Abstract
ADAMTS (a disintegrin and metalloprotease with thrombospondin motifs) proteases comprise the most recently discovered branch of the extracellular metalloenzymes. Research during the last 15 years, uncovered their association with a variety of physiological and pathological processes including blood coagulation, tissue repair, fertility, arthritis and cancer. Importantly, a frequent feature of ADAMTS enzymes relates to their effects on vascular-related phenomena, including angiogenesis. Their specific roles in vascular biology have been clarified by information on their expression profiles and substrate specificity. Through their catalytic activity, ADAMTS proteases modify rather than degrade extracellular proteins. They predominantly target proteoglycans and glycoproteins abundant in the basement membrane, therefore their broad contributions to the vasculature should not come as a surprise. Furthermore, in addition to their proteolytic functions, non-enzymatic roles for ADAMTS have also been identified expanding our understanding on the multiple activities of these enzymes in vascular-related processes.
Keywords: ADAMTS, angiogenesis, extracellular proteolysis, vasculature
Introduction
Our understanding of the mechanisms that regulate vascular growth has expanded significantly in the last three decades. The morphological and rather rudimentary knowledge of the vasculature in mid-1980s has been quickly filled with information on specific genes, signaling pathways and heterotypic cellular interactions that trigger growth, pattern, and provide differentiation cues to distinct segments of the vascular tree regulating the emergence of arteries, veins and capillaries. It is in this context that we review the function of ADAMTS proteases. Our goal is to provide a cohesive summary of how these extracellular enzymes contribute to the morphogenesis and maintenance of blood vessels and participate in the promotion or resolution of vascular pathology.
Extracellular proteases, particularly members of the MMP family are essential to the process of angiogenesis [1]. They participate in the initial degradation of the basement membrane facilitating sprout formation and promoting invasion of connective tissue through restricted degradation of extracellular matrix (ECM) proteins [2]. In addition, bioactive fragments resulting from processing of ECM proteins have uncovered important layers of regulatory control, both inhibitory and stimulatory in nature [3]. In this manner, posttranslational modifications of the extracellular milieu have emerged as a basic mechanism for regulation of vessel repair, promotion of atherosclerosis, as well as, stimulation and inhibition of angiogenesis. In this context, much of our recent understanding on the specific molecular landscape of substrates and proteases has benefited from technological advancements that enabled a detailed profile of the repertoire of proteolytic events in the extracellular milieu, as per initiatives such as the Degradome Project [4].
The first link between ADAMTS proteases and angiogenesis emerged early on during the cloning of the first human members of the family [5]. The original strategy was to identify proteins that harbored “anti-angiogenic domains” first identified in thrombospondin-1, i.e., the thrombospondin type I repeats (TSR) [6]. This effort resulted in the cloning of ADAMTS1 and 8 (originally named METH-1 and METH-2, respectively, to refer to a chimeric protein that contained both metalloprotease (ME) and thrombospondin (TH) domains). Experiments using in vivo and in vitro assays, confirmed that the TSR domains in ADAMTS1 and 8 harbored antiangiogenic function [5]. Additional members of this family were subsequently discovered and associated with multiple biological functions; however, their potential contribution to neovascularization was not always evaluated. The presence of TSR motifs within the structure of ADAMTS reiterates the possibility for a latent function in the regulation of vascular growth. Subsequently, it also became clear that in addition to the TSR motifs, the catalytic function of ADAMTS proteases could also suppress vascular growth, as it will be discussed.
To date the field has identified 19 members with similar overall domain structure, but with relatively unique properties and functions [7–10]. The earlier identification of ADAMTS1 and ADAMTS8 as endogenous angiogenic inhibitors was followed by reports implicating the potential contribution of other members, such as ADAMTS2, 3, 4, 5, 9, 12, 13, 15 and 18. Moreover, some reports also reported pro-angiogenic activities for a number of ADAMTS proteases, as it would be reviewed here. This highlights the delicate equilibrium and spatiotemporal complexity required to form and preserve the vasculature.
Multi-domain structure of ADAMTS: a range of multi-functional possibilities
As other matrix metalloenzymes, ADAMTS proteases exhibit a common multi-domain structure (Fig. 1A). The backbone organization consists of a prodomain, a catalytic motif and a disintegrin-like module, linked to an additional C-terminal sequence, referred to as ancillary domain. This region includes at least one TSR, a cysteine-rich domain, and a spacer fragment, that may or may not be followed by a variable number of additional TSR domains and other motifs (Cub, Gon1-like, mucin-like, lacunin). The composition of this C-terminal region offers a distinctive feature to each individual member of the family and provides cues as to their potential functional abilities, binding and anchoring properties, substrate recognition, half-life, and evolutionary trajectory, as reviewed previously [8]. Conversion of the zymogen form into an active ADAMTS protease is facilitated by proprotein convertases that activate the enzymes by removing the prodomain [11]. Furthermore, most ADAMTS are modified by proteolysis at their C-terminal region, with consequences to their respective affinities to ECM and cell surface proteins [11]. This additional modification offers expansion of functional properties, as it might alter binding and recognition of alternative partners. Besides, some of the released C-terminal fragments might exhibit autonomous biological activities, although their identification in vivo has not been widely determined.
