Abstract
Severe acute respiratory syndrome coronavirus 2 causes coronavirus disease 2019 (Covid-19), which has been declared as a pandemic by the World Health Organization. The aim of the study described here was to determine the severity of pneumonia and the clinical parameters related to a modified lung ultrasound score (mLUS) in patients with COVID-19 pneumonia. The study included 44 patients with proven COVID-19 pneumonia. Patients were divided into three groups on the basis of pneumonia severity: mild/moderate pneumonia (group I), severe pneumonia (group II) and critically ill patients (group III). It was determined that mLUS values in groups I–III were 6.51 ± 4.12, 23.5 ± 5.9 and 24.7 ± 3.9, respectively. mLUS values were significantly higher in group II and III patients than in group I patients. There was a positive relationship between mLUS and age and N-terminal pro-brain natriuretic peptide level and a negative relationship with PaO2/FiO2 (p = 0.032, β = 0.275 vs. p = 0.012, β = 0.315 vs. p = 0.001, β = –0.520, respectively). In patients with COVID-19 pneumonia, mLUS increases significantly with the severity of the disease.
Key Words: COVID-19 pneumonia, Ultrasonography, Modified lung ultrasound score
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19), which has been declared a pandemic by the World Health Organization. According to the World Health Organization report, 80% of those who contract the disease have mild symptoms. Pneumonia occurs in 14% of patients and progresses in 6% of patients, causing serious illness (Liu et al. 2020; Mattiuzzi and Lippi 2020). Pneumonia can progress to acute respiratory distress syndrome (ARDS), multi-organ dysfunction and death (Liu et al. 2020). Many demographic, clinical, laboratory and imaging method data related to disease severity have been collected. These can be summarized as advanced age, history of cardiovascular disease or risk factors, increased high-sensitivity cardiac troponin I (hs-cTnI), D-dimer, N-terminal pro-brain natriuretic peptide (NT-proBNP), high-sensitivity C-reactive protein (hs-CRP) and inflammatory cytokine levels, decreased lymphocyte level, hypoxia, hypotension, vasopressor requirement, low peripheral oxygen saturation (SpO2) and thorax computed tomography (CT) findings (Monastesse et al. 2017; Bhatraju et al. 2020; Du et al. 2020; Liu et al. 2020; Lyu et al. 2020)
Lung ultrasound (US) is an examination that can be performed at the bedside in patients with COVID-19 pneumonia and provide information on the severity of pneumonia. There are a few articles in the literature on the evaluation of lung US in patients with COVID-19 pneumonia, many of which share experience and images of their cases or suggest a review (Mongodi et al. 2020; Musolino et al. 2020; Peng et al. 2020; Poggiali et al. 2020; Sofia et al. 2020; Vetrugno et al. 2020). We use the lung US examination very frequently in both intensive care and inpatient services in our own clinics, and with this experience, after COVID-19 we obtained very good follow-up results with this examination in these patients. The lung ultrasound score (LUS) obtained as a result of examination of both lungs in 12 zones with lung US is a useful examination in the evaluation of patients with ARDS and bacterial pneumonia. In some previous publications, it has been reported that this examination can also be used in COVID-19 patients (Zhao et al. 2015; Vetrugno et al. 2020). However, this LUS assessment has not been evaluated in COVID-19 pneumonia. Modified LUS (mLUS) is a more specific assessment obtained by adding new parameters to the LUS score (Monastesse et al. 2017). We considered the hypothesis that the mLUS value, which is an objective parameter obtained with 12-zone lung US, could be more useful in terms of determining disease severity and follow-up, especially in cases with COVID-19 pneumonia, because of peripheral, bilateral and widespread lung involvement and the presence of ARDS in a significant number of cases. Therefore, our study was aimed at determining the severity of pneumonia and the clinical parameters related to the mLUS obtained with lung US in patients with COVID-19 pneumonia.
