Abstract
The diversity of signalling traits within and across taxa is vast and striking, prompting us to consider how novelty evolves in the context of animal communication. Sexual selection contributes to diversification, and here we endeavour to understand the initial conditions that facilitate the maintenance or elimination of new sexual signals and receiver features. New sender and receiver variants can occur through mutation, plasticity, hybridization and cultural innovation, and the initial conditions of the sender, the receiver and the environment then dictate whether a novel cue becomes a signal. New features may arise in the sender, the receiver or both simultaneously. We contend that it may be easier than assumed to evolve new sexual signals because sexual signals may be arbitrary, sexual conflict is common and receivers are capable of perceiving much more of the world than just existing sexual signals. Additionally, changes in the signalling environment can approximate both signal and receiver changes through a change in transmission characteristics of a given environment or the use of new environments. The Anthropocene has led to wide-scale disruption of the environment and may thus generate opportunity to directly observe the evolution of new signals to address questions that are beyond the reach of phylogenetic approaches.
Keywords: signal, sender, receiver, sexual selection, animal communication, environmental change
1. Introduction
Animals have evolved an astonishing variety of communication systems, spanning many signalling modalities, contexts and levels of complexity [1]. Novelty in animal communication is often striking (figure 1) and is characterized by abrupt or discontinuous change including new mechanisms of signal production, detection or perception (e.g. the use of a new organ and/or stimulation of a different sensory organ). How is communication sustained when such changes occur? Here, we describe alternative scenarios for the evolutionary origins of novelty in communication and analyse how initial conditions of the sender, receiver and the environment influence the evolutionary consequences (table 1). We focus on intersexual communication—mating signals, mating preferences and mate choice—because it provides countless opportunities for novelty to proliferate. Environmental selection on signals and receiver mechanisms, preferences for arbitrary traits and sexual conflict generate fitness surfaces that are dynamic over time and space [13–15], likely making mating signals, mating preferences and mate choice especially prone to novelty. Nevertheless, some of these characteristics are common components of other types of signalling, so the scenarios described may generalize beyond mating contexts.
Figure 1.
Novelty in animal signals and receiver features: (i) Odorrana tormota evolved ultrasonic calling songs, while ancestral species' songs are audible [2]; picture credit: A.S. Feng (https://phys.org/news/2008-05-female-concave-eared-frogs-ultrasonic.html); (ii) recently evolved morphs of the Pacific field cricket (Teleogryllus oceanicus) produce new sexual signals using modified wing morphology ([3]; picture credit: E.D. Broder); (iii) coevolution between male signals and female preference functions across the Enchenopa binotata complex [4]; picture credit: R.L. Rodríguez; (iv) in Drosophila, temperature impacts sender signalling behaviour but has minimal effects on female preferences [5]; picture credit: Hannah Davis/CC BY-SA (https://creativecommons.org/licenses/by-sa/4.0); (v) vervet monkeys use blue scrotal colour in sexual communication, which differs among species [6]; picture credit: Bjørn Christian Tørrissen/CC BY-SA (https://creativecommons.org/licenses/by-sa/3.0); (vi) visual signal variation in closely related Habronattus (top; picture credit Marshal Hedin) and Maratus (bottom; picture credit Madeline Girard) jumping spiders [7]; (vii) A. Origin of female foraging bias for male terminal yellow bands. B. Origin of terminal yellow band in males. C. Origin of female resistance to foraging costs in goodeid fishes [8]; picture credit: Wolfgang Gessl; (viii) satin bower birds (Ptilinorhynchus nuchalis) incorporate discarded blue items like pens, clothespins and bottlecaps into their displays [9,10]; picture credit: Gail Hampshire CC BY 2.0; (ix) frequency characteristics of vocal songs (produced via the syrinx) and feather sounds (produce via aeroelastic flutter) are similar across the ‘bee’ hummingbird clade ([11]; picture credit: Anand Varma); (x) electric mating signals have evolved independently in multiple lineages including elephant fish (Paramormyrops spp.) [12]; picture credit: C. Hopkins (https://www.nature.com/articles/467159a).
Table 1.
Key terms. Terms are italicized the first time they appear in the text.
| Associative learning: the development of a new behavioural response to a stimulus as a consequence of its pairing with positive or negative stimuli or experiences |
| Bias: reaction by a receiver that did not evolve in the context of the signal |
| Co-opt: an existing trait evolves to have a new function |
| Cue: trait that provides incidental information to a receiver |
| Disruption: (in communication) a change in the environment that distorts or masks the production, transmission, detection or reception of an existing mating signal |
| Novelty: (in communication) abrupt or discontinuous change in signal component or receiver feature, including new mechanisms of signal production, detection or perception (e.g. the use of a new organ, and/or stimulation of new perceptual mechanisms) |
| Receiver: an individual that attends to and evaluates signals |
| Response: reaction from a receiver that is prompted by a signal |
| Sender: individual producing and transmitting a signal or cue |
| Signal: trait that evolved to provide information to a receiver that benefits the sender |
In this review, we first describe contemporary examples of novelty in a mating context (figure 1; electronic supplementary material, table S1) and the mechanisms underlying the origin of such traits. We suggest that novel signal components and receiver features arise easily. Then, we reimagine the classic framework (e.g. [1]) of whether change occurs first in the signal or the receiver. We use a schematic (figure 2) to unpack four scenarios by which the interactions among the sender, the receiver and the environment facilitate the maintenance or elimination of novel animal communication features. Even in the case of no change occurring in the sender or the receiver, environmental change (figure 2, scenario 4) may approximate the scenarios of change in either signals or receivers (figure 2, scenarios 1–3). We conclude with four open questions about novelty in animal communication.
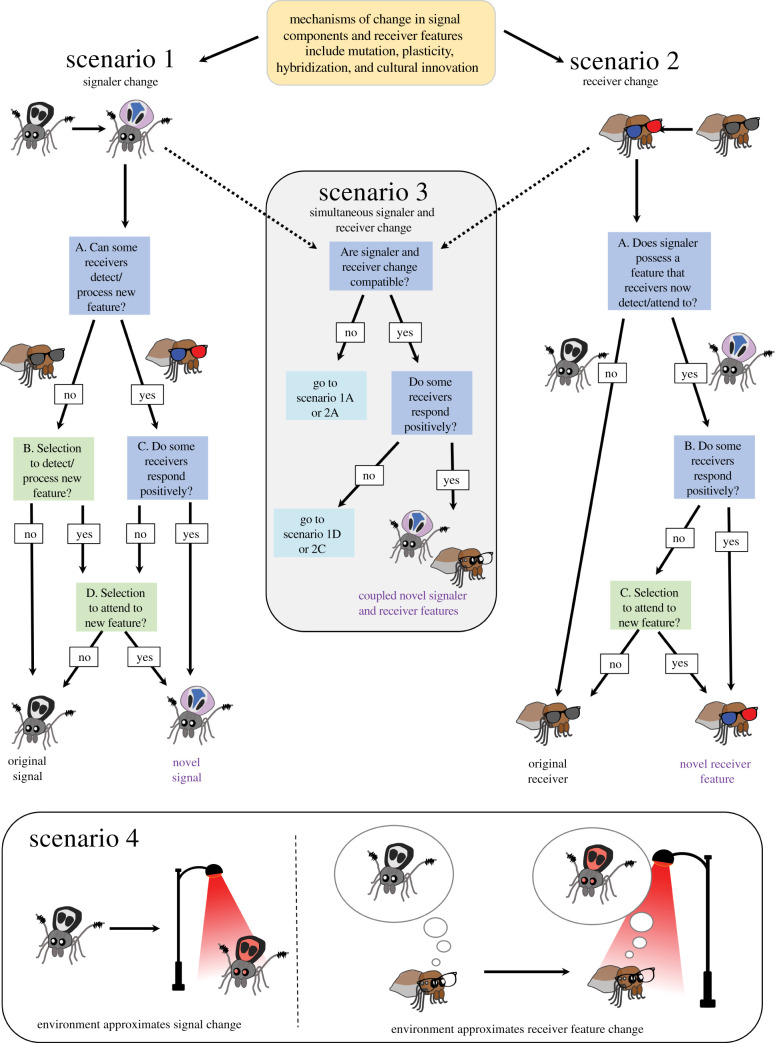
Figure 2.
