Abstract
Background
Severe fever with thrombocytopenia syndrome (SFTS) was listed as one of the most severe infectious disease by world health organization in 2017. It can mostly be transmitted by tick bite, while human-to-human transmission has occurred on multiple occasions. This study aimed to explore the epidemiological and clinical characteristics and make risk analysis of SFTS human-to-human transmission.
Methods
Descriptive and spatial methods were employed to illustrate the epidemiological and clinical characteristics of SFTS human-to-human transmission. The risk of SFTS human-to-human transmission was accessed through secondary attack rate (SAR) and basic reproductive number (R0). Logistic regression analysis was used to identify the associated risk factors.
Results
A total of 27 clusters of SFTS human-to-human transmission were reported in China and South Korea during 1996–2019. It mainly occurred among elder people in May, June and October in central and eastern China. The secondary cases developed milder clinical manifestation and better outcome than the index cases. The incubation period was 10.0 days (IQR:8.0–12.0), SAR was 1.72%-55.00%, and the average R0 to be 0.13 (95%CI:0.11–0.16). Being blood relatives of the index case, direct blood/bloody secretion contact and bloody droplet contact had more risk of infection (OR = 6.35(95%CI:3.26–12.37), 38.01 (95%CI,19.73–73.23), 2.27 (95%CI,1.01–5.19)).
Conclusions
SFTS human-to-human transmission in China and South Korea during 1996–2019 had obvious spatio-temporal distinction. Ongoing assessment of this transmission risk is crucial for public health authorities though it continues to be low now.
Author summary
This study is first to explore the epidemiological and clinical characteristics of SFTS human-to-human transmission. We found SFTS human-to-human transmission had obvious characteristic temporal and spatial distinction, which mainly occurred among elder people in May, June and October in the central and eastern China. The secondary cases developed milder clinical manifestation and better outcome than the index cases. The incubation period was 10.0 days (IQR:8.0–12.0). The risk of SFTS human-to-human transmission continues to be low, with the secondary attack rate (SAR) among humans was 1.72%-55.00% and basic reproductive number (R0) to be 0.13 (95%CI:0.11–0.16). Being blood relatives of the index case, direct blood/bloody secretion contact and bloody droplet contact had more risk of acquiring infection.
Introduction
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging infectious disease, which was listed as one of the most severe infectious disease by World Health Organization in 2017 [1]. It was first detected among humans in Henan Province in December 2009 [2]. As of October 2019, 7419 SFTS cases were identified in China, with 355 deaths, who mainly occurred in central and eastern China [3]. SFTS has also reported in South Korea, Japan and Vietnam [4–6]. It characterized by sudden onset of fever, respiratory and gastrointestinal symptoms, followed by the progressive thrombocytopenia and leukocytopenia. A dynamic study was conducted on SFTS cases in Shandong Province reveals 81.35% (48/59) SFTS cases recovered within 2 weeks, and others died due to multiple organ failure (MOF) [7].
SFTS is caused by severe fever with thrombocytopenia syndrome bunyavirus (SFTSV), which can mostly be transmitted by ticks bite (Haemaphysalis longicornis and Rhipicephalus microplus) [8]. Meanwhile, the secondary cases can be infected through human-to-human transmission of direct contact with blood or bloody secretions bearing SFTSV and probably inhalation of virus-containing droplet and aerosol [9–11]. To date, SFTS human-to-human transmission has been separately reported in South Korea and several provincesin China [9–48]. Nonetheless, no study has depicted overall epidemiological and clinical characteristics of this transmission.
SFTS human-to-human transmission has occurred on multiple occasions. Particularly, the SFTS cases of these transmission events represented a small fraction of the total, and sustained SFTS human-to-human transmission has not been documented. Thus far, no quantitative analysis of SFTS human-to-human transmission risk has been reported. Previous studies have showed that, some blood relatives of SFTS cases and medical personnel were infected among all the contacts, but some were not. Previous studies have assessed the risk factors of SFTS human-to-human transmission and drew inconsistent conclusions, which may be related to the different methods, standards of contact and the small sample size. For example, Gai et.al [10] classified the 63 contacts into 3 types of contact (blood, droplet and possible airborne) and showed that blood contact was the most likely risk factors using multivariate logistic regression analysis. Tang et.al [14] classified the 31 contacts into 4 types of contact (blood, respiratory secretion, urine and feces) and showed that contact with the index patient’s blood on mucous membranes or skin wounds and not wearing personal protective equipment while providing care were significantly associated with disease risk using the χ 2 test.