Methods
In this cross-sectional study, conducted between 15 March 2020 and 30 April 2020, 92 patients with pneumonia who were diagnosed with COVID-19, confirmed by detection of SARS-CoV-2 RNA in nasopharyngeal swab samples in the intensive care and inpatient services of our hospital, were evaluated. Before being included in the study, the patients were evaluated by two separate internal disease, chest disease and infectious disease specialists. In the case of indecision on the diagnosis, opinions of doctors with more experience from the relevant branches were obtained. COVID-19 patients with mild disease; pediatric patients (<18 y); those with a history of pulmonary disease (e.g., asthma, chronic obstructive pulmonary disease or restrictive pulmonary disease), severe kidney and liver dysfunction, acute myocardial infarction, heart failure, pulmonary embolism, previous heart or lung surgery, thyroid diseases, pregnancy or possibility of pregnancy active malignancy; and those who chose not to participate in the study were excluded from the study. The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee (Adana Health Practice and Research Center, Ethics Committee, 2020). All patients were included in the study after their written consent was obtained. After exclusion of patients on the basis of the criteria, 23 COVID-19 patients with mild to moderate pneumonia (group I: 13 men, 10 women, mean age 58.8 ± 5.1), 15 COVID-19 patients with severe pneumonia (group II: 7 men, 8 women, mean age 70.7 ± 9.2) and 6 critically ill COVID-19 patients (group III: 6 men, mean age 74.3 ± 7.2) were grouped; a total of 44 COVID-19 patients were included in the study (Fig. 1 ). Before the lung US and other examinations, the safety of personnel and devices was ensured to prevent COVID-19 contamination and spread in the light of the algorithms reported by our country's ministry of health. These safety measures were repeated before and after each lung US examination. Demographic features of all patients were obtained. During the lung US examination, all patients underwent for electrocardiography, pulse oximetry and non-invasive blood pressure measurements. At this stage, baseline heart rate, systolic blood pressure, diastolic blood pressure and SpO2 were recorded. Routine biochemistry parameters, white blood cell count, hemogram, blood glucose level, kidney function tests, and aspartate aminotransferase, alanine aminotransferase, hs-CRP, D-dimer, NT-proBNP and hs-cTnI levels were measured using an automated chemistry analyzer (Aeroset, Abbott, St. Paul, MN, USA) with appropriate commercial kits (Abbott).
Fig. 1.
Study flowchart illustrating patient selection
Arterial blood gas analysis
Patients were switched from the prone position to the supine position before blood gas analysis. Then, the area surrounding radial or femoral arteries was sterilized, and a 2-cm3 arterial blood gas sample was taken into the injector washed with 2 cm3 of heparin. The sampling was repeated when there was a suspicion about obtaining arterial blood gas or when a venous blood gas sample was suspected in the blood gas analysis. The ABL90 FLEX machine and micromode measurement method were used for blood gas analysis (Radiometer Medical ApS, Brønshøj, Denmark). Arterial partial pressure of carbon dioxide (PaCO2) and arterial partial pressure of oxygen (PaO2) were measured, and the PaO2/FiO2 (partial pressure of arterial oxygen/fractional inspired oxygen) ratio was calculated.
Reverse transcription real-time polymerase chain reaction
We enrolled 30 patients with COVID-19, as confirmed by detection of SARS-CoV-2 RNA in nasopharyngeal swab samples using a SARS-CoV-2 nucleic acid detection kit according to the manufacturer's protocol (Bioeksen, İstanbul, Turkey).
Lung ultrasound
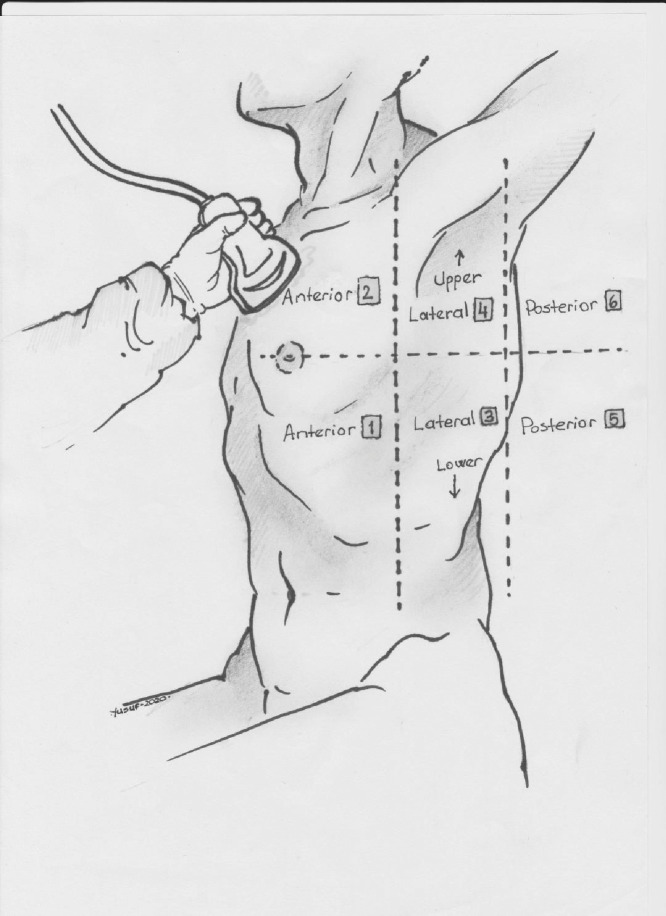