Simplified schematic showing alternative scenarios by which novelty may evolve in mating communication. Our example is a generalized peacock Jumping spider (Maratus spp.) mating system where drab females choose males based on many traits including abdomen colouration. Change is initiated by mechanisms including mutation, hybridization, plasticity and cultural innovation. If change is in a signal component only, scenario 1 follows. If change is in a receiver feature only, scenario 2 unfolds. Change may be simultaneously initiated in both sender and receiver and if compatible, scenario 3 follows. Scenario 4 occurs when environmental change approximates signal change (left) or receiver change (right). In this example, there is a change in the light environment. Blue boxes indicate proximate considerations, while green indicate ultimate considerations. Glasses on the receivers represent receiver features (i.e. sunglasses indicating monochromatic and 3D red/blue glasses indicating colour vision). There are some simplifications here. For instance, in all scenarios, new signal components and receiver features may not be costly and could be maintained in the genome and later co-opted for a communication function. Additionally, new traits are subject to costs and benefits, including those unrelated to mating communication, and we should expect them to persist only when net benefits outweigh costs.
2. Conceptualizing novelty in mating
Novelty can be conceptualized in terms of how new variants originate [16], or in terms of new functional and evolutionary consequences of those variants [17–19]. New variants in communication can arise through changes in senders, receivers and the environment in any combination. Novelty in signals includes changes in production (how signals are made), in signal properties (the distribution of energy in signals) or in the transmission features of the environment (how signals are modified during transmission). Receivers may change in sensory transduction (which organs are used to detect signal energy and their sensitivity spectrum with respect to signals), integration (how the central nervous system integrates responses from the periphery) and evaluation (how signals are interpreted); all of these changes are hereafter referred to as receiver features.
Contemporary examples will help to illustrate the striking novelty in mating communication. Because many examples of change in senders exist, we focus this paragraph on senders. New sender variants can arise when signal production changes and structures that did not previously serve a communicative function evolve or are co-opted to produce new signals. For example, club-winged manakins (Machaeropterus deliciosus) and hummingbirds (Stellula calliope) evolved novel acoustic signals (sonations) via modification of feather morphology ([11,20]; figure 1(ix)). These new sounds are produced using a mechanism that differs from the more widespread vocalizations produced in the syrinx, yet the song frequencies produced by both mechanisms overlap [21,22]. Similarly, in ghost crabs (Ocypode quadrata), co-opted gastric stridulation produces an acoustic signal that overlaps with sounds produced through claw stridulation [23]. Alternatively, structures currently used in communication can be refined to produce a signal with different attributes, such as changes in the aforementioned syrinx, which have produced much of the diversity found in song birds [24]. Changes to an existing structure can lead to radically new sender variants (novelty) by shifting the signal parameter space relative to the ancestral signal. For example, dramatic shifts to the ultrasonic range evolved in Odorrana frogs; calling songs in ancestral species are in the audible range (100–6000 Hz), while in Odorrana tormota, calling songs are in the ultrasonic range (greater than 20 000 Hz) ([2,25]; figure 1(i)). Such shifts in the signal parameter space could include changes in signalling modalities (e.g. from visual to vibration [26]; or from airborne to substrate-borne sound [27,28]). Similarly, new sender variants may also dramatically expand or contract the variation in parameter space. For example, ancestral Pacific field cricket (Teleogryllus oceanicus) calls are highly tonal in the 5 kHz range, while recently evolved ‘purring’ crickets (Teleogryllus oceanicus) use distinct wing morphology to produce broadband songs with peak frequencies spanning approximately 2–30 kHz ([3]; figure 1(ii)).
3. Novelty arises easily through many mechanisms
Novelty in senders and receivers can originate in a surprising diversity of ways, including through mutation (e.g. gene duplication), hybridization and cultural innovation (figure 2). New mutations can arise that change signal and receiver phenotypes [29,30] usually by directly changing metabolic networks, regulatory circuits or macromolecules [31] or through the recruitment or co-option of genes [32]. Hybridization can similarly generate novel signals [33–35] or signal combinations [36,37] through transgressive segregation [38] or new epistatic interactions [39].
Importantly, novelty can also arise very quickly without genetic change and could be incorporated into the genome at a later time, for instance via genetic accommodation [16]. Both signal expression and receiver preference often depend on the environment in which animals develop [40] and may be especially affected by early learning or imprinting (reviewed in [41]). Developmental plasticity, for instance, produces novel phenotypes when environments change, exposing hidden genetic variation to the novel selection regimes [16,42,43]. Developmental plasticity caused dramatic changes in receivers in cross-fostering experiments, for example [44,45]. Culturally transmitted signals and receiver features also change rapidly; meaningful differences in the attractiveness of conspecific songs arose recently in the white-throated sparrow (Zonotrichia albicollis) as a result of cultural evolution of bird song over only 20 years [46]. Associative learning may provide an even more general source of change in mating signals, mate preferences and mate choice; all that is required here is that an arbitrary stimulus be paired with a sexual reward. For example, rats and quail can both be trained to exhibit sexual arousal in conjunction with arbitrary inanimate objects present during sexual interactions [47,48]. Any stimulus that is associated with a positive sexual experience could thereby become a sexual signal. Finally, even without learning, changes in the availability of signal-building resources in the environment may result in novel signals. For example, orchid bees (Euglossa viridissima) incorporate compounds from herbicides in courtship chemical cues [49].
Novelty in animal communication is readily detectable at a macroevolutionary scale, and phylogenetic comparative methods (pioneered by Maddison & Maddison [50] and Felsenstein [51]) are useful for reconstructing changes in traits like signals or receiver features along the branches of an evolutionary tree. We often detect signal novelty because it is restricted to particular taxa and absent from close relatives (e.g. bowers in pufferfish (Torquigener spp.) [52] and bowerbirds (Ptilinorhynchus nuchalis) [9], electric pulses in gymnotiforms and mormyriform fishes [53]; figure 1). Characterizing new receiver variants requires behavioural and/or physiological work, but is also tractable at a macroevolutionary scale. For example, terminal yellow bands were a new sexual signal in male goodeid fishes that initially elicited a prey-approach response from females [8]. The authors went on to show a new receiver response in the lineage with yellow bands: females decoupled their sexual response from their prey-approach response (figure 1(vii)). Phylogenetic comparative methods likely underestimate the extent to which novel communication phenotypes arise, however. Longitudinal studies of mating signals and preferences, and experimental tests of responses to new signals, suggest that cultural transmission or rapid evolution can lead to loss or spread of novelty over just a few generations [46,54,55]. Such traits are expected to be gained and lost along individual branches of a phylogeny without leaving a trace in terms of interspecific variation in communication. Since mating traits and preferences have the potential to change assortative mating and population structure [56], such undetected changes in communication may have longer-reaching consequences for local adaptation and hybridization.
4. Novelty and the maintenance of communication
Here, we focus on the immediate evolutionary context that facilitates the maintenance or elimination of new features of communication when new variants arise (figure 2). To keep the framework simple, we consider situations in which a single sender sends a signal and a single receiver responds, rather than situations in which receivers also send signals (i.e. duetting). This rationale should nevertheless be applicable to more complex cases. We suggest that the characteristics of the sender and the receiver, and the environmental conditions present when the novelty arises, dictate whether and how coevolution between sender and receiver progresses. It is important to note here that the pre-existing characteristics of sender and receiver have been shaped by historic evolution, including forces outside of the context of communication (e.g. abiotic and biotic selection, drift); such characteristics (e.g. cues, receiver biases) can be co-opted for communication function via the processes we describe. Novelty in animal communication may involve initial changes in both the sender and the receiver, only the sender, only the receiver or neither (in which case the environment may approximate sender or receiver change (see below); figure 2 [1]). We use these four scenarios to frame our analysis, but we recognize that there is some overlap. For example, for both scenarios 1 and 2, there is one path in which receivers can already detect the new signal—whether we refer to this as sender first or receiver first depends on our starting point and is less important than understanding the possible evolutionary paths to novelty.
In the case of novelty occurring first in a signal component (figure 2, scenario 1), the immediate evolutionary dynamics depend on the environment and receivers. A novel signal is unlikely to be maintained if it is undetectable to receivers, detectable but unattractive to receivers or costly in that given environment (e.g. because of predation and food limitations). However, even if initially undetectable or unattractive, there may be selection for receivers to start attending to a novel signal component (e.g. if it reduces search costs; figure 2, scenario 1). On the other hand, it may be that receivers can already detect novel signal components when they arise, even if the signal changes are abrupt and discontinuous (figure 2, scenario 1 boxes A,C). Some receivers may respond positively if they have perceptual biases (so-called hidden preferences) [57–61] or general biases for novelty.
