Abstract
Objective:
Determine seizure frequency and association with neurologic outcomes in infants undergoing extracorporeal membrane oxygenation (ECMO). Identify patient or clinical factors associated with seizures or brain injury on imaging.
Methods:
Retrospective, single-center study including infants less than 1 year of age, who underwent ECMO between 2012–2017.
Results:
A total of 104 infants met study criteria including 45 patients with continuous EEG monitoring during their ECMO run and 59 infants without EEG. Seizures (electrographic-only or electro-clinical) were identified in 18 of the 45 (40%). Among the 18 infants with seizures, 14 (78%) had moderate to severe brain injury, while only 44% of those without seizures (12 of 27) on EEG had moderate to severe brain injury (p=0.03). Cardiopulmonary resuscitation prior to ECMO (ECPR), mode of ECMO, length of stay, survival to discharge, and congenital heart disease (CHD) were not associated with seizures. One of 10 patients with cyanotic CHD due to HLHS had seizures compared to 7 of 10 patients with non-HLHS lesions (p=0.02). Seizures were associated with moderate to severe brain injury, after adjusting for ECPR and CHD (p = 0.04).
Conclusions:
Electrographic seizures were common in patients undergoing ECMO and higher than previously reported. Seizures were associated with moderate to severe abnormalities on imaging, after adjusting for ECPR and CHD. This study adds to recent literature describing the risk of seizures in patients on ECMO and highlights the presence of brain injuries which may be identified by routine EEG surveillance.
Keywords: extracorporeal membrane oxygenation, electroencephalography, neonatal, congenital heart disease, neurologic outcomes
INTRODUCTION
Extracorporeal membrane oxygenation (ECMO) is a rescue modality for life-threatening cardiac or respiratory failure in neonatal and pediatric populations. Neonates compose the highest proportion (60%) of the pediatric ECMO population, with diverse etiologies including congenital heart disease (CHD).1 ECMO is also associated with an increased risk of seizures and neurologic injury.2–4 Mode of ECMO is associated with brain injury, with venous-arterial (VA) access having increased rates of brain injury as compared to venous-venous (VV) support.5,6 The need for ECMO in the infant population has been associated with worsening developmental outcomes; for example, infants with congenital diaphragmatic hernia who required ECMO due to severe pulmonary hypoplasia and pulmonary hypertension have been shown to have worse cognitive and motor outcomes than those who did not require ECMO.7
During ECMO, head ultrasound is used as a routine neuroimaging for infants with open fontanelle to identify new and evolving brain injury.8 Younger infants and those with cardiac indications for ECMO are more likely to have intracranial hemorrhage compared to older patients and non-cardiac indications.9 Similarly, infants have a higher rate of seizures and neurologic injury than older critically ill children, which may impact neurologic outcomes.10–12 CHD and the need for ECMO are known risk factors for seizures in critically ill pediatric patients.13 Specifically, infants with HLHS (hypoplastic left heart syndrome) and variants have a higher risk of seizures in the post-operative period compared to other CHD lesions.14 A majority of these seizures are electrographic and associated with higher mortality rates.15 Seizures may also be more common in infants with CHD with low cardiac output.16 Infants with CHD and those who undergo reparative surgery also have a risk of neurologic injury, including stroke.17,18
Recently, several studies have demonstrated the ability of neuromonitoring with continuous EEG (cEEG) to identify seizures in the pediatric ECMO population.16,19,20 However, there remains uncertainty about the importance of EEG-identified seizures on more immediate outcomes, as the presence of seizures has not consistently shown an association with increased mortality or morbidity in the pediatric ECMO population.16,20,21
In this study, we sought to quantify the rate of neurological complications, including electrographic seizures, brain injury, and short-term outcomes, in infants who underwent ECMO. In this population, we anticipated a subset of our patients would have seizures on EEG, and this would be associated with an increased proportion of brain injury in the congenital heart disease population, highlighting the importance of EEG as a screening tool for neurologic injury in this population. We chose to study the infant and neonatal population specifically because of the known increased risk of seizures in critically ill infants and the higher proportion of underlying cardiac disease.
METHODS
Design
This was a single center retrospective study of infants (aged less than 1 year) who underwent ECMO in the Neonatal, Cardiac and Pediatric Intensive Care Units at St. Louis Children’s Hospital, from January of 2012 through September 2017. Across this cohort, we investigated cEEG during ECMO course, neuroimaging, and clinical and patient factors associated with the risk of seizure and neurologic injury. This retrospective study was approved by the Washington University School of Medicine Institutional Review Board.
