Hypertension, the leading cause of death among all cardiovascular disease (CVD) risk factors, affects >1.1 billion adults worldwide.1,2 Fetal growth restriction (FGR), generally defined as fetal growth <10th percentile for gestational age and sex, is associated with increased risk of perinatal death and increases the risk of CVD, the leading cause of mortality worldwide.3–6 The annual incidence of FGR varies between 5% and 15%, depending on country, thus making FGR-associated CVD a major public health concern.7
Systematic reviews and meta-analyses have confirmed the inverse association between lower birth weight (BW) and higher blood pressure (BP) in later life, across age groups and independent of body mass index.8,9 BP tracking develops quite early in life. A longitudinal study in 1797 infants noted that BP tracking became stronger with age to 4 years.10,11 Although experimental data strongly support the notion that FGR specifically programs hypertension, clinical studies remain equivocal.12,13 Furthermore, the underlying mechanisms are incompletely understood. Understanding these mechanisms will inform therapeutic approaches toward preventing or treating hypertension and attenuating CVD. Mechanistic links between FGR and hypertension include accelerated vascular aging, programming of the renin-angiotensin system (RAS), maladaptive heart/kidney structure and physiology, sympathetic nervous system hyperactivity, and epigenetics.
Several studies noted the inverse association between BW and large artery stiffness across age groups (neonates through to ~30-year-old adults).14–19 Disrupted arterial elastin synthesis and deposition leads to stiffer vessels. The rate of elastin synthesis falls sharply after birth, leading to a low elastin reserve in affected individuals.20 The RAS, through its various metabolites, closely regulates arterial vasculature, and heart and kidney functions. FGR in human pregnancies and animal experiments of undernutrition have also been associated with decreased numbers of nephrons.21,22 Other possible contributors to the prenatal programming of hypertension include superimposed hyperfiltration with activated intrarenal RAS and the sympathetic nervous system.23 Last, the timing, severity, and duration of decreased substrate supply also impacts cardiovascular adaptations associated with FGR.24
This review summarizes each of these mechanisms with a focus on prevention and therapeutic strategies across the entire life course to mitigate hypertension and CVD.
Early Vascular Aging
Effects initiated in utero (abnormal fetoplacental blood flow) and amplified after birth (infancy to old age) owing to decreased arterial compliance, have an etiologic role in primary hypertension.25 The stiffened arteries may affect cardiac structure and function proximally, and high pulsatile stress may accelerate microvascular organ disease distally. Meta-analyses place arterial stiffness as an independent risk marker for future CVD risk (including hypertension) after adjusting conventional risk factors.26,27 Longitudinal appraisal of temporal relationships between arterial stiffness and incident hypertension suggests the precursor role for arterial properties.28
Interlinking FGR, Arterial Remodeling, and Endothelial Dysfunction
Alterations in the extracellular matrix of major arteries are influenced by their elastin content.20,29 Multiple pathways mediate vascular pathology (Figure 1; available at www.jpeds.com). Replacement of elastin with collagen, which is 100 times stiffer, occurs in FGR and permanently alters arterial compliance.20,30 With normal aging, the proportion of elastin is decreased and collagen is increased, making infants with FGR a classic cohort for studying early vascular ageing. Superimposed on “normal arterial aging,” this manifests in increased large artery stiffness and clinically as high BP.
Figure 1.
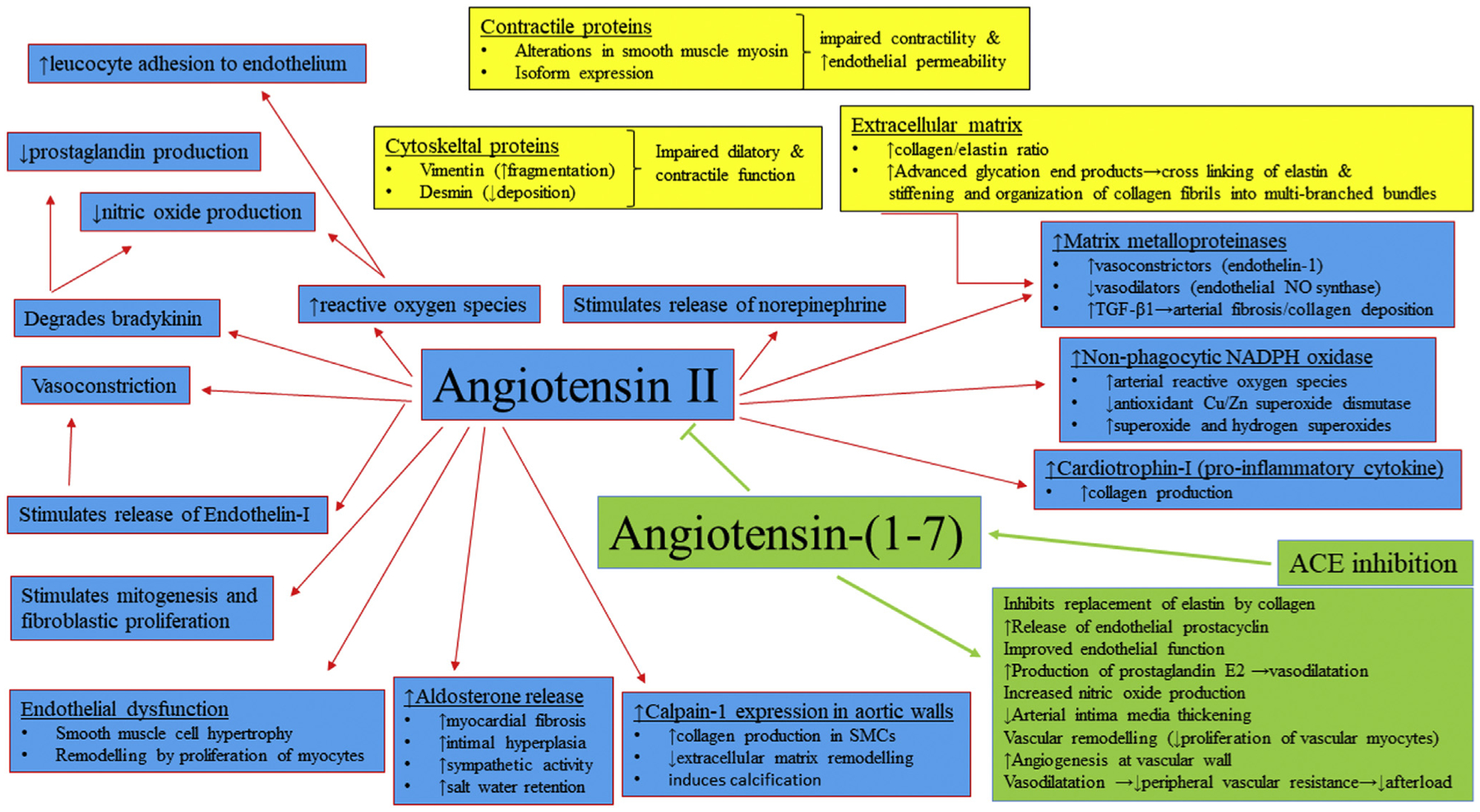
Mechanisms involved in increased arterial thickness and stiffness (central role of the RAS). ACE2, angiotensin-converting enzyme 2; Ang-(1–7), angiotensin-(1–7); AT1R, angiotensin II type 1 receptor; MasR, Mas receptor; NO, nitric oxide; TGF, transforming growth factor.
