Abstract
Although it is well established that human cytochrome P450 1 family enzymes are induced by cigarette smoking through activation of the Ah receptor, it is not known whether this leads to increased metabolic activation or detoxification of carcinogenic polycyclic aromatic hydrocarbons (PAH), which are present in cigarette smoke and the general environment. We gave oral doses of deuterated phenanthrene ([D10]Phe), a non-carcinogenic surrogate of carcinogenic PAH such as benzo[a]pyrene, to smokers (N = 170, 1 or 10 μg doses) and non-smokers (N = 57, 1 μg dose). Bioactivation products (dihydrodiol and tetraol) and detoxification products (phenols) of [D10]Phe were determined in 6-h urine to obtain a comprehensive metabolic profile. Cigarette smoking increased the bioactivation of [D10]Phe and decreased its detoxification resulting in significantly different metabolic patterns between smokers and non-smokers (P < 0.01), consistent with increased cancer risk in smokers. The Phe bioactivation ratios ([D10]PheT/total [D9]OHPhe) were significantly higher (2.3 (P < 0.01) to 4.8 (P < 0.001) fold) in smokers than non-smokers. With solid human in vivo evidence, our results for the first time demonstrate that cigarette smoking enhances the metabolic activation of Phe, structurally representative of carcinogenic PAH, in humans, strongly supporting their causal role in cancers caused by smoking. The results suggest potential new methods for identifying smokers who could be at particularly high risk for cancer.
This study demonstrates for the first time that induction of CYP1 enzymes by cigarette smoking significantly enhances the bioactivation of PAH in humans, highlighting their role as causes of cancer and suggesting new approaches to cancer prevention.
Introduction
More than 50 years ago, Welch et al. investigated the metabolism of benzo[a]pyrene (BaP) to 3-hydroxyBaP in placenta from smokers and non-smokers, demonstrating for the first time that cigarette smoking induced the human metabolism of BaP, a prototypic highly carcinogenic polycyclic aromatic hydrocarbon (PAH), now considered “carcinogenic to humans” by the International Agency for Research on Cancer (1,2). Decades of subsequent research extended and refined this initial finding as summarized in Supplementary data, available at Carcinogenesis Online, but it is still not known whether the induction of human PAH metabolism by cigarette smoking results in increased PAH metabolic activation or detoxification. This question, addressed in the study reported here, is clearly critical to our understanding of mechanisms of lung cancer etiology by cigarette smoking, and to the potential causative role in human lung cancer of the multiple carcinogenic PAH in cigarette smoke.
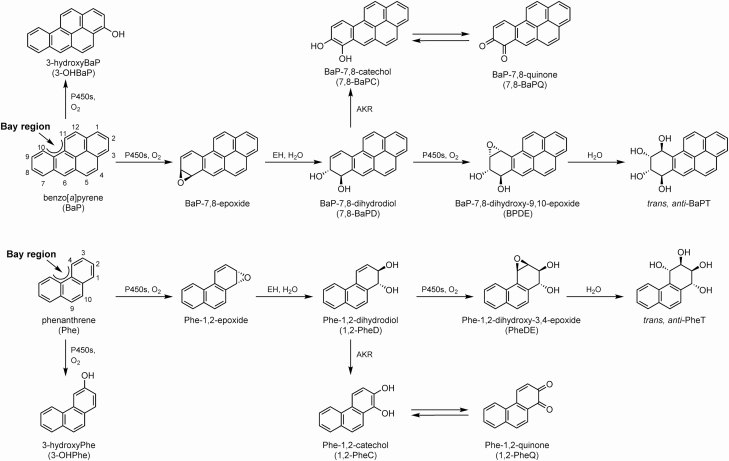
The metabolism of PAH is catalyzed by cytochromes P450 1A1, 1A2 and 1B1 (CYP1A1, CYP1A2 and CYP1B1), which are induced by cigarette smoking via activation of the Ah receptor and are involved in both the metabolic activation and detoxification of BaP and other PAH, as illustrated in Figure 1 for BaP and phenanthrene (Phe). A major pathway of metabolic activation proceeds through BaP-7,8-epoxide to the proximate carcinogen 7,8-BaPD, then to the ultimate carcinogen BPDE, which reacts with DNA to produce adducts that are critical in carcinogenesis by BaP because they cause mutations in the TP53 tumor suppressor gene and other critical genes (2,3). Redox cycling between BaP-7,8-catechol and BaP-7,8-quinone has also been associated with metabolic activation, whereas 3-hydroxyBaP (and other OHBaPs) are products of detoxification (4).
Figure 1.
Metabolism pathways of BaP and Phe to epoxides (BaP-7,8-epoxide and Phe-1,2-epoxide), dihydrodiols (7,8-BaPD and 1,2-PheD), diol epoxides (BPDE and PheDE), tetraols (trans,anti-BaPT and trans,anti-PheT), phenols (3-OHBaP and 3-OHPhe), catechols (7,8-BaPC and 1,2-PheC) and quinones (7,8-BaPQ and 1,2-PheQ). The major pathway of metabolic activation proceeds through the epoxides to the diols to the diol epoxides and is measured as 1,2-PheD and trans,anti-PheT while detoxification is measured as 3-OHPhe.
Extensive studies in laboratory animals have investigated the role of Cyp1a1, Cyp1a2 and Cyp1b1 in the metabolism of BaP in mice using genetic engineering tools (5–7). The results of these studies are complex and indicate that experimental design is a key factor and that these enzymes may have a more important role in carcinogen detoxification than activation in laboratory animals. For example, after reviewing studies involving targeted gene disruption methodologies, Reed et al. concluded that in vitro studies using hepatic enzyme systems showed that BaP was metabolically activated by cytochrome P450s, while studies in vivo indicated that hepatic P450 enzymes played a more pivotal role in BaP detoxification rather than in its activation (5).