Clinical Data
We identified infants (aged birth to 1 year of age) who underwent ECMO during the study dates using an internal ECMO patient registry. Data was extracted from the ECMO registry and the electronic medical record (EMR), including age, sex, ICU location, diagnosis, congenital heart lesion including type, survival, length of stay, and discharge disposition. ECMO data collected included: indication (e.g., cardiac [CHD and non-CHD etiologies], pulmonary, infection, meconium aspiration syndrome, pulmonary hypertension, and congenital diaphragmatic hernia), ECMO mode, run number, time of cannulation, decannulation time, and need for cardiopulmonary resuscitation prior to ECMO (ECPR). ECMO mode, or type, indicates the cannulation sites used for the procedure. For this study, this was simplified to either venous-arterial (VA) or venous-venous (VV). The VA cohort included infants converted from VV to VA (or vice versa). We reviewed EEG and imaging reports, including those from head ultrasound (HUS), computed tomography (CT), and magnetic resonance imaging (MRI) studies, either during (HUS/CT) or after (CT/MRI) the ECMO run. Inclusion criteria included Infants less than 1 year of age at time of ECMO cannulation, without EEG studies during the hospital course, and those with EEG while on ECMO. To minimize potential confounding due to non-ECMO related seizures, infants with EEG studies during the admission (either prior to or after their ECMO course, but not during ECMO were excluded from the analysis22 (Figure 1).
Figure 1:
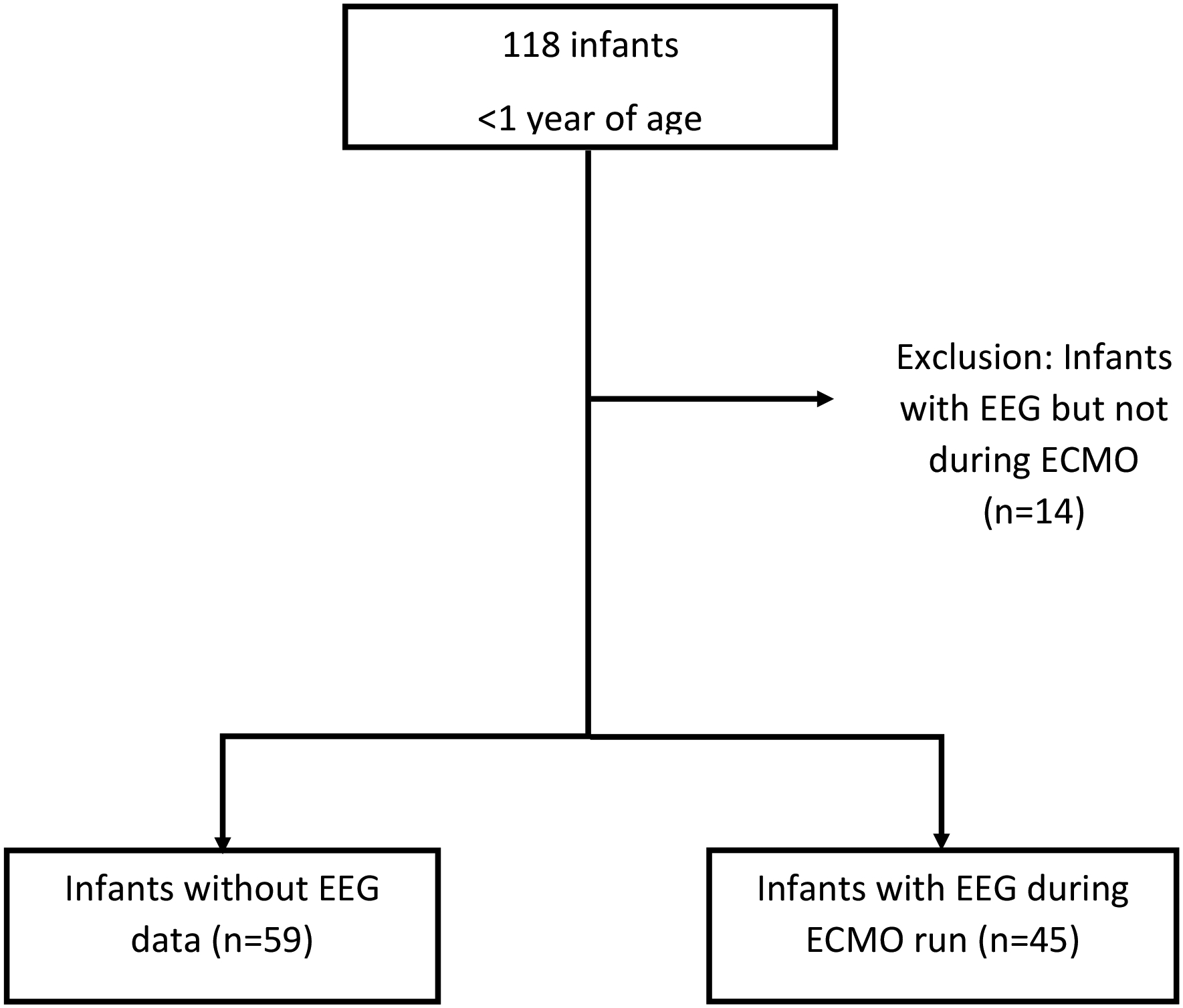
Patient identification
Cyanotic vs. acyanotic CHD determination
We determined the cardiac lesion for patients with CHD based on documentation in the EMR (Supplementary Table 1). A cardiac intensivist (AS) designated complex lesions as “cyanotic” or “acyanotic”. A cyanotic cardiac lesion describes defects where the blood pumped to the body has less oxygen than normal. Acyanotic lesions, while still representing abnormalities in the morphology of the heart, do not result in deoxygenated blood flow to the body.
