Abstract
Objectives:
To test whether ischemia-mediated contractile dysfunction underlying the mitral valve impacts functional mitral regurgitation (FMR), and prognostic impact of FMR.
Background:
FMR results from left ventricular (LV) remodeling, which can stem from myocardial tissue alterations. Stress-CMR can assess ischemia and infarction in the LV and papillary muscles; relative impact on FMR is uncertain.
Methods:
Vasodilator stress-CMR was performed in known or suspected CAD patients at seven sites. Images were centrally analyzed for MR etiology/severity, mitral apparatus remodeling and papillary ischemia.
Results:
8,631 patients (60.0±14.1 years, 55% male) were studied: FMR was present in 27%, among whom 16% (n=372) had advanced (≥moderate) FMR. Patients with ischemia localized to sub-papillary regions were more likely to have advanced FMR (p=0.003); those with ischemia localized to other areas were not (p=0.17). Ischemic/dysfunctional sub-papillary myocardium (OR=1.24 per 10% sub-papillary myocardium, [CI 1.17–1.31], p<0.001) was associated with advanced FMR controlling for infarction. Among a sub-group with (n=372) and without (n=744) advanced FMR matched (1:2) on infarct size/distribution, advanced FMR patients had increased adverse mitral apparatus remodeling, paralleled by greater ischemic/dysfunctional sub-papillary myocardium (p<0.001). While posteromedial papillary ischemia was more common with advanced FMR (p=0.006), sub-papillary ischemia with dysfunction remained associated (p<0.001) adjusting for posteromedial papillary ischemia (p=0.074). During follow-up (median=5.1 years), 1,473 deaths occurred in the overall cohort; advanced FMR conferred increased mortality risk (HR=1.52 [CI 1.25–1.86], p<0.001) controlling for LV ejection fraction, infarction, and ischemia.
Conclusions:
Ischemic and dysfunctional sub-papillary myocardium provides a substrate for FMR, which predicts mortality independent of key mechanistic substrates.
Keywords: mitral regurgitation, ischemia, cardiac magnetic resonance
Introduction
Functional mitral regurgitation (FMR) affects nearly 3 million people,(1) conferring increased risk for heart failure, arrhythmia, and death.(2–5) Whereas FMR has been associated with left ventricular (LV) geometric and functional remodeling, similar remodeling can occur in the context of different LV tissue substrates – including irreversible and reversible myocardial injury. Myocardial infarction is an established nidus for FMR.(6,7) Ischemia is known to impact LV function and geometry, but its influence on FMR is less clear. Animal studies have shown coronary occlusion to acutely produce FMR(8,9) but uncertainty exists whether this persists chronically, varies based on perfusion pattern, and whether animal data reflect clinical FMR physiology – in which ischemia can be diffuse and co-exist with infarction. Given that ischemia is potentially reversible, elucidating its impact on FMR is of substantial importance.
Cardiac magnetic resonance (CMR) enables LV ischemia to be assessed together with infarction and remodeling. Stress perfusion CMR provides high diagnostic accuracy for obstructive coronary artery disease (CAD).(10–12) Beyond the LV chamber wall, stress CMR enables perfusion to be assessed in the papillary muscles – key components of the mitral apparatus. Prior studies by our group(7) and others(13) have used late gadolinium enhancement (LGE) CMR to test impact of LV and papillary muscle infarction on FMR. To date, stress CMR has yet to be used to evaluate association of ischemia with FMR. Additionally, whereas FMR has been linked to adverse prognosis,(2–5,14) it is unknown whether this is independent of myocardial tissue alterations responsible for FMR itself.
This multicenter study encompassed 8,631 patients undergoing stress CMR for evaluation of known or suspected CAD. In all patients, MR etiology and severity was confirmed by a central core lab – papillary muscle ischemia and mitral apparatus remodeling was assessed in a sub-cohort of patients with and without advanced FMR (matched on infarct size/distribution). Goals were to test (1) relative association of LV ischemia and infarction on FMR, (2) modulatory influence of papillary muscle ischemia on FMR, and (3) magnitude to which FMR augments mortality risk after controlling for LV ischemia and infarction.
Methods
Population
The population comprised adults (≥18 years old) undergoing stress perfusion CMR to evaluate known or suspected CAD at seven U.S. medical centers between May 2005 and October 2018. Patients with prior mitral repair/replacement, primary mitral pathologies (prolapse, rheumatic, leaflet perforation), clinically documented non-ischemic cardiomyopathy, or CMR-evidenced conditions that could alter LV remodeling (infiltrative/hypertrophic cardiomyopathy, congenital heart disease) were excluded. For patients with multiple exams, the initial CMR was used for data analysis.
This research was conducted using the Society for Cardiovascular Magnetic Resonance (SCMR) Registry; registry details have previously been reported.(15) Briefly, each participating site in this study routinely performs stress perfusion CMR and uses the same software (Precession [Heart Imaging Technologies, Durham, NC]) for image interpretation and data collection. Imaging data are stored in an internal database together with structured reports (inclusive of demographics) prior to de-identification and transmission to an external web-based platform. Each local system determines mortality status quarterly via the Social Security Death Index, supplemented by local electronic medical records.
For this study, de-identified CMR images were queried from the registry and analyzed (in all patients) by a core lab to confirm MR severity and exclude degenerative pathology. To further test associations of LV and papillary muscle ischemia with advanced FMR, additional core lab analyses – including assessment of papillary muscle ischemia and quantification of mitral apparatus remodeling indices – were performed in a “nested case-control” cohort comprised of patients with and without advanced FMR matched 1:2 based on (sub-papillary) infarct size.
Figure 1 illustrates the study protocol, including data acquisition, follow-up, and centralized data analyses. Institutional review board approval for use of data encompassed in this study was obtained at each participatory site.
Figure 1. Study Design.
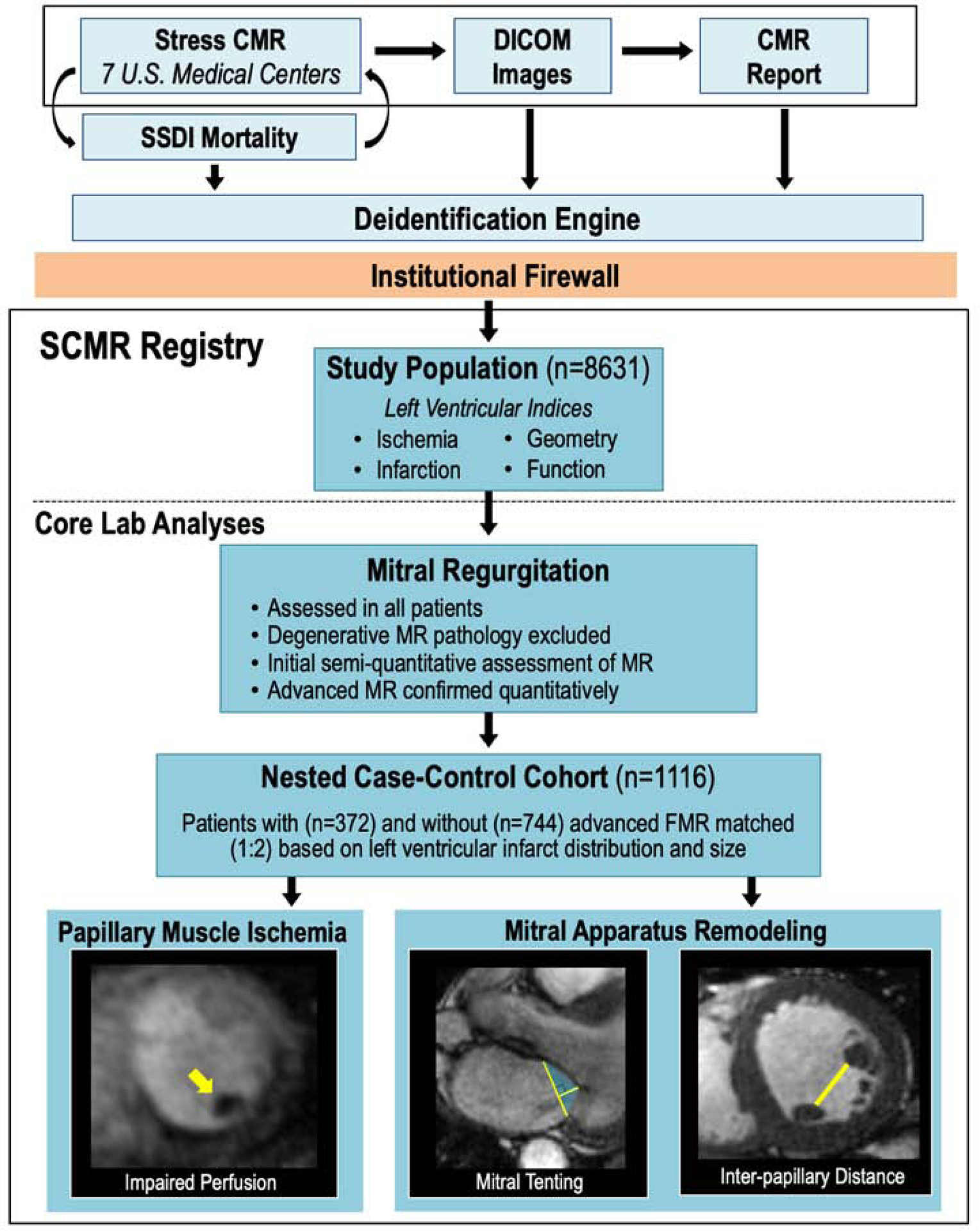
Schematic of multicenter image/data acquisition, de-identification, and centralized core lab analysis. All sites employed a similar stress CMR protocol. Ancillary clinical data were collected using a standardized reporting system, as were global LV chamber size/function, regional contractility, infarction, and ischemia. Mortality was obtained via the SSDI. Data were de-identified prior to core lab analyses, including assessment of MR etiology and severity in all patients, and papillary muscle ischemia and mitral apparatus geometric indices among a nested case-control cohort of patients with and without advanced FMR (bottom left: yellow arrow denotes papillary muscle ischemia, middle: yellow lines denote annular diameter and tenting height, fill denotes tenting area, right: yellow line denotes inter-papillary muscle distance).
