unchanged | 68 (69%) | 82 (75%) | 42 (52%) | 58 (75%) |
| Deterioration | 3 (3%) | 1 (1%) | 1 (1%) | 3 (4%) |
| VAS score (% ± SE): | ||||
| Dyspnea | −9.6 ± 2.4 | −6.4 ± 2.3 | NR | NR |
| Fatigue | −8.7 ± 2.6 | −8.7 ± 2.1 | NR | NR |
| General symptoms change | −7 ± 8.5 | −7 ± 8.6 | NR | NR |
| 6MWT [m] | +25 ± 60 | +20 ± 71 | +19 ± 75 | +2 ± 102 |
| Heart rate [bpm] | 0 (72 ± 15) | 0 (74 ± 14) | 0 (72 ± 13) | −1 (73 ± 14) |
| Systolic BP [mmHg] | 0 (127 ± 23) | 0 (121 ± 18) | 0 (127 ± 21) | −3 (124 ± 20) |
| Diastolic BP [mmHg] | −3 (74 ± 12) | −1 (75 ± 12) | 0 (74 ± 10) | 0 (75 ± 11) |
| Left ventricular EF [%] | +3 (36 ± 13) | +1 (34 ± 12) | +6 (39 ± 12)** | +2 (35 ± 14) |
| Left ventricular EDD [mm] | −2 (62 ± 10) | 0 (62 ± 10) | −1 (61 ± 9) | −1 (61 ± 11) |
| Left ventricular ESD [mm] | −2 (49 ± 11) | −2 (48 ± 12) | −2 (47 ± 13) | 0 (48 ± 15) |
Values given are ± standard deviation unless otherwise stated.
p = 0.003,
p = 0.021 for CoQ10 vs. placebo at 2 years,
BP — blood pressure; CoQ10 — coenzyme Q10; EDD — end-diastolic diameter; EF — ejection fraction; ESD — end-systolic diameter; 6MWT — 6-min walk test; NR — not recorded; NYHA — New York Heart Association; SE — standard error; VAS — Visual Analogue Scale
Effect on long-term endpoints
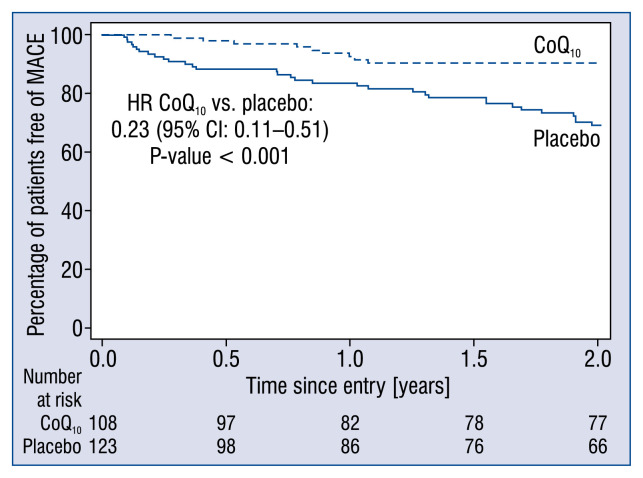
The long-term primary endpoint MACE was reached by significantly fewer patients in the CoQ10 group (n = 10, 9%) compared to the placebo group (n = 33, 27%, p = 0.001; Table 4). A significant risk reduction in MACE with CoQ10 compared to placebo was found from a Cox proportional hazards regression analysis stratified by center (hazard ratio [HR] 0.23; 95% confidence interval [CI] 0.11–0.51; p < 0.001; Fig. 1).
Table 4.
Major adverse cardiovascular events at 2 years.
| Endpoint | CoQ10 (n = 108) | Placebo (n = 123) |
|---|---|---|
| Death due to MI | 2 | 3 |
| Death due to HF | 1 | 6 |
| Sudden cardiac death | 4 | 8 |
| Hospitalization due to acute HF and PE | 0 | 1 |
| Hospitalization due to worsening HF | 3 | 15 |
| Total | 10 (9%)* | 33 (27%) |
p = 0.001.
CoQ10 — coenzyme Q10; HF — heart failure; MI — myocardial infarction; PE — pulmonary embolism
Figure 1.