Cord blood samples from FGR deliveries indicate activated RAS, especially increased angiotensin, contributing to increased fetoplacental vascular resistance.31 Pregnancies complicated by FGR with Doppler alterations are associated with higher placental angiotensin-converting enzyme (ACE) activity compared with those with normal fetal growth or low BW without Doppler abnormalities.24,32 RAS activation contributes to age-related arterial remodeling, whereas chronic ACE inhibition (beginning at an early age) delays the progression, making it a monitoring/therapeutic target that requires more investigation.33 Decreased endothelial-derived nitric oxide bioavailability is also linked to vascular pathologies and lower BW infants show early, persistent signs of endothelial dysfunction.34,35
Vascular Measures as Predictors of Long-term Cardiovascular Health
The advent of high-resolution B-mode ultrasound imaging has led to cardiac intima media thickness (cIMT) being widely used as a measure of subclinical atherosclerosis in pediatric research. Children with familial hypercholesterolemia have an increased cIMT.36,37 In adults, cIMT predicts myocardial infarction, stroke or death, and has been used as an end point in clinical trials assessing the impact of antihypertensive medications on cardiovascular risk.38–41 In adults, arterial stiffness has a strong predictive value for CVD events beyond that of classical risk factors such as pulse pressure.42 Such use of cIMT in pediatric populations is recent. A double-blind, placebo-controlled randomized controlled trial (RCT) recruited 214 children with familial hypercholesterolemia (age range, 8–18 years). After 2 years of pravastatin therapy, the mean cIMT was decreased, whereas placebo therapy increased cIMT.43 Recent studies in neonates have found aortic intima media thickness (aIMT) equally useful, with increased aIMT, stiffness, and increased BP in infants with FGR early in the postnatal period.14–16,44 Relevant cardiac and vascular ultrasound features that are useful for understanding this maladaptation have been summarized elsewhere.45 At 32 weeks of gestation, growth-restricted fetuses have higher maximum aIMT and there is a correlation between fetal assessments and those 18 months after birth.46 Systolic BP was significantly higher in the FGR subjects, correlating with both prenatal and postnatal aIMT values.46 This differential trajectory, identified before birth, predisposes to later hypertension and CVD risk. Arterial assessments in FGR cohorts aged 8–13 years and young adults also noted increased stiffness, impedance, and higher central pulse pressure.47,48
Pulse wave velocity (PWV) measures arterial stiffness and is a measure of transit time and distance between the carotid and femoral arteries. A faster aortic PWV is related to the disruption in deposition of elastin and compounded by its replacement with stiffer collagen. In 707 young adults (~30 years of age), BW inversely correlated with PWV and pulse pressure, making PWV a plausible link between BW and elevated systolic BP.19 Carotid-femoral PWV is a good surrogate CVD end point and is recognized for its excellent predictive value for CVD complications by the European Society of Cardiology guidelines.19,41 The Baltimore Longitudinal Study of Ageing as well as a 2014 analysis of 27 longitudinal studies noted the importance of PWV predictive value.26,28 Whether PWV can be used to identify individuals that were born FGR, and thus, be a target for preventive strategies to delay the progression of subclinical arterial stiffening and the onset of hypertension should be studied prospectively.
Vascular Stiffness as a Therapeutic Target
Preventive strategies to decrease the burden of adult CVD are more likely to be beneficial when implemented in early life, rather than in adulthood (after subclinical disease is established or after the onset of overt disease). In a RCT, the inverse association of BW with cIMT was present in control children (~8 years), but abrogated in those receiving a docosahexaenoic acid-rich supplement from 6 months to 5 years of age.49 Dietary consumption of eicosapentaenoic acid and docosahexaenoic acid has specific vascular benefits in children and adolescents who were born FGR (eg, lower BP, lower cIMT, and aIMT).50 Furthermore, breastfeeding and postnatal nutrition improved FGR-induced cardiovascular remodeling (reduced heart sphericity and cIMT).51 RAS blockade has a significant impact on arterial structure and function independent of BP control.52–55 ACE inhibitors reset the balance between vasoconstriction/proliferation and vasodilatation/antiproliferation.56,57 In rodents, ACE inhibitors (but not β-blockers) caused regression of the media-to-lumen ratio.58 Clinical human studies confirmed these findings, indicating that structural arterial wall remodeling (rather than increased BP alone) underlies arterial stiffening, and RAS inhibitors may slow down vascular ageing.59–61 In infants with severe bronchopulmonary dysplasia associated hypertension ACE inhibition decreased the aIMT and increased pulsatility.62 The influence of prematurity and FGR on arterial structure and function may be intertwined. Comparing preterm born adolescent females (~16 years of age) with controls, BP was significantly higher in the former, whereas carotid stiffness and PWV were comparable with controls.63 In contrast, brachioradial artery stiffness and BP was significantly higher amongst preterm FGR born children but not those born preterm or term appropriate for gestation.64
RAS Maladaptation: Increased Angiotensin II and Decreased Angiotensin-(1–7)
Increased activation of the RAS and the vasoconstricting, proliferative, inflammatory, and fibrotic actions of angiotensin II on the vasculature may have an important role to play.
Mechanisms
There are 2 primary pathways within the RAS; the first consists of ACE-angiotensin II-angiotensin II type 1 receptor. Renin converts angiotensinogen into angiotensin I, which ACE then converts into angiotensin II, which acts at the angiotensin II type 1 receptor to increase BP via vasoconstriction, kidney salt, and water retention (via stimulating aldosterone release), and augmenting sympathetic nervous system tone.65,66 The counter-regulatory ACE2-angiotensin-(1–7)-Mas receptor pathway protects against angiotensin II-mediated increased BP.67 ACE2 converts angiotensin II into angiotensin-(1–7), which acts at the Mas receptor to promote vasodilation, increased kidney salt and water excretion, and increased parasympathetic tone.65,68–70 RAS is also immunomodulatory: angiotensin II promotes oxidative stress leading to inflammation and ultimately fibrosis, whereas angiotensin-(1–7) promotes nitric oxide production and mitigates angiotensin II-mediated inflammation.71 ACE and ACE2 also inactivate angiotensin-(1–7) and angiotensin I by converting them into less bioactive peptides.72,73 Thus, the balance between these RAS pathways is an important mediator in the pathogenesis of organ injury, as loss of angiotensin-(1–7) and/or increased angiotensin II can promote organ injury and hypertension development.74
Maternal/placental RAS dysregulation causing increased ACE-angiotensin II pathway expression and activity can contribute to placental insufficiency and resultant FGR, as well as mediate FGR-induced programmed hypertension in the offspring.75 The same holds true for ACE2-angiotensin-(1–7) pathway suppression as an important contributor to FGR-induced hypertension.67 FGR likely impairs beneficial cardiovascular and renal effects mediated via the angiotensin II type 2 receptor in offspring.76 Betamethasone-exposed sheep fetuses demonstrated higher BP, higher circulating ACE activity, lower ACE2 activity, and lower renal angiotensin-(1–7) levels associated with increased oxidative stress.77 Prenatal glucocorticoid-induced models of FGR in sheep consistently demonstrate that RAS programming in the brain and kidneys contribute to development of hypertension and can precede overt disease.78–81
Prediction of Later Disease
In humans, clinical evidence is lacking regarding the short- and long-term effects of FGR on offspring RAS expression, in part owing to the lack of a widely accepted research and clinical definition of FGR. Being born small for gestational age (BW <10th percentile for gestational age and sex) is a commonly used surrogate measure of FGR.5,82,83 Recent evidence supports the notion that perinatal RAS programming persists across the life span. Among children born term, low BW was associated with increased circulating ACE activity and angiotensin II compared with those born appropriate for gestational age; both circulating ACE activity and angiotensin II also positively correlated with BP.84 Compared with term deliveries, adolescents who were born preterm with very low BW had lower plasma angiotensin-(1–7) relative to angiotensin II concentrations, lower estimated glomerular filtration rate and increased proportion of high BP. High BP persisted into young adulthood; obesity and female sex magnified these programming effects.85–89
Therapeutic Targets
Further investigations are required to establish these causal mechanisms and to develop preventative and therapeutic strategies aimed at blocking or reversing abnormal RAS programming to shift the balance away from angiotensin II and back toward angiotensin-(1–7). RAS blockade with ACE inhibitors to block angiotensin II production, and angiotensin II receptor blockers to block angiotensin II’s actions via its receptor are mainstays in treating hypertension. Clinical trials are needed to investigate if increasing angiotensin-(1–7), in addition to decreasing angiotensin II, could reverse or attenuate these programmed alterations over the life span to prevent hypertension and CVD or to reverse subclinical disease. Novel therapeutics, such as angiotensin-(1–7) itself or orally available analogues as well as ACE2, could be administered in the neonatal or early childhood period to upregulate angiotensin-(1–7) at the expense of angiotensin II, especially during periods of active injury.90–92 For example, angiotensin-(1–7) attenuated vasoconstriction and induce renal vasodilation in adults.93,94 However, to date no trials have attempted to reverse programmed RAS alterations to prevent or attenuate disease.
Substrate Delivery and Fetal Heart Maladaptation
Fetal acute hypoxia leads to increased circulating noradrenaline, peripheral vasoconstriction and hypertension in the fetus, resulting in redistribution of blood flow are key adaptations to chronic hypoxemia.95,96
Mechanisms
Although FGR sheep with chronic hypoxemia have no change in blood flow to the brain, heart, and adrenals (Figure 2; available at www.jpeds.com), they do have a decrease in oxygen and glucose delivery to the heart.97–99 This decreased substrate delivery to the heart is associated with a delay in cardiomyocyte maturation and a lesser number of fetal cardiomyocytes in the fetus that is sustained into adolescence.100–103 In addition, the mature cardiomyocytes that contribute to heart growth by hypertrophy are relatively larger in the FGR heart compared with the normally grown fetus.100 This factor is mediated by changes in signaling pathways that promote cardiac hypertrophy, such as angiotensin II, insulin-like growth factor 1 and 2 receptor, and noradrenalin.104,105
Figure 2.