Image Acquisition
Stress CMR was performed on 1.5T (77%) or 3.0T (23%) scanners. Cine-CMR utilized a steady-state free precession pulse sequence acquired at baseline in contiguous LV short, and standard (2-, 3-, 4-chamber) long axis orientations. Stress CMR was then performed using vasodilators (adenosine/regadenoson) concordant with established methods validated by our group(10) and outcomes research from this registry(15). A gradient-echo sequence (typical TR 1.6msec [1.5T], 2.9msec [3T] |TE 1.2msec [1.5T], 1.0msec [3T] | flip angle 15° [1.5T], 18° [3T] |in-plane resolution: 2.3mm × 1.9mm) was used to assess first-pass perfusion during gadolinium infusion; 3–5 LV short-axis images were acquired per heartbeat. Imaging was repeated 10-minutes thereafter if perfusion defects were seen with stress, or routinely per site protocol. Otherwise, a second gadolinium dose was administered without perfusion imaging (total 0.13–0.20 mmol/kg). LGE-CMR was acquired ~5 minutes following the second gadolinium infusion in orientations matched to cine-CMR. Phase-contrast CMR was acquired for valvular flow assessment at the discretion of participatory sites.
Image Analysis
Conventional Indices
LV volumes were quantified on cine-CMR via endocardial border planimetry at LV end-diastole and end-systole, from which stroke volume and ejection fraction were calculated. Regional function was graded using a 17-segment/4-point scoring system (0=normal; 1=mild/moderate hypokinesia; 2=severe hypokinesia; 3=akinesia; 4=dyskinesia). Myocardial infarction was identified on LGE-CMR; transmurality was graded per segment (0=no hyperenhancement; 1=1–25%; 2=26–50%; 3=51–75%; 4=76–100%). LGE in non-coronary arterial patterns (mid-wall, epicardial) was not included in analyses. Ischemia was identified on stress CMR based on impaired first-pass perfusion and localized using segmental partitions corresponding to cine and LGE analyses (apex not imaged): Segments were scored on a binary scale (present/absent). Concordant with established criteria,(10,15) perfusion defects larger than areas of LGE were considered indicative of ischemia.
To test relationships between mitral apparatus infarction/ischemia and FMR, an established algorithm(16) was used to partition segments into sub-papillary (basal-mid inferior/inferolateral | basal-mid anterior/anterolateral) and non-mitral valve adjacent (apical/septal) territories (Figure 2). Global infarct size was calculated by summing segmental scores (weighted by midpoint of hyperenhancement range) and dividing by total number of segments;(15) regional infarct size was calculated by summing segmental scores and dividing by number of regional segments. Global ischemia was calculated by dividing number of ischemic segments by total number of segments; regional ischemia extent was calculated by dividing number of ischemic segments by number of regional segments.
Figure 2. Mitral Apparatus Partitions.
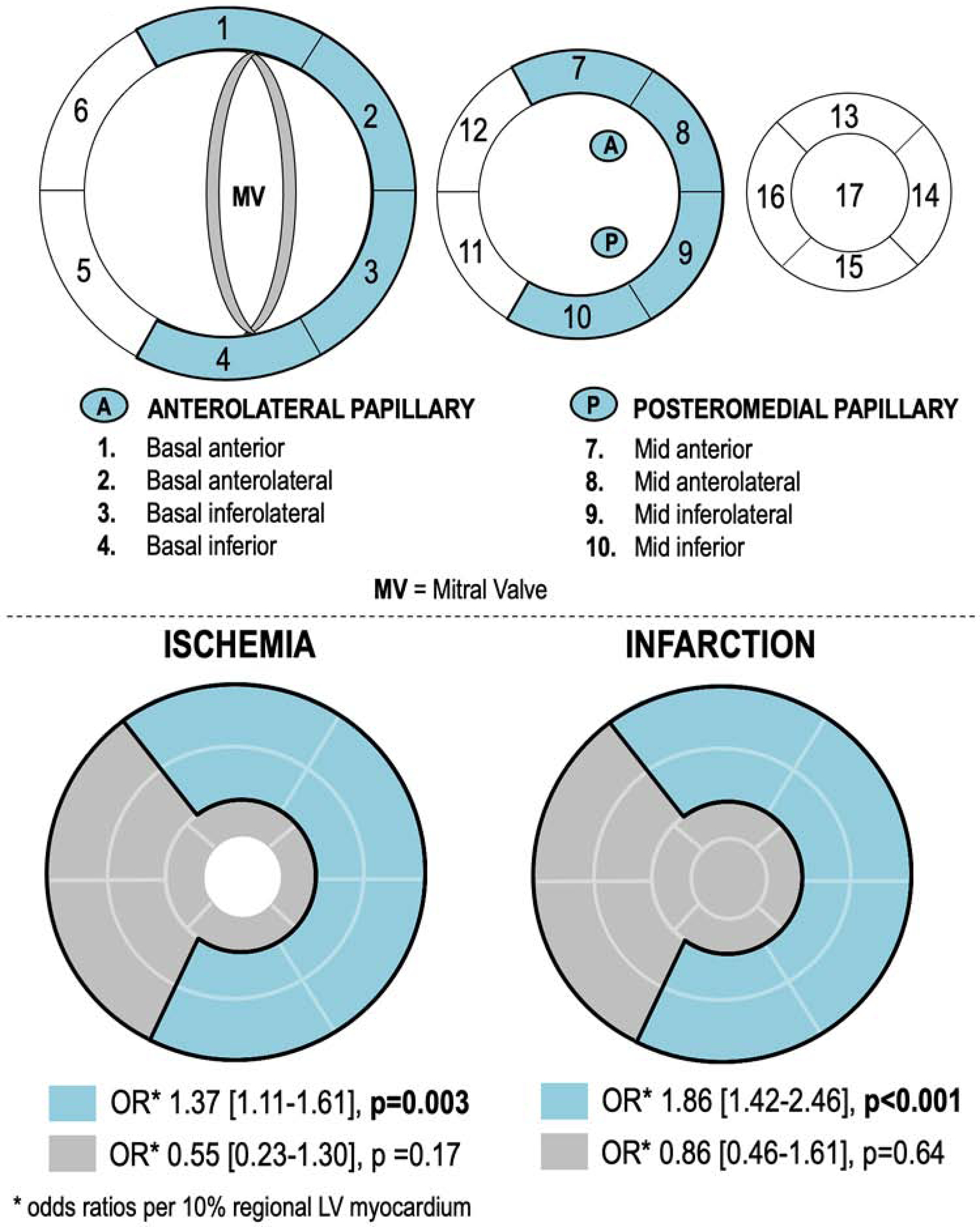
2A. Bullseye plot (17-segment model) illustrating LV wall myocardium subtended within the mitral apparatus, which was defined as segments adjacent to the anterolateral and posteromedial papillary muscles (blue denotes sub-papillary regions [basal-mid anterior/anterolateral, inferior/inferolateral walls]).
2B. Converged bullseye plots depicting associations of FMR with LV wall ischemia (left) and infarction (right) localized to sub-papillary regions (blue) or regions anatomically distant from the papillary muscles (grey): Patients with ischemia or infarction isolated to sub-papillary regions were more likely to have advanced FMR (both p<0.01), whereas those with ischemia or infarction isolated to other regions were not (p=NS).
Core Lab Analyses
Overall Population:
All cases were reviewed to exclude primary mitral valve pathology. Advanced (≥moderate) FMR was first identified based on semi-quantitative criteria (jet >1/3 left atrial [LA] area in at least 2 long axis orientations),(17) and confirmed quantitatively (regurgitant volume ≥30ml, regurgitant fraction ≥30%, or anatomic regurgitant orifice area ≥0.2cm2):(18,19) regurgitant volume was assessed via differential aortic forward stroke volume/LV stroke volume when phase contrast imaging of the aortic valve was available, or via differential RV/LV stroke volume when advanced tricuspid or aortic regurgitation was absent.
