Abstract
A recent experiment (Sadoon AA, Wang Y. 2018 Phys. Rev. E 98, 042411. (doi:10.1103/PhysRevE.98.042411)) has revealed that nucleoid-associated proteins (i.e. DNA-binding proteins) exhibit highly heterogeneous diffusion processes in bacteria where not only the diffusion constant but also the anomalous diffusion exponent fluctuates for the various proteins. The distribution of displacements of such proteins is observed to take a q-Gaussian form, which decays as a power law. Here, a statistical model is developed for the diffusive motion of the proteins within the bacterium, based on a superstatistics with two variables. This model hierarchically takes into account the joint fluctuations of both the anomalous diffusion exponents and the diffusion constants. A fractional Brownian motion is discussed as a possible local model. Good agreement with the experimental data is obtained.
Keywords: two-variable superstatistics, joint fluctuations, q-Gaussians, protein diffusion dynamics, bacteria
1. Introduction
Diffusion phenomena in living bacteria pose challenging scientific problems (e.g. [1–5]). There is a rapidly growing development of the experimental techniques of single-particle tracking (see [6] and references therein) which has made new interesting experimental results available. Recently, highly heterogeneous diffusion processes have been observed in various experiments (e.g. [7] and references therein), for example, in the experiment of [8] (see also [9] for a recent update), for the dynamics of histonelike nucleoid-structuring proteins in living Escherichia coli bacteria. Such nucleoid-associated proteins interact with DNA as well as with themselves and they are uniformly distributed over the bacterium. In the experiment, to measure the diffusion properties, the trajectories of these proteins, fused to fluorescent proteins, have been analysed at individual level. An interesting observation of [8] is that the displacement, Δx, is not Gaussian-distributed but obeys a q-Gaussian distribution [10] (sometimes also called a Pearson-type VII distribution [11])
| 1.1 |
where w is a positive quantity having the dimension of space and m is a positive exponent. This density asymptotically decays with a power law, This behaviour is a priori unexpected, since it implies large probabilities of large displacements (but note that the distribution still has a finite second moment since unlike the case of Lévy flights [12]). Accordingly, the behaviour is in marked contrast to the results obtained in other experimental studies, for example, in [13–15], where the distribution of displacements of mRNA or chromosomal locus in living bacteria was found to be of exponential form, with a characteristic displacement (see also [16] for other cell types). Thus, the result of [8] sheds new interesting aspects onto the complexity of protein diffusion in living bacteria.
The analysis of [8] has shown that there is actually a distribution of different anomalous diffusion exponents, as well as a distribution of different diffusion constants in the bacterium. In this paper, we will provide a theoretical framework for analysing these distributions.
1.1. Experimental observations
Let us write for the mean square displacement of a given protein
| 1.2 |
where Dα is the diffusion constant, α is the (anomalous) diffusion exponent and t is the elapsed time. (Here and hereafter, the notations we use are slightly different from those in [8].) Normal diffusion implies α = 1, whereas the case with (greater than 0) corresponds to anomalous diffusion [17–19].
A fundamental experimental observation of [8] is that not only the diffusion constants but also the diffusion exponents fluctuate in a wide range. The distribution of the diffusion constants is observed to asymptotically follow a power law
| 1.3 |
with whereas the diffusion exponents obey a rather broad distribution in the range ; see figures 1 and 2.
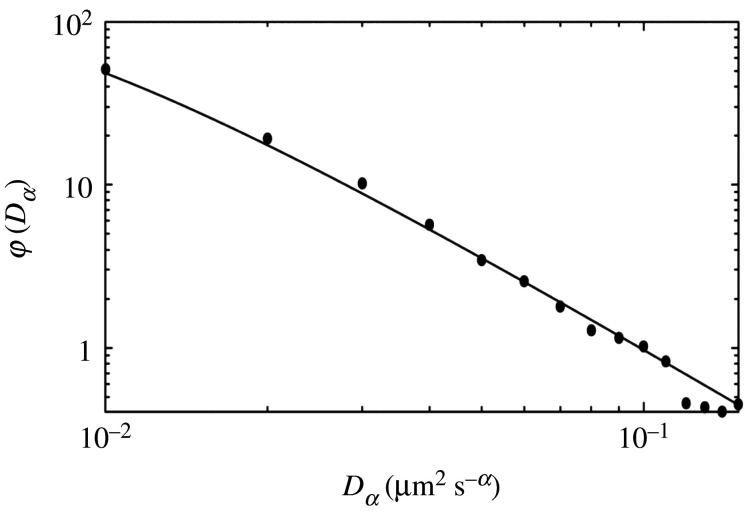
Figure 1.
Log–log plot of the normalized distribution of the numerical values of Dα . The histogram is based on the experimental data of fig. 2(d) in [8]. The solid line shows the inverse gamma distribution, equation (B 2), with in the interval with and Dimensionless numerical values of all quantities are used.
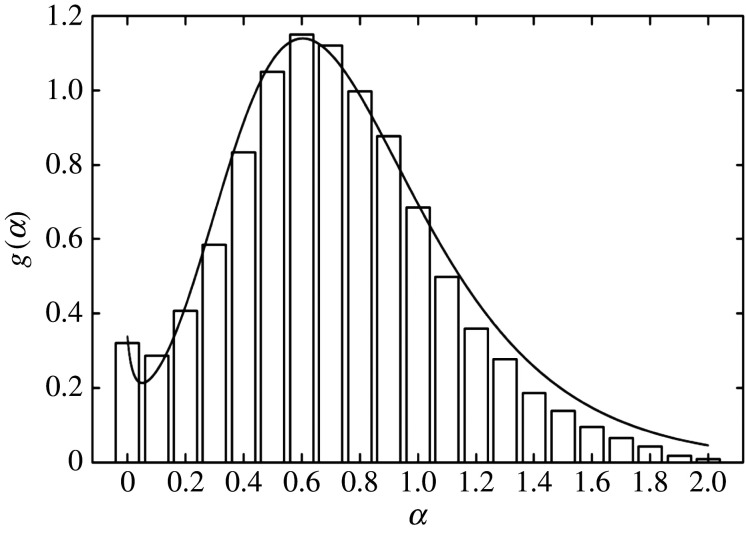
Figure 2.
The normalized marginal distribution g(α). The histogram describes the probability to find a given value of the anomalous diffusion coefficient α and is based on the experimental data of fig. 2(c) in [8]. The solid line shows the distribution of equation (2.5) with equations (2.12) and (2.13): and .