Perceptual biases in receivers are ubiquitous. Receivers generally perceive and attend to many more aspects of their surroundings than the signals of potential mates (e.g. they navigate the world attending to indications of the presence of food, natural enemies, etc.), and their sensory systems are more broadly tuned than only to courtship signals [62,63]. A novel signal will be favoured if it is detectable and elicits a favourable response as a consequence of latent biases. Importantly, although sensory systems are broadly tuned, receiver responses may be negative (e.g. if the new signal mimics something receivers have been selected to avoid, like a predator). And, novel signals may trigger biases in receivers even if they are initially harmful to receivers if they elicit responses that are beneficial to senders. However, responding to novel signals may instead bring about benefits (e.g. increased salience [58,64,65]). Goodeid fish (figure 1(vii)) are an example of the potential for receivers to evolve context-dependent responses following the evolution of a novel signal. Here, the terminal yellow band (TYB) of courting males mimics an insect larva, causing naive females to lose weight as they chase after male tails in lieu of prey. Females that have coevolved with the TYB, however, exhibit a more sophisticated response, attending to TYBs only in the context of mating [8]. Similarly, swordtail fish (Xiphophorus birchmanni) exhibit decoupled responses to body size and fin elongation once the latter evolves [66]. When novel signals evolve, receiver responses may thus be refined and further diversified, rather than eliminated [27,67–69].
Along with perceptual biases, a general bias for novelty can also cause novel signal components to be initially attractive. There are widespread mechanisms that allow new or rare signals to be salient such as comparative evaluation (mate assessment via comparison to other possible mates rather than assigning each mate an individual score [14]), which sometimes favours rare phenotypes [70], and release from habituation (receivers typically begin to tune out stimuli that repeatedly trigger the same sensor, but new signals may circumvent that [71]). So-called novelty or ‘rare-male’ effects, where unusual phenotypes are more attractive, are also widespread. Choosers often exhibit the Coolidge effect, preferring unfamiliar mates [72]. In addition to being easier to detect, novel signals may be easier to recognize and distinguish in memory [14,73]. It is important to note that even though many receivers likely have general biases for novelty, the expression of those biases still depends on their being presented in an appropriate context. This is suggested by studies of female starlings (Sturnus vulgaris) [74,75], which only respond to novel song elements as enhancements of already attractive long-bout songs. Similarly, female túngara frogs (Engystomops pustulosus) prefer songs with any of a vast array of novel acoustic ornaments, but only when paired with the species-typical ‘whine’ call [76].
Once receivers attend to a novel signal component, provided that they also respond positively to it, the component and its processing evolve through the cue-to-signal pathway [77]. Receiver features, including novel receiver features (see scenario 2 below), exert selection on signal components, favouring signal variants that match receiver features. The widespread presence of ritualized signals that arose as cues, e.g. from grooming movements [1,78], suggests that this pathway for novelty is not uncommon or constrained. In figure 2—scenario 1, we have focused on the role of the receiver in whether a novel signal component persists, but this path to novelty is also dependent on the broader environmental context. Specifically, if the cost of possessing the novel signal component or responding to it outweighs the benefits (e.g. by attracting predators, inhibiting mobility and impeding foraging), the new signal will not evolve.
If novelty arises first in a receiver feature (figure 2, scenario 2), the immediate evolutionary dynamics depend strongly on the properties of the signal. When novel receiver features arise, if the existing signal lacks an aspect to which receivers are now attentive the ancestral signal will persist (figure 2, scenario 2). However, if the novel receiver feature is not costly, it could be maintained in the genome, generating a hidden preference [58,59]. On the other hand, when novel receiver features arise, senders may already have a trait that the novel receiver feature can now detect. If so, receivers may immediately respond positively to the newly perceptible sender trait, which could then be refined into a signal (figure 2, scenario 2 [58]). As with novel signal components, when novelty arises only in a receiver feature, it will be subject to selection both within and outside the context of communication; there must be a net benefit for individuals possessing the novel receiver feature in order for it to be incorporated into a communication system. In the existing literature, we often know less about the receiver than the sender (electronic supplementary material, table S1), perhaps because the behavioural and physiological data required to answer questions about the receiver change are absent. But phylogenetic comparative methods have revealed several cases in which receivers appear to lead in the evolution of novelty: female Xiphophorous preference for swords predates the evolution of swords [79], and trichromacy (colour vision) evolved in old world monkeys before colourful male mating signals arose (e.g. red fur and skin) [80].
When there is a simultaneous novel change in both senders and receivers (figure 2, scenario 3), the evolutionary dynamics depend on whether or not the changes are compatible (i.e. in the same direction and modality). If changes are not compatible, the scenario would simply be equivalent to scenario 1 or 2 (figure 2). By contrast, simultaneous, congruent changes in both the sender and the receiver are surprisingly common. Communication systems can change rapidly if receivers respond favourably to signal changes (figure 2, scenario 3). In a number and diversity of cases, mating signals and mate preferences change near simultaneously via apparent pleiotropy, close physical linkage or associated with polyploidy (e.g. [81–88]). A similar pattern may arise when juveniles learn or imprint on their parents' signals and preferences [41]. Broader causes of plasticity (e.g. social interactions) have also been predicted to have the ability to generate signal-preference codivergence [89,90], and even non-social sources of phenotypic variation can result in striking signal-preference matching covariance (e.g. host plant developmental environments [91]). We recognize that the differences among scenarios 1, 2 and 3 depend on the starting point. On a phylogenetic scale, most examples of novelty in communication will appear to have evolved via scenario 3, resulting in coupled signaller and receiver traits. However, the initial path to novelty may begin with the sender, the receiver or both, and without the opportunity to directly watch this process play out, we may miss this nuance. These details are important for exploring the initial microevolutionary patterns facilitating novel mating signals and may be best studied using detailed investigations of organisms currently undergoing dynamic change in mating communication.
Finally, even if there is no change in signal components or receiver features (figure 2, scenario 4), novel communication can still occur through changes in the dynamics of the communication environment. We expand on this idea in the next section.
5. Disruptions through environmental effects
Environmental variation may catalyse the evolution of novel signals and responses to those signals through genetic accommodation and coevolution. When production and transmission are disrupted by changes in the environment, this would approximate a change in the sender, while a change in the environment impacting detection would approximate a new feature in the receiver (figure 2, scenario 4). To illustrate these points, we focus on temperature as an example. Temperature could impact sender behaviour and have minimal to no effect on receivers (figure 2, approximating scenario 1). For example, in some flies, temperature impacts sender signalling behavior but has minimal effects on female preferences for signals [5]. Temperature could also theoretically impact receivers and not senders (figure 2, approximating scenario 2). While we could not identify studies that showed this exact pattern, it has been demonstrated repeatedly that some components of signalling are temperature invariant, but are subject to varying receiver preferences across temperatures [92,93]. Additionally, temperature has been demonstrated to affect receiver sensory abilities [94,95], suggesting the possibility of this pattern. Temperature could also have effects on both senders and receivers (figure 2, approximating scenario 3) in ways that are equivalent (i.e. ‘temperature coupling’ [96,97]) or not [92,98].
While the above examples measure immediate adult responses to temperature, developmental exposure to environmental variation may also affect signals and receiver features through developmental plasticity [99]. In Bicyclus anynana, rearing temperature affects the development of sender behaviour (pheromone production, eyespot and colouration patterns [100,101]) and receiver mate choice behaviours [102,103]. Developmental plasticity can promote the evolution of novelty through several mechanisms, including, for example, genetic accommodation and expansion of targets for selection [40]. Importantly, even non-adaptive plasticity can facilitate a rapid evolutionary change by increasing the strength of selection, as has been demonstrated in guppies (Poecilia reticulata) [104].
A contemporary anthropogenic change may offer opportunity for the evolution of novelty in animal communication through changes to the abiotic (e.g. transmission properties and signal detection, and human-manufactured objects) and biotic (community composition, eavesdropping natural enemies and species distributions) environments as described above. Changes in the biotic and abiotic environments can also clearly alter the way natural selection acts on both sexual signals and receiver features [105,106]. Widespread habitat fragmentation, for example, alters the distributions and abundance of predators and parasitoids [107]. Changes in the predator community and eavesdropping natural enemies can then exert selection on sexual signals (e.g. a silent male morph evolved in response to selection from an introduced acoustic eavesdropper [108,109]) and receiver features (e.g. female guppies shift preferences in the presence of a predator [110]).