Nested Case-Control:
To test whether impact of LV ischemia on advanced FMR is associated with adverse mitral apparatus remodeling, cine-CMR was used to quantify mitral geometry:
Mitral annular diameter: diameter between junctions of anterior and posterior valve leaflets with the LA wall in 3-chamber (long axis) orientation.
Tenting height: diameter between leaflet coaptation and the mitral annular plane (measured in 3-chamber orientation, perpendicular to annulus); tenting area encompassed by the annulus and mitral leaflets.(7)
Interpapillary muscle distance: diameter between endocardial border of anterolateral and posteromedial papillary muscles in the basal-most LV short axis image encompassing both papillary muscles. Interpapillary diameters at LV end-diastole and end-systole were used to calculate papillary shortening.
Core lab analyses included assessment of papillary muscle ischemia, which was defined on stress CMR using equivalent criteria to that for the LV chamber wall (hypoperfusion ≥4 consecutive cardiac cycles in regions of viable papillary myocardium on LGE-CMR). Papillary ischemia was differentiated from artifact (see Appendix for representative examples) based on duration and timing of appearance (ischemic defects evident with contrast arrival in LV myocardium and persistent beyond time point of peak enhancement of uninvolved LV regions; dark-rim artifact evident with contrast arrival in LV chamber with transient duration), size (ischemic defect > 2 pixels in depth), and differential perfusion between stress and rest (nearly all exams [98%] had rest datasets to compare differential perfusion). Papillary ischemia was scored as present or absent, and categorized by location; no exams were excluded based on image quality. Prior to application in the broader nested-case control cohort, papillary ischemia analysis was evaluated in a sub-group (n=100) in which results demonstrated high inter- and intra-reader reproducibility (κ= 0.800, 0.804 respectively). Papillary muscle infarction was scored if any papillary hyperenhancement was visually evident on LGE-CMR, in accordance with established methods.(7)
Statistical Analysis
Continuous variables are summarized as means ± standard deviations when normally distributed, and otherwise as medians [interquartile range]; categorical variables as frequencies and percents. Generalized linear models were fit to characterize associations of patient characteristics with FMR; linear tests of trends were done by specifying relevant contrasts. Categorical variables were compared using chi-square tests. Intra- and inter-rater reproducibility of papillary ischemia assessment was tested using the kappa statistic. Logistic regression was performed to evaluate associations between imaging parameters and FMR in the overall cohort. Conditional logistic regression was used to evaluate associations between imaging parameters and FMR in the nested-case control cohort. Survival was estimated with the Kaplan-Meier estimator; patients were considered at risk starting at the date of imaging study until death, with censoring at the last date Social Security Death Index records were queried. Cox proportional hazards analysis was used to evaluate associations of clinical and imaging parameters with mortality. Two separate multivariable models using mortality as an outcome were constructed, one with clinical predictors and one with imaging predictors; variables with p<0.05 were retained in the models and retained variables were then combined in a single model: Incremental value of FMR to the model was tested using a likelihood ratio test. A 2-sided p<0.05 was considered statistically significant. Statistical calculations were performed using SPSS version 25.0 (SPSS, Chicago IL) and Stata version 15.0 (Stata Corp., College Station TX).
Results
Population Characteristics
The overall population encompassed 8,631 patients undergoing stress perfusion CMR for evaluation of known or suspected CAD, among whom FMR was present in 27% (n=2364). Among patients with FMR, severity was advanced (≥moderate) in 16% (n=372 [83% [n=309] moderate, 17% [n=63] severe]).
Table 1 reports demographic and LV geometric/functional remodeling characteristics among the overall population, including comparisons among patients stratified by FMR severity. FMR severity increased in parallel with age and prevalence of clinically established CAD (all p≤0.001). Notably, 54% of patients with advanced FMR had clinically reported heart failure at time of stress CMR. Regarding symptoms, dyspnea was more common among patients with FMR (p<0.001), paralleled by increased usage of heart failure medications. Consistent with this, adverse LV remodeling was strongly related to FMR, evidenced by an over 2.5-fold increment in LV end-systolic volume among patients with advanced compared to those without FMR, as well as lesser (albeit significant) increments in LV end-diastolic volume and dysfunction (all p<0.001).
TABLE 1.
Population Characteristics
| Mitral Regurgitation Severity | |||||
|---|---|---|---|---|---|
| Overall (N = 8,631) |
Absent (n = 6,272) |
Mild (n = 1,992) |
Advanced* (n = 372) |
p Value | |
| Clinical | |||||
| Age. yrs | 60.0 ± 14.1 | 58.7 ± 14.1 | 63.4 ± 13.4 | 64.5 ± 14.1 | <0.001 |
| Sex | 0.061 | ||||
| Male | 55 (4,765) | 55 (3,461) | 56 (1,118) | 50 (186) | |
| Female | 45 (3,866) | 45 (2,806) | 44 (874) | 50 (186) | |
| Body surface area. m2† | 1.81 ± 0.23 | 1.82 ± 0.23 | 1.80 ± 0.23 | 1.75 ± 0.24 | <0.001 |
| Pharmacological stress protocol | <0.001 | ||||
| Adenosine | 71 (6,034) | 70 (4,320) | 72 (1,416) | 80 (298) | |
| Regadenoson | 29 (2,498) | 30 (1,872) | 28 (553) | 20 (73) | |
| Atherosclerosis risk factors | |||||
| Hypertension | 68 (5,830) | 65 (4,099) | 72 (1,440) | 78 (291) | <0.001 |
| Hypercholesterolemia | 56 (4,841) | 55 (3,436) | 59 (1,181) | 60 (224) | 0.032 |
| Diabetes mellitus | 28 (2,399) | 27 (1,674) | 31 (609) | 31 (116) | 0.053 |
| Current or prior tobacco use | 32 (2,719) | 31 (1,955) | 33 (652) | 30 (112) | 0.662 |
| Family history of coronary artery disease | 31 (2,650) | 31 (1,940) | 30 (604) | 29 (106) | 0.326 |
| Known coronary artery disease | 30 (2,583) | 28 (1,743) | 35 (701) | 37 (139) | <0.001 |
| Myocardial infarction | 19 (1,642) | 17 (1,083) | 23 (457) | 28 (104) | <0.001 |
| Percutaneous coronary intervention | 7 (579) | 7 (429) | 7 (134) | 4 (16) | 0.062 |
| Coronary artery bypass graft | 2 (200) | 2 (140) | 3 (49) | 3 (11) | 0.364 |
| Clinical heart failure | 18 (1,515) | 13 (801) | 26 (513) | 54 (201) | <0.001 |
| Symptoms | |||||
| Chest pain | 48 (4,166) | 51 (3,174) | 42 (844) | 40 (148) | <0.001 |
| Dyspnea | 28 (2,458) | 27 (1,674) | 32 (635) | 40 (149) | <0.001 |
| Palpitations | 8 (672) | 8 (525) | 7 (131) | 4 (16) | 0.007 |
| Cardiovascular medications | |||||
| Aspirin | 57 (4,907) | 55 (3,472) | 60 (1,200) | 63 (235) | 0.001 |
| HMG-CoA reductase inhibitor | 50 (4,283) | 48 (3,008) | 54 (1,072) | 55 (203) | 0.009 |
| Beta-blocker | 36 (3,097) | 33 (2,055) | 43 (856) | 50 (186) | <0.001 |
| ACE inhibitor/ARB | 47 (4,040) | 45 (2,795) | 52 (1,026) | 59 (219) | <0.001 |
| Aldosterone antagonist | 5 (414) | 3 (212) | 8 (150) | 14 (52) | <0.001 |
| Loop diuretic | 31 (2,689) | 28 (1,753) | 37 (729) | 56 (207) | <0.001 |
| CMR | |||||
| Left ventricular function/geometry* | |||||
| End-diastolic volume, ml/m2 | 83.2 ± 30.9 | 78.1 ± 24.9 | 90.9 ± 34.5 | 130.9 ± 49.0 | <0.001 |
| End-systolic volume, ml/m2 | 39.6 ± 28.5 | 34.3 ± 20.9 | 47.6 ± 33.7 | 88.4 ± 48.7 | <0.001 |
| Ejection fraction, % | 55.6 ± 14.1 | 57.9 ± 12.0 | 51.9 ± 16.3 | 36.7 ± 16.4 | <0.001 |
Values are mean ± SD or % (n). unless otherwise indicated.
Advanced functional mitral regurgitation was first identified by using established semi-quantitative criteria (17) and then confirmed quantitatively based on differential aortic forward flow (phase contrast - cardiac magnetic resonance [CMR]) to left ventricular stroke volume (cine-CMR): 26.6% (n = 99). differential right ventricular/left ventricular stroke volume (cine-CMR): 50.8% (n = 189), and/or anatomic regurgitant office area (cine-CMR): 22.6% (n = 84).
Body surface area unavailable for 2.5% (n = 216). left ventricular volume unavailable for 4% (n = 374 [not quantifiable due to technical artifact or arrhythmia]). The p values are for linear tests of trends. The p values bolded where p < 0.05.
