Abstract
Background
Devices currently used to achieve hemostasis of the femoral artery following percutaneous cardiac catheterization are associated with vascular complications and remnants of artificial materials are retained at the puncture site. The Secure arterial closure Device induces hemostasis by utilizing thermal energy, which causes collagen shrinking and swelling. In comparison to established devices, it has the advantage of leaving no foreign material in the body following closing. This study was designed to evaluate the efficacy and safety of the Secure Device to close the puncture site following percutaneous cardiac catheterization.
Methods
The Secure Device was evaluated in a prospective non-randomized single-center trial with patients undergoing 6 F invasive cardiac procedures. A total of 67 patients were enrolled and the device was utilized in 63 patients. Fifty diagnostic and 13 interventional cases were evaluated. Femoral artery puncture closure was performed immediately after completion of the procedure. Time to hemostasis (TTH), time to ambulation (TTA) and data regarding short-term and 30-day clinical follow-up were recorded.
Results
Mean TTH was 4:30 ± 2:15 min in the overall observational group. A subpopulation of patients receiving anticoagulants had a TTH of 4:53 ± 1:43 min. There were two access site complications (hematoma > 5 cm). No major adverse events were identified during hospitalization or at the 30 day follow-up.
Conclusions
The new Secure Device demonstrates that it is feasible in diagnostic and interventional cardiac catheterization. With respect to safety, the Secure Device was non-inferior to other closure devices as tested in the ISAR closure trial.
Keywords: catheterization, vascular closure device, thermal vascular occlusion, Secure Device System, femoral vascular access
Introduction
Interventional cardiology has become a leader in both diagnosing and treating coronary artery disease. Although radial access seems to be favoured in acute coronary syndrome, femoral access is commonly used worldwide in both acute and chronic settings.
Following catheterization, the standard procedure for closing the common arteria femoralis is mechanic compression to achieve hemostasis. Applying pressure to the puncture site and prolonging the time to mobilization leads to discomfort for the patient and promotes side effects of immobilization. Furthermore, manual compression in combination with compression devices usage is associated with increased personnel requirements and considerable financial burden for health care institutions [1].
Hemostatic devices are categorized as being pressure devices, hemostatic pads or active vascular closure devices. Currently available active closure devices are divided into three groups: collagen plugs, suture based devices and plugs/clips. Clips are most frequently made of bio-resorbable materials or metal applied to both the inner and/or outer layer of the arterial wall [2, 3].
Generally, the majority of closure devices leave foreign materials either inside or outside the blood vessel, which are either permanently left in place or gradually dissolve over time. Objects that are left inside the body could potentially lead to the development of limb ischemia, occlude an artery, be a source of infection or disable re-puncture at the same site for months. Taking these potential adverse effects into consideration, a vascular closure device that achieves hemostasis without requiring any components to be left in the patient’s body would be desirable. The principle of thermal vessel occlusion is currently under investigation [4].
The secure arterial closure device was developed to enable hemostasis using thermal energy to achieve vessel occlusion without leaving any foreign materials inside the patient. A clinical study was conducted in Georgia on 42 patients, using an earlier version of the device. The earlier version of the device was Conformité Européenne approved and used in Germany on 50 patients. Clinical data regarding performance and safety have not yet been published.
The hypothesis was that the Secure Device System is non-inferior in terms of vascular access site complication to other vascular closure devices [5].
Methods
The study was a prospective single center single group study designed to evaluate the safety and performance of the Secure Device (Model: SE-HE-A2) powered by an external power supply. The study was performed from September 2016 to January 2017 after receiving approval from the Austrian Federal Office of Safety and Health Care (BASG). All procedures and investigations were accomplished in accordance to the Declaration of Helsinki and approved by the local ethics committee (ref. no: 28-364 ex15/16). Written informed consent was obtained from every individual participating.
The patients included were undergoing either coronary angiography and/or coronary intervention procedures using 6 French sheaths. Detailed inclusion and exclusion criteria are shown in the Supplementary materials. An angiogram was performed at the access site to evaluate puncture location and artery morphology. Puncture locations above the femoral bifurcation were defined as feasible for the Secure Device occlusion (Fig. 1) and only arteries with a diameter above 6 mm and an artery depth greater than 10 mm to the surface of the skin were accepted for inclusion. Exclusion criteria included heavy calcification visualized at the angiogram or a medical history of obstructive peripheral artery disease.