Fig. 1.
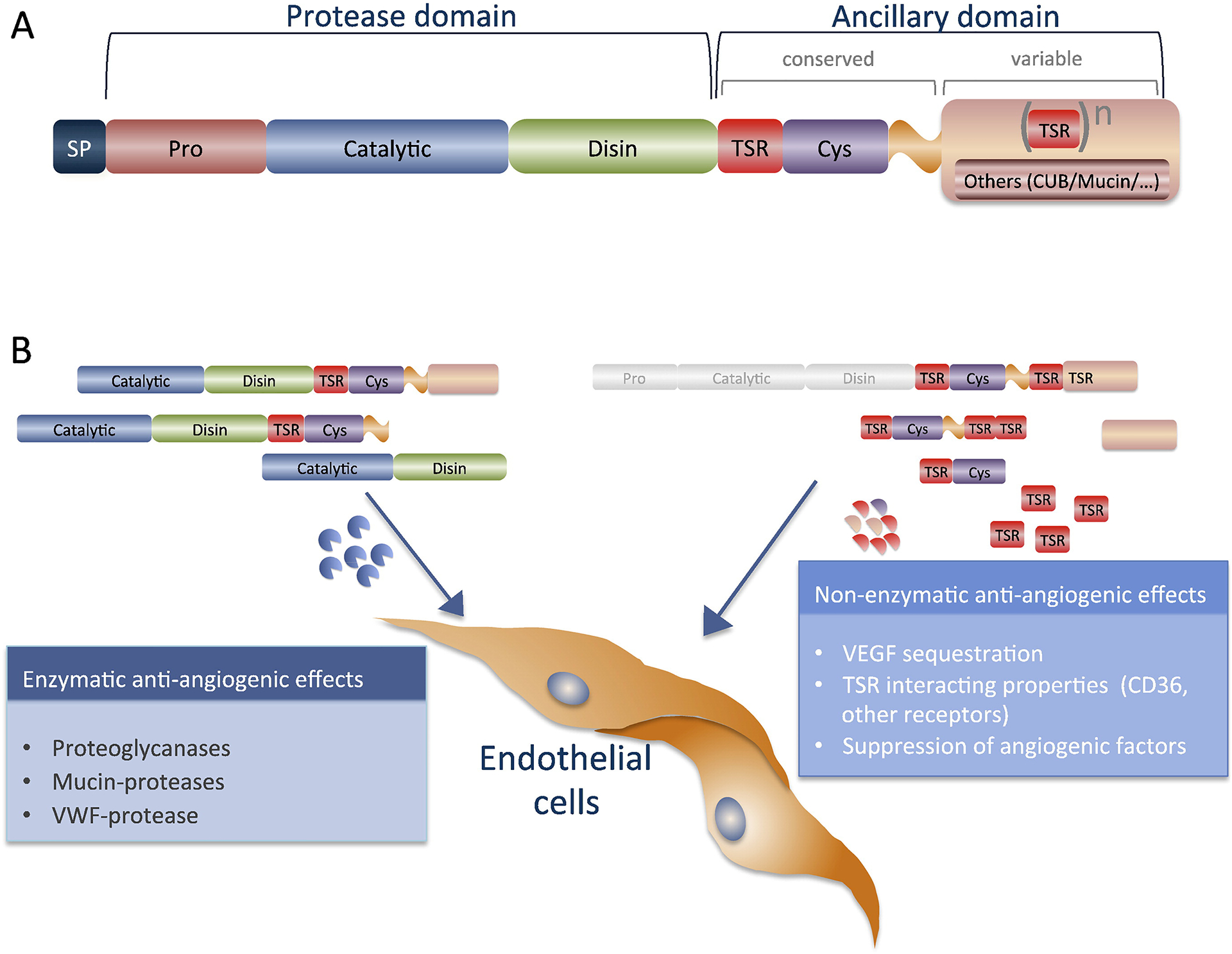
Schematic diagram of ADAMTS proteases and anti-angiogenic effects. A. The structure of ADAMTS proteases includes a protease domain and an ancillary region. The protease domain contains a pro-domain, a catalytic motif and a disintegrin-like module. The ancillary region includes at least one TSR, a cysteine-rich domain, and a spacer fragment, that may or may not be followed by a variable number of additional TSR domains and other motifs. B. The anti-angiogenic effects of ADAMTS are classified in non-enzymatic (right) and enzymatic (left). Non-enzymatic effects are mediated mainly by the TSRs but additional motifs might also contribute. The enzymatic effects are naturally linked to the relevance of the substrate to vascular function. ADAMTS proteases whose catalytic activities provoke anti-angiogenic effects include: proteoglycanases, mucin-proteoglycanases and vWF-protease. Abbreviations: Cys — cysteine-rich domain; Disin — disintegrin-like module; TSR — thrombospondin type I repeat.
To date several studies have addressed the contribution of both the catalytic domain, as well as the C-terminal ancillary regions in the modulation of angiogenesis. Here, we review the non-catalytic and subsequently the catalytic functions of ADAMTS proteases in vascular function.
