Abstract
Anthropogenic environmental change affects organisms by exposing them to enhanced sensory stimuli that can elicit novel behavioural responses. A pervasive feature of the built environment is artificial nocturnal lighting, and brightly lit urban areas can influence organism abundance, distribution and community structure within proximate landscapes. In some cases, the attractive or disorienting effect of artificial light at night can draw animals into highly unfavourable habitats, acting as a macroscale attractive ecological sink. Despite their significance for animal ecology, identifying cases of these phenomena and determining their effective scales and the number of organisms impacted remains challenging. Using an integrated set of remote-sensing observations, we quantify the effect of a large-scale attractive sink on nocturnal flights of an outbreak insect population in Las Vegas, USA. At the peak of the outbreak, over 45 million grasshoppers took flight across the region, with the greatest numbers concentrating over high-intensity city lighting. Patterns of dusk ascent from vegetated habitat toward urban areas suggest a daily pull toward a time-varying nocturnal attractive sink. The strength of this attractor varies with grasshopper density. These observations provide the first macroscale characterization of the effects of nocturnal urban lighting on the behaviour of regional insect populations and demonstrate the link between insect perception of the built environment and resulting changes in spatial and movement ecology. As human-induced environmental change continues to affect insect populations, understanding the impacts of nocturnal light on insect behaviour and fitness will be vital to developing robust large-scale management and conservation strategies.
Keywords: artificial lights at night, aeroecology, global change, radar entomology, Orthoptera, grasshopper
1. Introduction
Anthropogenic activity is dramatically altering landscapes across the globe and exposing organisms to novel environments. Strong stimuli in human-modified environments can prompt enhanced behavioural responses, resulting in ecological sinks where low-quality habitat attracts large numbers of individuals due to a mismatch between common behaviours and modified environments [1]. Levels of artificial light at night (ALAN) are rapidly increasing at rates up to 20% per year [2], causing widespread disruptions in animal physiology, behaviour and fitness [3–6]. Increases in artificial lights are an understudied form of anthropogenic change [7], the pace of which exceeds that of many organisms' capacity to adapt [8]. In particular, many taxa rely heavily on light stimuli as cues in orientation and navigation [9] and artificial lighting in the built environment can result in phototaxis, drawing organisms into potentially unfavourable habitats [10]. While understanding of sensory effects of ALAN on organisms has improved (see [4], for a review), many studies focus on localized point light sources (i.e. less than 1 km; [11]). By contrast, it is largely unknown what effects this disruption has at regional scales where ALAN manifests as skyglow. The spatio-temporal scale, number of individuals affected, and potential population effects of this disruption remain largely unknown.
During the summer of 2019, an outbreak population of grasshoppers (predominantly Orthoptera: Trimerotropis pallidipennis—the pallid-winged grasshopper; figure 1a) descended on Las Vegas, USA, and remarkable images of massive swarms surrounding the iconic illuminated cityscape flooded global news coverage (e.g. [12], figure 1b–d). Las Vegas is unrivaled as a night-time light source, with more radiance per unit area than any other city in the United States (56.2 nW cm−2 sr−1; [13]), and speculation surrounding the role of city lights in swarm aggregation became a common topic in reporting. Grasshopper outbreaks are generally studied through experimental plots and monitoring at small scales, with limited ability to quantify and describe movement phenomena at the magnitude of outbreak events such as the one in Las Vegas.
Figure 1.
Nocturnal urban lighting as a macroscale sensory stimulus for an outbreak population of grasshoppers. (a) The pallid-winged grasshopper (Trimerotropis pallidipennis). (b) An aerial view of the Las Vegas strip, illustrating an extreme case of large-scale artificial light at night. (c) Mass attraction of grasshoppers to the high-intensity light column of the Luxor Hotel and Casino on the Las Vegas strip. (d) An example of the widespread nocturnal grasshopper aggregations that were characteristic of this outbreak event. (e) A snapshot of the regional airspace taken by the Las Vegas weather surveillance radar (red circle) at the peak of the outbreak event on 27 July, 2019 07:07 UTC (00:07 local time), detecting approximately 45 million grasshoppers aloft. Downtown Las Vegas is denoted by a white circle. (f) Landscape-scale variability in vegetation index at the time of the grasshopper outbreak. (g) Landscape-scale variability in nocturnal radiance at the time of the grasshopper outbreak. (h–l) Dusk ascent and within-night movement dynamics of grasshopper aggregations across southern Nevada on the night of 25 July, 2019. (h–j) The abundance and distribution of grasshoppers aloft with respect to downtown Las Vegas (white circle) measured by the Las Vegas weather surveillance radar (red circle) at 03:35, 05:35 and 07:38 UTC. Colour maps in (h–j) are the same as (e) and weather signals are denoted by greyscale. (k) Time series of within-night variability in grasshopper abundance across the full radar surveillance domain (black). (l) Time series of within-night variability in Pearson correlation coefficient between the spatial distribution of grasshoppers and vegetation index (green) and nocturnal light intensity (blue) throughout the night. The yellow dotted lines indicate the times of local sunset and sunrise and filled circles correspond with the times shown in (h–j).
