Abstract
The extinction of species before they are discovered and named (dark extinction, DE) is widely inferred as a significant part of species loss in the ‘pre-taxonomic’ period (approx. 1500–1800 CE) and, to some extent, in the ‘taxonomic period’ (approx. 1800–present) as well. The discovery of oceanic islands and other pristine habitats by European navigators and the consequent introduction of destructive mammals, such as rats and goats, started a process of anthropogenic extinction. Much ecosystem change happened before systematic scientific recording, so has led to DE. Statistical methods are available to robustly estimate DE in the ‘taxonomic period’. For the ‘pre-taxonomic period’, simple extrapolation can be used. The application of these techniques to world birds, for example, suggests that approximately 56 DEs occurred in the ‘taxonomic period’ (1800–present) and approximately 180 in the ‘pre-taxonomic period’ (1500–1800). Targeting collection activities in extinction hotspots, to make sure organisms are represented in collections before their extinction, is one way of reducing the number of extinct species without a physical record (providing that collection efforts do not themselves contribute to species extinction).
Keywords: extinction rate, oceanic island, island extinction, St Helena, dark extinction, extinction debt
1. The problem of dark extinction
Some have complained that inscriptions on tomb-stones convey no general information except that individuals were born and died, accidents which must happen alike to all men. But the death of a species is so remarkable an event in natural history, that it deserves commemoration [1].
Sir Charles Lyell wrote this on the dodo (Raphus cucullatus), a species that was extirpated by humans so early that it escaped scientific attention for many years and is now largely known only from subfossil bones, and a single specimen, most of which was discarded in 1755.
The early extinction of the dodo highlights the likelihood of there being species that have become recently extinct without being scientifically recorded, and perhaps leaving no physical trace. For convenience, we use the term ‘dark extinction’ (DE) for this, i.e. the extinction of undescribed species. These species may be inferred or suggested but remain unknown to science. Some may be brought to light in future by the discovery of subfossil remains or unrecognized early collections, but many will remain completely unknowable. The name is a nod to cosmological ‘dark matter’, i.e. matter that does not interact with the electromagnetic force and so cannot be directly observed. Like DE it is almost certain to exist but remains theoretical.
The anthropogenic extinction of unrecognized species may be divided into phases as set out below.
2. The phases of anthropogenic dark extinction
(a). Prehistoric dark extinction
This period is excluded from analysis in this review although the concepts discussed here are potentially equally applicable, even though data are sparse. For our purposes here, the ‘historical period’ is defined as the early modern period from 1500 CE onwards.
Anthropogenic extinction certainly predates even the written record, going back as far as 10 000 BP driven by early human colonization of continental regions, likely including megafaunal extinctions of North America and Australia [2–6] and early extinctions in Polynesia [7]. These prehistoric extinctions are known from fossil and subfossil evidence, but given the incompleteness of the fossil record we can expect some number of still unknown extinctions.
(b). Dark extinction in early modern (pre-taxonomic) times
A new phase of extinction started in the early modern historical period: the period from 1500 onwards when improvements in European maritime technology brought European peoples and their domestic animals into contact with vulnerable oceanic islands with no native mammals. The environmental change that ensued was rapid and considerable. The year 1500 is a reasonable cut-off for defining ‘recently extinct’ [8], and 1500 to the present is therefore the main focus of this paper. However, there is a very important distinction between the taxonomic period approximately 1800 to present (for which there are enough data to allow direct modelling of DE) and the ‘pre-taxonomic period’, 1500 to approximately 1800, when although there are insufficient data to model DE satisfactorily, DE can be estimated by extrapolation.
Unfortunately, the ‘pre-taxonomic period’, although critical for island extinction, predated the development of sophisticated descriptive natural history, so understanding the early stages of human-driven island environmental change remains deeply problematic. Taking the dodo as an example, the last recorded sighting was pre-1700 and recorded information was fragmentary [9,10]. So although its DNA has been sequenced [11], our knowledge of its habits and natural history is rudimentary. Yet scientific knowledge of the dodo is extensive by comparison with some other early extinctions. The Ascension crake (Mundia elpenor) is known from subfossil bones, but the only record of the living organism comes from a small sketch by the early traveller Peter Mundy in 1656 [12]. Even more wispy is the basis for our knowledge of the extinct endemic cuckoo of St Helena, Nannococcyx psix, likely to have become extinct shortly after the discovery of the island in 1502, and known only from a single incomplete humerus [13]. We may therefore hypothesize that other island organisms may have become extinct around the same time without fossils being preserved and discovered, and while escaping the notice of early travellers.