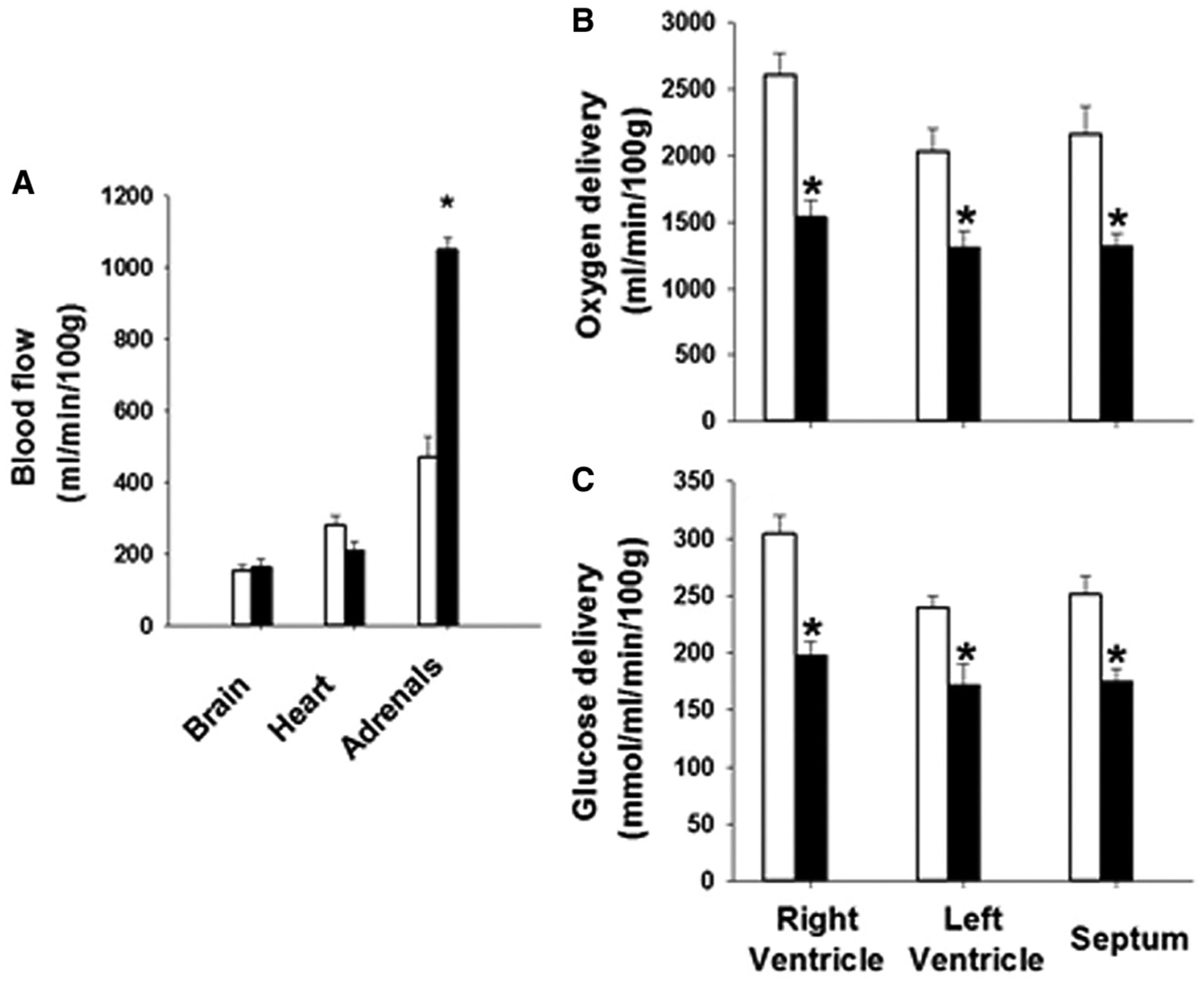
There is no change in blood flow to the brain or heart but an increase in blood flow to the adrenal glands in the chronically hypoxemic intrauterine growth restriction (IUGR) (black bars) compared with the control (white bars) fetus, A. However, there is a decrease in oxygen and glucose delivery to the heart of the IUGR fetus compared with controls, B and C.
Prediction of Later Disease
The timing, severity, and duration of reduced substrate supply leading to FGR negatively influences the developing fetus’s cardiovascular development and physiology.24 FGR directly affects the heart by way of altered myofiber architecture, reduction in cardiac sarcomeric proteins, increased glycogen and collagen deposition, and interstitial fibrosis.106,107 These effects are complemented by increased myocardial workload in the face of elevated placental resistance. FGR-induced fetal hypertension also contributes to cardiac hypertrophy. Human placental histopathology has recently noted vascular changes in FGR placentae.108 In utero cardiac remodeling has been noted by multiple investigators previously.109–111 Whether these early cardiovascular changes predict later-onset hypertension is as yet unknown.
Therapeutic Targets
Alterations in cardiac morphology are accompanied by subclinical cardiac dysfunction that can be demonstrated by fetal echocardiography.109–111 Several pathways have been identified as targets of intervention to improve heart health in the growth-restricted fetus, but must be tested in preclinical models to determine optimal timing and targets. In sheep models of chronic hypoxemia and FGR, fetal mean arterial BP is maintained but femoral artery blood flow is decreased compared with the normally grown fetus owing to greater dependence on the RAS and sympathetic tone.24,112–115 Infusion of an ACE inhibitor after approximately 135 days of gestation resulted in a greater hypotensive response in chronically hypoxemic FGR sheep fetuses when compared with normoxemic fetuses.113 High habitual fish intake (n-3 poly unsaturated fatty acid content) has shown to increase BW, possibly owing to the ratio of biologically active prostacyclins to thromboxanes, reducing blood viscosity, and thereby facilitating placental blood flow.116–118 In an RCT of 533 pregnant women, supplementation of 2.7 g/day of n-3 poly unsaturated fatty acid affected maternal thromboxane and prostacyclin production and pregnancies in the n-3 poly unsaturated fatty acid group weighed 107 g more (95% CI, 1–214).119,120 Although promising, the data are not yet sufficient to support a dietary supplementation recommendation. Oxidant injury is one of the proposed mechanisms. A double-blind, placebo-controlled RCT in FGR pregnancies is currently underway to assess the impact of maternal antenatal melatonin supplementation on early childhood neurodevelopmental (ACTRN12617001515381). Whether melatonin has the potential to alter the cardiometabolic milieu remains to be seen.
Kidney Maladaptation and Influence of the Autonomic Nervous System
Kidneys undergo maladaptation in response to uteroplacental insufficiency and FGR with reduction in nephron numbers and hyperfiltration injury through the remaining nephrons. Delivery of high-pressure waveform, undampened by stiff major arteries, affects glomerular filtration. FGR has been shown to be associated with persistent aortic wall thickening and higher microalbuminuria during infancy.46
Mechanisms
Nephron number correlates positively with BW, and decreased in nephron numbers may increase the risk of hypertension.121,122 Maternal protein restriction reduced numbers of glomeruli, increased glomerular size, reduced glomerular filtration rate, and higher BP.21 In sheep, prenatal glucocorticoid administration increased offspring BP, a decreased number of nephrons, and reduced glomerular filtration rate related to alterations to the renal RAS and sodium handling, in a sex-specific manner.81,123–125 Glucocorticoid exposure from gestational days 15 to 19 in the rat programmed higher BP associated with a decreased number of nephrons in male offspring, whereas early exposure from gestational days 13 to 14 programmed higher BP that was not associated with a change in nephron number.126 A decreased glomerular filtration rate was associated with decreased numbers of nephrons in male rats exposed to glucocorticoids but not maternal protein restriction.21,127 Once again, the timing and the type of insult seems critical. The relative contribution of the sympathetic and/or the parasympathetic nervous system to increased hypertension or CVD risk originating in early life remains unclear. Furthermore, a significant increase in heart rate in response to stress is associated with increased sympathetic and decreased parasympathetic nervous system activity in low BW women but not men, suggesting that discrepancies in sex-specific outcomes may be due to age or puberty status.128 Increased sympathetic nervous system activity is a mediator of increased heart rate in response to stress in low BW children and adolescents.129
Prediction of Later Disease and Therapeutic Targets
The role of the kidney in the developmental origins of hypertension extends beyond the contribution of nephron endowment. BP is regulated by activation of the RAS and sympathetic nervous systems. The reliance on either of these systems differs in growth-restricted FGR fetuses compared with normally grown fetuses. Many regulatory systems contribute to sodium and fluid balance and vascular and nervous system tone in the long-term BP control, including the RAS, and are therefore potential targets for therapeutic interventions.67,86,130,131 The promising potential of ACE inhibition has already been indicated elsewhere in this article. Prenatal exposure to maternal high salt intake programs sympathetic activation and increased BP response to stress in female but not male littermates, indicating that sex alters the developmental origins of sympathetic nervous system activation in a manner that is insult-specific.132 Infusion of an α-adrenergic antagonist, phentolamine, resulted in a greater fall in fetal BP in the FGR vs control fetuses that was related to the degree of hypoxemia.112 An inverse relationship between the magnitude of the fetal hypotensive response to α-adrenergic blockade and arterial PO2 suggested increased reliance on sympathetic tone to regulate BP in FGR. Chronic maintenance of BP through α-adrenoceptor activation in fetal life has long-term consequences for cardiovascular health. Renal denervation abolishes placental insufficiency- or perinatal dexamethasone-exposed programmed hypertension, implicating a role for the renal nerves.133–135 A summary of therapeutic constructs is presented in Figure 3 (available at www.jpeds.com).
