Abstract
The role of telomerase reverse transcriptase has been widely investigated in the contexts of ageing and age-related diseases. Interestingly, decreased telomerase activities (and accelerated telomere shortening) have also been reported in patients with emotion-related disorders, opening the possibility for subjective appraisal of stressful stimuli playing a key role in stress-driven telomere shortening. In fact, patients showing a pessimistic judgement bias have shorter telomeres. However, in humans the evidence for this is correlational and the causal directionality between pessimism and telomere shortening has not been established experimentally yet. We have developed and validated a judgement bias experimental paradigm to measure subjective evaluations of ambiguous stimuli in zebrafish. This behavioural assay allows classification of individuals in an optimistic–pessimistic dimension (i.e. from individuals that consistently evaluate ambiguous stimuli as negative to others that perceive them as positive). Using this behavioural paradigm we found that telomerase-deficient zebrafish (tert−/−) were more pessimistic in response to ambiguous stimuli than wild-type zebrafish. The fact that individuals with constitutive shorter telomeres have pessimistic behaviours demonstrates for the first time in a vertebrate model a genetic basis of judgement bias.
Keywords: judgement bias, pessimistic, telomere shortening, telomerase reverse transcriptase, zebrafish
1. Introduction
Telomeres are complexes of repetitive DNA sequences and proteins that together act as caps at chromosome ends protecting them from deterioration. Telomere shortening during mitosis is prevented by telomerase, an enzyme that adds 6 bp DNA repeat sequences [(TTAGGG)n] to telomeres. In humans, telomerase expression is restricted in somatic cells so that telomeres shorten during lifespan [1]. Telomere shortening is also accelerated by chronic psychological stress and may serve as an indicator for stress-related disease susceptibility (e.g. [2]). The subjective appraisal of stressful stimuli has been suggested to play a key role in stress-driven telomere shortening, since pessimistic individuals have shorter telomeres [3]. However, the available evidence in humans is correlational and the causal inference of pessimism driving the shortening of telomeres has not been established experimentally yet. On the other hand, decreased telomerase activities (and accelerated telomere shortening) have been also reported in patients with emotion-related disorders [4,5], which opens the possibility for a bidirectional link between pessimism and telomere attrition.
Judgement biases of ambiguous stimuli also occur in animals, with some individuals consistently evaluating them as negative (a.k.a. pessimists) and others as positive (a.k.a. optimists) [6]. Judgement bias has been conceptualized as a decision-making process that is modulated by the affective state of the individual, such that a negative emotional state is predicted to induce a pessimistic assessment of ambiguous stimuli [7,8]. Therefore, judgement bias has been seen mainly as a phenotypic state influenced by the current affective state of the individual, which depends to a great extent on the environment to which the individual is exposed, rather than a constitutive phenotypic trait, while research on the genetic component of judgement bias is scarce (e.g. [9,10]). In this respect, the availability of mutant lines for the telomerase reverse transcriptase (TERT) [11], which is the core catalytic protein component of telomerase, opens the possibility of testing the hypothesis that pessimism may be causally driven by telomere attrition (i.e. TERT mutants with shorter telomeres have constitutive pessimistic bias). In fact, a recent study in mice has reported that specific-valenced emotions, such as depressive-like states, are deeply influenced by the action of TERT [11]. Considering the emotional modulation of judgement biases, these intriguing findings on TERT open the possibility for telomerase-deficient individuals exhibiting also alterations in the judgement biases that produce subjective evaluations. We have tested this hypothesis by using a tert−/− mutant in zebrafish (Danio rerio), which offers an ideal model to unravel this question since: (i) its stress axis is well characterized and is highly conserved as compared with that of mammals [12]; (ii) pharmacological validation of standard behavioural tests of anxiety-like behaviour have been reported in this species [13]; and (iii) it is a short-lived fish species with human-like telomere sizes that, like in humans, requires telomerase for a normal lifespan and tissue homeostasis [14].