(c). Dark extinction in the current taxonomic period, 1800–present
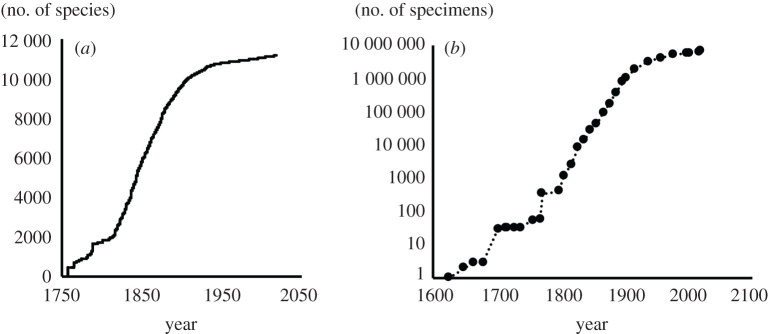
Although modern scientific nomenclature and methods trace their roots to the Linnaean revolution of the 1750s, the inventory of organisms outside Europe was slow to ramp up, and it is reasonable for our purposes to take the start of the ‘taxonomic period’ as 1800. In most groups, the majority of species were described after 1850, even for relatively conspicuous organisms like birds (figure 1). There is, therefore, scope for many organisms to have become extinct after 1800 but before being named, especially in the early years of the taxonomic period.
Figure 1.
(a) Plot of cumulative numbers of named species of birds based on the HBW checklist [14]. A similar plot for is given by Pimm et al. [15] based on earlier data. (b) Plot of cumulative numbers (log scale) of bird preserved specimen collections in museums based on records in the Global Biodiversity Information Facility (gbif.org).
What is even more important than description and naming is collection. Even if a species becomes extinct before being named, for instance because of the slow pace of taxonomic work, if it has been collected and is in a museum or herbarium collection, it can eventually be brought to light and named as an extinct organism. However, collecting too was slow to take off, with the overwhelming majority of specimens collected after 1900 (figure 1 gives an example for birds). Nevertheless, there are numerous recently extinct organisms in museums that are only now being described and named as species [16,17]. In groups characterized by high extinction rates or low taxonomic capacity, taxonomic activity lags behind extinction. This begs the question of whether the extinction of these species could have been prevented if taxonomic recognition had come earlier. While this is possible, these DEs are often in remote places with severe conservation threats and limited conservation resources.
(d). Future dark extinction: the capacity of taxonomy in the face of high extinction rates in the Anthropocene
Recent estimates of numbers of species globally [18] suggest that there are many groups, particularly arthropods, for which the numbers of undescribed species are very large. While taxonomic effort is steadily reducing this, it is likely that it will take many years (probably centuries) to complete the inventory at current rates of species discovery [19]. As anthropogenic extinction is an apparently ubiquitous feature of organisms, some future extinction of undescribed and undiscovered species is inevitable [19]. Fortunately, the opening up of many parts of the world to biological exploration by improved transport links has led to an acceleration in collecting, so even if species are not named until after they are extinct, their presence in collections will allow the documentation of extinction some time in the future.
3. Estimation of dark extinction in the taxonomic period
A number of methods can estimate the DE rate from the rate of species discovery and observed species extinctions. These methods are therefore limited: they cannot extend back to a time before species were described or before extinctions were observed. Thus, it is necessary to divide the study of DE into the ‘taxonomic period’ and the ‘pre-taxonomic period’.
(a). Dark extinction from E/MSY
Pimm et al. [20] realized that to express extinction as a rate, it is necessary to take into account both the number of species and time (scaled to millions of years for convenience when considering geological extinction rates). This resulted in the widely used metric of ‘extinction per million species-years' (E/MSY). In applying this to extinctions during the period in which extant species have been taxonomically known [21], it is necessary to calculate ‘species-years’ from the date of first taxonomic description. As Pimm et al., writing in 2014 [21, p. 1], put it: ‘For recent extinctions, we follow cohorts from the dates of their scientific description … for example, taxonomists described 1230 species of birds after 1900, and 13 of them are now extinct or possibly extinct. This cohort accumulated 98 334 species-years—meaning that an average species has been known for 80 years. The extinction rate (μ) is (13/98 334) × 106 = 132 E/MSY’. Or more generally:
where Et is the number of extinctions occurring within year t, St is the number of extant species known within year t and τ is the final year from which persistence/extinction data were collected. A corollary of this is that all these species would have been present at 1900, increasing the total number of species-years since 1900 to around 122 800 (using the numbers in the example above). Applying the estimated extinction rate of 132 E/MSY to this number gives 16 extinctions, of which we know of only 13, suggesting that there have been three DEs of birds (at a minimum) since 1900, as predicted by the analysis of this example cohort. It is easy to see how this thinking could be extended to all birds. We should note that this will provide only a lower bound on DEs for the time period considered, because the figure of 122 800 SY does not include species that were never seen. We can, however, provide an estimated overall percentage extinction rate. The extinction percentage calculated from E/S here is 13/1230 = 0.011 or 1.1%. We can estimate cumulative extinction percentage (p) between years t and τ by:
where (1 − μ)τ−t is the cumulative probability of persistence between years t and τ. Using this method, we obtain an extinction percentage of 0.015 or 1.5%. This approach could be extended to estimate the absolute number of extinctions. Indeed, the Tedesco and SEUX methods (below) incorporate annual persistence probabilities to achieve this. The E/MSY method can therefore be viewed as the simplest member of a family of methods for calculating DE during the taxonomic period, using information on species discovery and persistence.