Estimates of the time to primary endpoint of major adverse cardiovascular events (MACE) in the placebo group (solid line) and the coenzyme Q10 (CoQ10) group (dashed line). The primary endpoint was composite MACE of hospital stay for worsening heart failure, cardiovascular death, mechanical support, or urgent cardiac transplantation; CI — confidence interval; HR — hazard ratio.
A significantly greater proportion of patients in the CoQ10 group improved by at least one grade in NYHA functional classification after 2 years (n = 39, 48%) compared to the placebo group (n = 19, 25%, p = 0.003; Table 3). In the CoQ10 group there was a significant improvement of 6% in LVEF compared to baseline (p = 0.021) but there was no significant change in the placebo group (p = 0.234; Table 3). In the CoQ10 group, compared to baseline, serum NT-proBNP was reduced by a mean of 702 pg/mL (28%) in the CoQ10 group and a reduction of 276 pg/mL (12%) in the placebo group. Neither of these values were significantly different from baseline nor were there differences between the two groups (Table 2). For heart rate and blood pressure there were no significant changes from baseline with treatment in either group nor were there any between-group differences (Table 3).
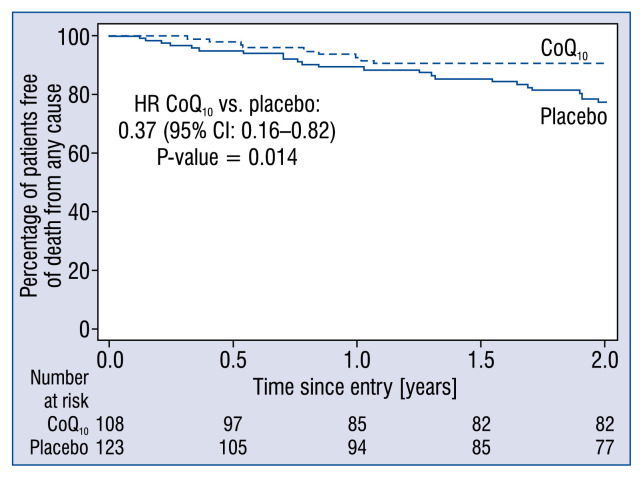
All-cause mortality was lower in the CoQ10 group, 10 (9%) patients vs. 24 (20%) patients in the placebo group, corresponding to a relative reduction of 53% (p = 0.040). Using a Cox proportional hazards regression analysis stratified by center revealed a significant reduction in all-cause mortality with CoQ10 compared to placebo (HR 0.37; 95% CI 0.16–0.82; p = 0.014; Fig. 2). The total number of cardiovascular deaths, was also lower in the CoQ10 group compared to the placebo group, 9 (8%) vs. 21 (17%) corresponding to a relative reduction of 51% (p = 0.052). From a Cox regression analysis stratified by center, the HR (CoQ10 vs. placebo) was 0.36 (95% CI 0.15–0.85; p = 0.020). Three (3%) patients were hospitalized due to worsening HF in the CoQ10 group vs. 16 (13%) patients in the placebo group (p = 0.007). The risk of unplanned hospitalization due to worsening HF counted as MACE was significantly lower in the CoQ10 group with a HR of 0.07 (95% CI 0.01–0.36; p = 0.001) using a Cox proportional hazards regression analysis stratified by center.
Figure 2.
Estimates of the secondary outcome death from any cause in the placebo group (solid line) and the coenzyme Q10 (CoQ10) group (dashed line); CI — confidence interval; HR — hazard ratio.
Adverse effects
There were no differences in the total number of adverse events in the CoQ10 group, 17 (16%) vs. 28 (23%) in the placebo group (p = 0.188).