Phe (Figure 1) is the simplest PAH with a bay region, a feature closely associated with carcinogenicity, but Phe, with only 3 aromatic rings, is noncarcinogenic (2). The metabolism of Phe closely resembles that of BaP, proceeding through 1,2-PheD and a bay region diol epoxide PheDE, which reacts with H2O to produce trans,anti-PheT (8,9). Phe is also metabolized to phenols (1-, 2-, 3-, 4-, 9-OHPhe) and quinones (1,2-; 3,4-; 9,10-PheQ) as illustrated (10,11).
In this study, we used a unique carcinogen phenotyping approach, in which smokers and non-smokers took a single oral dose of [D10]Phe. The use of [D10]Phe allowed us to profile critical metabolic pathways in smokers and non-smokers, without any interference from exposure to environmental Phe or its metabolites which are ubiquitous in the diet and polluted air among others. We have previously shown that orally administered [D10]Phe has a similar metabolic profile with respect to formation of trans,anti-PheT as [D10]Phe administered in a cigarette (8). Thus, we were able to determine the effect of cigarette smoking on the metabolic activation versus detoxification of [D10]Phe, as measured by the relative amounts of urinary [D10]trans,anti-PheT, [D10]1,2-PheD and total [D9]OHPhe in 170 smokers and 57 non-smokers.
Materials and methods
Chemicals
[D10]Phe (98%) was purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA). [D10]Phe for oral dosing was repurified in the University of Minnesota Molecular and Cellular Therapeutics GMP facility by normal-phase high-performance liquid chromatography followed by recrystallization from ethanol. It had >99% chemical purity and 98% isotopic purity. [D10]Phe oral dose solutions were prepared by dissolving 1 mg/4 mg of purified [D10]Phe (employing a balance that will accurately weigh 200 μg and an anti-static gun) in 1000 ml/400 ml of 100% ethanol and delivering 1-ml aliquots of the solution into amber dosing bottles at the Fairview Compounding Pharmacy (Minneapolis, MN), such that each bottle contained 1 μg/10 μg of [D10]Phe. All oral doses were stored at 4 °C before administration. On the day of dosing, 4 ml of drinking water was added to the amber dosing bottle, which was swirled to achieve a composition of 20% ethanol (this was necessary because storage in 20% ethanolic/80% H2O resulted in decreased concentrations of [D10]Phe).
Clinical study design
The study was approved by the U.S. Food and Drug Administration, Health Canada, and the University of Minnesota and Mayo Clinic Institutional Review Boards. Healthy volunteers including current cigarette smokers smoking at least 10 cigarettes per day for at least 1 year and non-smokers (age 18–65 years) were invited into the clinic for an orientation visit and informed consent was obtained. Eligible subjects must have been in good physical health (no history of aerodigestive cancer; no unstable medical condition such as liver, renal or cardiac dysfunction) and stable, good mental health (not currently or within the past 6 months experiencing unstable or untreated psychiatric diagnosis including substance abuse disorder as determined by the licensed medical professional). Subjects using tobacco or nicotine-containing products other than cigarettes greater than twice weekly, who were taking other investigational agents or medications that alter relevant metabolic enzymes, and/or women who were pregnant or nursing were also excluded.
One hundred thirty-five current smokers (65 females) received 10 μg (53.2 nmol) of [D10]Phe orally and another 35 current smokers (14 females) and 57 non-smokers (29 females) received 1 μg (5.32 nmol) of [D10]Phe orally once after fasting a minimum of 4 h to empty the stomach. We did not have regulatory permission to give 10 µg [D10]Phe to non-smokers. The smoking status of each subject was determined by questionnaire and confirmed by measurement of urinary cotinine. Those subjects who had ≥30 ng/ml of urinary cotinine were identified as current smokers (12). Urine collections were obtained from 0 to 6 h after dosing with [D10]Phe. Participants were encouraged to drink water during the collection period. The volume of the 6-h urine collection was measured, and aliquots (15 ml) of the urine samples were frozen at −20 °C until analysis. The collection period was based on our previous study demonstrating an excellent correlation between 6 and 48 h urinary excretion of [D10]PheT (r = 0.95, P < 0.001) (8). Thus analysis of 6-h urine is an accurate reflection of [D10]Phe metabolism in humans.
Determination of urinary metabolites
The analyses of [D10]PheT, [D10]PheD and [D9]OHPhe were performed essentially as described previously (8,13,14). Briefly, for the analysis of [D10]PheT, a 100 μL aliquot of urine containing 100 fmol [13C6]PheT as internal standard was incubated with β-glucuronidase (1050 units) and arylsulfatase (8400 units) overnight with shaking at 37 °C. After solid-phase extraction and silylation, the samples were analyzed by gas chromatography-negative chemical ionization-tandem mass spectrometry (GC-NCI-MS/MS). For the analysis of [D10]1,2-PheD, a 300 μL (10 μg dose group)/1 ml (1 μg dose group) aliquot of urine containing 5 pmol [13C6]1,2-PheD as internal standard was incubated with β-glucuronidase (1000 units) and arylsulfatase (8000 units) overnight with shaking at 37 °C. After solid-phase extraction and silylation, the samples were analyzed by GC-NCI-MS/MS. For the analysis of [D9]OHPhe ([D9]1-, 2-, 3-, 4- and 9-OHPhe), a 3-ml aliquot of urine containing 1 ng [13C6]3-OHPhe as internal standard was incubated with β-glucuronidase (2000 units) and arylsulfatase (16 000 units) overnight with shaking at 37 °C. After solid-phase extraction and silylation, the samples were analyzed by GC-electron impact (EI)-MS/MS with a TSQ Quantum instrument (Thermo Scientific, San Jose, CA). [D8]Phe ortho-quinones ([D8]PheQ) including [D8]1,2-PheQ, [D8]3,4-PheQ and [D8]9,10-PheQ were also determined in the urine of 50 subjects (16 1-μg dose group non-smokers, 9 1-μg dose group smokers and 25 10-μg dose group smokers) by LC-ESI-MS/MS using the method described previously (10). Typical GC/LC-MS/MS chromatograms of [D10]Phe metabolites and corresponding 13C-labeled internal standards are shown in Figure S1 (see Supplementary data, available at Carcinogenesis Online).