(b). The Tedesco method
A more formal solution to the estimation problem was put forward by Tedesco et al. [22]. This study proposed a parametric model based on a geometric series to estimate E[Xt], the expected number of undescribed species that have gone extinct (DE) at year t, with the assumption that both described and undescribed species have an invariant annual extinction probability, μ, and that undescribed species have an invariant annual description probability, d. Because μ and d can be calculated from observed extinctions and detections (similar to E/MSY), these variables together with the initial number of extant species (N0) can be used to estimate the number of DEs between years t = 1 and t = n (as in [22]):
using the geometric series with 1 – (μ + d) as the common ratio y, and μN0 as the series coefficient.
With this model, they calculated that pE (the proportion of undescribed extinct species among all extinct ones) varied, in their examples, between 0.15 and 0.59. Their model provides an important insight: that pE is mainly driven by the description rate, which places the focus squarely on taxonomic activity, or the lack of it. In their discussion, the authors invert the statement ‘the more diversity that is discovered the more urgent becomes putting additional resources into understanding [it]’ [22, p. 1368;23, p. 3846], as ‘the more diversity still to be discovered, the more urgent it becomes to put additional resources into understanding [it]’ (our italics). It is relevant to note that in many groups, the majority of species remain undescribed [24].
(c). Chisholm's SEUX
Subsequently, Chisholm et al. [25], noting that the Tedesco method depends on parametric (exponential decay) assumptions, developed a non-parametric approach. This model breaks species into four groups: detected extant species (S); undetected extant species (U); detected extinct species (E); and undetected extinct species (X). The sum of all these groups is always N (the total number of species). At the start point S = E = X = 0 and U = N. Over time, there are state transitions between the categories, as taxonomic effort moves species from undetected to detected, and ecosystem disturbance moves species from extant to extinct. Modelling the state transitions, with input data consisting of the first and last date when species were recorded, allows the calculation of the proportion of species that have gone extinct (detected and undetected). Consequently, the extinction rate (μt) can vary between time steps (t), which distinguishes this method from those previous, which model a constant rate. Also, the SEUX method allows the species discovery rate to vary over time. The authors originally applied their method to the birds of Singapore, where 195 species have been recorded over the last 200 years, of which 58 have been extirpated. The authors estimate a DE (local extirpation) of an additional 9.6 unrecorded species. Subsequent studies applied the method to plants [26] and butterflies [27].
(d). Comparison of methods and the future
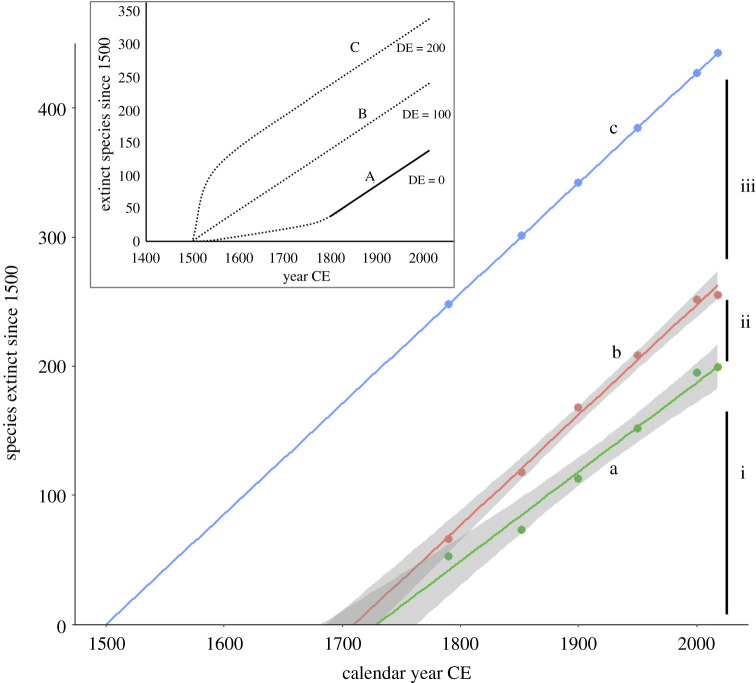
The two methods, parametric (Tedesco) and non-parametric (SEUX), are in many respects complementary and both should be applied more widely. A recent paper [28] conducted a quantified comparison of the Tedesco and SEUX models using simulated and actual data and found that the two models are in broad agreement. When common species are detected at a higher rate than rare ones (a reasonable assumption in many circumstances), both models underestimate DE (assuming common species are less liable to extinction). More generally, the problem is in detection and extinction rates being negatively correlated across species (e.g. rare species are harder to detect and have higher extinction rates). The SEUX model frequently delivered estimates that were somewhat lower than the Tedesco method, which is attributed to the SEUX assumption that there are no undetected extant species at the present. That is a likely assumption for well-known groups but not for some others. It should also be borne in mind that changes in taxonomic concept can have a problematic effect on these estimates. Recently, a number of cryptic bird taxa have been described, at the species level, largely or solely on the basis of DNA information (e.g. [29,30]). If this cluster of very recent taxonomic products of the DNA revolution is included, it has the effect of greatly inflating the estimate of DE. In our example (figure 2), we accordingly exclude new species discoveries of birds post-1995 to exclude species described as splits on molecular evidence.
Figure 2.