EEG monitoring
EEG studies were not routinely performed on all patients on ECMO during the study period. Indications (based on the report) included: ECMO, paralysis, spell evaluation, or clinical concern increasing risk for seizure (including encephalopathy, history of clinical seizure, meningitis, cardiac arrest, intracranial bleed, infarction, hypoperfusion event, post transplant). Studies were performed using Nihon Kohden digital EEG systems (Nihon Kohden, Tokyo, Japan) with an extended international 10–20 electrode placement with minor modifications when necessary due to head positioning. The EEG reports provided information regarding duration of EEG monitoring, background elements, seizure presence, and time of seizure onset.
Imaging data
Two authors (SLBH and RMG) assigned an injury severity score (normal, mild, moderate, or severe) for each patient based upon collective clinical imaging data (e.g., head ultrasound, head CT, brain MRI). Normal, mild, moderate, or severe assignments by diagnosis are provided in Supplementary Table 2. The mild designation was assigned in cases with low volume of injury (e.g., punctate hemorrhages), unrelated structural findings designated by the radiologist (hypomyelination, delayed sulcation, ventriculomegaly, etc.), or likely normal variants. Moderate or severe designations involved larger injury volume, injuries requiring neurosurgical intervention (thrombosis or progressive ventriculomegaly), and two or more mild category injuries. SLBH initially reviewed imaging reports with scores independently confirmed by RMG. Disagreement regarding injury severity was reconciled by review of neuroimaging by both authors together for consensus. MRI or CT findings superseded the imaging grade of earlier HUS studies if both study types were available. Due to the inability for patients to be acutely evaluated by MRI after ECMO, MRI findings may represent subacute and/or chronic evolution of injury.
Analysis
Statistical analyses were performed using Prism 8 (La Jolla, California) and SAS 9.4 (Cary, North Carolina). Descriptive data are presented as means, medians or proportions. We used Chi-Square analysis or Fisher Exact test for categorical variables and Mann-Whitney U test (Wilcoxin Rank Sum) for continuous variables. A multivariable logistic regression model was used to examine the relative influence of seizures versus ECPR or CHD on the dependent variables of imaging and outcome.
RESULTS
During the study period, 118 infants underwent ECMO cannulation (Figure 1). Fourteen patients were excluded due to EEG occurring outside of the ECMO course as seizures and injury in this population may precede or be remote to the ECMO course. Of the 104 patients remaining, the median (IQR) age at cannulation was 8 days of life (3, 103). CHD was the most common primary diagnosis, occurring in 45 (43%) infants. Of the 45 CHD lesions, 33 were cyanotic lesions, of which 14 had hypoplastic left heart syndrome (HLHS). Other indications included cardiac (non-CHD) or pulmonary etiologies, congenital diaphragmatic hernia, meconium aspiration syndrome complicated by pulmonary hypertension, and infection (Table 1, Supplementary Table 3). The most common mode of ECMO included VA cannulation, seen in 93 of the 104 patients (89%), either peripherally via right neck vessels or centrally via median sternotomy. ECPR occurred in 19 (18%) of the patients.