Figure 1.
X-ray examination of patient prior to Secure arterial closure Device implantation; puncture site location control; red arrow — 6 F sheath; white arrow — common femoral artery.
Eighty-two individuals were screened during the study procedures and 67 subjects were enrolled in the clinical trial after final intra-procedural inclusion (Fig. 2).
Figure 2.
Flowchart of patients included in the trial.
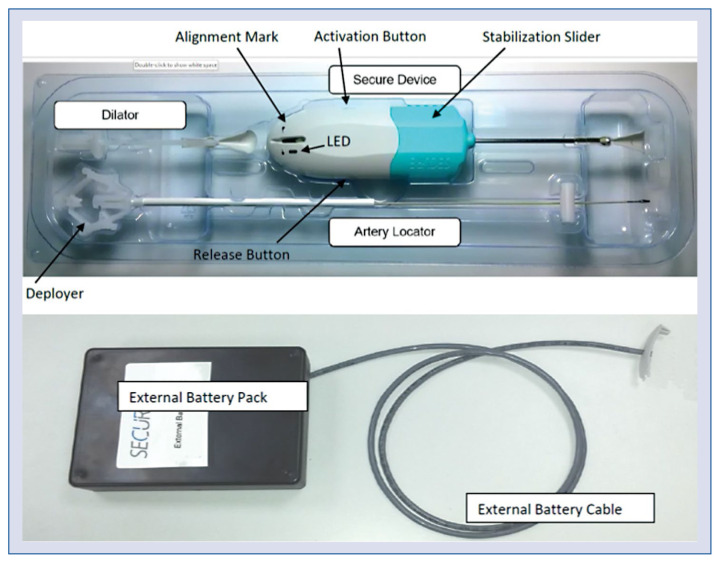
The system included an artery locator, a dilator and the Secure Device itself (Fig. 3). After finishing the procedure, the artery locator was introduced via the sheath with the help of the deployer at the proximal end. After successful introduction, the deployer was disconnected and removed. The sheath can be withdrawn while the deployed artery locator remains within the artery. To enlarge the tissue canal, the dilator was “screwed” in until the green mark was visible behind the dilator at the artery locator shaft. After removing the dilator, the Secure Device was inserted and advanced until the green indicator was visible on the same level as the alignment mark on the device to ensure optimal positioning of the heating dome (Fig. 4). Gentle permanent tension at the artery locator seals the hole in the artery during closing procedure. A stabilization slider at the base of the Secure Device ensured the correct pressure was applied.
Figure 3.
Secure Device including artery locator, dilator and the Secure artery closure Device. The external battery pack is not sterile and reusable. The tip of the artery locator with the deploying unit is made of nitinol and is covered by an elastic membrane made by PolyBlend (Advan Source biomaterials, Wilmington, MA). The shaft of the artery locator, made of Polyether Ether Ketone, served as a guide for the secure device. The dilator is made of a high-density plastic and should be inserted at an angle of approximately 45° to the artery. The tip of the Secure Device is 4.5 mm in diameter and made of silicone coated silver plated copper. A plastic wire protruded 1–2 mm from the stainless-steel tube at the end of the locator, indicating a fully deployed artery locator tip.
Figure 4.
A. Artery locator insertion and deploying the artery locator in the vessel; removing the sheath over the artery locator shaft; B. Pre-dilating the tissue tract; C. Inserting the Secure arterial closure Device to the alignment mark; D. Activate the Secure arterial closure Device to close the vessel.
The heating process was activated manually by pressing the activation button on the handle. A built-in test monitored the device while it was being activated. While the heating process was occurring, the artery locater was automatically un-deployed and retracted into the Secure Device handle. The heating process was regularly terminated within 5 to 7 s. Arterial closure LED light and vibration signal indicated the end of the procedure. After being rotated 90 degrees’, the Secure Device and the artery locater were easily removed from the patient. Minimal oozing from subcutaneous vessels may have occurred. Light compression was performed to prevent local subcutaneous bleeding on an as needed basis.
