Abstract
Background
Gene COPINE III may be related to a phosphoprotein with intrinsic kinase activity and belongs to an unconventional kinase family. The CPNE3 gene may be used as a biomarker for assessment of occurrence and prognosis of various tumors.
Methods
Peripheral blood was collected from 87 stable coronary artery disease (CAD) patients and 91 acute myocardial infarction (AMI) patients. Real-time quantitative polymerase chain reaction test and the western blot method were adopted to measure expression quantity of CPNE3 gene at the mRNA level and the protein level.
Results
The expression of the CPNE3 gene in peripheral blood of AMI patients was significantly lower than those in peripheral blood of stable CAD patients. Low expression of CPNE3 gene was found to be unrelated to level of fasting blood glucose and serum blood lipid of patients, quantity of cardiac troponin and time of onset but was found to be correlated to the Gensini score for coronary artery. When the expression of CPNE3 gene at the mRNA level in peripheral blood was used as the criterion for diagnosing AMI, its sensitivity, specificity, positive predictive value and negative predictive value were 69%, 64.8%, 68.6% and 65.2%, respectively.
Conclusions
Compared to stable CAD patients, AMI patients have a lower expression of CPNE3 gene in their peripheral blood. Patients who have low CPNE3 expression in peripheral blood are more likely to suffer from AMI than those with stable CAD. Low expression of CPNE3 gene serves as an potential independent risk factor of AMI.
Keywords: CPNE3 gene, acute myocardial infarction, stable coronary artery disease, independent risk, peripheral blood, genetic marker
Introduction
In the United States, 40% of all cases of death every year are related to coronary artery disease (CAD) and it is estimated that such disease will be a major cause of death around the world in the next decades [1]. In particular, there is a potential and fatal risk of acute myocardial infarction (AMI) in the progression of CAD [2, 3]. In patients with CAD, conventional risk factors were present at a much higher prevalence than commonly believed, with 15% to 20% of patients lacking any of the conventional risk factors for the disease [4]. Just like other chronic diseases, CAD is a complex polygenic disease, based on the incidence rate of AMI which increases with age, which is jointly caused by genetic factors and environmental factors [5].
According to various literature, gene expression level in peripheral blood can reflect changes of various complex diseases including cardiovascular disease and serves as an extremely significant biomarker for detection and verification of diseases [6, 7]. For example, serving as a potential inflammation marker, an increase in expression of α-defensin in peripheral blood may serve to predict the risk of CAD occurrence among hyperlipidemia patients [8].
Gene COPINE III may be related to a phosphoprotein with intrinsic kinase activity and belongs to an unconventional kinase family [9]. It may have an influence on membrane protein, lipid and other activities [10]. CPNE3 gene can be used to predict metastatic potential of small cell lung cancer and serves as an index for prognosis of patients or a therapeutic target [11]. Moreover, the CPNE3 gene also plays a role with high correlation in carcinogenesis of prostate cancer [12]. It has also been proved that CPNE3 gene is correlated to schizophrenia [13].
The result of a previous analysis on profile of differential gene expression (2 pools, 3 subjects in each pool) in peripheral blood for AMI conducted by this research team indicates that AMI patients have a lower expression in their peripheral blood than stable CAD patients, for which the time of expression is 0.484 time. Therefore, by using the method of expanding the sample size of clinical data, this research aims to verify the relationship of CPNE3 gene AMI.
Methods
Subjects
Among patients admitted into Department of Cardiovascular Medicine, China-Japan Union Hospital of Jilin University from April 2016 to September 2016 and subject to coronary angiography, 91 patients definitively diagnosed with AMI according to the global uniform definition of myocardial infarction issued in 2012 [14] were selected to form the AMI group and 87 patients diagnosed with stable CAD [15] based on 2013 European Society of Cardiology guidelines were selected to form the control group (1. Narrowing of ≥ 50% in the left main coronary artery and ≥ 70% in one or several of the major coronary arteries. 2. The duration of discomfort is brief — no more than 10 min in the majority of cases and more commonly even several minutes or less).
Statement of ethical approval
The Ethics Committee, China-Japan Union Hospital of Jilin University approved this research. Collection of samples and information about all subjects was approved by the subjects, from whom informed consent forms were signed.
