Abstract
The objective of this review of the literature is to summarize the physiology of orofacial pain in dentistry, particularly physiology of the pain pathway and molecular mechanisms on pathophysiology of pain, on account of new insights into classification of orofacial pain related diseases. This article will also focus on possible mechanisms of neuropathic orofacial pain which is distinguished from other types of pain.
Keywords: dentistry, neurophysiology, orofacial pain, pain, sensation, sensory function
Introduction
Pain is a sensory modality that is an unpleasant and emotional experience (Pak et al., 2018). Painful sensation is subjective and biologically useful, necessary for survival, being a warning sign and response of damaged tissue in the body (John, 1990; Rivera-Morales, 1986). Pain in the facial area is the most common reason which brings patients to see a dentist (Piovesan et al., 2003). Orofacial pain is pain associated with the hard and soft tissues of the head, face, and oral cavity (Sessle, 1987). There are diverse and several mechanisms related to this pathology (Sessle, 1987). Therefore, orofacial pain physiology should be elucidated and applied to clinical practice in the future. This review will define the physiology of orofacial pain and classification of orofacial pain in dentistry. In this review, the strategy for the search in terms of pain, pathophysiology of pain, orofacial pain, dental pain, nociceptive pain, neuropathic pain, pain pathway, and the publication year is 1980–2021.
Physiology of the Orofacial Pain Pathway
The orofacial region is composed of the oral cavity (teeth, gingiva, and oral mucosa), face, jaw bone, and temporomandibular joint (Messlinger and Handwerker, 2015). Physiology of orofacial pain pathways includes primary afferent neurons, pathologic changes in trigeminal ganglion, brainstem nociceptive neurons, and higher brain function regulating orofacial nociception.
Primary Afferent Neurons and Pathologic Changes in Trigeminal Ganglion
The trigeminal nerve or cranial nerve V (CN V) is a sensory nerve which innervates this region. The trigeminal pain pathway from the orofacial area is represented in Figure 1. At the peripheral nociceptors in the orofacial region, after receiving repetitive noxious stimuli or an excessive uncontrollable inflammation, the first order neurons in the trigeminal nerve will develop increased pain signals that are projected to the trigeminal ganglia (Huff and Daly, 2020). The trigeminal ganglia is similar to the dorsal root ganglia (Armstrong and Herr, 2020).
Figure 1.
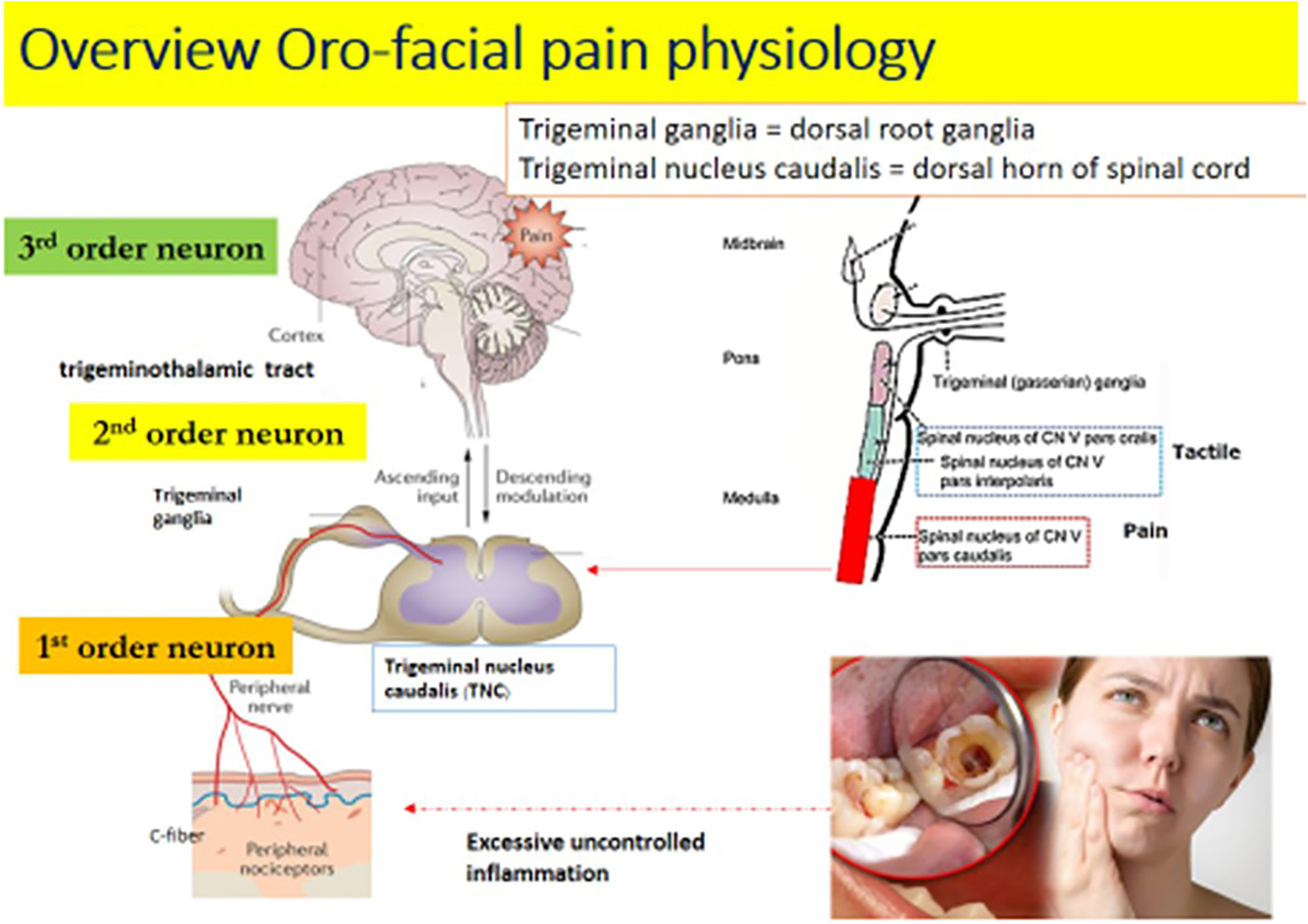
Overview of orofacial pain physiology.