This study first summarized the overall epidemiological and clinical characteristics of SFTS human-to-human transmission in the first and the second part of results section, respectively. Meanwhile, this study assessed the risk of SFTS human-to-human transmission through the secondary attack rate (SAR) and the basic reproductive number (R0), and identified associated risk factors in the third part.
Methods
Case definition
According to the national guideline for prevention and control of SFTS issued by the Chinese Ministry of Health, suspected SFTS case is defined as the person who presents with acute onset of fever (≥38°C) and other symptoms (e.g., gastrointestinal symptoms, bleeding), epidemiological exposure factors (e.g., being exposed to ticks or similar cases about 2 weeks before illness onset) and laboratory data showing thrombocytopenia or leukopenia. Laboratory-confirmed SFTS case is defined as suspected case who meets one or more of the following criteria: (1) detection of SFTSV RNA, (2) seroconversion or a 4-fold increase of anti-SFTSV immunoglobulin G (IgG) titers between acute and convalescent phase sera, and (3) isolation of SFTSV [49].
In this study, the cluster of SFTS human-to-human transmission was defined as one or more laboratory-confirmed SFTS cases among contacts of suspected/ laboratory-confirmed SFTS cases within 15 days. The index case was identified as the first case in the onset of an epidemiological investigation, who acquired SFTSV infection from an exposure to ticks. The secondary case was identified as the person who got infection from an exposure to the suspected/ laboratory-confirmed SFTS cases, among them, second-generation of secondary case was infected by the index case, and third-generation of secondary case was infected by the second-generation of secondary case.
Data collection
The databases including PubMed, Web of Science, China National Knowledge Infrastructure (CNKI) and Wanfang Data, were systematically searched for publications about the clusters of SFTS human-to-human transmission. The search was restricted to publications published from January 2010 to December 2019, using a combination of search strings consisting of terms with the exclusion of animal studies (S1 Text).
Publications were selected through four steps, involving duplication, the screening of the title and abstract, the screening of the full text, and further screening during the data extraction phase. The first step excluded duplicate publications. The second step excluded publications which were not related to SFTS human-to-human transmission through reviewing their title and abstract. The third step excluded publications which merely mentioned the number of clusters without details through reviewing their full text. We also checked the references of these publications for relevant publications possibly missed in the search. Additionally, we added 4 original cluster reports in Jiangsu Province. The fourth step combined the results of the publications which reported the same cluster, then excluded publications with important missing information(e.g. the demographic information and the date of onset of all SFTS cases in that cluster) (Fig 1).
Fig 1. Flow chart of search and selection procedure.
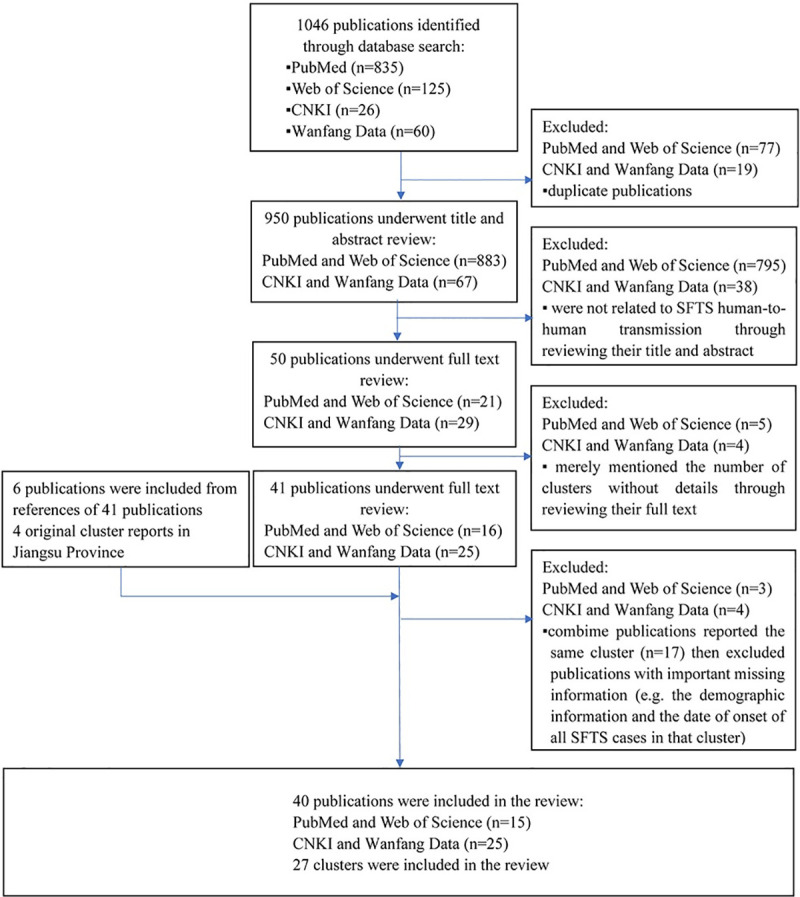
Note: n is the number of items in each category.