Consider signal transmission and detection, which are dependent on the abiotic environment through which senders transfer signals [1,111–113]. Most signal types are transmitted through the environment, and the environment can attenuate, amplify, filter and otherwise distort signals in a variety of ways. Human alteration of the environment (artificial light, acoustic anthropogenic noise and chemical pollutants [105,106]) has accelerated disruption to communication systems, potentially generating opportunity for the evolution of new signals [114]. Following the environmental change, we expect natural selection to favour signals that effectively transmit through the changed environment (e.g. through sensory drive [115]) and thus could favour the evolution of novelty in many instances. If a newly evolved cue cannot be transmitted through a particular environment, it likely will not be maintained. Numerous examples have been identified where anthropogenic effects mask signals, thus constraining the signal properties available to receivers [98,106,116–126] and sender and/or receiver behaviour itself [127–131]. For example, in turbulent water, female cichlids can no longer use male colouration to choose mates [126], and in polluted water with high concentrations of humic acid, female swordtails (Xiphophorus birchmanni) no longer preferred conspecific male chemical cues to congener species [125]. Alternatively, the changed environments (e.g. habitat change) could relax constraints on signal evolution and reveal different aspects of signals to selection, allowing for the evolution of novelty in communication. Though not directly linked to human impacts, microhabitat specialization on oak litter habitats is hypothesized to have relaxed restrictions on signal form in the wolf spider Schizocosa floridana, allowing a novel signal component, the tonal ‘chirp’, to evolve in this particular microhabitat [132,133]. ‘Sensory drive’ mechanisms driven by the environmental change such as these are expected to have wide ranging impacts on the evolution of novel signals. The impacts of environmental change will, in part, depend on how widespread and predictable the change is. Anthropogenic land-use change tends to homogenize environments across multiple scales including sensory environments [106,134–137]. Widespread, homogeneous alterations of the sensory environments driven by anthropogenic change are thus expected to lead to widespread changes in senders and receivers, although strong constraints may exist for some taxa [120,138].
Finally, human-manufactured objects have also created new environments to be exploited (metal and glass used as anchor points for webs of orb-weaving spiders (Araneus diadematus) [136]) and even new signals themselves (objects such as pens and clothespins used as bower bird ornaments or incorporation of compounds for herbicides in orchid bee courtship chemical cues [49]). Human-manufactured chemicals that are released into the environment also appear to affect receiver preferences; synthetic oestrogen affects female [139,140] and male preferences [141]. It is important to ascertain at what level (i.e. production, transmission or detection) and to what extent the environment disrupts signals as it has important implications for potential origins of novelty.
6. Conclusion
Understanding how biological diversity comes to be requires appreciation of evolutionary novelty, and novelty is readily detectable in mating communication (figure 1; electronic supplementary material, table S1). We argue above that novelty may arise easily, and we discuss how initial conditions of the sender, receiver and environment dictate how coevolution between the sender and the receiver progresses and whether a new cue becomes a signal. Our review reveals four open questions that require further investigation. Finding the answers to these questions is inherently multidisciplinary and will require studies integrating genetics, development, physiology, neurobiology, behaviour and phylogenetics. First, how widespread are each of the scenarios we outlined (figure 2), and does the evolutionary outcome differ depending on the scenario? While there are many compelling contemporary examples of novelty in mating communication (electronic supplementary material, table S1), for most of these there remain open questions, particularly regarding receivers. We will need more detailed behavioural and physiological work on receivers in systems that have documented a signal change, particularly in a robust phylogenetic context. We would gain a great insight by carefully identifying and documenting sender and receiver behaviours in study systems that are currently experiencing change in signal and/or receiver features (e.g. purring crickets [3]; see also question four). Second, which systems and which types of signals (e.g. courtship signals and warning colouration) are more resistant to novelty in animal communication? Theory predicts that some types of communication (e.g. warning and aggressive signals) may be more stable, but these types of signalling systems are also incredibly variable [142]. Additionally, how often is novelty in mating and courtship signals favoured per se? Future work needs to examine how the type of signalling system influences how (and what type of) novelty arises, the stability of the communication system to novelty and the evolutionary trajectory of novel signals for different communication systems. Third, it seems especially challenging to explain seemingly abrupt, discontinuous changes; e.g. new mechanisms of signal production or detection like new organs. Are the mechanisms most often involved in gradual versus abrupt changes in communication the same or different? We know that drift and selection are always important, but perhaps discontinuous change is associated with particular mechanisms (i.e. mutation, hybridization and pleiotropy). Fourth, how important is the environmental change in catalysing novelty in animal communication and under what circumstances do novel traits/novel responses follow from ecological change? One way to approach this question is to ask whether and what type of environmental change accompanies the emergence of novel communication features, but this is difficult to determine when looking back on past evolutionary change. The Anthropocene may offer increased opportunity to directly observe sender and receiver responses to the environmental change (the less detectable ephemeral ‘blips’ along branches of a phylogeny), allowing us to ask detailed behavioural and mechanistic questions that were heretofore inaccessible using phylogenetic approaches.
Acknowledgements
These ideas were developed when all authors participated in a symposium, ‘The what, when and how of new animal conversations', at the 2019 Animal Behavior Society meeting. We thank the symposium participants M. Morris and M. Rosenthal and symposium attendants for stimulating conversation. We especially thank M. Morris for her contributions to an earlier version of this manuscript and University of Denver's DUEEBs (University of Denver Ecologists and Evolutionary Biologists) group for feedback. We would like to acknowledge the Indigenous Peoples who lived and worked from time immemorial on the unceded lands where our universities currently reside, including the Cheyenne, Arapaho, Kiikaapoi, Ohlone, Osage, and Caddo Nations and tribes.
Data accessibility
This article has no additional data.
Authors' contributions
E.D.B. and R.M.T. conceived of the topic, invited the coauthors and led the writing. All authors met regularly to discuss ideas and contribute to the writing. All authors made substantial intellectual contributions, wrote sections of the manuscript and edited throughout. The authors have read and approved the manuscript, and agree to be held accountable for all aspects of the work.
Competing interests
We declare we have no competing interests.
Funding
NSF supported R.M.T. (IOS-1846520), D.O.E. (IOS-1556421), R.L.R. (IOS-1855962) and G.G.R. (IOS-1755327).