Brainstem Nociceptive Neurons
After that, the pain signals are sent to the second order neurons in the trigeminal nucleus caudalis located in the brainstem. The trigeminal nucleus caudalis is similar to the dorsal horn of the spinal cord and contains second order neurons.
For the trigeminal nucleus, there are three groups of nuclei located inside the brainstem (Joseph, 1982; Rivera-Morales, 1986; John, 1990; Wilkinson, 2014). The first spinal nucleus of the CN V is the pars oralis and the second is the par interpolaris. Both of them convey a tactile sensation in the orofacial area. The third spinal nucleus of the CN V is the pars caudalis or trigeminal nucleus caudalis (Christoforou, 2018; Klasser et al., 2018) which carries the pain perception of this involved area. Then the signals will be further projected to the third order neurons in the thalamus via the ventral trigeminothalamic tract (Joseph, 1982; Rivera-Morales, 1986; John, 1990; Roberts, 1991; van der Bilt et al., 2006; Wilkinson, 2014; Badel et al., 2019).
Higher Brain Function Regulating Orofacial Nociception
In the everse mechanism, there is a descending pathway or pain modulation process from the somatosensory cortex to the trigeminal nucleus caudalis (Sessle, 1987). Naturally, the brainstem perceives ascending sensory input and sends the signal to the thalamus and cortex respectively (Woolf and American Physiological Society, 2004). However, the neurons of the brainstem can also modulate the pain pathway by sending somatosensory signals through the periaqueductal gray, locus coeruleus and rostral ventromedial medulla. Periaqueductal gray is the central midbrain neurons and modulates pain pathways indirectly via other brainstem nucleus, including the locus coeruleus and rostral ventromedial medulla. The locus coeruleus is composed of noradrenergic neurons which are projected to the trigeminal nucleus caudalis. The rostral ventromedial medulla is a large region of medulla which is placed by serotoninergic neurons (Arendt-Nielsen et al., 2018). The descending pathway sends signals to the trigeminal nucleus caudalis. Either serotonin and norepinephrine are released or enkephalin or opioid peptides are produced: this process leads to pain reduction (Fields, 1987; Espinosa-Sanchez et al., 2020). A clinical study reported that patients experiencing temporomandibular joint pain might have a decrease in neurons on both sides of the brainstem, especially at the rostral ventromedial, which is responsible for descending pain pathways or pain modulation. Therefore, the reduction of neurons in the descending pain modulation might increase pain sensation in patients with painful temporomandibular disorders (Wilcox et al., 2015).
Therefore, numerous studies supported that the pathophysiology of orofacial pain is related to the trigeminal nerve and trigeminal pathway leading to alter pain perception in the relevant area of the somatosensory cortex and associated somatosensory cortex.
Classification of Orofacial Pain and Possible Mechanisms
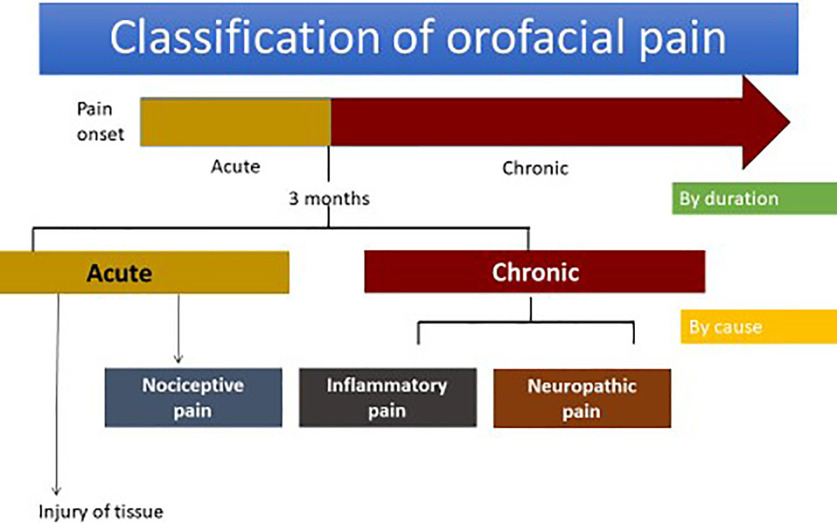
The classification of orofacial pain concerned by durations can be divided into acute pain and chronic pain. By causes, it can be categorized into nociceptive, inflammatory and neuropathic pain (Lee et al., 2005; Fig. 2)
Figure 2.