‘Extinction diagram’ for birds 1500–present: plots of cumulative extinction of birds since 1500 against time (calendar year, CE), showing the effect of DE. (line a) The lowest line shows the cumulative extinctions from 1500 to present that we know about (E = 199), plotted over the time period (1800–present) for which the data are most credible. This line has an intercept at 1728. (line b) The middle line shows the addition of DE estimated for the taxonomic period by the program seux (increment ii, approximately 56). This line has an intercept at 1710. (line c) Adding an additional 180 extinct species (increment iii) to the numbers (pre-taxonomic DE = 180) gives an intercept at 1500 (see the text). Here, the points are shown on the line to indicate that this is not a new regression but simply an upward displacement of the line below. Inset: Conceptual models of pre-1800 extinction. (line A) Slow-start model (no DE): this is equivalent to the actual known extinctions post-1500. Solid line: post-1800 known extinction; dotted line: pre-1800 with no DE (rather few extinctions are known from this period). (line B) Constant extinction (linear) model. Cumulative post-1500 extinction curve with the addition of pre-1800 DE (pre-1800 DE = 100). (line C) Filter model (see the text): high early DE filters out susceptible species (pre-1800 DE = 200).
Lastly, it may be noted that the estimation of DE is of a class of Laplacian problem that includes capture–recapture and epidemiological inference (where an unknown is estimated from more or less related knowns). Laplace suggested this type of inference in 1783 to estimate the population of France from the number of births registered in France and the ratio of births to population in certain parishes [31,32]. As the capture–recapture and epidemiological fields have well developed methods of statistical inference, in the future these fields may provide interesting insights for the study of DE.
4. Estimation of dark extinction in the pre-taxonomic period
(a). Extrapolation from cumulative extinction curves
The simplest of all methods for estimating DE in the period between 1500 and 1800 is extrapolation, which, although it makes some crude assumptions, provides a useful base model against which other estimates can be compared, and can be presented in the form of an ‘extinction diagram’ (figure 2). If the cumulative extinction of birds [33] since 1500 is taken as an example, we can take the period for which we have reasonable data on known extinctions (approx. 1800–present) to calculate an extinction curve and then extrapolate this back to the early period for which we may have ‘missing’ extinctions (figure 2, line a). To improve the accuracy, the DE for the period 1800 to the present, as calculated by SEUX or Tedesco, may be added and the combined cumulative extinction curve may be plotted (figure 2, line b)
Birds are suitable for this sort of analysis as they are well known taxonomically [14] and in terms of conservation status [33–36]. Also, their remains are frequently found subfossilized in arid environments [7,13], so their extinction (even if early) is generally well documented. They disperse well to isolated oceanic islands and evolve endemic species there, and as these islands are highly vulnerable to anthropogenic degradation, extinction rates have been comparatively high. According to one standard source, there have been 134 documented recent (post-1500) unequivocal species extinctions, of which 43 took place between 1500 and 1800 [33]. A more recent source notes 178 known bird extinctions since 1500 [37]. Bird taxonomy was well developed in the nineteenth century, and so the majority of post-1800 extinctions are likely to be documented.
A dataset of all birds with data on their date of first description and extinct/extant status was constructed from multiple sources [14,33,34], filtered to exclude extinctions of infraspecific taxa and species dubiously extinct (see Data accessibility statement). This extinction dataset (11 157 species of which 199 extinct) was then imported into R v.4.0.2 [38] for analysis. The recent extinction curve (post-1800) is linear and relatively steep and when extrapolated has an intercept at the year approximately 1728, falsely implying no extinction before 1728 if this extinction rate is constant (figure 2). The formula is:
where Ne is the cumulative number of extinctions. Per-year DEs were then estimated from the dataset using the R package seux [39] and added (per-year) to the extinction curve above. With these DE added, the fitted linear model becomes:
meaning that extinctions begin at year 1710, which is slightly closer to the beginning of the early modern period (year 1500).
To make the intercept 1500 (i.e. zero extinctions at year 1500), it is necessary to add extra extinctions (i.e. DEs) to the cumulative extinction data (figure 2, line c). The number required is given by:
or in this case (1710.2 – 1500)/1.1697 = 179.7 (figure 2). In other words, if the extinction rate from 1800 to present is constant back in time, then it implies approximately 180 additional undocumented extinctions from 1500 to 1800. Similar reasoning was used to extrapolate 10 DEs post-1502 in the endemic vascular flora of St Helena [40].
5. Extinction rates and extrapolation
(a). Constant extinction rate model
The graphical extrapolation presented in figure 2 assumes a constant rate of extinction (extinctions/year). Over the period for which we have reasonable data (1800–present), bird extinction does indeed appear to have a roughly constant rate, and this phenomenon has been found in other systems for which historical or subfossil data exist such as Pleistocene small mammals [3] and island plants [40]. It is most parsimonious to take this known rate as the hypothetical rate for the period about which we have few if any data (1500–1800). Under this assumption DEs merely result from the steady accumulation of extinctions since 1500 without fluctuations in the extinction rate.