Comparison of the European population with the total population
Baseline patient characteristics and management
In comparison with the whole population of Q-SYMBIO, the Europeans were slightly older (mean 65 vs. 62 years), heavier (84.3 vs. 77.5 kg) with a lower heart rate (73 vs. 81 bpm), and a higher prevalence of atrial fibrillation (26% vs 18%) (Table 5). The majority of both populations were classified as NYHA class III (89% and 87%). Almost half as many of the European patients were classified as NYHA IV compared to the total population (5% vs. 9%). The lower percentage of patients with end-stage HF in the European population was in accordance with a greater average performance in 6MWT (325 m vs. 287 m) and a slightly higher mean LVEF (33% vs. 31%). The Europeans were more frequently treated with beta-blockers (88% vs. 73%), statins (57% vs. 36%) and anticoagulants (37% vs. 25%). Patients treated with device-based therapy in Q-SYMBIO were all European.
Table 5.
Comparison of baseline characteristics in European and total population.
| Characteristic | European population (n = 231) | Total population (n = 420) | P |
|---|---|---|---|
| Age [years] | 64.8 ± 11 | 62.2 ± 12 | 0.007 |
| Male sex | 77% | 73% | 0.205 |
| Weight [kg] | 84.3 ± 17.8 | 77.5 ± 17 | < 0.001 |
| BMI [kg/m2] | 28.9 ± 6 | 28 ± 6 | 0.049 |
| Heart rate [bpm] | 73 ± 12 | 81 ± 15 | < 0.001 |
| Systolic BP [mmHg] | 124 ± 20 | 123 ± 17 | 0.805 |
| Diastolic BP [mmHg] | 75 ± 11 | 78 ± 11 | < 0.001 |
| Sinus rhythm | 62% | 74% | 0.003 |
| Atrial fibrillation | 26% | 18% | 0.019 |
| Rhythm, other (pace) | 12% | 9% | 0.138 |
| Ischemic heart disease | 66% | 70% | 0.333 |
| Dilated cardiomyopathy | 32% | 27% | 0.153 |
| Valvular heart disease | 3% | 3% | 1.000 |
| Duration of HF [months] | 41 ± 50 | 37 ± 41 | 0.271 |
| NYHA class II | 6% | 3% | |
| NYHA class III | 89% | 87% | 0.077 |
| NYHA class IV | 5% | 9% | |
| Left ventricular EF [%], [range] | 33 ± 12 [10–70] | 31 ± 10 [10–70] | 0.013 |
| Left ventricular EDD [mm] | 63 ± 11 | 65 ± 9 | 0.016 |
| Left ventricular ESD [mm] | 51 ± 13 | 54 ± 11 | < 0.001 |
| 6MWT [m], [range] | 325 ± 91 [25–525] | 287 ± 98 [25–525] | < 0.001 |
| Serum CoQ10 [μg/mL] | 0.92 ± 0.07 | 0.92 ± 0.05 | 0.211 |
| NT-proBNP [pg/mL]† | 2399 ± 272, p50: 1196 | 1783 ± 276, p50: 782 | 0.051 |
| Use of medications: | |||
| ACEI/ARBs | 91% | 89% | 0.476 |
| Beta-blockers | 88% | 73% | < 0.001 |
| Digoxin | 32% | 45% | 0.002 |
| Diuretics | 84% | 79% | 0.138 |
| Aldosterone antagonists | 54% | 56% | 0.934 |
| Statin derivatives | 57% | 36% | < 0.001 |
| Anticoagulants | 37% | 25% | 0.001 |
| Diabetes treatment | 26% | 23% | 0.441 |
Values are mean or number.
To convert values for NT-proBNP to picomoles per liter, divide by 8.457.
ACE/ARB — angiotensin converting enzyme inhibitor/angiotensin receptor blocker; BMI — body-mass index; BP — blood pressure; CoQ10 — coenzyme Q10; EDD — end-diastolic diameter; ESD — end-systolic diameter; EF — ejection fraction; HF — heart failure; NT-proBNP — N-terminal pro–B-type natriuretic peptide; NYHA — New York Heart Association; 6MWT — 6-min walk test
Event rate and treatment effects
The serum CoQ10 levels and overall event rates in the CoQ10 treated group of the European sub-population were similar or better than in the total population (Table 6). In the European sub-population, there was a significant improvement of 6% in LVEF compared to baseline (p = 0.021) in the CoQ10 group but no significant change in the placebo group (p = 0.234; Table 3). Whereas in the total population there were no significant between-group differences or changes from baseline in any of the echocardiographic measurements [12].
Table 6.