Figure 3.
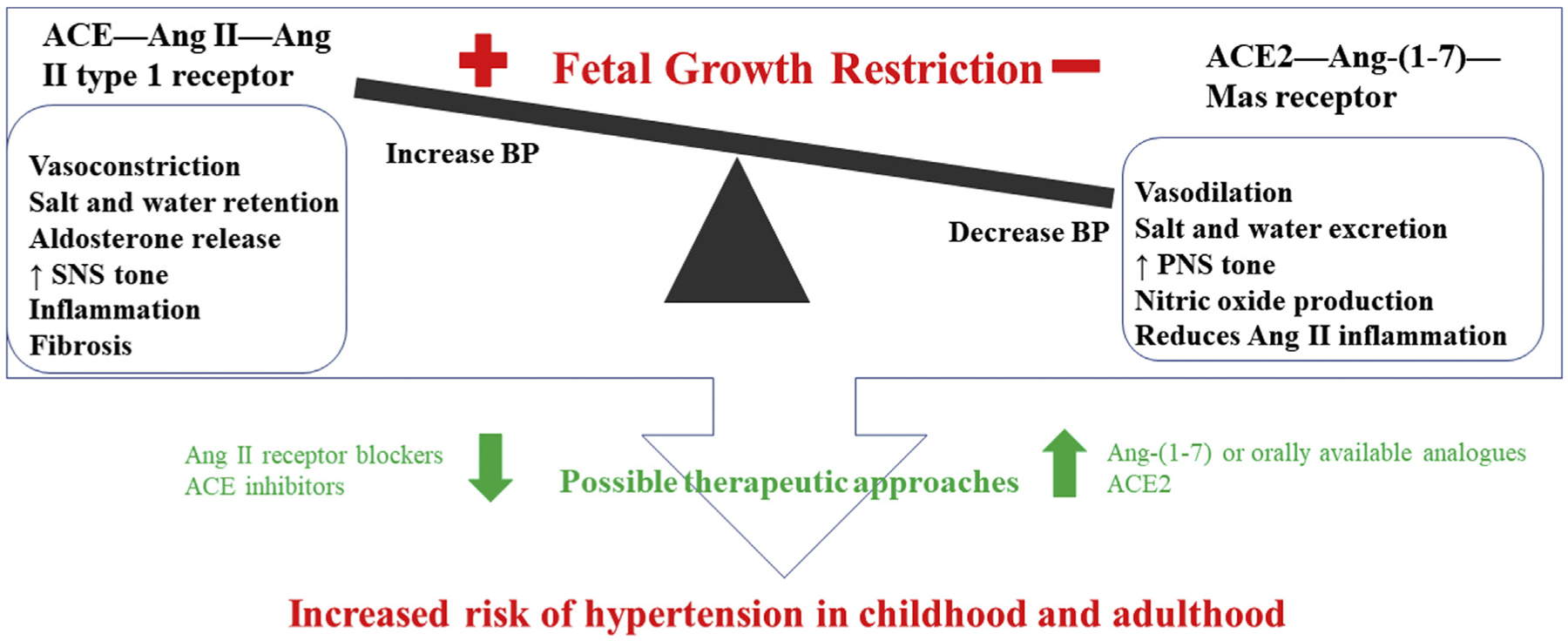
Summary of therapeutic constructs. Ang, angiotensin; SNS, sympathetic nervous system; PNS, parasympathetic nervous system.
Collectively, these studies indicate a critical role for the sympathetic nervous system in the etiology of increased BP in offspring exposed to FGR and other perinatal risk factors. Despite differences in the type of developmental insult, common mechanistic pathways contribute to the developmental origins of hypertension. Further investigation into the mechanisms involved in sympathetic activation and hypertension in individuals with FGR may lead to therapeutics and pharmacological targets to diminish CVD risk in this population.
Epigenetics: Generational Maladaptations
The role of epigenetic mechanisms in the developmental origins of cardiorenal disease is not yet clearly understood. Epigenetics implies inherited changes that do not alter the underlying DNA sequence, but ensure minute regulation of gene expression that may influence disease susceptibility in later life. Alcohol exposure during pregnancy is an example that alters the methylation patterns of several imprinted genes.136 Programming is initiated very early via epigenetic phenomena that occur preconception, periconception, or during gestation.105,137,138 Dietary protein restriction of pregnant rats induces, and folic acid supplementation prevents, epigenetic gene expression modification in offspring.139 FGR rats have changes in lung expression of specific microRNA that increase RAS molecules.140 Epigenetic processes can have a graded effect (as opposed to all-or-none), similar to the graded association between BW and chronic disease.141 In a study done on maternal low-protein rats, Bogdarina et al noted that alteration of DNA methylation of one or more RAS component genes might result in the development of hypertension. angiotensin AT1b receptor gene expression is highly dependent on promoter methylation; upregulation by the first week of life resulted in increased receptor protein expression.142
Limitations to Current Knowledge
The relatively low prevalence of FGR in most birth cohorts, the heterogeneity in definitions of FGR, and the heterogeneity of causes of FGR have limited clinical studies to date.4,13,82,83 Furthermore, it is crucial to accurately and consistently measure BP and define hypertension and related cardiac, kidney, and vascular outcomes to aid in comparing different clinical studies and pooling analyses. Developing reliable biomarkers to identify early or subclinical alterations in these mechanisms is vital. Inherent to this point is the reproducible measurement of components of the RAS that depend upon appropriate and rigorous blood and urine sample collection, processing, storage, and analytic methodologies.143 An improved understanding of the epidemiology of developmental origins of disease will aid in differentiating the risk imparted by FGR relative to other perinatal factors. This process should be the precursor to define that risk and enact therapeutic strategies to prevent or attenuate the early development of disease throughout the life span.
Moving Forward: Prevention and Therapeutics to Attenuate Maladaptation
Improving nutrition and living conditions between conception and early childhood and avoiding rapid increased in body size is key.144 In effect, the concept of mitigation through nutrition starts much earlier. Increased breast milk intake improved the metabolic and cardiovascular outcomes of growth-restricted animals.145 FGR was the strongest predictor of cardiovascular remodeling and BP at 4–5 years of age, breastfeeding for >6 months, and healthy fat dietary intake were associated with improved cardiac geometry and lower cIMT. Furthermore, overweight/obesity was associated with higher cIMT in FGR children compared with children born appropriate for gestational age.51 Evidently, postnatal nutrition ameliorates FGR-associated cardiovascular remodeling, identifying itself as an intervention option. A recent RCT indicates that micronutrient intervention in infancy (such as iron supplements) may modify the inverse association between BW and risk of hypertension.146
Monitoring childhood body weight and BP are essential for those whose BW were toward the lower end of the normal range or <10th percentile. The 2017 American College of Cardiology/American Heart Association and the American Academy of Pediatrics Clinical Practice Guidelines for High Blood Pressure in Adults and Children recommend screening BP in patients with a significant perinatal history; however, FGR per se is not included as a perinatal risk factor.147,148 This high-risk population includes those whose body mass index increases across percentiles. Prevention is paramount as the BP of patients with hypertension who had lower BW may be more difficult to control, often requiring multiple antihypertensive medications.149,150
Conclusions
Through the multiple mechanistic links outlined above, FGR is an important risk factor for later-onset hypertension. Better maternal periconception nutrition and health as well as management of preeclampsia, a common cause of FGR, are key toward prevention. Low-cost interventions such as breastfeeding and targeted early nutritional interventions need to be reinforced, and large-scale population-based studies are needed to assess their impact on future incidence of hypertension and CVD. Establishment of clinical normative values of arterial thickness and PWV could aid in identifying subclinical disease and identify appropriate therapeutic windows. Existing drugs (ACE inhibitors, angiotensin II receptor blockers) or novel therapies that can target the RAS to decrease angiotensin II’s actions and promote angiotensin-(1–7) may be useful in preventing hypertension or CVD or treating subclinical disease. Whether ACE inhibitors, with their arterial wall-modulating properties, may be beneficial to nephron preservation in this population remains to be determined. Measures of vasculature tone/function as end points for clinical assessments and therapeutics seem plausible as biomarkers, as are RAS measurements. Physical activity (avoidance of sedentary lifestyle) and improved lean-fat ratio may be key to attenuate the development of the metabolic syndrome and hypertension. Age-appropriate interventions, covering the life course nature of the disease (and its progression), may have a significant impact on preventing or delaying adult-onset CVD in this population.
