Abstract
The default mode network (DMN) and dorsal attention network (DAN) demonstrate an intrinsic “anticorrelation” in healthy adults, which is thought to represent the functional segregation between internally and externally directed thought. Reduced segregation of these networks has been proposed as a mechanism for cognitive deficits that occurs in many psychiatric disorders, but this association has rarely been tested in pre-adolescent children. The current analysis used data from the Adolescent Brain Cognitive Development study to examine the relationship between the strength of DMN/DAN anticorrelation and psychiatric symptoms in the largest sample to date of 9- to 10-year-old children (N = 6543). The relationship of DMN/DAN anticorrelation to a battery of neuropsychological tests was also assessed. DMN/DAN anticorrelation was robustly linked to attention problems, as well as age, sex, and socioeconomic factors. Other psychiatric correlates identified in prior reports were not robustly linked to DMN/DAN anticorrelation after controlling for demographic covariates. Among neuropsychological measures, the clearest correlates of DMN/DAN anticorrelation were the Card Sort task of executive function and cognitive flexibility and the NIH Toolbox Total Cognitive Score, although these did not survive correction for socioeconomic factors. These findings indicate a complicated relationship between DMN/DAN anticorrelation and demographics, neuropsychological function, and psychiatric problems.
Keywords: anticorrelation, default mode network, dorsal attention network, functional connectivity, attention deficit hyperactivity disorder
Introduction
Containing the medial prefrontal cortex, the posterior cingulate cortex, the temporoparietal junction, and the lateral and medial temporal lobes, the default mode network (DMN) was first identified through its deactivation during active functional magnetic resonance imaging (fMRI) tasks but has become studied in its own right through resting fMRI and task fMRI (Buckner and DiNicola 2019). The DMN is activated during spontaneous cognition (i.e., “rest”) and during many internally focused tasks (Buckner et al. 2008; Andrews-Hanna, Reidler, Huang, et al. 2010; Buckner and DiNicola 2019). It is also activated during externally focused tasks that require recall of internally stored information such as memories (Spreng et al. 2014; Crittenden et al. 2015; Konishi et al. 2015; Murphy et al. 2018; Smith et al. 2018), externally focused tasks requiring rule switching (Crittenden et al. 2015; Smith et al. 2018), and during task transitions (Smith et al. 2018). This has led to hypotheses that the DMN is critical in internally directed cognitions such as recalling memories or imagining future events (Andrews-Hanna, Reidler, Huang, et al. 2010) and that it is critical to encoding environmental context (Smith et al. 2018).
In contrast, the dorsal attention network (DAN), made up of the frontal eye fields and inferior parietal sulcus, is thought to be involved in top-down visual attention (Fox et al. 2006; Vossel et al. 2014). The DAN is sometimes grouped together with the frontoparietal network that includes the dorsolateral prefrontal cortex, supplementary motor area, and insula (i.e., cognitive control network; Cole and Schneider 2007; Niendam et al. 2012) to form what is sometimes called the task-positive network (Fox et al. 2005). However, most network parcellation schemes consider it to be a distinct neural network (Power et al. 2011; Yeo et al. 2015; Gordon et al. 2016). Additionally, the DAN is distinguishable from the ventral attention network, made up of the ventrolateral prefrontal cortex and temporoparietal junction, which engages stimulus-driven (or bottom-up) attention (Vossel et al. 2014). It is also distinct from the cingulo-opercular network and salience networks, which are made up of the caudal anterior cingulate and anterior insula and are often combined into a single network. However, some work has suggested that these networks are separable and fulfill distinct roles (Power et al. 2011).
The relationship between the DMN and DAN has become an active area of study. The time series of these two networks shows a negative correlation during resting fMRI, which has been termed “anticorrelation” (Greicius et al. 2003; Fox et al. 2005). Additionally, the degree to which the DAN becomes more active and the DMN becomes less active during an externally oriented task represents another index of DMN/DAN anticorrelation. This anticorrelation between the DMN and DAN has been identified in children as young as 1 year of age (Gao et al. 2013), increases as children and adolescents mature (Anderson et al. 2011), and decreases into older adulthood (Spreng et al. 2016). DMN/DAN anticorrelation has been proposed to represent the functional segregation of these two networks (Fox et al. 2005), and it has been suggested that it may be moderated by the frontoparietal (Gao and Lin 2012) or salience/cingulo-opercular networks (Goulden et al. 2014). Anticorrelation of the DMN and DAN is considered to be a helpful property of the brain, as appropriate segregation of these networks allows for more optimal allocation of mental resources and, consequently, superior neurocognitive performance (Kelly et al. 2008). In other words, in most cases, it is ideal for just one of these networks to be active at a time. Activation of the DMN during an externally oriented task has been suggested to represent an indicator of attention lapse (Weissman et al. 2006), which is consistent with reports that less DMN deactivation is related to poorer performance on demanding cognitive tasks (Kelly et al. 2008; Owens et al. 2018). One interpretation of these findings is that less DMN deactivation may represent difficulty disengaging from internal, self-generated thoughts impacting one’s ability to attend to the physical environment, as the DMN has been previously linked to mind wandering (Mason et al. 2007) and daydreaming (Kucyi and Davis 2014). Notably, DMN activation can be helpful during certain tasks that require functions subserved by the DMN. For example, DMN activation has been seen during tasks requiring recall of semantic memories (Spreng et al. 2014; Konishi et al. 2015) and rule switching (Crittenden et al. 2015; Smith et al. 2018), and activation in the DMN has been positively linked to performance on a target detection task with unpredictable experimental conditions (Hahn et al. 2007). However, these tasks seem to reflect exceptions to the broader rule of DMN activation (i.e., failure to deactivate) being negative for task performance.
Consistent with the assumption that DMN/DAN anticorrelation represents a feature of healthy neural connectivity, reduced DMN/DAN anticorrelation (i.e., reduced negative correlation between these networks) has been linked to numerous neurological and psychiatric disorders such as schizophrenia (Hu et al. 2017; Whitfield-Gabrieli et al. 2018), attention-deficit hyperactivity disorder (ADHD; Sun et al. 2012), conduct disorder and psychopathic traits (Pu et al. 2017), major depression (Posner et al. 2016), post-traumatic stress disorder (Patriat et al. 2016), and Alzheimer’s disease (Wang et al. 2007). Disrupted DMN/DAN anticorrelation has been induced in healthy adults by sleep deprivation (De Havas et al. 2012), and it has been found in recent cannabis users (Owens et al. 2019). Recent work has even found that patients with schizophrenia can modulate their DMN/DAN anticorrelation using real-time neurofeedback resulting in reductions in auditory hallucinations (Whitfield-Gabrieli et al. 2017).
However, despite this research, there remain questions regarding the reliability of previous work on DMN/DAN anticorrelation, the generalizability of prior findings to children, and the specificity of DMN/DAN anticorrelation to individual disorders. Most research linking DMN/DAN anticorrelation to neurocognition and to psychiatric disorders has been conducted in small studies (N < 50). Given recent work showing that small samples create major risk for type I error (Button et al. 2013), it is critical that findings such as these be replicated in larger samples. Related to this, given the small sample sizes of most existing work, it is difficult to determine the size of these effects, as small samples are also known to inflate effect sizes (Button et al. 2013). Additionally, most work on the relationships of DMN/DAN anticorrelation to neurocognition and psychiatric disorder has been conducted in adults. Given noted differences in the DMN during childhood (Fair et al. 2008), more work is needed to determine if abnormal patterns of anticorrelation are linked to cognitive ability and disease states in children. Furthermore, while there have been many cognitive and psychiatric variables that have been linked to DMN/DAN anticorrelation, many of these variables are highly associated with each other, as well as to demographic factors such as socioeconomic status, gender, and age. Thus, further disentanglement of the multicollinearity of these factors is needed to determine if these factors relate to DMN/DAN anticorrelation independently of each other. The current study aims to fill these gaps by using data from children participating in the Adolescent Brain Cognitive Development (ABCD) study to test associations of DMN/DAN anticorrelation during resting-state fMRI with numerous neurocognitive and psychiatric variables, both directly and while controlling for other variables likely to confound these associations.
Methods
Procedures
The ABCD is an ongoing multi-site, longitudinal neuroimaging study following a cohort of 11 880 youths for 10 years (www.ABCDstudy.org). Each year, the child and their parent/guardian complete a lab-based visit. Children answer questions about friends, family, school, substance use, and life events; parents complete electronic questionnaires about their children, their family, and themselves (Barch et al. 2018). Children also complete questionnaires and cognitive tasks (Luciana et al. 2018). Every other year (e.g., baseline visit, Year-2, Year-4), the children also complete an MRI scan, providing resting-state fMRI, task fMRI, and structural MRI data. One or both parents/guardians provided informed consent and youth assented to study procedures. For the baseline data used in the current study, all data (i.e., fMRI, questionnaire, neuropsychological tasks) were collected at a single visit or across two visits that occurred within 30 days of each other.