Time to hemostasis (TTH) was determined as the time between Secure Device removal and observed hemostasis in minutes and seconds. Device malfunctions were recorded and analysed regarding their relation to safety. Device malfunction included device related malfunction due to indicated inactivity or premature stopping of the heating process as well as mechanical obstacles and user errors using the device. The primary readout for this study was vascular access site complications. These complications included palpable hematoma measuring at least 5 cm in size, pseudo aneurysms, arteriovenous fistula formation, major bleeding related to the access site, acute leg ischemia, the need for vascular surgical or interventional treatment and local infections at 30 days after the procedure. Adverse events not previously described were investigated. Time to ambulation (TTA) was measured as the time from the Secure Device being removed from the patient to the subjects being able to walk at least 10 m.
Follow up of the patients took place at 4 time points: 2–4 h after Secure Device use, at ambulation, prior to discharge and a final examination 30 days after the procedure.
Statistical analysis
All parameters are expressed as mean ±standard deviation of the mean, as number of patient counts or percentage. TTH (primary endpoint) and TTA are expressed as mean ± standard deviation of the mean, as minutes (TTH) or hours (TTA). The statistical analysis of the complication rate was designed to reject the null hypothesis that the Secure Device is inferior in terms of vascular access-site complications to vascular closure devices (FemoSeal and Exoseal) as described in the ISAR-CLOSURE trial [5].
The ISAR-CLOSURE trial reported a complication rate of 6.9% in patients that were treated with a vascular closure devices. This was considered to be the null-hypothesis proportion for this trial. As in the ISAR-CLOSURE trial, considering that the majority of expected complications are not severe in nature, a non-inferiority margin that represents 140% of the null-hypothesis proportion was chosen, which results in a 3% margin of noninferiority. The expected complication rate for the Secure Device was estimated to be at most 5%. Given a type I error rate of α = 5%, a power of 80%, a null-hypothesis proportion of 6.9%, an expected true proportion of 5%, and a non-inferiority margin of 3%, the necessary sample size was determined to be 123 subjects.
However, the study was terminated early as the manufacturers board decided to discontinue the investment in Secure Device on December, 13 2016. Until then 67 patients were enrolled into the trial and all follow-up visits were performed.
Results
The average age of enrolled patients was 64.8 ± 15.6 years with an average body mass index of 28. Final activation of the Secure Device was performed in 63 cases. In 4 cases the device was installed but due to user error it was not activated. In these cases, manual compression was applied with no clinical disadvantage. Patient characteristics are listed in Table 1.
Table 1.
Clinical characteristics of patients included in the study.
| Basic characteristics | |
| Patients enrolled | 67 |
| Mean age | 64.8 ± 15.6 |
| Body mass index | 28.2 ± 4.2 |
| Female | 19.4% |
| Intervention | 13 |
| Blood pressure [mmHg] | 136.5 ± 25.6 / 73.7 ± 11.7 |
| Heart rate [/min] | 78.5 ± 11.7 |
| Concommitend medication | |
| ACE-I | 10.4% |
| Beta-blocker | 58.2% |
| Statin | 56.7% |
| Aspirin | 64.2% |
| Anticoagulant | 29.9% |
| P2Y12 inhibitor | 23.9% |
| Heparin | 17.9% |
| Baseline und follow-up lab | |
| Hemoglobin [g/dL] | 14.0 ± 1.6 |
| Platelet count [109/L] | 207.5 ± 71.2 |
| INR | 1.1 (0.85–1.46) |
| PTT [s] | 33.3 ± 4.9 |
| FU hemoglobin [g/dL] | 13.3 ± 1.8 |
| FU PLTC [109/L] | 234.6 ± 88.1 |
ACE-I — angiotensin converting enzyme inhibitor; FU — follow-up; INR — international normalized ratio; PTT — partial thromboplastin time
Time to hemostasis
Time to haemostasis (primary performance endpoint) was statistically evaluated in 51 subjects. The mean TTH was 4:30 ± 2:15 min in the overall observational group with a maximum time of 13:00 min and a minimum time of 2:00 min. Eleven of the subjects undergoing statistical analysis regarding TTH received anticoagulation medications (all unfractionated heparin; 70 IU/kg). In this subpopulation, TTH was 4:53 ± 1:43 min. TTH was not documented in 12 patients in which a Cathofix® compression system was applied if TTH was not achieved after > 5 min. One of these patients had a haematoma prior to the closing procedure. In 11 patients hemostasis was not primarily reached within 3–5 min and the physician decided to apply a compression assist system (Femostop®). In these cases TTH was not acquired.