2. Material and methods
(a). Fish and housing
Two independent experiments were carried out in this study: Experiment 1 aimed to validate a go/no-go judgement bias task in zebrafish, and Experiment 2 assessed the effect of telomerase deficiency on judgement bias. Fish used for Experiment 1 were four-months-old male wild-type (WT; Tübingen strain) zebrafish (D. rerio) (n = 14 fish). In Experiment 2, age-matched individuals of the telomerase mutant line tertAB/hu3430 (see electronic supplementary material for further details of this mutant line) and of the WT line were used to assess the role of telomerase in the modulation of judgement biases at two different ages (four or nine months old; n = 10–12 male fish per Genotype (WT or tert−/−) and Age). A between-individuals design with respect to age was used (i.e. fish from different breeding sources were tested at four and nine months old). All fish were bred and held at Instituto Gulbenkian de Ciência (IGC, Oeiras, Portugal) (see electronic supplementary material for more details on housing procedures). After behavioural testing, fish were kept in their housing tank to be reused in future experiments since procedures described here did not cause significant impairment of the wellbeing or general condition of the animals. All procedures were performed in accordance with Institutional and National regulations and guidelines, reviewed by the Ethics Committee of the Instituto Gulbenkian de Ciência, and approved by the competent Portuguese authority (Direcção Geral de Alimentação e Veterinária).
(b). Experimental procedures
(i). Experiment 1: validation of the judgement bias test
In this study, we have developed and validated a judgement bias test for zebrafish, which has been designed as a go/no-go task, based on the judgement bias experimental paradigm published by Harding et al. [6]. The behavioural apparatus consisted of a half arm radial maze (figure 1a) with guillotine doors linking the starting box with each arm. The two reference arms (P and N) were positioned 180° from each other. Coloured cards (green or red) were associated with each of these arms. The three ambiguous arms (NP, A and NN) were positioned at equidistant angles between the two reference arms and associated with mixed coloured cards (colour proportions of 3 : 1, 1 : 1 and 1 : 3, respectively). After a habituation period to the apparatus, fish were trained in the two reference arms to perform one response (positive (P)) when one cue was presented (specific location/colour cue) in order to experience a positive event (food reward; see electronic supplementary material for further details). Furthermore, fish were also trained to perform a different response (negative (N)) when presented with a different cue in order to avoid a negative event (chasing with net; see electronic supplementary material for further details). For technical reasons, the responses used in this study were lower (P) or higher (N) latencies to enter the experimental arms of the behavioural apparatus. Once fish were able to discriminate between P and N arms (as indicated by different latencies to enter each one), their responses to ambiguous arms between P and N (NP, A and NN) were tested (i.e. ambiguous probe trials). This type of experimental paradigm has been used in a wide range of species to assess judgement bias (e.g. [15–22]) (see electronic supplementary material for the detailed protocol of judgement bias assay for zebrafish).