Acknowledgments
J.M. holds an Australian Research Council Future Fellowship (FT170100431); A.S. has funding from NIH NHLBI R01HL146818; B.T.A. has funding from the NIH NHLBI R01HL143459. The authors declare no conflicts of interest.
Glossary
- ACE
Angiotensin-converting enzyme
- aIMT
Aortic intima media thickness
- BW
Birth weight
- BP
Blood pressure
- cIMT
Carotid intima media thickness
- CVD
Cardiovascular disease
- FGR
Fetal growth restriction
- PWV
Pulse wave velocity
- RAS
Renin-angiotensin system
- RCT
Randomized controlled trial
References
- 1.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT, et al. Potential US population impact of the 2017 ACC/AHA High Blood Pressure Guideline. Circulation 2018;137:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Virani SS, Callaway CW, Chang AR, Cheng S, Chiuve SE, et al. Heart disease and stroke statistics-2018 Update: a report from the American Heart Association. Circulation 2018;137:e67–492. [DOI] [PubMed] [Google Scholar]
- 3.Lees C, Marlow N, Arabin B, Bilardo CM, Brezinka C, Derks JB, et al. Perinatal morbidity and mortality in early-onset fetal growth restriction: cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (TRUFFLE). Ultrasound Obstet Gynecol 2013;42: 400–8. [DOI] [PubMed] [Google Scholar]
- 4.Battaglia FC, Lubchenco LO. A practical classification of newborn infants by weight and gestational age. J Pediatr 1967;71:159–63. [DOI] [PubMed] [Google Scholar]
- 5.Nardozza LMM, Caetano ACR, Zamarian ACP, Mazzola JB, Silva CP, Marçal VMG, et al. Fetal growth restriction: current knowledge. Arch Gynecol Obstet 2017;295:1061–77. [DOI] [PubMed] [Google Scholar]
- 6.Evensen KAI, Steinshamn S, Tjønna AE, Stølen T, Høydal MA, Wisløff U, et al. Effects of preterm birth and fetal growth retardation on cardiovascular risk factors in young adulthood. Early Hum Dev 2009;85:239–45. [DOI] [PubMed] [Google Scholar]
- 7.Lausman A, Kingdom J. Intrauterine growth restriction: screening, diagnosis, and management. J Obstet Gynaecol Can 2013;35:741–8. [DOI] [PubMed] [Google Scholar]
- 8.Gamborg M, Byberg L, Rasmussen F, Andersen PK, Baker JL, Bengtsson C, et al. , NordNet Study Group. Birth weight and systolic blood pressure in adolescence and adulthood: meta-regression analysis of sex- and age-specific results from 20 Nordic studies. Am J Epidemiol 2007;166:634–45. [DOI] [PubMed] [Google Scholar]
- 9.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens 2000;18:815–31. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation 2008;117:3171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Swiet M, Fayers P, Shinebourne EA. Blood pressure in first 10 years of life: the Brompton study. BMJ 1992;304:23–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keijzer-Veen MG, Finken MJ, Nauta J, Dekker FW, Hille ET, Frölich M, et al. Is blood pressure increased 19 years after intrauterine growth restriction and preterm birth? A prospective follow-up study in The Netherlands. Pediatrics 2005;116:725–31. [DOI] [PubMed] [Google Scholar]
- 13.Steen E, Bonamy A-K, Norman M, Hellström-Westas L. Preterm birth may be a larger risk factor for increased blood pressure than intrauterine growth restriction. Acta Paediatr 2015;104:1098–103. [DOI] [PubMed] [Google Scholar]
- 14.Skilton MR, Evans N, Griffiths KA, Harmer JA, Celermajer DS. Aortic wall thickness in newborns with intrauterine growth restriction. Lancet 2005;365:1484–6. [DOI] [PubMed] [Google Scholar]
- 15.Sehgal A, Doctor T, Menahem S. Cardiac function and arterial biophysical properties in small for gestational age infants: postnatal manifestations of fetal programming. J Pediatr 2013;163:1296–300. [DOI] [PubMed] [Google Scholar]
- 16.Sehgal A, Allison B, Gwini S, Miller S, Polglase G. Cardiac morphology and function in preterm growth restricted infants: relevance for clinical sequelae. J Pediatr 2017;188:128–34. [DOI] [PubMed] [Google Scholar]
- 17.Rondo PH, Lemos JO, Pereira JA, Oliveira JM, Innocente LR. Relationship between birthweight and arterial elasticity in childhood. Clin Sci (Lond) 2008;115:317–26. [DOI] [PubMed] [Google Scholar]
- 18.Broyd C, Harrison E, Raja M, Millasseau SC, Poston L, Chowienczyk PJ. Association of pulse waveform characteristics with birthweight in young adults. J Hypertens 2005;23:1391–6. [DOI] [PubMed] [Google Scholar]
- 19.Mzayek F, Sherwin R, Hughes J, Hassig S, Srinivasan S, Chen W, et al. The association of birth weight with arterial stiffness at mid-adulthood: the Bogalusa Heart Study. J Epidemiol Community Health 2009;63: 729–33. [DOI] [PubMed] [Google Scholar]
- 20.Martyn CN, Greenwald SE. Impaired synthesis of elastin in walls of aorta and large conduit arteries during early development as an initiating event in pathogenesis of systemic hypertension. Lancet 1997;350:953–5. [DOI] [PubMed] [Google Scholar]
- 21.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res 2001;49:460–7. [DOI] [PubMed] [Google Scholar]
- 22.Vehaskari VM, Aviles DH, Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int 2001;59:238–45. [DOI] [PubMed] [Google Scholar]
- 23.Habib S, Zhang Q, Baum M. Prenatal programming of hypertension in the rat: effect of postnatal rearing. Nephron Extra 2011;1:157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison JL. Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol 2008;35:730–43. [DOI] [PubMed] [Google Scholar]
- 25.Law CM, de Swiet M, Osmond C, Fayers PM, Barker DJ, Cruddas AM, et al. Initiation of hypertension in utero and its amplification throughout life. BMJ 1993;306:24–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 2014;63: 636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Sloten TT, Sedaghat S, Laurent S, London GM, Pannier B, Ikram MA, et al. Carotid stiffness is associated with incident stroke: a systematic review and individual participant data meta-analysis. J Am Coll Cardiol 2015;66:2116–25. [DOI] [PubMed] [Google Scholar]
- 28.Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Ageing. J Am Coll Cardiol 2008;51: 1377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lurbe E, Torro MI, Carvajal E, Alvarez V, Redón J. Birth weight impacts on wave reflections in children and adolescents. Hypertension 2003;41: 646–50. [DOI] [PubMed] [Google Scholar]
- 30.Lakatta EG. Arterial and cardiac ageing: major shareholders in cardiovascular disease enterprises. Part III: cellular and molecular clues to heart and arterial ageing. Circulation 2003;107:490–7. [DOI] [PubMed] [Google Scholar]
- 31.Kingdom JC, McQueen J, Connell JM, Whittle MJ. Fetal angiotensin II levels and vascular (type I) angiotensin receptors in pregnancies complicated by intrauterine growth retardation. Br J Obstet Gynaecol 1993;100:476–82. [DOI] [PubMed] [Google Scholar]
- 32.Cosmi E, Saccardi C, Zanardo V, Chiarelli S. Serum levels, placental expression and localization of angiotensin converting enzyme in pregnant women with uncomplicated pregnancy and in women with preeclampsia and IUGR foetuses. Am J Obstet Gynecol 2008;217:6. [Google Scholar]
- 33.Michel JB, Heudes D, Michel O, Poitevin P, Philippe M, Scalbert E, et al. Effect of chronic ANG I-converting enzyme inhibition on ageing processes. II. Large arteries. Am J Physiol 1994;267:R124–35. [DOI] [PubMed] [Google Scholar]
- 34.Martin H, Gazelius B, Norman M. Impaired acetylcholine-induced vascular relaxation in low birth weight infants: implications for adult hypertension? Pediatr Res 2000;47:457–62. [DOI] [PubMed] [Google Scholar]
- 35.Leeson CP, Whincup PH, Cook DG, Donald AE, Papacosta O, Lucas A, et al. Flow-mediated dilation in 9 to 11-year-old children: the influence of intrauterine and childhood factors. Circulation 1997;96:2233–8. [DOI] [PubMed] [Google Scholar]
- 36.Tonstad S, Joakimsen O, Stensland-Bugge E, Leren TP, Ose L, Russell D, et al. Risk factors related to carotid intima-media thickness and plaque in children with familial hypercholesterolemia and control subjects. Arterioscler Thromb Vasc Biol 1996;16:984–91. [DOI] [PubMed] [Google Scholar]
- 37.Aggoun Y, Bonnet D, Sidi D, Girardet JP, Brucker E, Polak M, et al. Arterial mechanical changes in children with familial hypercholesterolemia. Arterioscler Thromb Vasc Biol 2000;20:2070–5. [DOI] [PubMed] [Google Scholar]