The search yielded 1046 publications from 4 databases. A total of 40 publications considered eligible, of which 15 were English publications and 25 were Chinese publications. Finally, 27 clusters of SFTS human-to-human transmission were included in this study (Fig 1).
Data analysis
Descriptive epidemiological methods and Geographic Information System were employed to illustrate the epidemiological and clinical characteristics of SFTS human-to-human transmission. To determine the difference between the index and secondary cases, Pearson χ2 test, Independent Sample T test and Wilcoxon W test were used to compare qualitative variables, normal and abnormal distribution continuous variables, respectively. They test a null hypothesis stating that two sets of identical variables were from the same population.
Subsequently, the risk of SFTS human-to-human transmission was assessed through SAR (i.e. the proportion of individual contacts in whom SFTS developed) and average R0 (i.e. the number of people that a SFTS case would infect in a completely susceptible population). The R0 of each cluster was first obtained [50], then Kolmogorov-Smirnov test was used to estimate R0 distribution, as its overall distribution was unknown, and the average R0 and confidence interval were obtained after adjustment for exposed population. If R0 <1, transmission chains are not self-sustaining and are unable to generate an epidemic. By contrast, an epidemic is likely to occur whenever R0 >1. The mathematical formula was specified as follows:
Finally, the univariate logistic regression was used to identify the risk factors of SFTS human-to-human transmission, which further incorporated into the multivariate logistic regression for quantitative analysis of adjustment. All-subsets regression was used to fit the optimum model with maximum adjusted R2 value. The risks were expressed as odds ratios (OR) and their 95% confidence intervals (CI) were calculated. Wald test was used for test assumptions of parameters, whose assumption was the value of parameter equal to 0. We included the relationship with the index case (medical personnel and blood relatives) and different types of contact as the possible risk factors. All contacts without complete personal protection equipment were classified into 4 types of contact (direct blood/bloody secretion contact, bloody droplet contact, airborne contact and urine/feces/sweat contact). Bloody droplet contact means involving in intubation and/or caring for the index case during bleeding period with close proximity. Airborne contact means staying in a confined space where aerosol may exist for a period of time, i.e., staying in the ward during bleeding period of the index case and (or) funeral room with bleeding corpse.
The methods above were performed in R (version 3.5.1) (S2 Text). The average R0 and confidence interval was calculated in Stata (version 12.0). Statistical significance was defined as P< 0.05. ArcGIS (version 10.4.1) was used for spatial visualization.