Participants
In this article, we use data from the baseline visits at which participants were 9 or 10 years old. Participants were excluded if they could not successfully complete a T1 MRI scan at their baseline visit (e.g., due to a physical condition, due to claustrophobia) or if they were not able to complete questionnaires or perform cognitive tasks. Participants were excluded who were missing one or more of the demographic, neuropsychological, or psychiatric measures. Furthermore, because of a data processing error identified in the ABCD version 2.0.1 release, all subjects scanned on Phillips MRI scanners were excluded. In controlling for resting-state fMRI scan quality, participants were included if they had at least one resting-state fMRI run, which passed a visual and automated quality control assessment (detailed in Hagler et al. 2019). In this process, an automated assessment checked for movement and poor signal-to-noise ratio while a manual assessment included visual inspection of images by two trained technicians for artifacts, poor image quality, or other irregularities. Participants were also required to have at least one structural MRI scan, which passed Freesurfer visual quality control inspection. In this assessment, trained technicians reviewed the severity of five categories of artifacts for cortical surface reconstruction. Because motion was a significant concern given its known associations with many of the psychiatric variables of interest (e.g., attention problems, psychosis), participants were excluded if they had fewer than 5 min of valid scan time (i.e., 375 remaining volumes) after censoring for motion (>0.9 mm per volume). The 5-min threshold was chosen based on research showing that 5 min of scan time is sufficient to achieve stable functional connectivity correlations among brain regions (Van Dijk et al. 2010). This resulted in a total of 1192 participants excluded for motion or poor scan quality; when compared to those included in the study, these excluded participants performed more poorly on average on all neuropsychological tests (P < 0.01) and showed more symptoms/higher likelihood of diagnosis for all psychiatric measures (P < 0.01) except for internalizing symptoms, depression symptoms, depression diagnosis, adolescent conduct disorder diagnosis, and sleep disorder diagnosis. A total of 6543 participants were included in analyses, and their demographics are reported in Table 1.
Table 1.
Descriptive statistics on the current sample
| Mean | Std | Min | 25% | 50% | 75% | Max | Kurtosis | Skewness | |
|---|---|---|---|---|---|---|---|---|---|
| Continuous variables | |||||||||
| Child age (months) | 119.42 | 7.53 | 108 | 113 | 120 | 126 | 131 | −1.31 | −0.01 |
| Thought problems | 1.58 | 2.13 | 0 | 0 | 1 | 2 | 18 | 6.86 | 2.26 |
| Attention Sx | 2.76 | 3.36 | 0 | 0 | 2 | 4 | 20 | 2.34 | 1.55 |
| Internalizing Sx | 5.06 | 5.52 | 0 | 1 | 3 | 7 | 51 | 5.24 | 1.96 |
| Externalizing Sx | 4.17 | 5.56 | 0 | 0 | 2 | 6 | 47 | 7.74 | 2.40 |
| Depression Sx | 1.25 | 2.00 | 0 | 0 | 0 | 2 | 19 | 8.80 | 2.56 |
| Conduct disorder Sx | 1.15 | 2.18 | 0 | 0 | 0 | 1 | 22 | 15.64 | 3.35 |
| Prodromal schizophrenia | 5.60 | 9.89 | 0 | 0 | 1 | 7 | 96 | 13.32 | 3.14 |
| Sleep problems | 36.41 | 8.04 | 26 | 31 | 34 | 40 | 126 | 6.58 | 1.87 |
| Card sort | 93.49 | 8.87 | 51 | 89 | 94 | 99 | 120 | 2.01 | −0.67 |
| Crystalized intelligence | 87.15 | 6.79 | 54 | 83 | 87 | 91 | 115 | 0.68 | 0.20 |
| Flanker | 94.87 | 8.47 | 55 | 90 | 96 | 101 | 116 | 1.54 | −0.96 |
| Fluid intelligence | 92.84 | 10.06 | 44 | 87 | 93 | 100 | 131 | 0.22 | −0.27 |
| List sort | 97.83 | 11.42 | 36 | 90 | 97 | 105 | 136 | 0.79 | −0.49 |
| Pattern matching | 88.79 | 14.34 | 30 | 80 | 90 | 99 | 140 | 0.00 | −0.19 |
| Picture matching | 103.83 | 12.06 | 76 | 95 | 103 | 112 | 136 | −0.43 | 0.22 |
| Picture vocabulary | 85.33 | 7.88 | 29 | 80 | 85 | 90 | 119 | 0.92 | 0.19 |
| Reading | 91.46 | 6.58 | 63 | 88 | 91 | 95 | 119 | 1.63 | 0.15 |
| Total composite | 87.46 | 8.57 | 46 | 82 | 88 | 93 | 117 | 0.31 | −0.23 |
| Count | % | ||||||||
| Categorical variables | |||||||||
| Child sex (female) | 3271 | 49.99% | |||||||
| Race (White) | 4499 | 68.76% | |||||||
| Race (Black) | 842 | 12.87% | |||||||
| Race (Asian) | 134 | 2.05% | |||||||
| Race (other) | 1068 | 16.32% | |||||||
| Income (<$50 K) | 1731 | 26.46% | |||||||
| Income ($50 K–$100 K) | 2894 | 44.23% | |||||||
| Income (>$100 K) | 1918 | 29.31% | |||||||
| <HS diploma | 189 | 2.89% | |||||||
| HS diploma/GED | 461 | 7.05% | |||||||
| Some college | 1671 | 25.54% | |||||||
| Bachelor | 1816 | 27.75% | |||||||
| Postgraduate degree | 2406 | 36.77% | |||||||
| ADHD Dx | 524 | 8.01% | |||||||
| Childhood conduct Dx | 143 | 2.19% | |||||||
| Adolescent conduct Dx | 21 | 0.32% | |||||||
| Sleep disorder Dx | 150 | 2.29% | |||||||
Std, standard deviation; Sx, symptoms; HS, high school; GED, general education diploma; Dx, diagnosis.
Measures
Neuropsychological Measures
NIH Toolbox
Assessments were selected from the National Institutes of Health (NIH) Toolbox for evaluating neurological and behavioral functions. The toolbox consists of several different tasks including 1) Picture Vocabulary Test, 2) Flanker Inhibitory Control and Attention Test (Flanker), 3) Dimensional Change Card Sort Test (Card Sort), 4) List Sorting Working Memory Test (List Sorting), 5) Pattern Comparison Processing Speed Test (Pattern Comparison), and 6) Oral Reading Recognition Test. These tests are designed, respectively, to assess 1) executive function, 2) attention, 3) episodic memory, 4) language, 5) working memory, and 6) processing speed performance (Gershon et al. 2013; Hodes et al. 2013; Luciana et al. 2018). The tasks were administered by trained research staff using an iPad. In addition to specific task scores, the NIH Toolbox produces Fluid and Crystallized Composite Scores, as well as a Total Cognition Composite (akin to “g” or full-scale IQ). In Supplementary Material 1, we briefly describe each test used.
Psychiatric Measures
Child Behavior Checklist
The Child Behavior Checklist (CBCL) is a checklist completed by parents about emotional and behavioral symptoms experienced by their children aged 6–18 years (Achenbach and Rescorla 2001). This measure has been shown to have good validity and reliability for its syndrome scales (Dopfner et al. 1994). In the current study, the empirically based syndrome scales used were the Attention Problems Scale, Thought Problems Scale, Externalizing Composite, and Internalizing Composite. Additionally, the DSM-oriented Conduct Disorder Scale and Depression Scale were used.
The Kiddie Schedule for Affective Disorders and Schizophrenia
The Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) is an interview for the diagnosis of psychiatric disorders in children aged 6–18 years. It was administered to children by trained ABCD research staff and was self-administered on an iPad by parents. The K-SADS has shown to be both valid and reliable for the diagnosis of psychiatric disorders, aligning well with other established diagnostic protocols (Kaufman et al. 1997). In the current study, parent data were utilized for ADHD, conduct disorder, major depressive disorder, and sleep disorder. These diagnoses were based on parent report.
Prodromal Psychosis Scale
The Prodromal Psychosis Scale is a 21-item yes/no inventory completed by children for symptoms indicating risk for psychosis and current evidence of subthreshold positive symptoms (Karcher et al. 2018). It is computed as the total number of symptoms endorsed with possible scores ranging from 0 to 21. This measure was used as the index of psychotic-like symptoms in the current report.
Sleep Disturbance Scale
The Sleep Disturbance Scale for Children is an inventory completed by parents that uses a 5-point Likert-type scale (Romeo et al. 2013). This assessment is used for categorizing sleep disorders in children during the previous 6 months and incorporates five subdomains: disorders of initiating and maintaining sleep, sleep breathing disorders, disorders of arousal/nightmares, sleep/wake transition disorders, disorders of excessive somnolence, and sleep hyperhidrosis (Esbensen et al. 2018). In the current study, only the total score across all domains was examined.