Peripheral blood collection, total RNA extraction and cDNA synthesis
The collection of 4 mL of peripheral venous blood of each subject and conducting total RNA extraction from the collected peripheral blood by using total RNA extraction reagent kit was carried out (RNAsimple Total RNA Kit, Tiangen Biotech (Beijing) Co. Ltd. Beijing) according to instructions on the reagent kit. Reverse transcription of 1 μg of the qualified total RNA was conducted by using reverse transcription reagent kit (TOYOBO Rever Tra Ace qPRC RT kit, Shanghai) and the obtained cDNA sample was stored under conditions with a temperature of −20°C for real-time (RT) quantitative polymerase chain reaction (PCR) in the next step.
Real-time quantitative PCR test
Polymerase chain reaction amplification was conducted with SYBR real-time quantitative PCR reagent kit (SYBR Premix Ex Taq TM, TaKaRa, Dalian). The 20 μL-reaction system was adopted, for which each reaction included: 10 μL of SYBR Premix Ex Taq TM, 0.5 μL of forward primer and 0.5 μL of reverse primer (concentration should be 10 μmol/L), 8 μL of nuclease-free double distilled water and 1 μL of cDNA template. Amplification was realized by using Mx3005P RT quantitative PCR system (Strata Gene). Relative expression quantity 2−ΔCt (ΔCt = Target Gene Ct Value — Reference Gene Ct Value) was used to express the obtained cycle thresholds (Ct) of each sample and a comparison was conducted [16]. Design of PCR primer was conducted according to CPNE3 gene sequence provided by NCBI Database and synthesis was completed by Shanghai-based Sangon Biotech. Primer sequence is indicated in Table 1.
Table 1.
Sequence of RT-PCR primers.
| Genes | Genes primer sequence (5′–3′) | |
|---|---|---|
| CPNE3 | Fa | GTCAGACCCTTTATGTGTGTTGT |
| Rb | TGGAAAATTGGGGATTCAAGCAA | |
| GAPDH | Fa | ACGGATTTGGTCGTATTGGGCG |
| Rb | CTCCTGGAAGATGGTGATGG |
Fa — sequence from sense strands; Rb — sequence from anti-sense strands
Statistical analysis
All data were statistically analyzed with SPSS24.0 software. When the measured data conformed to normal distribution, statistical description was conducted by using X̄ ± S and intergroup difference comparison was realized through using independent T test analysis. When measurement data did not conform to normal distribution, median and inter-quartile range was adopted to conduct statistical description and inter-group difference comparison was realized with rank-sum test. Statistical description of enumeration data was realized through the use of frequency number and χ2 test to analyze inter-group differences. Binary logistic regression analysis was conducted to analyze relevant risk factors of AMI. As for the correlation between relative expression quantity of CPNE3 gene, cardiac troponin I and Gensini score, double-variable correlation analysis was conducted to analyze any correlation. Statistical result characterized by p < 0.05, bilaterally, was deemed as having statistical significance.
Results
Clinical data analysis
It is indicated by the results of clinical data analysis on the subjects that: There is no significant statistical difference between the two groups in the following aspects: Age, sex, body mass index, history of hypertension, family history of coronary heart disease, systolic pressure, triglyceride (TG), total cholesterol (TC), low density lipoprotein cholesterol (LDL-C) and high density lipoprotein cholesterol (HDL-C). Those patients in the AMI group are older than patients in the stable CAD group and such difference is statistically significant (t = 2.063, p = 0.041). It was also found that left ventricular ejection fraction (EF) was significantly different between the two groups (Z = −3.530, p = 0.000; see Table 2 for details).
Table 2.
Comparison of clinical data between the acute myocardial infarction (AMI) group and the stable coronary artery disease (CAD) group.