Classification of orofacial pain.
Acute orofacial pain is the sudden onset of pain related to physical sensations and possibly of limited duration as well as being temporary because of tissue injury causes. Chronic orofacial pain is long lasting pain beyond three months, which is the normal healing period of time (Bell, 1989; Buxbaum, 1994; Christopher, 1998; Monheim, 2019). The difference of acute and chronic orofacial pain is shown in Table 1.
Table 1.
Difference in acute orofacial pain and chronic pain orofacial pain
| Characteristics | Acute orofacial pain | Chronic orofacial pain |
|---|---|---|
| Duration | Onset | Sustained, persistent >3 months in humans |
| Cause | Caused by inflammation or injury of tissue | Caused by inflammation, nerve damage and excessive or uncontrolled inflammation |
| Cause has gone away or healed | No pain when normal healing occurs or is only temporary (pain disappears once stimulus is removed) | Persistent pain and excessive, uncontrolled causes |
| Signs and symptoms | Sudden, sharp, intense, localized | Aching, diffused |
| Physiologic response | Acute pain affects increased cardiovascular functions such as increased blood pressure and heart rate via sympathetic response | Chronic pain affects physiological responses with adaptation behaviors or psychological responses such as depression and anxiety |
| Examples in the orofacial area | (1) Dental pain: pulpitis (2) Mucogingival pain |
(1) Neuropathic pain: trigeminal neuralgia, peripheral trigeminal nerve injury, postherpetic neuralgia (2) Chronic inflammatory pain: chronic pulpitis and apical lesions, temporomandibular disorder pain (3) Neurovascular pain: migraines, tension-type headaches |
Another classification of orofacial pain is classified by three causative groups; nociceptive, inflammatory and neuropathic pain. Nociceptive pain is pain that occurs when noxious stimuli such as heat, cold, intense mechanical force, and chemical irritants directly stimulate nociceptive sensory neurons. Then the nociceptors send signals to the central nervous system leading to pain response such as withdrawal reflex. Therefore, nociceptive pain mechanisms obviously act as a vital physiological sensation (Ananthan and Benoliel, 2020). Inflammatory pain is a pain caused by damaged tissues. Once tissues are injured, the release of inflammatory mediators subsequently occurs and activates pain perception (Shinoda et al., 2019). Neuropathic pain is a pain caused by defects in the peripheral or central nervous system (Fehér et al., 2019; Jaaskelainen, 2019). Thus, the differences between nociceptive, inflammatory and neuropathic pain are shown in Table 2.
Table 2.
Difference between nociceptive, inflammatory, and neuropathic pain
| Characteristics | Nociceptive orofacial pain | Inflammatory orofacial pain | Neuropathic pain |
|---|---|---|---|
| Causes and mechanism of pain pathway | Noxious stimulation at the peripheral nerve and transmitted by normal components of the sensory trigeminal nerve | Strong noxious stimulus causes lesions in the tissue leading to local inflammation responses and increased inflammatory mediators | Caused by nerve damage or injury and increased peripheral sensitization, structure change by increased sodium activation, calcium activity of nerves leading to ectopic discharges, and glia cell activation |
| Nerve condition | Normal nerve structure | Normal nerve structure | Abnormal nerve structure |
| Stimulation | Response to noxious stimulus for protective and withdrawal response | Response to noxious stimulus and increase of activity of peripheral nociceptors | -Response to non-noxious and noxious stimulation -Spontaneous pain without stimulation because ectopic discharges occurred in damaged nerves |
| Example | Hot soup contacting the oral mucosa immediately caused pain perception (heat/hot), and then they threw away this hot soup | -Pulp necrosis with apical abscess -Temporomandibular joint capsulitis or synovitis is caused by joint inflammation. Joint pain and limitation of jaw movement develops afterward |
Peripheral trigeminal nerve injury is caused by nerve damage such as facial trauma accident or trigeminal neuralgia contributing to abnormal nerve structure and expression of severe shooting pain, intermittent patterns, and feels like electric shocks |
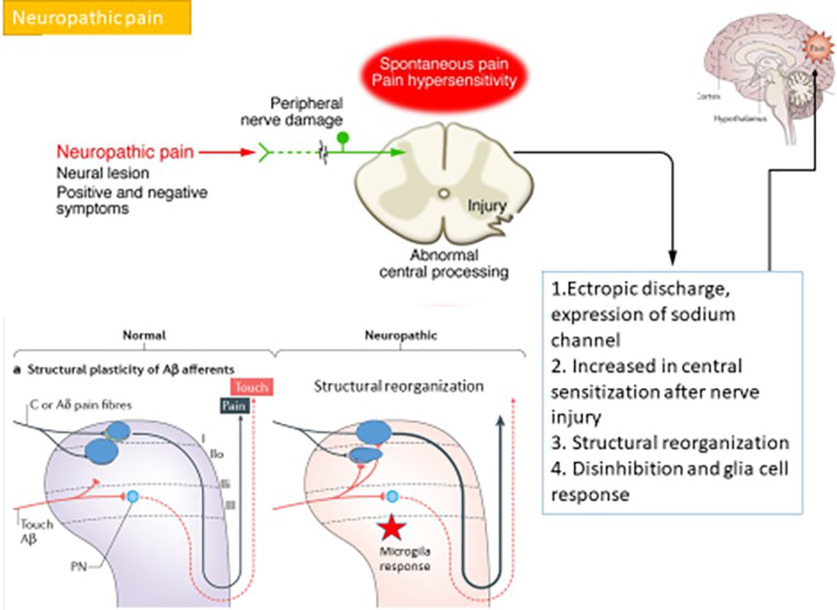
Orofacial neuropathic pain originates from disorders of the somatosensory system. Results of injury to the nerves involved the pain pathway and maybe considered as chronic pain conditions (Jaaskelainen, 2004). The mechanisms of neuropathic pain are distinct from other types of orofacial pain (Fig. 3).