Demographic Covariate Measures
Demographic questionnaire
A demographics questionnaire was also administered to children’s parents to determine demographic information including the child’s sex, age, race, and parental education.
Resting-State fMRI Acquisition and Preprocessing
In most cases, four 5-min runs of resting-state fMRI were obtained. However, some data collection sites collected just three 5-min runs of resting-state fMRI data for subjects shown to have at least 13 min of usable data based on real-time fMRI analysis. Resting-state data were recorded while participants lay with eyes open viewing a cross hair. Twenty six different scanners from two vendors (Siemens and General Electric) were utilized, employing harmonized protocols (Casey et al. 2018; Hagler et al. 2019). Sixty slices were collected using a 2.4 mm × 2.4 mm × 2.4 mm voxel size, field of view of 216 × 216, and flip angle of 52°. The time repetition was 800 ms and the time echo was 30 ms, and data were acquired using MultiBand Acceleration with a factor of 6.
Resting-state data were preprocessed by the Data Analysis and Informatics Core of ABCD, which is detailed in the study of Hagler et al. (2019). fMRI data were registered to the first frame to account for head motion, corrected for spatial and intensity distortions, and co-registered with structural MRI scans. Subsequent preprocessing steps specific to resting-state fMRI included removal of initial volumes, normalization and demeaning, censoring of volumes with >0.2 mm motion, band-pass filtering (between 0.009 and.08 Hz), and within-subject (first level) regression to remove quadratic trends, motion, and mean time courses of cerebral white matter, ventricles, and whole brain (as well as their derivatives). Data were sampled onto the cortical surfaces and divided into the 422 cortical parcels that make up the 13 functionally defined networks described in the study of Gordon et al. (2016) (see Fig. 1). Correlations were calculated for the average timeseries of vertices in each ROI pair, and these correlations were z-transformed. For within-network connectivity (i.e., coherence of networks), the average was taken of the z-transformed correlations of all ROIs in that network. For between-network connectivity (i.e., connectivity or anticorrelation of networks), the average was taken of z-transformed correlations between each ROI pair across the networks. Anticorrelation of the DMN and the DAN represents the between-network correlation of these two networks. DMN coherence and DAN coherence represent the within-network correlations of these two networks.
Figure 1.
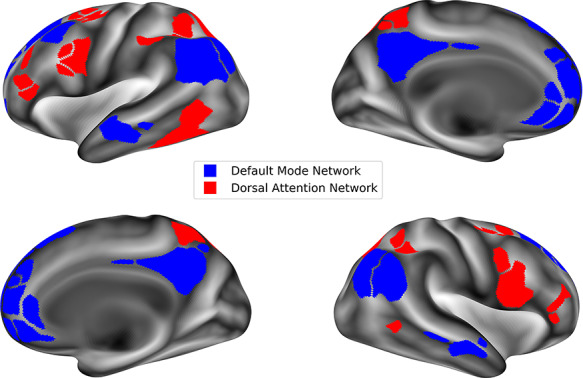
Map of the default mode network and dorsal attention network as defined in the Gordon Parcellation used in the current study.
Analyses
Data Download and Analysis Software
Data were downloaded from the ABCD Data Repository on the National Institute of Mental Health Data Archive, using data from ABCD release 2.0.1. Analyses were conducted in R software version 3.6.1 using the Rstudio integrated development environment version 1.1.456. The Rscript for the analyses conducted is available on Github (https://github.com/owensmax/DMNDAN_Correlates).
Modeling Approach
Relationships between DMN/DAN anticorrelation (the dependent variable in all models) and neuropsychological and psychiatric variables of interest (as independent variables in separate models) were assessed using linear mixed effects models. Mixed effect models were used instead of simpler regression models in order to control for the large numbers of siblings included in the ABCD dataset and for the fact that data were collected at 18 different sites on 26 unique scanners. To account for these factors, sibling status was modeled as a random effect nested inside of a random effect of scanner. In addition to these random effects, primary analyses of DMN/DAN anticorrelation with psychiatric and neuropsychological variables were conducted with six demographic covariates modeled as fixed effects: age, sex, race, parental education, household income, and in-scanner motion. Preliminary and supplementary analyses use fixed effect covariates in some cases, as described below.
Preliminary Analyses
Evidence of DMN/DAN anticorrelation
First, we examined if anticorrelation between the DMN and DAN was present in the current sample using a one sample t-test.
Relationship of DMN/DAN anticorrelation to within-network coherence
We also examined the relationship of DMN network coherence and DAN network coherence with DMN/DAN anticorrelation in a mixed effect model without including demographic covariates.
Relationship of DMN/DAN anticorrelation to demographics
Next, each of the demographic covariates was tested individually for its relationship to DMN/DAN anticorrelation using a linear mixed effects model that included only the demographic variable (i.e., covariate) as an independent variable and DMN/DAN anticorrelation as the dependent variable with scanner and family as random effects.
Relationship of DMN/DAN anticorrelation to scanner type
Additionally, to determine the degree to which scanner differences reflected differences in anticorrelation, scanner (i.e., individual MRI device serial number) was examined as an independent variable with DMN/DAN anticorrelation as the dependent variable (and only family ID as a random effect). To further understand scanner effects, several other related variables were tested as independent variables in separate mixed effect models for their relationship to DMN/DAN anticorrelation: scanner manufacturer (i.e., SIEMENS or GE), scanner model (i.e., GE Discovery MR750, SIEMENS Prisma, or SIEMENS Prisma Fit), and data collection site (i.e., the city in which data collection occurred). To help disentangle the degree to which the scanner’s manufacturer was driving these effects, scanner, scanner model, and site were all examined in three separate models for participants whose data were collected on SIEMENS scanners (n = 4899) and in three more separate models for participants whose data were collected on GE scanners (n = 1644). Each of these preliminary analyses was not considered as additional tests in the primary multiple comparison correction. However, multiple comparison correction was used within these analyses, with each preliminary analysis considered as a separate family of tests (6 tests for the demographic covariate analyses and 1 test for site analysis).
Primary Analyses
To best assess the robustness and replicability of results both split-half and full-sample approaches were used. In both approaches, mixed effects models were used with each psychiatric and neuropsychological variable as an independent variable in its own model, also including covariates as independent variables; DMN/DAN anticorrelation was the dependent variable.
Relationship of DMN/DAN anticorrelation to psychiatric and neuropsychological variables, full sample
In the full-sample analyses, false discovery rate (FDR) correction was used as the primary correction to minimize type I error. This was done correcting for the number of psychiatric and neuropsychological variables tested for association with DMN/DAN anticorrelation using alpha set to 0.05 (M/23*0.05 = 0.0022 for first test, 0.0044 for second test, etc.).
Relationship of DMN/DAN anticorrelation to psychiatric and neuropsychological variables, split-half
In addition to the full-sample analyses, to assess the replicability of the results, we conducted 500 split-half replications (i.e., random samplings without replacement) in which we repeated the primary analyses in two approximately equal split-halves of the data. In each iteration, the full sample of 6543 participants was randomly split into two approximately equal halves, with the constraint that members of each family be categorized exclusively into one of the two halves (i.e., no sibling pairs were split between halves). To identify relationships that were replicable across independent samples, an alpha of <0.05 in both halves was considered significant, and the percent of samplings in which this occurred was recorded, as well as the average regression coefficient, P-value, and R2∆. In primary analyses, a finding was considered robust if it was significant beyond FDR in the full-sample analyses and was significant in both halves in at least 95% of the split-half replication tests.
Follow-Up Analyses
Relationship of DMN/DAN anticorrelation to psychiatric and neuropsychological variables without accounting for demographic covariates
Additional analyses were undertaken to understand the covariates’ effects on the relationship of DMN/DAN anticorrelation and the psychiatric and cognitive variables of interest. To examine if the inclusion of demographic covariates was reducing the number of robust associations identified, mixed effect analyses were conducted in the full sample 1) without fixed effect (demographic) covariates and 2) using only age, sex, and scanner motion as covariates. The analyses using only age, sex, and motion were conducted as an attempt to isolate the effect of socioeconomic status as a covariate. The question addressed by these analyses was “Are the use of demographic covariates causing fewer psychiatric or neuropsychological correlates of DMN/DAN anticorrelation to be identified?”
Relationship of DMN/DAN anticorrelation to attention problems accounting for all other psychiatric and neuropsychological variables
Furthermore, to see if significant associations were being driven by multicollinearity among psychiatric and neuropsychological variables, we examined all independent variables in one mixed effect model, along with demographic covariates. The question addressed by these analyses was “Are associations of independent variables with DMN/DAN anticorrelation significant beyond their association with other independent variables?”
Relationship of DMN/DAN anticorrelation to interactions between demographic variables and variables of interest
Furthermore, to determine if there were relevant interactions between demographic covariates and variables of interest (i.e., neuropsychological and psychiatric variables), interaction terms were created of each covariate and independent variable pair; these were tested in a separate model for each independent variable with DMN/DAN anticorrelation as the dependent variable and with covariates, the independent variable, and the interaction terms of that independent variable and each covariate as independent variables.