The remote-sensing capability of weather radar enables real-time monitoring of animal movement events at regional scales and provides quantitative information on abundance as well as the spatial flow of individuals and biomass. This tool has expanded our ability to track bird migration and aerial insect movements [13–17] and can provide unique system-level insight into large-scale dynamics of migration systems [18,19]. In particular, radar observations have yielded some of the first insights into how free-flying animals respond to ALAN across the landscape [9,13,20,21]. While the effects of light on avian wildlife at the landscape scale are becoming better understood, the macroscale response of high-flying insects is still unknown. Although making direct observations of insects aloft is challenging, rich literature has demonstrated the utility of radar in observing flights of locusts [16,17,22]. Adapting these techniques, we use weather surveillance radar to examine the spatial dynamics of a swarm of grasshoppers during a region-wide outbreak centred on the highly illuminated Las Vegas strip.
Using 75 days of remote-sensing observations, we quantify the abundance and biomass of aerial grasshopper activity and explore the spatio-temporal distribution of flight activity in comparison to artificially lit environments and the vegetated landscape during the outbreak (electronic supplementary material, Methods). Mass movements of Trimerotropis pallidipennis frequently occur at night [23], when artificial light may constitute a nocturnal attractive cue, but this cue is unlikely to persist during the day, when signals related to resource quality may drive grasshopper behaviour on the ground. Diurnal ground-feeding behaviour of this species is difficult to track at large scales, but dusk ascent and aerial movement after take-off is visible on radar. We expect nocturnal patterns in grasshopper aerial aggregation to correspond with landscape patterns of artificial light while aggregation patterns during dusk ascent likely reflect the diurnal distribution and the presence of vegetation. We expect that the behaviour of conspecifics may affect grasshopper behaviour, resulting in density-dependent patterns of flight. We investigate the relationship between these fluctuating landscape cues and grasshopper activity aloft, revealing an unprecedented view of the collective behavioural responses of an insect population to their surrounding anthropogenic environment. Moreover, we provide the first estimates of the scale of biomass transport into a macroscale attractive sink, demonstrating the potential of nocturnal city lighting to act as a time-varying attractive sink.
2. Results
(a). Nocturnal flight patterns
Urban areas with high light levels attracted grasshoppers in distinct daily patterns throughout the 2.5-month analysis period, consisting of dusk ascent from vegetated habitat and nocturnal flight toward lit urban areas (figure 1; electronic supplementary material, movie S1; figure S1 and S2). Prior to dusk, aerial grasshopper abundance was low and weakly spatially correlated with the presence of vegetation (mean 02–04 UTC correlation coefficient: 0.0903; electronic supplementary material, Methods). Shortly after sunset aerial abundance increased as grasshoppers ascended (figure 1h; figure 1k,l, timepoint H). Grasshoppers continued to fill the airspace, attaining maximum flight altitudes around 2 km above ground level. At this time, the spatial correlation between grasshopper aerial abundance and artificial light increased (mean 07–08 UTC correlation coefficient: 0.3648), suggesting convergence of grasshoppers in illuminated urban areas (figure 1i; figure 1k,l, timepoint I). Concurrently, the spatial correlation with vegetation decreased (mean 07–08 UTC correlation coefficient: 0.004). Shortly before midnight, grasshopper aerial abundance peaked (figure 1j; figure 1k,l, timepoint J). This daily pattern of ascent in vegetated areas and subsequent peak correlations above nocturnally illuminated areas was apparent across much of the study period (electronic supplementary material, figures S1 and S2). Overall, we found that the spatial pattern in ALAN across the landscape strongly influenced the distribution of aerial grasshopper activity throughout the night, whereas the locations of initial dusk ascent showed grasshopper distribution prior to sunset more strongly correlated with vegetation greenness.