The apparent robustness of recent extinction rates, at first look, seems rather extraordinary, but not without precedent. The supposed tendency of extinction to be roughly constant over geological time has been much debated in the palaeobiological literature since it was first postulated, as Van Valen's Law [41,42]. The relative stability of recent extinction rates may result from the circumstance that census extinction rarely follows immediately from disturbance, but instead results from the paying down of extinction debt [43,44] over a long period of time (the speed of which will be a taxon- and ecosystem-specific characteristic). The resolution of extinction debt may be an exponential process, with a half-life, so absolute extinctions are expected to decline as the debt is paid off. But disturbance is rarely ‘one off’ but rather is cumulative, so the extinction debt is continually being topped up.
The combination of (i) an intrinsic rate of accumulation of environmental insult over time, and (ii) an intrinsic rate of extinction debt resolution, when combined, may well lead to an approximately linear extinction curve. This is reasonable in large groups, such as world birds, where idiosyncratic fluctuations in the extinction rate due to particular events in time and space will average out at large scales. Mechanistically, it may seem more reasonable to plot the extinction rate as a relative rate (e.g. percentage of species going extinct per unit time), rather than absolute numbers of extinctions. However, where the extinction rate is low there will be little difference, and when plotted graphically (figure 2), the use of absolute number conveys important information that would be hidden as a percentage. However, percentages are an alternative, and may under certain circumstances provide greater linearity.
(b). The filter model
An alternative conceptual model suggests an extinction crunch (very high rate of extinction in the early years, in which sensitive species are filtered out) followed by the establishment of a slower but steady rate at least by 1800 (figure 2, inset line C). This would mean that we are dramatically underestimating the amount of DE. This is the ‘extinction filter’ hypothesis of Balmford [45], where the ‘filter’ is a rapid anthropogenic environmental change, which removes extinction-sensitive species, by analogy with a physical filter removing solid particles from liquid.
Balmford takes the observation of Greuter [46] that Mediterranean-type ecosystems that have been disturbed more recently (California, Western Australia) have a higher rate of plant extinction than the European/North African Mediterranean region which has been heavily disturbed by forest clearing and goat browsing since at least the Bronze Age. This suggests that the European Mediterranean has had a DE episode that has filtered out the extinction-prone species, so that the extinction rate has declined in recent times. However, this potentially overlooks the fact that the patterns of disturbance have been different in the three areas, in both swiftness and type. The European Mediterranean ecosystems co-evolved with those browsing animals (rabbits, goats and sheep) which were also a major source of anthropogenic disturbance. The lower rate of extinction in the European Mediterranean may thus be intrinsic, and it is therefore reasonable to suppose that even the prehistoric period had a similar rate to that seen today. For groups like birds, there is little evidence currently of a dramatic extinction crunch 1500–1800. Certainly, there were early extinctions during this period, especially on islands. But again, it is important to recognize that extinction takes time and rather than ‘instant extinction’, an extinction debt is produced by disturbance, which is gradually resolved (often over a period of hundreds of years) by subsequent extinctions [44].
(c). Slow-start extinction model
The third possibility is the ‘slow-start’, i.e. very little extinction in the early years after 1500 but followed by the gradual establishment of an approximately constant extinction rate by 1800 (figure 2, inset line A). An example of a slow-start model that omits consideration of DE is given in Fig. 1 of Ceballos et al. [47]. If this scenario was correct, it would mean that we are overestimating pre-taxonomic DE, which is actually minimal or non-existent, but this scenario seems unlikely. Much of the early extinction after 1500 was on oceanic islands, many of which were discovered in the 1500s. The evidence points to rapid ecological catastrophe following the introduction of rats, pigs and goats, which would have led to extinction debt and its steady and inexorable resolution by extinction from the beginning. Indeed, for groups that leave fossil evidence, such as birds, there are signs of significant early extinction. The endemic hoopoe and ground dove of St Helena have no historical record and must have been extinct before colonization in 1648. Introduced mammals, especially rodents and pigs (Sus scrofa), would doubtless be responsible for devastating predation on the nestlings of ground-nesting birds.
6. Avoiding dark extinction in the future
There has been much discussion on preventing extinction, and the extent to which our efforts can be successful. Our focus here is not on preventing extinction per se, but on ensuring that when extinction happens we at least know about it, i.e. on preventing DE. In some species-rich but poorly known groups, it seems inevitable that undescribed species will become extinct in the near future. One way of mitigating this would be to concentrate taxonomic efforts on vulnerable, endemic-rich sites such as islands, distinctive habitats such a cave-systems (arthropods), ultramafic or limestone mountains in the tropics (plants), or endemic-rich habitats threatened by mining, logging or other resource extraction. A particular focus should be on collecting in these areas, so even if these species cannot be described before their extinction, a physical record will at least exist.