Relationship of attention problems and connectivity of other networks
Finally, to ensure that results did not reflect generalized effects of heightened connectivity across the entire brain, we examined the relationship of each robust psychiatric or neuropsychological correlate of DMN/DAN anticorrelation to the connectivity of the DMN with each of the other 11 brain networks in the Gordon Parcellation Atlas: frontoparietal, ventral attention, cingulo-opercular, salience, sensory motor hand, sensory motor mouth, auditory, visual, retrosplenial, cingulo-parietal, and “None” (i.e., a network of all regions not falling in an established network). We also examined the connectivity of the DAN with each other brain network, plus the internal coherence of the DMN and the internal coherence of the DAN (in total, 24 functional connectivity pairs). The question addressed by these analyses was “Are the psychiatric and neuropsychological correlates of DMN/DAN anticorrelation also related to the connectivity within and between other networks?”
Each of these supplementary analyses was not considered additional tests in the primary multiple comparison correction given their post hoc, descriptive nature. However,multiple comparison correction was used within supplementary analyses, with each analysis considered as a separate family of tests (23 tests for the psychiatric and neuropsychological variables without covariates, 23 tests for the psychiatric and neuropsychological variables covarying for age, sex, and scanner motion only, 1 test for the single mixed model with all independent variables and covariates, 115 tests for the interaction analyses, and 24 tests for the analyses of other network connections).
Results
Preliminary Analyses
Evidence of DMN/DAN Anticorrelation
DMN/DAN anticorrelation was identified in the current sample, with the mean connectivity (z-scored correlation) between the DMN and the DAN being −0.13 (one-sample t-test: t = 160.99, P = 2E-16).
Relationship of DMN/DAN Anticorrelation to Within-Network Coherence
DMN/DAN anticorrelation was negatively associated with the coherence of the DMN (B = −0.48, standard error [SE] = 0.009, t = 54.50, P = 2E-16, R2Δ = 31.2%) and coherence of the DAN (B = −0.48, SE = 0.008, t = 60.91, P = 2E-16, R2Δ = 36%).
Relationship of DMN/DAN Anticorrelation to Demographics
DMN/DAN anticorrelation was associated with all covariates tested (P < 0.01) except for parental education (P > 0.19). See Table 2 for full statistics of associations with covariates.
Table 2.
Associations of demographic covariates with anticorrelation of DMN and DAN
| Phenotype (IV) | B | SE | t | P | R2∆ |
|---|---|---|---|---|---|
| Child age | −0.0006 | 0.0001 | −5.25 | 1.55E-07 | 0.004 |
| Child sex (male) | 0.0151 | 0.0016 | 9.50 | <2e-16 | 0.014 |
| Scanner motion | 0.0990 | 0.0050 | 19.84 | <2e-16 | 0.057 |
| Parent’s highest education (high school) | 0.0033 | 0.0056 | 0.59 | 0.5560 | 0.005 |
| Parent’s highest education (some college) | 0.0065 | 0.0050 | 1.30 | 0.1940 | |
| Parent’s highest education (bachelor’s) | −0.0015 | 0.0051 | −0.30 | 0.7650 | |
| Parent’s highest education (graduate) | −0.0053 | 0.0050 | −1.06 | 0.2910 | |
| Child race (Black) | 0.0178 | 0.0026 | 6.96 | 3.83E-12 | 0.008 |
| Child race (Asian) | −0.0041 | 0.0058 | −0.71 | 0.4767 | |
| Child Race (Other) | 0.0056 | 0.0023 | 2.48 | 0.0131 | |
| Parent’s income ($50 000–$100 000) | −0.0126 | 0.0021 | −6.03 | 1.79E-09 | 0.006 |
| Parent’s income (>$100 000) | −0.0063 | 0.0022 | −2.86 | 0.0042 |
B, unstandardized regression coefficient; SE, standard error of regression coefficient; t, t−statistic of regression coefficient; P, alpha value of regression coefficient; R2, variance explained by that variable. For multi-category variables (i.e., race and income), R2 represents the variance explained by all dummy variables for a given category. For race, “White” was used as the reference category; for income, “<$50 000” was used as the reference category, for parent’s highest education, “Less than a high school degree” was used as the reference category.
Relationship of DMN/DAN Anticorrelation to Scanner Type
Additionally, in separate mixed effect models, DMN/DAN anticorrelation was linked to scanner (i.e., individual MRI device serial number, R2Δ = 7.37%), scanner manufacturer (R2Δ = 4.33%), scanner model (R2Δ = 4.66%), and data collection site (R2Δ = 7.13%). Within Siemens scanners only, DMN/DAN anticorrelation was still linked to scanner (R2Δ = 3.62%), scanner model (R2Δ = 0.56%), and data collection site (R2Δ = 3.71%), though with a smaller effect size. Within GE scanners only, DMN/DAN anticorrelation was still linked to scanner (R2Δ = 2.09%) and site (R2Δ = 1.35%); scanner model was not examined as there was only one model of GE scanner included. Scanner (individual MRI device serial number) was accounted for in all other analyses as a random effect.
Primary Analyses
Relationship of DMN/DAN Anticorrelation to Psychiatric and Neuropsychological Variables, Full Sample
Attention problems, ADHD diagnosis, thought problems, depression symptoms, internalizing symptoms, and externalizing problems were associated with DMN/DAN anticorrelation beyond FDR correction after accounting for demographic covariates (i.e., age, sex, race, parents’ income, parents’ education, and in-scanner motion). Effect sizes were small, with the largest effect size being attention problems (∆R2 = 0.4%). No other psychiatric symptoms were related to the DMN/DAN anticorrelation. Additionally, no neuropsychological variables were related to DMN/DAN anticorrelation beyond FDR after accounting for demographic covariates. See Table 3 for full statistics of primary analysis associations between psychiatric and neuropsychological variables with DMN/DAN anticorrelation.
Table 3.
Associations of neuropsychological and psychiatric variables with functional connectivity of the DMN and DAN
| Phenotype (IV) | B | SE | t | P | R 2Δ |
|---|---|---|---|---|---|
| Attention problems | 0.0011 | 0.0002 | 4.79 | 1.69E-06 | 0.004 |
| ADHD diagnosis | 0.0111 | 0.0028 | 3.91 | 9.27E-05 | 0.002 |
| Thought problems | 0.0012 | 0.0004 | 3.39 | 0.0007 | 0.002 |
| Depression symptoms | 0.0011 | 0.0004 | 2.94 | 0.0033 | 0.001 |
| Internalizing symptoms | 0.0004 | 0.0001 | 2.91 | 0.0036 | 0.001 |
| Externalizing symptoms | 0.0004 | 0.0001 | 2.70 | 0.0069 | 0.001 |
| Sleep problems | 0.0002 | 0.0001 | 2.39 | 0.0167 | 0.001 |
| Conduct disorder symptoms | 0.0008 | 0.0004 | 2.28 | 0.0224 | 0.001 |
| Card sort | −0.0002 | 0.0001 | −2.11 | 0.0348 | 0.001 |
| Prodromal schizophrenia | 0.0001 | 0.0001 | 1.74 | 0.0812 | 0.000 |
| Picture matching | 0.0001 | 0.0001 | 1.58 | 0.1147 | 0.000 |
| Adolescent conduct disorder | 0.0201 | 0.0136 | 1.48 | 0.1387 | 0.000 |
| Sleep disorder | 0.0074 | 0.0051 | 1.45 | 0.1477 | 0.000 |
| Pattern matching | −0.0001 | 0.0001 | −1.34 | 0.1806 | 0.000 |
| Childhood conduct disorder | 0.0056 | 0.0053 | 1.07 | 0.2849 | 0.000 |
| Depression diagnosis | 0.0174 | 0.0186 | 0.94 | 0.3485 | 0.000 |
| Reading | 0.0001 | 0.0001 | 0.77 | 0.4435 | 0.000 |
| Flanker | −0.0001 | 0.0001 | −0.67 | 0.5041 | 0.000 |
| Fluid intelligence | 0.0000 | 0.0001 | −0.56 | 0.5760 | 0.000 |
| List sort | 0.0000 | 0.0001 | 0.51 | 0.6093 | 0.000 |
| Crystalized intelligence | 0.0001 | 0.0001 | 0.39 | 0.6995 | 0.000 |
| Total composite | 0.0000 | 0.0001 | −0.25 | 0.8068 | 0.000 |
| Picture vocabulary | 0.0000 | 0.0001 | −0.08 | 0.9342 | 0.000 |
Model includes covariates age, sex, race, parental income, parental education, and in-scanner motion. Bold associations indicate significant beyond multiple comparison correction (FDR). B = unstandardized regression coefficient; SE = standard error of regression coefficient; t = t-statistic of regression coefficient; P = alpha value of regression coefficient; R2 = variance explained by that variable.