(b). Density-dependent patterns
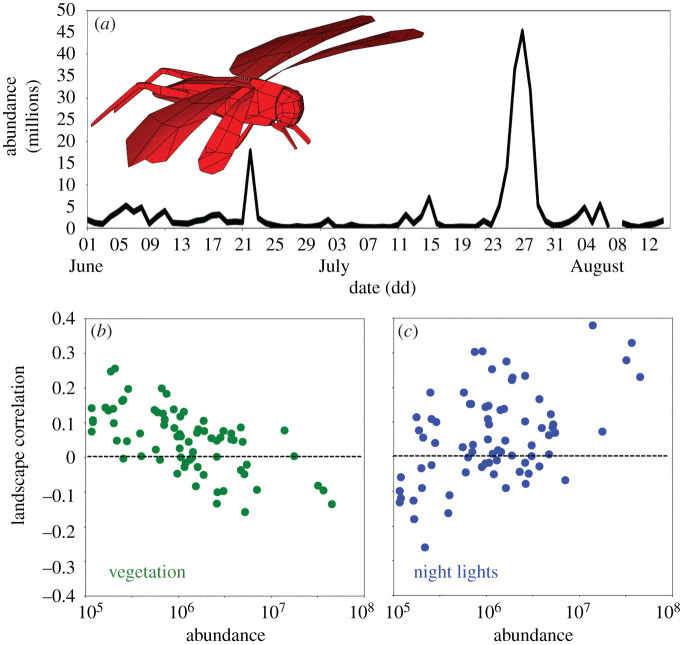
Grasshopper density in the air over Las Vegas built up throughout June and July and peaked on 27 July at an estimated 45.8 million grasshoppers (figure 2a). At this peak, approximately 30.2 metric tons of insect biomass was in motion over the region, concentrating over the lit metro area. The relationship between grasshopper aerial distribution and the underlying landscape varied with grasshopper abundance. With increasing aerial activity, the correlation between the spatial distribution of grasshoppers and vegetation greenness decreased (regression, adj. R2 = 0.19, p < 0.001, figure 2b). By contrast, the correlation between ALAN and the spatial distribution of grasshoppers strengthened with overall grasshopper abundance (regression, adj. R2 = 0.15, p < 0.001, figure 2c), suggesting that grasshopper density influences the behavioural response to landscape cues.
Figure 2.
Variability in abundance of grasshoppers across the 75-day analysis period and resulting impacts on landscape associations. (a) Across-night variability in mean grasshopper abundance over the full radar surveillance domain (black) from 1 June to 14 August. ((a), inset) The electromagnetic scattering model of a pallid-winged grasshopper. (b) Nightly mean Pearson correlation coefficient between the spatial distribution of grasshoppers and vegetation index as a function of grasshopper abundance for the analysis period. (c) Nightly mean Pearson correlation coefficient between the spatial distribution of grasshoppers and nocturnal light intensity as a function of grasshopper abundance for the analysis period. Nightly means are calculated from 1 h before sunset to 1 h after sunrise.
3. Discussion
We document for the first time that anthropogenic light acts as a macroscale attractive sink for nocturnal insects. Positive phototaxis in grasshoppers has not previously been documented in the literature on artificial lights at night [4], and we find that nocturnal swarming increases grasshopper density in highly lit areas. While the effects of skyglow extend far beyond the local environment [24,25], previous ALAN research on insects focused on effects in close proximity to point sources (e.g. [26–28]). Uniquely, our results demonstrate behavioural impacts of anthropogenic environments on large numbers of insects at the macroscale.
The strength of using weather surveillance radar to analyse animal movements lies in its ability to follow large numbers of individuals across significant distances. However, our inference is restricted to aerial behaviour, and we cannot confirm grasshopper behaviour on the ground. Nonetheless, the recurring nature of dusk departure flights from vegetated areas towards artificial light suggests that either each day's aerial movement involves new individuals that land and persist in urban areas, or alternatively that the artificial light cue is absent during the day, and the grasshoppers gradually redistribute across the landscape. In the latter case, the characteristic pattern that we describe in the nightly distributions of grasshoppers in the air would be generated by a diurnal back-and-forth movement, with nocturnal swarming towards anthropogenic light and gradual diurnal redistribution to areas of vegetative productivity. While the latter scenario may be more likely, without following individual grasshoppers, we cannot distinguish between the two.