Figure 1.
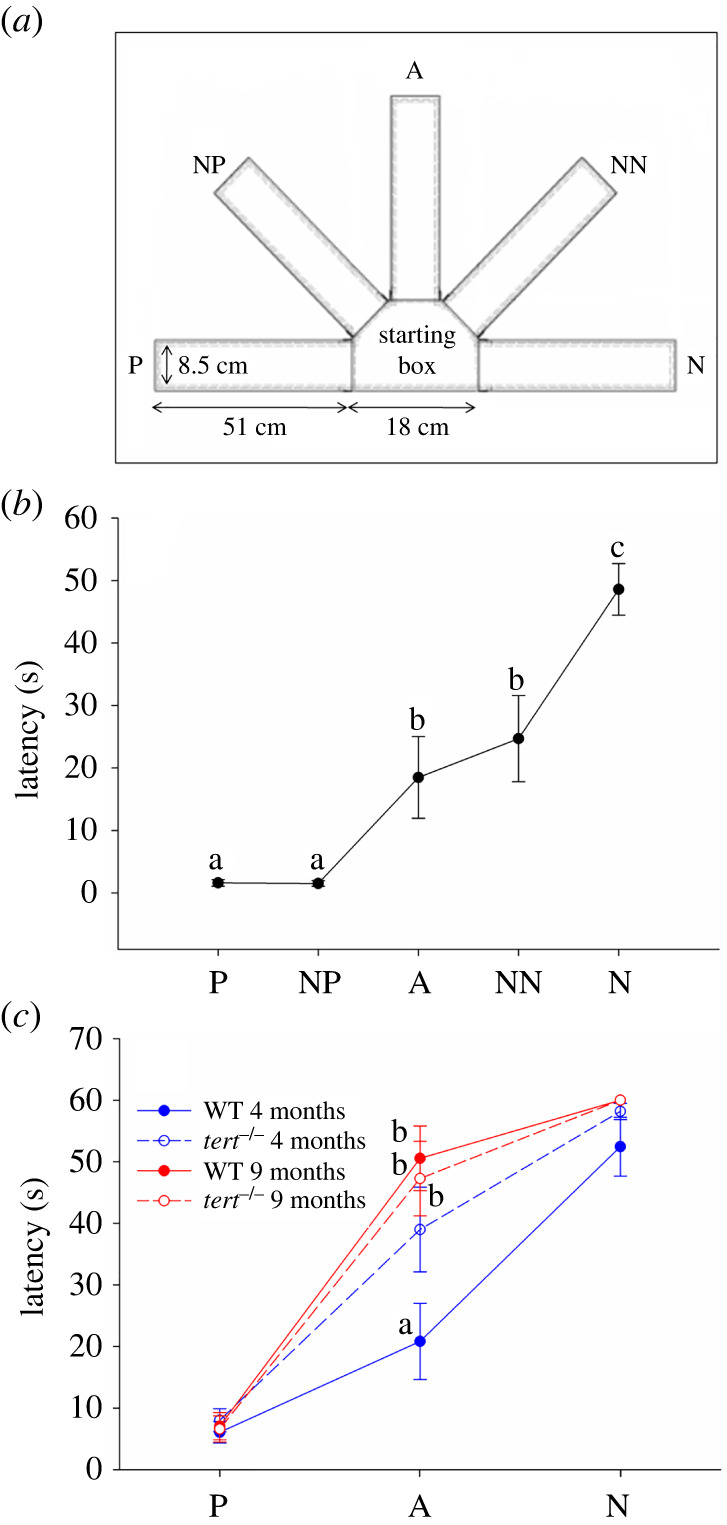
Judgement bias in zebrafish. (a) Diagram of the experimental setup showing the two reference locations (i.e. positive/rewarded (P) and negative/aversive (N)) and the three ambiguous locations (i.e. near-positive (NP), ambiguous (A) and near-negative (NN)). Each location is associated with a specific colour cue. The test consists in training the fish to discriminate between the P and the N location/colour cue. Once fish are able to discriminate between them (as indicated by different latencies in entering into each one), their responses to ambiguous locations/colour cues between the positive and the negative are tested. (b) Mean latencies during the test phase (Experiment 1) on trials performed for the P and N training locations/colour cues, and for the three ambiguous locations/colour cues (NP, A and NN; n = 14 male fish); different letters indicate significant differences between groups following post hoc multiple comparisons tests. (c) Performance of tert−/− mutants and WT siblings (Experiment 2) at different ages (n = 10–12 male fish per Genotype (WT or tert−/−) and Age (four or nine months old)) in the judgement bias paradigm. Different letters indicate significant differences between genotype and age groups for each Treatment (P, A, N) following planned comparisons tests. Data are expressed as mean ± s.e.m.
(ii). Experiment 2: effect of telomerase deficiency on judgement bias
The experimental procedure validated in Experiment 1 was used in Experiment 2 to assess the effect of telomerase deficiency on judgement bias. Since an accurate discrimination performance between stimuli (presumably by a generalization response) was demonstrated in Experiment 1, a shorter test phase omitting NP and NN cue testing was implemented in Experiment 2 (see electronic supplementary material for further details). A shorter test phase and, consequently, a lower number of training trials in this phase could have a number of advantages in terms of minimizing potential events affecting the categorization of the ambiguous cue. For instance, a higher number of positive outcomes (i.e. food rewards) may lead to a decrease in appetite, which could affect the performance of optimistic behaviours independently of the affective state. Appetite impact on judgement bias tasks has been already reported [23,24]. On the other hand, a higher confounding influence of stress could be achieved by increasing the number of negative outcomes (i.e. punishments) and/or the overall duration of the test phase. The effects of stress on task learning in judgement bias tests have also been previously reported [25,26].