Access site complication rate
Two out of 63 (3.2%) individuals experienced access site associated complications (primary safety endpoint). A palpable haematoma of > 5 cm was the only complication in these 2 subjects. In terms of severity, both hematomas were categorized as mild since they were only superficial but were neither indurated nor visibly swollen. By the 30 day follow-up, both hematomas had completely resolved. Since there were only 63 patients actively treated with the device, instead of the 123 subjects originally planned for according to the statistical sample size calculation, the confidence interval of the results was larger than expected.
Device malfunction rate
Sixty-nine devices were used in 63 patients. Two devices had malfunctions (secondary endpoint) that hindered activation of the Secure Device itself. In 14 devices there was either major (activation of the device) or minor (artery locator protruding) problems during the vascular closing procedure, representing a 20.3% malfunction rate. Additionally, another 8.7% of the Secure Device applications failed due to user errors and device misuse. In these cases, either parts of the closing procedure or the whole procedure failed. In 1 case the missing fixation of the artery locator during insertion of the device did not work, and made it necessary to also change the system with the deployed artery locator. In some cases an incomplete artery locator retraction at the end of the closing procedure hindered complete vascular occlusion, at which point manual compression was applied.
Adverse events and severe adverse events
In addition to main access site complication rate, additional adverse events as secondary endpoint were analyzed. The most frequent adverse events observed were small hematomas. All small hematomas were totally resolved within a short time and not detected 30 days after the procedure. One patient experienced a decrease in blood pressure, local infection (elevated C-reactive protein, inconspicuous access site, normal chest X-ray and unremarkable urine test) and developed bruising. An acute allergic reaction to the contrast agent used was investigated in 2 patients and an initial manifestation of diabetes was detected in another patient. The only reported severe adverse event was an unexpected ST-segment elevation myocardial infarction with stent thrombosis after percutaneous coronary intervention (PCI), 10 min after the closing procedure was completed, which was then followed by a second PCI.
Time to ambulation
The time between Secure Device System activation and patient ambulation was assessed. Mean time to ambulation was 8:04 ± 6:32 h. In the subgroup of patients who received anticoagulants, the mean time to ambulation was 12:45 ± 8:54 h.
Discussion
This study was initiated to investigate the safety and efficacy performance of the Secure Device System in clinical routine. While manual compression is still the gold standard following femoral access vascular intervention, vascular closure devices have been found to be safe and associated with less complications in femoral access after coronary angiography [6]. Nevertheless, the incidence of complications such as hematomas and pseudoaneurysms are often discussed with controversy [7]. Relevant complications include groin hematomas, bleeding, arteriovenous fistulas and pseudoaneurysms developing at the puncture site as shown at meta-analysis level. In a PCI setting the rate of complications were higher compared to diagnostic catheters [8]. More recent data on a large cohort of patients investigated intravascular and extravascular devices compared to manual compression. Vascular closure devices showed non-inferiority to manual compression in terms of vascular access site complications [5]. Comparable results were found with the polymer disc based device FemoSeal®, which accomplished faster haemostasis with less hematoma formation [6]. Suture based closure device and plug based devices, regardless of whether they use metal or collagen, ultimately leave foreign materials either directly in the arterial wall or within the surrounding tissue [5, 9].
Foreign materials may be a problem for a patient in long-term if re-puncture is necessary or potentially a source for local reactions in the arterial wall or the adventitial surrounding. In a small number of patients, the presence of a foreign material could lead to limb ischemia. Although interventional treatment of limb ischemia seems feasible [10], these are major adverse events. In clinical practice, repetitive use of devices at the same puncture site is avoided. Since there is no human data regarding fibrosis at the site of puncture, investigating the local effects of collagen based devices has been limited to animal models. In tissue samples and histological analysis of the collagen plug vessel narrowing and peri-adventitial inflammation inducing extravascular scarring was found in a dog model [11]. A recent trial in minipigs also described vessel stenosis influencing blood flow and histological alterations following closure using collagen plugs [12].
Research into active vascular closure devices that do not leave foreign materials at the puncture site is a growing field. The idea of using thermal energy to achieve arterial access occlusion is becoming a focus. The pathophysiological mechanism of thermal occlusion is the result of the local collagen shrinkage outside the vessel and local swelling to achieve hemostasis [4]. In this small trial, TTH was accomplished after 3 min for diagnostic procedures and 4 min for interventional catheterization, and no severe adverse events were reported.