- 38.Terpstra WF, May JF, Smit AJ, Graeff PA, Meyboom-de Jong B, Crijns HJ. Effects of amlodipine and lisinopril on intima-media thickness in previously untreated, elderly hypertensive patients (the ELVERA trial). J Hypertens 2004;22:1309–16. [DOI] [PubMed] [Google Scholar]
- 39.Zanchetti A, Bond MG, Hennig M, Neiss A, Mancia G, Dal Palu C, et al. European Lacidipine Study on Atherosclerosis Investigators. Calcium antagonist lacidipine slows down progression of asymptomatic carotid atherosclerosis: principal results of the European Lacidipine Study on Atherosclerosis (ELSA), a randomized, double-blind, long-term trial. Circulation 2002;106:2422–7. [DOI] [PubMed] [Google Scholar]
- 40.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006;27:2588–605. [DOI] [PubMed] [Google Scholar]
- 41.Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS). Stroke 2006;37:87–92. [DOI] [PubMed] [Google Scholar]
- 42.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension 2002;39:10–5. [DOI] [PubMed] [Google Scholar]
- 43.Wiegman A, Hutten BA, de Groot E, Rodenburg J, Bakker HD, Buller HR, et al. Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized controlled trial. JAMA 2004;292:331–7. [DOI] [PubMed] [Google Scholar]
- 44.Sehgal A, Allison B, Gwini S, Menahem S, Miller SL, Polglase GR. Vascular ageing and cardiac maladaptation in growth restricted preterm infants. J Perinatol 2018;38:92–7. [DOI] [PubMed] [Google Scholar]
- 45.Sehgal A, Skilton MR, Crispi F. Human fetal growth restriction: a cardiovascular journey through to adolescence. J Dev Orig Health Dis 2016;6:626–35. [DOI] [PubMed] [Google Scholar]
- 46.Zanardo V, Fanelli T, Weiner G, Fanos V, Zaninotto M, Visentin S, et al. Intrauterine growth restriction is associated with persistent aortic wall thickening and glomerular proteinuria during infancy. Kidney Int 2011;80:119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miles KA, McDonnell BJ, Maki-Petaja KM, Yasmin, Cockcroft JR, Wilkinson IB, et al. The impact of birth weight on blood pressure and arterial stiffness in later life: the Enigma Study. J Hypertens 2011;29:2324–31. [DOI] [PubMed] [Google Scholar]
- 48.Boutouyrie P, Bussy C, Hayoz D, Hengstler J, Dartois N, Laloux B, et al. Local pulse pressure and regression of arterial wall hypertrophy during long-term antihypertensive treatment. Circulation 2000;101:2601–6. [DOI] [PubMed] [Google Scholar]
- 49.Skilton MR, Ayer JG, Harmer JA, Webb K, Leeder SR, Marks GB, et al. Impaired fetal growth and arterial wall thickening: a randomized trial of omega-3 supplementation. Pediatrics 2012;129:e698–703. [DOI] [PubMed] [Google Scholar]
- 50.Skilton MR, Raitakari OT, Celermajer DS. High intake of dietary long chain omega-3 fatty acids is associated with lower blood pressure in children born with low birth weight: NHANES 2003–2008. Hypertension 2013;61:972–6. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez-Lopez M, Osorio L, Acosta-Rojas R, Figueras J, Cruz-Lemini M, Figueras F, et al. Influence of breastfeeding and postnatal nutrition on cardiovascular remodelling induced by fetal growth restriction. Pediatr Res 2016;79:100–6. [DOI] [PubMed] [Google Scholar]
- 52.Neutel JM. Effect of the renin–angiotensin system on the vessel wall: using ACE inhibition to improve endothelial function. J Hum Hypertens 2004;18:599–606. [DOI] [PubMed] [Google Scholar]
- 53.London GM. Arterial stiffness, wave reflections and myocardial protection: the REASON project. Drugs 2003;63:9–17. [PubMed] [Google Scholar]
- 54.Kengne AP, Czernichow S, Huxley R, Grobbee D, Woodward M, Neal B, et al. Blood pressure variables and cardiovascular risk: new findings from ADVANCE. Hypertension 2009;54:399–404. [DOI] [PubMed] [Google Scholar]
- 55.Wu CF, Liu PY, Wu TJ, Hung Y, Yang SP, Lin GM, et al. Therapeutic modification of arterial stiffness: an update and comprehensive review. World J Cardiol 2015;7:742–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ceconi C, Fox KM, Remme WJ, Fox KM, Bertrand M, Ferrari R, et al. ACE inhibition with perindopril and endothelial function. Results of a sub-study of the EUROPA study: PERTINENT. Cardiovasc Res 2007;73:237–46. [DOI] [PubMed] [Google Scholar]
- 57.Bots ML, Remme WJ, Luscher TF, Fox KM, Bertrand M, Ferrari R, et al. ACE inhibition and endothelial function: main findings of PERFECT, a sub-study of the EUROPA trial. Cardiovasc Drugs Ther 2007;21:269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schiffrin EL, Deng LY. Comparison of effects of angiotensin I-converting enzyme inhibition and beta-blockade for 2 years on function of small arteries from hypertensive patients. Hypertension 1995;25:669–703. [DOI] [PubMed] [Google Scholar]
- 59.Thybo NK, Stephens N, Cooper A, Aalkjaer C, Heagerty AM, Mulvany MJ. Effect of antihypertensive treatment on small arteries of patients with previously untreated essential hypertension. Hypertension 1995;25:474–81. [DOI] [PubMed] [Google Scholar]
- 60.Zhuo JL, Froomes P, Casley D, Liu JJ, Murone C, Chai SY, et al. Perindopril chronically inhibits angiotensin converting enzyme in both the endothelium and adventitia of the internal mammary artery in patients with ischemic heart disease. Circulation 1997;96:174–82. [PubMed] [Google Scholar]
- 61.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288:2981–97. [DOI] [PubMed] [Google Scholar]
- 62.Sehgal A, Krishnamurthy MB, Clark M, Menahem S. ACE inhibition for severe bronchopulmonary dysplasia-An approach based on physiology. Physiol Rep 2018;6:e13821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonamy AK, Bendito A, Martin H, Andolf E, Sedin G, Norman M. Preterm birth contributes to increased vascular resistance and higher blood pressure in adolescent girls. Pediatr Res 2005;58:845–9. [DOI] [PubMed] [Google Scholar]
- 64.Cheung YF, Wong KY, Lam BC, Tsoi NS. Relation of arterial stiffness with gestational age and birth weight. Arch Dis Child 2004;89:217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arnold AC, Gallagher PE, Diz DI. Brain renin–angiotensin system in the nexus of hypertension and aging. Hypertens Res 2013;36:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Masi S, Uliana M, Virdis A. Angiotensin II and vascular damage in hypertension: role of oxidative stress and sympathetic activation. Vascul Pharmacol 2019;115:13–7. [DOI] [PubMed] [Google Scholar]
- 67.South AM, Shaltout HA, Washburn LK, Hendricks AS, Diz DI, Chappell MC. Fetal programming and the angiotensin-(1–7) axis: a review of the experimental and clinical data. Clin Sci 2019;133:55–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1–7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension 2007;49:185–92. [DOI] [PubMed] [Google Scholar]
- 69.Yousif MHM, Benter IF, Diz DI, Chappell MC. Angiotensin-(1–7)-dependent vasorelaxation of the renal artery exhibits unique angiotensin and bradykinin receptor selectivity. Peptides 2017;90:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DelliPizzi A, Hilchey Sean D, Bell-Quilley Caroline P. Natriuretic action of angiotensin (1–7). Br J Pharmacol 1994;111:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heitsch H, Brovkovych S, Malinski T, Wiemer G. Angiotensin-(1–7)-stimulated nitric oxide and superoxide release from endothelial cells. Hypertension 2001;37:72–6. [DOI] [PubMed] [Google Scholar]
- 72.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 2000;87:E1–9. [DOI] [PubMed] [Google Scholar]
- 73.Chappell MC, Pirro NT, Sykes A, Ferrario CM. Metabolism of angiotensin-(1–7) by angiotensin-converting enzyme. Hypertension 1998;31:362–7. [DOI] [PubMed] [Google Scholar]
- 74.Varagic J, Ahmad S, VonCannon JL, Moniwa N, Brosnihan KB, Wysocki J, et al. Predominance of AT1 blockade over Mas–mediated angiotensin-(1–7) mechanisms in the regulation of blood pressure and renin–angiotensin system in mRen2.Lewis rats. Am J Hypertens 2013;26:583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grigore D, Ojeda NB, Robertson EB, Dawson AS, Huffman CA, Bourassa EA, et al. Placental insufficiency results in temporal alterations in the renin angiotensin system in male hypertensive growth restricted offspring. Am J Physiol Regul Integr Comp Physiol 2007;293:R804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamauchi T, Mogi M, Kan-No H, Shan BS, Higaki A, Min LJ, et al. Roles of angiotensin II type 2 receptor in mice with fetal growth restriction. Hypertens Res 2018;41:157–64. [DOI] [PubMed] [Google Scholar]