Effects of ADAMTS in the vasculature
Non-enzymatic functions of ADAMTS in angiogenesis/vascular function
As mentioned previously, the approach used to clone human ADAMTS1 and ADAMTS8 genes was based on a search for TSR-containing modules [5], as this single TSR motif by itself exhibits antiangiogenic properties [6,12]. Since all ADAMTS members contain at least one TSR (1 to 15), multiple reports in the literature have hone on exploring their relevance to vascular sprouting. Following this rationale, it would be expected that all ADAMTS members display some degree of anti-angiogenic activity. However, to date this type of inhibitory properties has been attributed to the TSR motifs present in ADAMTS1, 2, 4, 5, 8, 12 and 13, although other domains were also implicated in these properties, as discussed below.
In several cases, the anti-angiogenic activity of the TSR domains of distinct ADAMTS proteases was determined as isolated fragments; that is in the contextual absence of the catalytic regions (Fig. 1B). While these experiments are of importance, the determination as to whether these domains exist independently is of relevance. Alternatively, evaluation of these domains in the context of a catalytically active or inactive full-length protein would offer additional validation and important biological relevance. For example, a recent report communicated both anti-tumorigenic and anti-angiogenic activity of the C-term ancillary region of ADAMTS4 in a mouse melanoma model. This was in contrast to the full length protease that was found to be pro-tumorigenic [13]. The findings with the C-fragment validated previous studies that used peptides derived from different ADAMTS proteins, displaying inhibitory properties on HUVEC cultures [14].
Studies on ADAMTS1 reported that the C-terminal spacer domain together with its 3 TSRs convey anti-angiogenic properties by sequestering VEGF and blocking its binding to VEGFRs [15]. Closest to ADAMTS1 in terms of structure, ADAMTS15 was also found to hold anti-angiogenic activities through its C-terminal motif; interestingly the full-length protein was shown to be physiologically cleaved releasing this C-terminal fragment [16]. A similar catalytic-independent function in the regulation of angiogenesis was attributed to ADAMTS2, with direct consequences to inhibition of tumor growth [17]. Analogous conclusions were also reported for ADAMTS5 using both cellular and tumor models [18]. In this case, xenograft assays indicated that the anti-angiogenic properties of this protease were associated with the downregulation of several growth factors such as VEGF, PlGF, and PD-ECGF [18]. This same group also showed that the in vitro inhibitory activity was exclusive to the first, but not the second TSR [19].
A more complex function for the C-terminal ancillary domain was reported for ADAMTS12 [20]. This region exhibits angio-inhibitory activity in a VEGF-induced tube formation assay. Truncated ADAMTS12, lacking the C-terminal region, was inhibitory, but less efficient perhaps because of impairment in protein–protein interactions, suggesting the contribution of both multiple domains in the angio-modulatory activity. Aortic ring assays with conditioned media in the presence of wild-type and catalytically inactive ADAMTS12 further supported these findings [21].
Finally, C-terminal region of ADAMTS13 [22] was also shown to display angio-modulatory properties (both pro- and anti-angiogenic), and it will be discussed later.
Catalytic functions of ADAMTS proteases in angiogenesis
As previously mentioned, most reports on the proteolytic-independent anti-angiogenic actions of ADAMTS proteases are based on the activity of isolated fragments or on the use of catalytically inactive mutants. While these results hold potential therapeutic/pharmacologic value, they might not reflect the biological properties of ADAMTS proteases in vivo. Therefore, a comprehensive identification of substrates and functional determination of the full-length proteases is of relevance.
The emerging findings on catalytic properties of distinct ADAMTS proteases allow us to further classify these proteases according to the specificity of their substrates [8]. The current classification includes: proteoglycanases, procollagen aminopeptidases, mucin-proteoglycanases, and vWF-protease. An assessment of the available literature on procollagenases (including ADAMTS2, 3 and 14) does not indicate a direct role for this subgroup in the regulation of angiogenesis and vascular-modulatory functions. In the previous section, it was mentioned that ADAMTS2 harbors anti-angiogenic properties, but this was independent of its catalytic domains. Importantly, a very recent report on ADAMTS3 revealed a distinct proteolytic activity with consequences as inducer of lymphangiogenesis (assessed in the next section).
In contrast to procollagenases, the subfamily of proteoglycanases has been shown to display multiple vascular-modulatory functions that are in fact linked to their catalytic properties (Fig. 1B). In addition, the identification of additional substrates not categorized as proteoglycans, but also relevant components of specialized vascular-related extracellular matrices, such as the basement membrane has further emphasized a role for these enzymes in vascular biology. Research on ADAMTS1 has provided the larger list of substrates, ranging from proteoglycans, such as: aggrecan, versican, biglycan and syndecan-4; to extracellular glycoproteins, such as: TSP1 and 2, nidogens, TFPI-2 and IGFBP2 [23–29].
Proteolysis of TSP1 and 2 by ADAMTS1 promoted the release of anti-angiogenic TSR fragments with remarkable inhibitory consequences to wound healing and tumor angiogenesis [23]. In a different scenario, cleavage of the basement membrane glycoproteins Nidogen-1 and −2 by ADAMTS1 promote vascular impairment and correlate with tumor suppressive activities in some cancers [29]. Using an in vitro approach, cleavage of syndecan-4 by ADAMTS1 was found to alter the adhesive properties of both tumor and endothelial cells, with inhibitory consequences to angiogenesis and tumor expansion [27]. The cleavage of versican by ADAMTS1 was also evaluated in the context of tumor neovascularization, tumor progression and ovulation. As both anti- and pro-angiogenic properties have been highlighted for this substrate, it will be appraised in the next section.