ACE - angiotensin-converting enzyme; ARB - angiotensin receptor blocker; MMG-CoA - 3-hydroxy-3-methylglutaryl coenzyme A.
LV Chamber Wall Ischemia
Figure 2 illustrates segmental partitions used to localized ischemia regionality (2A) and analyses of localized sub-papillary ischemia on advanced FMR (2B). As shown, patients with ischemia localized only to sub-papillary regions were more likely to have advanced FMR (OR 1.37 per 10% sub-papillary LV, [CI 1.11–1.61], p=0.003), as were those with infarction localized to these regions (OR 1.86 per 10% sub-papillary LV, [CI 1.42–2.46], p<0.001). Conversely, ischemia or infarction localized only to non-papillary muscle adjacent territories were not associated with advanced FMR (both p=NS). Similarly, multivariable analysis among the overall population demonstrated sub-papillary LV wall ischemia to be associated with advanced FMR (OR 1.15 per 10% sub-papillary LV, [CI 1.07–1.23], p<0.001) when controlling for ischemia in non-papillary muscle adjacent territories (OR 1.00 per 10% sub-papillary LV, [CI 0.93–1.08], p=0.919).
Sub-papillary ischemia is further detailed in Table 2A. As shown, prevalence of ischemia increased in relation to FMR (p<0.001) and was present in 31% of patients with advanced FMR. Among patients with sub-papillary ischemia, ischemia extent increased in relation to graded severity of FMR (p=0.004); differences between strata were more marked when assessed based on extent of ischemic segments with concomitant LV dysfunction (p<0.001) as evidenced by a 2-fold greater median extent of ischemia between patients with advanced FMR vs. those with mild FMR.
TABLE 2.
LV Subpapillary Ischemia and Infarction in Relation to FMR in Overall Population
| Mitral Regurgitation Severity | ||||
|---|---|---|---|---|
| Absent (n=6,267) | Mild (n=1,992) | Advanced (n=372) | p value | |
| LV SubPapillary Ischemia | ||||
| Present/Absent | ||||
| Anterior/Anterolateral | 10 (628) | 16 (325) | 19 (70) | <0.001 |
| Inferior/Inferolateral | 12 (770) | 20 (389) | 24 (89) | <0.001 |
| Aggregate | 17 (1,047) | 26 (510) | 31 (114) | <0.001 |
| Ischemia Extent (% subpapillary left ventricle)* | 25 (12.5–50.0) | 25 (12.5–50.0) | 37.5 (25.0–62.5) | 0.004 |
| Ischemia with dysfunction Extent (% subpapillary LV)* | 0 (0.0–12.5) | 12.5 (0.0–25) | 25 (0.0–50) | <0.001 |
| LV Sub-Papillary Infarction | ||||
| Present/dbsent | ||||
| Anterior/anterolateral | 12 (744) | 20 (393) | 31 (115) | <0.001 |
| Inferior/inferolateral | 17 (1,046) | 27 (538) | 43 (160) | <0.001 |
| Aggregate | 22 (1,369) | 34 (677) | 50 (186) | <0.001 |
| Infarct Extent (% subpapillary left ventricle)† | 7.8 (3.1–15.6) | 9.4 (4.7–16) | 15.6 (4.7–26.6) | <0.001 |
Values are % (n) or median (interquartile range), unless otherwise indicated. The p values are for linear tests of trends. Subpapillary regions partitioned such that total myocardium subtended by each territory was equivalent (anterior/anterolateral = basal-mid anterior/anterolateral walls; inferior/inferolateral = basal inferior/inferolateral walls).
Extent among patients with subpapillary ischemia.
Extent among patients with subpapillary infarction.
LV = left ventricular; FMR = functional mitral regurgitation.
Ischemia severity and distribution paralleled associations between FMR and LV infarction. As shown in Table 2A, presence and extent of sub-papillary infarction increased stepwise with FMR severity (p<0.001). Patients with FMR often had substantial viable myocardium in infarcted territories; among patients with advanced FMR, 47% of infarcts were fully subendocardial, demonstrating comparable degree of viability to patients without or with mild FMR (49% and 56% respectively). Multivariable analysis (Table 2B) demonstrated sub-papillary infarct size (OR 1.55 per 10% sub-papillary LV, [CI 1.43–1.69]) and ischemic/dysfunctional sub-papillary myocardium (OR 1.24 per 10% sub-papillary LV, [CI 1.17–1.31]) to each be associated with advanced FMR (both p<0.001) in a model composed of the two variables. Figure 3 provides representative examples of patients with inferior ischemia in regions with substantial viable myocardium, for whom presence or absence of regional contractile function in ischemic territories correlated with presence or absence of FMR.
Figure 3. Representative Examples.
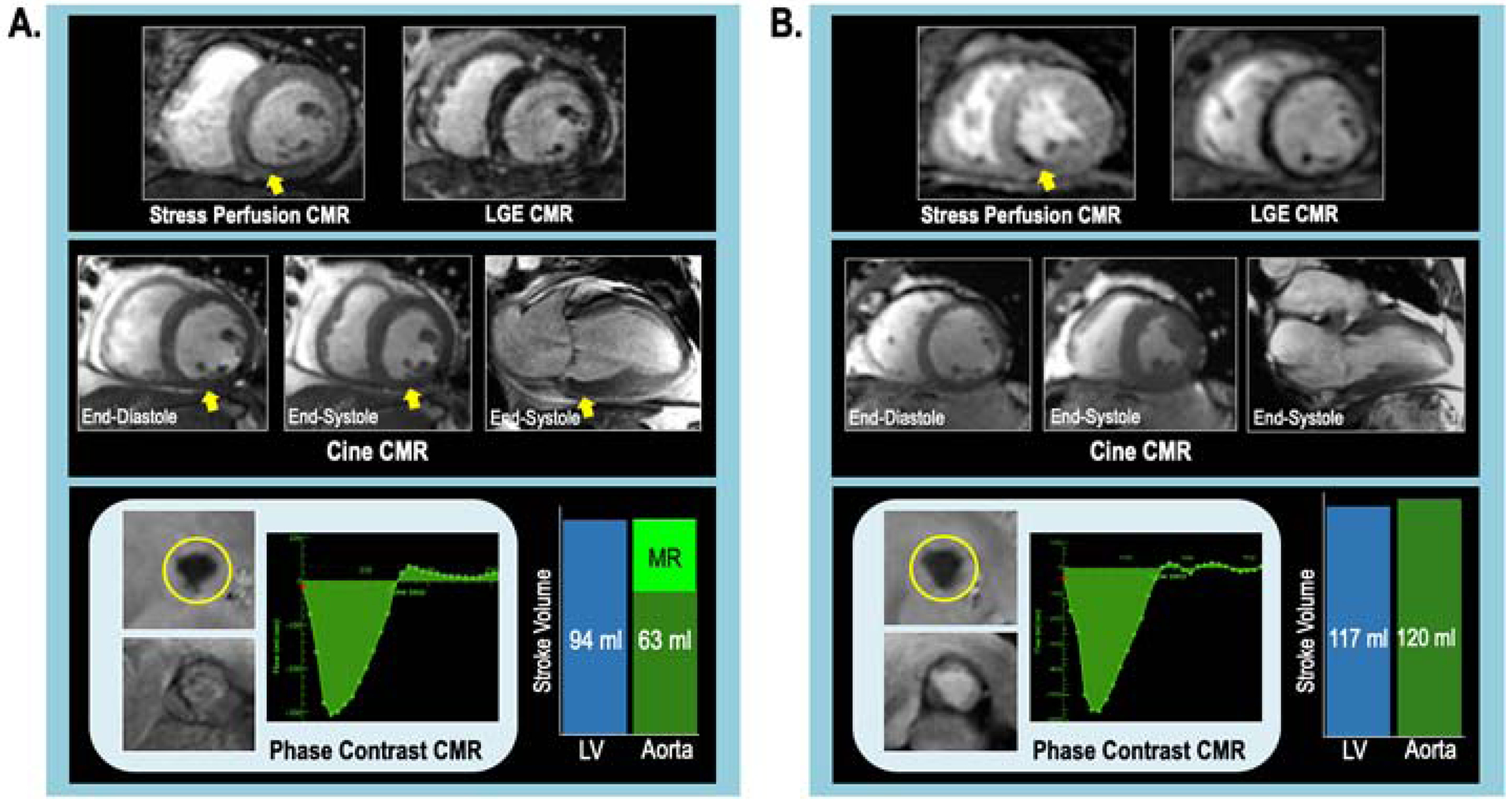
Examples of LV sub-papillary ischemia in patients with and without advanced FMR, illustrating hypothesized modulatory impact of contractile dysfunction.
3A. Advanced FMR: Stress CMR-evidenced ischemia involving the LV inferior, inferoseptal, and inferolateral walls (top left [arrow denotes perfusion defect]), which were viable on LGE-CMR (top right). Note impaired contractile function in ischemic territories as discerned by cine-CMR (middle left: short axis, middle right: 2-chamber orientation [arrow denotes dysfunctional region]). Quantitative analysis demonstrated advanced FMR (regurgitant fraction 33%, volume 31ml) [bottom left: phase contrast CMR, bottom right: differential LV/aortic stroke volume).