Table 1:
Patient demographics
| Patient cohort n = 104 | EEG during ECMO n = 45 (%) | No EEG during ECMO n = 59 (%) | p-value | |
|---|---|---|---|---|
| Age of cannulation in days; median (IQR)* | 8 days (3, 103) | 14 days (4, 89) | 7 days (3, 107.5) | 0.53 |
| Female | 43 (41%) | 26 (58%) | 17 (29%) | 0.005 |
| Location | 0.003 | |||
| Neonatal ICU | 34 (33 %) | 7 (16%) | 27 (46%) | |
| Pediatric ICU | 14 (13 %) | 6 (13%) | 8 (13%) | |
| Cardiac ICU | 56 (54 %) | 32 (71%) | 24 (41%) | |
| Reason for ECMO | 0.01 | |||
| Congenital Heart Disease (CHD) | 45 (43%) | 26 (58%) | 19 (32%) | |
| Other than CHD | 59(57%) | 19 (42%) | 40 (68%) | |
| Congenital Heart Disease | 0.73 | |||
| Cyanotic (n, % of CHD) | 33 (73%) | 20 (77%) | 13 (68%) | |
| Acyanotic (n, % of CHD) | 12 (27%) | 6 (23%) | 6 (32%) | |
| Cyanotic heart disease | 0.31 | |||
| Hypoplastic left heart syndrome (n, % of Cyanotic) | 14 (42%) | 10 (50%) | 4 (31%) | |
| Non- Hypoplastic left heart syndrome (n, % of Cyanotic) | 19 (58%) | 10 (50%) | 9 (69%) | |
| ECMO Mode | 0.11 | |||
| VA | 93 (89%) | 43 (96%) | 50 (85%) | |
| VV | 11 (11%) | 2 (4%) | 9 (15%) | |
| ECPR | 19 (18%) | 13 (29%) | 6 (10%) | 0.02 |
| Outcomes | ||||
| Survival to discharge | 58 (56%) | 19 (42%) | 39 (66%) | 0.02 |
ECMO: Extracorporeal membrane oxygenation
ECPR: Cardiopulmonary resuscitation prior to ECMO
BSID: Bayley Scales of Infant Development
VA: venous-arterial; VV: venous-venous
excluding cases with ECMO cannulation at OSH, or operative report not consistent with ECMO dataset
Statistics: Chi-square (Location), Fisher exact test (CHD, gender, etiology, HLHS, mode, ECPR, Survival), Mann-Whitney (Age of cannulation)
Forty-five of the 104 patients (43%) underwent clinically indicated continuous EEG monitoring (cEEG) during ECMO. There was an overall increase in cEEG monitoring during ECMO throughout the study period, increasing from 16% to 78% during the study period. The percentage of EEG with electrographic seizures ranged between 25% (in 2016) to 57% (in 2017) during this time, but no clear trend was seen over time (Supplementary Figure 1). Patients undergoing cEEG were more likely to be female (p=0.005), located in the CICU (p=0.003), and have undergone ECPR (p=0.02). Only 26 of the 45 patients (58%) with CHD underwent EEG during ECMO; 13 of these (50%) underwent ECPR at time of ECMO initiation. The distribution of cyanotic versus acyanotic CHD lesions (p=0.73) and HLHS versus non-HLHS cyanotic lesions (p=0.31) did not differ between patients with or without cEEG monitoring (Table 1). Those patients who did not undergo EEG during ECMO were more likely to survive to discharge (p=0.02).