ADAMTS4, described as the primary aggrecanase, was later found to cleave versican and brevican, with a similar pattern to that shown by ADAMTS1 [30,31]. In fact, their related spatiotemporal distribution suggests both overlapping and distinct functions during ovulation, also in conjunction with ADAMTS5 [32]. Additional substrates were also reported for ADAMTS4 with interesting features related to angiogenesis. In particular, members of the Matrilins, described as inhibitors of neovascularization, were shown to be cleaved by ADAMTS4 and 5 [33], but this information has not been followed up with studies that directly evaluate the contribution of this proteolysis to vascular morphogenesis.
Importantly versican and aggrecan are also substrates for ADAMTS9 [34], another proteoglycanase that displays a peculiar expression pattern associated with microvascular endothelial cells [35]. The anti-angiogenic properties of ADAMTS9 have been evaluated in the context of the adamts9+/− mouse, that develops an increased corneal neovascularization and excessive tumor neovascularization [35]. Common to other ADAMTS members, downregulation of ADAMTS9 was noted in breast cancer [36], and its angio-inhibitory functions were also described in matrigel plug assays with esophageal squamous and nasopharyngeal cell carcinomas [37]. These studies showed that the overexpression of ADAMTS9 correlated with repression of proangiogenic factors, such as MMP9 and VEGF [37].
A recent report has also attributed anti-angiogenic properties to the proteoglycanase ADAMTS15 in relation to its activity on syndecan-4 [38]. This protease was also reported to act as a tumor suppressor [39], a function that could be linked to these anti-angiogenic properties.
As for the mucin-proteases, such as ADAMTS12, angio-inhibitory activity has been attributed to their TSRs (mentioned in previous section) although those studies were based on the truncation of the C-term domain. It is possible that this structural modification might simply affect the interacting ability of the protease to its specific binding partners [20].
Finally, ADAMTS13, the sole member of the vWF-protease group, which displays clear structural differences from other ADAMTS members, also impacts the vasculature. Most of the publications on ADAMTS13 have focused on its central role in thrombotic thrombocytopenic purpura (TTP) [40]. The main substrate of ADAMTS13 is von Willebrand’s Factor (vWF), an important participant of the clotting cascade and a component of the vascular basement membrane. Importantly, vWF displays hemostatic properties during angiogenesis [41]. In this context, both pro- and anti-angiogenic activities have been described for ADAMTS13, therefore we will resume this discussion later in the review.
Angiomodulatory properties of ADAMTS proteases
Pro-angiogenic actions of ADAMTS proteases have been limited to very few members of the family. However, a close evaluation of those reports reveals the need to gain additional molecular and mechanistic insights into the properties of these proteases in the context of complex biological scenarios. As more studies convey specific nuances and alternative functional effects, it has become critical to reassess the initial anti- and pro-angiogenic classification to incorporate a more complex, but functionally appropriate, label for ADAMTS proteases in relation to vascular function. In particular, the contribution of these proteases to the vascular niche and proangiogenic activities might suggest a broader role in homeostatic regulation of vascular function.
In the case of ADAMTS1, the protease has been identified as a “tip-cell” specific protease and it was also shown to promote endothelial cell sprouting in collagen invasion assays [42]. Accordingly, the expressing cell type and functional context are likely important as to whether it exhibits pro- or anti-angiogenic properties. All other studies that have attributed anti-angiogenic functions to this protease included either delivery on site or through expression by non-endothelial cells. Interestingly ADAMTS1 transcripts are elevated in endothelial cells under hypoxic conditions and upon treatment with VEGF [43,44]. In tumors, ADAMTS1 transcripts were correlated with suppression of tumor development, as suggested in Down’s syndrome individuals with an extra copy of chromosome 21 [45]. In addition, its potential contribution to induce the acquisition of an endothelial-like phenotype has been reported in the context of highly plastic tumor populations [46]. Likewise, ADAMTS1 expression was associated with changes in vascular density of some tumors. For example, increase in ADAMTS1 levels in a prostate cancer model correlated directly with an increase of vessel diameter without apparent changes in microvascular density and pericyte coverage [47]. A similar vascular phenotype was observed in HEK293-derived xenografts with altered levels of ADAMTS1, although in this case vascular density was also affected and the authors implied a role for the ADAMTS1 substrates Nidogen-1 and −2 [29].
Similar to ADAMTS1, both pro- and anti-angiogenic activities have been attributed to ADAMTS4. Using the same melanoma model, where anti-angiogenic properties were associated with TSR domains (reviewed in the section above), these authors showed that full-length ADAMTS4 promoted tumor angiogenesis and, probably as a consequence, tumor growth [13]. In contrast, Hsu and colleagues previously reported anti-angiogenic effects using a recombinant ADAMTS4 that lacks the spacer region [48]. These authors also confirmed endogenous expression of ADAMTS4 by endothelial cells, previously found to be regulated by VEGF [49]. Significant, overlapping functions for both ADAMTS1 and ADAMTS4 were suggested during ovulation [32], although the adamts4 KO did not display a remarkable reproductive phenotype, in sharp contrast to adamts1 deficient mice.