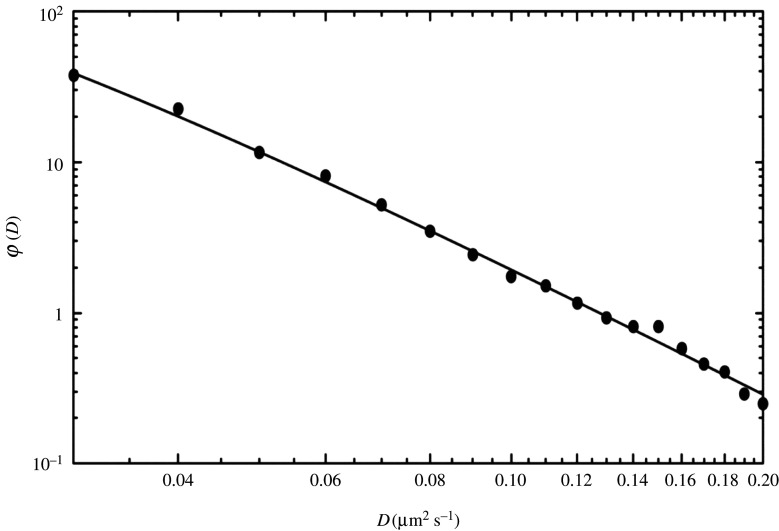
Regarding the distribution in equation (1.3), the following points should be noted: it has been obtained for numerical values of Dα in the sense that the dimension is neglected, since the dimension of Dα changes depending on the values of α, as can be seen in equation (1.2). For small elapsed time, only normal diffusion is observed and, remarkably, the diffusion constant, D, in this case has also been found to asymptotically obey the distribution in equation (1.3), denoted as where the exponent, turns out to take the value ; see figure 3.
Figure 3.
Log–log plot of the normalized distribution of the diffusion constant D in units of (µm2 s−1). The histogram is based on the experimental data in the left-bottom inset in fig. 2(d) of [8]. The solid line shows the inverse gamma distribution of equation (B 1) with A = 0.011 µm2 s−1 in the interval with ɛ = 0.025 µm2 s−1 and κ = 0.205 µm2 s−1.
It may be worth pointing out that the power-law nature in equation (1.3) is non-trivial, since it is apparently different from the exponential law reported, for example, in [14] (see also [20–22] for an entropic approach to this law).
The mean square displacement in an ensemble average, i.e. an average of square displacement over all of the individual trajectories, has also been obtained in the measurements of [8], where both an average diffusion constant and an average diffusion exponent are determined. Then, the bacteria have been classified into three groups based on their cell age (or, equivalently cell length). As can be seen in fig. 6(c) and (d) in [8], it has been observed for such groups that, in terms of the cell age, the average diffusion constant increases significantly, whereas the average diffusion exponent is approximately constant (it increases only slightly).
1.2. Two-variable superstatistical treatment
Generally, in non-equilibrium statistical physics, the diffusion constant is proportional to temperature through the Einstein relation [23]; hence, a theory of fluctuating diffusion constants is mathematically equivalent to a theory of fluctuating temperatures. This leads us naturally to the concept of superstatistics [24]. Superstatistics is a ‘statistics of statistics’ with largely separated time scales: a prototype example is a Brownian motion in a fluid environment in a variety of non-equilibrium stationary states [25]. The marginal distribution of the Brownian particle is written as a superposition of the statistics describing the local Brownian motion on a short time scale (where inverse temperature is locally fixed) with respect to the statistics associated with slowly fluctuating inverse temperatures on a long time scale. The superstatistical idea has been widely used for a variety of complex systems/phenomena organized by different dynamics with such a separation of time scales (see [26] for a review and [27–33] for very recent developments). Among others, the discussions in [7,34,35] are suitable for biological application: in the former [7], a fluctuating quantity is the diffusion constant, whereas it is the diffusion exponent in the latter [34,35].
The basic idea of this paper is to develop a two-variable superstatistical formalism which is then applied to model the stochastic motion of histonelike nucleoid-structuring proteins in living Escherichia coli bacteria. In our approach, the time scale of variation of the fluctuations of both the diffusion exponent and the inverse temperature is much larger than that of the dynamics of the protein in local areas of the bacterium, leading to associated statistics with two largely separated time scales. In fact, the superstatistical probability densities can simply be associated with the different types of proteins that diffuse in the bacterium, thus there is naturally an ensemble of different diffusion constants and different scaling exponents of anomalous diffusion. We describe the statistical property of the protein over the bacterium as a superposition of these different statistics. For a given protein in given local areas, fractional Brownian motion [36] (a simple Gaussian stochastic process where the mean square displacement exhibits anomalous diffusion) is used as a simple local stochastic process; see also [7] and references therein. Our approach can be easily generalized to other local stochastic models, for example, along the lines discussed in [37], where a Langevin equation with fluctuations of both friction and noise intensity has been studied. We show that the present theory gives rise to q-Gaussian (Pearson-type) distributions, in agreement with the experimental measurements. We also propose a particular form of the distribution of the anomalous diffusion exponents, again in agreement with the experimental data. Overall, our approach leads to a general characterization and effective thermodynamic description of the most important properties of complex biomolecule diffusion processes, applicable in many different contexts (see also [38–41] for further possible applications, where fluctuations of both the diffusion constant and the diffusion exponent have been experimentally observed).
2. Results and discussion
As mentioned earlier, for the experiments discussed here both the diffusion constant and the diffusion exponent can fluctuate in a spatio-temporal way. These fluctuations are fundamental; they do not just come from insufficient sampling of trajectories.
Generally, it is well known that the diffusion constant for ordinary Brownian motion is proportional to temperature, the proportionality constant being the mobility of the particle. Thus, a distribution of different protein diffusion constants is formally equivalent to a distribution of different temperatures, which, by transformation of random variables, can be re-formulated as a distribution of inverse temperatures. It is well known [25] that a χ2 distribution of inverse temperatures leads to q-Gaussian distributions. This we now apply to the case of protein diffusion in the bacterium with different diffusion constants. Instead of talking about a distribution of diffusion constants, we talk about the corresponding distribution of (effective) inverse temperatures, which is mathematically equivalent.
