Abstract
Intralocus sexual conflict arises when the expression of shared alleles at a single locus generates opposite fitness effects in each sex (i.e. sexually antagonistic alleles), preventing each sex from reaching its sex-specific optimum. Despite its importance to reproductive success, the relative contribution of intralocus sexual conflict to male pre- and post-copulatory success is not well-understood. Here, we used a female-limited X-chromosome (FLX) evolution experiment in Drosophila melanogaster to limit the inheritance of the X-chromosome to the matriline, eliminating possible counter-selection in males and allowing the X-chromosome to accumulate female-benefit alleles. After more than 100 generations of FLX evolution, we studied the effect of the evolved X-chromosome on male attractiveness and sperm competitiveness. We found a non-significant increase in attractiveness and decrease in sperm offence ability in males expressing the evolved X-chromosomes, but a significant increase in their ability to avoid displacement by other males' sperm. This is consistent with a trade-off between these traits, perhaps mediated by differences in body size, causing a small net reduction in overall male fitness in the FLX lines. These results indicate that the X-chromosome in D. melanogaster is subject to selection via intralocus sexual conflict in males.
Keywords: male attractiveness, sperm competition, male fitness, intralocus sexual conflict, X-chromosome
1. Introduction
Sperm competition theory [1] predicts that males partition their energy investment between pre- and post-copulatory success when resources are limited, leading to a trade-off between sexual attractiveness and sperm competitiveness [2,3]. Selection on pre- and post-copulatory success in males can also lead to sexual conflict, because traits that improve male paternity success can reduce the fitness of their mates [4,5]. Interlocus sexual conflict (IRSC) arises when these sex-specific strategies are mutually incompatible [6]. Reproductive proteins that prevent selective sperm use by females and increase male fertilization success relative to rivals at a cost to female fecundity are a classic example of interlocus sexual conflict in Drosophila [7,8].
By contrast, intralocus sexual conflict (IASC) can arise when the expression of shared alleles at a single locus generates opposite fitness effects in each sex (i.e. sexually antagonistic alleles) [9], preventing them from reaching their respective optima [10]. The unequal inheritance pattern of the X-chromosome between the two sexes has led theoretical models to predict an accumulation of sexually antagonistic alleles on the X-chromosome [11]. Some empirical data support this model in Drosophila melanogaster [11–13], but not all [14,15].
IASC and IRSC are two distinct forms of sexual conflict that can interact over a given trait (e.g. sperm competitiveness), since IASC may constrain the response to IRSC [16,17]. How IASC contributes to IRSC is still not well-understood [16,18], although there is evidence that sexually antagonistic loci may often be sex-biased to some extent [19,20]. In this study, we attempted to investigate how IASC affects male reproductive success using a female-limited X-chromosome (FLX) evolution experiment, which has resulted in feminization of body size and development time in males [15]. After more than 100 generations of FLX evolution, we measured three male fitness components and analysed how male pre- and post-copulatory success responded to the FLX evolution experiment. We expected to see a reduction in both pre- and post-copulatory fitness components in males expressing a feminized FLX X-chromosome, assuming that the feminization we observed in other traits is detrimental to male-specific fitness (e.g. via pleiotropic effects, or if body size trades-off with male-limited reproductive traits).