References
- 1.Bradbury JW, Vehrencamp SL. 2011. Principles of animal communication, 2nd edn. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 2.Feng AS, Narins PM, Xu C-H, Lin W-Y, Yu Z-L, Qiu Q, Xu Z-M, Shen J-X. 2006. Ultrasonic communication in frogs. Nature 440, 333-336. ( 10.1038/nature04416) [DOI] [PubMed] [Google Scholar]
- 3.Tinghitella RM, Broder ED, Gurule-Small GA, Hallagan CJ, Wilson JD. 2018. Purring crickets: the evolution of a novel sexual signal. Am. Nat. 192, 773-782. ( 10.1086/700116) [DOI] [PubMed] [Google Scholar]
- 4.Rodríguez RL, Ramaswamy K, Cocroft RB. 2006. Evidence that female preferences have shaped male signal evolution in a clade of specialized plant-feeding insects. Proc. R. Soc. B 273, 2585-2593. ( 10.1098/rspb.2006.3635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritchie MG, Saarikettu M, Livingstone S, Hoikkala A. 2001. Characterization of female preference functions for Drosophila montana courtship song and a test of the temperature coupling hypothesis. Evolution 55, 721-727. ( 10.1554/0014-3820(2001)055[0721:COFPFF]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 6.Cramer JD, Gaetano T, Gray JP, Grobler P, Lorenz JG, Freimer NB, Schmitt CA, Turner TR. 2013. Variation in scrotal color among widely distributed vervet monkey populations (Chlorocebus aethiops pygerythrus and Chlorocebus aethiops sabaeus). Am. J. Primatol. 75, 752-762. ( 10.1002/ajp.22156) [DOI] [PubMed] [Google Scholar]
- 7.Leduc-Robert G, Maddison WP. 2018. Phylogeny with introgression in Habronattus jumping spiders (Araneae: Salticidae). BMC Evol. Biol. 18, 24. ( 10.1186/s12862-018-1137-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia CM, Ramirez E. 2005. Evidence that sensory traps can evolve into honest signals. Nature 434, 501-505. ( 10.1038/nature03363) [DOI] [PubMed] [Google Scholar]
- 9.Kelley LA, Endler JA. 2017. How do great bowerbirds construct perspective illusions? R. Soc. Open Sci. 4, 160661. ( 10.1098/rsos.160661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endler JA, Day LB. 2006. Ornament colour selection, visual contrast and the shape of colour preference functions in great bowerbirds, Chlamydera nuchalis. Anim. Behav. 72, 1405-1416. ( 10.1016/j.anbehav.2006.05.005) [DOI] [Google Scholar]
- 11.Clark CJ, Elias DO, Prum RO. 2011. Aeroelastic flutter produces hummingbird feather songs. Science 333, 1430-1433. ( 10.1126/science.1205222) [DOI] [PubMed] [Google Scholar]
- 12.Leal M, Losos JB. 2010. Communication and speciation. Nature 467, 159-160. ( 10.1038/467159a) [DOI] [PubMed] [Google Scholar]
- 13.Chaine AS, Lyon BE. 2008. Adaptive plasticity in female mate choice dampens sexual selection on male ornaments in the lark bunting. Science 319, 459-462. ( 10.1126/science.1149167) [DOI] [PubMed] [Google Scholar]
- 14.Rosenthal GG. 2017. Mate choice: the evolution of sexual decision making from microbes to humans. Princeton, NJ: Princeton University Press. [Google Scholar]
- 15.Pfennig KS. 2007. Facultative mate choice drives adaptive hybridization. Science 318, 965-967. ( 10.1126/science.1146035) [DOI] [PubMed] [Google Scholar]
- 16.West-Eberhard MJ. 2003. Developmental plasticity and evolution. New York, NY: Oxford University Press. [Google Scholar]
- 17.Mayr E. 1963. Animal species and evolution. Cambridge, MA: Harvard University Press. [Google Scholar]
- 18.Pigliucci M. 2008. Is evolvability evolvable? Nat. Rev. Genet. 9, 75-82. ( 10.1038/nrg2278) [DOI] [PubMed] [Google Scholar]
- 19.Hallgrimsson B, Hall BK. 2011. Epigenetics: linking genotype and phenotype in development and evolution. Berkley and Los Angeles, CA: University of California Press. [Google Scholar]
- 20.Bostwick KS, Elias DO, Mason A, Montealegre-Z F.. 2010. Resonating feathers produce courtship song. Proc. R. Soc. B 277, 835-841. ( 10.1098/rspb.2009.1576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bostwick KS. 2000. Display behaviors, mechanical sounds, and evolutionary relationships of the club-winged manakin (Machaeropterus deliciosus). Auk 117, 465-478. ( 10.1093/auk/117.2.465) [DOI] [Google Scholar]
- 22.Clark CJ, McGuire JA, Bonaccorso E, Berv JS, Prum RO. 2018. Complex coevolution of wing, tail, and vocal sounds of courting male bee hummingbirds. Evolution 72, 630-646. ( 10.1111/evo.13432) [DOI] [PubMed] [Google Scholar]
- 23.Taylor JRA, deVries MS, Elias DO. 2019. Growling from the gut: co-option of the gastric mill for acoustic communication in ghost crabs. Proc. R. Soc. B 286, 20191161. ( 10.1098/rspb.2019.1161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kingsley EP, et al. 2018. Identity and novelty in the avian syrinx. Proc. Natl Acad. Sci. USA 115, 10 209-10 217. ( 10.1073/pnas.1804586115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arch VS, Grafe TU, Narins PM. 2008. Ultrasonic signalling by a Bornean frog. Biol. Lett. 4, 19-22. ( 10.1098/rsbl.2007.0494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elias DO, Hebets EA, Hoy RR, Mason AC. 2005. Seismic signals are crucial for male mating success in a visual specialist jumping spider (Araneae: Salticidae). Anim. Behav. 69, 931-938. ( 10.1016/j.anbehav.2004.06.024) [DOI] [Google Scholar]
- 27.Ter Hofstede HM, Schöneich S, Robillard T, Hedwig B. 2015. Evolution of a communication system by sensory exploitation of startle behavior. Curr. Biol. 25, 3245-3252. ( 10.1016/j.cub.2015.10.064) [DOI] [PubMed] [Google Scholar]
- 28.Belwood JJ, Morris GK. 1987. Bat predation and its influence on calling behavior in neotropical katydids. Science 238, 64-67. ( 10.1126/science.238.4823.64) [DOI] [PubMed] [Google Scholar]
- 29.Tinghitella RM. 2008. Rapid evolutionary change in a sexual signal: genetic control of the mutation ‘flatwing’ that renders male field crickets (Teleogryllus oceanicus) mute. Heredity 100, 261-267. ( 10.1038/sj.hdy.6801069) [DOI] [PubMed] [Google Scholar]
- 30.Niehuis O, et al. 2013. Behavioural and genetic analyses of Nasonia shed light on the evolution of sex pheromones. Nature 494, 345-348. ( 10.1038/nature11838) [DOI] [PubMed] [Google Scholar]
- 31.Wagner A. 2011. The molecular origins of evolutionary innovations. Trends Genet. 27, 397-410. ( 10.1016/j.tig.2011.06.002) [DOI] [PubMed] [Google Scholar]
- 32.Shirai LT, Saenko SV, Keller RA, Jerónimo MA, Brakefield PM, Descimon H, Wahlberg N, Beldade P. 2012. Evolutionary history of the recruitment of conserved developmental genes in association to the formation and diversification of a novel trait. BMC Evol. Biol. 12, 21. ( 10.1186/1471-2148-12-21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrera-Guzmán AO, Aleixo A, Shawkey MD, Weir JT. 2018. Hybrid speciation leads to novel male secondary sexual ornamentation of an Amazonian bird. Proc. Natl Acad. Sci. USA 115, E218-E225. ( 10.1073/pnas.1717319115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamichhaney S, Han F, Webster MT, Andersson L, Grant BR, Grant PR. 2018. Rapid hybrid speciation in Darwin's finches. Science 359, 224-228. ( 10.1126/science.aao4593) [DOI] [PubMed] [Google Scholar]
- 35.Akopyan M, Gompert Z, Klonoski K, Vega A, Kaiser K, Mackelprang R, Rosenblum EB, Robertson JM. 2020. Genetic and phenotypic evidence of a contact zone between divergent color morphs of the iconic red-eyed treefrog. Mol. Ecol. 29, 4442-4446. ( 10.1111/mec.1563) [DOI] [PubMed] [Google Scholar]
- 36.Maddison W, McMahon M. 2000. Divergence and reticulation among Montane populations of a jumping spider (Habronattus pugillis Griswold). Syst. Biol. 49, 400-421. ( 10.1080/10635159950127312) [DOI] [PubMed] [Google Scholar]
- 37.Fisher JW, Siracusa M, Tieu K. 2009. Estimation of signal information content for classification. In 2009 IEEE 13th Digital Signal Processing Workshop and 5th IEEE Signal Processing Education Workshop. Marco Island, FL, 2009, pp. 353–358. ( 10.1109/dsp.2009.4785948) [DOI] [Google Scholar]