Relationship of DMN/DAN Anticorrelation to Psychiatric and Neuropsychological Variables, Split-Half
In the split-half analyses, attention problems were found to be significantly linked to DMN/DAN anticorrelation in 95% of the 500 split-half replication tests and ADHD diagnosis was linked in 77%. Thought problems were linked to DMN/DAN anticorrelation in 44% of the split-half replications. Depression symptoms were linked in 13% of the replications and internalizing symptoms were linked in 9%. No other independent variables were linked to DMN/DAN anticorrelation in at least 1% of the replication tests. Full statistics for the split-half analysis are in Supplementary Table 1. In summary, these analyses identify attention problems as the only robust psychiatric association with DMN/DAN anticorrelation across full-sample and split-half analyses.
Follow-Up Analyses
Relationship of DMN/DAN Anticorrelation to Psychiatric and Neuropsychological Variables without Accounting for Demographic Covariates
When only age, sex, and in-scanner motion were accounted for, conduct disorder symptoms, card sort task performance, sleep problems, prodromal schizophrenia symptoms, and total cognitive score were also associated with DMN/DAN anticorrelation, in addition to all variables identified in the primary analyses (Supplementary Table 2). When no demographic covariates were accounted for, all neuropsychological and psychiatric measures tested were associated with DMN/DAN anticorrelation except for adolescent conduct disorder and depression diagnosis (Supplementary Table 3). Effect sizes were small, although larger than in analyses with covariates accounted for; the largest R2 was 0.9% for attention problems. See Table 4 for comparison of effects with each covariate scheme. See Figure 2 for a visualization of the relationship between DMN/DAN anticorrelation and attention problems with and without covariates accounted for.
Table 4.
Associations of neuropsychological and psychiatric variables with functional connectivity of the DMN and DAN
| No Cov Stats | Some Cov Stats | All Cov Stats | |||||
|---|---|---|---|---|---|---|---|
| Phenotype (IV) | B | P | B | P | B | P | |
| Sig All Covs | Attention problems | 0.002 | 1.58E-14 | 0.001 | 7.56E-08 | 0.001 | 1.69E-06 |
| ADHD diagnosis | 0.016 | 1.67E-08 | 0.012 | 3.84E-05 | 0.011 | 9.27E-05 | |
| Thought problems | 0.002 | 4.48E-07 | 0.001 | 0.0003 | 0.001 | 0.0007 | |
| Externalizing problems | 0.001 | 3.55E-07 | 0.001 | 0.0006 | 0.000 | 0.0069 | |
| Depression symptoms | 0.002 | 0.0001 | 0.001 | 0.0012 | 0.001 | 0.0033 | |
| Internalizing symptoms | 0.000 | 0.0018 | 0.000 | 0.0016 | 0.000 | 0.0036 | |
| Sig Some Covs | Conduct symptoms | 0.002 | 1.15E-06 | 0.001 | 0.0016 | 0.001 | 0.0224 |
| Card sort | −0.001 | 4.05E-10 | 0.000 | 0.0016 | 0.000 | 0.0348 | |
| Sleep problems | 0.000 | 0.0004 | 0.000 | 0.0022 | 0.000 | 0.0167 | |
| Prodromal schizophrenia | 0.000 | 4.18E-05 | 0.000 | 0.0073 | 0.000 | 0.0812 | |
| Total composite | −0.001 | 1.42E-11 | 0.000 | 0.0167 | 0.000 | 0.8068 | |
| Sig No Covs | Fluid intelligence | 0.000 | 1.96E-09 | 0.000 | 0.0358 | 0.000 | 0.576 |
| Picture vocabulary | −0.001 | 9.80E-08 | 0.000 | 0.031 | 0.000 | 0.9342 | |
| Crystalized intelligence | −0.001 | 1.07E-07 | 0.000 | 0.0678 | 0.000 | 0.6995 | |
| Pattern matching | 0.000 | 4.19E-07 | 0.000 | 0.0704 | 0.000 | 0.1806 | |
| Reading | 0.000 | 0.0001 | 0.000 | 0.3574 | 0.000 | 0.4435 | |
| Flanker | 0.000 | 0.0035 | 0.000 | 0.1312 | 0.000 | 0.5041 | |
| List sort | 0.000 | 0.0092 | 0.000 | 0.3888 | 0.000 | 0.6093 | |
| Picture matching | 0.000 | 0.011 | 0.000 | 0.7643 | 0.000 | 0.1147 | |
| Child conduct Dx | 0.013 | 0.0187 | 0.008 | 0.1515 | 0.006 | 0.2849 | |
| Sleep disorder Dx | 0.011 | 0.0393 | 0.008 | 0.1069 | 0.007 | 0.1477 | |
| Non-Sig | Adolescent conduct Dx | 0.023 | 0.109 | 0.023 | 0.0871 | 0.020 | 0.1387 |
| Depression diagnosis | 0.023 | 0.24 | 0.021 | 0.2672 | 0.017 | 0.3485 | |
First section from top (“Sig All Covs”) indicates significant beyond FDR when controlling for age, sex, race, income, education, and in-scanner motion. Second section from top (“Sig Some Covs”) indicates significant beyond FDR when controlling for age, sex, and in-scanner motion. Third section from top (“Sig No Covs”) indicates significant beyond FDR with no fixed effect covariates included. Fourth section (“Non-sig”) indicates not significant under any covariate scheme. B = unstandardized regression coefficient; P = alpha value of regression coefficient; Dx = diagnosis.
Figure 2.
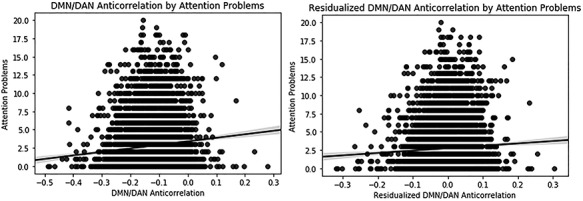
Scatterplots illustrating robust results: the association of attention problems with DMN and DAN anticorrelation. Scatter plot is shown for association with covariates not accounted for (left) and with covariates accounted for (right).
Relationship of DMN/DAN Anticorrelation to All Psychiatric, Neuropsychological, and Demographic Variables in a Single Model
When all independent variables (i.e., psychiatric and neuropsychological) were considered in a single model along with all demographic covariates, attention problems was the only psychiatric or neuropsychological variable significantly related to DMN/DAN anticorrelation (Supplementary Table 4). Additionally, scanner motion, sex, race, and age were each associated with DMN/DAN anticorrelation.
Relationship of DMN/DAN Anticorrelation to Interactions between Demographic Variables and Variables of Interest
No interactions were found between demographic covariates and independent variables in their relationship to DMN/DAN anticorrelation.
Relationship of Attention Problems and Connectivity of Other Networks
When the relationship of attention problems with the connectivity of other networks with the DMN and DAN was explored, the only network identified was the cingulo-opercular network (Table 5). Activation in the DAN and cingulo-opercular network was positively correlated across the resting-state fMRI timeseries (rz = 0.08, P < 0.001) and activation in the DMN and cingulo-opercular network was negatively correlated across the timeseries (rz = −.1, P < 0.001). The functional connectivity of the cingulo-opercular network and the DAN was inversely associated with attention problems beyond FDR correction, accounting for all demographic covariates. Likewise, the anticorrelation of the cingulo-opercular network and the DMN was positively associated with attention problems beyond FDR correction, accounting for all demographic covariates. Additionally, the coherence of the DAN and the coherence of the DMN were inversely linked to attention problems. No other network connections of the DAN or DMN were linked to attention problems.
Table 5.