| Categories of data | The AMI group (n = 91) | The stable CAD group (n = 87) | t/χ2/z | P |
|---|---|---|---|---|
| Age [years] | 64.55 ± 11.464 | 61.32 ± 8.452 | 2.063 | 0.041 |
| Sex: | 0.186 | 0.666 | ||
| Male | 60 (65.9) | 69 (69.0) | ||
| Female | 31 (34.1) | 27 (31.0) | ||
| BMI [kg/m2] | 24.802 (22.477–27.357) | 25.799 ± 3.209 | −1.711 | 0.087 |
| Hypertension | 44 (48.4) | 45 (51.7) | 0.202 | 0.653 |
| Family medical history | 7 (7.7) | 4 (4.6) | 0.718 | 0.397 |
| Systolic pressure [mmHg] | 133.73 ± 23.488 | 136.04 ± 20.524 | −0.350 | 0.727 |
| Diastolic pressure [mmHg] | 81 (75.75–91) | 80.84 ± 12.61 | −1.136 | 0.256 |
| Smoking history | 41 (45.1) | 40 (46.0) | 0.015 | 0.902 |
| Diabetes | 20 (22.0) | 28 (32.2) | 2.352 | 0.125 |
| Fasting blood-glucose [mmol/L] | 6.03 (5.085–8.235) | 5.81 (5.240–8.170) | −0.360 | 0.719 |
| Total cholesterol [mmol/L] | 4.553 ± 1.358 | 4.334 ± 1.010 | 1.194 | 0.234 |
| Triglyceride [mmol/L] | 1.57 (1.160–2.303) | 1.74 (1.140–2.500) | −0.500 | 0.617 |
| LDL-C [mmol/L) | 3.091 ± 1.101 | 2.855 ± 0.844 | 1.569 | 0.118 |
| HDL-C [mmol/L] | 0.94 (0.805–1.133) | 0.88 (0.770–1.070) | −0.896 | 0.370 |
| Ejection fraction [%] | 63 (42–65) | 65 (63–67.5) | −3.530 | 0.000 |
BMI — body mass index; HDL-C — high density lipoprotein cholesterol; LDL-C — low density lipoprotein cholesterol
Comparative analysis on relative expression quantity of CPNE3 gene at the mRNA level between the AMI group and the stable CAD group
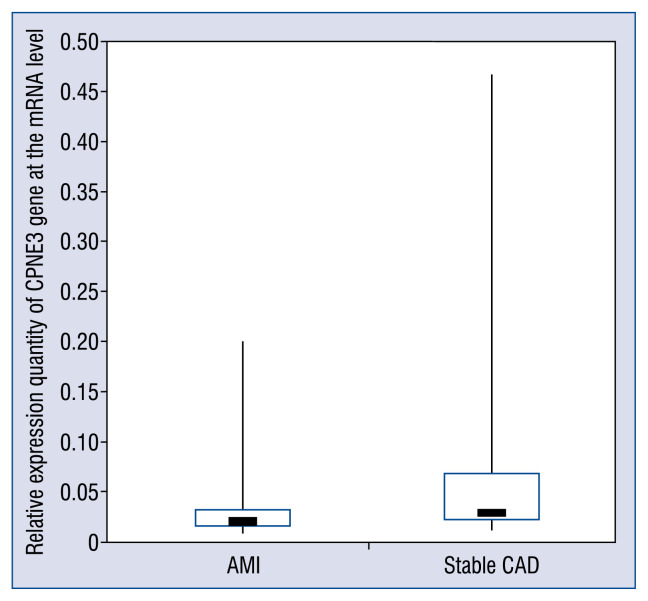
ΔCt value of each sample obtained through RT-PCR was the mean value obtained through three repeated measurements on each sample. The results indicate that 2−ΔCt of the AMI group is 0.020 (0.016–0.032) and 2ΔΔCt of the stable CAD group was 0.029 (0.021–0.067), for which there is a significant statistical difference (Z = −4.231, p = 0.000) between these two groups. The relative expression quantity of CPNE3 gene at the mRNA level in peripheral blood of patients in the AMI group was significantly lower than that of patients in the stable CAD group, for which the relative expression quantity of CPNE3 gene of the AMI group is 0.69 times that of the stable CAD group (see Figure 1 for details).
Figure 1.
Comparative analysis on relative expression quantity of CPNE3 gene at the mRNA level between the acute myocardial infarction (AMI) group and the stable coronary artery disease (CAD) group.