There is a conundrum here. If undescribed species are in danger of extinction, do we refrain from collection (which might inadvertently cause extinction) in the hope (perhaps never to be realized) that by doing so the species will not become extinct? Or do we make sure to collect specimens so that a physical record remains after the (perhaps unavoidable) extinction? A case in point is the St Helena giant earwig (Labidura herculeana). An expedition in the 1960s [48,49] collected all the specimens they could find, a total of 40. They were the last people ever to see the species alive (although subfossil pincers occasionally turn up). It is not unreasonable to think that this rather zealous collecting may have accelerated the extinction of the species. On the other hand, the abundance of introduced predators such as mice and large centipedes (Scolopendra morsitans) may have meant that extinction was inevitable, and at least the collection effort has led to a fuller museum representation of ‘what once was’. A compromise would be to apply the well-known ‘rule of 20’ [50] for collecting from populations on the verge of extinction. In this, a population of under 20 individuals would not be collected from. For larger populations 1 in 20 could be collected, on the basis that a one-time 5% predation rate is survivable. The exceptions would be for large clonal organisms, such as trees or shrubs, for which a specimen is only a small part of the organism. A recent plea for the importance of museum specimens as the extinction crisis gathers pace states: ‘As many as half of the specimens likely represent populations that no longer exist, and an increasing number of species as well. What is in our collections will often turn out to be all that remains of organisms that once thrived’ [51, p. 149].
Finally, the issue needs to be addressed as to whether we need to know about, or have a specimen of, something that is extinct: the ‘what's gone is gone’ argument. However, accurate knowledge of past extinction can potentially inform conservation of other species. A case can be made that future generations should have a clear record of the consequences of current environmental destruction, and that means bringing DE to the light.
Acknowledgements
We thank William L. Harrower (Department of Forest and Conservation Sciences, University of British Columbia (UBC)) and Ailene MacPherson (Department of Zoology, UBC) for discussion. We also thank two anonymous reviewers for helpful comments. In particular, we wish to acknowledge Dr Ryan Chisholm (National University of Singapore) for constructive criticism of an early version of the manuscript and for generously sharing important insights on extinction.
Data accessibility
Data extracted from published sources used in the preparation of figures have been archived (with explanatory metadata) in Dryad under https://doi.org/10.5061/dryad.wstqjq2kb [52]. R code used in analyses is available at: https://github.com/mannfred/extinctions.
Authors' contributions
M.M.A.B. performed analyses, contributed ideas and contributed to writing the paper. Q.C.B.C. conceived the study, performed analyses and wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
We acknowledge the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants and Postgraduate Scholarships Programs for funding (grant no. RGPIN-2014-05820 to Q.C.B.C. and grant no. GC-2017-Q4-00199 to M.M.A.B.).
References
- 1.Lyell C. 1832. Changes caused by the progress of human population. In Principles of geology: being an attempt to explain the former changes of the Earth's surface, by reference to causes now in operation. London: John Murray. [Google Scholar]
- 2.Bartlett LJ, Williams DR, Prescott GW, Balmford A, Green RE, Eriksson A, Valdes PJ, Singarayer JS, Manica A. 2016. Robustness despite uncertainty: regional climate data reveal the dominant role of humans in explaining global extinctions of Late Quaternary megafauna. Ecography 39, 152-161. ( 10.1111/ecog.01566) [DOI] [Google Scholar]
- 3.Martin PS. 1984. Prehistoric overkill: the global model. In Quaternary extinctions: a prehistoric revolution (eds Martin PS, Klein RG), pp. 354-403. Tucson, AZ: University of Arizona Press. [Google Scholar]
- 4.Gillespie R. 2008. Updating Martin's global extinction model. Q. Sci. Rev. 27, 2522-2529. ( 10.1016/j.quascirev.2008.09.007) [DOI] [Google Scholar]
- 5.Brook BW, Bowman DMJS. 2002. Explaining the Pleistocene megafaunal extinctions: models, chronologies, and assumptions. Proc. Natl Acad. Sci. USA 99, 14 624-14 627. ( 10.1073/pnas.232126899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rule S, Brook BW, Haberle SG, Turney CSM, Kershaw AP, Johnson CN. 2002. The aftermath of megafaunal extinction: ecosystem transformation in Pleistocene Australia. Science 335, 1483-1486. ( 10.1126/science.1214261) [DOI] [PubMed] [Google Scholar]
- 7.Steadman DW. 1995. Prehistoric extinctions of Pacific island birds – biodiversity meets zooarchaeology. Science 267, 1123-1131. ( 10.1126/science.267.5201.1123) [DOI] [PubMed] [Google Scholar]
- 8.Fisher DO, Blomberg SP. 2011. Correlates of rediscovery and the detectability of extinction in mammals. Proc. R. Soc. B 278, 1090-1097. ( 10.1098/rspb.2010.1579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nowak-Kemp M, Hume JP. 2017. The Oxford dodo. Part 1: the museum history of the Tradescant dodo: ownership, displays and audience. Hist. Biol. 29, 234-247. ( 10.1080/08912963.2016.1152471) [DOI] [Google Scholar]
- 10.Hume JP. 2006. The history of the dodo Raphus cucullatus and the penguin of Mauritius. Hist. Biol. 18, 69-93. ( 10.1080/08912960600639400) [DOI] [Google Scholar]
- 11.Shapiro B, Sibthorpe D, Rambaut A, Austin J, Wragg GM, Bininda-Emonds OR, Lee PL, Cooper A. 2002. Flight of the dodo. Science 295, 1683. ( 10.1126/science.295.5560.1683) [DOI] [PubMed] [Google Scholar]
- 12.Bourne WRP, Ashmole NP, Simmons KEL. 2003. A new subfossil night heron and a new genus for the extinct rail from Ascension Island, central tropical Atlantic Ocean. Ardea 91, 45-51. [Google Scholar]
- 13.Olson SL. 1975. Paleornithology of St. Helena Island, South Atlantic Ocean. Smithson. Contrib. Paleobiol. 23, 1-49. [Google Scholar]
- 14.BirdLife. 2019. Handbook of the Birds of the World and BirdLife International digital checklist of the birds of the world (Version 4). See http://datazone.birdlife.org/species/taxonomy (accessed 26 March 2020).