The time scale of variation of the fluctuations of both the diffusion exponent, and the inverse temperature, is naturally much larger than that of the dynamics of the protein in a given local block (spatial region); see appendix A. In fact, different inverse temperatures can correspond to an ensemble of different proteins with different diffusion constants. Let us denote the joint distribution of both and by which we can generally write as
| 2.1 |
Here, is the conditional distribution describing the probability of given a value of and is the marginal distribution describing the probability of i.e. For the protein (or ensemble of proteins) in a given local block, we denote the probability of finding the protein in the interval at time t by The protein moves from one block to another on a long time scale. Therefore, for the entire bacterium, let us introduce a superstatistical ensemble of all proteins by defining the following integrated probability distribution:
| 2.2 |
The above equation is in conformity with the viewpoint of a superstatistics with two variables: the probability distribution is expressed as a superposition of with respect to the distribution Thus, a statistical treatment of the fluctuations of both and is hierarchically introduced in this way. Equation (2.2) describes a kind of superstatistical partition function, characteristic for each bacterium.
Before proceeding, we mention the following: the correlation between and is supposed to exist at a general statistical level. Accordingly, one may wonder if the correlation is connected with a kind of fluctuation-dissipation-like relation, which is meant in the sense that is proportional to temperature and is inversely proportional to the friction constant depending on α. Such a relation is motivated by a similar approach described in [42]. If such a relation holds, then one can obtain a superposition of joint fluctuations in which correlations are taken into account. In the case where an ensemble of different proteins of different shapes is considered, the correlation may be non-negligible. But as will be seen in later sections, for the data studied here, the correlation turns out to be weak.
Superstatistical techniques are an approximation, and one has to be clear about what types of approximations are done, and how well these are experimentally justified. In [43], a large separation of two time scales was explicitly taken into account by the use of conditional probabilities, in which the integration over the fast variable (i.e. an effective energy concerned with a local region) was taken after that of the slow variable (i.e. the inverse temperature). In [44], it was then pointed out that this procedure is opposite to the one in the adiabatic scheme (see [45] for a relevant discussion, where a dynamical equation for a slowly fluctuating quantity was studied). In this respect, the present procedure of the integration associated with the joint fluctuations in equation (2.2) is seen to be consistent with the adiabatic scheme.
So far, equation (2.2) is formal, without having determined the distributions appearing there. In the following, we discuss distributions that are consistent with the experimental data. Since D is distributed according to as noted earlier, is found to be a power-law distribution given by where the relation is assumed and the prime denotes, throughout the present work, differentiation with respect to β. In contrast with the explicit form of is unspecified. We will propose in the next subsection an example of such a form, allowing us to evaluate the marginal distribution describing the fluctuations of i.e. which can be compared with distributions observed in the experiment (see equation (2.12) and appendix D).
In the following, we use the fact that the above experimentally observed power-law distribution in bacteria is only describing the behaviour for small values of the inverse temperature (i.e. large values of temperature T). We are free to assume suitable behaviour for larger values of to get agreement with q-Gaussians of the displacement distributions, by integrating over all This is the standard formalism of superstatistics for one variable as described in [25], assuming a χ2-distributed β.
Therefore, let us suppose that is given by the following χ2 distribution:
| 2.3 |
in the whole range of where is the average of and is the Euler gamma function. The above functional form exhibits power-law behaviour for small β, as required, but also provides a cut-off for large β. We also mention that the experimental data are seen to be consistent with equation (2.3); see figure 3 as well as appendix B.
Regarding let us write it as where is a suitable function. As mentioned earlier, the average anomalous diffusion exponent increases only slightly with respect to the cell age, in contrast with the average diffusion constant. This suggests the existence of a weak correlation between and at the statistical level, i.e. the two variables are not fully statistically independent. Accordingly, has a weak dependence on in the sense that is approximately constant in the whole range of and accordingly its first derivative with respect to is small. So, we expand around at up to the first order of where and Thus, with we have the following conditional distribution:
| 2.4 |
and the weak correlation is described in this way. The weakness of dependence is guaranteed if is assumed to be small.
From equations (2.3) and (2.4), the marginal distribution is immediately calculated to be
| 2.5 |
where the quantity has been assumed to be positive (which is indeed confirmed in the case of equation (2.12); see appendix D).
2.1. A possible model
For as supported, for example, by the data in [8] and examined for the present case in appendix C, we apply the approach of fractional Brownian motion [36] as a possible stochastic process. By this, we mean that the probability density is given as follows [19]:
| 2.6 |
where denotes the diffusion constant. As discussed in appendix A, it is given by the relation with s and c being a characteristic time and a positive constant, respectively, and it corresponds to in equation (1.2). The effective temperature dependence of can be non-trivial in experiments. Therefore, it is of interest to experimentally examine how this dependence is realized.
Substituting equations (2.1), (2.3), (2.4) and (2.6) into equation (2.2), we obtain the following distribution:
| 2.7 |
where the symbol denotes the average with respect to the conditional distribution i.e. and is defined by
| 2.8 |
provided that, following the formalism in [25], the integration over has been performed.
We here discuss some further experimental features of the data in [8]. Based on the experimental data presented in fig. 6(a), (c) and (d) in [8] and equation (1.2), we estimate the typical spatial scale of the local block as at the elapsed time of 0.2 s which gives the first five data points (of the total of 10 data points) in the mean square displacement. is found to be of the order of 100 nm for the three age groups mentioned earlier. Then, as can be seen in fig. 4(a) in [8], the displacements of have been examined via equation (1.1) for a larger time, which is supposed to approximately be 0.41 s in fig. 2(b) of [8], and the cell size of bacteria studied in [8] is in the range between 1 and 6 µm. Therefore, it seems natural to consider, for such a large time, that the protein diffuses over a region of a few local blocks in the bacterium. This implies that the fluctuations of rather than those of dominantly contribute to the protein dynamics due to its power-law nature. In the latter, we employ the average value, of the diffusion exponent for the bacteria in each group based on the experimental data in fig. 6(d) in [8].