Among the 45 patients undergoing cEEG, electrographic seizures occurred in 18 (40%). Patients with CHD did not have increased seizures and those with HLHS had less seizures, with only one of the 10 infants with HLHS and EEG monitoring having a seizure (Table 2, Figure 2). In these patients with cEEG, the frequency of ECPR (p=0.74) and VA or VV mode of ECMO (p>0.99) was similar between those with and those without electrographic seizures (Table 2).
Table 2:
Electrographic seizure in patients with EEG data (n=45)
| Seizure (n=18) | No seizure (n=27) | p-value | |
|---|---|---|---|
| Patient factors | |||
| CHD | |||
| Cyanotic (% of CHD) | 8 (73 %) | 12 (80 %) | >0.99 |
| Acyanotic (% of CHD) | 3 (27 %) | 3 (20 %) | |
| Cyanotic | |||
| non-HLHS (% of cyanotic) | 7 (88 %) | 3 (25 %) | 0.02 |
| HLHS (% of cyanotic) | 1 (12 %) | 9 (75 %) | |
| ECMO factors | |||
| ECPR | 6 (33%) | 7 (26%) | 0.74 |
| VA | 17 (94%) | 26 (96 %) | >0.99 |
HLHS: Hypoplastic left heart syndrome
ECPR: Cardiopulmonary resuscitation prior to ECMO
Statistics: Fisher’s exact (CHD, Cyanotic, ECPR, VA)
Figure 2a:
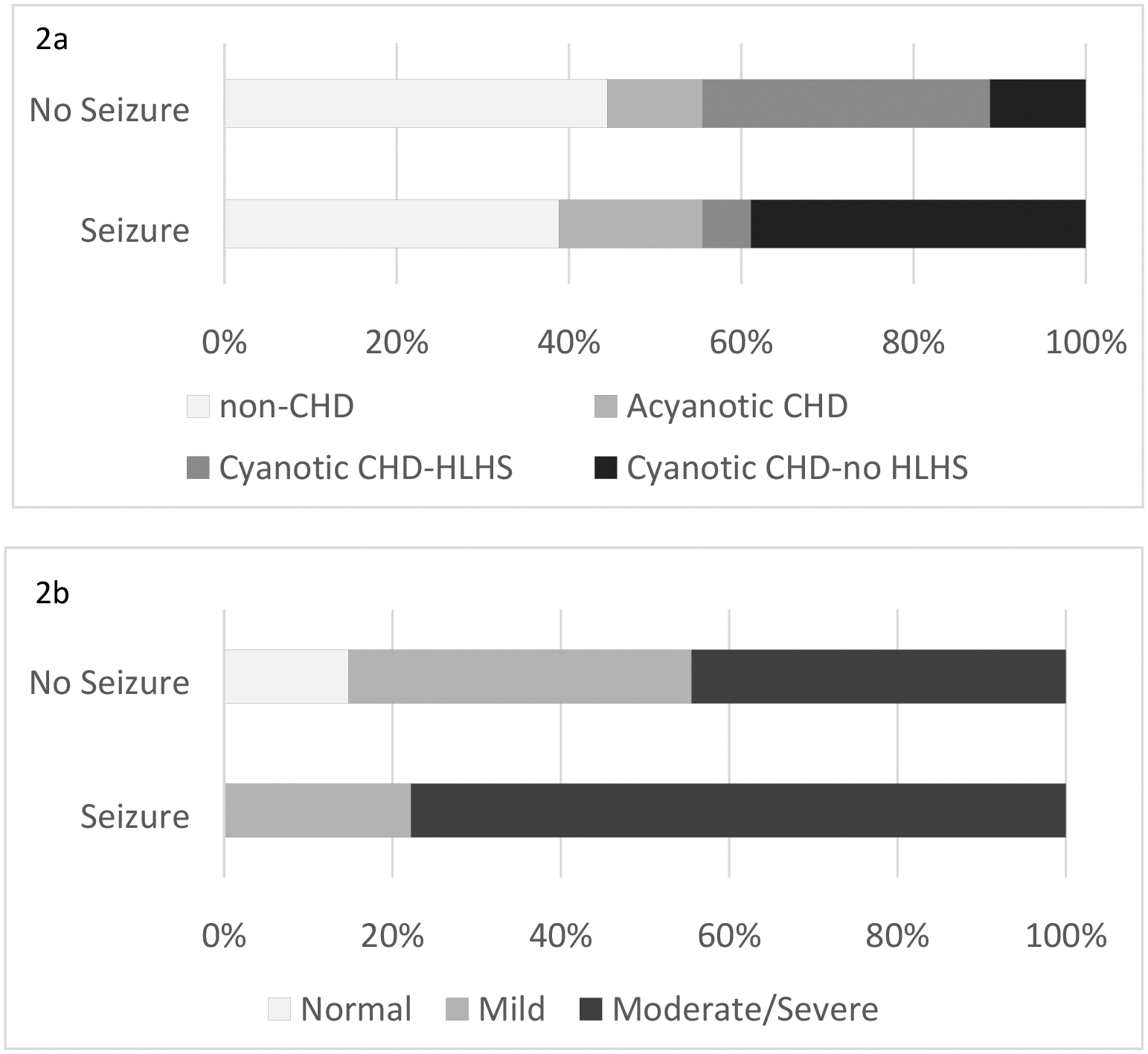
In patients with CHD, electrographic seizures are more likely to be associated with cyanotic non-HLHS lesions. 2b: Electrographic seizures are associated with moderate to severe brain imaging findings.
CHD: congenital heart disease
HLHS: Hypoplastic left heart syndrome
Neuroimaging data (HUS, CT, or MRI) were available in 103 of the 104 patients, of whom 68 (66%) underwent either CT or MRI. Moderate to severe brain injury occurred in 41 (40%) infants. Of the 45 patients who underwent EEG monitoring, 35 (77%) underwent CT or MRI during or after ECMO (Supplementary Table 2). Among those with EEG data available, infants with electrographic seizures had a higher frequency of moderate to severe brain injury (p=0.03, Table 3, Figure 2). In a multivariable logistic regression including ECPR and CHD in the model, seizures still remained a significant predictor of moderate to severe neuroimaging (OR 4.3, 95% CI 1.1, 16.6).
Table 3:
Outcomes in patients with EEG data (n=45)
| Seizure (n=18) | No seizure (n=27) | p-value | |
|---|---|---|---|
| Normal neuroimaging | 0 | 4 (9%) | 0.14 |
| Moderate to Severe Brain injury on neuroimaging | 14 (78%) | 12 (44%) | 0.03 |
| Death during hospital stay | 11 (61%) | 15 (56%) | 0.76 |
| LOS (median days, IQR) | 41 (9,67) | 41 (27, 84) | 0.26 |
LOS: Length of stay including patients with death during hospital stay
Statistics: Fisher’s exact (Normal neuroimaging, moderate to severe brain injury),Mann-Whitney (LOS)
Electrographic seizures did not correlate with mortality (p=0.76) or length of hospital stay (p=0.26, Table 3). Seizures remained unassociated with mortality in multivariate analysis after adjusting for ECPR (OR 1.2 95% CI 0.3, 4.1).