Figure 3.
The possible mechanisms of neuropathic pain.
The possible mechanisms of orofacial neuropathic pain are distinguishable from nociceptive and inflammatory pain. After trigeminal nerves are damaged, the neuroma or injured axon around the damaged site proliferate heterotopic sodium channels such as voltage gated channel types 1.3, 1.7, and 1.8 (Allard et al., 2006; Hargus and Patel, 2007). The proliferation of sodium channels may cause lower stimulation thresholds and provoke ectopic discharges (Li et al., 2015). The spread of sodium channels triggers central sensitization at the trigeminal nucleus caudalis and leads to an enhanced barrage of nociceptive signals, which results in spontaneous pain and pain hypersensitivity (Piovesan et al., 2003; Bernal and Roza, 2018).
The alteration of the calcium channel in the primary afferent nociceptors leads to enhanced transmitters which are released into the trigeminal nucleus caudalis (Li et al., 2019). In addition, AMPA receptors activate trigeminal nucleus caudalis neurons that are related to common responses to painful stimuli, but NMDA receptors are physiologically blocked by a magnesium ion. This blockage is likely removed by accumulation or repetitive depolarization from ectopic discharges. Alteration of the calcium channel results in an amplification and prolongation of the noxious input in the trigeminal nucleus caudalis inside the brainstem (Yam et al., 2018; Armstrong and Herr, 2019).
Furthermore, after nerve injury, the peripheral nerve lesion causes axon and myelin sheath degradation which is later followed by infiltration of macrophages and other types of immune cells such as neutrophils and T cells at the injured site (Spencer and Gremillion, 2007; Lebrilla and Mahal, 2009; Todd, 2010). Moreover, nerve damage subsequently generates functional modulation and modification of the central nervous system (Boadas-Vaello et al., 2017).
Functional modulation after nerve injury leads to increased posttranslational processing such as c-fos in the trigeminal nucleus caudalis. Moreover, the coupling between sympathetic postganglionic neurons and afferent neurons under pathophysiological conditions can occur to maintain pain. Modification after nerve injury is altered in connectivity and cell death by some adjacent uninjured nerve fibers which become excited because of non-synaptic or cross talk of electrical transfers called ephaptic transmission. Finally, this modulation and modification function causes an increase in central sensitization in the trigeminal nucleus caudalis and subsequently the recruitment of secondary messenger pathways after central sensitization results in a rise of neuron excitability or nociceptors or pain signals to the thalamus and somatosensory cortex (Yam et al., 2018; Dolphin et al., 2020).
Non-injury state in the area of the trigeminal nucleus caudalis and dorsal horn of the spinal cord, Aβ fibers penetrate to the dorsal horn, travel ventrally and terminate in Lamina II and deeper into the spinal cord. However, after the peripheral nerve is damaged or injured (Nelson, 2019), there is c-fiber terminal atrophy and A fiber terminal sprouting into the superficial dorsal horn, which conducts easier stimulation of pain signals in the dorsal horn of the spinal cord, and is the reason why non-painful stimulators can produce pain responsiveness in allodynia (Song et al., 2013).
In addition, after nerve injury, a loss of inhibition occurs because of dysfunction of GABA production and apoptosis of inhibitory interneurons resulting in reduction of inhibition pain signals (Tashiro et al., 2014). Lack of or a decrease in inhibition pain signals cause an increased excitability of neurons (Yin et al., 2018). Moreover, after prolonged nerve injury, there follows a decrease in pain modulation of descending pain pathways which result in more nociceptive signals in the pain pathway (Sessle, 2011).
Therefore, the possible pain mechanisms related to glia cell response after nerve injury are caused by microglial activation. Microglia can then release pro-inflammatory mediators such as interleukin 6, interleukin 1β, and tumor necrosis factor α, and activate astrocytes (Han et al., 2012; Won et al., 2012; Lemos et al., 2018). Activated astrocytes or reactive astrocytes directly trigger NMDA receptors (Zhou et al., 2019) leading to excess glutamate release to the trigeminal nucleus caudalis and increase pain signaling projected to the brain and development of neuropathic pain (Jancalek, 2011; Mamoru et al., 2011; Eftekhari et al., 2015).
Chronic neuropathic orofacial pain manifests many distinct functions and possible mechanism apart from inflammatory pain (Tandon et al., 2003) Nevertheless, the differential diagnosis should be performed and defined these types of pain because of different pain management (Allegri et al., 2012).