As a common substrate of several ADAMTS (mainly 1, 4 and 5), the matricellular proteoglycan versican has been widely linked to vascular function, as well as to tumorigenesis. However, its proteolysis does not clearly result in either pro- or anti-angiogenic activities and deserves additional experimental evaluation.
As discussed previously, studies on the vWR-protease ADAMTS13 also reported ambiguous contributions of this protease in angiogenesis and vascularization [22,50]. Although anti-angiogenic properties were attributed to its TSRs motifs, later studies support a dose-dependent angio-modulatory action. While the authors did not correlate ADAMTS13 activities with its proteolytic functions, the fact that vWF itself also regulates angiogenesis [41] is suggestive of a role for this protease in vascular homeostasis.
Finally, a recent report on ADAMTS3 provided a new activity for this protease with a significant contribution to lymphangiogenesis [51]. These authors identified VEGF-C as a novel substrate for ADAMTS3 supporting roles on bioavailability and regulation VEGFR3 activation.
Final consideration and future directions
A comprehensive evaluation of the published literature related to the effects of ADAMTS proteases in vascular biology reveals a complex picture that cannot be encapsulated into a single theme or function. Clearly, the multi-domain structure of these proteases and the fact that different fragments with autonomous functions could be physiologically released, emphasize the importance to investigate the presence of these motifs in biological samples. The presence of these fragments could hold prognostic/diagnostic significance by either highlighting the existence of endogenous anti-angiogenic mechanisms or to reveal a particular pathological condition. Most results on non-enzymatic actions of ADAMTS display anti-angiogenic properties and clearly pharmacological exploration of these domains might hold therapeutic value.
The catalytic effects of ADAMTS proteases on vascular-related processes require further investigation. Although a significant number of reports have contributed to clarify substrates and functions, the complexity and dynamic nature of extracellular microenvironments provide a challenging landscape in need of molecular dissection. Along these lines, it is encouraging to witness the identification of several vascular-specific molecules as targets of ADAMTS proteases (Fig. 2). This information will pave the way to elucidate their contributions to vascular biology. Clearly additional studies, coupled with new technological advances in the fields of proteomics and large-data analysis, will be required to correlate the catalog of specific substrates with intracellular signaling, cellular effects and tissue responses.
Fig. 2.
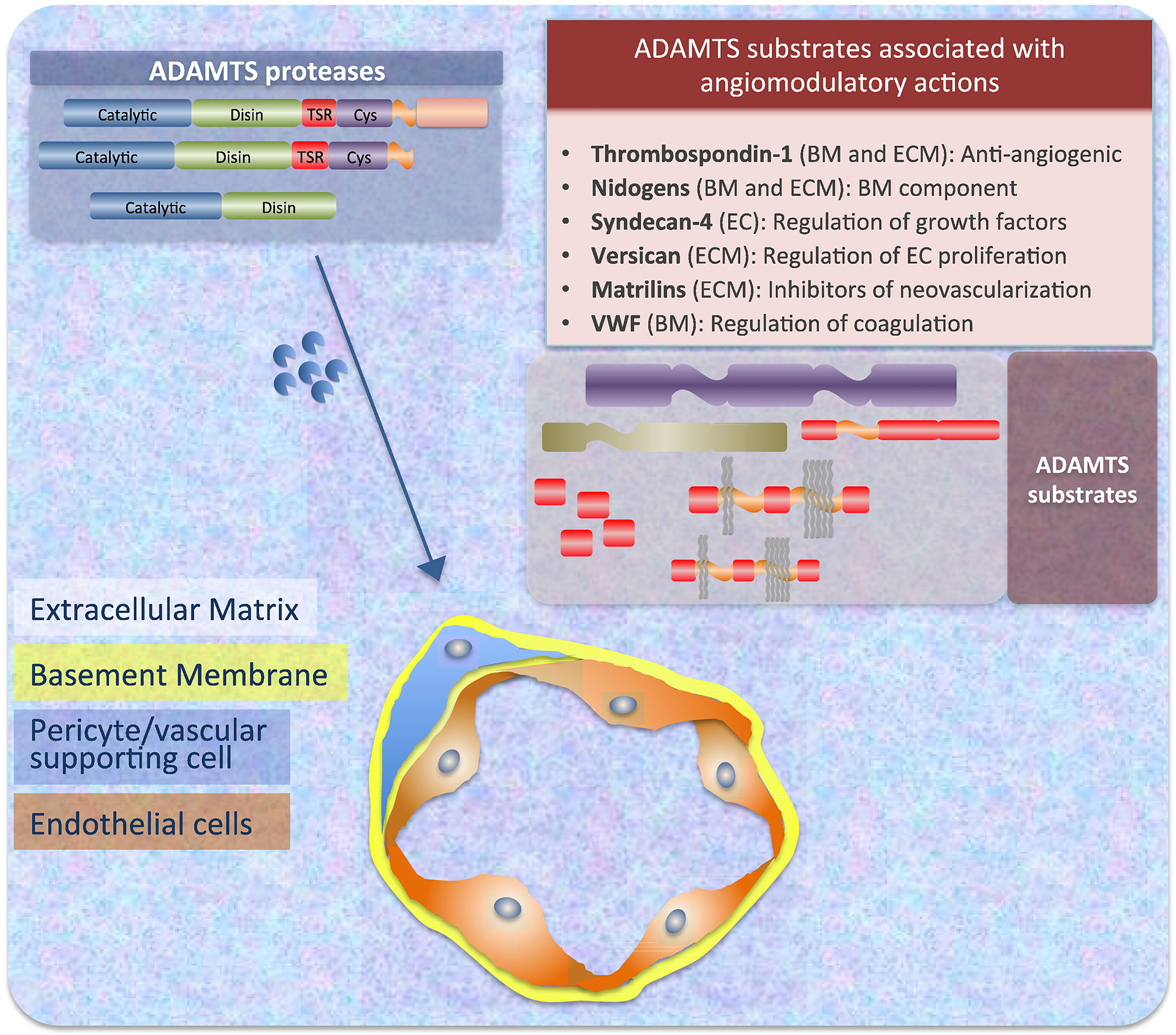
Substrates of ADAMTS proteases, distribution in relation to blood vessels and functions. ADAMTS proteases can be catalytically active either as full-length proteins or as truncated versions of enzymatically active fragments, after the release of their C-terminal regions. Most of ADAMTS substrates are components of the extracellular matrix, associated to specific cell types, or present in the vascular basement membrane (as indicated in panel). The modification of these substrates by proteolysis affects the activities of endothelial cells and pericytes. Abbreviations: BM — basement membrane; EC — endothelial cell; ECM — extracellular matrix.
Acknowledgments
This work was supported by grants from the Ministerio de Economía y Competitividad and Instituto de Salud Carlos III from Spain, co-financed by FEDER (PI13/00168), from the Consejería de Economía, Innovación y Ciencia-Junta de Andalucía (P10-CTS5865) to JCRM, and from the National Institutes of Health (R01HL114086) to MLIA.
References
- 1.Handsley MM, Edwards DR. Metalloproteinases and their inhibitors in tumor angiogenesis. Int J Cancer 2005;115: 849–60. 10.1002/ijc.20945. [DOI] [PubMed] [Google Scholar]