DISCUSSION
In our infant ECMO population, electrographic seizures occurred in 40% of patients who underwent EEG monitoring. This is higher than in other studies of pediatric patients undergoing ECMO.16,20 This difference is likely due to a combination of factors, including this being an exclusively infant cohort as other studies include older pediatric populations with differing etiologies of cardiorespiratory failure. Previous studies have also shown that younger pediatric patients have higher rates of seizures than older children in the pediatric ICU.12,10 In addition, the acuity of critical illness may have contributed to the number of seizures. Because our cohort had a lower proportion of patients monitored on EEG compared to other studies, we may have over-representation of critically ill patients. For example, in patients with CHD undergoing cardiac surgery with routine post-operative EEG surveillance, seizures were identified in 18% of patients,23 while in more acutely ill neonates undergoing therapeutic hypothermia, at high risk for hypoxic ischemic injury, the incidence of seizures was higher at 34–65%.24–26
In the present study, electrographic seizures were associated with imaging findings of moderate to severe brain injury on MRI and CT. These findings were variable, including: ventriculomegaly, hemorrhagic and ischemic infarctions, edema, hemorrhage, subdural hematoma, thrombus, and global atrophy. This is consistent with previous work that illustrated an association between seizures and abnormalities on neuroimaging in older children.27 Specifically, pediatric patients with ischemic stroke, intraparenchymal hemorrhage, or venous thrombosis are at particularly high risk for seizures, ranging from 19–44%.13 Patients on ECMO are also at high risk for these neurologic injuries, including, ischemic, hemorrhagic, or venous infarction,4,28,29 with head ultrasound identified as an insufficient screening tool for infarction.28 Lin and colleagues also showed 71% of patients with electrographic seizures in their ECMO cohort had acute imaging findings.16 Thus by screening for seizures (a common indication for patients on ECMO), EEG may also be a reasonable, non-invasive, serial monitor for neurologic injury, particularly in young patients with congenital heart disease.30,31
Patients with CHD represented the largest group within our cohort. While we did not find increased seizures in our CHD population overall, our HLHS population was less likely to have seizures. This is counter to prior studies, which have found an increased risk of brain injury and seizures in CHD patients, including those with HLHS.16,32–35 This difference may be related to multiple factors, including an evolution in the management of patients with HLHS over time or intrinsic characteristics of brain oxygenation physiology and a tolerance to hypoxemia in this population. Further studies with prospective and routine EEG are necessary to explore these hypotheses.
We did not find a difference in mortality or length of stay in patients with seizures. Prior studies that include older patients have shown a relationship between seizures and increased mortality.16 It is possible that excluding older pediatric patients with different indications for ECMO may have impacted the risk for death in our cohort and/or seizures may herald more significant injury or illness in older patients. Consideration of length of stay in neonatal populations may also be confounded by postnatal discharge requirements, in addition to the clinical course.
Our study does not address the impact of seizure burden or seizure treatment on clinical outcomes. The Boston Circulatory Arrest Study group showed that postoperative seizures are associated with worse developmental outcomes at 1 and 2½ years of age,35 although another study has suggested the opposite with post-operative seizures associated with favorable outcomes and no recurrent seizures in patients with CHD.36 In critically ill children, in general, status epilepticus and high seizure burden are associated with worse short and long-term outcomes.37–39 Specifically, in neonates, seizure burden has been associated with worse outcomes in neonatal hypoxic ischemic encephalopathy24,40 and with lower IQ and increased rates of cerebral palsy and speech delay in the neonatal ECMO population.41 Conversely, the consequence of post-operative seizures on neurodevelopment in the CHD population is less clear.42 Our study had only a small proportion undergo both EEG monitoring and developmental testing (4 of 45), from which we could not draw definitive conclusions.
Limitations of our study include the retrospective nature and EEG monitoring being performed for clinical concerns. Even if 100% of patients in this study who did not undergo EEG monitoring had no seizures, the frequency of seizures seen in our cohort would still be 17% (18 of 104 patients), which would be similar to prior studies.16,20 Our EEGs were performed based on clinical concerns, which may have selected for a higher risk population. It is more likely that the lower number of EEGs performed during the early portion of the study period may have underestimated the number of seizures instead of overestimating them. Our institutional practice has since evolved to standardized cEEG monitoring of all ECMO patients. Another limitation is the reliance on non-uniform neuroimaging studies (CT, MRI or HUS) and radiology interpretations that were not blinded to the clinical risk for injury. Finally, we did not have sufficient follow-up data to make any conclusions about the role of seizures on patient neurodevelopmental outcomes. With the development of standardized post-ICU follow-up clinics including the ECMO and CHD patients, future studies will be able to further characterize the neurodevelopmental outcomes of ECMO patients.
This study has highlighted the importance of cEEG as part of a multifaceted approach to neuromonitoring for brain injury in this at-risk population and has shown that seizures during ECMO are associated with brain injury. Future studies with prospective cEEG will continue to explore the seizure and CNS injury differences in the ECMO CHD population, evaluate the significance of other EEG electrographic elements (e.g. EEG background) in predicting outcomes, as well as further characterize the neurodevelopmental outcomes of these patients.
CONCLUSIONS
Electrographic seizures occur frequently among infants who undergo ECMO and are associated with moderate to severe brain injuries on neuroimaging.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the DHREAMS study team for providing the developmental assessments for our congenital diaphragmatic hernia subgroup.