(c). Behavioural observations
The latency to enter the target arm (60 s maximum) was recorded for each trial. Video recordings were analysed using a multi-event recorder software (Observer XT, Noldus Technology, v. 9). Behavioural data were analysed by one researcher and scored blindly to Genotype, Age and Treatment.
(d). Statistical analyses
For the analyses of both experiments—validation of the judgement bias paradigm (Experiment 1) and the judgement bias test in the tert−/− mutants (Experiment 2)—we used the R software [27] packages ‘afex' [28] and ‘lme4’ [29] for the linear mixed-effects models (GLMMs), and the ‘emmeans' package [30] for planned comparisons. Details of R codes used and the original datasets can be found online [31]. The response variables were the latencies to respond to stimuli, that is, the time it took the fish to enter the experimental arms (positive (P), near-positive (NP), ambiguous (A), near-negative (NN), negative (N) in Experiment 1, and P, A and N in Experiment 2) of the behavioural apparatus. Latencies were restricted to the interval between 0 and 60 s and were log-transformed. In the model of Experiment 1, the fixed effect was Treatment, with five groups (P, NP, A, NN, N). In the model of Experiment 2, the fixed effects were Treatment (with three groups: P, N, A) in interaction with Age (with two groups: four and nine months of age) and Genotype (with two groups: wild-type and tert−/−). In both models, the random effect was the individual fish, since the same individuals were tested in all treatments within each experiment and for each age group (but different sets of individuals were used for the two age groups). Inspection of model residuals from both experiments showed satisfactory normal distributions. All p-values are two-tailed except when indicated otherwise (i.e. when an a priori directional hypothesis is provided).
3. Results
(a). Experiment 1: validation of the judgement bias test in zebrafish
WT zebrafish showed significant differences in the latency for each Treatment (P, NP, A, NN, N) (figure 1b; GLMM: F4,52 = 27.626, p < 0.001), reflecting a generalization response with fish showing a lower latency to enter the P arm and progressively increasing the latency as the colour cue/location neared N. This stimuli generalization suggests that individuals are categorizing the different cues as predicting the event associated with each one, and hence displaying appropriate responses.
(b). Experiment 2: effect of telomerase deficiency in judgement bias
tert−/− zebrafish mutants were tested for judgement bias and compared with age-matched WT fish for two different ages (four or nine months old; figure 1c). Statistical analysis of Experiment 2 showed that only Treatment had a significant main effect (table 1), reflecting the generalization response described in Experiment 1. There was also an interaction effect between Genotype and Age (table 1), with tert−/− mutants displaying a more pessimistic bias than WT at younger age, and such differences disappearing in older fish (figure 1c). There was also an interaction between Age and Treatment (table 1), with older WT showing more pessimistic bias (figure 1c). It is important to note that both WT and tert−/− mutants showed similar latencies to enter the P and N reference arms (figure 1c), indicating that the observed differences in the response towards the A arm result from judgement biases rather than from altered sensorimotor abilities, which may also be compromised in tert−/− mutants.
Table 1.