- 15.Pimm S, Raven P, Peterson A, Sekercioglu CH, Ehrlich PR. 2006. Human impacts on the rates of recent, present, and future bird extinctions. Proc. Natl Acad. Sci. USA 103, 10 941-10 946. ( 10.1073/pnas.0604181103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawksworth DL, Cowie RH. 2013. The discovery of historically extinct, but hitherto undescribed, species: an under-appreciated element in extinction-rate assessments. Biodivers. Conserv. 22, 2429-2432. ( 10.1007/s10531-013-0542-0) [DOI] [Google Scholar]
- 17.Richling I, Bouchet P. 2013. Extinct even before scientific recognition: a remarkable radiation of helicinid snails (Helicinidae) on the Gambier Islands, French Polynesia. Biodivers. Conserv. 22, 2433-2468. ( 10.1007/s10531-013-0496-2) [DOI] [Google Scholar]
- 18.Stork NE. 2018. How many species of insects and other terrestrial arthropods are there on Earth? Annu. Rev. Entomol. 63, 31-45. ( 10.1146/annurev-ento-020117-043348) [DOI] [PubMed] [Google Scholar]
- 19.Costello MJ, May RM, Stork NE. 2013. Can we name Earth's species before they go extinct? Science 339, 413-416. ( 10.1126/science.1230318) [DOI] [PubMed] [Google Scholar]
- 20.Pimm SL, Russell GJ, Gittleman JL, Brooks TM. 1995. The future of biodiversity. Science 269, 347-350. ( 10.1126/science.269.5222.347) [DOI] [PubMed] [Google Scholar]
- 21.Pimm SL, Jenkins CN, Abell R, Brooks TM, Gittleman JL, Joppa LN, Raven PH, Roberts CM, Sexton JO. 2014. The biodiversity of species and their rates of extinction, distribution, and protection. Science 344, 1246752. ( 10.1126/science.1246752) [DOI] [PubMed] [Google Scholar]
- 22.Tedesco PA, Bigorne R, Bogan AE, Giam X, Jezequel C, Hugueny B. 2014. Estimating how many undescribed species have gone extinct. Conserv. Biol. 28, 1360-1370. ( 10.1111/cobi.12285) [DOI] [PubMed] [Google Scholar]
- 23.Ceballos G, Ehrlich PR. 2009. Discoveries of new mammal species and their implications for conservation and ecosystem services. Proc. Natl Acad. Sci. USA 106, 3841-3846. ( 10.1073/pnas.0812419106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mora C, Tittensor DP, Adl S, Simpson AGB, Worm B. 2011. How many species are there on Earth and in the ocean? PLoS Biol. 9, e1001127. ( 10.1371/journal.pbio.1001127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chisholm RA, Giam XL, Sadanandan KR, Fung T, Rheindt FE. 2016. A robust nonparametric method for quantifying undetected extinctions. Conserv. Biol. 30, 610-617. ( 10.1111/cobi.12640) [DOI] [PubMed] [Google Scholar]
- 26.Kristensen NP, Seah WW, Chong KY, Yeoh YS, Fung T, Berman LM, Tan HZ, Chisholm RA. 2020. Extinction rate of discovered and undiscovered plants in Singapore. Conserv. Biol. 34, 1229-1240. ( 10.1111/cobi.13499) [DOI] [PubMed] [Google Scholar]
- 27.Theng M, Jusoh WFA, Jain A, Huertas B, Tan DJX, Tan HZ, Kristensen NP, Meier R, Chisholm RA. 2020. A comprehensive assessment of diversity loss in a well-documented tropical insect fauna: almost half of Singapore's butterfly species extirpated in 160 years. Biol. Conserv. 242, 108401. ( 10.1016/j.biocon.2019.108401) [DOI] [Google Scholar]
- 28.Lum D, Tedesco PA, Hugueny B, Giam XL, Chisholm RA. In press. Quantifying the relative performance of two undetected-extinction models. Conserv. Biol. ( 10.1111/cobi.13562) [DOI] [PubMed] [Google Scholar]
- 29.Saitoh T, et al. 2015. DNA barcoding reveals 24 distinct lineages as cryptic bird species candidates in and around the Japanese Archipelago. Mol. Ecol. Resour. 15, 177-186. ( 10.1111/1755-0998.12282) [DOI] [PubMed] [Google Scholar]
- 30.Shakya SB, Lim HC, Moyle RG, Rahman MA, Lakim M, Sheldon FH. 2019. A cryptic new species of bulbul from Borneo. Bull. Br. Ornithol. Club 139, 46-55. ( 10.25226/bboc.v139i1.2019.a3) [DOI] [Google Scholar]
- 31.Laplace PS. 1786. Sur les naissances, les mariages et les morts [On births, marriages and deaths]. Mém. Acad. R. Sci. Paris 1783, 693-702. [In French.] [Google Scholar]
- 32.Kuusela V. 2012. Laplace – a pioneer of statistical inference. J. Électron. Hist. Probab. Stat. 8, 1-24. [Google Scholar]
- 33.Szabo JK, Khwaja N, Garnett ST, Butchart SHM. 2012. Global patterns and drivers of avian extinctions at the species and subspecies level. PLoS ONE 7, e47080. ( 10.1371/journal.pone.0047080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butchart SHM, Lowe S, Martin RW, Symes A, Westrip JRS, Wheatley H. 2018. Which bird species have gone extinct? A novel quantitative classification approach. Biol. Conserv. 227, 9-18. ( 10.1016/j.biocon.2018.08.014) [DOI] [Google Scholar]
- 35.Monroe MJ, Butchart SHM, Mooers AO, Bokma F. 2019. The dynamics underlying avian extinction trajectories forecast a wave of extinctions. Biol. Lett. 15, 20190633. ( 10.1098/rsbl.2019.0633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.IUCN. 2020. The IUCN Red List of Threatened Species (Version 2020-1). See https://www.iucnredlist.org (accessed 26 March 2020).