Comparison of serum coenzyme Q10 (CoQ10) and overall event rates and risk reduction at 2 years in the European and total population.
| Endpoint | European sub-population | Total population | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| CoQ10 (n = 108) | Placebo (n = 123) | RRR | P | CoQ10 (n = 202) | Placebo (n = 218) | RRR | P | |
| CoQ10-S [μg/mL] | 3.55 | 0.76 | < 0.001 | 2.01 | 0.81 | < 0.001 | ||
| MACE | 9% | 27% | 66% | 0.001 | 15% | 26% | 43% | 0.005 |
| Death from any cause | 9% | 20% | 53% | 0.040 | 10% | 18% | 42% | 0.036 |
| Cardiovascular death | 8% | 17% | 51% | 0.052 | 9% | 16% | 43% | 0.039 |
| Hospitalization for HF | 3% | 13% | 79% | 0.007 | 8% | 14% | 41% | 0.067 |
HF — heart failure; MACE — major adverse cardiovascular events; RRR — relative risk reduction
Discussion
Summary
The beneficial effect of CoQ10 in the landmark Q-SYMBIO study of 420 international patients was reflected in the more racially homogeneous, more intensively treated subgroup of 231 European patients in terms of a significant improvement in NYHA class and a significant risk reduction for the primary composite MACE endpoint and reductions in the secondary endpoints of all-cause mortality, cardiovascular mortality and hospitalization for HF. The improvements in major clinical endpoints were supported by a significant increase in LVEF in the European population which had not been found in the larger cohort [12]. It was concluded that the therapeutic efficacy of CoQ10 demonstrated in the original Q-SYMBIO study was confirmed and even enhanced in the European sub-population.
Despite a careful selection of patients and an apparently homogenous population in clinical studies there are inherent hidden factors in HF trials that may affect outcomes. These factors include ethnicity, medical preferences of physicians, financing of medical care and drug availability [13–15, 19]. This study aimed to investigate if the therapeutic efficacy of CoQ10 found in a total international population of Q-SYMBIO (n = 420) also applied to a more homogeneous European sub-population (n = 231). Compared to the total population of Q-SYMBIO, the European sub-population was slightly older, with a lower heart rate, a higher LVEF and a higher percentage with atrial fibrillation. Similar differences in baseline characteristics have recently been found in trials of chronic HF (PARADIGM-HF, EMPHASIS-HF) and acute HF (ASCEND-HF, ASTRONAUT) with patients enrolled from 5–6 global regions including Asia and Europe [14, 16, 20]. The European sub-population of Q-SYMBIO showed a higher adherence to guideline recommended medical and device therapies when compared to the entire study population. The Europeans were more frequently prescribed beta-blockers, statins and anticoagulants and less frequently digoxin. Furthermore, all patients receiving device-based therapy in Q-SYMBIO were European.
A sub-optimal use of guideline-directed medical therapy in Asian countries compared to Western countries has been described previously in registries for HF and recent large-scale HF trials [14, 20, 21]. Global differences in adherence to guideline-directed therapy in PARADIGM-HF and ASTRONAUT correspond to differences found in Q-SYMBIO including a higher rate in the prescription of beta-blockers, anticoagulation and a lower rate of digoxin in European populations compared to Asian-Pacific populations [16, 20]. A lower use of lipid-lowering agents such as statins in Asian countries is also well known [22]. Similarly, to Q-SYMBIO, analyses of PARADIGM-HF, ASTRONAUT and ASCEND-HF have demonstrated a markedly lower use of device-based therapy in Asian-Pacific regions compared to other regions, probably reflecting economic differences [16, 20, 21]. The more frequent prescription of anti-coagulants reflects the higher occurrence of atrial fibrillation in the European sub-population of Q-SYMBIO. However, not all differences in medication can be explained by differences in baseline characteristics. Differences in medication and device therapy may be related to medical practice patterns, resources in medical care and perceptions of drug tolerability in Asian populations [21].
The findings in this analysis showing no major differences in outcomes in the European sub-population despite differences in medical therapy and baseline characteristics were in accordance with PARADIGM-HF and EMPHASIS. In contrast, regional differences in outcome have been found in the acute HF trials ASTRONAUT and ASCEND-HF and may be a result of differences in the management and duration of hospitalization for acute HF patients having a greater impact on outcome [14].