Based on these observations, in the region of the local blocks with constant diffusion exponent, we can take the conditional distribution in equation (2.4) in good approximation as follows: for which From this, as well as equations (2.7) and (2.8), we immediately derive the following distribution:
| 2.9 |
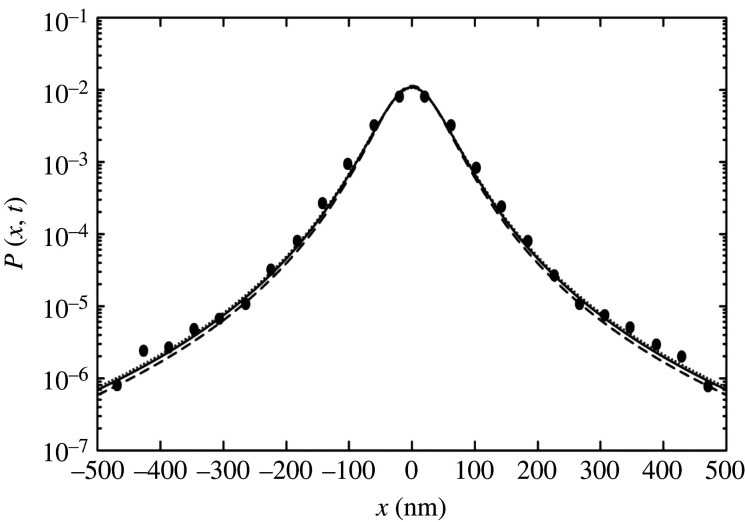
Clearly, equation (2.9) has the form of a q-Gaussian distribution. In figure 4, we present the plot of in equation (2.9). The experimental data are nicely described by the q-Gaussian distribution in equation (2.9). Upon renaming, in equation (1.1), we therefore have the following relations:
| 2.10 |
and
| 2.11 |
Figure 4.
Semi-log plot of the normalized distribution P(x, t) with variables x (nm) and t (s). This histogram uses the experimental data of fig. 4(a) in [8]. The three lines show the q-Gaussian distributions of equation (2.9): for the dashed line, for the solid line and for the dotted line [8]. and are considered.
Equation (2.10) tells us how the power-law exponent, the value of which is seen to be about (or, in terms of the exponent q in q-Gaussians), originates from the diffusion-constant fluctuations, whereas equation (2.11) determines the time evolution of w.
Let us briefly pause here to mention the experimental importance of fractional Brownian motion models. Golding & Cox [1] experimentally studied the diffusion of mRNA molecules inside E. coli bacteria, and it seems that they already at that early stage proposed the superposition of fractional Brownian motion processes as a suitable model, which was compared with measurements. In [46], a suitable distribution of the diffusivity was employed, and anomalous diffusion with ergodicity breaking [19] was found to emerge. The work in [47] has shown that Weibull-type distributions of the diffusivity give rise to mean square displacements similar to those experimentally observed for mRNA molecules. Whereas Molina-García et al. [46] mainly clarified the mathematical setting, Maćkała & Magdziarz [47] dealt with the experimental fine-tuning.
So far, we have focused our attention on the protein diffusion process in a small region of a few local blocks/areas with given local diffusion exponent. On a sufficiently long time scale, the protein will diffuse over the entire cytoplasm of the bacterium, and it is reasonable to consider that the contributions from the fluctuations of α are important as well, in addition to the fluctuations of β. This describes––in a superstatistical way—additional fluctuations in the anomalous diffusion exponent. The idea is that in some regions, e.g. in very crowded areas with obstacles, subdiffusion is dominant, whereas in other regions, nearly ballistic motion (superdiffusion) may be possible. These possibilities fluctuate spatially over the bacterium, and this also depends on the complexity and shape of the particular protein chosen, i.e. the ensemble of all biomolecules. (For a possible relevance to the latter, see a recent work in [48], where fluctuations of the size of a polymer have been discussed in connection with fluctuating diffusivity.)
To examine equation (2.7) in this situation, all kinds of conditional distributions can be considered. In the following, we wish to discuss a particular example form of the conditional distribution As discussed in appendix D, this is given by
| 2.12 |
where is a positive quantity depending weakly on and is the normalization factor. Only dimensionless numerical values of all quantities appearing are treated, since equation (2.12) is obtained from the distribution of dimensionless numerical values of is calculated in this case to be
| 2.13 |
where a1 (less than 0) is a small constant, representing a weak correlation.
In figure 2, we show the plot of the marginal distribution as given in equation (2.5) together with equations (2.12) and (2.13). There, it is observed that this distribution fits the experimental data quite well: in particular, it takes on a maximum value near and a local minimum value near respectively.
We think that for the data we use, there is evidence for sufficient sampling of the trajectories. According to Sadoon & Wang [8], trajectories with a minimum length of 10 frames (from the total of 20 000 frames in the resulting movies) were analysed for calculating the mean square displacements, some of which are based on trajectories that are quite long; see fig. 3(a) in [8]. Fluctuations may sometimes just originate from a lack of precision and insufficient statistics, as discussed in [49–51], where estimation techniques for this have been developed. We believe that in our case here we have more fundamental fluctuations, not caused by insufficient sampling. The function in equation (2.5) fits quite well with the experimental data as mentioned above, under the assumption (see appendix D) that given a value of the distribution of Dα takes the form in equation (B 2) leading to equation (1.3). There is robust and reproducible behaviour of the diffusion-constant fluctuations caused by medium heterogeneity (similarly, also diffusion-exponent fluctuations), with a possible additional correction from ensemble heterogeneity.
Further experimental data of protein anomalous diffusion dynamics can help to build the optimum model for a given experiment, possibly checking for universal and non-universal properties, i.e. properties that are observed for all bacteria and other properties that are very specific to a given specific experiment.
Importantly, when using superstatistical descriptions, one has to check under which conditions such a description is a valid approximation for a given dynamics or experimental realization. In [52] (see also [7,53]), a superstatistical description based on a gamma distribution of diffusivity was used for modelling the non-Gaussianity of displacements. For the particular dynamical model studied in [52], it was shown that such a description is appropriate only for short time scales, on which the diffusivity is considered not to be changing too much. Now, in the present work, we see that superstatistical modelling seems to make sense also for longer time scales, fitting experimental data well, in fact, for two simultaneous observables such as the diffusion constant and the diffusion exponent, although one still needs to investigate in more detail what the microscopic origin of the observed two-variable superstatistical behaviour in bacteria is. Further studies along these lines, and further comparison with further sets of experimental data, are needed to ultimately answer this question.