All patients and controls underwent lung US screening using a portable US device (Philips EPIQ 7) with a 2- to 5-MHz convex probe (Philips Health Care, Bothell, WA, USA). Participants were evaluated by two radiology specialists experienced in lung US. Specialists had more than 5 y of experience in pulmonary disease studies and had performed at least 500 lung US procedures per year. US examinations were approximately 15–20 min long. Lung US was performed using the B-mode technique, with the patient in sitting or supine position. The anteroposterolateral approach from the intercostal space was employed. Each hemithorax was divided into six zones in the supine position. First, the anterior and posterior axillary lines were identified, and the thorax was divided into three separate zones: front, middle and rear. A single line was drawn from the nipple or fourth intercostal space perpendicular to the anteroposterior axillary line. In this frame, a total of six zones were obtained by creating upper and lower zones (Fig. 2 ) (Soummer et al. 2012; Monastesse et al. 2017; Koh et al. 2019; Liu et al. 2020). The presence of pleural effusion was recorded. During the procedure, participants were asked to pause breathing for a few seconds to minimize pulmonary movement occurring with respiration. In each subject, 12 valid measurements from different pulmonary zones were obtained.
Fig. 2.
Each hemithorax was divided into six quadrants in patients in the supine position. First, anterior axillary and posterior axillary lines were determined; in this way, the thorax was separated into three zones: anterior, lateral, posterior. A single line was drawn from the nipple or fourth intercostal space perpendicular to the anterior–posterior axillary line, thus creating the upper and lower zones and resulting in a total of six zones. When this was applied to both hemithoraxes, a total of 12 zones were scored.
In lung US, the mLUS score was used for simple lung aeration loss assessment (Soummer et al. 2012; Monastesse et al. 2017). The previously mentioned 12 zones were scored between 0 and 3 with a simple grading system: 0 points for normal aeration (0–2 B-lines), 1 point for mild loss of aeration (≥3 B-lines or 1 or multiple small subpleural consolidations separated by a normal pleural line), 2 points for moderate loss of aeration (multiple coalescent B-lines or multiple small subpleural consolidations separated by a thickened or irregular pleural line) (Fig. 3 a–d) and 3 points for severe loss of aeration (consolidation or small subpleural consolidation >1 × 2 cm) (Fig. 4 a–d). A total of 12 pulmonary zone scores were obtained for both lungs, with scores between 0 and 36. The total modified LUS score of 0 was accepted as “no aeration loss,” and the modified LUS score of 36 was accepted as “complete aeration loss.”
Fig. 3.
Patient with mild to moderate pneumonia and a modified lung ultrasound score of 7 on lung ultrasonography examination. (a) Normal ventilation in the left upper anterior lung zone. (b) Two B-lines indicative of mild aeration disorder in the left lower posterior lung zone. (c) More than three B-lines indicative of mild aeration disorder in the left middle posterior lung zone. (d) Multiple small subpleural consolidations indicative of moderate aeration disorder in the right lower posterior lung zone.
Fig. 4.
Patient with severe pneumonia and a modified lung ultrasound score of 23 on lung ultrasonography examination. (a) Multifocal B-lines and severe aeration disorder in the left upper posterior lung zone. (b) Subpleural consolidations indicative of severe aeration disorder in the left lower anterior lung zone. (c) Consolidation in right middle anterior lung zone. (d) White lung pattern in the right lower posterior lung zone.
Statistical analysis
All analyses were performed using the SPSS 22.0 (SPSS for Windows 22.0, IBM, Armonk, NY, USA) statistical software package. Continuous variables in group data were expressed as the mean ± standard deviation. Categorical variables were expressed as the number (%). Continuous variables that were normally distributed were compared using the analysis of variance test, whereas the Kruskal–Wallis test was used to compare non-normally distributed samples. The χ2-test was used to compare categorical variables. Pearson's and Spearman's correlation analyses was performed to determine parameters related to mLUS. Linear regression analysis was performed for parameters that were more closely related to the parameters related to PaO2/FiO2 and mLUS score in the univariate analysis. Statistical significance was accepted as p < 0.05 for all comparisons.
Results
As previously mentioned, the study data were divided into three groups and compared. Lung US measurements were successfully obtained from all patients included in the study. Inter-observer agreement was determined as 96%.