Statistical analysis
For continuous variables, the Wilcoxon rank sum test was used since they were not normally distributed. To compare the pie charts, a multivariate test for compositional data was used (15,16) because the percentages in each pie chart are linearly correlated (i.e. the sum of the three percentages is 100%) and can be regarded as compositional data. A value of P < 0.05 was considered statistically significant. The data were analyzed by SPSS Version 26.0 for Windows (IBM Inc., Armonk, NY) and R package Compositional Version 3.9 under R Version 4.0.0.
Results
Smokers received doses of either 1 or 10 µg of [D10]Phe, while non-smokers received only 1 µg doses of [D10]Phe, due to regulatory limitations. To determine the possible effect of the amount of an oral dose of [D10]Phe on the metabolic pattern, five smokers took two different oral doses of [D10]Phe (1 or 10 μg) 1-year apart. The percentages of [D10]1,2-PheD (P = 0.55), [D10]PheT (P = 0.31), total [D9]OHPhe (P = 0.15) as well as the compositional test (P = 0.35) were not significantly different between the two doses in these 5 subjects, confirming that these different oral doses of [D10]Phe did not affect the metabolic pattern in the same person. Thus, comparison of the metabolic patterns of [D10]Phe between the 1 μg dose in non-smokers and the 10 μg dose in smokers was valid.
Concentrations of urinary [D10]Phe metabolites (nmol/6-h urine) are summarized in Table 1. All the [D10]Phe metabolites in the 10 µg dose group were significantly higher than in the 1 µg dose group (P < 0.01) as they received different oral doses of [D10]Phe. Thus, the mean values of total [D10]Phe metabolites (∑([D10]1,2-PheD, [D10]PheT and total [D9]OHPhe)) in the 10 µg dose group (9.94 nmol/6-h urine) were 12.7 and 14.8 times higher than that in the 1 µg dose non-smokers (0.78 nmol/6-h urine, P < 0.001) and smokers (0.67 nmol/6-h urine, P < 0.001). Although there were no significant differences between the 1 µg dose non-smokers and smokers (P = 0.472) in total [D10]Phe metabolites, the concentration of total [D9]OHPhe in the 1 µg dose smokers (0.18 nmol/6-h urine) was significantly lower than that in non-smokers (0.28 nmol/6-h urine, P < 0.01), indicating cigarette smoke affected the metabolic pattern, as further shown below. The concentrations of total [D8]PheQ were 0.0044 ± 0.0079 (mean ± SD) nmol/6-h urine and 0.072 ± 0.11 nmol/6-h urine in the 1 µg dose subjects (N = 25) and 10 µg dose subjects (N = 25), respectively, accounting for <1% of total deuterated Phe metabolites. The average 6-h conversion rate (6-h total [D10]Phe metabolites (nmol)/oral dose of [D10]Phe (nmol)) were 14.7 % in the 1 µg dose non-smokers, 12.7% in the 1 µg dose smokers and 18.7% in the 10 µg dose smokers. Our earlier study demonstrated that levels of [D10]Phe metabolites at 6 h were about half the amount excreted in 48 h (8).
Table 1.
Concentrations of [D10]Phe metabolites in 6-h urine (nmol/6-h urine)
| [D10]Phe metabolites | ||||
|---|---|---|---|---|
| [D10]1,2-PheD | [D10]PheT | Total [D9]OHPhe | ||
| Non-smokers (1 μg dose, N = 57) | Mean (range) | 0.39 (ND ~1.61) | 0.11 (0.02–0.25) | 0.28 (0.05–1.55) |
| SD | 0.40 | 0.06 | 0.23 | |
| CV (%) | 102 | 52.0 | 80.8 | |
| Median (25th, 75th) | 0.23 (0.12, 0.44) | 0.11 (0.07, 0.14) | 0.24 (0.15, 0.34) | |
| Smokers (1 μg dose, N = 35) | Mean (range) | 0.36 (ND ~1.29) | 0.14 (0.003–0.29) | 0.18 a(ND ~0.68) |
| SD | 0.33 | 0.07 | 0.14 | |
| CV (%) | 90.2 | 55.2 | 77.5 | |
| Median (25th, 75th) | 0.23 (0.14, 0.56) | 0.14 (0.07, 0.17) | 0.16 (0.07, 0.24) | |
| Smokers (10 μg dose, N = 135) | Mean (range) | 6.55 a(ND ~28.63) | 2.26 a(ND ~7.75) | 1.13 a(ND ~6.41) |
| SD | 4.37 | 1.67 | 0.75 | |
| CV (%) | 66.7 | 73.9 | 66.7 | |
| Median (25th, 75th) | 5.49 (3.29, 9.33) | 1.82 (0.88, 3.38) | 1.01 (0.65, 1.47) | |
aBolded values, P < 0.01 compared with non-smokers, Wilcoxon rank-sum test.