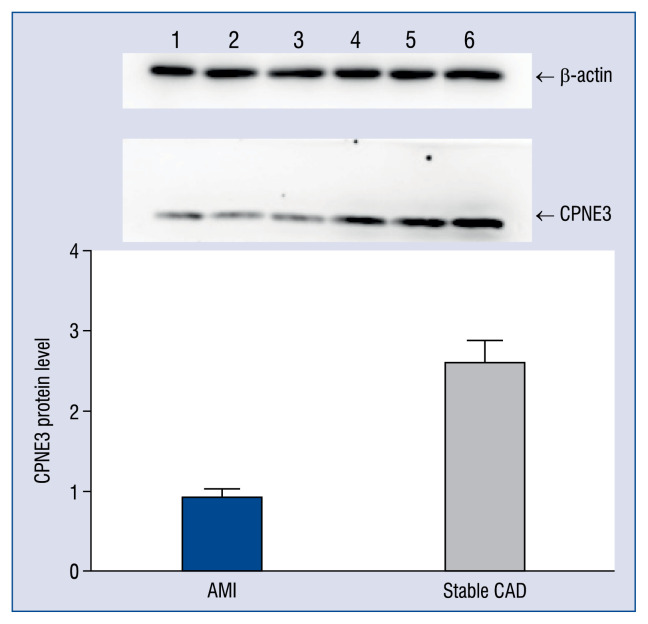
Analysis on the result of expression of CPNE3 gene at the protein level in peripheral blood
Western blot was conducted on peripheral blood of patients with β-actin as the reference. The results indicated that the relative expression of CPNE3 was 0.925 ± 0.118 in the AMI group and 2.599 ± 0.261 in the stable CAD group. Compared to stable CAD patients, AMI patients have a low expression of CPNE3 at the protein level in their peripheral blood and the expression of CPNE3 of the AMI group at the protein level is 0.356 times that of the stable CAD group (see Figure 2 for details).
Figure 2.
Comparison of expression level of CPNE3 gene at the protein level. For the acute myocardial infarction (AMI) group, sample numbers are 1, 2 and 3; For the stable coronary artery disease (CAD) group, sample numbers are 4, 5 and 6.
Analysis on correlation between expression quantity of CPNE3 gene and age, serum blood lipid and blood glucose, ejection fraction of patients
Based on stratification standard for blood lipid level [17], all subjects included were divided into groups: with normal TC (< 5.18 mmol/L), with increased TC (≥ 5.18 mmol/L), with normal TG (< 1.76 mmol/L) with increased TG (≥ 1.76 mmol/L), with normal LDL-C (< 3.37 mmol/L) with increased LDL-C (≥ 3.37 mmol/L), with normal HDL-C (≥ 0.907 mmol/L) and the group with decreased HDL-C (< 0.907 mmol/L). Based on the standard level of fasting blood glucose [18], all subjects were divided into groups with normal level of fasting blood glucose (≤ 6.0) and a group with an increased level of fasting blood glucose (> 6.0). Based on standard age groups of Chinese people, all subjects were divided into groups at an advanced age (> 65) and at a younger age (≤ 65). All the subjects were divided into groups with normal EF (≥ 50%) and with low EF (< 50%). The result indicates that there is a difference (p = 0.008) in the expression quantity of the CPNE3 gene at the mRNA level between the group at an advanced age and the group at a younger age (see Table 3 for detail of the results).
Table 3.
Analysis on correlation between expression quantity of CPNE3 gene at the MRNA and age of patient, blood glucose, levels of serum TC, HDL-C and LDL-C, ejection fraction of patients.
| Group | Number of patients | Relative expression quantity of CPNE3 | Z | P |
|---|---|---|---|---|
| Normal blood glucose | 71 | 0.029 (0.019–0.064) | −0.739 | 0.460 |
| Increased blood glucose | 72 | 0.024 (0.018–0.045) | ||
| Normal total cholesterol | 133 | 0.025 (0.018–0.048) | −0.554 | 0.580 |
| Increased total cholesterol | 38 | 0.028 (0.018–0.076) | ||
| Normal triglyceride | 101 | 0.028 (0.019–0.064) | −0.768 | 0.442 |
| Increased triglyceride | 70 | 0.025 (0.0017–0.043) | ||
| Normal LDL-C | 121 | 0.025 (0.018–0.046) | −0.503 | 0.615 |
| Increased LDL-C | 50 | 0.030 (0.017–0.084) | ||
| Normal HDL-C | 94 | 0.025 (0.018–0.045) | −1.318 | 0.188 |
| Decreased HDL-C | 77 | 0.026 (0.018–0.059) | ||
| Younger age | 116 | 0.030 (0.019–0.070) | −2.635 | 0.008 |
| Advanced age | 62 | 0.022 (0.017–0.030) | ||
| Normal ejection fraction | 108 | 0.026 (0.018–0.042) | −1.434 | 0.152 |
| Low ejection fraction | 23 | 0.046 (0.021–0.070) |
HDL-C — high density lipoprotein cholesterol; LDL-C — low density lipoprotein cholesterol; TC — total cholesterol
Analysis on correlation between expression quantity of CPNE3 gene at mRNA level and age of patient and AMI using logistic regression analysis
Based on the cut-off value of relative expression quantity of CPNE3 gene, all the subjects were divided into a group with high expression quantity (2−ΔCt > 0.013) and a group with low expression quantity (2−ΔCt ≤ 0.013). The result indicates that: Low expression of CPNE3 is a factor closely related with AMI, the OR value increased 3.845 fold respectively. Relatively advanced age is also a factor closely related with AMI, the OR value was 2.605 fold, respectively. Correlation of estimate between low expression and relatively advanced age is (rs = −0.451, p = 0.000; see Table 4 for details).