In one fifth of the device applications, either device activation issues or intra-procedural obstacles resulted in changing the device or switching to manual compression. Although malfunctions are a common problem in vascular closure devices, malfunction rates are highly variable depending on the device. The StarClose System, a clip based device, has a malfunction rate around 1.1%. AngioSeal, a well-established collagen plug induced vascular closure device, has been found to have malfunction rates up to 10% [13, 14]. The majority of the device malfunctions occurred at the beginning of the Secure Device activation, so the device was able to be changed and did not influence the study procedure. None of the device malfunctions were related to patient safety.
The average time to hemostasis in the 54 assessed individuals was slightly above 4 min (2 to 13 min). The complication rate was low for the Secure Device. Clinical follow-up included physical examination focussing on the access site. No duplex was performed unless auscultation or palpation were suspicious for an access site complication. Although physical examinations were all performed by experienced clinicians it cannot be ruled out that a vascular complication only detectable by duplex might have been present. However, there was no case of an access site related problem within the 30 day follow-up, supporting the idea that no additional complications developed. The only access site associated complication was the occurrence of a hematoma larger than 5 cm. In 2 cases, a relevant hematoma was detected. The device was successfully applied in both cases and time to hemostasis was obtained in 5 and 6 min after device activation, respectively.
Although the study was terminated after only 63 patients with active use of the Secure Device, since the measured proportion of access site complications was 3.2%, lower than the expected proportion of up to 5%, the data from the 63 patients was sufficient to reject the predefined null-hypothesis. However, it should be noted that baseline characteristics between this trial and the ISAR-CLOSURE trial revealed some differences. The percentage of female participants as well as average age was higher in the ISAR CLOSURE trial increasing the risk for bleeding whereas body mass index was higher in the present study. With respect to anticoagulation and antithrombotic medication there was comparable use of acetylsalicylic acid in both trials (~2/3 of all patients) but P2Y12 inhibitors were used more often in the ISAR-CLOSURE trial whereas anticoagulation was more frequent in the present trial. An important difference regarding the follow-up of the patients is the systematic duplex-sonographic follow-up in ISAR-CLOSURE. Although it cannot be ruled out that routine duplex was more sensitive to detect access site complications (predefined complications were the composite of hematoma ≥ 5 cm, pseudoaneurysm, arterio-venous fistula, access-site-related major bleeding, acute ipsilateral leg ischemia, need for vascular surgical/interventional treatment or local infection) these should have been detected with this two step approach.
It is known that minor vascular events have been found to occur frequently with different devices [15]. Besides the two primary safety endpoint events of groin hematomas > 5 cm, 9 small local hematomas were reported as adverse events. Three other anticipated adverse events were reported. Either blood pressure decrease, infection or bruising was observed in 3 different patients. All of the investigated events might be potentially device related, although they were evaluated only descriptively in this trial.
In the subgroup of patients that received manual compression after Secure Device activation, as decided by the physician, the time to hemostasis was not evaluated.
The present study investigated a moderate rate of user failure, although extensive training had been performed. In prior clinical experience with the application system, especially the artery locator and the handling of the Secure Device itself, it requires additional practice in order to become adept. Compared to other vascular closure devices, further development of the device might increase user friendly application.
Conclusions
Vascular occlusion devices are commonly used for femoral access catheterization.
The new Secure Device uses thermal energy and has been found to be feasible in both diagnostic and interventional cardiac catheterization in this small and prematurely concluded trial. In comparison to established devices, it has the advantage of leaving no foreign material in the body following closure.
Based on the limited data available it seems to be non-inferior to other closure devices in terms of safety.
However, the rate of malfunctions was still significant and requests further development of the technique.
Further clinical investigation will be needed regarding the Secure Device thermal vascular closure technique in larger patient populations.
Supplementary Information
Footnotes
Funding: The study was funded by Calore Medical Ltd., 14 HaIlan st., Or Akiva, Israel, 30600.
Conflict of interest: Michael Sacherer, Olev Luha and Robert Zweiker were sub-investigators, Karin Brandner was study nurse and Dirk von Lewinski was principle investigator in this funded trial.