3B. No FMR: Stress CMR-evidenced ischemia involving LV inferior wall and inferoseptum (top left), which were viable on LGE-CMR (top right). Cine-CMR demonstrated preserved contractility in ischemic territories (middle left: short axis, middle right 2-chamber orientation). Quantitative analysis demonstrated equivalent LV-aortic stroke volume, consistent with absence of MR.
Papillary Muscle Ischemia
To elucidate mechanisms by which LV chamber wall ischemia impacted FMR, mitral apparatus remodeling and papillary muscle perfusion pattern were assessed in nested case-control cohort (n=1,116) including all patients with advanced FMR (n=372) as well as in unaffected (advanced FMR -) patients (n=744) matched (1:2) on sub-papillary infarct size (LV infarct size: 9.0±13.7%). Infarct size was near two-fold greater in the inferior/inferolateral as compared to the anterior/anterolateral sub-papillary region (5.9±10.1% vs. 3.1±6.6%, p<0.001), paralleling differential prevalence of papillary infarction; anterolateral and posteromedial papillary infarction were equivalent between groups ([anterolateral: 13.4% vs 13.3%, p=0.95] and [posteromedial: 6.2% vs 6.6%, p=0.80]), consistent with matching on sub-papillary infarct size.
As shown in Table 3A, patients with advanced FMR had greater adverse mitral apparatus geometric remodeling, including increased tenting area, mitral annular diameter, and interpapillary distances – paralleling impaired functional remodeling as evidenced by decreased interpapillary fractional shortening (all p<0.001). Additionally, despite the fact that groups were matched based on sub-papillary LV infarction, patients with advanced FMR had greater extent of sub-papillary ischemia (p=0.049) for which differences were more marked with respect to extent of ischemia with concomitant contractile dysfunction (p<0.001). Table 3A also demonstrates that papillary muscle ischemia paralleled ischemia in the LV wall; stress-induced perfusion defects involving the posteromedial papillary muscle were more common among patients with, vs. those without, advanced FMR (22% vs. 16%, p=0.006) whereas prevalence of anterolateral papillary muscle perfusion defects was nearly equivalent between groups (13% vs 12%, p=0.603). Multivariable analysis testing ischemic/dysfunctional sub-papillary myocardium and posteromedial papillary muscle ischemia together (Table 3B) demonstrated that advanced FMR was associated with sub-papillary LV ischemia with dysfunction (OR 1.16 per 10% sub-papillary LV, [CI 1.07–1.25], p<0.001) even after controlling for posteromedial papillary muscle ischemia (OR 1.37, [CI 0.97–1.95], p=0.074).
TABLE 3.
LV Subpapillary Ischemia and Infarction in Relation to FMR in Overall Population: Multivariable Analysis
| Univariable Odds Ratio* | 95% CI | p value | Multivariable Odds Ratio | 95% CI | p value | |
|---|---|---|---|---|---|---|
| Subpapillary Ischemia with dysfunction† | 1.33 | 1.27–1.41 | <0.001 | 1.24 | 1.17–1.31 | <0.001 |
| Subpapillary infarction† | 1.65 | 1.53–1.79 | <0.001 | 1.55 | 1.43–1.69 | <0.001 |
Subpapillary ischemia with dysfunction and subpapillary infarction tested in relation to advanced FMR.
Odds ratios per 10% subpapillary LV myocardium; p values for logistic regression.
Cl = confidence interval; other abbreviations as in Table 2.
Clinical Prognosis
Mortality was assessed after CMR in the overall cohort to determine whether FMR impacted survival risk after adjustment for clinical determinants of cardiovascular prognosis, and substrates for FMR itself (ischemia, infarction, adverse LV remodeling). Follow-up occurred over a median interval of 5.1 years (IQR 2.5, 8.2 years), during which 1,473 deaths occurred (aggregate mortality 17%). All-cause mortality over 5 years was 32% (n=135) among patients with advanced FMR, 19% (n=437) with mild FMR, and 11% (n=901) among patients without MR. Figure 4 provides Kaplan-Meier survival curves for patient groups partitioned in relation to FMR severity. As shown, MR severity showed a stepwise relationship with mortality: patients with mild FMR had a >1.5-fold increase (HR 1.64, [CI 1.46–1.83], p<0.001) and patients with advanced FMR had a >2.5-fold increase (HR 2.79, [CI 2.34–3.34], p<0.001) in mortality compared to those without FMR. Notably, prognostic impact of advanced FMR (compared to patients without FMR) was demonstrable for different quantitative methods, as evidenced by augmented mortality risk among patients in whom advanced FMR was confirmed via phase contrast/cine-CMR differential LV stroke volume (HR=1.89 [CI 1.2–2.98]; p=0.006), cine-CMR differential LV/RV stroke volume (HR=3.19 [CI 2.55–3.99]; p<0.001), and cine-CMR anatomic regurgitant orifice area (HR=2.68 [CI=1.89–3.79]; p<0.001).
Figure 4. Mortality in Relation to FMR Severity.
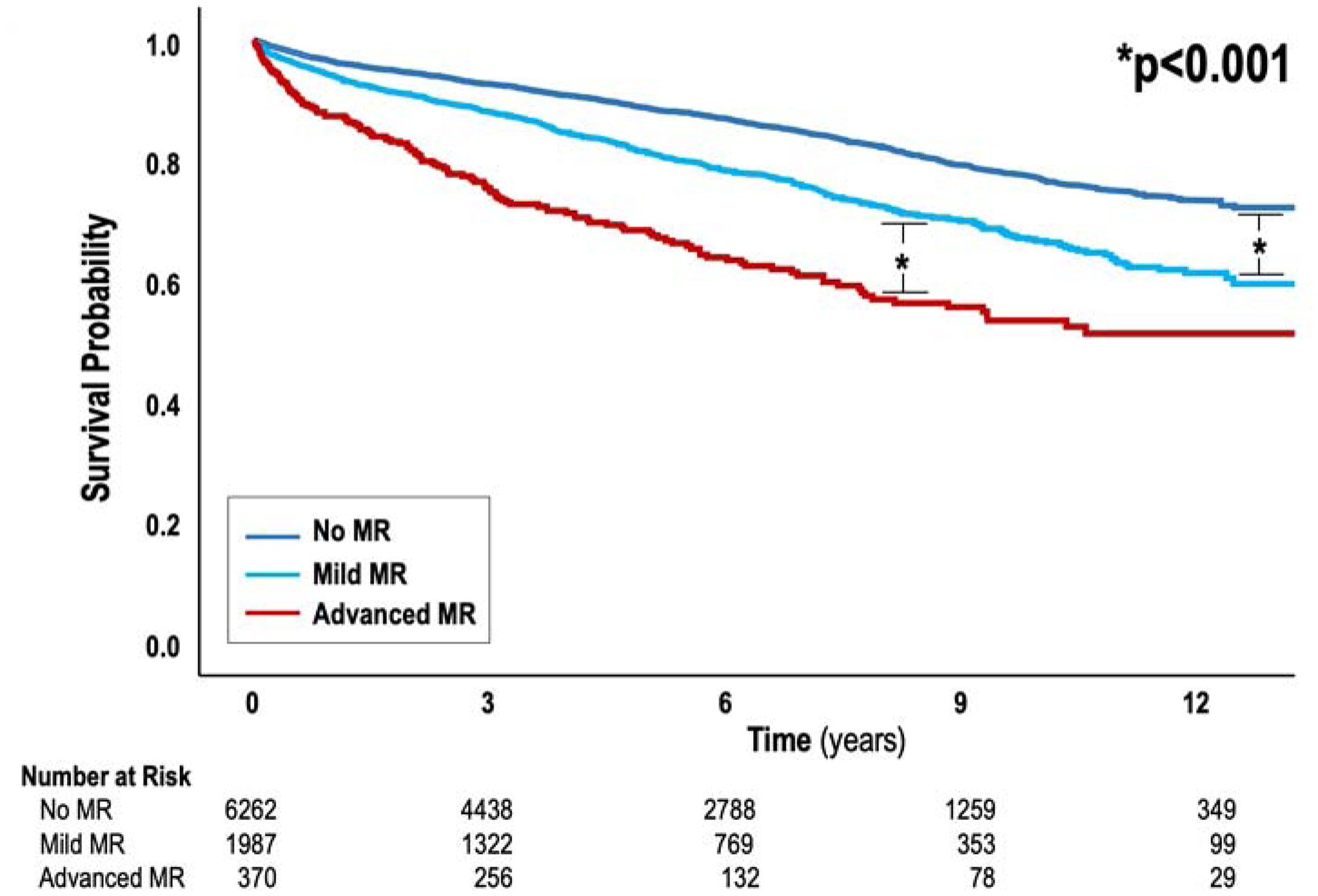
Kaplan-Meier survival curves depicting all-cause mortality among groups stratified based on FMR severity. Mortality increased in relation to FMR severity (p<0.001), including when patients with mild FMR were compared to those with no FMR as well as among patients with advanced FMR in relation to all other groups.