Table 4.
Result of logistic regression analysis on independent risk factors of acute myocardial infarction.
| Regression coefficient | Standard error | Wald | P | Odds ratio | 95% confidence interval | |
|---|---|---|---|---|---|---|
| Low expression of CPNE3 gene | 1.347 | 0.326 | 17.022 | 0.000 | 3.845 | 2.028–7.292 |
| Relatively advanced age | 0.957 | 0348 | 7.583 | 0.006 | 2.605 | 1.318–5.149 |
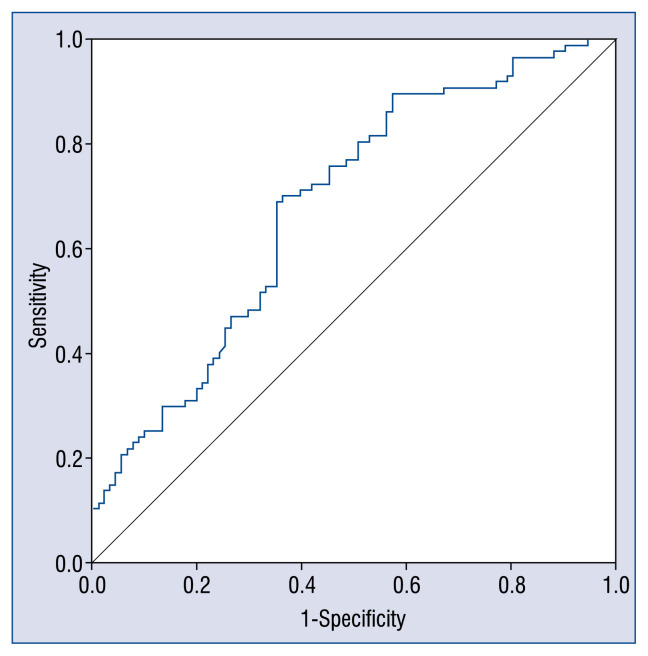
ROC curve and cut-off value of relative expression quantity of CPNE3 gene in diagnosis of AMI
Based on the relative expression quantity of CPNE3 gene, the 2−ΔCt value, acquired through PCR, receiver operating characteristic (ROC) curve was created, as indicated in Figure 3. It is known from Figure 3 that the area under curve (AUC) is 0.684 ± 0.040 and the cut-off value of relative expression quantity of CPNE3 gene determined is based on the maximum value of Youden index is 0.024, for which sensitivity and specificity for diagnosis of AMI are 0.690 and 0.648, respectively. Positive predictive value and negative predictive value are 68.6% and 65.2%, respectively (see Figure 3 for details).
Figure 3.
Receiver operating characteristic (ROC) curve CPNE3 gene in diagnosis of acute myocardial infarction. Diagonal segments are produced by ties.
Double-variable correlation analysis between relative expression quantity of CPNE3 gene and degree of severity of coronary artery lesion, cardiac troponin I, interval from timespan of onset of AMI of patients to blood collection
The result of Gensini score of all subjects was 25 (9.5–52). The expression quantity of CPNE3 at the mRNA level was negatively correlated to Gensini score (rs = −0.200, p = 0.018).
The test result of cardiac troponin for the AMI group is 0.790 (0.138–8.760) ng/mL. Concentration of serum troponin I (TnI) can reflect the scope of AMI. The expression quantity of CPNE3 gene in peripheral blood is unrelated to serum TnI concentration (rs = −0.144, p = 0.364).