CV, coefficient of variation.
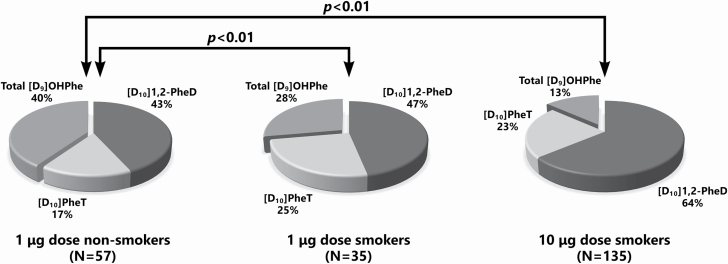
The percentages of each metabolite of the total [D10]Phe metabolites quantified are presented in Table 2 and Figure 2. The composition of the metabolite pattern was significantly different in smokers and non-smokers for [D10]Phe metabolites ((P < 0.01). This was most clearly evident when comparing smokers (10 µg dose, N = 135) and non-smokers (1 µg dose, N = 57) in Figure 2. Considering the individual metabolites, the percentages of the bioactivation end products [D10]PheT were significantly higher in smokers (P = 0.02 in 1 µg dose smokers and P < 0.01 in 10 µg dose smokers) than non-smokers (P < 0.01). As for [D10]1,2-PheD, an intermediate metabolite in the bioactivation pathway, its percentage was higher in all the smokers than non-smokers while the difference was statistically significant in the 10 µg dose smokers versus 1 µg dose non-smokers (P < 0.01). The percentage of total [D9]OHPhe, representing detoxification, was lower in all current smokers than non-smokers (P < 0.01). Males and females had similar non-smoker versus smoker effects on the metabolic pattern (see Supplementary data Table 1, available at Carcinogenesis Online). The Phe bioactivation ratios (PBR): [D10]PheT/total [D9]OHPhe were significantly different in smokers and non-smokers as shown in Table 3 and Figure 3.
Table 2.
Percentage of each metabolite of the total [D10]Phe metabolites
| Non-smokers (1 μg dose, N = 57) | Smokers (1 μg dose, N = 35) | Smokers (10 μg dose, N = 135) | |||
|---|---|---|---|---|---|
| Mean % (SD) | Mean % (SD) | P-valuea | Mean % (SD) | P-valuea | |
| [D10]1,2-PheD | 43.15 (23.35) | 46.74 (19.73) | 0.35 | 63.89 (14.95) | <0.01 |
| [D10]PheT | 16.52 (10.18) | 25.48 (16.61) | 0.02 | 22.76 (13.46) | <0.01 |
| Total [D9]OHPhe | 40.32 (20.46) | 27.78 (13.89) | <0.01 | 13.35 (9.58) | <0.01 |
| Compositionalb | P-value < 0.01 | P-value < 0.01 | |||
aBolded values, statistically significant compared to non-smokers, using the Wilcoxon rank-sum test for each percentage.
bThe test for compositional data was used, as in the pie charts of Figure 2.
Figure 2.
Pie charts representing the pattern of metabolites of [D10]Phe in smokers and non-smokers.
Table 3.
PBR in smokers and non-smokers
| Non-smokers (1 μg dose [D10]Phe) | Smokers (1 μg dose[D10]Phe) | Smokers (10 μg dose[D10]Phe) | |
|---|---|---|---|
| PBR ([D10]PheT/[D9]3-OHPhe) | 2.05 | 3.56a | 3.92c |
| PBR ([D10]PheT/total [D9]OHPhe) | 0.40 | 0.91b | 1.91c |
a P < 0.05.
b P < 0.01.
c P < 0.001 compared with non-smokers. Mann–Whitney U-test was used since the data were not normally distributed.
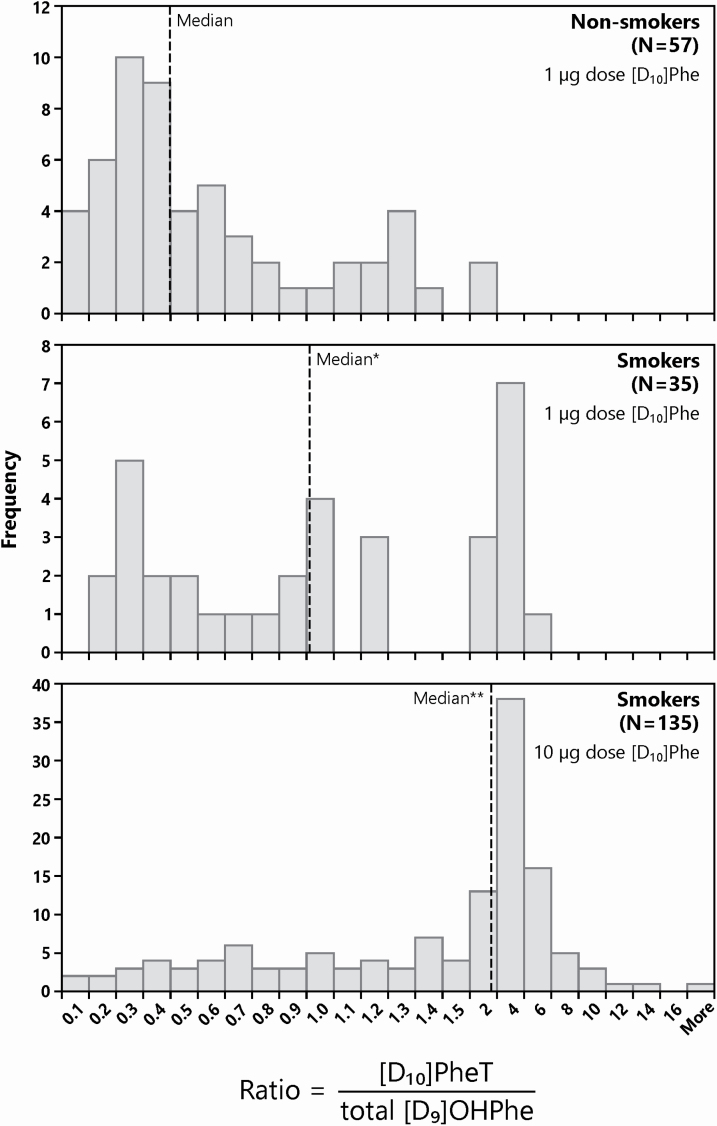
Figure 3.