- 38.Kagawa K, Takimoto G. 2018. Hybridization can promote adaptive radiation by means of transgressive segregation. Ecol. Lett. 21, 264-274. ( 10.1111/ele.12891) [DOI] [PubMed] [Google Scholar]
- 39.Powell DL, et al. 2020. Natural hybridization reveals incompatible alleles that cause melanoma in swordtail fish. Science 368, 731-736. ( 10.1126/science.aba5216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moczek AP, Sultan S, Foster S, Ledón-Rettig C, Dworkin I, Nijhout HF, Abouheif E, Pfennig DW.. 2011. The role of developmental plasticity in evolutionary innovation. Proc. R. Soc. B 278, 2705-2713 ( 10.1098/rspb.2011.0971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verzijden MN, ten Cate C, Servedio MR, Kozak GM, Boughman JW, Svensson EI. 2012. The impact of learning on sexual selection and speciation. Trends Ecol. Evol. 27, 511-519. ( 10.1016/j.tree.2012.05.007) [DOI] [PubMed] [Google Scholar]
- 42.Le Rouzic A, Carlborg O.. 2008. Evolutionary potential of hidden genetic variation. Trends Ecol. Evol. 23, 33-37. ( 10.1016/j.tree.2007.09.014) [DOI] [PubMed] [Google Scholar]
- 43.Meier JI, Marques DA, Mwaiko S, Wagner CE, Excoffier L, Seehausen O. 2017. Ancient hybridization fuels rapid cichlid fish adaptive radiations. Nat. Commun. 8, 14363. ( 10.1038/ncomms14363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verzijden MN, Etman E, van Heijningen C, van der Linden M, ten Cate C.. 2007. Song discrimination learning in zebra finches induces highly divergent responses to novel songs. Proc. R. Soc. B 274, 295-301. ( 10.1098/rspb.2006.3728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kozak GM, Head ML, Boughman JW. 2011. Sexual imprinting on ecologically divergent traits leads to sexual isolation in sticklebacks. Proc. R. Soc. B 278, 2604-2610. ( 10.1098/rspb.2010.2466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Otter KA, Mckenna A, LaZerte SE, Ramsay SM. 2020. Continent-wide shifts in song dialects of white-throated sparrows. Curr. Biol. 30, 3231-3235.e3. ( 10.1016/j.cub.2020.05.084) [DOI] [PubMed] [Google Scholar]
- 47.Pfaus JG, Erickson KA, Talianakis S. 2013. Somatosensory conditioning of sexual arousal and copulatory behavior in the male rat: a model of fetish development. Physiol. Behav. 122, 1-7. ( 10.1016/j.physbeh.2013.08.005) [DOI] [PubMed] [Google Scholar]
- 48.Cetinkaya H, Domjan M. 2006. Sexual fetishism in a quail (Coturnix japonica) model system: test of reproductive success. J. Comp. Psychol. 120, 427-432. ( 10.1037/0735-7036.120.4.427) [DOI] [PubMed] [Google Scholar]
- 49.Ramírez SR, Eltz T, Fritzsch F, Pemberton R, Pringle EG, Tsutsui ND. 2010. Intraspecific geographic variation of fragrances acquired by orchid bees in native and introduced populations. J. Chem. Ecol. 36, 873-884. ( 10.1007/s10886-010-9821-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maddison WP, Maddison DR. 1992. Macclade: analysis of phylogeny and character evolution. Sunderland, MA: Sinauer Associates. [DOI] [PubMed] [Google Scholar]
- 51.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1-15. ( 10.1086/284325) [DOI] [Google Scholar]
- 52.Kawase H, Okata Y, Ito K. 2013. Role of huge geometric circular structures in the reproduction of a marine pufferfish. Sci. Rep. 3, 2106. ( 10.1038/srep02106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arnegard ME, Zwickl DJ, Lu Y, Zakon HH.. 2010. Old gene duplication facilitates origin and diversification of an innovative communication system—twice. Proc. Natl Acad. Sci. USA 107, 22 172-22 177. ( 10.1073/pnas.1011803107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Svensson EI, Gosden TP. 2007. Contemporary evolution of secondary sexual traits in the wild. Funct. Ecol. 21, 422-433. ( 10.1111/j.1365-2435.2007.01265.x) [DOI] [Google Scholar]
- 55.Svensson EI. 2019. Eco-evolutionary dynamics of sexual selection and sexual conflict. Funct. Ecol. 33, 60-72. ( 10.1111/1365-2435.13245) [DOI] [Google Scholar]
- 56.Schumer M, Powell DL, Delclós PJ, Squire M, Cui R, Andolfatto P, Rosenthal GG.. 2017. Assortative mating and persistent reproductive isolation in hybrids. Proc. Natl Acad. Sci. USA 114, 10 936-10 941. ( 10.1073/pnas.1711238114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mendelson TC, Martin MD, Flaxman SM. 2014. Mutation-order divergence by sexual selection: diversification of sexual signals in similar environments as a first step in speciation. Ecol. Lett. 17, 1053-1066. ( 10.1111/ele.12313) [DOI] [PubMed] [Google Scholar]
- 58.Ryan MJ, Akre KL, Baugh AT, Bernal XE, Lea AM, Leslie C, Still MB, Wylie DC, Rand AS. 2019. Nineteen years of consistently positive and strong female mate preferences despite individual variation. Am. Nat. 194, 125-134. ( 10.1086/704103) [DOI] [PubMed] [Google Scholar]
- 59.Moehring AJ, Boughman JW. 2019. Veiled preferences and cryptic female choice could underlie the origin of novel sexual traits. Biol. Lett. 15, 20180878. ( 10.1098/rsbl.2018.0878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carl Gerhardt H, Humfeld SC, Marshall VT.. 2007. Temporal order and the evolution of complex acoustic signals. Proc. R. Soc. B 274, 1789. ( 10.1098/rspb.2007.0451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reichert MS, Finck J, Ronacher B. 2017. Exploring the hidden landscape of female preferences for complex signals. Evolution 71, 1009-1024. ( 10.1111/evo.13202) [DOI] [PubMed] [Google Scholar]
- 62.Kolm N, Amcoff M, Mann RP, Arnqvist G. 2012. Diversification of a food-mimicking male ornament via sensory drive. Curr. Biol. 22, 1440-1443. ( 10.1016/j.cub.2012.05.050) [DOI] [PubMed] [Google Scholar]
- 63.Espmark Y, Amundsen T, Rosenqvist G. 2000. Animal signals: signalling and signal design in animal communication. Trondheim, Norway: Tapir Academic Press. [Google Scholar]
- 64.Lynch KS, Ryan MJ. 2020. Understanding the role of incentive salience in mate choice decision-making. Integr. Comp. Biol. 60, 712-721. ( 10.1093/icb/icaa054) [DOI] [PubMed] [Google Scholar]
- 65.Zhang K, Chen CD, Monosov IE. 2019. Novelty, salience, and surprise timing are signaled by neurons in the basal forebrain. Curr. Biol. 29, 134-142.e3. ( 10.1016/j.cub.2018.11.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fisher HS, Mascuch SJ, Rosenthal GG. 2009. Multivariate male traits misalign with multivariate female preferences in the swordtail fish, Xiphophorus birchmanni. Anim. Behav. 78, 265-269. ( 10.1016/j.anbehav.2009.02.029) [DOI] [Google Scholar]
- 67.Greenfield MD, Rodriguez RL. 2004. Genotype–environment interaction and the reliability of mating signals. Anim. Behav. 68, 1461-1468. ( 10.1016/j.anbehav.2004.01.014) [DOI] [Google Scholar]
- 68.Rodríguez RL, Snedden WA. 2004. On the functional design of mate preferences and receiver biases. Anim. Behav. 68, 427-432. ( 10.1016/j.anbehav.2003.08.031) [DOI] [Google Scholar]
- 69.Rodríguez RL. 2009. Trait duplication by means of sensory bias. Behav. Ecol. 20, 1376-1381. ( 10.1093/beheco/arp130) [DOI] [Google Scholar]
- 70.Janif ZJ, Brooks RC, Dixson BJ. 2014. Negative frequency-dependent preferences and variation in male facial hair. Biol. Lett. 10, 20130958. ( 10.1098/rsbl.2013.0958) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ryan MJ, Page RA, Hunter KL, Taylor RC. 2019. ‘Crazy love’: nonlinearity and irrationality in mate choice. Anim. Behav. 147, 189-198. ( 10.1016/j.anbehav.2018.04.004) [DOI] [Google Scholar]
- 72.Kelley JL, Graves JA, Magurran AE. 1999. Familiarity breeds contempt in guppies. Nature 401, 661-662. ( 10.1038/44314) [DOI] [PubMed] [Google Scholar]
- 73.Dawkins MS, Guilford T. 1991. The corruption of honest signalling. Anim. Behav. 41, 865-873. ( 10.1016/s0003-3472(05)80353-7) [DOI] [Google Scholar]