- 77.Shaltout HA, Figueroa JP, Rose JC, Diz DI, Chappell MC. Alterations in circulatory and renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 in fetal programmed hypertension. Hypertension 2009;53:404–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marshall AC, Shaltout HA, Nautiyal M, Rose JC, Chappell MC, Diz DI. Fetal betamethasone exposure attenuates angiotensin-(1–7)-Mas receptor expression in the dorsal medulla of adult sheep. Peptides 2013;44: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marshall AC, Shaltout HA, Pirro NT, Rose JC, Diz DI, Chappell MC. Antenatal betamethasone exposure is associated with lower ANG-(1–7) and increased ACE in the CSF of adult sheep. Am J Physiol Regul Integr Comp Physiol 2013;305:R679–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Su Y, Bi J, Pulgar VM, Chappell MC, Rose JC. Antenatal betamethasone attenuates the angiotensin-(1–7)-Mas receptor-nitric oxide axis in isolated proximal tubule cells. Am J Physiol Renal Physiol 2017;312: F1056–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Su Y, Bi J, Pulgar VM, Figueroa J, Chappell M, Rose JC. Antenatal glucocorticoid treatment alters Na+ uptake in renal proximal tubule cells from adult offspring in a sex-specific manner. Am J Physiol Renal Physiol 2015;308:F1268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gordijn SJ, Beune IM, Thilaganathan B, Papageorghiou A, Baschat AA, Baker PN, et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol 2016;48:333–9. [DOI] [PubMed] [Google Scholar]
- 83.McCowan LM, Figueras F, Anderson NH. Evidence-based national guidelines for the management of suspected fetal growth restriction: comparison, consensus, and controversy. Am J Obstet Gynecol 2018;218:S855–68. [DOI] [PubMed] [Google Scholar]
- 84.Franco MCP, Casarini DE, Carneiro-Ramos MS, Sawaya AL, Barreto-Chaves ML, Sesso R. Circulating renin–angiotensin system and catecholamines in childhood: is there a role for birthweight? Clin Sci 2008;114:375–80. [DOI] [PubMed] [Google Scholar]
- 85.South AM, Nixon PA, Chappell MC, Diz DI, Russell GB, Shaltout HA, et al. Obesity is associated with higher blood pressure and higher levels of angiotensin II but lower angiotensin-(1–7) in adolescents born preterm. J Pediatr 2019;205:55–60. e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.South AM, Nixon PA, Chappell MC, Diz DI, Russell GB, Snively BM, et al. Antenatal corticosteroids and the renin-angiotensin-aldosterone system in adolescents born preterm. Pediatr Res 2017;81:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.South AM, Nixon PA, Chappell MC, Diz DI, Russell GB, Jensen ET, et al. Association between preterm birth and the renin-angiotensin system in adolescence: influence of sex and obesity. J Hypertens 2018;36:2092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.South AM, Nixon PA, Chappell MC, Diz DI, Russell GB, Jensen ET, et al. Renal function and blood pressure are altered in adolescents born preterm. Pediatr Nephrol 2019;34:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.South AM, Jensen ET, Nixon PA, Washburn LK. Individuals born preterm have an increased rate of hypertension from adolescence to adulthood [abstract]. J Am Soc Nephrol 2019;30:298–9. [Google Scholar]
- 90.Machado-Silva A, Passos-Silva D, Santos RA, Sinisterra RD. Therapeutic uses for angiotensin-(1–7). Expert Opin Ther Pat 2016;26: 669–78. [DOI] [PubMed] [Google Scholar]
- 91.Epelman S, Shrestha K, Troughton RW, Francis GS, Sen S, Klein AL, et al. Soluble angiotensin-converting enzyme 2 in human heart failure: relation with myocardial function and clinical outcomes. J Card Fail 2009;15:565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wester A, Devocelle M, Tallant EA, Chappell MC, Gallagher PE, Paradisi F. Stabilization of Angiotensin-(1–7) by key substitution with a cyclic non-natural amino acid. Amino Acids 2017;49: 1733–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Twist DJL, Houben AJHM, de Haan MW, Mostard GJM, Kroon AA, de Leeuw PW. Angiotensin-(1–7)–induced renal vasodilation in hypertensive humans is attenuated by low sodium intake and angiotensin II co-infusion. Hypertension 2013;62:789–93. [DOI] [PubMed] [Google Scholar]
- 94.Ueda S, Masumori-Maemoto S, Wada A, Ishii M, Brosnihan KB, Umemura S. Angiotensin (1–7) potentiates bradykinin-induced vasodilatation in man. J Hypertens 2001;19:2001–9. [DOI] [PubMed] [Google Scholar]
- 95.Duan AQ, Darby JRT, Soo JY, Lock MC, Zhu MY, Flynn LV, et al. Feasibility of phase-contrast cine magnetic resonance imaging for measuring blood flow in the sheep fetus. Am J Physiol Regul Integr Comp Physiol 2019;317:R780–92. [DOI] [PubMed] [Google Scholar]
- 96.Morrison JL, Berry MJ, Botting KJ, Darby JRT, Frasch MG, Gatford KL, et al. Improving pregnancy outcomes in humans through studies in sheep. Am J Physiol RIC 2018;315:R1123–53. [DOI] [PubMed] [Google Scholar]
- 97.Miller SL, Supramaniam VG, Jenkin G, Walker DW, Wallace EM. Cardiovascular responses to maternal betamethasone administration in the intrauterine growth-restricted ovine fetus. Am J Obstet Gynecol 2009;201:613. e1–8. [DOI] [PubMed] [Google Scholar]
- 98.Poudel R, McMillen IC, Dunn SL, Zhang S, Morrison JL. Impact of chronic hypoxemia on blood flow to the brain, heart, and adrenal gland in the late-gestation IUGR sheep fetus. Am J Physiol Regul Integr Comp Physiol 2015;308:R151–62. [DOI] [PubMed] [Google Scholar]
- 99.Mizuno M, Siddique K, Baum M, Smith SA. Prenatal programming of hypertension induces sympathetic over activity in response to physical stress. Hypertension 2013;61:180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morrison JL, Botting KJ, Dyer JL, Williams SJ, Thornburg KL, McMillen IC. Restriction of placental function alters heart development in the sheep fetus. Am J Physiol 2007;293:R306–13. [DOI] [PubMed] [Google Scholar]
- 101.Botting KJ, McMillen IC, Forbes H, Nyengaard JR, Morrison JL. Chronic hypoxemia in late gestation decreases cardiomyocyte number but does not change expression of hypoxia-responsive genes. J Am Heart Assoc 2014;28:e000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vranas S, Heinemann GK, Liu H, De Blasio MJ, Owens JA, Gatford KL, et al. Small size at birth predicts decreased cardiomyocyte number in the adult ovine heart. J Dev Orig Health Dis 2017;8:618–25. [DOI] [PubMed] [Google Scholar]
- 103.Botting KJ, Loke XY, Zhang S, Andersen JB, Nyengaard JR, Morrison JL. IUGR decreases cardiomyocyte endowment and alters cardiac metabolism in a sex- and cause-of-IUGR-specific manner. Am J Physiol Regul Integr Comp Physiol 2018;315:R48–67. [DOI] [PubMed] [Google Scholar]
- 104.Wang KC, Botting KJ, Zhang S, McMillen IC, Brooks DA, Morrison JL. Akt signalling as a mediator of cardiac adaptation to low birth weight. J Endocrinol 2017;233:R81–94. [DOI] [PubMed] [Google Scholar]
- 105.Wang KC, Tosh DN, Zhang S, McMillen IC, Duffield JA, Brooks DA, et al. IGF-2R-Galphaq signalling and cardiac hypertrophy in the low-birth-weight lamb. Am J Physiol Regul Integr Comp Physiol 2015;308:R627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tintu A, Rouwet E, Verlohren S, Brinkmann J, Ahmad S, Crispi F, et al. Hypoxia induces dilated cardiomyopathy in the chick embryo: mechanism, intervention, and long-term consequences. PLoS One 2009;4: e5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Verburg BO, Jaddoe VW, Wladimiroff JW, Hofman A, Witteman JC, Steegers EA. Fetal hemodynamic adaptive changes related to intrauterine growth: the Generation R Study. Circulation 2008;117:649–59. [DOI] [PubMed] [Google Scholar]
- 108.Sehgal A, Dahlstrom J, Chan Y, Allison BJ, Miller S, Polglase GR. Placental histopathology in preterm fetal growth restriction. J Pediatr Ch Health 2019;55:582–7. [DOI] [PubMed] [Google Scholar]
- 109.Tsyvian P, Malkin K, Wladimiroff JW. Assessment of fetal left cardiac isovolumic relaxation time in appropriate and small-for gestationalage fetuses. Ultrasound Med Biol 1995;21:739–43. [DOI] [PubMed] [Google Scholar]
- 110.Crispi F, Gratacos E. Fetal cardiac function: technical considerations and potential research and clinical applications. Fetal Diagn Ther 2012;32:47–64. [DOI] [PubMed] [Google Scholar]
- 111.Hernandez-Andrade E, Benavides-Serralde JA, Cruz-Martinez R, Welsh A, Mancilla-Ramirez J. Evaluation of conventional Doppler fetal cardiac function parameters: E/A ratios, outflow tracts, and myocardial performance index. Fetal Diagn Ther 2012;32:22–9. [DOI] [PubMed] [Google Scholar]