3. Conclusion
We have developed an effective superstatistical kinetic theory for describing the diffusion dynamics of an ensemble of complex biomolecules, in our case applied to the specific example of histonelike nucleoid-structuring proteins in living Escherichia coli bacteria. This model hierarchically takes into account both the diffusion-exponent fluctuations and the temperature (diffusion constant) fluctuations. We have shown that the theory naturally contains the q-Gaussian (Pearson-type) distributions often observed in experiments, for which the temperature fluctuations play a crucial role, representing local changes of the diffusion constant. The approach of fractional Brownian motion has been applied as a local stochastic process, representing the presence of an additional spectrum of anomalous diffusion exponents, which is important to consider in the most general and most complex cases describing the full contents of biomolecules in the bacterium. Proposing a concrete statistical form of the diffusion-exponent fluctuations, we have discussed the protein diffusion dynamics on a long time scale, for which the existence of the weak correlation between both the fluctuations is essential, described by a generalized two-parameter superstatistical formalism. We believe this formalism is quite generally applicable to a large variety of complex anomalous diffusion processes in small biological systems, and experimentally testable in future experiments.
Acknowledgements
The present research has been designed jointly by Y.I. and C.B. and it was completed while Y.I. stayed at the Institut für Computerphysik, Universität Stuttgart. Y.I. would like to thank the Institut für Computerphysik for its warm hospitality.
Appendix A. Superstatistical fluctuations of the diffusion constant
Consider the stochastic motion of the protein over the bacterium, which is regarded as a complex medium for the diffusion of the proteins. This medium is then divided into many small spatial regions or ‘blocks’, in each of which the protein exhibits heterogeneous diffusion according to equation (1.2), leading to variations of both α and β depending on the local blocks. These superstatistical fluctuations are considered to give rise to the fluctuations of Dα as follows. The fact noted earlier that D is distributed according to suggests that this fluctuation comes from displacement of the protein rather than the characteristic time being required for displacement, since such a characteristic time is constant in the random walk picture for normal diffusion [54]. The situation may be the same for Dα due to the same power-law nature of diffusivity. The power-law nature implies that D and Dα share a similar origin in their fluctuations. In fact, it is clear that one can define diffusion constants for both normal and anomalous diffusion, just their dimension is different. The diffusion constant for normal diffusion is proportional to temperature, i.e. as in the Einstein relation [23], such an origin is expected to be formally related to temperature. To investigate this point in more detail for anomalous diffusion, let us note the experimental fact [8] that for the mean square displacement in the ensemble average, the numerical value of the diffusion constant in the case of normal diffusion observed for small elapsed time is three times larger than that in the case of anomalous diffusion observed for large elapsed time, at least for the dataset that we study here. This indicates, for a given individual trajectory, that where () stands for displacement. (This symbol for the displacement should not be mixed with the one in equation (1.1).) Therefore, denoting and with s being a positive constant describing the characteristic time mentioned above, these observations allow us to evaluate Thus, Dα is given by
| A 1 |
with c being a positive constant.
From the above, the value of which turns out to be relevant through the present work, is estimated as follows. In the case of normal diffusion, the average of over the distribution in equation (2.3) is given by
| A 2 |
This should take on approximately the value of one-third of for the example experiments we look at here, which is the average value of D, and [8]; hence, it is found that Also, the average of over the joint fluctuations given in equations (2.3) and (2.4) can be calculated and is given by
| A 3 |
Using equations (2.12) and (2.13), the value turns out to be approximately given by This value is close to the average value of Dα measured in the experiment, which is [8]. This observation is seen to support the relation in equation (A 1).
Appendix B. The fluctuation distribution of the diffusion constant
The left-bottom inset in fig. 2(d) in [8] is seen to imply that the experimental data of the diffusion constant D (µm2 s−1) can be fitted with a slightly curved line, since the data point at D = 0.03 µm2 s−1 is below the red dashed line presented there, whereas the data points around at D = 0.1 µm2 s−1 are above the red dashed line. Accordingly, we suppose that such a curved line is described by the following inverse gamma distribution:
| B 1 |
in the interval where A is a positive constant having the dimension of the diffusion constant, and are lower and upper bounds on D, respectively; see figure 3. Clearly, this distribution decays as a power law for large D.
Here, we employ the assumption that the range of in equation (2.3) is unbounded, although the values of and in practice (i.e. in true experiments) are of course small and large, respectively, but still finite. To illustrate this aspect, we examine the influence of in our present theory. Let us compare the average value of D in the two cases with A = 0.011 µm2 s−1 and (figure 3), which is given by 0.051 µm2 s−1 in the case of and by 0.062 µm2 s−1 in the limit showing that these two values are close to each other. Since D appears in the denominator in the exponential factor in equations (2.4), (2.6) and (2.12), the contribution from D becomes small as it increases. From this, we conclude that the influence of is quite negligible.
From the experimental data in fig. 2(d) in [8], the situation seems to be similar for the dimensionless numerical values of Dα (). That is, it is seen that the data point at Dα = 0.01 µm2 s−α is below the red dashed line presented there, whereas almost all of the data points between Dα = 0.02 µm2 s−α and Dα = 0.1 µm2 s−α are above the red dashed line, implying that the experimental data can be fitted by a slightly curved line. As this curved line, we shall take the inverse gamma distribution given by
| B 2 |
in the interval where is a dimensionless positive constant, and are lower and upper bounds of Dα, respectively. As shown in figure 1, this distribution is seen to fit well, exhibiting the power-law behaviour in equation (1.3) for large Dα.
As will be shown in appendix D, the distribution in equation (B 2) turns out to play a key role for obtaining the form of the conditional distribution
Appendix C. A possible local stochastic process
Fractional Brownian motion [36,55] has been employed as the underlying process describing anomalous diffusion observed in a wide class of crowded fluid/biological systems; see [56–59], for example. This fact naturally motivates us to examine if the approach of fractional Brownian motion is appropriate as a model for the typical local diffusion dynamics in a bacterium, providing a concrete formula for
A process of fractional Brownian motion is described––based on that of the ordinary Brownian motion denoted by B(t)––in such a way that past increments of B(t) are incorporated into the process: where t0 is a positive constant having the dimension of time, is the unbiased Gaussian white noise satisfying and is the Riemann–Liouville fractional integral operator [60] with . Here, H is referred to as the Hurst exponent and it satisfies 0 < H < 1. Normal diffusion is realized in the case of H = 1/2, whereas the case of describes anomalous diffusion. So, if describes the individual trajectory in a given local region, then should hold in equation (1.2) (where it is understood that the case of α = 0 (α = 2) is realized in the limit ()). In our present discussion, the following three features of the process of fractional Brownian motion are relevant. Firstly, the velocity autocorrelation function in the process becomes negative (positive) if 0 < H < 1/2 (1/2 < H < 1) [19]. This is consistent with the experimental result presented in fig. 5(a) in [8], where it is clearly seen that the velocity autocorrelation function of the proteins becomes negative for small elapsed time. It should be also noted that the case of 0 < α < 1 has been predominantly observed. Secondly, the diffusion processes are assumed to be ergodic, since the protein can diffuse almost anywhere, in the sense [19,61] that the mean square displacement obtained through the position autocorrelation function is equivalent to the one derived based on the probability distribution. Lastly, the process does not exhibit any ageing phenomenon, since the sequence of the increments of is stationary [19]. Regarding this point, we should say that for the experimental data [8] there is a dependence on cell age, but this can be incorporated in the choice of the effective temperature relevant for each cell age group.