- 74.Sockman KW, Gentner TQ, Ball GF. 2005. Complementary neural systems for the experience-dependent integration of mate-choice cues in European starlings. J. Neurobiol. 62, 72-81. ( 10.1002/neu.20068) [DOI] [PubMed] [Google Scholar]
- 75.Sockman KW, Gentner TQ, Ball GF.. 2002. Recent experience modulates forebrain gene-expression in response to mate-choice cues in European starlings. Proc. R. Soc. Lond. B 269, 2479-2485. ( 10.1098/rspb.2002.2180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ryan MJ, Stanley Rand A. 1990. The sensory basis of sexual selection for complex calls in the Tungara Frog, Physalaemus pustulosus (sexual selection for sensory exploitation). Evolution 44, 305. ( 10.2307/2409409) [DOI] [PubMed] [Google Scholar]
- 77.Greenfield MD. 2002. Signalers and receivers: mechanisms and evolution of arthropod communication. New York, NY: Oxford University Press. [Google Scholar]
- 78.Scott JL, Kawahara AY, Skevington JH, Yen S-H, Sami A, Smith ML, Yack JE. 2010. The evolutionary origins of ritualized acoustic signals in caterpillars. Nat. Commun. 1, 4. ( 10.1038/ncomms1002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Basolo AL. 1990. Female preference predates the evolution of the sword in swordtail fish. Science 250, 808-810. ( 10.1126/science.250.4982.808) [DOI] [PubMed] [Google Scholar]
- 80.Fernandez AA, Morris MR. 2007. Sexual selection and trichromatic color vision in primates: statistical support for the preexisting-bias hypothesis. Am. Nat. 170, 10-20. ( 10.1086/518566) [DOI] [PubMed] [Google Scholar]
- 81.Kronforst MR, Young LG, Kapan DD, McNeely C, O'Neill RJ, Gilbert LE. 2006. Linkage of butterfly mate preference and wing color preference cue at the genomic location of wingless. Proc. Natl Acad. Sci. USA 103, 6575-6580. ( 10.1073/pnas.0509685103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sæther SA, Sætre GP, Borge T, Wiley C. 2007. Sex chromosome-linked species recognition and evolution of reproductive isolation in flycatchers. Science 318, 95-97. ( 10.1126/science.1141506) [DOI] [PubMed] [Google Scholar]
- 83.Shaw KL, Lesnick SC.. 2009. Genomic linkage of male song and female acoustic preference QTL underlying a rapid species radiation. Proc. Natl Acad. Sci. USA 106, 9737-9742. ( 10.1073/pnas.0900229106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pryke SR, Rollins LA, Griffith SC. 2010. Females use multiple mating and genetically loaded sperm competition to target compatible genes. Science 329, 964-967. ( 10.1126/science.1192407) [DOI] [PubMed] [Google Scholar]
- 85.Wiley C, Shaw KL. 2010. Multiple genetic linkages between female preference and male signal in rapidly speciating Hawaiian crickets. Evolution 64, 2238-2245. ( 10.1111/j.1558-5646.2010.01007.x) [DOI] [PubMed] [Google Scholar]
- 86.McNiven VTK, Moehring AJ. 2013. Identification of genetically linked female preference and male trait. Evolution 67, 2155-2165. ( 10.1111/evo.12096) [DOI] [PubMed] [Google Scholar]
- 87.Xu M, Shaw KL. 2019. Genetic coupling of signal and preference facilitates sexual isolation during rapid speciation. Proc. R. Soc. B 286, 20191607. ( 10.1098/rspb.2019.1607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tucker MA, Gerhardt HC. 2012. Parallel changes in mate-attracting calls and female preferences in autotriploid tree frogs. Proc. R. Soc. B 279, 1583-1587. ( 10.1098/rspb.2011.1968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bailey NW, Moore AJ. 2012. Runaway sexual selection without genetic correlations: social environments and flexible mate choice initiate and enhance the Fisher process. Evolution 66, 2674-2684. ( 10.1111/j.1558-5646.2012.01647.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bailey NW, Kölliker M. 2019. Social runaway: Fisherian elaboration (or reduction) of socially selected traits via indirect genetic effects. Evolution 73, 1549-1563. ( 10.1111/evo.13791) [DOI] [PubMed] [Google Scholar]
- 91.Rebar D, Rodriguez RL. 2015. Insect mating signal and mate preference phenotypes covary among host plant genotypes. Evolution 69, 602-610. ( 10.1111/evo.12604) [DOI] [PubMed] [Google Scholar]
- 92.Rosenthal MF, Elias DO.. 2019. Nonlinear changes in selection on a mating display across a continuous thermal gradient. Proc. R. Soc. B 286, 20191450. ( 10.1098/rspb.2019.1450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brandt EE, Rosenthal MF, Elias DO. 2020. Complex interactions between temperature, sexual signals and mate choice in a desert-dwelling jumping spider. Anim. Behav. 170, 81-87. ( 10.1016/j.anbehav.2020.10.010) [DOI] [Google Scholar]
- 94.Fonseca PJ, Correia T. 2007. Effects of temperature on tuning of the auditory pathway in the cicada Tettigetta josei (Hemiptera, Tibicinidae). J. Exp. Biol. 210, 1834-1845. ( 10.1242/jeb.001495) [DOI] [PubMed] [Google Scholar]
- 95.Eberhard MJB, Schleimer J-H, Schreiber S, Ronacher B. 2015. A temperature rise reduces trial-to-trial variability of locust auditory neuron responses. J. Neurophysiol. 114, 1424-1437. ( 10.1152/jn.00980.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jocson DMI, Smeester ME, Leith NT, Macchiano A, Fowler-Finn KD. 2019. Temperature coupling of mate attraction signals and female mate preferences in four populations of Enchenopa treehopper (Hemiptera: Membracidae). J. Evol. Biol. 32, 1046-1056. ( 10.1111/jeb.13506) [DOI] [PubMed] [Google Scholar]
- 97.Pires A, Hoy RR. 1992. Temperature coupling in cricket acoustic communication. J. Comp. Physiol. A 171, 69-78. ( 10.1007/bf00195962) [DOI] [PubMed] [Google Scholar]
- 98.Brandt EE, Kelley JP, Elias DO. 2018. Temperature alters multimodal signaling and mating success in an ectotherm. Behav. Ecol. Sociobiol. 72, 191. ( 10.1007/s00265-018-2620-5) [DOI] [Google Scholar]
- 99.Gurule-Small GA, Tinghitella RM. 2018. Developmental experience with anthropogenic noise hinders adult mate location in an acoustically signalling invertebrate. Biol. Lett. 14, 20170714. ( 10.1098/rsbl.2017.0714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brakefield PM, Gates J, Keys D, Kesbeke F, Wijngaarden PJ, Montelro A, French V, Carroll SB. 1996. Development, plasticity and evolution of butterfly eyespot patterns. Nature 384, 236-242. ( 10.1038/384236a0) [DOI] [PubMed] [Google Scholar]
- 101.Dion E, Monteiro A, Yew JY. 2016. Phenotypic plasticity in sex pheromone production in Bicyclus anynana butterflies. Sci. Rep. 6, 39002. ( 10.1038/srep39002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Prudic KL, Jeon C, Cao H, Monteiro A. 2011. Developmental plasticity in sexual roles of butterfly species drives mutual sexual ornamentation. Science 331, 73-75. ( 10.1126/science.1197114) [DOI] [PubMed] [Google Scholar]
- 103.Bear A, Monteiro A. 2013. Male courtship rate plasticity in the butterfly Bicyclus anynana is controlled by temperature experienced during the pupal and adult stages. PLoS ONE 8, e64061. ( 10.1371/journal.pone.0064061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ghalambor CK, Hoke KL, Ruell EW, Fischer EK, Reznick DN, Hughes KA. 2015. Non-adaptive plasticity potentiates rapid adaptive evolution of gene expression in nature. Nature 525, 372-375. ( 10.1038/nature15256) [DOI] [PubMed] [Google Scholar]
- 105.Delhey K, Peters A. 2017. Conservation implications of anthropogenic impacts on visual communication and camouflage. Conserv. Biol. 31, 30-39. ( 10.1111/cobi.12834) [DOI] [PubMed] [Google Scholar]
- 106.Dominoni DM, et al. 2020. Why conservation biology can benefit from sensory ecology. Nat. Ecol. Evol. 4, 502-511. ( 10.1038/s41559-020-1135-4) [DOI] [PubMed] [Google Scholar]
- 107.Murphy SM, Battocletti AH, Tinghitella RM, Wimp GM, Ries L. 2016. Complex community and evolutionary responses to habitat fragmentation and habitat edges: what can we learn from insect science? Curr. Opin. Insect Sci. 14, 61-65. ( 10.1016/j.cois.2016.01.007) [DOI] [PubMed] [Google Scholar]