2. Material and methods
(a). Fly stocks
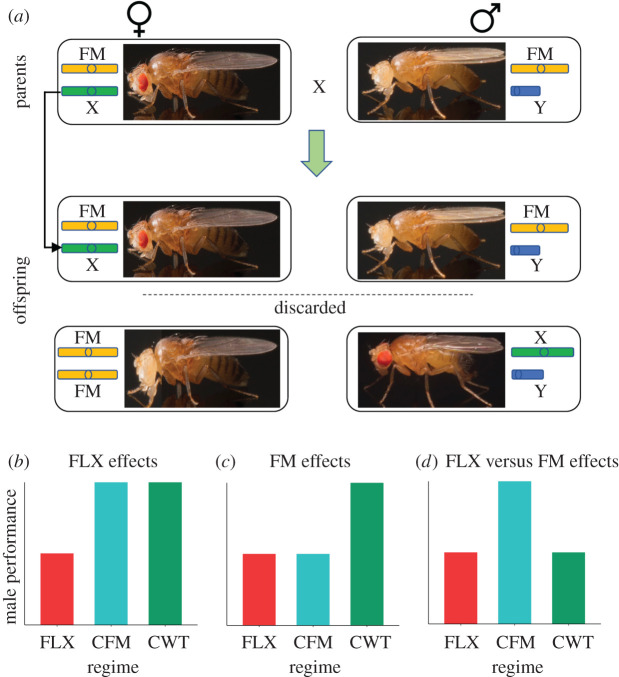
All experimental populations were established from a large outbred LHM stock population and kept under standard conditions (25°C; 12 h L : 12 h D light–dark cycle; 60% humidity; cornmeal–molasses–yeast food [21]). The experimental protocol for the evolution experiment is described in full detail in Lund-Hansen et al. [15]. Briefly, an FM balancer (FM7a) was used to control and limit the inheritance of the selected X-chromosomes to the matriline in the female-limited X-chromosome (FLX) selection regime (figure 1a). We also included two control regimes: (i) Control FM (CFM), which was added to control for any unexpected effects of the FM balancer, and (ii) Control wild type (Cwt), which controls for other aspects of the experimental protocol. The three regimes were kept in four replicate populations each (see electronic supplementary material).
Figure 1.
Protocol for the female-limited X-chromosome (FLX) evolution experiment and graphical interpretation of the selection regimes' effects on male performance. (a) The FLX evolution protocol. The evolving X-chromosome (green bar) is passed from mother to daughter with the help of an FM balancer chromosome (yellow bar). The parental cross produces four genotypes, of which the offspring above the dashed line are crossed to produce the next generation, and the offspring under the dashed line are discarded. At generations 107, 143 and 160, males expressing the evolved X-chromosome (male offspring under the dashed line) were used to study male pre- and post-copulatory success. The FM balancer carries several phenotypic markers, which can be used to phenotype offspring, as illustrated by the pictures next to the genotypes (fly pictures by Qinyang Li). (b,c,d) Potential outcomes of the experiments. (b) FLX effects. If FLX males perform worse than CFM and Cwt males, this should be a result of the FLX selection. (c) FM effects. If FLX and CFM males both perform worse than Cwt males, this suggests an effect of the FM balancer. (d) FLX versus FM effects. If males from the FLX regime perform worse than CFM males but similarly to Cwt males, this suggests that the deleterious effects of a feminized X-chromosome seem to outweigh the FM effect.
All target X/Y males carried the evolved genome (X, Y and autosomal chromosomes) and were collected directly from the selection regimes (figure 1a, male offspring under the dashed line) using light CO2 anaesthesia (see electronic supplementary material).
(b). Male attractiveness
We measured male attractiveness as copulation latency (the time for a female to accept a mating) and mating frequency (the frequency with which the target male is accepted). One 5-day-old virgin LHM female was paired with a 5-day-old target virgin male. The female mating responses were observed over a period of 30 min and the time until copulation (copulation latency), and whether it occurred at all (mating frequency) were noted. This experiment was carried out at generation 107, in 12 blocks within a day, with 20 flies per selection regime per replicate population in each block, estimating the attractiveness of a total of 80 males per regime (see electronic supplementary material).
(c). Sperm competition
Sperm competitiveness of D. melanogaster males is defined by both the ability to displace sperm that is resident in the female's reproductive tract (sperm offence) and the ability to resist displacement by sperm from subsequent males (sperm defence) [1,22]. Details of the protocol can be found in the electronic supplementary material. Briefly, virgin females are provided with the opportunity to mate with a target or competitor male on consecutive days. For sperm defence, the target male is the first to mate, and for offence, the target male is the second to mate. Competitor males carry a brown eye mutation (bw−) while target males are wild-type. Because the wild-type red eye colour is dominant over the brown, the number of target and non-target offspring produced by the female is used as a measure of paternity share.
Both the sperm defence and offence experiments were carried out at generation 143, in 15 experimental replicates per regime per replicate population. Females that produced no offspring after the first mating were removed from the experiments. To ensure sufficient sample sizes, we performed the offence experiment again at generation 160, with an average of 26 experimental replicates per regime per replicate population.