The serum level of CoQ10 in the CoQ10 treated European sub-population remained constant and above 3 μg/mL throughout the study period (Tables 2, 6). In contrast, the serum level of CoQ10 in CoQ10 treated patients in the total population decreased from 3.01 ± 0.17 μg/mL at 3 months to 2.01 ± 0.20 μg/mL at 2 years (Table 6). This could indicate a problem with compliance in the non-European patient population towards the end of the study period. The higher serum levels of CoQ10 of the European HF patients during the full study period may have contributed to the slightly increased CoQ10 efficacy (increased LVEF; Tables 3, 6) compared with the efficacy found in the total population [12], despite the fact that the European cohort was better medicated and smaller sample size.
Current drug therapy for HF predominately targets the secondary consequences of the failing heart by blocking overactivated neurohormonal pathways. While this therapy provides some relief of symptoms, improves prognosis and prevents some degree of cardiac remodeling, it does not target the basic energy depletion of the failing myocardium [23]. Significantly decreased tissue levels of CoQ10 have been found in patients with failing hearts such as dilated cardiomyopathy, restrictive cardiomyopathy and toxic myocardial disease [9]. In patients with HF of mixed etiology, a deficiency of CoQ10 in serum and tissue is more pronounced in the severest stages of HF. After oral supplementation with CoQ10 of selected patients with cardiomyopathy undergoing repeat biopsies after 5 months of treatment, tissue deficiency was reduced significantly and this was accompanied by an improvement in clinical and hemodynamic parameters [9, 10, 24]. The therapeutic efficacy of CoQ10 is primarily ascribed to its important role as electron carrier in the electron transport chain and strong anti-oxidative properties thus increasing bioenergetics and preventing oxidative damage of the failing myocardium [25, 26]. Other beneficial actions of CoQ10 include stabilization of cell membranes and the mitochondrial membrane transition pore thus protecting the myocardium from apoptotic events [27]. Further evidence suggests that endothelial function is improved [28, 29] and cardiac contractility increased by CoQ10 [30, 31]. In concert, these actions by CoQ10 may halt the vicious cycle of HF and protect the myocardium from further deterioration and perhaps facilitate a potential for myocardial recovery [32].
Limitations of the study
In comparing the European subgroup with the main Q-SYMBIO group it was not possible to ascribe differences between European vs. non-European to ethnic or geographic differences. The main Q-SYMBIO group of 420 included 231 patients from Europe, 178 patients from Asia and 11 patients from Australia. Thus, it was not possible in this subgroup analysis to elucidate ethnic differences but rather to study and confirm the efficacy of CoQ10 in a sub-group where standard therapy was more closely applied. The present study was not powered to assess between-population differences. Measurements of LVEF have a varying intra- and interobserver variance from 3% to 7% depending on how trained the observer is, nevertheless, an absolute improvement of 6% in LVEF is likely to be genuine and clinically relevant.
Conclusions
It was concluded that in the European subgroup of the Q-SYMBIO study the evidence of therapeutic efficacy of CoQ10 found in the original study was confirmed, despite higher adherence to guideline directed therapy than that of the whole group. In addition, CoQ10 therapy was associated with an increase in LVEF in the European population which had not been found in the larger cohort. This subgroup analysis provides confirmatory evidence for the conclusion of the original study that the treatment of patients with moderate to severe HF with CoQ10 in addition to standard therapy is safe, well tolerated and is associated with a reduction in symptoms, MACE and with improved survival.
Acknowledgements
We gratefully acknowledge the contribution from the investigators of Q-SYMBIO: Australia: F. Rosenfeldt; Austria: P. Dolliner, G. Steurer; Denmark: S.A. Mortensen; Hungary: V. Nagy, J. Feher (deceased), G. Paragh, P. Fülop; India: A. Kumar, H. Kaur; Malaysia: C.S. Ping, A.A.A. Rahim; Poland: K.J. Filipiak, M. Bronisz, M. Stopinski, M. Marchel, A. Kaplon-Cieslicka, W. Sinkiewicz, B. Wozakowska-Kaplon, M. Bzymek, H. Wysocki, M. Krzciuk; Slovakia: D. Pella, I. Lazurova; Sweden: U. Alehagen. We express our appreciation to F. Skjøth, Center for Cardiovascular Research, Aalborg University Hospital, Aalborg, Denmark for the statistical analysis and the core biochemical laboratory of G.P. Littarru, Ancona, Italy.