Table 4 provides multivariable analysis testing FMR in relation to all-cause mortality while controlling for clinical and imaging indices known to impact both prognosis and FMR. Table 4A presents a multivariable model of clinical risk factors, demonstrating male sex, age, and multiple cardiovascular risk factors to predict mortality. Table 4B presents a multivariable model of imaging indices, demonstrating LV ejection fraction (LVEF), global ischemia and infarction to predict mortality. The combined imaging and clinical model is shown with the addition of FMR in Table 4C, demonstrating FMR to be associated with mortality even after adjusting for clinical and imaging factors. Consistent with this, FMR remained associated with mortality (HR 2.23 for advanced FMR [1.34–1.69] | HR 1.51 for mild FMR [1.34–1.69]) when tested with sub-papillary ischemia with dysfunction (HR 1.12 per 10% sub-papillary LV [1.09–1.16]) and sub-papillary infarction (HR 1.20 per 10% sub-papillary LV [1.15–1.26], p<0.001 for all).
TABLE 4.
LV/Papillary Muscle Ischemia and Mitral Apparatus Remodeling in Nested Case-Control Cohort of Infarct-Matched Patients With and Without Advanced FMR
| Advanced Mitral Regurgitation | |||
|---|---|---|---|
| Absent (n = 744) | Present (n = 372) | p Value | |
| Mitral apparatus geometry | |||
| Mitral annular diameter, cm | 3.13 ± 0.48 | 3.48 ± 0.53 | <0.001 |
| Mitral tenting height, cm | 0.82 ± 0.29 | 1.09 ± 0.31 | <0.001 |
| Mitral tenting area, cm2 | 1.50 ± 0.68 | 2.37 ± 0.96 | <0.001 |
| LV sphericity index | 0.23 ± 0.09 | 0.30 ± 0.08 | <0.001 |
| Interpapillary end-diastolic diameter, cm | 2.62 ± 0.62 | 3.14 ± 0.75 | <0.001 |
| Interpapillary end-systolic diameter, cm | 1.50 ± 0.68 | 2.17 ± 0.85 | <0.001 |
| Papillary fractional shortening, % | 44.8 ± 15.6 | 33.2 ± 14.7 | <0.001 |
| Left atrial geometry | |||
| Left atrial area, cm2 | 23.4 ± 6.8 | 31.3 ± 8.9 | <0.001 |
| Left atrial diameter, cm | 3.75 ±1.10 | 4.51 ± 0.72 | <0.001 |
| Left ventricular/papillary ischemia | |||
| Subpapillary ischemia (present/absent) | |||
| Anterior/anterolateral | 17 (126) | 19 (70) | 0.421 |
| Inferior/inferolateral | 21 (159) | 24 (89) | 0.310 |
| Aggregate | 27 (204) | 31 (114) | 0.228 |
| Subpapillary ischemia extent (% subpapillary left ventricle)* | 25 (12.5–50.0) | 37.5 (25.0–62.5) | 0.049 |
| Subpapillary ischemia with dysfunction extent (% subpapillary left ventricle)* | 12.5 (0.0–25.0) | 25 (12.5–50.0) | <0.001 |
| Papillary muscle ischemia | |||
| Posteromedial | 16 (115) | 22 (81) | 0.006 |
| Anterolateral | 12 (88) | 13 (48) | 0.603 |
| Bilateral | 7 (53) | 10 (37) | 0.109 |
Values are mean ± SD, % (n), or median (interquartile range), unless otherwise indicated. Patients with and without advanced FMR matched (1:2) based on subpapillary infarct size; p values for conditional logistic regression.
Extent among patients with subpapillary ischemia.
Abbreviations as in Table 2.
Discussion
This study yields new insights regarding mechanism and prognostic utility of FMR (Central Illustration). First, LV ischemia underlying the mitral valve was strongly associated with FMR: Patients with ischemia localized to sub-papillary regions were more likely to have advanced FMR (p=0.003), whereas those with ischemia localized to other areas were not (p=NS). Ischemic and dysfunctional sub-papillary LV myocardium augmented risk of FMR even after controlling for infarct size (both p<0.001). Second, among groups matched on sub-papillary infarct size, patients with advanced FMR had greater adverse mitral apparatus remodeling – including larger mitral tenting area and reduced inter-papillary fractional shortening (both p<0.001) – paralleled by more extensive ischemic and dysfunctional sub-papillary myocardium. Whereas posteromedial papillary muscle ischemia was more common among patients with advanced FMR, sub-papillary ischemia with dysfunction remained associated with advanced FMR (p<0.001) after controlling for posteromedial papillary ischemia (p=0.074). Third, during 5.1 years median follow-up, mortality increased proportionally to FMR severity: FMR predicted mortality even after controlling for LVEF, ischemia and infarction.
Central Illustration: Sub-Papillary Ischemia is Associated with Functional Mitral Regurgitation.
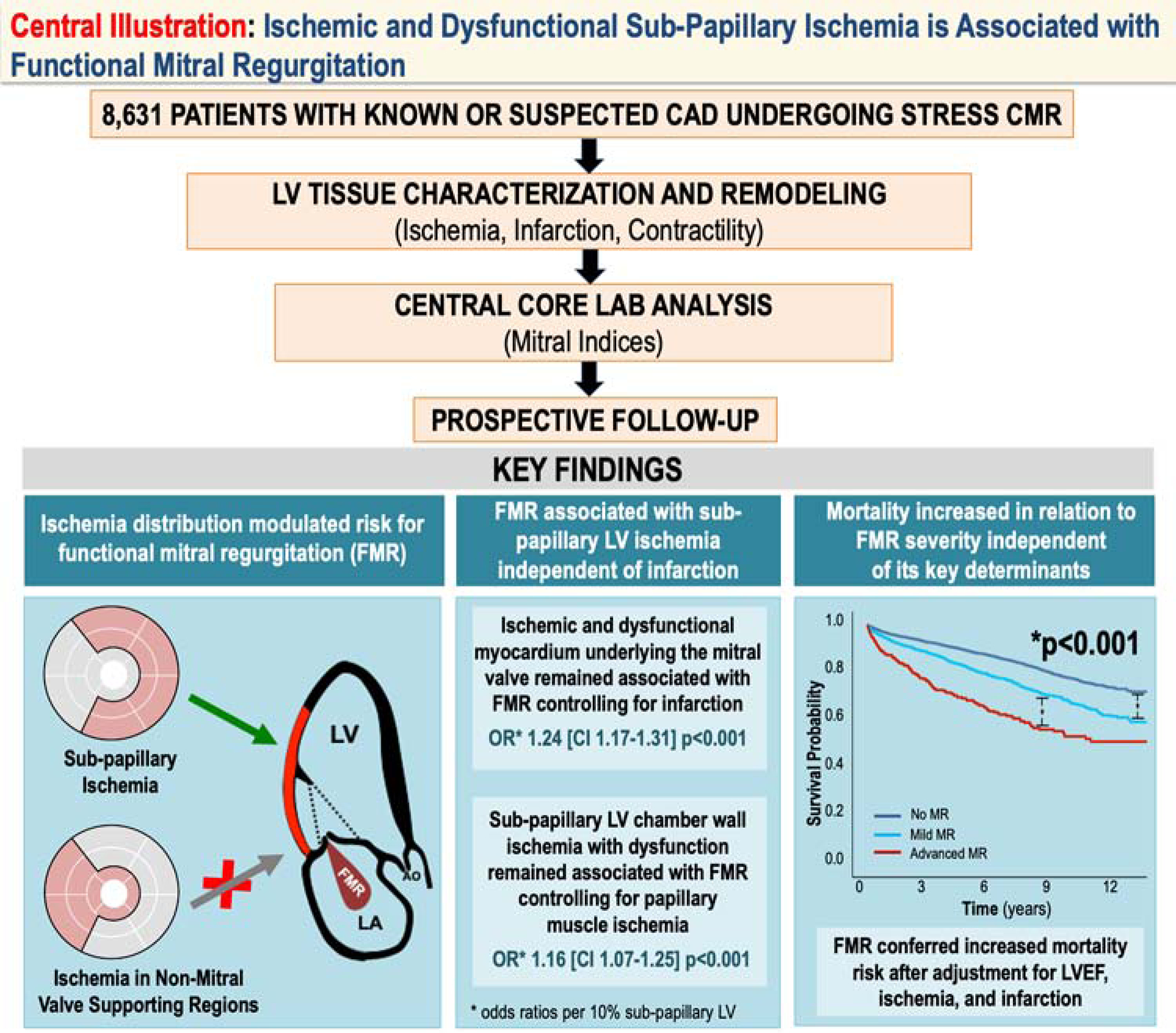
Multicenter data from the SCMR registry demonstrated stress CMR evidenced LV ischemia underlying the mitral valve to be a key determinant of FMR. Extent of ischemic and dysfunctional LV myocardium in sub-papillary segments remained associated with FMR when controlling for LV infarction in corresponding territories or papillary muscle ischemia. During follow-up, mortality risk increased in proportion to severity of FMR: FMR severity remained associated with increased mortality even after adjustment for conventional prognostic indices and determinants of FMR (LV ischemia, infarction, dysfunction).