In [62], the displacement autocorrelation function has been calculated for the process of fractional Brownian motion. For large elapsed time, the function becomes negative (positive) if 0 < α < 1 (1 < α < 2). Therefore, the corresponding fractional approach is suitable if such a behaviour is observed for the experimental data.
Taking into account all of these considerations, we see that the model of local fractional Brownian motion is a good one for the local dynamics modelling in bacteria and other small complex systems. It simply needs to be amended by the detailed properties of the diffusion-constant distribution and diffusion-exponent distribution.
Appendix D. The conditional fluctuation distribution of the diffusion exponent
Here, we present a particular form of the distribution which allows us to derive the form of the marginal distribution Given a value of an allowed range of dimensionless numerical values of Dα can be determined through the diffusion exponent α in equation (A 1). Let us assume that the normalized probability distribution of such values takes on the form of the inverse gamma distribution in equation (B 2). Accordingly, is given by where α is considered to be distributed in the interval 0 < α < 2 due to the fact [8] that the average diffusion exponent is approximately constant in terms of the cell age, which implies that the interval to be taken does not drastically change. Since should have a weak dependence on we consider that in this case depends on in such a way that where with and Here, a1 should be small, realizing the weakness. Thus, can be expressed as the distribution in equation (2.12) with being the normalization factor given by
| D 1 |
where is the incomplete gamma function defined by Using this, in equation (2.13) is found to be given by
| D 2 |
in equation (2.12) is peaked at and the centre of this distribution should tend to approach the origin α = 0 as increases, since the average diffusion exponent slightly increases with respect to the cell age [8]. Accordingly, the condition requires a1 to be negative.
Keeping this in mind, it is found that the marginal distribution in equation (2.5) with equations (2.12) and (2.13) takes on a maximum value and a local minimum value, respectively, at and
| D 3 |
and
| D 4 |
where and () are defined by
| D 5 |
and
| D 6 |
where the quantity inside the square root in equations (D 3) and (D 4) is assumed to be positive (which is confirmed in the present case, see below).
In figure 2, a0 and a1 are taken in such a way that and which is very similar to the experimental result [8]; see the histogram in figure 2.
The experimental data imply that the quantity with equation (2.13) in equations (2.5), (2.8) and (A 3) takes on values between 0.12 and 1.16, whereas the quantity inside the square root in equations (D 3) and (D 4) has approximately the value of 38, i.e. it is positive, as required.
Data accessibility
This article has no additional data.
Authors' contributions
Y.I. and C.B. jointly designed research and wrote the paper.
Competing interests
The authors declare no conflict of competing interest.
Funding
We received no funding for this study.
References
- 1.Golding I, Cox EC. 2006. Physical nature of bacterial cytoplasm. Phys. Rev. Lett. 96, 098102. ( 10.1103/PhysRevLett.96.098102) [DOI] [PubMed] [Google Scholar]
- 2.Weber SC, Spakowitz AJ, Theriot JA. 2010. Bacterial chromosomal loci move subdiffusively through a viscoelastic cytoplasm. Phys. Rev. Lett. 104, 238102. ( 10.1103/PhysRevLett.104.238102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.English BP, Hauryliuk V, Sanamrad A, Tankov S, Dekker NH, Elf J. 2011. Single-molecule investigations of the stringent response machinery in living bacterial cells. Proc. Natl Acad. Sci. USA 108, E365-E373. ( 10.1073/pnas.1102255108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parry BR, Surovtsev IV, Cabeen MT, O'Hern CS, Dufresne ER, Jacobs-Wagner C. 2014. The bacterial cytoplasm has glass-like properties and is fluidized by metabolic activity. Cell 156, 183-194. ( 10.1016/j.cell.2013.11.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapanidis AN, Uphoff S, Stracy M. 2018. Understanding protein mobility in bacteria by tracking single molecules. J. Mol. Biol. 430, 4443-4455. ( 10.1016/j.jmb.2018.05.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manley S, Gillette JM, Patterson GH, Shroff H, Hess HF, Betzig E, Lippincott-Schwartz J. 2008. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat. Methods 5, 155-157. ( 10.1038/nmeth.1176) [DOI] [PubMed] [Google Scholar]
- 7.Chechkin AV, Seno F, Metzler R, Sokolov IM.. 2017. Brownian yet non-Gaussian diffusion: from superstatistics to subordination of diffusing diffusivities. Phys. Rev. X 7, 021002. ( 10.1103/physrevx.7.021002) [DOI] [Google Scholar]
- 8.Sadoon AA, Wang Y.. 2018. Anomalous, non-Gaussian, viscoelastic, and age-dependent dynamics of histonelike nucleoid-structuring proteins in live Escherichia coli. Phys. Rev. E 98, 042411. ( 10.1103/PhysRevE.98.042411) [DOI] [Google Scholar]
- 9.Sadoon AA, et al. 2020. Silver ions caused faster diffusive dynamics of histone-like nucleoid-structuring proteins in live bacteria. Appl. Environ. Microbiol. 86, e02479–19. ( 10.1128/AEM.02479-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsallis C. 2009. Introduction to nonextensive statistical mechanics: approaching a complex world. New York, NY: Springer. [Google Scholar]