Demographic and clinical data
When the demographic data were compared with respect to study group, the gender distribution was similar between the groups, but the mean age for group I was significantly lower than those for groups II and III. Although the frequencies of hypertension and diabetes mellitus were similar, the smoking rate in group III was higher. It was determined that among the clinical parameters, systolic and diastolic blood pressures were the lowest in group III and were significantly lower than those for the other two groups (Table 1 ). The baseline pulse increased from group I to group III, and group III patients had significantly higher baseline pulse values than group I (Table 1).
Table 1.
Clinical, demographic and laboratory findings of the three study groups
| Variable | Group I (n = 23) | Group II (n = 15) | Group III (n = 6) | p |
|---|---|---|---|---|
| Age (y) | 58.8 ± 5.1*,† | 70.7 ± 9.2 | 74.3 ± 7.2 | <0.001 |
| Sex (male/female) | 13/10 | 7/8 | 6/0 | 0.334 |
| Hypertension, n (%) | 12 (52%) | 9 (60%) | 4 (67%) | 0.776 |
| Diabetes mellitus, n (%) | 4 (17%) | 3 (20%) | 4 (67%) | 0.061 |
| Current smoker, n (%) | 12 (52%) | 6 (40%) | 6 (100%) | 0.014 |
| Systolic blood pressure (mm Hg) | 131 ± 21* | 128 ± 23‡ | 94 ± 15 | 0.002 |
| Diastolic blood pressure (mm Hg) | 76 ± 15* | 75 ± 12‡ | 71± 7.4 | 0.038 |
| Pulse (bpm) | 73 ± 12* | 77 ± 15 | 85 ± 18 | 0.049 |
| White blood cells (μL) | 5787 ± 1951 | 7673 ± 1486 | 7458 ± 1164 | 0.140 |
| Hemoglobin (g/dL) | 13.5 ± 1.44 | 12.2 ± 2.12 | 12.6 ± 1.48 | 0.067 |
| Glucose (mg/dL) | 106 ± 21* | 143 ± 42 | 186 ± 124 | 0.012 |
| Blood urea nitrogen (mg/dL) | 33.9 ± 15.1 | 36.2 ± 18.9 | 50.7 ± 6.6 | 0.358 |
| Creatinine (mg/dL) | 0.85 ± 0.38 | 0.96 ± 0.27 | 1.01 ± 0.18 | 0.243 |
| Aspartate aminotransferase (u/L) | 27.8 ± 6.6 | 29.1 ± 7.39 | 31.7 ± 15.1 | 0.595 |
| Alanine aminotransferase (u/L) | 20.9 ± 4.1* | 28.8 ± 15.2 | 35.3 ± 23.2 | 0.027 |
| hs-CRP (mg/dL) | 3.16 ± 0.32* | 4.23 ± 0.93 | 6.9 ± 4.5 | 0.012 |
| D-Dimer (ng/mL) | 445 ± 225* | 690 ± 507‡ | 3,090 ± 1,500 | <0.001 |
| NT-proBNP (pg/mL) | 75 ± 8.1* | 3,444 ± 6,485‡ | 18,733 ± 15,147 | <0.001 |
| hs-cTnI (ng/L) | 3.05 ± 1.70*,† | 9.07 ± 6.7 | 10.1 ± 9.74 | 0.019 |
hs-CRP = high-sensitivity C-reactive protein; hs-cTnI = high-sensitivity cardiac troponin I; NT-proBNP = N-terminal pro-brain natriuretic peptide; group I = COVID-19 patients with mild to moderate pneumonia; group II = COVID-19 patients with severe pneumonia; group III = COVID-19 critically ill patients.
Values are expressed as the mean ± standard deviation or n (%).
Significant association between groups I and III (p < 0.05).
Significant association between groups I and II (p < 0.05).
Significant association between groups II and III p < 0.05).
Laboratory data
Glucose, alanine aminotransferase and hs-CRP levels were found to be the highest in group III, significantly higher than the values in group I (Table 1). D-Dimer and NT-proBNP levels were found to be the highest in group III and were significantly higher than those in the other two groups (Table 1). The hs-cTnI level increased from group I to group III and was significantly higher in groups II and III than in group I (Table 1). Other laboratory parameters were found to be similar across all groups.
Blood gas and pulmonary ultrasonography data
When blood gas data were compared between study groups, PaO2, PaO2/FiO2 and SpO2 values were found to decrease from group I to group III, and these values significantly differed among all groups (Table 2 ). PaCO2 values were found to increase from group I to group III and significantly differed between groups (Table 2). On the basis of the lung US data, mLUS and mean score values were higher in groups II and III, and the latter groups significantly differed from group I (Table 2). These parameters were similar in group II and III patients.