Consequence, if orofacial pain (acute/chronic stages), which is not neuropathic pain, does not produce an ectopic discharge, structural reorganization and glia cell response. This process of inflammatory pain is sustained by chemical inflammation at the primary trigeminal afferents neuron such as substance P, bradykinin, and calcitonin related peptide proteins leading to peripheral sensitization of peripheral nociceptors at the lesion (Tandon et al., 2003; Allegri et al., 2012). However, repetitive stimulation of c-fibers via the inflammation or nerve injury stimulates a gradual increase of neuron excitability or winds them up, causing central sensitization. Evidence showed that blockading NMDA receptors could attenuate chronic inflammatory or neuropathic pain in animal models (Wong et al., 2014; Lin et al., 2019; Neyama et al., 2020). Subsequently, its mechanism can drive central hyper excitability by chemical mediators and other peptides that remove the magnesium-ion block of NMDA receptors at the trigeminal nucleus caudalis, which could possibly increase pain perception (Koichi et al., 2011).
Currently, there is another name of pain classification which called “nociplastic pain.” Nociplastic pain is meant an un-classification of pain or not properly covered by nociceptive pain or neuropathic pain. Therefore, nociplastic pain is caused by an altered nociceptive function but not inflammatory responses. An example of nocociplastic pain is persistent idiopathic dentoalveolar pain, previously known as atypical odontalgia. Persistent idiopathic dentoalveolar pain is a chronic pain condition that shows as a persistent tooth, alveolar bone, gingiva although undetectable pathology during clinical or radiologic examination (Aydede and Shriver, 2018). However, the pathophysiology of nociplastic pain is still unclear, further study about nociplastic pain should be investigated.
Conclusion
Orofacial pain has become more problematic among the general population. The anatomic complexity of the orofcial region contributes to challenging diagnosis and treatment for many clinicians. A better understanding of underlying physiological mechanisms on orofacial pain may support to improve a clinician’s clarification and perception in the aspect of non-odontogenic or dental pain origin.
Synthesis
Reviewing Editor: Karen Szumlinski, University of California at Santa Barbara
Decisions are customarily a result of the Reviewing Editor and the peer reviewers coming together and discussing their recommendations until a consensus is reached. When revisions are invited, a fact-based synthesis statement explaining their decision and outlining what is needed to prepare a revision will be listed below. The following reviewer(s) agreed to reveal their identity: Yuichi Ogino.
After consultation with two expert reviewers, we have reached the decision of Revise-Rereview. This decision is based primarily on the comments of Reviewer 1, who expressed concerns regarding the organization of the review and the level of detail in each subsection. As no experiments are required, two months should be sufficient to revise the report. Should you require any extra time, please inform eNeuro and we can extend the time. Please find below the specific comments of both reviewers.
Reviewer 1:
n the present review author tried to rereviews the literature to have the better understanding of the Physiology of Orofacial Pain in dentistry.
Major:
1. Author tried to present the review on the Physiology of Orofacial Pain in dentistry. However, author must the describe the strategy for the search ie search terms...... Year of search ie 1990- 20121.
2. Authors must present the thorough review of the topic.
One of the major comment is that, there are no defined sections in the review to present the better understanding of the topic, to understand the central pathway or peripheral pathway or any markers what's latest and specific receptors are involved. For example:
Primary Afferent Neurons
Pathological Changes in Trigeminal Ganglion
Brainstem Nociceptive Neurons
Receptor Mechanisms of Trigeminal Primary Afferent Neurons
Higher Brain Function Regulating Orofacial Nociception
3. Authors are suggested to refer to few of the articles
J. Oral Biosci. 53(4):318-329, 2011; Indian J Physiol Pharmacol 2003; 47 (3) : 247-269; Journal of Clinical Neurophysiology: November 2019 - Volume 36 - Issue 6 - p 422-429; International Review of Neurobiology Volume 97, 2011, Pages 227-250
Reviewer 2
This review manuscript is an easy-to-understand in pain field in dentistry with comprehensible figures and tables, that will help every dentist in clinical practice.
Minor:
Insert Abbreviation of cranial nerve at the first appearance: (CN).
Major:
As a review literature regarding orofacial pain in dentistry, it would be preferable to refer nociplastic pain (Aydede and Shriver. PAIN 2018). My recommendation is adding a short reference to the nociplastic pain in chronic pain section in your own manner, for example, PIDAP (persistent idiopathic dentoalveolar pain; 6.3 in International Classification of Orofacial Pain, 1st edition) previously known as atypical odontalgia.
Author Response
Response to reviewers
We thanks to raising point of two reviewers and revised followed these
recommendations
Reviewer 1:
the present review author tried to rereviews the literature to have the better
understanding of the Physiology of Orofacial Pain in dentistry.
Major:
1. Author tried to present the review on the Physiology of Orofacial Pain in
dentistry. However, author must the describe the strategy for the search ie
search terms...... Year of search ie 1990- 2021.
Response: We have added this points in page 1, paragraph 1 of subheading
introduction
2. Authors must present the thorough review of the topic.
One of the major comment is that, there are no defined sections in the review to
present the better understanding of the topic, to understand the central pathway
or peripheral pathway or any markers what's latest and specific receptors are
involved. For example:
Primary Afferent Neurons
Pathological Changes in Trigeminal Ganglion
Brainstem Nociceptive Neurons
Receptor Mechanisms of Trigeminal Primary Afferent Neurons
Higher Brain Function Regulating Orofacial Nociception
Response: We have changes followed the recommendation in blue color in text.