Whereas FMR is commonly designated as “ischemic” when LV contractile dysfunction underlying the mitral valve is present, knowledge gaps persist regarding magnitude to which ischemia contributes to FMR. Experimental animal studies support a link between ischemia and FMR.(8,9) However, coronary anatomy may not reflect altered myocardial tissue substrate, including ischemia regionality and concomitant infarction. Additionally, findings derived from focal coronary occlusion may not apply to chronic CAD patients, in whom ischemia is often diffuse and coexists with infarction. Regarding clinical studies, it should be recognized that prior studies have shown that FMR can increase with exercise. For example, Giga et al(20) reported that FMR increase during stress echo correlated with change in wall motion: While these data suggest a link between ischemia and FMR, it should be noted that the population was comprised of post-MI patients and that MI-associated LV function can dynamically change during exercise (resulting in altered remodeling and FMR), raising questions as to whether exercise-induced MR is primarily due to ischemia. To further test links between ischemia and FMR, our group used radionuclide imaging,(16) and demonstrated perfusion defects in sub-papillary myocardium to be associated with FMR. However, data were acquired without dedicated viability imaging, which prohibited assessment of whether SPECT-evidenced perfusion defects represented ischemic or infarcted myocardium – providing a rationale for CMR in the current study.
While this the first CMR study to examine impact of LV and papillary muscle ischemia on FMR, our results extend logically upon prior animal data. For example, using a sheep model, Messas et al found that LV inferior ischemia acutely produced FMR, which was paradoxically diminished by concomitant posteromedial papillary muscle ischemia.(8) Similarly, prior CMR studies have shown that papillary infarction does not independently impact FMR. (7,13) In this study, posteromedial papillary ischemia was more common with advanced FMR, but the association between FMR and papillary ischemia was attenuated (p=0.074) when controlling for ischemic and dysfunctional myocardium in the adjacent LV (p<0.001). Our analyses demonstrated advanced FMR patients to have greater ischemic and dysfunctional myocardium in sub-papillary regions – paralleled by greater remodeling – compared to patients without advanced FMR but with equivalent infarct size and distribution. Taken together, our data support the concept that ischemia-mediated contractile dysfunction and adverse remodeling in mitral valve controlling regions contribute to pathogenesis of FMR.
Applied clinically, our findings provide insight on heterogeneous FMR response to coronary revascularization reported in clinical trials. For example, in the Cardiothoracic Surgical Trials Network trial,(21) 32% of patients with moderate MR undergoing coronary bypass surgery had advanced FMR at 2-year follow-up: Patients free of advanced FMR had greater improvement in inferior and lateral contractility (indicative of reverse remodeling in mitral valve supporting regions), consistent with our finding that ischemic and dysfunctional myocardium underlying the mitral valve was associated with FMR. In this context, our study supports utility of stress CMR to identify patients in whom FMR stems from ischemia (a potentially reversible substrate) who would be most likely to respond to revascularization.
Regarding outcomes, this study tested impact of FMR when controlling for LV ischemia, infarction, and LVEF – each of which are associated with mortality.(15) In prior studies that have examined FMR in relation to prognosis, LV injury was assessed indirectly via LVEF or biomarkers.(2–4) More recently, CMR has been used to test prognostic impact of FMR: Cavalcante et al reported FMR to confer increased mortality risk independent of LGE-CMR-evidenced infarct size.(5) However, lack of stress perfusion data prohibited assessment of ischemia as a confounder that could impact FMR and prognosis. Our data – demonstrating a link between FMR and mortality after controlling for ischemia and infarction – extends on prior literature by demonstrating that prognostic impact of FMR is incremental to that of key mechanistic determinants.
Several limitations should be noted. First, our study assessed ischemia on stress perfusion CMR using a standardized scoring algorithm, rather than quantitative assessment. However, our approach has been validated in prior research,(10,15) and parallels approaches in prior large-scale studies.(11,12) Second, it is important to recognize that our study leveraged “real world” clinical CMR data acquired at several experienced sites, encompassing over 8,500 patients over a 13-year interval. While stress CMR was acquired using a standardized protocol and data were transferred to a centralized core lab for dedicated analyses of key mitral remodeling indices, site-specific practices and variability in patient tolerance of additional CMR pulse sequences (e.g. phase velocity encoded) limited our ability to quantify advanced FMR in a uniform manner. It should also be noted that optimal criteria for definition of advanced FMR are uncertain, and that our findings might have been stronger had a different, standardized, cutoff been used. On the other hand, it should be noted that different approaches for MR assessment on CMR have been validated,(18,22) and that all advanced FMR patients had MR grade confirmed quantitatively. More broadly, our finding that graded FMR severity predicted mortality even after adjustment for an array of conventional prognostic indices adds support to the analytic approach used in our study. Regarding population, not all patients had established heart failure or advanced LV dysfunction and thus, FMR prevalence was lower than would be expected in a uniform heart failure cohort. Additionally, a registry limitation is that it did not enable assessment of alterations in medical regimen, coronary revascularization, targeted mitral interventions, or temporal changes in regurgitant severity to elucidate natural history of FMR.
In conclusion, this study demonstrates that ischemic and dysfunctional LV myocardium underlying the mitral valve is associated with FMR independent of infarction or papillary muscle ischemia. Given our finding that FMR strongly predicts mortality, future research is warranted to test whether coronary revascularization to resolve ischemia-mediated contractile dysfunction improves FMR and its clinical consequences.
Supplementary Material
Typical Examples of Papillary Muscle Ischemia and Artifact
Representative videos demonstrating stress-CMR evidenced (a) papillary ischemia (involving posteromedial papillary muscle) and (b) dark-rim artifact. Note that papillary muscle ischemia was distinguishable from artifact by timing of appearance (ischemic perfusion defect develops concomitantly with contrast arrival in LV myocardium), persistent relative hypoenhancement, and differential perfusion between stress and rest (top images: stress, bottom images: rest).
TABLE 5.
LV/Papillary Muscle Ischemia and Mitral Apparatus Remodeling in Nested Case-Control Cohort of Infarct-Matched Patients With and Without Advanced FMR: Multivariable Analysis
TABLE 6.
FMR-Related Clinical Predictors of All-Cause Mortality in Overall Cohort
| Univariable HR | 95% CI | p Value | Multivariable HR | 95% CI | p Value | |
|---|---|---|---|---|---|---|
| Male | 1.52 | 1.36–1.69 | <0.001 | 1.48 | 1.33–1.66 | <0.001 |
| Age (per 10 yrs) | 1.50 | 1.44–1.56 | <0.001 | 1.48 | 1.42–1.55 | <0.001 |
| Hypertension | 1.51 | 1.34–1.71 | <0.001 | 1.03 | 0.90–1.17 | 0.69 |
| Hyperlipidemia | 1.24 | 1.12–1.38 | <0.001 | 0.83 | 0.74–0.93 | 0.002 |
| Diabetes mellitus | 1.63 | 1.47–1.81 | <0.001 | 1.44 | 1.29–1.60 | <0.001 |
| Current or prior tobacco use | 0.89 | 0.79–0.99 | 0.04 | 1.11 | 0.99–1.25 | 0.08 |
| Family history | 1.15 | 1.03–1.30 | 0.02 | 0.90 | 0.80–1.01 | 0.07 |
| Known coronary artery disease | 1.49 | 1.34–1.66 | <0.001 | 1.13 | 1.00–1.26 | 0.04 |
| Clinical heart failure | 2.22 | 1.98–2.48 | <0.001 | 1.83 | 1.63–2.05 | <0.001 |
TABLE 7.
FMR-Related Imaging Predictors of All-Cause Mortality in Overall Cohort
| Univariable HR | 95% CI | p Value | Multivariable HR | 95% CI | p Value | |
|---|---|---|---|---|---|---|
| LV end-diastolic volume (per 10 ml/m2) | 1.09 | 1.07–1.10 | <0.001 | 1.01 | 0.99–1.03 | 0.46 |
| Ejection fraction (per 5% decrement) | 1.15 | 1.13–1.16 | <0.001 | 1.11 | 1.09–1.14 | <0.001 |
| Global LV infarction (per 10% increment) | 1.29 | 1.24–1.36 | <0.001 | 1.10 | 1.04–1.16 | 0.001 |
| Global LV ischemia (per 10% increment) | 1.13 | 1.10–1.15 | <0.001 | 1.09 | 1.07–1.12 | <0.001 |
TABLE 8.