Footnotes
Funding: Grant from International Coenzyme Q10 Association, a non-profit association formed in 1997 to promote research and educational activities related to CoQ10.
Conflict of interest: None declared
References
- 1.Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347(18):1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 2.Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta. 2004;1660(1–2):171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Littarru GP, Tiano L. Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Mol Biotechnol. 2007;37(1):31–37. doi: 10.1007/s12033-007-0052-y. [DOI] [PubMed] [Google Scholar]
- 4.Bhagavan HN, Chopra RK. Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res. 2006;40(5):445–453. doi: 10.1080/10715760600617843. [DOI] [PubMed] [Google Scholar]
- 5.Kalén A, Appelkvist EL, Dallner G. Age-related changes in the lipid compositions of rat and human tissues. Lipids. 1989;24(7):579–584. doi: 10.1007/BF02535072. [DOI] [PubMed] [Google Scholar]
- 6.Bentinger M, Tekle M, Dallner G. Coenzyme Q-biosynthesis and functions. Biochem Biophys Res Commun. 2010;396(1):74–79. doi: 10.1016/j.bbrc.2010.02.147. [DOI] [PubMed] [Google Scholar]
- 7.Littarru GP, Tiano L. Clinical aspects of coenzyme Q10: an update. Nutrition. 2010;26(3):250–254. doi: 10.1016/j.nut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Folkers K, Littarru GP, Ho L, et al. Evidence for a deficiency of coenzyme Q10 in human heart disease. Int Z Vitaminforsch. 1970;40(3):380–390. [PubMed] [Google Scholar]
- 9.Folkers K, Vadhanavikit S, Mortensen SA. Biochemical rationale and myocardial tissue data on the effective therapy of cardiomyopathy with coenzyme Q10. Proc Natl Acad Sci U S A. 1985;82(3):901–904. doi: 10.1073/pnas.82.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vadhanavikit S, Morishita M, Duff GA, et al. Micro-analysis for coenzyme Q10 in endomyocardial biopsies of cardiac patients and data on bovine and canine hearts. Biochem Biophys Res Commun. 1984;123(3):1165–1169. doi: 10.1016/s0006-291x(84)80255-7. [DOI] [PubMed] [Google Scholar]
- 11.Sharov VG, Todor AV, Silverman N, et al. Abnormal mitochondrial respiration in failed human myocardium. J Mol Cell Cardiol. 2000;32(12):2361–2367. doi: 10.1006/jmcc.2000.1266. [DOI] [PubMed] [Google Scholar]
- 12.Mortensen SA, Rosenfeldt F, Kumar A, et al. The effect of co-enzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail. 2014;2(6):641–649. doi: 10.1016/j.jchf.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Poole-Wilson PA. Global differences in the outcome of heart failure: implications for clinical practice. J Am Coll Cardiol. 2008;52(20):1649–1651. doi: 10.1016/j.jacc.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Egwim C, Dixon B, Ambrosy AP, et al. Global variations in patient populations and outcomes in heart failure clinical trials. Curr Heart Fail Rep. 2017;14(1):30–39. doi: 10.1007/s11897-017-0316-1. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira JP, Girerd N, Rossignol P, et al. Geographic differences in heart failure trials. Eur J Heart Fail. 2015;17(9):893–905. doi: 10.1002/ejhf.326. [DOI] [PubMed] [Google Scholar]
- 16.Kristensen SL, Martinez F, Jhund PS, et al. Geographic variations in the PARADIGM-HF heart failure trial. Eur Heart J. 2016;37(41):3167–3174. doi: 10.1093/eurheartj/ehw226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Littarru GP, Mosca F, Fattorini D, et al. Assay of coenzyme Q10 in plasma by a single dilution step. Methods Enzymol. 2004;378:170–176. doi: 10.1016/S0076-6879(04)78014-3. [DOI] [PubMed] [Google Scholar]