Table 2.
Comparison of arterial blood gas analyses and pulmonary ultrasonography findings with respect to study group
| Variable | Group I (n = 23) | Group II (n = 15) | Group III (n = 6) | p |
|---|---|---|---|---|
| PaO2 (mm Hg) | 96.1 ± 2.3*,† | 83.7 ± 4.3‡ | 64.3 ± 2.9 | <0.001 |
| PaCO2 (mm Hg) | 40.9 ± 2.8*,† | 55.8 ± 5.5‡ | 70.7 ± 4.9 | <0.001 |
| PaO2/FiO2 (mm Hg) | 425 ± 17*,† | 351 ± 32‡ | 254 ± 33 | <0.001 |
| SpO2 (%) | 98 ± 1.7*,† | 92 ± 1.3‡ | 76.3 ± 4.6 | <0.001 |
| mLUS score | 6.5 ± 7.1*,† | 23.5 ± 5.9 | 24.7 ± 3.9 | <0.001 |
| Mean score | 0.54 ± 0.58*,† | 1.97 ± 0.51 | 2.05 ± 0.33 | <0.001 |
mLUS = modified lung ultrasound; PaO2/FiO2 = partial pressure of arterial oxygen/fractional inspired oxygen ratio; PaCO2 = arterial partial pressure of carbon dioxide; PaO2 = arterial partial pressure of oxygen; SpO2 = peripheral oxygen saturation; group I = COVID-19 patients with mild to moderate pneumonia; group II = COVID-19 patients with severe pneumonia; group III = critically ill COVID-19 patients.
Values are expressed as the mean ± standard deviation or n (%).
Significant association between groups I and III (p < 0.05).
Significant association between groups I and II (p < 0.05).
Significant association between groups II and III (p < 0.05).
Determination of parameters related to mLUS score
Correlation analysis was performed to determine the parameters associated with mLUS. These parameters are summarized in Table 3 . Linear regression analysis was performed to determine the presence of closer relationships with the parameters associated with mLUS in the correlation analysis (Table 3). As a result of this analysis, a positive and significant relationship was found between mLUS and age and NT-proBNP level. mLUS was found to be significantly and negatively associated with PaO2/FiO2 levels (Table 3). The relationship between mLUS and PaO2/FiO2 level is illustrated in Figure 5 .
Table 3.
Correlation and linear regression analysis for parameters significantly associated with modified lung ultrasound scores
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| p | r | p | β | |
| Age (y) | <0.001 | 0.591 | 0.032 | 0.275 (0.114–0.674) |
| D-Dimer (ng/mL) | 0.044 | 0.305 | 0.318 | 0.132 (0.098–0.451) |
| NT-proBNP (pg/mL) | 0.005 | 0.415 | 0.012 | 0.315 (0.154–0.685) |
| hs-cTnI (ng/L) | 0.022 | 0.344 | 0.184 | 0.158 (0.084–0.254) |
| PaO2/FiO2 (mm Hg) | <0.001 | – 0.697 | 0.001 | – 0.520 (–0.354 to –0.760) |
hs-cTnI = high-sensitivity cardiac troponin I; NT-proBNP = N-terminal probrain natriuretic peptide; PaO2/FiO2 = partial pressure of arterial oxygen/fractional inspired oxygen ratio
in multivariate analysis.
Fig. 5.
Scatterplot of the relationship of modified lung ultrasound scores to partial pressure of arterial oxygen/fractional inspired oxygen ratio (PaO2/FiO2).
Discussion
Our study has added important information to the literature on COVID-19 pneumonia. First, in patients with COVID-19 pneumonia, mLUS evaluation with lung US was performed for the first time, and it was thought that this examination may be useful in evaluating the severity of the disease. It is determined that there is a close and significant relationship between the PaO2/FiO2 value, which is an important parameter for ARDS and respiratory support requirements, and mLUS. Another important finding is the positive, significant relationship between mLUS score and NT-proBNP level, which is considered important for determining the severity and prognosis of the disease in COVID-19 patients. Our study is the first study to evaluate the mLUS score in patients with COVID-19 pneumonia and share the results.
Typical CT manifestations of the disease in cases with pneumonia are ground-glass opacity (GGO) or mixed GGO, consolidation and vascular dilatation (Pan et al. 2020; Shi et al. 2020). For this reason, lung CT is widely used in the diagnosis of COVID-19 pneumonia in our country and hospital. In COVID-19 pneumonia, lesions are characterized by bilateral involvement and peripheral distribution, and predominantly lower lobes are involved (Pan et al. 2020; Shi et al. 2020).