Results of the general linear mixed model to assess the effects of Treatment (positive versus negative versus ambiguous), Genotype (wild-type versus tert mutant), Age (four months old versus nine months old), and the double and triple interactions among these variables. *Indicates a significant effect.
| main effects and interactions | F-value | p (>F) |
|---|---|---|
| Genotype | F1,40 = 2.14 | p = 0.15 |
| Age | F1,40 = 3.22 | p = 0.08 |
| Treatment | F2,80 = 169.04 | p < 0.001* |
| Genotype × Age | F1,40 = 4.35 | p < 0.05* |
| Genotype × Treatment | F2,80 = 1.48 | p = 0.23 |
| Age × Treatment | F2,80 = 7.63 | p < 0.001* |
| Genotype × Age × Treatment | F2,80 = 2.10 | p = 0.13 |
Since other judgement bias studies have used a judgement bias score in their analyses (e.g. [7,32]), we also ran such an analysis in parallel, which yielded similar results (see electronic supplementary material for further details; electronic supplementary material, figure S1A). Based on the judgement bias score, animals can be classified into optimistic and pessimistic. In line with the GLMM analysis presented above, the proportion of pessimistic individuals was significantly higher in tert−/− mutants than in WT fish at four months old (0.60, n = 10 versus 0.25, n = 12; z = −1.66, one-tailed p = 0.049, electronic supplementary material, figure S1B), and in WT at nine months than in WT at four months old (0.818, n = 11 versus 0.25, n = 12; z = −2.72, one-tailed p < 0.01; electronic supplementary material, figure S1B).
4. Discussion
In this study, we have measured optimism/pessimism for individual zebrafish by using a judgement bias task that has been designed to measure expectations of positive (reward) and negative (punishment) outcomes when fish are exposed to ambiguous stimuli intermediate between two stimuli previously associated with reward and punishment. Before discussing the results obtained from the judgement bias paradigm, a number of issues related to our behavioural task need to be considered. Cues commonly used in judgement bias paradigms are spatial (e.g. [7]), visual (e.g. [19]), olfactory (e.g. [17]) or auditory (e.g. [20]). However, we have used a combination of different stimulus classes (i.e. spatial and visual), which has been successfully operated in bumblebees [15]. The use of more than one class of stimulus may facilitate the acquisition of the available information related to the task, and hence improve discrimination learning. This fact could be critical to overcome the difficulties showed by zebrafish in categorizing more than one stimulus simultaneously and, consequently, in successfully performing judgement bias paradigms [33]. On the other hand, our results suggest the occurrence of a basic psychological mechanism, namely stimulus generalization, which has been proven to play an important role in responses to ambiguous stimuli in judgement bias paradigms (e.g. [15–19]). The occurrence of this mechanism is indicative of an accurate discrimination between a stimulus (or set of stimuli) that predicts a positive consequence and a stimulus (or set of stimuli) that predicts a negative one.
The majority of the studies conducted to date on judgement bias have focused on the effects of manipulations that are expected to induce a negative affective state (e.g. [34,35]). In fact, Baciadonna & McElligott [36] suggest that judgement bias tasks are highly sensitive to manipulations that produce negative emotions. Here, we show that at four months of age zebrafish tert−/− mutants display more pessimistic-like behaviours in response to ambiguous cues as compared with WT zebrafish. Considering the above-mentioned studies, it can be hypothesized that the pessimistic-like judgement bias displayed by telomerase-deficient zebrafish could be indicative of a negative affective state associated with tert silencing. The terthu3430 homozygous mutant strain in zebrafish (tert−/−) has been shown to have shorter telomeres than WT siblings as a consequence of the absence of telomerase [14]. These mutant zebrafish develop degenerative phenotypes from four to six months onwards and die prematurely. The development of such early phenotypic alterations (e.g. increased inflammation), which are common in aged organisms, may be responsible for the altered judgement bias performances of the telomerase-deficient mutants. In fact, a correlational link between pessimism and inflammation has already been reported in humans [37]. Similarly, the increased pessimism in older WT fish is also paralleled by an ageing related shortening of telomeres that WT fish experience. In fact, Henriques et al. [14] also found that telomeres decrease in length over time during the first year of life of tert+/+ zebrafish, which is accompanied by deterioration of physical state. However, at nine months of age tert−/− mutants and WT fish still differ in their telomere lengths, and hence the most plausible explanation for the lack of a difference in pessimism at older age is the existence of a threshold in terms of tissue homeostasis, above which the judgement bias phenotype is similarly affected. Together these results suggest that a decrease in the physical state and/or in lifespan signalled by telomerase is associated with a pessimistic judgement bias. This result is in line with the current theories that link life-history strategy to affective states in animals and humans, according to which the adaptive function of mood (and their dependent judgement bias) is to integrate information about the recent state of the environment and of the physical condition of the organism in order to optimize behavioural decision-making [38]. Accordingly, individuals that are physically compromised are less able to cope with undetected threats if they should arise, and should behave more cautiously towards ambiguous stimuli [38]. Similarly, individuals with short lifespans are expected to follow a fast life-history strategy, which has been associated with depression syndromes in humans [39,40]. Together, this evidence supports our initial hypothesis, that tert mutants with shorter telomeres have constitutive pessimistic bias, which may be interpreted in the scope of adaptive life-history theory.