Histograms of the Phe bioactivation ratios (PBR: ratios of [D10]PheT to total [D9]OHPhe) in smokers and non-smokers. *P < 0.01; **P < 0.001 compared with non-smokers. Mann–Whitney U-test was used since the data were not normally distributed.
Non-deuterated Phe metabolites were also simultaneously detected. For each of the non-deuterated Phe metabolites, the mean urinary concentrations in smokers were 1.61- to 3.56-fold higher than those in non-smokers (all P < 0.01 except for 1,2-PheD). The mean values of total non-deuterated Phe metabolites (∑(1,2-PheD, PheT and total OHPhe)) in the 1 µg dose group of smokers (5.36 nmol/6-h urine, P < 0.01) and 10 µg dose group of smokers (5.98 nmol/6-h urine, P < 0.001) were also significantly higher than that in non-smokers (2.89 nmol/6-h urine). This result indicates that Phe metabolites are reliable and effective biomarkers of human exposure to PAH in cigarette smoke, generally consistent with previous studies (9,14,17).
Discussion
In this study, we used a novel approach featuring [D10]Phe as an indicator of human PAH metabolism. The use of [D10]Phe eliminates possible complications from environmental exposure to metabolites of Phe, a ubiquitous PAH. In our previous study, we demonstrated that the pharmacokinetics of [D10]Phe administered to smokers in a cigarette were essentially identical to [D10]Phe given orally to the same subjects (8). Thus, oral administration of [D10]Phe, as in the present study, reflects exposure to PAH in cigarette smoke, even though the oral dose is likely metabolized in the liver by CYP1A2 while the smoking dose is metabolized in the lung by CYP1A1 and CYP1B1. All three enzymes are induced by cigarette smoking (18–20).
The results of this unique study demonstrate for the first time in humans that the induction of CYP1A1, 1A2 and 1B1 by cigarette smoking via the Ah receptor, a phenomenon that has been known and investigated for more than 50 years, causes an increase in the metabolic activation of Phe, as clearly shown in the metabolic profile of [D10]Phe (Figure 2). The structural similarity of Phe and BaP, a highly carcinogenic PAH considered “carcinogenic to humans” by the IARC, and the close relationship of their metabolic pathways as shown in our previous studies, support the conclusion that cigarette smoking increases the metabolic activation of carcinogenic PAH in humans, resulting in increased cancer risk, as discussed further below. While the increased exposure to PAH in smokers compared with non-smokers has been repeatedly demonstrated (9,10,14,17), the increased metabolic activation of these PAH in smokers arguably increases cancer risk and supports the critical role of these potent carcinogens as causes of lung cancer and other PAH-related cancers in smokers.
We developed methods to panoramically characterize [D10]Phe metabolites in human urine (8,10,13,14). The use of Phe metabolites has inherent advantages: it has a bay region—a feature closely associated with carcinogenicity, its metabolism is very similar to that of BaP while its metabolite concentrations in human urine are ~ 10 000 times higher than those of BaP, making it much more practical for large studies. A unique superiority of the [D10]Phe metabolite phenotyping approach is that it integrates all human enzymatic and genetic factors that impact carcinogenic PAH metabolism without any interference from environmental exposure by giving a precise dose to the subjects. Non-deuterated Phe metabolites detected in human urine can be influenced by extraneous environmental factors such as dietary intake. Hydroxyphenanthrenes (mainly 2-OHPhe and 3-OHPhe) and some other metabolites like epoxides, ortho-quinones and glucuronide conjugates have been identified from cow/goat milk, marine products (fish and crab) and egg yolk (21–23). Consumption of these animal products could lower the credibility of results of non-deuterated Phe in human in vivo metabolism analysis.
PAH such as BaP requires metabolic activation to exert their carcinogenic effects. Thus, cancer risk may be related to the metabolic imbalance between bioactivation and detoxification pathways. Differences in individual susceptibility to the adverse effects of carcinogenic PAHs may, in part, be due to differences in the metabolic enzymes, especially CYP enzymes. The CYP1 enzymes—CYP1A1, CYP1A2 and CYP1B1—are responsible for both metabolically activating and detoxifying BaP (24–27). This raises the question: is the induction of CYP1 enzymes more important in bioactivation or detoxification of carcinogenic PAH in humans? Previously, animal research and human in vitro studies produced inconsistent results. The Ah receptor-mediated induction of CYP1A1 was found to be related to both the enhancement and suppression of BaP-DNA adduct formation in mouse/rat organs (6,28–33) and human cells (34–39). The biggest limitation of these studies is that they cannot precisely simulate the complex situation in vivo in humans. Thus, these previous paradoxical results can hardly be used in extrapolation to describe and predict the authentic role of CYP1 enzymes in PAH carcinogenesis and cancer susceptibility in humans due to the lack of direct evidence from human in vivo research. Although the relationship between CYP1 enzyme induction and increased cancer risk has been proposed (26,40,41), no previous studies have precisely examined the consequences of induction of CYP 1A1, 1A2 and 1B1 with respect to bioactivation versus detoxification of carcinogenic PAH in humans. From the metabolic perspective, the relationship between induction of CYP1 enzymes and human cancer risk was still unclear.