There is a general view and consensus that lung CT and lung US examination may be useful in these patients and there is growing evidence in the literature that support this opinion (Mongodi et al. 2020; Musolino et al. 2020; Poggiali et al. 2020; Peng et al. 2020; Sofia et al. 2020; Vetrugno et al. 2020). Particularly the disease's involvement of peripheral lung tissue more frequently is an important advantage for lung US examination. Lung US is an easy-to-learn (≥25 supervised exams) and safe examination that can be performed in about 5 min and requires no advanced software (Rouby et al. 2018). X-Ray and CT scans used in this disease cannot be repeated because of the problems involved with transport of critically ill patients and to limit the spread of the disease by patients infected with COVID-19. Because the latter scans also use ionized radiation, their repetition would cause further radiation exposure. Therefore, it is necessary to use lung US examination more widely.
In normal lung US evaluation, there is normal aeration in the lungs and A-lines are observed. These A-lines are parallel to the pleural line; the skin–pleural line and pleural line–A-line distances are very close. The main lung US finding in COVID-19 pneumonia is hyperechoic artifacts resulting from acute interstitial damage, perpendicular to the pleural line and extending deep and exhibiting vertical extension; these are called B-lines (Testa et al. 2012; Poggiali et al. 2020; Sofia et al. 2020; Vetrugno et al. 2020). The number, thickness and shape of B-lines are very important. For example, in lung US evaluation, 0–2 B-lines are scored 0 points, while ≥3 B-lines are scored 1 point (Monastesse et al. 2017; Koh et al. 2019). The number of existing B-lines may increase further, and multiple B-lines may form a single hyperechoic image, which is specified as "white lung." The white lung image in the lung US examination is the equivalent of GGO, which is the CT finding in pneumonia patients and is considered typical of COVID-19. The next stage is lung consolidations and is specified as the hepatization of the lung parenchyma. At this stage, lung aeration also suffers a major loss. However, none of these findings are specific for COVID-19. In the studies conducted, the lung US examination has 80% sensitivity in determining whether pneumonia is caused by bacterial or viral pathogens. However, available data are limited in terms of specific lung US examinations in viral pneumonia in patients with critical disease (Testa et al. 2012). In our study, all participants were considered probable COVID-19 on admission to the emergency department and confirmed with a positive nasopharyngeal swab specimen. For this reason, we did not perform lung US evaluation for the diagnosis of COVID-19-induced viral pneumonia in our study.
Very recently, a letter published by Peng et al. (2020) mentions their experience with lung US in 20 patients with COVID-19 pneumonia. In this very valuable article, the importance of 12-zone lung evaluation was mentioned (Koh et al. 2019; Peng et al. 2020). Important findings for COVID-19 in these articles are as follows: (i) pleural line thickening accompanied by irregularity; (ii) focal, multifocal and confluent B-lines; (iii) multifocal small, non-translobar and translobar consolidation; (iv) presence of A-lines in the recovery phase; and (v) infrequent pleural effusion. Although these data are very valuable, there is no objective classification and no scoring system with 12 zones. However, in the letter by Peng et al. (2020) and previous similar articles (Poggiali et al. 2020; Sofia et al. 2020; Vetrugno et al. 2020), only experience and case presentation information was shared, and no objective algorithm and scoring system was mentioned. In another study, Bonadia et al. (2020) scanned a total of 14 areas (3 posterior, 2 lateral and 2 anterior for each lung) and found that LUS, performed in the emergency department by emergency physicians, is able to predict at first evaluation the overall prognosis of COVID-19 patients, recognizing those needing ICU admission and those at higher risk of death. In our study, we used the mLUS score as an objective parameter. As a result, we obtained information on the severity of lung involvement in COVID-19 pneumonia over a 36-point scoring.