FUNDING / IRB
This study was not supported by any outside or institutional grant funding. The study is included under our institutional IRB protocol for critically ill infants at risk for seizures (RMG, WUSTL IRB 201611081). KPG is supported by a K23 grant from NINDS/NIH (NS099472). CDS Is supported by a K02 grant from NINDS/NIH (NS089852)
Footnotes
Financial disclosure statement: No financial disclosures.
Bibliography
- 1.https://www.elso.org/Registry/Statistics/InternationalSummary.aspx. Updated January 2020. Accessed March 31, 2020.
- 2.Boyle K, Felling R, Yiu A, et al. Neurologic Outcomes After Extracorporeal Membrane Oxygenation: A Systematic Review. Pediatr Crit Care Med. 2018;19(8):760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Bahr V, Kalzen H, Hultman J, et al. Long-Term Cognitive Outcome and Brain Imaging in Adults After Extracorporeal Membrane Oxygenation. Crit Care Med. 2018;46(5):e351–e358. [DOI] [PubMed] [Google Scholar]
- 4.Wien MA, Whitehead MT, Bulas D, et al. Patterns of Brain Injury in Newborns Treated with Extracorporeal Membrane Oxygenation. AJNR Am J Neuroradiol. 2017;38(4):820–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpenter JL, Yu YR, Cass DL, et al. Use of venovenous ECMO for neonatal and pediatric ECMO: a decade of experience at a tertiary children’s hospital. Pediatr Surg Int. 2018;34(3):263–268. [DOI] [PubMed] [Google Scholar]
- 6.Polito A, Barrett CS, Rycus PT, Favia I, Cogo PE, Thiagarajan RR. Neurologic injury in neonates with congenital heart disease during extracorporeal membrane oxygenation: an analysis of extracorporeal life support organization registry data. ASAIO J. 2015;61(1):43–48. [DOI] [PubMed] [Google Scholar]
- 7.Danzer E, Hoffman C, D’Agostino JA, et al. Short-Term Neurodevelopmental Outcome in Congenital Diaphragmatic Hernia: The Impact of Extracorporeal Membrane Oxygenation and Timing of Repair. Pediatr Crit Care Med. 2018;19(1):64–74. [DOI] [PubMed] [Google Scholar]
- 8.Gray B, Rintoul NE. Guidelines for Neonatal Respiratory Failure. ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support Web site. https://www.elso.org/Portals/0/ELSOGuidelinesNeonatalRespiratoryFailurev1_4_1.pdf. Published 2017. Accessed May 5, 2020. [DOI] [PubMed]
- 9.Hervey-Jumper SL, Annich GM, Yancon AR, Garton HJ, Muraszko KM, Maher CO. Neurological complications of extracorporeal membrane oxygenation in children. J Neurosurg Pediatr. 2011;7(4):338–344. [DOI] [PubMed] [Google Scholar]
- 10.Abend NS, Arndt DH, Carpenter JL, et al. Electrographic seizures in pediatric ICU patients: cohort study of risk factors and mortality. Neurology. 2013;81(4):383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain. 2014;137(Pt 5):1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Nonconvulsive seizures are common in critically ill children. Neurology. 2011;76(12):1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abend NS, Dlugos DJ, Clancy RR. A review of long-term EEG monitoring in critically ill children with hypoxic-ischemic encephalopathy, congenital heart disease, ECMO, and stroke. J Clin Neurophysiol. 2013;30(2):134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaynor JW, Nicolson SC, Jarvik GP, et al. Increasing duration of deep hypothermic circulatory arrest is associated with an increased incidence of postoperative electroencephalographic seizures. J Thorac Cardiovasc Surg. 2005;130(5):1278–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naim MY, Gaynor JW, Chen J, et al. Subclinical seizures identified by postoperative electroencephalographic monitoring are common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2015;150(1):169–178; discussion 178–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin JJ, Banwell BL, Berg RA, et al. Electrographic Seizures in Children and Neonates Undergoing Extracorporeal Membrane Oxygenation. Pediatr Crit Care Med. 2017;18(3):249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Zimmerman RA, Jarvik GP, et al. Perioperative stroke in infants undergoing open heart operations for congenital heart disease. Ann Thorac Surg. 2009;88(3):823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clancy RR, McGaurn SA, Wernovsky G, et al. Risk of seizures in survivors of newborn heart surgery using deep hypothermic circulatory arrest. Pediatrics. 2003;111(3):592–601. [DOI] [PubMed] [Google Scholar]
- 19.Okochi S, Cheung EW, Barton S, et al. An Analysis of Risk Factors for Hemolysis in Children on Extracorporeal Membrane Oxygenation. Pediatr Crit Care Med. 2018;19(11):1059–1066. [DOI] [PubMed] [Google Scholar]
- 20.Piantino JA, Wainwright MS, Grimason M, et al. Nonconvulsive seizures are common in children treated with extracorporeal cardiac life support. Pediatr Crit Care Med. 2013;14(6):601–609. [DOI] [PubMed] [Google Scholar]