In this study, we characterized urinary [D10]Phe metabolite profiles in cigarette smokers and non-smokers. Cigarette smoke is a strong inducer of CYP1 enzymes. It has been reported that the CYP1A1 enzyme activity is induced by cigarette smoke up to 100-fold in human lung (19,42,43). The inducing effects of smoking on CYP1A2 and CYP1B1 activity in humans have also been confirmed (20,44). We found that bioactivation was the dominant pathway induced in the metabolism of Phe in smokers. PheT, the bioactivation end product of Phe, was significantly and positively correlated with BaP-tetraol in our previous studies (45) and was significantly related to increased lung cancer risk in humans (46). In this study, smokers not only had a significantly higher proportion of activation products ([D10]1,2-PheD and [D10]PheT, P < 0.01), but also a significantly lower proportion of detoxification products (total [D9]OHPhe, P < 0.01) with a significant difference in the whole distribution pattern compared with non-smokers (P < 0.01; Figure 2). This is the first solid human in vivo evidence to demonstrate that induction of CYP1 enzymes by cigarette smoke contribute significantly to the metabolic activation of PAH. As critical drivers in the bioactivation pathway, variations of CYP1 enzyme activity must play an important role in individual differences in carcinogenic PAH metabolism and cancer susceptibility. These variations can be summarized in the PBR, the ratio of the metabolic activation end product [D10]PheT to the detoxification end products total [D9]OHPhe. These two end products succinctly summarize the comprehensive effects of P450s on bioactivation and detoxification pathways. The PBR could be characteristic of a given individual’s bioactivation capability of carcinogenic PAH. We observed a large interindividual variation among the subjects in their PBR: the PBR varied by as much as 23-fold among 1 µg dose non-smokers, 32-fold among 1 µg dose current smokers and 271-fold among 10 µg dose current smokers. This approach allows us to directly and accurately assess in vivo interindividual variation in PAH metabolism in humans. The PBR also represents the relative dominance of one pathway over the other and may be modifiable by the induction of CYP1 enzymes. In this study, PBR were 2.28- (P < 0.01) to 4.78 (P < 0.001)-fold higher in smokers than that in non-smokers (Table 3 and Figure 3). Our previous studies of non-deuterated Phe metabolites have shown that this ratio is induced by cigarette smoking, consistent with the present results. However, as noted above, those results might have been compromised by environmental or dietary exposure to Phe metabolites (21–23). We propose that the PBR can be used to identify cigarette smokers and ex-smokers who are at high risk for lung cancer and other tobacco-related cancers. The PBR can be readily determined by analysis of a urine sample 6 h after a dose of 1 µg [D10]Phe as in this study. This could be particularly important for ex-smokers who remain at high risk for lung cancer for years after quitting. A high PBR would signal immediate participation in a lung cancer screening program to detect cancer at a relatively early stage while it is still amenable to treatment.
The formation of ortho-quinones and reactive oxygen species has been proposed as an important bioactivation pathway of carcinogenic PAH such as BaP (47,48). BaP-7,8-dione can not only generate reactive oxygen species resulting in oxidative DNA damage but can also react with DNA to form stable B[a]P-7,8-dione-DNA adducts (47,49,50). The results obtained upon analysis of the urine of 50 subjects in this study indicate that total [D8]PheQs make up only a small percentage (<1%) of [D10]Phe metabolites. In contrast, our previous study found that unlabeled total PheQs can reach 14.5% of unlabeled Phe metabolites in humans (10). These results indicate that most of the PAH ortho-quinones in human urine originate from external exposures in the environment but not internal metabolism of parent PAH. Thus, [D8]PheQs are not further discussed in the human bioactivation studies described here.
One limitation of the study was that, while we confirmed current cigarette smoking or non-smoking status by analysis of urinary cotinine, we could not positively identify former smokers and had to rely on questionnaire data. It is possible that some subjects identified as non-smokers were actually former smokers, and that the induction of cytochrome P450s may have persisted into the time frame of the current study. This would tend to decrease our observed effects by potentially increasing the PBR in non-smokers. The persistence of induction of CYP enzymes by smoking is unclear and requires further study.
Conclusion
The results presented in this study demonstrate that cigarette smoking increases the metabolic activation of Phe, a structurally representative PAH, via the well-established induction of CYP1 enzymes. With solid human in vivo evidence, this study for the first time revealed that cigarette smoking raises a dual risk of carcinogenic PAH in humans: both higher exposure and higher metabolic activation. The results of this study significantly improve our understanding of PAH metabolism in cigarette smokers and are applicable to investigations of lung cancer susceptibility and prevention, potentially through the simple measurement of the urinary PBR.
Supplementary Material
Acknowledgement
We thank Bob Carlson for his editorial assistance.
Glossary
Abbreviations
- 3-OHBaP
3-hydroxyBaP
- CYP1
cytochrome P450 1
- BaP
benzo[a]pyrene
- PAH
polycyclic aromatic hydrocarbons
- PBR
Phe bioactivation ratios
- Phe
phenanthrene
Funding
This study was supported by P01-CA-138338 from the U.S. National Cancer Institute. Mass spectrometry was carried out in the Analytical Biochemistry Shared Resource and statistical analysis in the Biostatistics Shared Resource of the Masonic Cancer Center, University of Minnesota, supported in part by Cancer Center Support Grant CA-77598. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
Conflict of Interest Statement: None declared.