COVID-19 pneumonia generally progresses in four stages, and with respect to these stages, we have expert opinions and our own experience with lung US findings (Poggiali et al. 2020). Stage I is the onset of the disease, at which point lung US findings are normal. Stage II is the period of mild pneumonia; these patients have multiple B-lines, which is a simple lung US finding. Stage III is severe pneumonia; in the clinical follow-up, tachypnea, severe breathlessness and SpO2 <90% is observed despite O2 support. During this period, there are diffuse or multifocal B-lines and initial subpleural consolidations on lung US. Stage IV is the ARDS or critical illness period in which patients have PaO2/FiO2 values between 200 and 300 and require respiratory support. In stage IV, there are diffuse or multifocal B-lines, in addition to lung consolidation and white lung pattern, on lung US. However, it would be more appropriate in COVID-19 disease to obtain the lung US findings used in staging from all segments of both lungs. In our study, we did not include patients with stage I COVID-19 pneumonia because of the lack of lung US findings. We performed lung US evaluation in stage II–IV patients. In addition, we performed the US according to this pneumonia staging with 12-zone evaluations, as COVID-19 pneumonia disseminates within the whole lung. Thus, we obtained an objective parameter of disease severity through mLUS evaluation.
There are data from COVID-19 patients indicating that advanced age; male gender; low lymphocyte count; increased CRP, inflammatory cytokine, NT-proBNP and hs-cTnI levels; hypotension; hypoxia; vasopressor requirement; concomitant cardiac disease; and cardiovascular risk factors are associated with disease severity (Monastesse et al. 2017; Bhatraju et al. 2020; Liu et al. 2020; Lyu et al. 2020). In our study, we found that the mLUS score increased significantly in critically ill patients with severe pneumonia. In addition, we found that there is a very close and significant relationship between mLUS and PaO2/FiO2, NT-proBNP level and advanced age, which are parameters proven to indicate disease severity precisely. Considering the previous studies on mLUS, our knowledge of the location and course of lung involvement in COVID-19 infection and our own experience in this study, we conclude that the evaluation of mLUS would be a useful review in assessing the severity of COVID-19 disease.
Limitations
There are some important limitations to our study. One is the small number of patients in the study. Another limitation is that LUS parameters cannot be re-evaluated in patients who have clinically improved or worsened because of lack of clinical follow-up. Prognosis evaluation and studies involving more patients may provide more significant information. In our study, the effect of treatment of patients with COVID-19 pneumonia could not be evaluated. In addition, lung US examination and mLUS score were evaluated by a single physician for each patient to reduce the risk of contact and transmission of the disease, and evaluation was not repeated. Another limitation is that disease severity is already captured by respiratory parameters, age and comorbidities, and this study was not designed to provide us with information on the possible prognostic role of LUS. At the time of the clinical study, we could not evaluate mLUS because there were no patients with asymptomatic and mild pneumonia in our hospital. These patients were followed up in the outpatient clinic.
Conclusions
Bedside lung US and mLUS represent an effective tool for determining the severity of pneumonia in the intensive care unit. The mLUS value obtained with lung US as a result of our examination may be useful in patients with COVID-19 pneumonia and in the decision to provide respiratory support. Lung US examination is a safe, reproducible, easily applicable, radiation-free, low-risk, easy-to-learn examination and can also reduce X-ray and CT scan frequency; therefore, the frequency of its use in diagnosis, follow-up and disease severity determination in COVID-19 pneumonia should be increased. We think that lung US and mLUS values are useful parameters in the evaluation of the severity of pulmonary involvement in patients diagnosed with COVID-19. It may be beneficial to carry out studies that involve more patients and evaluate the regression and progression of the disease.
Acknowledgments
Conflict of interest disclosure
The authors have no conflicts of interest to disclose.