3. Authors are suggested to refer to few of the articles
J. Oral Biosci. 53(4):318-329, 2011; Indian J Physiol Pharmacol 2003;
47 (3) : 247-269; Journal of Clinical Neurophysiology: November 2019 -
Volume 36 - Issue 6 - p 422-429; International Review of Neurobiology
Volume 97, 2011, Pages 227-250
Response: We have changes followed the recommendation in blue color in text2
Reviewer 2
This review manuscript is an easy-to-understand in pain field in dentistry with
comprehensible figures and tables, that will help every dentist in clinical
practice.
Minor:
Insert Abbreviation of cranial nerve at the first appearance: (CN).
Response: We insert abbreviation of cranial nerve at the first appearance in
page II, paragraph II (red color in text)
Major:
1. As a review literature regarding orofacial pain in dentistry, it would be
preferable to refer nociplastic pain (Aydede and Shriver. PAIN 2018). My
recommendation is adding a short reference to the nociplastic pain in chronic
pain section in your own manner, for example, PIDAP (persistent idiopathic
dentoalveolar pain; 6.3 in International Classification of Orofacial Pain, 1st
edition) previously known as atypical odontalgia.
Response: We thank to raising points. We have added in page 5 paragraph 3
(red color in text)
References
- Allard B, Magloire H, Couble ML, Maurin JC, Bleicher F (2006) Voltage-gated sodium channels confer excitability to human odontoblasts: possible role in tooth pain transmission. J Biol Chem 281:29002–29010. 10.1074/jbc.M601020200 [DOI] [PubMed] [Google Scholar]
- Allegri M, Clark MR, De Andrés J, Jensen TS (2012) Acute and chronic pain: where we are and where we have to go. Minerva Anestesiol 78:222–235. [PubMed] [Google Scholar]
- Ananthan S, Benoliel R (2020) Chronic orofacial pain. J Neural Transm (Vienna) 127:575–588. 10.1007/s00702-020-02157-3 [DOI] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Morlion B, Perrot S, Dahan A, Dickenson A, Kress HG, Wells C, Bouhassira D, Mohr Drewes A (2018) Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur J Pain 22:216–241. 10.1002/ejp.1140 [DOI] [PubMed] [Google Scholar]
- Armstrong SA, Herr MJ (2019) Physiology, nociception. Treasure Island: StatPearls Publishing LLC. [PubMed] [Google Scholar]
- Armstrong SA, Herr MJ (2020) Physiology, nociception. Treasure Island: StatPearls Publishing LLC. [PubMed] [Google Scholar]
- Aydede M, Shriver A (2018) Recently introduced definition of “nociplastic pain” by the International Association for the Study of Pain needs better formulation. Pain 159:1176–1177. 10.1097/j.pain.0000000000001184 [DOI] [PubMed] [Google Scholar]
- Badel T, Zadravec D, Bašić Kes V, Smoljan M, Kocijan Lovko S, Zavoreo I, Krapac L, Anić Milošević S (2019) Orofacial pain - diagnostic and therapeutic challenges. Acta Clin Croat 58:82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell WE (1989) Orofacial pains; classification, diagnosis, management, Ed, 4. Chicago: Year Book Medical Publishers Inc. [Google Scholar]
- Bernal L, Roza C (2018) Hyperpolarization-activated channels shape temporal patterns of ectopic spontaneous discharge in C-nociceptors after peripheral nerve injury. Eur J Pain 22:1377–1387. 10.1002/ejp.1226 [DOI] [PubMed] [Google Scholar]
- Boadas-Vaello P, Homs J, Reina F, Carrera A, Verdú E (2017) Neuroplasticity of supraspinal structures associated with pathological pain. Anat Rec (Hoboken) 300:1481–1501. 10.1002/ar.23587 [DOI] [PubMed] [Google Scholar]
- Buxbaum JD (1994) Dental management of orofacial pain. In: Hand book of pain management (Tollison CDSJ, Tollison JW, eds), 2nd Ed 2, pp 306–327. Baltimore: Williams and Wikins. [Google Scholar]
- Christoforou J (2018) Neuropathic orofacial pain. Dent Clin North Am 62:565–584. 10.1016/j.cden.2018.05.005 [DOI] [PubMed] [Google Scholar]
- Christopher LB (1998) Applied oral physiology, Ed 2. London: Wright. [Google Scholar]
- Dolphin AC, Insel PA, Blaschke TF, Meyer UA (2020) Introduction to the theme “ion channels and neuropharmacology: from the past to the future”. Annu Rev Pharmacol Toxicol 60:1–6. 10.1146/annurev-pharmtox-082719-110050 [DOI] [PubMed] [Google Scholar]
- Eftekhari S, Salvatore CA, Johansson S, Chen TB, Zeng Z, Edvinsson L (2015) Localization of CGRP, CGRP receptor, PACAP and glutamate in trigeminal ganglion. Relation to the blood-brain barrier. Brain Res 1600:93–109. 10.1016/j.brainres.2014.11.031 [DOI] [PubMed] [Google Scholar]