Associations of attention problems with connectivity between default mode and other networks and with connectivity between dorsal attention and other networks
| Network connection | B | SE | t | P | ∆R 2 |
|---|---|---|---|---|---|
| Default Mode and Cingulo-opercular Conn | 0.0010 | 0.0002 | 4.26 | 2.09E-05 | 0.003 |
| Dorsal Attention and Cingulo-opercular Conn | −0.0008 | 0.0002 | −3.65 | 0.0003 | 0.002 |
| Dorsal Attention Coherence | −0.0010 | 0.0003 | −3.56 | 0.0004 | 0.002 |
| Default Mode Coherence | −0.0008 | 0.0003 | −2.88 | 0.0039 | 0.001 |
| Default Mode and Sensory Motor Hand Conn | 0.0005 | 0.0002 | 2.52 | 0.0117 | 0.001 |
| Dorsal Attention and Ventral Attention Conn | 0.0005 | 0.0002 | 2.33 | 0.0197 | 0.001 |
| Default Mode and Frontoparietal Conn | 0.0004 | 0.0002 | 2.12 | 0.0337 | 0.001 |
| Default Mode and Ventral Attention Conn | −0.0005 | 0.0002 | −2.04 | 0.0413 | 0.001 |
| Dorsal Attention and Frontoparietal Conn | −0.0004 | 0.0002 | −1.94 | 0.0529 | 0.001 |
| Dorsal Attention and None Conn | 0.0002 | 0.0001 | 1.66 | 0.0977 | 0.000 |
| Default Mode and Auditory Conn | 0.0003 | 0.0002 | 1.48 | 0.1403 | 0.000 |
| Default Mode and Retrosplenial Conn | −0.0004 | 0.0003 | −1.20 | 0.2297 | 0.000 |
| Default Mode and None Conn | −0.0002 | 0.0001 | −1.14 | 0.2555 | 0.000 |
| Default Mode and Sensory Motor Mouth Conn | 0.0003 | 0.0003 | 1.10 | 0.2730 | 0.000 |
| Dorsal Attention and Cingulo-parietal Conn | 0.0004 | 0.0003 | 1.06 | 0.2891 | 0.000 |
| Dorsal Attention and Sensory Motor Mouth Conn | 0.0003 | 0.0003 | 1.05 | 0.2952 | 0.000 |
| Dorsal Attention and Sensory Motor Hand Conn | −0.0002 | 0.0002 | −0.98 | 0.3274 | 0.000 |
| Dorsal Attention and Retrosplenial Conn | 0.0003 | 0.0003 | 0.88 | 0.3775 | 0.000 |
| Dorsal Attention and Auditory Conn | −0.0002 | 0.0002 | −0.74 | 0.4587 | 0.000 |
| Dorsal Attention and Salience Conn | −0.0002 | 0.0003 | −0.59 | 0.5580 | 0.000 |
| Dorsal Attention and Visual Conn | 0.0001 | 0.0003 | 0.57 | 0.5658 | 0.000 |
| Default Mode and Cingulo-parietal Conn | 0.0001 | 0.0004 | 0.21 | 0.8324 | 0.000 |
| Default Mode and Visual Conn | 0.0000 | 0.0003 | 0.17 | 0.8621 | 0.000 |
| Default Mode and Salience Conn | 0.0000 | 0.0003 | −0.13 | 0.8945 | 0.000 |
Model includes covariates age, sex, race, parental income, parental education, and in-scanner motion. Bold associations indicate significant beyond FDR correction (24 tests). The “None” network in the Gordon Parcellation represents the brain regions not included in any other established network. Conn, connectivity; B, unstandardized regression coefficient; SE, standard error of regression coefficient; t, t-statistic of regression coefficient; P, alpha value of regression coefficient; R2, variance explained by that variable.
Discussion
The current study tested several prior findings relating to the anticorrelation of the DMN and DAN, which has been theorized to be an index of functional segregation of these two networks that relates to numerous cognitive and psychiatric variables. Consistent with the literature, significant anticorrelation was found between these two networks in children aged 9–10 years, and this anticorrelation was significantly, inversely correlated to the coherence of the DMN and DAN. The anticorrelation of the DMN and DAN was tested for association with six demographic covariates, the individual and composite scales of the NIH toolbox, and symptoms and diagnoses of ADHD, schizophrenia, conduct disorder/externalizing problems, depression/internalizing problems, and sleep problems. Interestingly, DMN/DAN anticorrelation was linked to all demographic covariates tested except education; this set the stage for analyses of the NIH toolbox neuropsychological tests, which were all related to DMN/DAN anticorrelation when considered without covariates but were all unrelated after covarying for these demographic variables. The only NIH toolbox measures to be linked to DMN/DAN anticorrelation beyond FDR correction using only age, sex, and in-scanner motion as covariates were the dimensional card sort task, which is a measure of executive function and cognitive flexibility, and the total cognitive composite. In terms of psychiatric associations, beyond FDR correction with all covariates included, DMN/DAN anticorrelation was inversely associated with attention problems, ADHD diagnosis, thought problems, depression symptoms, internalizing symptoms, and externalizing symptoms. In split-half analyses, only the attention problem scale was identified in both split halves at least 95% of the time; the only other variable linked to DMN/DAN anticorrelation more than half the time was ADHD diagnosis. This suggests that several variables identified in full-sample analyses were not replicable across smaller samples.
The only findings to meet the criteria established as robust or come close were for ADHD, as attention problems (R2Δ = 0.4%) and ADHD diagnosis (R2Δ = 0.2%) were negatively linked to DMN/DAN anticorrelation after controlling for demographic factors in the full sample and were found to be linked in both split-halves in the majority of replication tests. In fact, attention problems were linked to DMN/DAN anticorrelation even after controlling for all other independent variables and covariates, providing very strong support for the hypothesis of reduced DMN/DAN segregation being a feature of attention problems and ADHD; likewise ADHD diagnosis was linked to DMN/DAN anticorrelation after controlling for all other independent variables and covariates if attention problems were not included in the model [analyses not reported]. These findings are consistent with existing theoretical accounts of DMN/DAN anticorrelation, which has been proposed as an index of the ability to maintain functional segregation between these two networks, reflecting superior ability to maintain separate modes of thought for externally and internally directed cognition (Whitfield-Gabrieli and Ford 2012). Furthermore, findings are consistent with existing hypotheses that attentional lapses in ADHD are in part a result of DMN interference during attempts to engage attention (Sonuga-Barke and Castellanos 2007; Cortese et al. 2012), which is buttressed by work showing that individuals with ADHD demonstrate lower levels of deactivation of the DMN during attentional tasks (Fassbender et al. 2009; Liddle et al. 2011; Metin et al. 2015) and that DMN deactivation during attentional tasks is associated with better performance (e.g., McKiernan et al. 2003; Singh and Fawcett 2008; Owens et al. 2018). Also of interest, supplementary analyses found that the relationship of the DMN and DAN did not represent a global relationship of functional connectivity to attention problems. Instead, the only other functional connections related to attention problems were the coherence of the DMN and DAN and each of these networks’ connectivity with the cingulo-opercular network, which has been suggested to be the network that oversees and modulates the anticorrelation between the two networks (Sridharan et al. 2008).
There were also several psychiatric phenomena that have been identified in the literature as being linked with reduced DMN/DAN anticorrelation, which were found in the full sample but that showed poor replicability in the split-half procedure. These included thought problems, depression symptoms, internalizing symptoms, and externalizing symptoms. Additionally, conduct disorder symptoms, prodromal psychosis symptoms, and sleep problems were linked to DMN/DAN anticorrelation when socioeconomic status was not accounted for. The effect sizes of these relationships were all small (<0.2% for R2Δ). These findings suggest mixed support for prior findings linking these psychiatric phenomena to DMN/DAN anticorrelation (De Havas et al. 2012; Posner et al. 2016; Pu et al. 2017) or reduced anticorrelation in adolescents with conduct disorder (Pu et al. 2017).
Furthermore, in the current study, the strongest neuropsychological correlate of DMN/DAN anticorrelation was the dimensional card sort task (R2Δ = 0.19%), which was linked to DMN/DAN anticorrelation beyond FDR correction after controlling for age, sex, and in-scanner motion. This task is a measure of executive functioning, specifically set shifting and cognitive flexibility, and has been shown to distinguish individuals with ADHD previously (Lawrence et al. 2004; Zelazo 2006). The only other neuropsychological measure to be linked to DMN/DAN anticorrelation beyond age, sex, and in-scanner motion was the total cognitive composite, derived by aggregating scores on all measures. This suggests that impaired DMN/DAN anticorrelation may have a global effect on cognition rather than a specific effect on one skill. These neurocognitive findings may represent a linkage between ADHD and DMN/DAN anticorrelation, potentially serving either as a consequence or mechanism of this linkage. However, their lack of association beyond income, education, and race suggests that they are intimately tied up in socioeconomic status.
That DMN/DAN anticorrelation was strongly linked to age, sex, and socio-economic status was among the clearest findings of the current study. Age and sex are common covariates (or as factors in group matching) in studies of resting-state anticorrelation in clinical populations (e.g., Sun et al. 2012; Posner et al. 2016; Pu et al. 2017; Whitfield-Gabrieli et al. 2018), though they are not universally used (e.g., De Havas et al. 2012); this is consistent with the broader neuroimaging literature in which age and sex are common covariates but socioeconomic factors are not (Hyatt et al. 2019). However, socioeconomic status was examined in a recent work for its link to DMN connectivity, which found that, in adults, income and education are linked to DMN, DAN, and cingulo-opercular network connectivity and that these factors may serve as a mediator of the link between anticorrelation of the networks and neuropsychological function (Shen et al. 2018). This mediational result is echoed in the current study by the finding that when socioeconomic factors were not covaried, total cognitive ability and card sort task performance were related to DMN/DAN anticorrelation beyond FDR correction, but when these factors were included as covariates this effect dissipated. Thus, to best compare with prior work on the DMN/DAN anticorrelation and neuropsychological function, it may be more appropriate to consider our supplementary results, which do not covary for income, education, and race, lest true associations mediated by socioeconomic status be overlooked. In short, this means that DMN/DAN anticorrelation may be linked to overall cognitive ability and, in specific, to set shifting, cognitive flexibility, and executive function. Likewise, conduct disorder symptoms, prodromal symptoms of schizophrenia, and sleep problems were all linked to DMN/DAN anticorrelation when socioeconomic status was not accounted for, suggesting a similar mediating effect may be at play. Fully exploring these potential mediation models goes beyond the scope of this paper, but it is an area that should be explored in future work. Thus, a key takeaway of the current study is that the covariate strategy used is critically important to whether effects from prior research will replicate for investigations in the role of DMN/DAN anticorrelation in clinical phenomena. As has been shown, the use of covariates in the neuroimaging literature is sporadic but can have major impacts on results (Hyatt et al. 2019). The current results highlight that it should be considered as a critical decision for studies testing associations of resting-state fMRI with cognitive or psychiatric variables.