- 108.Zuk M, Rotenberry JT, Tinghitella RM. 2006. Silent night: adaptive disappearance of a sexual signal in a parasitized population of field crickets. Biol. Lett. 2, 521-524. ( 10.1098/rsbl.2006.0539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zuk M, Kolluru GR. 1998. Exploitation of sexual signals by predators and parasitoids. Q. Rev. Biol. 73, 415-438. ( 10.1086/420412) [DOI] [Google Scholar]
- 110.Godin J-GJ, Briggs SE. 1996. Female mate choice under predation risk in the guppy. Anim. Behav. 51, 117-130. ( 10.1006/anbe.1996.0010) [DOI] [Google Scholar]
- 111.Endler JA. 1990. On the measurement and classification of colour in studies of animal colour patterns. Biol. J. Linn. Soc. Lond. 41, 315-352. ( 10.1111/j.1095-8312.1990.tb00839.x) [DOI] [Google Scholar]
- 112.Endler JA, Basolo AL. 1998. Sensory ecology, receiver biases and sexual selection. Trends Ecol. Evol. 13, 415-420. ( 10.1016/S0169-5347(98)01471-2) [DOI] [PubMed] [Google Scholar]
- 113.Boughman JW. 2002. How sensory drive can promote speciation. Trends Ecol. Evol. 17, 571-577. ( 10.1016/S0169-5347(02)02595-8) [DOI] [Google Scholar]
- 114.Rosenthal GG, Stuart-Fox D. 2012. Environmental disturbance and animal communication. In Behavioural responses to a changing world: mechanisms and consequences (eds Candolin U, Wong BBM), pp. 16-31. Oxford, UK: Oxford University Press. [Google Scholar]
- 115.Endler JA. 1992. Signals, signal conditions, and the direction of evolution. Am. Nat. 139, S125-S153. ( 10.1086/285308) [DOI] [Google Scholar]
- 116.Francis CD, Barber JR. 2013. A framework for understanding noise impacts on wildlife: an urgent conservation priority. Front. Ecol. Environ. 11, 305-313. ( 10.1890/120183) [DOI] [Google Scholar]
- 117.Kight CR, Swaddle JP. 2011. How and why environmental noise impacts animals: an integrative, mechanistic review. Ecol. Lett. 14, 1052-1061. ( 10.1111/j.1461-0248.2011.01664.x) [DOI] [PubMed] [Google Scholar]
- 118.Halfwerk W, Slabbekoorn H. 2009. A behavioural mechanism explaining noise-dependent frequency use in urban birdsong. Anim. Behav. 78, 1301-1307. ( 10.1016/j.anbehav.2009.09.015) [DOI] [Google Scholar]
- 119.Shannon G, et al. 2016. A synthesis of two decades of research documenting the effects of noise on wildlife. Biol. Rev. Camb. Philos. Soc. 91, 982-1005. ( 10.1111/brv.12207) [DOI] [PubMed] [Google Scholar]
- 120.Raboin M, Elias DO. 2019. Anthropogenic noise and the bioacoustics of terrestrial invertebrates. J. Exp. Biol. 222, jeb178749, ( 10.1242/jeb.178749) [DOI] [PubMed] [Google Scholar]
- 121.Owens ACS, Lewis SM. 2018. The impact of artificial light at night on nocturnal insects: a review and synthesis. Ecol. Evol. 8, 11 337-11 358. ( 10.1002/ece3.4557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Seymoure BM. 2018. Enlightening butterfly conservation efforts: the importance of natural lighting for butterfly behavioral ecology and conservation. Insects 9, 22. ( 10.3390/insects9010022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Owens ACS, Cochard P, Durrant J, Farnworth B, Perkin EK, Seymoure B. 2020. Light pollution is a driver of insect declines. Biol. Conserv. 241, 108259. ( 10.1016/j.biocon.2019.108259) [DOI] [Google Scholar]
- 124.Simpson SD, Munday PL, Wittenrich ML, Manassa R, Dixson DL, Gagliano M, Yan HY. 2011. Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol. Lett. 7, 917-920. ( 10.1098/rsbl.2011.0293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fisher HS, Wong BBM, Rosenthal GG. 2006. Alteration of the chemical environment disrupts communication in a freshwater fish. Proc. R. Soc. B 273, 1187-1193. ( 10.1098/rspb.2005.3406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Seehausen O, Van Alphen JJM, Witte F.. 1997. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277, 1808-1811. ( 10.1126/science.277.5333.1808) [DOI] [Google Scholar]
- 127.Lurling M, Scheffer M. 2007. Info-disruption: pollution and the transfer of chemical information between organisms. Trends Ecol. Evol. 22, 374-379. ( 10.1016/j.tree.2007.04.002) [DOI] [PubMed] [Google Scholar]
- 128.Delpuech J-M, Gareau E, Terrier O, Fouillet P. 1998. Sublethal effects of the insecticide chlorpyrifos on the sex pheromonal communication of Trichogramma brassicae. Chemosphere 36, 1775-1785. ( 10.1016/S0045-6535(97)10071-6) [DOI] [Google Scholar]
- 129.Beyers DW, Farmer MS. 2001. Effects of copper on olfaction of Colorado pikeminnow. Environ. Toxicol. Chem. 20, 907-912. ( 10.1002/etc.5620200427) [DOI] [PubMed] [Google Scholar]
- 130.Zhou H, Du J, Huang Y.. 2005. Effects of sublethal doses of malathion on responses to sex pheromones by male Asian corn borer moths, Ostrinia furnacalis (Guenée). J. Chem. Ecol. 31, 1645-1656. ( 10.1007/s10886-005-5804-1) [DOI] [PubMed] [Google Scholar]
- 131.Wei H-Y, Du J-W.. 2004. Sublethal effects of larval treatment with deltamethrin on moth sex pheromone communication system of the Asian corn borer, Ostrinia furnacalis. Pestic. Biochem. Physiol. 80, 12-20. ( 10.1016/j.pestbp.2004.05.001) [DOI] [Google Scholar]
- 132.Rosenthal MF, Hebets EA, Kessler B, McGinley R, Elias DO. 2019. The effects of microhabitat specialization on mating communication in a wolf spider. Behav. Ecol. 30, 1398-1405. ( 10.1093/beheco/arz091) [DOI] [Google Scholar]
- 133.Rosenthal MF, Hebets EA, McGinley R, Raiza C, Starrett J, Yan L, Elias DO. 2020. Exploring a novel substrate-borne vibratory signal in the wolf spider Schizocosa floridana. Ethology 127, 135-144. ( 10.1111/eth.13114) [DOI] [Google Scholar]
- 134.Falchi F, Cinzano P, Duriscoe D, Kyba CCM, Elvidge CD, Baugh K, Portnov BA, Rybnikova NA, Furgoni R. 2016. The new world atlas of artificial night sky brightness. Sci. Adv. 2, e1600377. ( 10.1126/sciadv.1600377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Seymoure B, Buxton R, White J, Linares C, Fristrup K, Crooks K, Wittemyer G, Angeloni L. 2019. Anthropogenic light disrupts natural light cycles in critical conservation areas. ( 10.2139/ssrn.3439670) [DOI]
- 136.Wu C-H, Elias DO. 2014. Vibratory noise in anthropogenic habitats and its effect on prey detection in a web-building spider. Anim. Behav. 90, 47-56. ( 10.1016/j.anbehav.2014.01.006) [DOI] [Google Scholar]
- 137.Schell CJ, Dyson K, Fuentes TL, Des Roches S, Harris NC, Miller DS, Woelfle-Erskine CA, Lambert MR.. 2020. The ecological and evolutionary consequences of systemic racism in urban environments. Science 369, eaay4497. ( 10.1126/science.aay4497) [DOI] [PubMed] [Google Scholar]
- 138.Seymoure BM, Linares C, White J. 2019. Connecting spectral radiometry of anthropogenic light sources to the visual ecology of organisms. J. Zool. 308, 93-110. ( 10.1111/jzo.12656) [DOI] [Google Scholar]
- 139.Dzieweczynski TL, Greaney NE. 2017. Sex and dose-dependent effects of an estrogen mimic on boldness in threespine stickleback, Gasterosteus aculeatus, from an anadromous population. J. Exp. Mar. Biol. Ecol. 497, 78-85. ( 10.1016/j.jembe.2017.09.013) [DOI] [Google Scholar]
- 140.Dzieweczynski TL, Kane JL. 2017. The bachelorette: female Siamese fighting fish avoid males exposed to an estrogen mimic. Behav. Process. 140, 169-173. ( 10.1016/j.beproc.2017.05.005) [DOI] [PubMed] [Google Scholar]
- 141.Saaristo M, Lagesson A, Bertram MG, Fick J, Klaminder J, Johnstone CP, Wong BBM, Brodin T. 2019. Behavioural effects of psychoactive pharmaceutical exposure on European perch (Perca fluviatilis) in a multi-stressor environment. Sci. Total Environ. 655, 1311-1320. ( 10.1016/j.scitotenv.2018.11.228) [DOI] [PubMed] [Google Scholar]
- 142.Briolat ES, Burdfield-Steel ER, Paul SC, Rönkä KH, Seymoure BM, Stankowich T, Stuckert AMM. 2018. Diversity in warning coloration: selective paradox or the norm? Biol. Rev. Camb. Philos. Soc. 94, 388-414. ( 10.1111/brv.12460) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.