The incipient long-time (≥ 20 min) rest pain of patients suffering from AMI in the last week was considered as the time of onset and the interval from onset of AMI of patients in the AMI group to the time when blood was collected should be the time interval of occurrence, for which the result was 24 (11–72) h. There was no correlation between the expression quantity of CPNE3 gene and timespan (rs = −0.098, p = 0.533).
Discussion
The role played by the Copine family in cells still remains unknown although some studies have indicated that it might have an influence on membrane protein, lipid and other activities [10]. It has been reported that the interaction between CPNE3 and ErbB2 could promote tumor metastasis, which played a role in non-small cell lung cancer, breast cancer, prostate cancer and ovarian tumor [11, 12, 19]. However, there is no study on the role played by CPNE3 in occurrence and progression of cardiovascular disease. This study focused on investigating the expression quantity of CPNE3 gene in peripheral blood of AMI patients and found that it was significantly lower than that of stable CAD patients at both the mRNA and protein levels.
CPNE3 mRNA expression with clinical variables were correlated, including blood glucose, serum TC, TG, LDL-C or HDL-C, age, and EF. As a result, a significantly different expression of CPNE3 was found between patients with AMI and patients with stable CAD. Further analysis indicated that abnormal expression quantity of CPNE3 gene was irrelevant to EF values. According to a previous study, patients with AMI had a preponderance to develop REF/HFREF due to irreversible myocardial damage. It was particularly true in large transmural/ST-segment elevation myocardial infarction infarcts [20]. Thus, the significant difference of CPNE3 gene expressions in the present study is not due to reduced EF since AMI leads to reduced EF.
Prevalence and fatality rate of AMI was reported to increase with age [21]. Currently, age remained a strong independent predictor of both in hospital and 1-year post-discharge mortality rates in patients with AMI [22]. Binary logistic analysis showed that low expression of CPNE3 gene was an independent risk factor for AMI (OR 3.845; p < 0.05) and age also was an independent risk factor for AMI (OR 2.605; p < 0.05). Regardless of age and other factors, low expression of CPNE3 gene could increase the risk of AMI in stable CAD patients by 3.845 times. While advanced age of patients alone could increase the risk of AMI by 2.605 times. Thus, it was speculated that older patients who have low CPNE3 expression would be more likely to suffer from AMI.
CPNE3 was shown to participate in immune-regulation and neutrophil degranulation, including metabolism of lipids such as fatty acid, triglyceride, lipoprotein and ketone bodies (from REACTOME). Conventional risk factors of CAD [23–25], hypertension [25], hypercholesterolemia [26], diabetes, [27] obesity, [28] smoking, [25, 29] and family history [30] were believed to have an adverse influence on prognosis in those with established disease, presumably through their effect on the progression of atherosclerotic disease processes. However, the present study demonstrated that low expression of CPNE3 was unrelated to blood glucose, serum TC, TG, LDL-C, and HDL-C. It was speculated that the mechanism of CPNE3’s role in promoting AMI may be not through lipid metabolism or blood glucose.
Although the mechanism of CPNE3’s role in promoting the occurrence of AMI is still unknown. Results herein indicate that the relative expression quantity of CPNE3 gene is correlated to Gensini score, which defines the severity of coronary artery lesion. Gensini score has been reported to be mainly correlated to the degree of coronary stenosis and scope of blood supply [31]. Therefore, lower expression of CPNE3 may lead to a higher severity of coronary artery lesion.
Quantity of cardiac troponin predicts the extent of myocardial infarction. This study showed that expression quantity of CPNE3 gene was unrelated to a concentration of TnI. Furthermore, the present study indicated that there was also no correlation between the expression quantity of CPNE3 gene in peripheral blood and timespan of AMI. If a low expression of CPNE3 had occurred with AMI, expression quantity of CPNE3 gene should be correlated to concentration of TnI and the timespan and vice versa. In our case, low expression of CPNE3 gene didn not occur with AMI and thus might serve as one of the causes of AMI which was supported by the ROC curve of CPNE3 mRNA expression in diagnosing AMI.