- 112.Danielson L, McMillen IC, Dyer JL, Morrison JL. Restriction of placental growth results in greater hypotensive response to a-adrenergic blockade in fetal sheep during late gestation. J Physiol 2005;563:611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Allison BJ, Brain KL, Niu Y, Kane AD, Herrera EA, Thakor AS, et al. Fetal in vivo continuous cardiovascular function during chronic hypoxia. J Physiol 2016;594:1247–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Edwards LJ, Simonetta G, Owens JA, Robinson JS, McMillen IC. Restriction of placental and fetal growth in sheep alters fetal blood pressure responses to angiotensin II and captopril. J Physiol 1999;515: 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Darby JRT, Varcoe TJ, Orgeig S, Morrison JL. Cardiorespiratory consequences of intrauterine growth restriction: influence of timing, severity and duration of hypoxaemia. Theriogenology 2020;150:84–95. [DOI] [PubMed] [Google Scholar]
- 116.Harper V, MacInnes R, Campbell D, Hall M. Increased birth weight in northerly islands: is fish consumption a red herring? BMJ 1991;303:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Olsen SF, Hansen HS, Sorensen TI, Jensen B, Secher NJ, Sommer S, et al. Intake of marine fat, rich in (n-3)-polyunsaturated fatty acids, may increase birthweight by prolonging gestation. Lancet 1986;2:367–9. [DOI] [PubMed] [Google Scholar]
- 118.Leaf A, Weber PC. Cardiovascular effects of n-3 fatty acids. N Engl J Med 1988;318:549–57. [DOI] [PubMed] [Google Scholar]
- 119.Sorensen JD, Olsen SF, Pedersen AK, Boris J, Secher J, FitzGerald GA. Effects of fish oil supplementation in the third trimester of pregnancy on prostacyclin and thromboxane production. Am J Obstet Gynecol 1993;168:915–22. [DOI] [PubMed] [Google Scholar]
- 120.Olsen SF, Sorensen JD, Secher NJ, Hedegaard M, Henriksen TB, Hansen HS, et al. Randomized controlled trial of effect of fish-oil supplementation on pregnancy duration. Lancet 1992;339:1003–7. [DOI] [PubMed] [Google Scholar]
- 121.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens 1988;1:335–47. [DOI] [PubMed] [Google Scholar]
- 122.Hughson M, Farris AB 3rd, Douglas-Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int 2003;63:2113–22. [DOI] [PubMed] [Google Scholar]
- 123.Wintour EM, Moritz KM, Johnson K, Ricardo S, Samuel CS, Dodic M. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J Physiol 2003;549:929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tang L, Carey LC, Bi J, Valego N, Sun X, Deibel P, et al. Gender differences in the effects of antenatal betamethasone exposure on renal function in adult sheep. Am J Physiol Regul Integr Comp Physiol 2009;296: R309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang J, Massmann GA, Rose JC, Figueroa JP. Differential effects of clinical doses of antenatal betamethasone on nephron endowment and glomerular filtration rate in adult sheep. Reprod Sci 2010;17:186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ortiz LA, Quan A, Weinburg A, Baum M. Effect of prenatal dexamethasone on rat development. Kidney Int 2001;59:1663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Boubred F, Daniel L, Buffat C, Tsimaratos M, Oliver C, Lelièvre-Pégorier M, et al. The magnitude of nephron number reduction mediates intrauterine growth-restriction-induced long term chronic renal disease in the rat. A comparative study in two experimental models. J Transl Med 2016;14:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jones A, Beda A, Ward AM, Osmond C, Phillips DI, Moore VM, et al. Size at birth and autonomic function during psychological stress. Hypertension 2007;49:548–55. [DOI] [PubMed] [Google Scholar]
- 129.Pirojsakul K, Thanapinyo A, Nuntnarumit P. Blood pressure and heart rate during stress in children born small for gestational age. Pediatr Nephrol 2017;32:1053–8. [DOI] [PubMed] [Google Scholar]
- 130.Baum M Role of the kidney in the prenatal and early postnatal programming of hypertension. Am J Physiol Renal Physiol 2010;298: F235–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Baum M Role of renal sympathetic nerve activity in prenatal programming of hypertension. Pediatr Nephrol 2018;33:409–19. [DOI] [PubMed] [Google Scholar]
- 132.Porter JP, King SH, Honeycutt AD. Prenatal high-salt diet in the Sprague-Dawley rat programs blood pressure and heart rate hyper-responsiveness to stress in adult female offspring. Am J Physiol Regul Integr Comp Physiol 2007;293:R334–42. [DOI] [PubMed] [Google Scholar]
- 133.Alexander B, Hendon A, Dwyer T. Renal denervation abolishes hypertension in low birth weight offspring from pregnant rats with reduced uterine perfusion. Hypertension 2005;45:754–8. [DOI] [PubMed] [Google Scholar]
- 134.Intapad S, Tull F, Brown A, Dasinger JH, Ojeda NB, Fahling JM, et al. Renal denervation abolishes the age-dependent increase in blood pressure in female intrauterine growth-restricted rats at 12 months of age. Hypertension 2013;61:828–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dagan A, Kwon HM, Dwarakanath V, Baum M. Effect of renal denervation on prenatal programming of hypertension and renal tubular transporter abundance. Am J Physiol Renal Physiol 2008;295:F29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kitsiou-Tzeli S, Tzetis M. Maternal epigenetics and fetal and neonatal growth. Curr Opin Endocrinol Diabetes Obes 2017;24:43–6. [DOI] [PubMed] [Google Scholar]
- 137.Wang KC, Brooks DA, Summers-Pearce B, Bobrovskaya L, Tosh DN, Duffield JA, et al. Low birth weight activates the renin-angiotensin system, but limits cardiac angiogenesis in early postnatal life. Physiol Rep 2015;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang S, Rattanatray L, MacLaughlin SM, Cropley JE, Suter CM, Molloy L, et al. Peri-conceptional undernutrition in normal and overweight ewes leads to increased adrenal growth and epigenetic changes in adrenal IGF2/H19 gene in offspring. FASEB J 2010;24:2772–82. [DOI] [PubMed] [Google Scholar]
- 139.Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr 2005;135:1382–6. [DOI] [PubMed] [Google Scholar]
- 140.Goyal R, Leitzke A, Goyal D, Gheorghe CP, Longo LD. Antenatal maternal hypoxic stress: adaptations in fetal lung Renin-Angiotensin system. Reprod Sci 2011;18:180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Barker DJ, Eriksson JG, Forsén T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 2002;31: 1235–9. [DOI] [PubMed] [Google Scholar]
- 142.Bogdarina I, Welham S, King PJ, Burns SP, Clark AJL. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ Res 2007;100:520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chappell MC. Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am J Physiol Heart Circ Physiol 2016;310: H137–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Eriksson Johan G, Forsén Tom J, Kajantie E, Osmond C, Barker David JP. Childhood growth and hypertension in later life. Hypertension 2007;49:1415–21. [DOI] [PubMed] [Google Scholar]
- 145.Briffa JF, O’Dowd R, Moritz KM, Romano T, Jedwab LR, McAinch AJ, et al. Uteroplacental insufficiency reduces rat plasma leptin concentrations and alters placental leptin transporters: ameliorated with enhanced milk intake and nutrition. J Physiol 2017;595:3389–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lindberg J, Norman M, Westrup B, Domellof M, Berglund SK. Lower systolic blood pressure at age 7 y in low-birth-weight children who received iron supplements in infancy: results from a randomized controlled trial. Am J Clin Nutr 2017;106:475–80. [DOI] [PubMed] [Google Scholar]
- 147.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2017;71:1269–324. [DOI] [PubMed] [Google Scholar]
- 148.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 2017;140:e20171904. [DOI] [PubMed] [Google Scholar]
- 149.Yliharsila H, Eriksson JG, Forsén T, Kajantie E, Osmond C, Barker DJ. Self-perpetuating effects of birth size on blood pressure levels in elderly people. Hypertension 2003;41:441–50. [DOI] [PubMed] [Google Scholar]
- 150.Lackland DT, Egan BM, Syddall HE, Barker DJ. Associations between birthweight and antihypertensive medication in black and white Medicaid recipients. Hypertension 2002;39:179–83. [DOI] [PubMed] [Google Scholar]