References
- 1. Welch, R.M., et al. (1968) Cigarette smoking: stimulatory effect on metabolism of 3,4-benzpyrene by enzymes in human placenta. Science, 160, 541–542. [DOI] [PubMed] [Google Scholar]
- 2. International Agency for Research on Cancer. (2010) Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, v. 92. IARC, Lyon, FR, pp. 35–818. [PMC free article] [PubMed] [Google Scholar]
- 3. Pfeifer, G.P., et al. (2002) Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene, 21, 7435–7451. [DOI] [PubMed] [Google Scholar]
- 4. Penning, T.M. (2014) Human aldo-keto reductases and the metabolic activation of polycyclic aromatic hydrocarbons. Chem. Res. Toxicol., 27, 1901–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reed, L., et al. (2018) The role of cytochrome P450 enzymes in carcinogen activation and detoxication: an in vivo-in vitro paradox. Carcinogenesis, 39, 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shiizaki, K., et al. (2017) Modulation of benzo[a]pyrene-DNA adduct formation by CYP1 inducer and inhibitor. Genes Environ., 39, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nebert, D.W., et al. (2013) Oral benzo[a]pyrene: understanding pharmacokinetics, detoxication, and consequences–Cyp1 knockout mouse lines as a paradigm. Mol. Pharmacol., 84, 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhong, Y., et al. (2011) Metabolism of [D10]phenanthrene to tetraols in smokers for potential lung cancer susceptibility assessment: comparison of oral and inhalation routes of administration. J. Pharmacol. Exp. Ther., 338, 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hecht, S.S., et al. (2005) Longitudinal study of urinary phenanthrene metabolite ratios: effect of smoking on the diol epoxide pathway. Cancer Epidemiol. Biomarkers Prev., 14, 2969–2974. [DOI] [PubMed] [Google Scholar]
- 10. Luo, K., et al. (2020) Identification and quantification of phenanthrene ortho-quinones in human urine and their association with lipid peroxidation. Environ. Pollut., 266(Pt 1), 115342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patel, Y.M., et al. (2016) Metabolites of the polycyclic aromatic hydrocarbon phenanthrene in the urine of cigarette smokers from five ethnic groups with differing risks for lung cancer. PLoS One, 11, e0156203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim, S. (2016) Overview of cotinine cutoff values for smoking status classification. Int. J. Environ. Res. Public Health, 13, 1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hecht, S.S., et al. (2013) Longitudinal study of [D10]phenanthrene metabolism by the diol epoxide pathway in smokers. Biomarkers, 18, 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luo, K., et al. (2020) Quantitation of phenanthrene dihydrodiols in the urine of smokers and non-smokers by gas chromatography-negative ion chemical ionization-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci., 1141, 122023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. James, G.S. (1954) Tests of linear hypotheses in univariate and multivariate analysis when the ratios of the population variances are unknown. Biometrika, 41, 19–43. [Google Scholar]
- 16. Aitchison, J. (1982) The statistical-analysis of compositional data. J. R. Stat. Soc. Ser. B Methodol., 44, 139–177. [Google Scholar]
- 17. U.S. Department of Health and Human Services, C.D.C. (2019) Fourth report on human exposure to environmental chemicals, Updated tables, January 2019, Vol. 2. https://www.cdc.gov/exposurereport/index.html. [Google Scholar]
- 18. Plowchalk, D.R., et al. (2012) Prediction of drug clearance in a smoking population: modeling the impact of variable cigarette consumption on the induction of CYP1A2. Eur. J. Clin. Pharmacol., 68, 951–960. [DOI] [PubMed] [Google Scholar]
- 19. McLemore, T.L., et al. (1990) Expression of CYP1A1 gene in patients with lung cancer: evidence for cigarette smoke-induced gene expression in normal lung tissue and for altered gene regulation in primary pulmonary carcinomas. J. Natl. Cancer Inst., 82, 1333–1339. [DOI] [PubMed] [Google Scholar]
- 20. Port, J.L., et al. (2004) Tobacco smoke induces CYP1B1 in the aerodigestive tract. Carcinogenesis, 25, 2275–2281. [DOI] [PubMed] [Google Scholar]
- 21. Sette, C.B., et al. (2013) Formation and identification of PAHs metabolites in marine organisms. Mar. Environ. Res., 91, 2–13. [DOI] [PubMed] [Google Scholar]
- 22. Fournier, A., et al. (2010) Transfer kinetics to egg yolk and modeling residue recovered in yolk of readily metabolized molecules: polycyclic aromatic hydrocarbons orally administered to laying hens. Chemosphere, 78, 1004–1010. [DOI] [PubMed] [Google Scholar]
- 23. Lapole, D., et al. (2007) Milk and urine excretion of polycyclic aromatic hydrocarbons and their hydroxylated metabolites after a single oral administration in ruminants. J. Dairy Sci., 90, 2624–2629. [DOI] [PubMed] [Google Scholar]
- 24. Uppstad, H., et al. (2010) Importance of CYP1A1 and CYP1B1 in bioactivation of benzo[a]pyrene in human lung cell lines. Toxicol. Lett., 192, 221–228. [DOI] [PubMed] [Google Scholar]
- 25. Gelboin, H.V. (1980) Benzo[a]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol. Rev., 60, 1107–1166. [DOI] [PubMed] [Google Scholar]