References
- 1.Walter J, Vogl M, Holderried M, et al. Manual Compression versus Vascular Closing Device for Closing Access Puncture Site in Femoral Left-Heart Catheterization and Percutaneous Coronary Interventions: A Retrospective Cross-Sectional Comparison of Costs and Effects in Inpatient Care. Value Health. 2017;20(6):769–776. doi: 10.1016/j.jval.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Hon LQ, Ganeshan A, Thomas SM, et al. An overview of vascular closure devices: what every radiologist should know. Eur J Radiol. 2010;73(1):181–190. doi: 10.1016/j.ejrad.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 3.Krishnasamy VP, Hagar MJ, Scher DJ, et al. Vascular closure devices: technical tips, complications, and management. Tech Vasc Interv Radiol. 2015;18(2):100–112. doi: 10.1053/j.tvir.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Davidson LJ, Luna J, Virmani R, et al. First human experience of thermal arterial closure. Catheter Cardiovasc Interv. 2014;84(1):30–36. doi: 10.1002/ccd.25152. [DOI] [PubMed] [Google Scholar]
- 5.Schulz-Schüpke S, Helde S, Gewalt S, et al. Comparison of vascular closure devices vs manual compression after femoral artery puncture: the ISAR-CLOSURE randomized clinical trial. JAMA. 2014;312(19):1981–1987. doi: 10.1001/jama.2014.15305. [DOI] [PubMed] [Google Scholar]
- 6.Holm NR, Sindberg B, Schou M, et al. Randomised comparison of manual compression and FemoSeal™ vascular closure device for closure after femoral artery access coronary angiography: the CLOSure dEvices Used in everyday Practice (CLOSE-UP) study. EuroIntervention. 2014;10(2):183–190. doi: 10.4244/EIJV10I2A31. [DOI] [PubMed] [Google Scholar]
- 7.Koreny M, Riedmüller E, Nikfardjam M, et al. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization: systematic review and meta-analysis. JAMA. 2004;291(3):350–357. doi: 10.1001/jama.291.3.350. [DOI] [PubMed] [Google Scholar]
- 8.Nikolsky E, Mehran R, Halkin A, et al. Vascular complications associated with arteriotomy closure devices in patients undergoing percutaneous coronary procedures: a meta-analysis. J Am Coll Cardiol. 2004;44(6):1200–1209. doi: 10.1016/j.jacc.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 9.Martin JL, Pratsos A, Magargee E, et al. A randomized trial comparing compression, Perclose Proglide and Angio-Seal VIP for arterial closure following percutaneous coronary intervention: the CAP trial. Catheter Cardiovasc Interv. 2008;71(1):1–5. doi: 10.1002/ccd.21333. [DOI] [PubMed] [Google Scholar]
- 10.Dong H, Peng M, Jiang X, et al. Endovascular therapy for Angioseal-related acute limb ischemia: Perioperative and long-term results. Catheter Cardiovasc Interv. 2017;89(S1):609–615. doi: 10.1002/ccd.26936. [DOI] [PubMed] [Google Scholar]
- 11.Gargiulo NJ, Veith FJ, Ohki T, et al. Histologic and duplex comparison of the perclose and angio-seal percutaneous closure devices. Vascular. 2007;15(1):24–29. doi: 10.2310/6670.2007.00004. [DOI] [PubMed] [Google Scholar]
- 12.Kabelitz L, Nonn A, Nolte KW, et al. Long term outcome after application of the angio-seal vascular closure device in minipigs. PLoS One. 2016;11(9):e0163878. doi: 10.1371/journal.pone.0163878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiliopoulos S, Katsanos K, Karnabatidis D, et al. Safety and efficacy of the StarClose vascular closure device in more than 1000 consecutive peripheral angioplasty procedures. J Endovasc Ther. 2011;18(3):435–443. doi: 10.1583/10-3277.1. [DOI] [PubMed] [Google Scholar]
- 14.Minko P, Katoh M, Gräber S, et al. Obesity: an independent risk factor for insufficient hemostasis using the AngioSeal vascular closure device after antegrade puncture. Cardiovasc Intervent Radiol. 2012;35(4):775–778. doi: 10.1007/s00270-011-0230-y. [DOI] [PubMed] [Google Scholar]
- 15.Schmelter C, Liebl A, Poullos N, et al. Suitability of Exoseal vascular closure device for antegrade femoral artery puncture site closure. Cardiovasc Intervent Radiol. 2013;36(3):659–668. doi: 10.1007/s00270-012-0501-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.