- Espinosa-Sanchez JM, Espinosa-Campos L, Batuecas-Caletrío Á (2020) From neuroanatomy to neurophysiology. Anat Rec (Hoboken) 303:1221–1231. 10.1002/ar.24190 [DOI] [PubMed] [Google Scholar]
- Fehér G, Nemeskéri Z, Pusch G, Zádori I, Bank G, Gurdán Z, Mészáros J, Mák K, Tibold A, Komoly S (2019) Chronic orofacial pain. Orv Hetil 160:1047–1056. 10.1556/650.2019.31432 [DOI] [PubMed] [Google Scholar]
- Han SR, Yang GY, Ahn MH, Kim MJ, Ju JS, Bae YC, Ahn DK (2012) Blockade of microglial activation reduces mechanical allodynia in rats with compression of the trigeminal ganglion. Prog Neuropsychopharmacol Biol Psychiatry 36:52–59. 10.1016/j.pnpbp.2011.10.007 [DOI] [PubMed] [Google Scholar]
- Hargus NJ, Patel MK (2007) Voltage-gated Na+ channels in neuropathic pain. Expert Opin Investig Drugs 16:635–646. 10.1517/13543784.16.5.635 [DOI] [PubMed] [Google Scholar]
- Fields HL (1987) Pain. New York: McGraw-Hill. [Google Scholar]
- Huff T, Daly DT (2020) Neuroanatomy, cranial nerve 5 (trigeminal). Treasure Island: StatPearls Publishing LLC. [PubMed] [Google Scholar]
- Jaaskelainen SK (2004) Clinical neurophysiology and quantitative sensory testing in the investigation of orofacial pain and sensory function. J Oro Pain 18:85–107. [PubMed] [Google Scholar]
- Jaaskelainen SK (2019) Differential diagnosis of chronic neuropathic orofacial pain: role of clinical neurophysiology. J Clin Neurophysiol 36:422–429. [DOI] [PubMed] [Google Scholar]
- Jancalek R (2011) Signaling mechanisms in mirror image pain pathogenesis. Ann Neurosci 18:123–127. 10.5214/ans.0972.7531.111810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John J (1990) The management of pain, Ed 2. Philadelphia: Lea and Febiger. [Google Scholar]
- Joseph G (1982) Correlative neuroanatomy and functional neurology, Ed 18. California: Lange Medical Publications. [Google Scholar]
- Klasser GD, Almoznino G, Fortuna G (2018) Sleep and orofacial pain. Dent Clin North Am 62:629–656. 10.1016/j.cden.2018.06.005 [DOI] [PubMed] [Google Scholar]
- Koichi I, Yoshiki I, Kuniya H, Masamichi S (2011) Physiological mechanisms of neuropathic pain: the orofacial region. Int Rev Neurobiol 97:227–250. [DOI] [PubMed] [Google Scholar]
- Lebrilla CB, Mahal LK (2009) Post-translation modifications. Curr Opin Chem Biol 13:373–374. 10.1016/j.cbpa.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Lee CH, Oh U (2005) Painful channels in sensory neurons. Mol Cells 20:315–324. [PubMed] [Google Scholar]
- Lemos GA, da Silva PLP, Batista AUD, Palomari ET (2018) Experimental model of temporomandibular joint arthritis: evaluation of contralateral joint and masticatory muscles. Arch Oral Biol 95:79–88. 10.1016/j.archoralbio.2018.07.003 [DOI] [PubMed] [Google Scholar]
- Li G, Liu X, Du J, Chen J, She F, Wu C, Li C (2015) Positive shift of Nav1.8 current inactivation curve in injured neurons causes neuropathic pain following chronic constriction injury. Mol Med Rep 12:3583–3590. 10.3892/mmr.2015.3839 [DOI] [PubMed] [Google Scholar]
- Li XH, Miao HH, Zhuo M (2019) NMDA receptor dependent long-term potentiation in chronic pain. Neurochem Res 44:531–538. 10.1007/s11064-018-2614-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Zhao Y, Cheng B, Zhao H, Miao L, Li Q, Chen Y, Zhang M (2019) NMDAR and JNK activation in the spinal trigeminal nucleus caudalis contributes to masseter hyperalgesia induced by stress. Front Cell Neurosci 13:495. 10.3389/fncel.2019.00495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamoru T, Shigeji M, Barry JS, Masamichi S, Koichi I (2011) Peripheral and central mechanisms of trigeminal neuropathic and inflammatory pain. J Oral Biosci 53:318–329. [Google Scholar]
- Messlinger K, Handwerker HO (2015) Physiology of pain. Schmerz 29:522–530. 10.1007/s00482-015-0052-y [DOI] [PubMed] [Google Scholar]
- Monheim LM (2019) Analgesia in dentistry now and in the future. Anesth Prog 66:227–231. 10.2344/0003-3006-66.4.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TS (2019) Dorsal horn PKCγ interneurons mediate mechanical allodynia through 5-HT2AR-dependent structural reorganization. J Neurosci 39:6221–6223. 10.1523/JNEUROSCI.0291-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyama H, Dozono N, Ueda H (2020) NR2A-NMDA receptor blockade reverses the lack of morphine analgesia without affecting chronic pain status in a fibromyalgia-like mouse model. J Pharmacol Exp Ther 373:103–112. 10.1124/jpet.119.262642 [DOI] [PubMed] [Google Scholar]