- 2.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 2011;3. 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bix G, Iozzo RV. Matrix revolutions: “tails” of basement-membrane components with angiostatic functions. Trends Cell Biol 2005;15:52–60. 10.1016/j.tcb.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Otin C, Overall CM. Protease degradomics: a new challenge for proteomics. Nat Rev Mol Cell Biol 2002;3:509–19. [DOI] [PubMed] [Google Scholar]
- 5.Vázquez F, Hastings G, Ortega MA, Lane TF, Oikemus S, Lombardo M, et al. METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. J Biol Chem 1999;274:23349–57. [DOI] [PubMed] [Google Scholar]
- 6.Iruela-Arispe ML, Lombardo M, Krutzsch HC, Lawler J, Roberts DD. Inhibition of angiogenesis by thrombospondin-1 is mediated by 2 independent regions within the type 1 repeats. Circulation 1999;100:1423–31. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Rao N, Ge R. Emerging roles of ADAMTSs in angiogenesis and cancer. Cancers (Basel) 2012;4:1252–99. 10.3390/cancers4041252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem 2009;284: 31493–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J 2005;386:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagstaff L, Kelwick R, Decock J, Edwards DR. The roles of ADAMTS metalloproteinases in tumorigenesis and metastasis. Front Biosci (Landmark Ed) 2011;16:1861–72. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Manzaneque JC, Milchanowski AB, Dufour EK, Leduc R, Iruela-Arispe ML. Characterization of METH-1/ ADAMTS1 processing reveals two distinct active forms. J Biol Chem 2000;275:33471–9. [DOI] [PubMed] [Google Scholar]
- 12.Tolsma SS, Volpert OP, Good DJ, Frazier WA, Polverini PJ, Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol 1993;122:497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao N, Ke Z, Liu H, Ho C-J, Kumar S, Xiang W, et al. ADAMTS4 and its proteolytic fragments differentially affect melanoma growth and angiogenesis in mice. Int J Cancer 2013;133:294–306. 10.1002/ijc.28037. [DOI] [PubMed] [Google Scholar]
- 14.Karagiannis ED, Popel AS. Anti-angiogenic peptides identified in thrombospondin type I domains. Biochem Biophys Res Commun 2007;359:63–9. 10.1016/j.bbrc.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luque A, Carpizo DR, Iruela-Arispe ML. ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J Biol Chem 2003;278: 23656–65. [DOI] [PubMed] [Google Scholar]
- 16.Molokwu CN, Adeniji OO, Chandrasekharan S, Hamdy FC, Buttle DJ. Androgen regulates ADAMTS15 gene expression in prostate cancer cells. Cancer Invest 2010;28:698–710. 10.3109/07357907.2010.489538. [DOI] [PubMed] [Google Scholar]
- 17.Dubail J, Kesteloot F, Deroanne C, Motte P, Lambert V, Rakic J-M, et al. ADAMTS-2 functions as anti-angiogenic and anti-tumoral molecule independently of its catalytic activity. Cell Mol Life Sci 2010;67:4213–32. 10.1007/s00018-010-0431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar S, Namini SS, Rao N, Ge R, Sharghi-Namini S. ADAMTS5 functions as an anti-angiogenic and antitumorigenic protein independent of its proteoglycanase activity. Am J Pathol 2012;181:1056–68. 10.1016/j.ajpath.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 19.Sharghi-Namini S, Fan H, Sulochana KN, Potturi P, Xiang W, Chong Y-S, et al. The first but not the second thrombospondin type 1 repeat of ADAMTS5 functions as an angiogenesis inhibitor. Biochem Biophys Res Commun 2008;371:215–9. 10.1016/j.bbrc.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 20.Llamazares M, Obaya AJ, Moncada-Pazos A, Heljasvaara R, Espada J, Lopez-Otin C, et al. The ADAMTS12 metalloproteinase exhibits anti-tumorigenic properties through modulation of the Ras-dependent ERK signalling pathway. J Cell Sci 2007;120:3544–52. [DOI] [PubMed] [Google Scholar]
- 21.El Hour M, Moncada-Pazos A, Blacher S, Masset A, Cal S, Berndt S, et al. Higher sensitivity of Adamts12-deficient mice to tumor growth and angiogenesis. Oncogene 2010;29:3025–32. [DOI] [PubMed] [Google Scholar]