Acknowledgements
The authors thank the Fish Facility Platform of the Instituto Gulbenkian de Ciência (Portugal) for animal care.
Ethics
All procedures were performed in accordance with Institutional and National regulations and guidelines, reviewed by the Ethics Committee of the Instituto Gulbenkian de Ciência, and approved by the competent Portuguese authority (Direcção Geral de Alimentação e Veterinária; permit no. 0421/000/000/2013).
Data accessibility
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.rv15dv46m [31].
Authors' contributions
F.E., M.G.F. and R.F.O. designed the experiment; F.E. and D.A.-T. ran the experiment; S.A.M.V. analysed the data; M.G.F. provided the test line; F.E. and R.F.O. wrote the first draft of the paper; all authors revised the manuscript, approved the final version of the manuscript, and agree to be held accountable for the content therein.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the BIAL Foundation through grant no. 130/12 to R.F.O. F.E. was supported by a Marie Skłodowska-Curie Actions—Individual Fellowship (H2020-MSCA-IF/703285) under the Horizon 2020 Framework Programme (H2020).
References
- 1.Blackburn EH. 2001. Switching and signaling at the telomere. Cell 106, 661-673. ( 10.1016/s0092-8674(01)00492-5) [DOI] [PubMed] [Google Scholar]
- 2.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. 2004. Accelerated telomere shortening in response to life stress. Proc. Natl Acad. Sci. USA 101, 17 312-17 315. ( 10.1073/pnas.0407162101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Donovan A, Lin J, Dhabhar FS, Wolkowitz O, Tillie JM, Blackburn E, Epel E. 2009. Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in post-menopausal women. Brain Behav. Immun. 23, 446-449. ( 10.1016/j.bbi.2008.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kao HT, et al. 2008. Rapid telomere erosion in schizophrenia. Mol. Psychiatry 13, 118-119. ( 10.1038/sj.mp.4002105) [DOI] [PubMed] [Google Scholar]
- 5.Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, Nierenberg AA, Fava M, Wong KK. 2006. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol. Psychiatry 60, 432-435. ( 10.1016/j.biopsych.2006.02.004) [DOI] [PubMed] [Google Scholar]
- 6.Harding EJ, Paul ES, Mendl M. 2004. Animal behaviour: cognitive bias and affective state. Nature 427, 312. ( 10.1038/427312a) [DOI] [PubMed] [Google Scholar]
- 7.Mendl M, Brooks J, Basse C, Burman O, Paul E, Blackwell E, Casey R. 2010. Dogs showing separation-related behaviour exhibit a ‘pessimistic' cognitive bias. Curr. Biol. 20, R839-R840. ( 10.1016/j.cub.2010.08.030) [DOI] [PubMed] [Google Scholar]
- 8.Enkel T, Gholizadeh D, und Halbach OVB, Sanchis-Segura C, Hurlemann R, Spanagel R, Gass P, Vollmayr B. 2010. Ambiguous-cue interpretation is biased under stress- and depression-like states in rats. Neuropsychopharmacology 35, 1008-1015. ( 10.1038/npp.2009.204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorato E, Zidar J, Garnham L, Wilson A, Løvlie H. 2018. Heritabilities and co-variation among cognitive traits in red junglefowl. Phil. Trans. R. Soc. B 373, 20170285. ( 10.1098/rstb.2017.0285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gott A, Andrews C, Bedford T, Nettle D, Bateson M. 2019. Developmental history and stress responsiveness are related to response inhibition, but not judgement bias, in a cohort of European starlings (Sturnus vulgaris). Anim. Cogn. 22, 99-111. ( 10.1007/s10071-018-1226-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou QG, et al. 2016. Reactivation of Tert in the medial prefrontal cortex and hippocampus rescues aggression and depression of Tert−/− mice. Transl. Psychiatry 6, e836. ( 10.1038/tp.2016.106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alsop D, Vijayan M. 2009. The zebrafish stress axis: molecular fallout from the teleost-specific genome duplication event. Gen. Comp. Endocrinol. 161, 62-66. ( 10.1016/j.ygcen.2008.09.011) [DOI] [PubMed] [Google Scholar]