FMR-Related Predictors of All-Cause Mortality in Overall Cohort: Combined Model
| Univariable HR | 95% CI | p Value | Multivariable HR | 95% CI | p Value | |
|---|---|---|---|---|---|---|
| FMR severity | <0.001* | |||||
| Mild vs. none | 1.64 | 1.46–1.84 | <0.001 | 1.21 | 1.07–1.36 | <0.01 |
| Advanced vs. none | 2.79 | 2.33–3.34 | <0.001 | 1.52 | 1.25–1.86 | <0.001 |
| Male | 1.52 | 1.36–1.69 | <0.001 | 1.36 | 1.22–1.52 | <0.001 |
| Age (per 10 yrs) | 1.50 | 1.44–1.56 | <0.001 | 1.47 | 1.41–1.53 | <0.001 |
| Hyperlipidemia | 1.24 | 1.12–1.38 | <0.001 | 0.83 | 0.74–0.94 | 0.002 |
| Diabetes mellitus | 1.63 | 1.47–1.81 | <0.001 | 1.48 | 1.32–0.165 | <0.001 |
| Known coronary artery disease | 1.49 | 1.34–1.66 | <0.001 | 0.99 | 0.88–1.12 | 0.88 |
| Clinical heart failure | 2.22 | 1.98–2.48 | <0.001 | 1.27 | 1.11–1.46 | <0.001 |
| Ejection fraction (per 5% decrement) | 1.15 | 1.13–1.16 | <0.001 | 1.08 | 1.05–1.10 | <0.001 |
| Global LV infarction (per 10% increment) | 1.29 | 1.24–1.36 | <0.001 | 1.08 | 1.01–1.15 | 0.03 |
| Global LV ischemia (per 10% increment) | 1.13 | 1.10–1.15 | <0.001 | 1.06 | 1.03–1.09 | <0.001 |
Perspectives: Core Clinical Competencies and Translational Implications.
Competency in Medical Knowledge 1:
Ischemic and dysfunctional left ventricular chamber wall myocardium subtending the papillary muscle is associated with functional mitral regurgitation (FMR) independent of infarct size and papillary muscle ischemia.
Competency in Medical Knowledge 2:
FMR is strongly associated with increased mortality even after controlling for established clinical cardiovascular prognostic indices or key mechanistic determinants (left ventricular ischemia, infarction, LV remodeling) of FMR itself.
Translational Outlook 1:
Future research is warranted to test whether targeted coronary revascularization to resolve ischemia-mediated contractile dysfunction improves FMR and associated adverse prognosis.
Acknowledgements:
The authors thank Dr. Seth Uretsky for valuable review and suggestions for this study.
Funding Sources: National Institutes of Health grants R01 HL128278 (JWW, MAR, RAL, JK), R01 HL128099 & R01 HL141917 (RAL), R01-HL63348 (MAR), K23 HL140092 (JK), K23 HL132011 (CS), T32 HL7854-23 (JDK). Glorney-Raisbeck Fellowship / NY Academy of Medicine (JDK).
Abbreviations
- CAD
Coronary Artery Disease
- CMR
Cardiac Magnetic Resonance
- FMR
Functional Mitral Regurgitation
- LA
Left Atrial
- LGE
Late Gadolinium Enhancement
- LV
Left Ventricle
- LVEF
Left Ventricular Ejection Fraction
- SCMR
Society for Cardiovascular Magnetic Resonance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interests / Disclosures: Dr. Judd has an equity interest, Dr. Raymond Kim serves on the Board of Directors, and Preston Cargile is an employee of for Heart Imaging Technologies. Dr. Klem is a consultant and a speaker honorarium for Bayer, and receives funding from Medtronic. Dr. Karmpaliotis receives funding from Abbott Vascular, Boston Scientific, Abiomed Equity: Saranas, Soundbite, and Traverse Vascular. Dr. Leon receives funding from Abbott Vascular, Boston Scientific, and Medtronic. Dr. Weinsaft has received speaker honoraria from GE Healthcare.
References
- 1.Groarke JD CB, O’Gara PT, Fuster V, Harrington RA, Narula J, Eapen ZJ. Ischemic Mitral Regurgitation. In: H JW, editor Hurst’s The Heart. 14 ed. New York, NY: McGraw-Hill Education, 2017. [Google Scholar]
- 2.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation 2001;103:1759–64. [DOI] [PubMed] [Google Scholar]
- 3.Bursi F, Enriquez-Sarano M, Nkomo VT et al. Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation 2005;111:295–301. [DOI] [PubMed] [Google Scholar]
- 4.Perez de Isla L, Zamorano J, Quezada M et al. Functional mitral regurgitation after a first non-ST-segment elevation acute coronary syndrome: contribution to congestive heart failure. Eur Heart J 2007;28:2866–72. [DOI] [PubMed] [Google Scholar]
- 5.Cavalcante JL, Kusunose K, Obuchowski NA et al. Prognostic Impact of Ischemic Mitral Regurgitation Severity and Myocardial Infarct Quantification by Cardiovascular Magnetic Resonance. JACC Cardiovasc Imaging 2019. [DOI] [PubMed] [Google Scholar]
- 6.Otsuji Y, Handschumacher MD, Liel-Cohen N et al. Mechanism of ischemic mitral regurgitation with segmental left ventricular dysfunction: three-dimensional echocardiographic studies in models of acute and chronic progressive regurgitation. J Am Coll Cardiol 2001;37:641–8. [DOI] [PubMed] [Google Scholar]
- 7.Chinitz JS, Chen D, Goyal P et al. Mitral apparatus assessment by delayed enhancement CMR: relative impact of infarct distribution on mitral regurgitation. JACC Cardiovasc Imaging 2013;6:220–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Messas E, Guerrero JL, Handschumacher MD et al. Paradoxic decrease in ischemic mitral regurgitation with papillary muscle dysfunction: insights from three-dimensional and contrast echocardiography with strain rate measurement. Circulation 2001;104:1952–7. [DOI] [PubMed] [Google Scholar]
- 9.Kono T, Sabbah HN, Rosman H et al. Mechanism of functional mitral regurgitation during acute myocardial ischemia. J Am Coll Cardiol 1992;19:1101–5. [DOI] [PubMed] [Google Scholar]
- 10.Klem I, Heitner JF, Shah DJ et al. Improved detection of coronary artery disease by stress perfusion cardiovascular magnetic resonance with the use of delayed enhancement infarction imaging. J Am Coll Cardiol 2006;47:1630–8. [DOI] [PubMed] [Google Scholar]
- 11.Greenwood JP, Maredia N, Younger JF et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet 2012;379:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagel E, Greenwood JP, McCann GP et al. Magnetic Resonance Perfusion or Fractional Flow Reserve in Coronary Disease. N Engl J Med 2019;380:2418–2428. [DOI] [PubMed] [Google Scholar]
- 13.Tanimoto T, Imanishi T, Kitabata H et al. Prevalence and clinical significance of papillary muscle infarction detected by late gadolinium-enhanced magnetic resonance imaging in patients with ST-segment elevation myocardial infarction. Circulation 2010;122:2281–7. [DOI] [PubMed] [Google Scholar]
- 14.Kwon DH, Kusunose K, Obuchowski NA et al. Predictors and Prognostic Impact of Progressive Ischemic Mitral Regurgitation in Patients With Advanced Ischemic Cardiomyopathy. Circulation: Cardiovascular Imaging 2016;9:e004577. [DOI] [PubMed] [Google Scholar]
- 15.Heitner JF, Kim RJ, Kim HW et al. Prognostic Value of Vasodilator Stress Cardiac Magnetic Resonance Imaging: A Multicenter Study With 48000 Patient-Years of Follow-up. JAMA Cardiol 2019;4:256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volo SC, Kim J, Gurevich S et al. Effect of myocardial perfusion pattern on frequency and severity of mitral regurgitation in patients with known or suspected coronary artery disease. Am J Cardiol 2014;114:355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heitner J, Bhumireddy GP, Crowley AL et al. Clinical application of cine-MRI in the visual assessment of mitral regurgitation compared to echocardiography and cardiac catheterization. PLoS One 2012;7:e40491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchner S, Debl K, Poschenrieder F et al. Cardiovascular magnetic resonance for direct assessment of anatomic regurgitant orifice in mitral regurgitation. Circ Cardiovasc Imaging 2008;1:148–55. [DOI] [PubMed] [Google Scholar]
- 19.Zoghbi WA, Adams D, Bonow RO et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303–371. [DOI] [PubMed] [Google Scholar]
- 20.Giga V, Ostojic M, Vujisic-Tesic B et al. Exercise-induced changes in mitral regurgitation in patients with prior myocardial infarction and left ventricular dysfunction: relation to mitral deformation and left ventricular function and shape. Eur Heart J 2005;26:1860–5. [DOI] [PubMed] [Google Scholar]
- 21.Michler RE, Smith PK, Parides MK et al. Two-Year Outcomes of Surgical Treatment of Moderate Ischemic Mitral Regurgitation. N Engl J Med 2016;374:1932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uretsky S, Gillam L, Lang R et al. Discordance between echocardiography and MRI in the assessment of mitral regurgitation severity: a prospective multicenter trial. J Am Coll Cardiol 2015;65:1078–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Typical Examples of Papillary Muscle Ischemia and Artifact
Representative videos demonstrating stress-CMR evidenced (a) papillary ischemia (involving posteromedial papillary muscle) and (b) dark-rim artifact. Note that papillary muscle ischemia was distinguishable from artifact by timing of appearance (ischemic perfusion defect develops concomitantly with contrast arrival in LV myocardium), persistent relative hypoenhancement, and differential perfusion between stress and rest (top images: stress, bottom images: rest).