(d). Statistical analysis
All statistical analyses were conducted in RStudio v. 1.1.463 (http://www.rstudio.com/). The mating frequency was analysed by fitting a generalized linear mixed-effects model (GLMM) from the R package lme4 [23], with the family set as binomial (mated/not mated), with regime and block as fixed factors, and with replicate population nested within the regime as a random effect. Copulation latency was analysed using the same model (GLMM), but the family set as gamma. These two models were tested for overdispersion using the dispersion_glmer command in the blmeco package [24].
For both the sperm defence and offence analyses, we first excluded all vials that had fewer than 10 offspring, then we used cbind() to create a response variable matrix of successes (number of first male's offspring) and failures (second male's offspring) as the dependent variables in a binomial zero-inflated GLMM (glmmTMB package in R) [25]. Regime and block (for offence) were fixed factors, and replicate population was nested within regime as a random effect. We checked for overdispersion by visual inspection of the plot of scaled residuals using the simulateResiduals() function in the DHARMa package [25]. The Anova command from the car package [26] was used for type 3 sums of squares significance testing. Post hoc analysis of effects was performed using the package emmeans [27].
Finally, to see if the differences we observed could be related to changes in body size, we used Pearson correlations to analyse the relationship between male body size (thorax size from [15]) and sperm competitive ability (both defence and offence), with population means as the unit of analysis [28].
3. Results and discussion
Limiting the inheritance of the X-chromosome to females for over 100 generations should allow X-linked female-beneficial alleles to increase in frequency, and we have previously found evidence of feminization of body size and development time in the FLX selection regime [15]. We therefore expected that expressing the experimentally evolved X-chromosomes would result in a reduction in pre- and/or post-copulatory fitness components in males, assuming phenotypic feminization is detrimental to males. Our results were consistent with this expectation for sperm offence, although the effect did not quite reach significance, but not for an attractiveness or sperm defence. We did not find any significant correlations between body size and sperm offence or defence on the replicate population level (electronic supplementary material, figures S1 and S2), but this may not reflect patterns on the individual level.
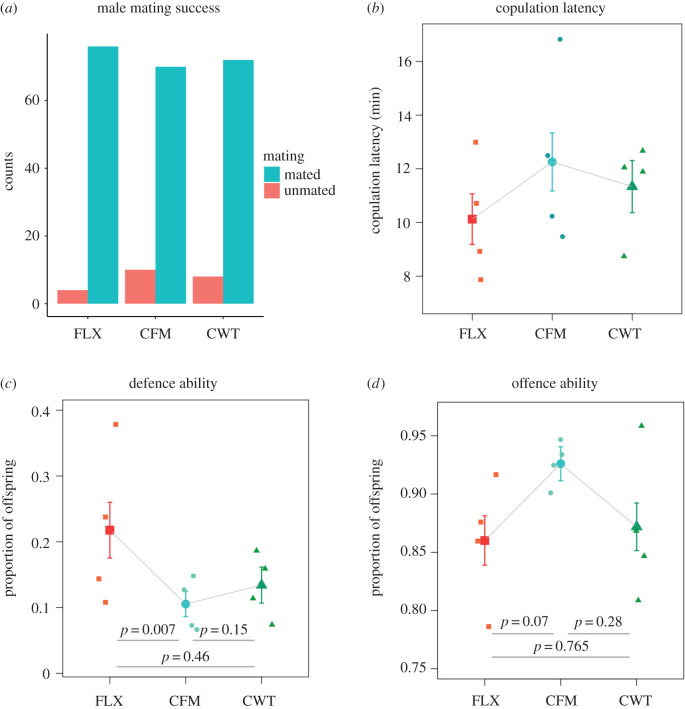
We found no significant difference between regimes in either mating frequency (p = 0.24, electronic supplementary material, table S1) or copulation latency (p = 0.48, electronic supplementary material, table S1) in the no-choice attractiveness experiment. This indicates that the FLX males were not refused more or less often than the control males (figure 2a) and were not more or less attractive than the control males (figure 2b), despite their expression of the evolved X-chromosome. If anything, FLX males were actually slightly more attractive than the other males (figure 2a,b), which is counter to our expectations, even if the difference is not significant. However, we have previously found that FLX males are larger than the other males [15], and being large increases male attractiveness and success in intrasexual competition for access to females [29,30]. This may have compensated for possible decreases in attractiveness in other traits (e.g. courtship behaviour), if any exist.