- 11.Pearson K. 1916. IX. Mathematical contributions to the theory of evolution.—XIX. Second supplement to a memoir on skew variation. Phil. Trans. R. Soc. Lond. A 216, 429-457. ( 10.1098/rsta.1916.0009) [DOI] [Google Scholar]
- 12.Shlesinger MF, Zaslavsky GM, Frisch U, eds. 1995. Lévy flights and related topics in physics. Heidelberg, Germany: Springer. [Google Scholar]
- 13.Stylianidou S, Kuwada NJ, Wiggins PA. 2014. Cytoplasmic dynamics reveals two modes of nucleoid-dependent mobility. Biophys. J. 107, 2684-2692. ( 10.1016/j.bpj.2014.10.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lampo TJ, Stylianidou S, Backlund MP, Wiggins PA, Spakowitz AJ. 2017. Cytoplasmic RNA-protein particles exhibit non-Gaussian subdiffusive behavior. Biophys. J. 112, 532-542. ( 10.1016/j.bpj.2016.11.3208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu S, Sheats J, Cicuta P, Sclavi B, Lagomarsino MC, Dorfman KD. 2018. Subdiffusion of loci and cytoplasmic particles are different in compressed Escherichia coli cells. Commun. Biol. 1, 176. ( 10.1038/s42003-018-0185-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witzel P, Götz M, Lanoiselée Y, Franosch T, Grebenkov DS, Heinrich D. 2019. Heterogeneities shape passive intracellular transport. Biophys. J. 117, 203-213. ( 10.1016/j.bpj.2019.06.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouchaud J-P, Georges A. 1990. Anomalous diffusion in disordered media: statistical mechanisms, models and physical applications. Phys. Rep. 195, 127-293. ( 10.1016/0370-1573(90)90099-N) [DOI] [Google Scholar]
- 18.Höfling F, Franosch T. 2013. Anomalous transport in the crowded world of biological cells. Rep. Prog. Phys. 76, 046602. ( 10.1088/0034-4885/76/4/046602) [DOI] [PubMed] [Google Scholar]
- 19.Metzler R, Jeon J-H, Cherstvy AG, Barkai E. 2014. Anomalous diffusion models and their properties: non-stationarity, non-ergodicity, and ageing at the centenary of single particle tracking. Phys. Chem. Chem. Phys. 16, 24 128-24 164. ( 10.1039/C4CP03465A) [DOI] [PubMed] [Google Scholar]
- 20.Itto Y. 2019. Time evolution of entropy associated with diffusivity fluctuations: diffusing diffusivity approach. Eur. Phys. J. B 92, 164. ( 10.1140/epjb/e2019-100054-9) [DOI] [Google Scholar]
- 21.EPJ. org. 2019. Entropy explains RNA diffusion rates in cells. EPJ B Highlight. See https://www.epj.org/epjb-news/1776-epjb-highlight-entropy-explains-rna-diffusion-rates-in-cells. [Google Scholar]
- 22.Itto Y. 2019. Entropy production rate of diffusivity fluctuations under diffusing diffusivity equation. J. Phys.: Conf. Ser. 1391, 012054. ( 10.1088/1742-6596/1391/1/012054) [DOI] [Google Scholar]
- 23.Nelson P. 2004. Biological physics: energy, information, life. New York, NY: W. H. Freeman and Company. [Google Scholar]
- 24.Beck C, Cohen EGD. 2003. Superstatistics. Physica A 322, 267-275. ( 10.1016/S0378-4371(03)00019-0) [DOI] [Google Scholar]
- 25.Beck C. 2001. Dynamical foundations of nonextensive statistical mechanics. Phys. Rev. Lett. 87, 180601. ( 10.1103/PhysRevLett.87.180601) [DOI] [PubMed] [Google Scholar]
- 26.Beck C, Cohen EGD. 2017. Superstatistics: superposition of Maxwell-Boltzmann distributions. In Kappa distributions: theory and applications in plasmas (ed. Livadiotis G). Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 27.Hanel R, Thurner S, Gell-Mann M. 2011. Generalized entropies and the transformation group of superstatistics. Proc. Natl Acad. Sci. USA 108, 6390-6394. ( 10.1073/pnas.1103539108) [DOI] [Google Scholar]
- 28.Yalcin GC, Rabassa P, Beck C. 2016. Extreme event statistics of daily rainfall: dynamical systems approach. J. Phys. A: Math. Theor. 49, 154001. ( 10.1088/1751-8113/49/15/154001) [DOI] [Google Scholar]
- 29.Schäfer B, Beck C, Aihara K, Witthaut D, Timme M.. 2018. Non-Gaussian power grid frequency fluctuations characterized by Lévy-stable laws and superstatistics. Nat. Energy 3, 119-126. ( 10.1038/s41560-017-0058-z) [DOI] [Google Scholar]
- 30.Colombo EH. 2019. Connecting metapopulation heterogeneity to aggregated lifetime statistics. Ecol. Complex. 39, 100777. ( 10.1016/j.ecocom.2019.100777) [DOI] [Google Scholar]
- 31.Iliopoulos A, Chorozoglou D, Kourouklas C, Mangira O, Papadimitriou E. 2019. Superstatistics, complexity and earthquakes: a brief review and application on Hellenic seismicity. Boll. Geof. Teor. Appl. 60, 531-548. ( 10.1016/j.chaos.2019.109511) [DOI] [Google Scholar]
- 32.Williams G, Schäfer B, Beck C. 2020. Superstatistical approach to air pollution statistics. Phys. Rev. Res. 2, 013019. ( 10.1103/PhysRevResearch.2.013019) [DOI] [Google Scholar]
- 33.Metzler R. 2020. Superstatistics and non-Gaussian diffusion. Eur. Phys. J. Spec. Top. 229, 711-728. ( 10.1140/epjst/e2020-900210-x) [DOI] [Google Scholar]
- 34.Itto Y. 2014. Heterogeneous anomalous diffusion in view of superstatistics. Phys. Lett. A 378, 3037-3040. ( 10.1016/j.physleta.2014.08.022) [DOI] [Google Scholar]
- 35.Itto Y. 2017. Virus infection pathway in living cell: anomalous diffusion, exponent fluctuations, and time-scale separation. In Frontiers in anti-infective drug discovery (eds Atta-ur-Rahman, Iqbal Choudhary M), vol. 5. Sharjah, UAE: Bentham Science Publishers. [Google Scholar]
- 36.Mandelbrot BB, van Ness JW.. 1968. Fractional Brownian motions, fractional noises and applications. SIAM Rev. 10, 422-437. ( 10.1137/1010093) [DOI] [Google Scholar]