- 37.Sayol F, Steinbauer MJ, Blackburn TM, Antonelli A, Faurby S. 2020. Anthropogenic extinctions conceal widespread evolution of flightlessness in birds. Sci. Adv. 6, eabb6095. ( 10.1126/sciadv.abb6095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.R-project.org. [Google Scholar]
- 39.Kristensen NP. 2020. SEUX for R (R package version 0.1.0). See https://github.com/nadiahpk/seux (accessed 1 December 2020).
- 40.Lambdon P, Cronk QC. 2020. Extinction dynamics under extreme conservation threat: the flora of St Helena. Front. Ecol. Evol. 8, 41. ( 10.3389/fevo.2020.00041) [DOI] [Google Scholar]
- 41.Van Valen L. 1973. A new evolutionary law. Evol. Theory 1, 1-30. [Google Scholar]
- 42.Raup DM. 1975. Taxonomic survivorship curves and Van Valen's Law. Paleobiology 1, 82-96. [Google Scholar]
- 43.Tilman D, May RM, Lehman CL, Nowak MA. 1994. Habitat destruction and the extinction debt. Nature 371, 65-66. ( 10.1038/371065a0) [DOI] [Google Scholar]
- 44.Halley JM, Monokrousos N, Mazaris AD, Newmark WD, Vokou D. 2016. Dynamics of extinction debt across five taxonomic groups. Nat. Commun. 7, 12283. ( 10.1038/ncomms12283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balmford A. 1996. Extinction filters and current resilience: the significance of past selection pressures for conservation biology. Trends Ecol. Evol. 11, 193-196. ( 10.1016/0169-5347(96)10026-4) [DOI] [PubMed] [Google Scholar]
- 46.Greuter W. 1994. Extinctions in Mediterranean areas. Phil. Trans. R. Soc. Lond. B 344, 41-46. ( 10.1098/rstb.1994.0049) [DOI] [Google Scholar]
- 47.Ceballos G, Ehrlich PR, Barnosky AD, García A, Pringle RM, Palmer TM. 2015. Accelerated modern human-induced species losses: entering the sixth mass extinction. Sci. Adv. 1, e1400253. ( 10.1126/sciadv.1400253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brindle A. 1970. La faune terrestre de l'île de Sainte-Hélène (première partie). 8.–Dermaptera [The terrestrial fauna of St Helena (part one). 8.–Dermaptera]. Ann. Mus. R. Afr. Cent. Tervuren Ser. 8 Sci. Zool. 181, 211-227. [In French.] [Google Scholar]
- 49.Basilewsky P. 1985. The South Atlantic island of St Helena and the origin of its beetle fauna. In Taxonomy, phylogeny, and zoogeography of beetles and ants: a volume dedicated to the memory of Philip Jackson Darlington, Jr. (1904–1983) (ed. Ball GE), pp. 257-275. Dordrecht: Dr W. Junk. [Google Scholar]
- 50.Wagner D. 1991. The ‘1-in-20 rule’ for plant collectors. Plant Sci. Bull. 37, 11. [Google Scholar]
- 51.Raven PH, Miller SE. 2020. Here today, gone tomorrow. Science 370, 149. ( 10.1126/science.abf1185) [DOI] [PubMed] [Google Scholar]
- 52.Boehm MMA, Cronk QCB. 2021. Data from: Dark extinction: the problem of unknown historical extinctions. Dryad Digital Repository. ( 10.5061/dryad.wstqjq2kb) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Boehm MMA, Cronk QCB. 2021. Data from: Dark extinction: the problem of unknown historical extinctions. Dryad Digital Repository. ( 10.5061/dryad.wstqjq2kb) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data extracted from published sources used in the preparation of figures have been archived (with explanatory metadata) in Dryad under https://doi.org/10.5061/dryad.wstqjq2kb [52]. R code used in analyses is available at: https://github.com/mannfred/extinctions.