Figure 2.
Male mating attractiveness and sperm competition. (a) Counts of mated (blue) and unmated (red) males in the mating experiment for the three regimes. (b) Comparison of copulation latency (mating speed) in minutes between regimes. (c) Sperm defence ability measured as proportion of offspring sired by the target male. (d) Sperm offence ability measured as proportion of offspring sired by the target male. Points with error bars represent overall means and standard errors, and individual points are the means for the four replicate populations. FLX: red, CFM: blue, CWT: green. p-values are from Tukey's HSD test.
For post-copulatory success, we found a significant difference between selection regimes in sperm defence (p = 0.009), where FLX males sired a larger proportion of the offspring than both CFM and Cwt males (figure 2c, table 1). This increase is attributable to the selection regime and not the presence of the FM balancer (figure 1b) and is consistent with the previously observed increase in body size, if larger males transfer more sperm or are preferred under cryptic female choice [6,31]. By contrast, there was a trend towards a significant difference between selection regimes in sperm offence (p = 0.08, table 1), where CFM males had higher performance in sperm offence than FLX males, which sired a similar proportion of offspring to that of Cwt males (figure 2d). The (non-significant) increase in sperm offence seen in CFM males is likely to be a result of autosomal and/or Y-linked adaptation to regain fitness in the presence of the balancer (figure 1d). However, the decreased offence ability in FLX males compared with CFM males suggests that any adaptation to regain fitness in the presence of the balancer seems to have been outweighed by the deleterious effects of a feminized X-chromosome in FLX males (figure 1c). This interpretation is supported by our previous finding that males carrying an FM balancer have substantially lower fitness than wild-type males [15]. We are currently carrying out follow-up experiments to disentangle these effects.
Table 1.
Summary of the results from ANOVA analysis of GLMM models.
| fixed effects |
χ2 | d.f. | p-value | |
|---|---|---|---|---|
| defence | intercept | 53.36 | 1 | <0.001 |
| regime | 9.52 | 2 | 0.009 | |
| offence | intercept | 41.6 | 1 | <0.001 |
| regime | 5.05 | 2 | 0.08 | |
| block | 198.77 | 1 | <0.001 | |
From previous work, we know that CFM males have a marginally higher total reproductive fitness than FLX and Cwt males [15]. Although this difference was not significant, the overall pattern was very similar to the pattern seen here in sperm offence. Collectively, these results suggest that under normal conditions, any potential increase in attractiveness or sperm defence in FLX males is outweighed by other negative effects, including in sperm offence. In addition, other research has shown that large males are more harmful to females [32,33]. This may also serve to decrease the post-copulatory success of the larger FLX males.
Several general conclusions can be drawn from our results. First, that IASC may affect sex-specific fitness in complex ways. We expected that release from intralocus sexual conflict by a feminization of the X-chromosome would result in a decrease in some or all male fitness components, under the assumption that feminization is detrimental to male fitness. Instead, we found evidence of an increase in sperm defence ability in FLX males. This illustrates how difficult it can be to predict the outcome of releasing one sex from IASC, which has also been shown in Timema stick insects, where a transition to asexuality actually resulted in masculinization of the female expression profile [34]. Second, the fact that sperm offence changed both in CFM males relative to Cwt males, and in the FLX males relative to the CFM males, suggests that this trait has been subject to conflicting selection pressures under our selection regimes. Specifically, the presence of the balancer seems to have resulted in compensatory evolution on the Y-chromosome and autosomes. Finally, although we cannot be sure of the exact genetic mechanism, our results are also suggestive of a trade-off between pre- and post-copulatory traits as predicted from sperm competition theory, specifically that an investment in increased body size may come at a cost to sperm offence ability (electronic supplementary material, figure S2). This type of effect (but in the opposite direction) was seen in Prasad et al. [28] and suggests that the large but fast-developing FLX males [15] may trade-off body size against gonad development [15,35,36], although further investigation is needed.