Results
Epidemiological characteristics
Demographic characteristics
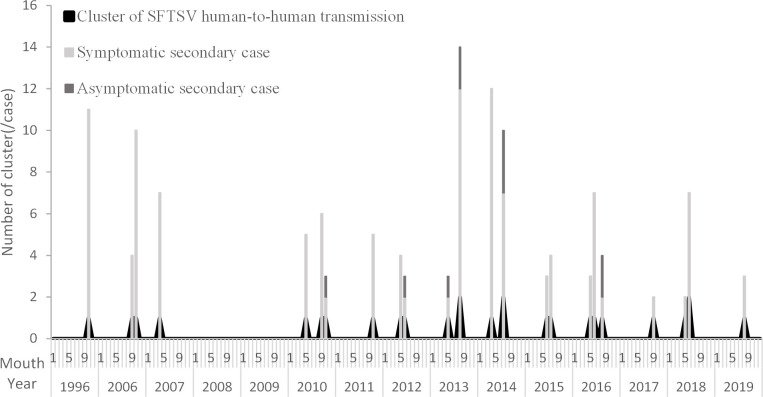
A total of 27 clusters of SFTS human-to-human transmission were reported in China and South Korea during 1996–2019, including 2 clusters of third-generation transmission in China. It contained 138 cases, with an average of 5 cases per cluster (range with 2–12 cases). The index cases were older than the secondary cases with the mean age of 65.4 years (t = 4.90, P<0.01), however, there were no significant difference in sex ratio between them (χ2 = 1.08, P = 0.30). Farmers accounted for 95.83% (23/24) of the index cases and 69.89% (65/93) of the secondary cases (Table 1). There overall 10 asymptomatic infections, of which 7 were second-generation secondary cases, and 3 were third-generation secondary cases. Asymptomatic infections accounted for 7.61% (7/92) and 33.33% (3/9) of all the second-generation and third-generation secondary cases, respectively (χ2 = 2.30, P = 0.13).
Table 1. Demographic and clinical characteristics of cases of SFTS human-to-human transmission in China and South Korea, 1996–2019.
| Characteristics | Index cases (n = 27) | Secondary cases (n = 111) | χ2 | t | P |
|---|---|---|---|---|---|
| Demographic features | |||||
| Sex, male | 16(n = 27) | 65(n = 93) | 1.08 | N/A | 0.30 |
| Age, y | 65.35±8.14(n = 26) | 51.76±13.35(n = 82) | N/A | 4.90 | <0.01 |
| Age>65y | 12(n = 26) | 13(n = 82) | 10.18 | N/A | <0.01 |
| Farmer | 23(n = 24) | 54(n = 94) | 12.43 | N/A | <0.01 |
| Time from onset to treatment, d | 4.00±2.81(n = 26) | 3.31±1.92(n = 45) | N/A | 976.50* | 0.62 |
| Time from onset to admission, d | 5.57±3.02(n = 26) | 3.31±1.92(n = 45) | N/A | 3.87 | <0.01 |
| General symptoms | |||||
| Fever | 27(n = 27) | 96(n = 106) | 1.57 | N/A | 0.21 |
| Fatigue | 13(n = 18) | 43(n = 64) | 0.16 | N/A | 0.69 |
| Headache | 10(n = 17) | 37(n = 79) | 0.81 | N/A | 0.37 |
| Myalgia | 13(n = 18) | 61(n = 85) | 0.002 | N/A | 0.97 |
| Gastrointestinal symptoms | 23(n = 24) | 53(n = 93) | 12.65 | N/A | <0.01 |
| Respiratory symptoms | 17(n = 19) | 25(n = 62) | 14.07 | N/A | <0.01 |
| CNS manifestation | 18(n = 21) | 10(n = 70) | 38.69 | N/A | <0.01 |
| Hemorrhagic symptoms | 23(n = 24) | 17(n = 90) | 49.25 | N/A | <0.01 |
| MODS | 16(n = 19) | 6(n = 69) | 45.31 | N/A | <0.01 |
| Fatal outcome | 27(n = 27) | 10(n = 110) | 90.89 | N/A | <0.01 |
Note: CNS is central nervous system, MODS is multi organ dysfunction syndrome, and n is the total number of cases for which information is available
* is T value (Wilcoxon W Test), y is year, d is day.
Among 27 index cases, 11 of them had definite history of tick bite. The average time from onset to admission of the index cases was longer than that in the secondary cases (5.6 days versus 3.3 days; P<0.01), while there was no difference of the average time from onset to treatment between them (T = 976.50, P = 0.62). Except for 4 retrospectively confirmed clusters, Laboratory-confirmed SFTS cases and those were confirmed before death accounted for 69.57% (16/23) and 43.48% (10/23) of all the index cases, respectively. Particularly, 3 index cases were misdiagnosed (be diagnosed and be in treatment as other disease rather than SFTS) with influenza and human granulocytic anaplasmosis, enteritis and infectious diarrhea, and blood disorder, respectively (Table 2).