Grasshoppers exhibited a density-dependent pattern with regard to nocturnal light and landscape greenness. One possible hypothesis for the positive density-dependent response to nocturnal lighting is that the behavioural response of conspecifics strengthens the response to nocturnal light cues, creating a positive feedback between nocturnal lighting and the number of grasshoppers attracted to those well-lit areas. Alternatively, increased aggregation towards ALAN at high densities may be related to local atmospheric conditions [10]. Grasshopper take-off generally decreases at high wind speeds [16], and wind, temperature or humidity may also affect their tendency to accumulate in flight near Las Vegas. Overall, our described patterns of density-dependent landscape associations motivate further research evaluating the interaction between grasshopper behaviour, weather and landscape to parse the underlying mechanisms.
Our results extend our understanding of the ways in which artificial light at night increasingly affect biodiversity and ecosystem function [7,29]. ALAN can prompt animals to alter their flight behaviour and navigate towards highly lit urbanized areas that do not contain sufficient quality habitat on the ground [9,13,20]. Invertebrates drawn to bright environments at night may be ‘trapped’ in the lit area and thereby unable to forage [30], mate [31] or disperse into suitable habitat [32,33]. For example, long-term abundances of phototactic moths in the UK show greater declines in environments with higher light pollution levels [34], and ALAN may play an underappreciated role in recent widespread declines in insect abundances [35,36]. These studies suggest that spatial redistribution in response to anthropogenic environmental cues can have negative effects on fitness.
Our results demonstrate impacts of human-induced environmental change on insect populations, illustrating the need for further research on artificial light at night effects on insect behaviour and fitness. Moreover, this foundational knowledge will illuminate new large-scale drivers of insect spatial ecology, revealing insights into the macroscale flows of organisms, biomass and nutrients across our shared landscape.
Acknowledgements
We thank Don Reynolds, Ellen Welti, Katharine Marske, Charlotte Wainwright and Melissa Sadir for comments on an early draft of the manuscript.
Data accessibility
All data used in this study are freely available at the following URLs: Radar https://s3.amazonaws.com/noaa-nexrad-level2/index.html; EVI https://modis.gsfc.nasa.gov/data/dataprod/mod13.php; ALAN https://eogdata.mines.edu/nighttime_light/v10/201907/vcmcfg/ (ALAN previously available at https://ngdc.noaa.gov/eog/viirs/download_dnb_composites.html).
Authors' contributions
Conceptualization was undertaken by A.M.D., A.I.S., B.A.C., E.K.T., J.F.K., P.M.C. and P.M.S; methodology and data acquisition were undertaken by D.M., E.K.T. and P.M.S.; analysis and interpretation were undertaken by A.M.D., A.I.S., B.A.C., D.M., E.K.T., J.F.K., P.M.C., P.M.S. and M.Z.; visualization was undertaken by P.M.S.; writing was undertaken by E.K.T., J.F.K., P.M.C. and P.M.S.; review and editing were undertaken by A.M.D., A.I.S., B.A.C., D.M., E.K.T., J.F.K., P.M.C., P.M.S. and M.Z.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by NSF–Division of Emerging Frontiers grant no. 1840230.