Therefore, it was speculated that low expression of CPNE3 gene may increase the risk of AMI by promoting formation of coronary artery lesion. Although it is not conclusive if low expression of CPNE3 causes AMI, it is speculated that low CPNE3 expression is a cause of AMI. To confirm this, a prospective randomized study is indispensable. More experiments of CPNE3 functions and investigation of its mechanism of molecular biology are also required.
Conclusions
Acute myocardial infarction patients have the lower expression of CPNE3 gene in their peripheral blood in comparison to stable CAD patients. There is an association between low expression of CPNE3 and AMI. Patients who have a lower CPNE3 expression in peripheral blood are more likely to suffer from AMI than other patients with stable CAD. Low expression of CPNE3 gene serves as a potential independent risk factor of AMI.
Acknowledgements
This research is funded by New Century Support Program for Outstanding Talents (2008), Science and Technology Department of Jilin Province (20090734) and Jilin Province Department of Finance (2012009). Many thanks to Professor Zhao Zhihui, School of Animal Husbandry and Veterinary Medicine, Jilin University and his team for their technical guidance in this research.
Footnotes
Conflict of interest: None declared
References
- 1.Shah PK. Pathophysiology of coronary thrombosis: role of plaque rupture and plaque erosion. Prog Cardiovasc Dis. 2002;44(5):357–368. doi: 10.1053/pcad.2002.123473. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 3.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111(25):3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 4.Khot UN, Khot MB, Bajzer CT, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290(7):898–904. doi: 10.1001/jama.290.7.898. [DOI] [PubMed] [Google Scholar]
- 5.Kraus WE. Genetic approaches for the investigation of genes associated with coronary heart disease. Am Heart J. 2000;140(4):S27–S35. doi: 10.1067/mhj.2000.109380. [DOI] [PubMed] [Google Scholar]
- 6.Aziz H, Zaas A, Ginsburg GS. Peripheral blood gene expression profiling for cardiovascular disease assessment. Genomic Med. 2007;1(3–4):105–112. doi: 10.1007/s11568-008-9017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elashoff MR, Wingrove JA, Beineke P, et al. Development of a blood-based gene expression algorithm for assessment of obstructive coronary artery disease in non-diabetic patients. BMC Med Genomics. 2011;4:26. doi: 10.1186/1755-8794-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maneerat Y, Prasongsukarn K, Benjathummarak S, et al. Increased alpha-defensin expression is associated with risk of coronary heart disease: a feasible predictive inflammatory biomarker of coronary heart disease in hyperlipidemia patients. Lipids Health Dis. 2016;15:117. doi: 10.1186/s12944-016-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caudell EG, Caudell JJ, Tang CH, et al. Characterization of human copine III as a phosphoprotein with associated kinase activity. Biochemistry. 2000;39(42):13034–13043. doi: 10.1021/bi001250v. [DOI] [PubMed] [Google Scholar]
- 10.Perestenko PV, Pooler AM, Noorbakhshnia M, et al. Copines-1, -2, -3, -6 and -7 show different calcium-dependent intracellular membrane translocation and targeting. FEBS J. 2010;277(24):5174–5189. doi: 10.1111/j.1742-4658.2010.07935.x. [DOI] [PubMed] [Google Scholar]
- 11.Lin Hc, Zhang Fl, Geng Q, et al. Quantitative proteomic analysis identifies CPNE3 as a novel metastasis-promoting gene in NSCLC. J Proteome Res. 2013;12(7):3423–3433. doi: 10.1021/pr400273z. [DOI] [PubMed] [Google Scholar]
- 12.Mo W, Zhang J, Li X, et al. Identification of novel AR-targeted microRNAs mediating androgen signalling through critical pathways to regulate cell viability in prostate cancer. PLoS One. 2013;8(2):e56592. doi: 10.1371/journal.pone.0056592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen OS, Mccoy SY, Middleton FA, et al. Transcriptomic analysis of postmortem brain identifies dysregulated splicing events in novel candidate genes for schizophrenia. Schizophr Res. 2012;142(1–3):188–199. doi: 10.1016/j.schres.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thygesen K, Alpert J, Jaffe A, et al. Third Universal Definition of Myocardial Infarction. Journal of the American College of Cardiology. 2012;60(16):1581–1598. doi: 10.1016/j.jacc.2012.08.001.. [DOI] [PubMed] [Google Scholar]