Table 2. Line lists of the index cases of SFTSV human-to-human transmission in China and South Korea, 1996–2019.
| No. | Reference No. | Location | Date of onset | Date of treatment | Date of admission | Date of death time | Date of confirmed | Time from onset to treatment, d | Time from onset to admission, d | Time from onset to death, d | Time from onset to SFTS diagnosis, d | Other diagnoses |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | [20] | Jiangsu | 1996/10/02 | 1996/10/12 | 1996/10/14 | 1996/10/14 | N/A | 11 | 13 | 13 | N/A | N/A |
| 2 | [11] | Anhui | 2006/09/28 | 2006/09/29 | 2006/09/29 | 2006/10/04 | N/A | 2 | 2 | 7 | N/A | N/A |
| 3 | [11] | Anhui | 2006/10/31 | 2006/11/03 | 2006/11/03 | 2006/11/05 | N/A | 4 | 4 | 6 | N/A | N/A |
| 4 | [9,24] | Jiangsu | 2007/04/18 | 2007/04/19 | 2007/04/20 | 2007/04/27 | N/A | 2 | 3 | 10 | N/A | N/A |
| 5 | [14,26] | Henan | 2010/05/20 | 2010/05/20 | 2010/05/25 | 2010/05/30 | N/A | 1 | 6 | 11 | N/A | influenza; human granulocytic anaplasmosis |
| 6 | [10] | Shandong | 2010/09/25 | 2010/09/28 | 2010/09/28 | 2010/10/05 | 2010/10/05 | 4 | 4 | 11 | 11 | N/A |
| 7 | [12,42] | Jiangsu | 2010/10/06 | 2010/10/07 | 2010/10/13 | 2010/10/21 | 2010/10/15 | 2 | 8 | 16 | 12 | N/A |
| 8 | [27–29,31] | Shandong | * | 2011/10/11 | 2011/10/11 | 2010/10/14 | N/A | * | * | * | N/A | N/A |
| 9 | [13,34] | Hubei | 2012/05/06 | 2012/05/07 | 2012/05/07 | 2012/05/12 | N/A | 2 | 2 | 7 | N/A | N/A |
| 10 | [15,30,43] | Liaoning | 2012/06/04 | 2012/06/06 | 2012/06/06 | 2012/06/12 | 2012/06/11 | 3 | 3 | 9 | 8 | N/A |
| 11 | [35] | Zhejiang | 2013/05/29 | 2013/05/30 | 2013/06/02 | 2013/06/04 | 2013/06/04 | 2 | 5 | 7 | 7 | enteritis; infectious diarrhea |
| 12 | [16,36] | Shandong | 2013/08/25 | 2013/08/25 | 2013/08/29 | 2013/09/04 | 2013/09/02 | 1 | 5 | 11 | 9 | N/A |
| 13 | [32,44] | Anhui | 2013/08/27 | 2013/09/02 | 2013/09/02 | 2013/09/07 | 2013/09/04 | 7 | 7 | 12 | 9 | N/A |
| 14 | [17,33,37] | Zhejiang | 2014/04/23 | 2014/04/25 | 2014/04/29 | 2014/05/01 | 2014/05/20 | 3 | 7 | 9 | 28 | N/A |
| 15 | [18,38] | Shandong | 2014/07/06 | 2014/07/08 | 2014/07/13 | 2014/07/14 | 2014/07/13 | 3 | 8 | 9 | 8 | N/A |
| 16 | [39] | Liaoning | 2014/07/28 | 2014/08/05 | 2014/08/05 | 2014/08/07 | 2014/08/06 | 9 | 9 | 11 | 10 | N/A |
| 17 | [40] | Shandong | 2015/06/10 | 2015/06/18 | 2015/06/18 | 2015/06/21 | 2015/06/18 | 9 | 9 | 12 | 9 | N/A |
| 18 | [23,41] | Jiangsu | 2015/07/10 | 2015/07/12 | 2015/07/12 | 2015/07/18 | N/A | 3 | 3 | 9 | N/A | blood disorder |
| 19 | [19,46,48] | Jiangsu | 2016/05/21 | 2016/05/21 | 2016/05/21 | 2016/05/28 | N/A | 1 | 1 | 8 | N/A | N/A |
| 20 | JS1 | Jiangsu | 2016/06/10 | 2016/06/12 | 2016/06/16 | 2016/06/17 | N/A | 3 | 7 | 8 | N/A | N/A |
| 21 | [45] | Anhui | 2016/08/09 | 2016/08/13 | 2016/08/14 | 2016/08/20 | 2016/08/23 | 5 | 6 | 12 | 15 | N/A |
| 22 | [21] | South Korea | 2017/09/22 | 2017/09/24 | 2017/09/27 | 2019/10/01 | 2019/10/01 | 3 | 6 | 10 | 10 | N/A |