- 26. Nebert, D.W., et al. (2004) Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J. Biol. Chem., 279, 23847–23850. [DOI] [PubMed] [Google Scholar]
- 27. Shimada, T., et al. (2004) Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci., 95, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu, Q., et al. (2008) Differences in gene expression and benzo[a]pyrene-induced DNA adduct formation in the liver of three strains of female mice with identical AhRb2 genotype treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin and/or benzo[a]pyrene. J. Appl. Toxicol., 28, 724–733. [DOI] [PubMed] [Google Scholar]
- 29. Harrigan, J.A., et al. (2004) DNA adduct formation in precision-cut rat liver and lung slices exposed to benzo[a]pyrene. Toxicol. Sci., 77, 307–314. [DOI] [PubMed] [Google Scholar]
- 30. Hodek, P., et al. (2013) The relationship between DNA adduct formation by benzo[a]pyrene and expression of its activation enzyme cytochrome P450 1A1 in rat. Environ. Toxicol. Pharmacol., 36, 989–996. [DOI] [PubMed] [Google Scholar]
- 31. Revel, A., et al. (2003) Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects lung from DNA damage and apoptosis caused by benzo[a]pyrene. J. Appl. Toxicol., 23, 255–261. [DOI] [PubMed] [Google Scholar]
- 32. Vanhees, K., et al. (2012) Maternal intake of quercetin during gestation alters ex vivo benzo[a]pyrene metabolism and DNA adduct formation in adult offspring. Mutagenesis, 27, 445–451. [DOI] [PubMed] [Google Scholar]
- 33. Shimizu, Y., et al. (2000) Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA, 97, 779–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Waard, P.W.J., et al. (2008) Influence of TCDD and natural Ah receptor agonists on benzo[a]pyrene-DNA adduct formation in the Caco-2 human colon cell line. Mutagenesis, 23, 67–73. [DOI] [PubMed] [Google Scholar]
- 35. Gelhaus, S.L., et al. (2011) Regulation of benzo[a]pyrene-mediated DNA- and glutathione-adduct formation by 2,3,7,8-tetrachlorodibenzo-p-dioxin in human lung cells. Chem. Res. Toxicol., 24, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shiizaki, K., et al. (2013) Dioxin suppresses benzo[a]pyrene-induced mutations and DNA adduct formation through cytochrome P450 1A1 induction and (±)-anti-benzo[a]pyrene-7,8-diol-9,10-epoxide inactivation in human hepatoma cells. Mutat. Res., 750, 77–85. [DOI] [PubMed] [Google Scholar]
- 37. Chang, K.W., et al. (1999) Differential response to benzo[a]pyrene in human lung adenocarcinoma cell lines: the absence of aryl hydrocarbon receptor activation. Life Sci., 65, 1339–1349. [DOI] [PubMed] [Google Scholar]
- 38. Takemura, H., et al. (2010) Inhibitory effects of chrysoeriol on DNA adduct formation with benzo[a]pyrene in MCF-7 breast cancer cells. Toxicology, 274, 42–48. [DOI] [PubMed] [Google Scholar]
- 39. Kang, Z.C., et al. (1999) Quercetin inhibits benzo[a]pyrene-induced DNA adducts in human Hep G2 cells by altering cytochrome P-450 1A1 gene expression. Nutr. Cancer, 35, 175–179. [DOI] [PubMed] [Google Scholar]
- 40. Guengerich, F.P. (2008) Cytochrome p450 and chemical toxicology. Chem. Res. Toxicol., 21, 70–83. [DOI] [PubMed] [Google Scholar]
- 41. Ioannides, C., et al. (1993) Induction of cytochrome P4501 as an indicator of potential chemical carcinogenesis. Drug Metab. Rev., 25, 485–501. [DOI] [PubMed] [Google Scholar]
- 42. Anttila, S., et al. (2003) Methylation of cytochrome P4501A1 promoter in the lung is associated with tobacco smoking. Cancer Res., 63, 8623–8628. [PubMed] [Google Scholar]
- 43. Dörrenhaus, A., et al. (2007) Increased CYP1A1 expression in human exfoliated urothelial cells of cigarette smokers compared to non-smokers. Arch. Toxicol., 81, 19–25. [DOI] [PubMed] [Google Scholar]
- 44. Boyle, J.O., et al. (2010) Effects of cigarette smoke on the human oral mucosal transcriptome. Cancer Prev. Res. (Phila.), 3, 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hochalter, J.B., et al. (2011) Quantitation of a minor enantiomer of phenanthrene tetraol in human urine: correlations with levels of overall phenanthrene tetraol, benzo[a]pyrene tetraol, and 1-hydroxypyrene. Chem. Res. Toxicol., 24, 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yuan, J.M., et al. (2014) Urinary metabolites of a polycyclic aromatic hydrocarbon and volatile organic compounds in relation to lung cancer development in lifelong never smokers in the Shanghai Cohort Study. Carcinogenesis, 35, 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Penning, T.M., et al. (1999) Dihydrodiol dehydrogenases and polycyclic aromatic hydrocarbon activation: generation of reactive and redox active o-quinones. Chem. Res. Toxicol., 12, 1–18. [DOI] [PubMed] [Google Scholar]
- 48. Bolton, J.L., et al. (2000) Role of quinones in toxicology. Chem. Res. Toxicol., 13, 135–160. [DOI] [PubMed] [Google Scholar]
- 49. Flowers-Geary, L., et al. (1996) Cytotoxicity and mutagenicity of polycyclic aromatic hydrocarbon ortho-quinones produced by dihydrodiol dehydrogenase. Chem. Biol. Interact., 99, 55–72. [DOI] [PubMed] [Google Scholar]
- 50. Huang, M., et al. (2013) Identification of stable benzo[a]pyrene-7,8-dione-DNA adducts in human lung cells. Chem. Res. Toxicol., 26, 685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.