Acknowledgements
We would like to say many thanks to Qinyang Li and Shahzad Hussain for their help during the project and data collection. Thanks as well to the SexGen group and three anonymous reviewers for constructive feedback on an earlier version of this manuscript.
Authors' contributions
Y.M. and G.K. designed experiments, collected data and analysed them with J.K.A.’s help. Y.M. wrote the first draft of this manuscript with input from J.K.A. and K.K.L.-H.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by H2020 ERC grant no. ERC-Stg-678148 to J.K.A. and a ‘Bolashak’ International Scholarship of Kazakhstan to Y.M.
References
- 1.Parker GA. 1970. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 45, 525-567. ( 10.1111/j.1469-185X.1970.tb01176.x) [DOI] [Google Scholar]
- 2.Evans JP. 2010. Quantitative genetic evidence that males trade attractiveness for ejaculate quality in guppies. Proc. R. Soc. B 277, 3195-3201. ( 10.1098/rspb.2010.0826). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long TAF, Pischedda A, Stewart AD, Rice WR. 2009. A cost of sexual attractiveness to high-fitness females. PLoS Biol. 7, e1000254. ( 10.1371/journal.pbio.1000254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edward DA, Stockley P, Hosken DJ. 2014. Sexual conflict and sperm competition. Cold Spring Harb. Perspect. Biol. 7, a017707. ( 10.1101/cshperspect.a017707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnqvist G, Rowe L. 2005. Sexual conflict. Princeton, NJ: Princeton University Press. [Google Scholar]
- 6.Parker GA. 2006. Sexual conflict over mating and fertilization: an overview. Phil. Trans. R. Soc. B 361, 235-259. ( 10.1098/rstb.2005.1785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowe L, Chenoweth SF, Agrawal AF. 2018. The genomics of sexual conflict. Am. Nat. 192, 274-286. ( 10.1086/698198) [DOI] [PubMed] [Google Scholar]
- 8.Wilburn DB, Swanson WJ. 2016. From molecules to mating: rapid evolution and biochemical studies of reproductive proteins. J. Proteomics 135, 12-25. ( 10.1016/j.jprot.2015.06.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonduriansky R, Chenoweth SF. 2009. Intralocus sexual conflict. Trends Ecol. Evol. 24, 280-288. ( 10.1016/j.tree.2008.12.005) [DOI] [PubMed] [Google Scholar]
- 10.Chippindale AK, Gibson JR, Rice WR. 2001. Negative genetic correlation for adult fitness between sexes reveals ontogenetic conflict in Drosophila. Proc. Natl Acad. Sci. USA 98, 1671-1675. ( 10.1073/pnas.041378098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rice WR. 1984. Sex chromosomes and the evolution of sexual dimorphism. Evolution 38, 735-742. ( 10.1111/j.1558-5646.1984.tb00346.x) [DOI] [PubMed] [Google Scholar]
- 12.Gibson JR, Chippindale AK, Rice WR. 2002. The X chromosome is a hot spot for sexually antagonistic fitness variation. Proc. R. Soc. Lond. B 269, 499-505. ( 10.1098/rspb.2001.1863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Innocenti P, Morrow EH. 2010. The sexually antagonistic genes of Drosophila melanogaster. PLoS Biol. 8, e1000335. ( 10.1371/journal.pbio.1000335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill S, Pennell TM, Flis I, Ingleby FC, Mott R, Fowler K, Morrow EH, Reuter M. 2019. Genome-wide sexually antagonistic variants reveal long-standing constraints on sexual dimorphism in fruit flies. PLoS Biol. 17, e3000244. ( 10.1371/journal.pbio.3000244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lund-Hansen KK, Abbott JK, Morrow EH. 2020. Feminization of complex traits in Drosophila melanogaster via female-limited X chromosome evolution. Evolution 74, 2703-2713. ( 10.1111/evo.14021) [DOI] [PubMed] [Google Scholar]