- 21.Hahn JS, Vaucher Y, Bejar R, Coen RW. Electroencephalographic and neuroimaging findings in neonates undergoing extracorporeal membrane oxygenation. Neuropediatrics. 1993;24(1):19–24. [DOI] [PubMed] [Google Scholar]
- 22.Wynn J, Aspelund G, Zygmunt A, et al. Developmental outcomes of children with congenital diaphragmatic hernia: a multicenter prospective study. J Pediatr Surg. 2013;48(10):1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clancy RR, Sharif U, Ichord R, et al. Electrographic neonatal seizures after infant heart surgery. Epilepsia. 2005;46(1):84–90. [DOI] [PubMed] [Google Scholar]
- 24.Kharoshankaya L, Stevenson NJ, Livingstone V, et al. Seizure burden and neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy. Dev Med Child Neurol. 2016;58(12):1242–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nash KB, Bonifacio SL, Glass HC, et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neurology. 2011;76(6):556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wusthoff CJ, Dlugos DJ, Gutierrez-Colina A, et al. Electrographic seizures during therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. J Child Neurol. 2011;26(6):724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saengpattrachai M, Sharma R, Hunjan A, et al. Nonconvulsive seizures in the pediatric intensive care unit: etiology, EEG, and brain imaging findings. Epilepsia. 2006;47(9):1510–1518. [DOI] [PubMed] [Google Scholar]
- 28.LaRovere KL, Vonberg FW, Prabhu SP, et al. Patterns of Head Computed Tomography Abnormalities During Pediatric Extracorporeal Membrane Oxygenation and Association With Outcomes. Pediatr Neurol. 2017;73:64–70. [DOI] [PubMed] [Google Scholar]
- 29.Pinto VL, Pruthi S, Westrick AC, Shannon CN, Bridges BC, Le TM. Brain Magnetic Resonance Imaging Findings in Pediatric Patients Post Extracorporeal Membrane Oxygenation. ASAIO J. 2017;63(6):810–814. [DOI] [PubMed] [Google Scholar]
- 30.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015;32(2):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part II: personnel, technical specifications, and clinical practice. J Clin Neurophysiol. 2015;32(2):96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Los Reyes E, Roach ES. Neurologic complications of congenital heart disease and its treatment. Handb Clin Neurol. 2014;119:49–59. [DOI] [PubMed] [Google Scholar]
- 33.Gaynor JW, Jarvik GP, Bernbaum J, et al. The relationship of postoperative electrographic seizures to neurodevelopmental outcome at 1 year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg. 2006;131(1):181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menache CC, du Plessis AJ, Wessel DL, Jonas RA, Newburger JW. Current incidence of acute neurologic complications after open-heart operations in children. Ann Thorac Surg. 2002;73(6):1752–1758. [DOI] [PubMed] [Google Scholar]
- 35.Rappaport LA, Wypij D, Bellinger DC, et al. Relation of seizures after cardiac surgery in early infancy to neurodevelopmental outcome. Boston Circulatory Arrest Study Group. Circulation. 1998;97(8):773–779. [DOI] [PubMed] [Google Scholar]
- 36.Ehyai A, Fenichel GM, Bender HW Jr. Incidence and prognosis of seizures in infants after cardiac surgery with profound hypothermia and circulatory arrest. JAMA. 1984;252(22):3165–3167. [PubMed] [Google Scholar]
- 37.Abend NS, Wagenman KL, Blake TP, et al. Electrographic status epilepticus and neurobehavioral outcomes in critically ill children. Epilepsy Behav. 2015;49:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Payne ET, Hahn CD. Continuous electroencephalography for seizures and status epilepticus. Curr Opin Pediatr. 2014;26(6):675–681. [DOI] [PubMed] [Google Scholar]
- 39.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic status epilepticus is associated with mortality and worse short-term outcome in critically ill children. Crit Care Med. 2013;41(1):215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fitzgerald MP, Massey SL, Fung FW, Kessler SK, Abend NS. High electroencephalographic seizure exposure is associated with unfavorable outcomes in neonates with hypoxic-ischemic encephalopathy. Seizure. 2018;61:221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parish AP, Bunyapen C, Cohen MJ, Garrison T, Bhatia J. Seizures as a predictor of long-term neurodevelopmental outcome in survivors of neonatal extracorporeal membrane oxygenation (ECMO). J Child Neurol. 2004;19(12):930–934. [DOI] [PubMed] [Google Scholar]
- 42.Gunn JK, Beca J, Hunt RW, Olischar M, Shekerdemian LS. Perioperative amplitude-integrated EEG and neurodevelopment in infants with congenital heart disease. Intensive Care Med. 2012;38(9):1539–1547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