Considerations and Future Directions
While many associations of DMN/DAN anticorrelation with neuropsychological performance and psychiatric problems were at least partially replicated from the literature, the effect sizes for all were small; even the most robust relationships explained only a quarter of 1% of the variance. One factor that may have contributed to small effect sizes is that by excluding subjects with excess motion, we also likely excluded some of the worst cases of psychiatric disorder, as subjects excluded for motion or scanner problems did show more severe psychopathology on almost all psychiatric measures. That noted, a major strength of this study was its sample size; this is the largest sample to date to be used to investigate any of the associations tested between DMN/DAN and a psychiatric or neuropsychological phenotype (the majority of existing work has sample sizes of fewer than 100). Because of its large sample size, the current study was able to adjust for demographic confounds, correct rigorously for multiple comparisons, and detect small effects. However, the use of a community sample rather than a clinical one is a limitation to the analyses of psychiatric symptoms/diagnosis in the current study. This means that most participants did not have clinical levels of psychiatric symptoms and those that did likely represent a less severe subset of individuals. These less severe psychiatric symptoms likely play a role in the small effect sizes seen. Furthermore, since schizophrenia diagnosis was an exclusion from the ABCD study, the current analysis used self-reported psychotic-like experiences and parent-reported thought problems as proxies for the effect shown in the literature that individuals with schizophrenia show reduced DMN/DAN anticorrelation.
Another consideration is in the processing and analysis of resting-state fMRI analyses. Processing was done using the standard ABCD preprocessing pipeline and the resting-state summary variables calculated by the Data Analysis and Informatics Core of ABCD (Hagler et al. 2019), which was specifically designed to be optimized for the sample of the current manuscript given what is known about movement and artifact during fMRI for children. For example, the current study used global signal regression as well as regression of BOLD signal in white matter and cerebrospinal fluid from resting-state fMRI timeseries. The current literature highlights the importance of removing systematic artifacts in the data; importantly, these global artifacts likely reflect not just movement but respiratory and other physiologic noise (Power et al. 2014). Without global signal regression (GSR), spurious artifacts ensue, particularly in studies of development, and GSR was recently shown to have the best success at reducing artifacts in youths of similar age to the current sample (Lydon-Staley et al. 2019). In considering this decision for the current analysis, DMN/DAN anticorrelation has been shown to be independent of any one strategy for accounting for global signal and has been found in studies in which global signal was not accounted for (Fox et al. 2009; Chai et al. 2012; Power et al. 2014), indicating that the anticorrelation is not itself an artifact of GSR. Furthermore, anticorrelations identified using GSR have been confirmed by concurrent electrophysiological signal, and GSR has been shown to improve correspondence between neuronal and hemodynamic signals (Keller et al. 2013). Work examining the effects of GSR on distinguishing clinical groups that show high levels of motion found that using GSR tends to reduce differences between groups that are driven by motion (Burgess et al. 2016). Furthermore, while no one strategy for accounting for global signal has been used exclusively in the literature, most work linking DMN/DAN anticorrelation to psychiatric and cognitive phenomena has used some correction for global signal in its analysis, making the current approach the closest possible analogue of prior studies that examined the relationship of anticorrelation and psychiatric and cognitive phenomena.
Another example of the multiple possible approaches to the analysis of DMN/DAN anticorrelation is the definition of the DMN. The current study examined the anticorrelation between the timeseries of the entire DMN and the entire DAN as defined by the Gordon network parcellation. While there are slight differences between the most popular parcellations, there is clear agreement among them regarding the hubs of the DMN and DAN (Power et al. 2011; Yeo et al. 2015; Gordon et al. 2016). Therefore, there is little reason to think that the parcellation scheme chosen was a relevant factor in the current results. However, in this domain, there are interesting avenues for future research. For example, there is evidence that the DMN is made up of several subsystems (Andrews-Hanna, Reidler, Sepulcre, et al. 2010) and may even represent two distinct functional networks (Buckner and DiNicola 2019). Future research might consider how these DMN subsystems may relate differentially to the DAN and if fine-grained fractionation of the DMN can help improve the understanding of the relationship of DMN/DAN anticorrelation to cognition and psychiatric disorders.
Conclusion
In summary, this study tested several associations found previously in the literature between the anticorrelation of the DMN and DAN with psychiatric problems in 9- to 10-year-old children. It did so in the largest sample to date for any of these questions, which allowed for the use of a split-half design, extensive consideration of covariates, and appropriate multiple comparison correction. The results indicated robust associations of attention problems with anticorrelation of the DMN and DAN. Future work should proceed with confidence that these represent reliable relationships, albeit with small effect sizes. The results suggest a more complicated relationship with prior findings linking anticorrelation of the DMN and DAN to psychosis, conduct disorder, depression, and sleep, which are likely intimately connected to demographic factors.
Funding
National Institutes of Health/National Institute of Drug Abuse T32DA043593. National Institutes of Health and additional federal partners (to ABCD Study under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, U24DA041147, U01DA041093, and U01DA041025). A full list of supporters is available at https://abcdstudy.org/federal-partners.html.
Notes
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children aged 9–10 and follow them over 10 years into early adulthood. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/scientists/workgroups/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from version 2.0.1.
Conflict of Interest
The authors have no conflicts of interest to declare.
Supplementary Material
References
- Achenbach TM, Rescorla L. 2001. Manual for the ASEBA school-age forms & profiles : an integrated system of multi-informant assessment. ASEBA, Burlington, VT. [Google Scholar]
- Anderson JS, Ferguson MA, Lopez-Larson M, Yurgelun-Todd D. 2011. Connectivity gradients between the default mode and attention control networks. Brain Connect. 1:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. 2010a. Evidence for the default network’s role in spontaneous cognition. J Neurophysiol. 104:322–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. 2010b. Functional-anatomic fractionation of the brain’s default network. Neuron. 65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Albaugh MD, Avenevoli S, Chang L, Clark DB, Glantz MD, Hudziak JJ, Jernigan TL, Tapert SF, Yurgelun-Todd D et al. 2018. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: rationale and description. Dev Cogn Neurosci. 32:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. 2008. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Buckner RL, DiNicola LM. 2019. The brain’s default network: updated anatomy, physiology and evolving insights. Nat Rev Neurosci. 20:593–608. [DOI] [PubMed] [Google Scholar]
- Burgess GC, Kandala S, Nolan D, Laumann TO, Power JD, Adeyemo B, Harms MP, Petersen SE, Barch DM. 2016. Evaluation of Denoising strategies to address motion-correlated artifacts in resting-state functional magnetic resonance imaging data from the Human Connectome Project. Brain Connect. 6:669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, Munafò MR. 2013. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 14:365–376. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, Soules ME, Teslovich T, Dellarco DV, Garavan H et al. 2018. The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Dev Cogn Neurosci. 32:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Castañán AN, Öngür D, Whitfield-Gabrieli S. 2012. Anticorrelations in resting state networks without global signal regression. Neuroimage. 59:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Schneider W. 2007. The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage. 37:343–360. [DOI] [PubMed] [Google Scholar]
- Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, Castellanos FX. 2012. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI sudies. Am J Psychiatry. 169:1038–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden BM, Mitchell DJ, Duncan J. 2015. Recruitment of the default mode network during a demanding act of executive control. Elife. 2015:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Havas JA, Parimal S, Soon CS, Chee MWL. 2012. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. Neuroimage. 59:1745–1751. [DOI] [PubMed] [Google Scholar]
- Dopfner M, Schmeck K, Berner W, Lehmkuhl G, Poustka F. 1994. Reliability and factorial validity of the child behavior checklist - an analysis of a clinical sample and a field sample. Z Kinder Jugendpsychiatr. 22:189–205. [PubMed] [Google Scholar]