- 22.Lee M, Rodansky ES, Smith JK, Rodgers GM. ADAMTS13 promotes angiogenesis and modulates VEGF-induced angiogenesis. Microvasc Res 2012;84:109–15. 10.1016/j.mvr.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Lee NV, Sato M, Annis DS, Loo JA, Wu L, Mosher DF, et al. ADAMTS1 mediates the release of antiangiogenic polypeptides from TSP1 and 2. EMBO J 2006;25:5270–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torres-Collado AX, Kisiel W, Iruela-Arispe ML, Rodriguez-Manzaneque JC. ADAMTS1 interacts with, cleaves, and modifies the extracellular location of the matrix inhibitor tissue factor pathway inhibitor-2. J Biol Chem 2006;281:17827–37. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Manzaneque JC, Westling J, Thai SN, Luque A, Knauper V, Murphy G, et al. ADAMTS1 cleaves aggrecan at multiple sites and is differentially inhibited by metalloproteinase inhibitors. Biochem Biophys Res Commun 2002;293:501–8. [DOI] [PubMed] [Google Scholar]
- 26.Brown HM, Dunning KR, Robker RL, Boerboom D, Pritchard M, Lane M, et al. ADAMTS1 cleavage of versican mediates essential structural remodeling of the ovarian follicle and cumulus-oocyte matrix during ovulation in mice. Biol Reprod 2010;83:549–57. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Manzaneque JC, Carpizo D, Plaza-Calonge Mdel C, Torres-Collado AX, Thai SN, Simons M, et al. Cleavage of syndecan-4 by ADAMTS1 provokes defects in adhesion. Int J Biochem Cell Biol 2009;41:800–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martino-Echarri E, Fernández-Rodríguez R, Bech-Serra JJ, Plaza-Calonge Mdel C, Vidal N, Casal C, et al. Relevance of IGFBP2 proteolysis in glioma and contribution of the extracellular protease ADAMTS1. Oncotarget 2014;5:4295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martino-Echarri E, Fernández-Rodríguez R, Rodríguez-Baena FJ, Barrientos-Durán A, Torres-Collado AX, Plaza-Calonge Mdel C, et al. Contribution of ADAMTS1 as a tumor suppressor gene in human breast carcinoma. Linking its tumor inhibitory properties to its proteolytic activity on nidogen-1 and nidogen-2. Int J Cancer 2013;133:2315–24. 10.1002/ijc.28271. [DOI] [PubMed] [Google Scholar]
- 30.Matthews RT, Gary SC, Zerillo C, Pratta M, Solomon K, Arner EC, et al. Brain-enriched hyaluronan binding (BEHAB)/Brevican cleavage in a glioma cell line is mediated by a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) family member. J Biol Chem 2000;275:22695–703. [DOI] [PubMed] [Google Scholar]
- 31.Westling J, Gottschall PE, Thompson VP, Cockburn A, Perides G, Zimmermann DR, et al. ADAMTS4 (aggrecanase-1) cleaves human brain versican V2 at Glu405–Gln406 to generate glial hyaluronate binding protein. Biochem J 2004; 377:787–95. 10.1042/BJ20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richards JS, Hernandez-Gonzalez I, Gonzalez-Robayna I, Teuling E, Lo Y, Boerboom D, et al. Regulated expression of ADAMTS family members in follicles and cumulus oocyte complexes: evidence for specific and redundant patterns during ovulation. Biol Reprod 2005;72:1241–55. [DOI] [PubMed] [Google Scholar]
- 33.Ehlen HWA, Sengle G, Klatt AR, Talke A, Müller S, Paulsson M, et al. Proteolytic processing causes extensive heterogeneity of tissue matrilin forms. J Biol Chem 2009;284:21545–56. 10.1074/jbc.M109.016568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Somerville RPT, Longpre J-M, Jungers KA, Engle JM, Ross M, Evanko S, et al. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J Biol Chem 2003;278:9503–13. 10.1074/jbc.M211009200. [DOI] [PubMed] [Google Scholar]
- 35.Koo B-H, Coe DM, Dixon LJ, Somerville RPT, Nelson CM, Wang LW, et al. ADAMTS9 is a cell-autonomously acting, anti-angiogenic metalloprotease expressed by microvascular endothelial cells. Am J Pathol 2010;176:1494–504. 10.2353/ajpath.2010.090655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porter S, Scott SD, Sassoon EM, Williams MR, Jones JL, Girling AC, et al. Dysregulated expression of adamalysin–thrombospondin genes in human breast carcinoma. Clin Cancer Res 2004;10:2429–40. [DOI] [PubMed] [Google Scholar]