- 18.Sokoll LJ, Baum H, Collinson PO, et al. Multicenter analytical performance evaluation of the Elecsys proBNP assay. Clin Chem Lab Med. 2004;42(8):965–972. doi: 10.1515/CCLM.2004.157. [DOI] [PubMed] [Google Scholar]
- 19.O’Connor CM, Fiuzat M, Swedberg K, et al. Influence of global region on outcomes in heart failure β-blocker trials. J Am Coll Cardiol. 2011;58(9):915–922. doi: 10.1016/j.jacc.2011.03.057. [DOI] [PubMed] [Google Scholar]
- 20.Greene SJ, Fonarow GC, Solomon SD, et al. Global variation in clinical profile, management, and post-discharge outcomes among patients hospitalized for worsening chronic heart failure: findings from the ASTRONAUT trial. Eur J Heart Fail. 2015;17(6):591–600. doi: 10.1002/ejhf.280. [DOI] [PubMed] [Google Scholar]
- 21.Mentz RJ, Roessig L, Greenberg BH, et al. Heart failure clinical trials in east and southeast asia: understanding the importance and defining the next steps. JACC Heart Fail. 2016;4(6):419–427. doi: 10.1016/j.jchf.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma KK, Gupta R, Agrawal A, et al. Low use of statins and other coronary secondary prevention therapies in primary and secondary care in India. Vasc Health Risk Manag. 2009;5:1007–1014. doi: 10.2147/vhrm.s8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovascular Research. 2009;81(3):412–419. doi: 10.1093/cvr/cvn301.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mortensen SA. Perspectives on therapy of cardiovascular diseases with oenzyme Q10 (Ubiquinone) Clinical Investigator. 1993;71(S8):S116–S123. doi: 10.1007/bf00226851.. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari R, Guardigli G, Mele D, et al. Oxidative stress during myocardial ischaemia and heart failure. Curr Pharm Des. 2004;10(14):1699–1711. doi: 10.2174/1381612043384718. [DOI] [PubMed] [Google Scholar]
- 26.Opie LH. The metabolic vicious cycle in heart failure. Lancet. 2004;364(9447):1733–1734. doi: 10.1016/S0140-6736(04)17412-6. [DOI] [PubMed] [Google Scholar]
- 27.Papucci L, Schiavone N, Witort E, et al. Coenzyme q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J Biol Chem. 2003;278(30):28220–28228. doi: 10.1074/jbc.M302297200. [DOI] [PubMed] [Google Scholar]
- 28.Belardinelli R, Muçaj A, Lacalaprice F, et al. Coenzyme Q10 and exercise training in chronic heart failure. Eur Heart J. 2006;27(22):2675–2681. doi: 10.1093/eurheartj/ehl158. [DOI] [PubMed] [Google Scholar]
- 29.Littarru GP, Tiano L, Belardinelli R, et al. Coenzyme Q(10), endothelial function, and cardiovascular disease. Biofactors. 2011;37(5):366–373. doi: 10.1002/biof.154. [DOI] [PubMed] [Google Scholar]
- 30.Rosenfeldt F, Marasco S, Lyon W, et al. Coenzyme Q10 therapy before cardiac surgery improves mitochondrial function and in vitro contractility of myocardial tissue. J Thorac Cardiovasc Surg. 2005;129(1):25–32. doi: 10.1016/j.jtcvs.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 31.Belardinelli R, Muçaj A, Lacalaprice F, et al. Coenzyme Q10 improves contractility of dysfunctional myocardium in chronic heart failure. Biofactors. 2005;25(14):137–145. doi: 10.1002/biof.5520250115. [DOI] [PubMed] [Google Scholar]
- 32.Wilcox JE, Fonarow GC, Ardehali H, et al. “Targeting the Heart” in Heart Failure: Myocardial Recovery in Heart Failure With Reduced Ejection Fraction. JACC Heart Fail. 2015;3(9):661–669. doi: 10.1016/j.jchf.2015.04.011. [DOI] [PubMed] [Google Scholar]