- Pak DJ, Yong RJ, Kaye AD, Urman RD (2018) Chronification of pain: mechanisms, current understanding, and clinical implications. Curr Pain Headache Rep 22:9. 10.1007/s11916-018-0666-8 [DOI] [PubMed] [Google Scholar]
- Piovesan EJ, Kowacs PA, Oshinsky ML (2003) Convergence of cervical and trigeminal sensory afferents. Curr Pain Headache Rep 7:377–383. 10.1007/s11916-003-0037-x [DOI] [PubMed] [Google Scholar]
- Rivera-Morales WC (1986) Orofacial pains; classification, diagnosis, management, Ed 4. Chicago: Year Book Medical Publishers Inc. [Google Scholar]
- Roberts GJ (1991) A colour atlas of dental analgesia and sedation. London: Wolfe Publishing Ltd. [Google Scholar]
- Sessle BJ (1987) Neurophysiology of orofacial pain. Dent Clin North Am 4:595–613. [PubMed] [Google Scholar]
- Sessle BJ (2011) Peripheral and central mechanisms of orofacial inflammatory pain. Int Rev Neurobiol 97:179–206. 10.1016/B978-0-12-385198-7.00007-2 [DOI] [PubMed] [Google Scholar]
- Shinoda M, Kubo A, Hayashi Y, Iwata K (2019) Peripheral and central mechanisms of persistent orofacial pain. Front Neurosci 13:1227. 10.3389/fnins.2019.01227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Zhang YM, Xu J, Wu JR, Qin X, Hua R (2013) [Effect of spontaneous firing of injured dorsal root ganglion neuron on excitability of wide dynamic range neuron in rat spinal dorsal horn.] Sheng Li Xue Bao 65:533–539. [PubMed] [Google Scholar]
- Spencer CJ, Gremillion HA (2007) Neuropathic orofacial pain: proposed mechanisms, diagnosis, and treatment considerations. Dent Clin North Am 51:209–224. 10.1016/j.cden.2006.09.006 [DOI] [PubMed] [Google Scholar]
- Tandon OP, Malhotra V, Tandon S, D’Silva I (2003) Neurophysiology of pain: insight to orofacial pain Indian. J Physiol Pharmacol 47: 247–269. [PubMed] [Google Scholar]
- Tashiro A, Bereiter DA, Thompson R, Nishida Y (2014) GABAergic influence on temporomandibular joint-responsive spinomedullary neurons depends on estrogen status. Neuroscience 259:53–62. 10.1016/j.neuroscience.2013.11.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ (2010) Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 11:823–836. 10.1038/nrn2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bilt A, Engelen L, Pereira LJ, van der Glas HW, Abbink JH (2006) Oral physiology and mastication. Physiol Behav 89:22–27. 10.1016/j.physbeh.2006.01.025 [DOI] [PubMed] [Google Scholar]
- Wilcox SL, Gustin SM, Macey PM, Peck CC, Murray GM, Henderson LA (2015) Anatomical changes within the medullary dorsal horn in chronic temporomandibular disorder pain. Neuroimage 117:258–266. 10.1016/j.neuroimage.2015.05.014 [DOI] [PubMed] [Google Scholar]
- Wilkinson JL (2014) Neuroanatony for medical students. London: Wright. [Google Scholar]
- Won KA, Kang YM, Lee MK, Park MK, Ju JS, Bae YC, Ahn DK (2012) Participation of microglial p38 MAPK in formalin-induced temporomandibular joint nociception in rats. J Orofac Pain 26:132–141. [PubMed] [Google Scholar]
- Wong H, Kang I, Dong XD, Christidis N, Ernberg M, Svensson P, Cairns BE (2014) NGF-induced mechanical sensitization of the masseter muscle is mediated through peripheral NMDA receptors. Neuroscience 269:232–244. 10.1016/j.neuroscience.2014.03.054 [DOI] [PubMed] [Google Scholar]
- Woolf CJ, American Physiological Society (2004) Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med 140:441–451. 10.7326/0003-4819-140-8-200404200-00010 [DOI] [PubMed] [Google Scholar]
- Yam MF, Loh YC, Tan CS, Khadijah Adam S, Abdul Manan N, Basir R (2018) General pathways of pain sensation and the major neurotransmitters involved in pain regulation. Int J Mol Sci 19:2164. 10.3390/ijms19082164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Yi MH, Kim DW (2018) Impaired autophagy of GABAergic interneurons in neuropathic pain. Pain Res Manag 2018:9185368. 10.1155/2018/9185368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou LJ, Peng J, Xu YN, Zeng WJ, Zhang J, Wei X, Mai CL, Lin ZJ, Liu Y, Murugan M, Eyo UB, Umpierre AD, Xin WJ, Chen T, Li M, Wang H, Richardson JR, Tan Z, Liu XG, Wu LJ (2019) Microglia are indispensable for synaptic plasticity in the spinal dorsal horn and chronic pain. Cell Rep 27:3844–3859.e6. 10.1016/j.celrep.2019.05.087 [DOI] [PMC free article] [PubMed] [Google Scholar]