- 13.Cachat J, et al. 2010. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat. Protocols 5, 1786-1799. ( 10.1038/nprot.2010.140) [DOI] [PubMed] [Google Scholar]
- 14.Henriques CM, Carneiro MC, Tenente IM, Jacinto A, Ferreira MG. 2013. Telomerase is required for zebrafish life span. PLoS Genet. 9, e1003214. ( 10.1371/journal.pgen.1003214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perry CJ, Baciadonna L, Chittka L. 2016. Unexpected rewards induce dopamine-dependent positive emotion-like state changes in bumblebees. Science 353, 1529-1531. ( 10.1126/science.aaf4454) [DOI] [PubMed] [Google Scholar]
- 16.Laubu C, Louâpre P, Dechaume-Moncharmont F-X. 2019. Pair-bonding influences affective state in a monogamous fish species. Proc. R. Soc. B 286, 20190760. ( 10.1098/rspb.2019.0760) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bateson M, Desire S, Gartside SE, Wright GA. 2011. Agitated honeybees exhibit pessimistic cognitive biases. Curr. Biol. 21, 1070-1073. ( 10.1016/j.cub.2011.05.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rygula R, Papciak J, Popik P. 2013. Trait pessimism predicts vulnerability to stress-induced anhedonia in rats. Neuropsychopharmacology 38, 2188-2196. ( 10.1038/npp.2013.116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salmeto AL, Hymel KA, Carpenter EC, Brilot BO, Bateson M, Sufka KJ. 2011. Cognitive bias in the chick anxiety–depression model. Brain Res. 1373, 124-130. ( 10.1016/j.brainres.2010.12.007) [DOI] [PubMed] [Google Scholar]
- 20.Murphy E, Nordquist RE, van der Staay FJ. 2013. Responses of conventional pigs and Göttingen miniature pigs in an active choice judgement bias task. Appl. Anim. Behav. Sci. 148, 64-76. ( 10.1016/j.applanim.2013.07.011) [DOI] [Google Scholar]
- 21.Matheson SM, Asher L, Bateson M. 2008. Larger, enriched cages are associated with ‘optimistic' response biases in captive European starlings (Sturnus vulgaris). Appl. Anim. Behav. Sci. 109, 374-383. ( 10.1016/j.applanim.2007.03.007) [DOI] [Google Scholar]
- 22.Doyle RE, Lee C, Deiss V, Fisher AD, Hinch GN, Boissy A. 2011. Measuring judgement bias and emotional reactivity in sheep following long-term exposure to unpredictable and aversive events. Physiol. Behav. 102, 503-510. ( 10.1016/j.physbeh.2011.01.001) [DOI] [PubMed] [Google Scholar]
- 23.Anderson MH, Munafò MR, Robinson ESJ. 2013. Investigating the psychopharmacology of cognitive affective bias in rats using an affective tone discrimination task. Psychopharmacology (Berl.) 226, 601-613. ( 10.1007/s00213-012-2932-5) [DOI] [PubMed] [Google Scholar]
- 24.Rygula R, Szczech E, Papciak J, Nikiforuk A, Popik P. 2014. The effects of cocaine and mazindol on the cognitive judgement bias of rats in the ambiguous-cue interpretation paradigm. Behav. Brain Res. 270, 206-212. ( 10.1016/j.bbr.2014.05.026) [DOI] [PubMed] [Google Scholar]
- 25.Mendl M, Burman OHP, Parker RMA, Paul ES. 2009. Cognitive bias as an indicator of animal emotion and welfare: emerging evidence and underlying mechanisms. Appl. Anim. Behav. Sci. 118, 161-181. ( 10.1016/j.applanim.2009.02.023) [DOI] [Google Scholar]
- 26.Conrad CD. 2010. A critical review of chronic stress effects on spatial learning and memory. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 742-755. ( 10.1016/j.pnpbp.2009.11.003) [DOI] [PubMed] [Google Scholar]
- 27.R Core Team. 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.R-project.org/. [Google Scholar]
- 28.Singmann H, Bolker B, Westfall J, Aust F, Ben-Shachar MS. 2020. afex: Analysis of factorial experiments. R package version 0.27-2. See https://CRAN.R-project.org/package=afex.