- 37.Vitali S, Sposini V, Sliusarenko O, Paradisi P, Castellani G, Pagnini G. 2018. Langevin equation in complex media and anomalous diffusion. J. R. Soc. Interface 15, 20180282. ( 10.1098/rsif.2018.0282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H, Dou S-X, Liu Y-R, Li W, Xie P, Wang W-C, Wang P-Y. 2015. Mapping intracellular diffusion distribution using single quantum dot tracking: compartmentalized diffusion defined by endoplasmic reticulum. J. Am. Chem. Soc. 137, 436-444. ( 10.1021/ja511273c) [DOI] [PubMed] [Google Scholar]
- 39.Stadler L, Weiss M. 2017. Non-equilibrium forces drive the anomalous diffusion of telomeres in the nucleus of mammalian cells. New J. Phys. 19, 113048. ( 10.1088/1367-2630/aa8fe1) [DOI] [Google Scholar]
- 40.Etoc F, et al. 2018. Non-specific interactions govern cytosolic diffusion of nanosized objects in mammalian cells. Nat. Mater. 17, 740-746. ( 10.1038/s41563-018-0120-7) [DOI] [PubMed] [Google Scholar]
- 41.Sabri A, Xu X, Krapf D, Weiss M. 2020. Elucidating the origin of heterogeneous anomalous diffusion in the cytoplasm of mammalian cells. Phys. Rev. Lett. 125, 058101. ( 10.1103/PhysRevLett.125.058101) [DOI] [PubMed] [Google Scholar]
- 42.Goychuk I. 2009. Viscoelastic subdiffusion: from anomalous to normal. Phys. Rev. E 80, 046125. ( 10.1103/PhysRevE.80.046125) [DOI] [PubMed] [Google Scholar]
- 43.Abe S, Beck C, Cohen EGD. 2007. Superstatistics, thermodynamics, and fluctuations. Phys. Rev. E 76, 031102. ( 10.1103/PhysRevE.76.031102) [DOI] [PubMed] [Google Scholar]
- 44.Abe S. 2014. Fokker-Planck theory of nonequilibrium systems governed by hierarchical dynamics. Found. Phys. 44, 175-182. ( 10.1007/s10701-014-9775-3) [DOI] [Google Scholar]
- 45.Reynolds AM. 2003. Superstatistical mechanics of tracer-particle motions in turbulence. Phys. Rev. Lett. 91, 084503. ( 10.1103/PhysRevLett.91.084503) [DOI] [PubMed] [Google Scholar]
- 46.Molina-García D, Pham TM, Paradisi P, Manzo C, Pagnini G. 2016. Fractional kinetics emerging from ergodicity breaking in random media. Phys. Rev. E 94, 052147. ( 10.1103/physreve.94.052147) [DOI] [PubMed] [Google Scholar]
- 47.Maćkała A, Magdziarz M. 2019. Statistical analysis of superstatistical fractional Brownian motion and applications. Phys. Rev. E 99, 012143. ( 10.1103/physreve.99.012143) [DOI] [PubMed] [Google Scholar]
- 48.Hidalgo-Soria M, Barkai E. 2020. Hitchhiker model for Laplace diffusion processes. Phys. Rev. E 102, 012109. ( 10.1103/PhysRevE.102.012109) [DOI] [PubMed] [Google Scholar]
- 49.Grebenkov DS. 2011. Probability distribution of the time-averaged mean-square displacement of a Gaussian process. Phys. Rev. E 84, 031124. ( 10.1103/PhysRevE.84.031124) [DOI] [PubMed] [Google Scholar]
- 50.Sikora G, Teuerle M, Wyłomańska A, Grebenkov D. 2017. Statistical properties of the anomalous scaling exponent estimator based on time-averaged mean-square displacement. Phys. Rev. E 96, 022132. ( 10.1103/physreve.96.022132) [DOI] [PubMed] [Google Scholar]
- 51.Lanoiselée Y, Sikora G, Grzesiek A, Grebenkov DS, Wyłomańska A. 2018. Optimal parameters for anomalous-diffusion-exponent estimation from noisy data. Phys. Rev. E 98, 062139. ( 10.1103/PhysRevE.98.062139) [DOI] [Google Scholar]
- 52.Lanoiselée Y, Grebenkov DS.. 2018. A model of non-Gaussian diffusion in heterogeneous media. J. Phys. A: Math. Theor. 51, 145602. ( 10.1088/1751-8121/aab15f) [DOI] [Google Scholar]
- 53.Lanoiselée Y, Grebenkov DS.. 2019. Non-Gaussian diffusion of mixed origins. J. Phys. A: Math. Theor. 52, 304001. ( 10.1088/1751-8121/ab2826) [DOI] [Google Scholar]
- 54.Einstein A. 1905. Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen. Ann. Phys. (Leipzig) 17, 549-560. ( 10.1002/andp.19053220806) [DOI] [Google Scholar]
- 55.Goychuk I, Kharchenko VO. 2013. Rocking subdiffusive ratchets: origin, optimization and efficiency. Math. Model. Nat. Phenom. 8, 144-158. ( 10.1051/mmnp/20138210) [DOI] [Google Scholar]
- 56.Szymanski J, Weiss M. 2009. Elucidating the origin of anomalous diffusion in crowded fluids. Phys. Rev. Lett. 103, 038102. ( 10.1103/PhysRevLett.103.038102) [DOI] [PubMed] [Google Scholar]
- 57.Magdziarz M, Weron A, Burnecki K, Klafter J. 2009. Fractional Brownian motion versus the continuous-time random walk: a simple test for subdiffusive dynamics. Phys. Rev. Lett. 103, 180602. ( 10.1103/PhysRevLett.103.180602) [DOI] [PubMed] [Google Scholar]
- 58.Weiss M. 2013. Single-particle tracking data reveal anticorrelated fractional Brownian motion in crowded fluids. Phys. Rev. E 88, 010101(R). ( 10.1103/PhysRevE.88.010101) [DOI] [PubMed] [Google Scholar]
- 59.Krapf D, et al. 2019. Spectral content of a single non-Brownian trajectory. Phys. Rev. X 9, 011019. ( 10.1103/PhysRevX.9.011019) [DOI] [Google Scholar]
- 60.Hilfer R ed. 2000. Applications of fractional calculus in physics. Singapore: World Scientific. [Google Scholar]
- 61.Deng W, Barkai E. 2009. Ergodic properties of fractional Brownian-Langevin motion. Phys. Rev. E 79, 011112. ( 10.1103/PhysRevE.79.011112) [DOI] [PubMed] [Google Scholar]
- 62.Burov S, Jeon J-H, Metzler R, Barkai E. 2011. Single particle tracking in systems showing anomalous diffusion: the role of weak ergodicity breaking. Phys. Chem. Chem. Phys. 13, 1800-1812. ( 10.1039/c0cp01879a) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.