- 16.Pennell TM, de Haas FJH, Morrow EH, van Doorn GS. 2016. Contrasting effects of intralocus sexual conflict on sexually antagonistic coevolution. Proc. Natl Acad. Sci. USA 113, E978-E986. ( 10.1073/pnas.1514328113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schenkel MA, et al. 2018. Making sense of intralocus and interlocus sexual conflict. Ecol. Evol. 8, 13 035-13 050. ( 10.1002/ece3.4629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pennell TM, Morrow EH. 2013. Two sexes, one genome: the evolutionary dynamics of intralocus sexual conflict. Ecol. Evol. 3, 1819-1834. ( 10.1002/ece3.540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lund-Hansen KK. 2017. Experimental manipulation of sexual antagonism in Drosophila melanogaster. PhD thesis, University of Sussex. [Google Scholar]
- 20.Abbott JK, Chippindale AK, Morrow EH. 2020. The microevolutionary response to male-limited X-chromosome evolution in Drosophila melanogaster reflects macroevolutionary patterns. J. Evol. Biol. 33, 738-750. ( 10.1111/jeb.13618) [DOI] [PubMed] [Google Scholar]
- 21.Chippindale AK, Rice WR. 2001. Y chromosome polymorphism is a strong determinant of male fitness in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 98, 5677-5682. ( 10.1073/pnas.101456898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark AG, Aguadé M, Prout T, Harshman LG, Langley CH. 1995. Variation in sperm displacement and its association with accessory gland protein loci in Drosophila melanogaster. Genetics 139, 189-201. ( 10.1093/genetics/139.1.189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1– 48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 24.Korner-Nievergelt F, von Felten S, Roth T, Guélat J, Almasi B, Korner-Nievergelt P. 2015. Bayesian data analysis in ecology using linear models with R, BUGS, and Stan. New York, NY: Academic Press. [Google Scholar]
- 25.Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Mächler M, Bolker BM. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378-400. ( 10.32614/RJ-2017-066) [DOI] [Google Scholar]
- 26.Fox J, Weisberg S. 2018. An R companion to applied regression, 3rd edn. Newbury Park, CA: SAGE Publications. [Google Scholar]
- 27.Lenth RV. 2016. Least-squares means: the R package lsmeans. J. Stat. Softw. 69, 1-33. ( 10.18637/jss.v069.i01) [DOI] [Google Scholar]
- 28.Prasad NG, Bedhomme S, Day T, Chippindale AK. 2007. An evolutionary cost of separate genders revealed by male-limited evolution. Am. Nat. 169, 29-37. ( 10.1086/509941) [DOI] [PubMed] [Google Scholar]
- 29.Partridge L, Ewing A, Chandler A. 1987. Male size and mating success in Drosophila melanogaster: the roles of male and female behaviour. Anim. Behav. 35, 555-562. ( 10.1016/S0003-3472(87)80281-6) [DOI] [Google Scholar]
- 30.Andersson MB. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 31.Moya-Laraño J, Fox CW. 2006. Ejaculate size, second male size, and moderate polyandry increase female fecundity in a seed beetle. Behav. Ecol. 17, 940-946. ( 10.1093/beheco/arl029) [DOI] [Google Scholar]
- 32.Rice WR. 1996. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature 381, 232-234. ( 10.1038/381232a0) [DOI] [PubMed] [Google Scholar]
- 33.Pitnick S, Garcia-Gonzalez F. 2002. Harm to females increases with male body size in Drosophila melanogaster. Proc. R. Soc. Lond. B 269, 1821-1828. ( 10.1098/rspb.2002.2090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker DJ, Bast J, Jalvingh K, Dumas Z, Robinson-Rechavi M, Schwander T. 2019. Sex-biased gene expression is repeatedly masculinized in asexual females. Nat. Commun. 10, 4638. ( 10.1038/s41467-019-12659-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonnier G. 1926. Temperature and time of development of the two sexes in Drosophila. J. Exp. Biol. 4, 186. [Google Scholar]
- 36.Nunney L. 1996. The response to selection for fast larval development in Drosophila melanogaster and its effect on adult weight: an example of a fitness trade-off. Evolution 50, 1193-1204. ( 10.1111/j.1558-5646.1996.tb02360.x) [DOI] [PubMed] [Google Scholar]