References
- 1.Delibes M, Gaona P, Ferreras P. 2001. Effects of an attractive sink leading into maladaptive habitat selection. Am. Nat. 158, 277-285. ( 10.1086/321319) [DOI] [PubMed] [Google Scholar]
- 2.Holker F, et al. 2010. The dark side of light: a transdisciplinary research agenda for light pollution policy. Ecol. Soc. 15, 13. ( 10.5751/ES-03685-150413) [DOI] [Google Scholar]
- 3.Tinkham ER. 1938. Western Orthoptera attracted to lights. J. N.Y. Entom. Sci. 46, 339-353. [Google Scholar]
- 4.Owens ACS, Lewis SM. 2018. The impact of artificial light at night on nocturnal insects: a review and synthesis. Ecol. Evol. 8, 11 337-11 358. ( 10.1002/ece3.4557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desouhant E, Gomes E, Mondy N, Amat I. 2019. Mechanistic, ecological, and evolutionary consequences of artificial light at night for insects: review and prospective. Entom. Ext. et Applic. 167, 37-58. ( 10.1111/eea.12754) [DOI] [Google Scholar]
- 6.Boyes DH, et al. 2020. Is light pollution driving moth population declines? A review of causal mechanisms across the life cycle. Insect Conserv. Div. 14, 167-187. [Google Scholar]
- 7.Davies TW, Smyth T. 2018. Why artificial light at night should be a focus for global change research in the 21st century. Glob. Chang. Biol. 24, 872-882. ( 10.1111/gcb.13927) [DOI] [PubMed] [Google Scholar]
- 8.Swaddle JP, et al. 2015. A framework to assess evolutionary responses to anthropogenic light and sound. Trends Ecol. Evol. 30, 550-560. ( 10.1016/j.tree.2015.06.009) [DOI] [PubMed] [Google Scholar]
- 9.Verheijen FJ. 1960. The mechanisms of the trapping effect of artificial light sources upon animals. Arch. Néerlandaises Zool. 13, 1-107. ( 10.1163/036551660X00017) [DOI] [Google Scholar]
- 10.Weishampel ZA, Cheng WH, Weishampel JF. 2016. Sea turtle nesting patterns in Florida vis-a-vis satellite-derived measures of artificial lighting. Remote Sens. Ecol. Conserv. 2, 59-72. [Google Scholar]
- 11.Bennie J, Davies TW, Cruse D, Inger R, Gaston KJ. 2015. Cascading effects of artificial light at night: resource-mediated control of herbivores in a grassland ecosystem. Phil. Trans. R. Soc. B 370, 20140131. ( 10.1098/rstb.2014.0131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vigdor N. 2019. Grasshopper invasion of Las Vegas may last weeks, experts say. The New York Times 27 July 2019, p. 21. (see https://www.nytimes.com/2019/07/27/us/grasshoppers-vegas.html)
- 13.Horton KG, Nilsson C, Van Doren BM, La Sorte FA, Dokter AM, Farnsworth A. 2019. Bright lights in the big cities: migratory birds' exposure to artificial light. Front. Ecol. Environ. 17, 209-214. ( 10.1002/fee.2029) [DOI] [Google Scholar]
- 14.Bridge ES, et al. 2011. Technology on the move: recent and forthcoming innovations for tracking migratory birds. Bioscience 61, 689-698. ( 10.1525/bio.2011.61.9.7) [DOI] [Google Scholar]
- 15.Stepanian PM, Entrekin SA, Wainwright CE, Mirkovic D, Tank JL, Kelly JF. 2020. Declines in an abundant aquatic insect, the burrowing mayfly, across major North American waterways. Proc. Natl Acad. Sci. USA 117, 2987-2992. ( 10.1073/pnas.1913598117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drake VA, Reynolds DR. 2012. Radar entomology: observing insect flight and migration. Wallingford, UK: CABI. [Google Scholar]
- 17.Rainey R. 1989. Migration and meteorology. Flight behaviour and the atmospheric environment of locusts and other migrant pests. New York, NY: Oxford University Press. [Google Scholar]
- 18.Kelly JF, Horton KG. 2016. Toward a predictive macrosystems framework for migration ecology. Glob. Ecol. Biogeogr. 25, 1159-1165. ( 10.1111/geb.12473) [DOI] [Google Scholar]
- 19.Bauer S, et al. 2019. The grand challenges of migration ecology that radar aeroecology can help answer. Ecography 42, 861-875. ( 10.1111/ecog.04083) [DOI] [Google Scholar]