- Esbensen AJ, Hoffman EK, Shaffer R, Chen E, Patel L, Jacola L. 2018. Reliability of parent report measures of behaviour in children with Down syndrome. J Intellect Disabil Res. 62:785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NUF, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. 2008. The maturing architecture of the brain’s default network. Proc Natl Acad Sci U S A. 105:4028–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, Schweitzer JB. 2009. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 1273:114–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. 2006. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 103:10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. 2009. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 101:3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Shen D, Smith JK, Zhu H, Lin W. 2013. The synchronization within and interaction between the default and dorsal attention networks in early infancy. Cereb Cortex. 23:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Lin W. 2012. Frontal parietal control network regulates the anti-correlated default and dorsal attention networks. Hum Brain Mapp. 33:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. 2013. NIH toolbox for assessment of neurological and behavioral function. Neurology. 80:S2–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. 2016. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb Cortex. 26:288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulden N, Khusnulina A, Davis NJ, Bracewell RM, Bokde AL, McNulty JP, Mullins PG. 2014. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage. 99:180–190. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. 2003. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Hatton S, Cornejo MD, Makowski C, Fair DA, Dick AS, Sutherland MT, Casey BJ, Barch DM, Harms MP et al. 2019. Image processing and analysis methods for the Adolescent Brain Cognitive Development study. Neuroimage. 202:116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Stein EA. 2007. Cingulate activation increases dynamically with response speed under stimulus unpredictability. Cereb Cortex. 17:1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes RJ, Insel TR, Landis SC, NIH Blueprint for Neuroscience Research . 2013. The NIH toolbox: setting a standard for biomedical research. Neurology. 80:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu ML, Zong XF, Mann JJ, Zheng JJ, Liao YH, Li ZC, He Y, Chen XG, Tang JS. 2017. A review of the functional and anatomical default mode network in schizophrenia. Neurosci Bull. 33:73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt CS, Owens MM, Crowe ML, Carter NT, Lynam DR, Miller JD. 2019. The quandary of covarying: a brief review and empirical examination of covariate use in structural neuroimaging studies on psychological variables. Neuroimage. 205:116225. [DOI] [PubMed] [Google Scholar]
- Karcher NR, Barch DM, Avenevoli S, Savill M, Huber RS, Simon TJ, Leckliter IN, Sher KJ, Loewy RL. 2018. Assessment of the prodromal questionnaire-brief child version for measurement of self-reported psychoticlike experiences in childhood. JAMA Psychiatry. 75:853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. 1997. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 36:980–988. [DOI] [PubMed] [Google Scholar]
- Keller CJ, Bickel S, Honey CJ, Groppe DM, Entz L, Craddock RC, Lado FA, Kelly C, Milham M, Mehta AD. 2013. Neurophysiological investigation of spontaneous correlated and anticorrelated fluctuations of the BOLD signal. J Neurosci. 33:6333–6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. 2008. Competition between functional brain networks mediates behavioral variability. Neuroimage. 39:527–537. [DOI] [PubMed] [Google Scholar]
- Konishi M, McLaren DG, Engen H, Smallwood J. 2015. Shaped by the past: the default mode network supports cognition that is independent of immediate perceptual input. PLoS One. 10:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Davis KD. 2014. Dynamic functional connectivity of the default mode network tracks daydreaming. Neuroimage. 100:471–480. [DOI] [PubMed] [Google Scholar]
- Lawrence V, Houghton S, Douglas G, Durkin K, Whiting K, Tannock R. 2004. Executive function and ADHD: a comparison of children’s performance during neuropsychological testing and real-world activities. J Atten Disord. 7:137–149. [DOI] [PubMed] [Google Scholar]
- Liddle EB, Hollis C, Batty MJ, Groom MJ, Totman JJ, Liotti M, Scerif G, Liddle PF. 2011. Task-related default mode network modulation and inhibitory control in ADHD: effects of motivation and methylphenidate. J Child Psychol Psychiatry Allied Discip. 52:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Bjork JM, Nagel BJ, Barch DM, Gonzalez R, Nixon SJ, Banich MT. 2018. Adolescent neurocognitive development and impacts of substance use: overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Dev Cogn Neurosci. 32:67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon-Staley DM, Ciric R, Satterthwaite TD, Bassett DS. 2019. Evaluation of confound regression strategies for the mitigation of micromovement artifact in studies of dynamic resting-state functional connectivity and multilayer network modularity. Netw Neurosci. 3:427–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. 2007. Wandering minds: the default network and stimulus-independent thought. Science (80-). 315:393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. 2003. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 15:394–408. [DOI] [PubMed] [Google Scholar]
- Metin B, Krebs RM, Wiersema JR, Verguts T, Gasthuys R, Van der Meere JJ, Achten E, Roeyers H, Sonuga-Barke E. 2015. Dysfunctional modulation of default mode network activity in attention-deficit/hyperactivity disorder. J Abnorm Psychol. 124:208. [DOI] [PubMed] [Google Scholar]
- Murphy C, Jefferies E, Rueschemeyer SA, Sormaz M, H ting W, Margulies DS, Smallwood J. 2018. Distant from input: evidence of regions within the default mode network supporting perceptually-decoupled and conceptually-guided cognition. Neuroimage. 171:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. 2012. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 12:241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MM, Duda B, Sweet L, MacKillop J. 2018. Distinct functional and structural neural underpinnings of working memory. Neurimage. 174:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MM, McNally S, Petker T, Amlung MT, Balodis IM, Sweet LH, MacKillop J. 2019. Urinary tetrahydrocannabinol is associated with poorer working memory performance and alterations in associated brain activity. Neuropsychopharmacology. 44:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriat R, Birn RM, Keding TJ, Herringa RJ. 2016. Default-mode network abnormalities in pediatric posttraumatic stress disorder. J Am Acad Child Adolesc Psychiatry. 55:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Cha J, Wang Z, Talati A, Warner V, Gerber A, Peterson BS, Weissman M. 2016. Increased default mode network connectivity in individuals at high familial risk for depression. Neuropsychopharmacology. 41:1759–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL et al. 2011. Functional network Organization of the Human Brain. Neuron. 72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. 2014. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu W, Luo Q, Jiang Y, Gao Y, Ming Q, Yao S. 2017. Alterations of brain functional architecture associated with psychopathic traits in male adolescents with conduct disorder. Sci Rep. 7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo DM, Bruni O, Brogna C, Ferri R, Galluccio C, De Clemente V, Di Jorio M, Quintiliani M, Ricci D, Mercuri E. 2013. Application of the sleep disturbance scale for children (SDSC) in preschool age. Eur J Paediatr Neurol. 17:374–382. [DOI] [PubMed] [Google Scholar]
- Shen X, Cox SR, Adams MJ, Howard DM, Lawrie SM, Ritchie SJ, Bastin ME, Deary IJ, McIntosh AM, Whalley HC. 2018. Resting-state connectivity and its association with cognitive performance, educational attainment, and household income in the UK biobank. Biol Psychiatry Cogn Neurosci Neuroimaging. 3:878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KD, Fawcett IP. 2008. Transient and linearly graded deactivation of the human default-mode network by a visual detection task. Neuroimage. 41:100–112. [DOI] [PubMed] [Google Scholar]
- Smith V, Mitchell DJ, Duncan J. 2018. Role of the default mode network in cognitive transitions. Cereb Cortex. 28:3685–3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Castellanos FX. 2007. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev. 31:977–986. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Dupre E, Selarka D, Garcia J, Gojkovic S, Mildner J, Luh WM, Turner GR. 2014. Goal-congruent default network activity facilitates cognitive control. J Neurosci. 34:14108–14114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Viviano JD, Schacter DL. 2016. Attenuated anticorrelation between the default and dorsal attention networks with aging: evidence from task and rest. Neurobiol Aging. 45:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. 2008. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 105:12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Cao Q, Long X, Sui M, Cao X, Zhu C, Zuo X, An L, Song Y, Zang Y et al. 2012. Abnormal functional connectivity between the anterior cingulate and the default mode network in drug-naïve boys with attention deficit hyperactivity disorder. Psychiatry Res Neuroimaging. 201:120–127. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. 2010. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 103:297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel S, Geng JJ, Fink GR. 2014. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 20:150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Liang M, Wang L, Tian L, Zhang X, Li K, Jiang T. 2007. Altered functional connectivity in early Alzheimer’s disease: a resting-state fMRI study. Hum Brain Mapp. 28:967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. 2006. The neural bases of momentary lapses in attention. Nat Neurosci. 9:971–978. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Bauer C, Okano K, Nestor P, Del Re E, Gosh S, Niznikiewicz M. 2017. M64. Real time fMRI feedback targeting default mode network (DMN) reduces auditory hallucinations. Schizophr Bull. 43:S233. [Google Scholar]
- Whitfield-Gabrieli S, Fischer AS, Henricks AM, Khokhar JY, Roth RM, Brunette MF, Green AI. 2018. Understanding marijuana’s effects on functional connectivity of the default mode network in patients with schizophrenia and co-occurring cannabis use disorder: a pilot investigation. Schizophr Res. 194:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. 2012. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 8:49–76. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Eickhoff SB, Yaakub SN, Fox PT, Buckner RL, Asplund CL, Chee MWL. 2015. Functional specialization and flexibility in human association cortex. Cereb Cortex. 25:3654–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD. 2006. The Dimensional Change Card Sort (DCCS): a method of assessing executive function in children. Nat Protoc. 1:297–301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