| 23 | [22] | South Korea | */10/01 | */10/05 | */10/05 | */10/10 | N/A | 5 | 5 | 10 | N/A | N/A |
| 24 | [47] | Shandong | 2018/05/24 | 2018/05/30 | 2018/05/30 | 2018/06/01 | 2018/06/01 | 7 | 7 | 9 | 9 | N/A |
| 25 | JS2 | Jiangsu | 2018/06/17 | 2018/06/17 | 2018/06/17 | 2018/07/07 | 2018/07/06 | 1 | 1 | 21 | 20 | N/A |
| 26 | JS3 | Jiangsu | 2018/06/25 | 2019/07/02 | 2019/07/05 | 2017/07/10 | 2018/07/11 | 8 | 11 | 16 | 17 | N/A |
| 27 | JS4 | Jiangsu | 2019/08/24 | 2019/08/26 | 2019/08/26 | 2019/08/30 | 2019/09/14 | 3 | 3 | 7 | 22 | N/A |
| Mean | N/A | N/A | N/A | N/A | N/A | N/A | 4.00 | 5.57 | 10.42 | 12.75 | N/A |
Note: JS1, JS2, JS3 and JS4 are the 4 original cluster reports in Jiangsu Province
* is missing from publications (We have attempted to contact the corresponding authors for missing information, however, it was missing at the time of the investigation and some of the investigations (e.g. investigation of close contacts) was not carried out at that time), d is day.
Temporal and Spatial distribution
Except for 4 retrospectively confirmed clusters (2 in 1996 and 2007 in Jiangsu Province and 2 in 2006 in Anhui Province), 1–3 clusters were reported annually from March to October (mainly in May, June and October) since 2010, when SFTSV was first identified (Fig 2).
Fig 2. Clusters and cases of SFTS human-to-human transmission in China and South Korea, 1996–2019.
Note:The time of 1 nosocomial cluster (6 cases) in South Korea is missing from publications.
A total of 25 clusters occurred in central and eastern China, involving 7 provinces, among which Jiangsu Province reported the most clusters (9 clusters), followed by Shandong Province (6 clusters), Anhui Province (4 clusters), Zhejiang Province and Liaoning Province (2 clusters each), and Henan Province and Hubei Province (1 cluster each). Especially, 2 clusters of third-generation transmission occurred in Shandong Province and Jiangsu Province. Meanwhile, 2 clusters were reported in South Korea.
Clinical characteristics
There was no statistical difference in general symptoms (fever, fatigue, headache and myalgia) between the index and secondary cases according to Pearson χ2 test. Notably, the index cases had more gastrointestinal symptoms, respiratory symptoms, CNS manifestation, hemorrhagic symptoms and MODS than the secondary cases. All of the index cases eventually died with the average course of illness of 10.4 days, while the mortality of the secondary case was only 9.09% (10/110) (χ2 = 90.89, P<0.01) (Table 1).
Risk analysis
Risk assessment
The index cases were highly contagious at least within 2–18 days of onset, leading to secondary infection. The incubation period of the secondary cases was 3–15 days, with the median of 10.0 days (IQR:8.0–12.0). The SAR was ranged 1.72%-55.0%. According to the Kolmogorov-Smirnov test, R0 was shown to be Poisson distributed (Z = 0.54, P = 0.93). The average R0 adjusted for exposed population was 0.13 (95%CI:0.11–0.16) (Table 3).