- 20.Cabrera-Cruz SA, Smolinsky JA, Buler JJ. 2018. Light pollution is greatest within migration passage areas for nocturnally-migrating birds around the world. Sci. Rep. 8, 3261. ( 10.1038/s41598-018-21577-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cabrera-Cruz SA, Cohen EB, Smolinsky JA, Buler JJ. 2020. Artificial light at night is related to broad- scale stopover distributions of nocturnally migrating landbirds along the Yucatan Peninsula, Mexico. Remote. Sens. 12, 395. [Google Scholar]
- 22.Achtemeier GL. 1992. Grasshopper response to rapid vertical displacements within a ‘clear air’ boundary layer as observed by Doppler radar. Environ. Entom. 21, 921-938. ( 10.1093/ee/21.5.921) [DOI] [Google Scholar]
- 23.Otte D. 1984. The North American grasshoppers. Vol. II, Acrididae: Oedipodinae. Cambridge, MA: Harvard University Press. [Google Scholar]
- 24.Kyba C, Ruhtz T, Fischer J, Hölker F. 2011. Cloud coverage acts as an amplifier for ecological light pollution in urban ecosystems. PLoS ONE 6, e17307. ( 10.1371/journal.pone.0017307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies TW, Bennie J, Inger R, Gaston KJ. 2013. Artificial light alters natural regimes of night-time sky brightness. Sci. Rep. 3, 1722. ( 10.1038/srep01722) [DOI] [Google Scholar]
- 26.Macgregor C, Evans DM, Fox R, Pocock MJ. 2017. The dark side of street lighting: impacts on moths and evidence for the disruption of nocturnal pollen transport. Glob. Chang. Biol. 23, 697-707. ( 10.1111/gcb.13371) [DOI] [PubMed] [Google Scholar]
- 27.Manríquez P, et al. 2019. Artificial light pollution influences behavioral and physiological traits in a keystone predator species, Concholepas concholepas. Sci. Total. Environ. 661, 543-552. ( 10.1016/j.scitotenv.2019.01.157) [DOI] [PubMed] [Google Scholar]
- 28.Gaston KJ, Visser ME, Hölker F. 2015. The biological impacts of artificial light at night: the research challenge. Phil. Trans. R. Soc. B 370, 20140133. ( 10.1098/rstb.2014.0133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longcore T, Rich C. 2004. Ecological light pollution. Front. Ecol. Environ. 2, 191-198. ( 10.1890/1540-9295(2004)002[0191:ELP]2.0.CO;2) [DOI] [Google Scholar]
- 30.van Langevelde F, Van Grunsven RH, Veenendaal EM, Fijen TP. 2017. Artificial night lighting inhibits feeding in moths. Biol. Lett. 13, 20160874. ( 10.1098/rsbl.2016.0874) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Firebaugh A, Haynes KJ. 2016. Experimental tests of light-pollution impacts on nocturnal insect courtship and dispersal. Oecologia 182, 1203-1211. ( 10.1007/s00442-016-3723-1) [DOI] [PubMed] [Google Scholar]
- 32.Degen T, Mitesser O, Perkin EK, Weiß NS, Oehlert M, Mattig E, Hölker F. 2016. Street lighting: sex-independent impacts on moth movement. J. Anim. Ecol. 85, 1352-1360. ( 10.1111/1365-2656.12540) [DOI] [PubMed] [Google Scholar]
- 33.Manfrin A, Singer G, Larsen S, Weiß N, van Grunsven RH, Weiß NS, Wohlfahrt S, Monaghan MT, Hölker F. 2017. Artificial light at night affects organism flux across ecosystem boundaries and drives community structure in the recipient ecosystem. Front. Environ. Sci. 5, 61. [Google Scholar]
- 34.Wilson J, et al. 2018. A role for artificial night-time lighting in long-term changes in populations of 100 widespread macro-moths in UK and Ireland: a citizen-science study. J. Insect Conserv. 22, 189-196. ( 10.1007/s10841-018-0052-1) [DOI] [Google Scholar]
- 35.Grubisic M, van Grunsven RH, Kyba CC, Manfrin A, Hölker F. 2018. Insect declines and agroecosystems: does light pollution matter? Ann. Appl. Biol. 173, 180-189. ( 10.1111/aab.12440) [DOI] [Google Scholar]
- 36.Owens ACS, Cochard P, Durrant J, Farnworth B, Perkin EK, Seymoure B. 2020. Light pollution is a driver of insect declines. Biol. Conserv. 241, 108259. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this study are freely available at the following URLs: Radar https://s3.amazonaws.com/noaa-nexrad-level2/index.html; EVI https://modis.gsfc.nasa.gov/data/dataprod/mod13.php; ALAN https://eogdata.mines.edu/nighttime_light/v10/201907/vcmcfg/ (ALAN previously available at https://ngdc.noaa.gov/eog/viirs/download_dnb_composites.html).