- 37.Lo PHY, Lung HL, Cheung AKL, Apte SS, Chan KW, Kwong FM, et al. Extracellular protease ADAMTS9 suppresses esophageal and nasopharyngeal carcinoma tumor formation by inhibiting angiogenesis. Cancer Res 2010;70:5567–76. 10.1158/0008-5472.CAN-09-4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelwick R, Wagstaff L, Decock J, Roghi C, Cooley LS, Robinson SD, et al. Metalloproteinase-dependent and -independent processes contribute to inhibition of breast cancer cell migration, angiogenesis and liver metastasis by A disintegrin and metalloproteinase with thrombospondin motifs-15 (ADAMTS-15). Int J Cancer 2014. 10.1002/ijc.29129. [DOI] [PubMed] [Google Scholar]
- 39.Viloria CG, Obaya AJ, Moncada-Pazos A, Llamazares M, Astudillo A, Capella G, et al. Genetic inactivation of ADAMTS15 metalloprotease in human colorectal cancer. Cancer Res 2009;69:4926–34. [DOI] [PubMed] [Google Scholar]
- 40.Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature 2001;413:488–94. 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 41.Starke RD, Ferraro F, Paschalaki KE, Dryden NH, McKinnon TAJ, Sutton RE, et al. Endothelial von Willebrand factor regulates angiogenesis. Blood 2011;117:1071–80. 10.1182/blood-2010-01-264507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su S-C, Mendoza EA, Kwak H-I, Bayless KJ. Molecular profile of endothelial invasion of three-dimensional collagen matrices: insights into angiogenic sprout induction in wound healing. Am J Physiol Cell Physiol 2008;295:C1215–29. 10.1152/ajpcell.00336.2008. [DOI] [PubMed] [Google Scholar]
- 43.Xu Z, Yu Y, Duh EJ. Vascular endothelial growth factor upregulates expression of ADAMTS1 in endothelial cells through protein kinase C signaling. Invest Ophthalmol Vis Sci 2006;47:4059–66. 10.1167/iovs.05-1528. [DOI] [PubMed] [Google Scholar]
- 44.Hatipoglu OF, Hirohata S, Cilek MZ, Ogawa H, Miyoshi T, Obika M, et al. ADAMTS1 is a unique hypoxic early response gene expressed by endothelial cells. J Biol Chem 2009;284:16325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reynolds LE, Watson AR, Baker M, Jones TA, D’Amico G, Robinson SD, et al. Tumour angiogenesis is reduced in the Tc1 mouse model of Down’s syndrome. Nature 2010;465:813–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Casal C, Torres-Collado AX, Plaza-Calonge MC, Martino-Echarri E, Ramon y Cajal S, Rojo F, et al. ADAMTS1 contributes to the acquisition of an endothelial-like phenotype in plastic tumor cells. Cancer Res 2010;70:4676–86. [DOI] [PubMed] [Google Scholar]
- 47.Gustavsson H, Tesan T, Jennbacken K, Kuno K, Damber JE, Welen K. ADAMTS1 alters blood vessel morphology and TSP1 levels in LNCaP and LNCaP-19 prostate tumors. BMC Cancer 2010;10:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsu Y-P, Staton CA, Cross N, Buttle DJ. Anti-angiogenic properties of ADAMTS-4 in vitro. Int J Exp Pathol 2012;93: 70–7. 10.1111/j.1365-2613.2011.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kahn J, Mehraban F, Ingle G, Xin X, Bryant JE, Vehar G, et al. Gene expression profiling in an in vitro model of angiogenesis. Am J Pathol 2000;156:1887–900. 10.1016/S0002-9440(10)65062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee M, Keener J, Xiao J, Long Zheng X, Rodgers GM. ADAMTS13 and its variants promote angiogenesis via upregulation of VEGF and VEGFR2. Cell Mol Life Sci 2014. 10.1007/s00018-014-1667-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeltsch M, Jha SK, Tvorogov D, Anisimov A, Leppänen V-M, Holopainen T, et al. CCBE1 enhances lymphangiogenesis via a disintegrin and metalloprotease with thrombospondin motifs-3-mediated vascular endothelial growth factor-C activation. Circulation 2014;129:1962–71. 10.1161/CIRCULATIONAHA.113.002779. [DOI] [PubMed] [Google Scholar]