- 29.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1-48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 30.Lenth R. 2020. emmeans: Estimated marginal means, aka least-squares means. R package version 1.4.7. See https://CRAN.R-project.org/package=emmeans.
- 31.Espigares F, Abad-Tortosa D, Varela SAM, Ferreira MG, Oliveira RF. 2021. Data from: Short telomeres drive pessimistic judgment bias in zebrafish. Dryad Digital Repository. ( 10.5061/dryad.rv15dv46m) [DOI] [PMC free article] [PubMed]
- 32.Kis A, Hernádi A, Kanizsár O, Gácsi M, Topál J. 2015. Oxytocin induces positive expectations about ambivalent stimuli (cognitive bias) in dogs. Horm. Behav. 69, 1-7. ( 10.1016/j.yhbeh.2014.12.004) [DOI] [PubMed] [Google Scholar]
- 33.Tan SLT. 2017. Cognitive bias as an indicator of emotional state and welfare in captive zebrafish. PhD thesis, School of Biosciences, University of Melbourne. [Google Scholar]
- 34.Bethell EJ, Koyama NF. 2015. Happy hamsters? Enrichment induces positive judgement bias for mildly (but not truly) ambiguous cues to reward and punishment in Mesocricetus auratus. R. Soc. Open Sci. 2, 140399. ( 10.1098/rsos.140399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neave HW, Daros RR, Costa JHC, von Keyserlingk MAG, Weary DM. 2013. Pain and pessimism: dairy calves exhibit negative judgement bias following hot-iron disbudding. PLoS ONE 8, e80556. ( 10.1371/journal.pone.0080556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baciadonna L, McElligott AG. 2015. The use of judgement bias to assess welfare in farm livestock. Anim. Welfare 24, 81-91. ( 10.7120/09627286.24.1.081) [DOI] [Google Scholar]
- 37.Roy B, Diez-Roux AV, Seeman T, Ranjit N, Shea S, Cushman M. 2010. The association of optimism and pessimism with inflammation and hemostasis in the Multi-Ethnic Study of Atherosclerosis (MESA). Psychosom. Med. 72, 134-140. ( 10.1097/PSY.0b013e3181cb981b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nettle D, Bateson M. 2012. The evolutionary origins of mood and its disorders. Curr. Biol. 22, R712-R721. ( 10.1016/j.cub.2012.06.020.) [DOI] [PubMed] [Google Scholar]
- 39.Han W, Chen B-B. 2020. An evolutionary life history approach to understanding mental health. Gen. Psychiatry 33, e100113. ( 10.1136/gpsych-2019-100113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Del Giudice M. 2014. An evolutionary life history framework for psychopathology. Psychol. Inq. 25, 261-300. ( 10.1080/1047840X.2014.884918) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Espigares F, Abad-Tortosa D, Varela SAM, Ferreira MG, Oliveira RF. 2021. Data from: Short telomeres drive pessimistic judgment bias in zebrafish. Dryad Digital Repository. ( 10.5061/dryad.rv15dv46m) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.rv15dv46m [31].