References
- Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, Greninger AL, Pipavath S, Wurfel MM, Evans L, Kritek PA, West TE, Luks A, Gerbino A, Dale CR, Goldman JD, O'Mahony S, Mikacenic C. Covid-19 in critically ill patients in the Seattle region—Case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadia N, Carnicelli A, Piano A, Buonsenso D, Gilardi E, Kadhim C, Torelli E, Petrucci M, Maurizio LD, Biasucci DG, Fuorlo M, Forte E, Zaccaria R, Franceschi F. Lung ultrasound findings are associated with mortality and need for intensive care admission in COVID-19 patients evaluated in the emergency department. Ultrasound Med Biol. 2020;46:2927–2937. doi: 10.1016/j.ultrasmedbio.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, Guo GY, Du J, Zheng CL, Zhu Q, Hu M, Li XY, Peng P, Shi HZ. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: A prospective cohort study. Eur Respir J. 2020;55 doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh JC, Hong JH, Kweon TD, Park JY, Ko E, Kim JY. Relationship between PaO2/FiO2 and number of regions with B-line on transthoracic lung ultrasound: A prospective, observational study. Anesth Pain Med. 2019;14:187–192. [Google Scholar]
- Liu X, Zhou H, Zhou Y, Wu X, Zhao Y, Lu Y, Tan W, Yuan M, Ding X, Zou J, Li R, Liu H, Ewing RM, Hu Y, Nie H, Wang Y. Risk factors associated with disease severity and length of hospital stay in COVID-19 patients. J Infect. 2020;81:e95–e97. doi: 10.1016/j.jinf.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu P, Liu X, Zhang R, Shi L, Gao J. The performance of chest CT in evaluating the clinical severity of COVID-19 pneumonia: Identifying critical cases based on CT characteristics. Invest Radiol. 2020;55:412–421. doi: 10.1097/RLI.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiuzzi C, Lippi G. Which lessons shall we learn from the 2019 novel coronavirus outbreak? Ann Transl Med. 2020;8:48. doi: 10.21037/atm.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastesse A, Girard F, Massicotte N, Chartrand-Lefebvre C, Girard M. Lung ultrasonography for the assessment of perioperative atelectasis: A pilot feasibility study. Anesth Analg. 2017;124:494–504. doi: 10.1213/ANE.0000000000001603. [DOI] [PubMed] [Google Scholar]
- Mongodi S, Orlando A, Arisi E, Tavazzi G, Santangelo E, Caneva L, Pozzi M, Pariani E, Bettini G, Maggio G, Perlini S, Preda L, Iotti GA, Mojoli F. Lung ultrasound in patients with acute respiratory failure reduces conventional imaging and health care provider exposure to COVID-19. Ultrasound Med Biol. 2020;46:2090–2093. doi: 10.1016/j.ultrasmedbio.2020.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musolino AM, Supino MC, Buonsenso D, Ferro V, Valentini P, Magistrelli A, Lombardi MH, Romani L, D'Argenio P, Campana A, Lung Roman. Ultrasound Study Team for Pediatric COVID-19 (ROMULUS COVID Team). Lung ultrasound in children with COVID-19: Preliminary findings. Ultrasound Med Biol. 2020;46:2094–2098. doi: 10.1016/j.ultrasmedbio.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Ye T, Sun P, Gui S, Liang B, Li L, Zheng D, Wang J, Hesketh RL, Yang L, Zheng C. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng QY, Wang XT, Zhang LN. Chinese Critical Care Ultrasound Study Group (CCUSG). Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med. 2020;46:849–850. doi: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggiali E, Dacrema A, Bastoni D, Tinelli V, Demichele E, Mateo Ramos P, Marcianò T, Silva M, Vercelli A, Magnacavallo A. Can lung US help critical care clinicians in the early diagnosis of novel coronavirus (COVID-19) pneumonia? Radiology. 2020;295:E6. doi: 10.1148/radiol.2020200847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouby JJ, Arbelot C, Gao Y, Zhang M, Lv J, An Y, Wang C, Bin D, Barbas CSV, DexheimerNeto FL, Prior Caltabeloti F, Lima E, Cebey A, Perbet S, Constantin JM, APECHO study group Training for lung ultrasound score measurement in critically ill patients. Am J Respir Crit Care Med. 2018;198:398–401. doi: 10.1164/rccm.201802-0227LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofia S, Boccatonda A, Montanari M, Spampinato M, D'ardes D, Cocco G, Accogli E, Cipollone F, Schiavone C. Thoracic ultrasound and SARS-COVID-19: A pictorial essay. J Ultrasound. 2020;23:217–221. doi: 10.1007/s40477-020-00458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soummer A, Perbet S, Brisson H, Arbelot C, Constantin JM, Lu Q, Rouby JJ, Lung Ultrasound Study Group Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress. Crit Care Med. 2012;40:2064–2072. doi: 10.1097/CCM.0b013e31824e68ae. [DOI] [PubMed] [Google Scholar]
- Testa A, Soldati G, Copetti R, Giannuzzi R, Portale G, Gentiloni-Silveri N. Early recognition of the 2009 pandemic influenza A (H1N1) pneumonia by chest ultrasound. Crit Care. 2012;16:R30. doi: 10.1186/cc11201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrugno L, Bove T, Orso D, Barbariol F, Bassi F, Boero E, Ferrari G, Kong R. Our Italian experience using lung ultrasound for identification, grading and serial follow-up of severity of lung involvement for management of patients with COVID-19. Echocardiography. 2020;37:625–627. doi: 10.1111/echo.14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Jiang L, Xi X, Jiang Q, Zhu B, Wang M, Xing J, Zhang D. Prognostic value of extravascular lung water assessed with lung ultrasound score by chest sonography in patients with acute respiratory distress syndrome. BMC Pulm Med. 2015;15:98. doi: 10.1186/s12890-015-0091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]