- 15.Montalescot G, Sechtem U, Achenbach S, et al. ESC Committee for Practice Guidelines, Document Reviewers. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson TA, Ito MK, Maki KC, et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: part 1 - executive summary. J Clin Lipidol. 2014;8(5):473–488. doi: 10.1016/j.jacl.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Chamberlain JJ, Rhinehart AS, Shaefer CF, et al. Management of Diabetes: Synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Ann Intern Med. 2016;164(8):542–552. doi: 10.7326/M15-3016. [DOI] [PubMed] [Google Scholar]
- 19.Heinrich C, Keller C, Boulay A, et al. Copine-III interacts with ErbB2 and promotes tumor cell migration. Oncogene. 2010;29(11):1598–1610. doi: 10.1038/onc.2009.456. [DOI] [PubMed] [Google Scholar]
- 20.Desta L, Jernberg T, Spaak J, et al. Heart failure with normal ejection fraction is uncommon in acute myocardial infarction settings but associated with poor outcomes: a study of 91,360 patients admitted with index myocardial infarction between 1998 and 2010. Eur J Heart Fail. 2016;18(1):46–53. doi: 10.1002/ejhf.416. [DOI] [PubMed] [Google Scholar]
- 21.Murakami H, Igarashi K, Igarashi Y, et al. [Influence of number of citizens greater than 50 years of age on prevalence of acute myocardial infarction: epidemiological study of Sapporo residents]. J Cardiol. 2007;50(3):167–174. [PubMed] [Google Scholar]
- 22.Rich MW, Bosner MS, Chung MK, et al. Is age an independent predictor of early and late mortality in patients with acute myocardial infarction? Am J Med. 1992;92(1):7–13. doi: 10.1016/0002-9343(92)90008-y. [DOI] [PubMed] [Google Scholar]
- 23.Bayturan O, Kapadia S, Nicholls SJ, et al. Clinical predictors of plaque progression despite very low levels of low-density lipoprotein cholesterol. J Am Coll Cardiol. 2010;55(24):2736–2742. doi: 10.1016/j.jacc.2010.01.050. [DOI] [PubMed] [Google Scholar]
- 24.Chhatriwalla AK, Nicholls SJ, Wang TH, et al. Low levels of low-density lipoprotein cholesterol and blood pressure and progression of coronary atherosclerosis. J Am Coll Cardiol. 2009;53(13):1110–1115. doi: 10.1016/j.jacc.2008.09.065. [DOI] [PubMed] [Google Scholar]
- 25.Kronmal RA, McClelland RL, Detrano R, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115(21):2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 26.Pekkanen J, Linn S, Heiss G, et al. Ten-year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N Engl J Med. 1990;322(24):1700–1707. doi: 10.1056/NEJM199006143222403. [DOI] [PubMed] [Google Scholar]
- 27.Bayturan O, Tuzcu EM, Uno K, et al. Comparison of rates of progression of coronary atherosclerosis in patients with diabetes mellitus versus those with the metabolic syndrome. Am J Cardiol. 2010;105(12):1735–1739. doi: 10.1016/j.amjcard.2010.01.359. [DOI] [PubMed] [Google Scholar]
- 28.Perk J, De Ba, Gohlke H, et al. European Guidelines on Cardiovascular Disease Prevention in Clinical Practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts)] Giornale italiano di cardiologia. 2013;14(5):328–92. doi: 10.1714/1264.13964. [DOI] [PubMed] [Google Scholar]
- 29.Frey P, Waters DD, DeMicco DA, et al. Impact of smoking on cardiovascular events in patients with coronary disease receiving contemporary medical therapy (from the Treating to New Targets [TNT] and the Incremental Decrease in End Points Through Aggressive Lipid Lowering [IDEAL] trials) Am J Cardiol. 2011;107(2):145–150. doi: 10.1016/j.amjcard.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Otaki Y, Gransar H, Berman DS, et al. Impact of family history of coronary artery disease in young individuals (from the CONFIRM registry) Am J Cardiol. 2013;111(8):1081–1086. doi: 10.1016/j.amjcard.2012.12.042. [DOI] [PubMed] [Google Scholar]
- 31.Huang G, Zhao JL, Du H, et al. Coronary score adds prognostic information for patients with acute coronary syndrome. Circ J. 2010;74(3):490–495. doi: 10.1253/circj.cj-09-0637. [DOI] [PubMed] [Google Scholar]