Table 3. Risk assessment of SFTS human-to-human transmission in China and South Korea, 1996–2019.
| No. | Reference No. | Secondary cases (No.) | Time of exposure since illness onset of index cases min, max (day) | Incubation period min, max, median (day) | Secondary attack rate (Secondary case/Exposure population) | R0 |
|---|---|---|---|---|---|---|
| 1 | [20] | 10 | *, *(n = 0) | *, *, *(n = 0) | 23.81% (10/42) | 5.0 |
| 2 | [11] | 3 | 6, 6(n = 3) | 7, 9, 7.0(n = 3) | 33.33% (3/9) | 3.0 |
| 3 | [11] | 9 | 5, 5(n = 9) | 6, 14, 8.0(n = 9) | 23.08% (9/39) | 9.0 |
| 4 | [9,24] | 6 | 9, 10(n = 6) | 7, 12, 9.0(n = 6) | 6.59% (6/91) | 6.0 |
| 5 | [10,14,26] | 4 | 11, 11(n = 4) | 8, 10, 9.0(n = 4) | 12.90% (4/31) | 4.0 |
| 6 | [10] | 5 | *, *(n = 0) | 7, 15, *(n = 0) | 7.94% (5/63) | 5.0 |
| 7 | [12,42] | 2 | 12, 12(n = 1) | 10, 10, 10.0(n = 1) | 22.2% (2/9) | 2.0 |
| 8 | [27–29,31] | 4 | 5,5(n = 4) | 4, 8,7.5(n = 4) | 50.00% (4/8) | 4.0 |
| 9 | [13,34] | 3 | *, *(n = 0) | 7, 12, *(n = 0) | 4.92% (3/61) | 3.0 |
| 10 | [15,30,43] | 2 | 12, 12(n = 1) | 12, 12, 12.0(n = 1) | 33.33% (2/6) | 2.0 |
| 11 | [35] | 2 | 2, 2(n = 1) | 7, 7, 7.0(n = 1) | 25.00% (2/8) | 2.0 |
| 12 | [16,36] | 8 | 12, 12(n = 8) | 7, 13, 9.0(n = 8) | 20.00% (8/40) | 8.0 |
| 13 | [32,44] | 4 | 7, 8(n = 2) | 11, 12, 11.5(n = 2) | 13.33% (4/30) | 4.0 |
| 14 | [17,33,37] | 11 | 10, 10(n = 11) | 10, 15, 12.0(n = 11) | 55.00% (11/20) | 11.0 |
| 15 | [18,38] | 5 | 9, 9(n = 2) | 8, 9, 8.5(n = 2) | * | 2.0 |
| 16 | [39] | 3 | 3, 8(n = 30) | 8, 12, 11.0(n = 3) | 33.33% (3/9) | 3.0 |
| 17 | [40] | 2 | 5, 6(n = 2) | 12, 14, 13.0(n = 2) | 33.33% (2/6) | 2.0 |
| 18 | [23,41] | 3 | 5, 10(n = 3) | 9, 14, 9.0(n = 3) | 21.43% (3/14) | 3.0 |
| 19 | [19,46,48] | 2 | 8, 8(n = 2) | 8, 12, 10.0(n = 2) | 6.25% (2/32) | 2.0 |
| 20 | JS1 | 6 | 8, 8(n = 6) | 4,14,8.0(n = 6) | 31.60% (6/17) | 6.0 |
| 21 | [45] | 3 | 4, 4(n = 1) | 10, 10, 10.0(n = 1) | 8.82% (3/35) | 3.0 |
| 22 | [21] | 1 | 9,9(n = 1) | 12,12,12.0(n = 1) | 7.10% (1/14) | 1.0 |
| 23 | [22] | 5 | 5,5(n = 5) | 8,11,9.0(n = 5) | 20.0% (5/25) | 5.0 |
| 24 | [47] | 1 | 9,9(n = 1) | 3,3,3.0(n = 1) | * | 1.0 |
| 25 | JS2 | 1 | 18,18(n = 1) | 14,14,14.0(n = 1) | 1.72% (1/58) | 1.0 |
| 26 | JS3 | 4 | 13,16(n = 4) | 9,12,10.0(n = 4) | 8.89% (4/45) | 4.0 |
| 27 | JS4 | 2 | 5,6(n = 2) | 10,11,10.5(n = 2) | 4.65% (2/43) | 2.0 |
| Total | N/A | 111 | 2, 18(n = 80) | 3, 15, 10.0(n = 84) | 1.72%, 55.00% | N/A |
Note: n is the total number of cases for whose information is available
* is missing from publications
Logistic analysis
Blood relatives of index case, medical personnel and other persons (including neighbors, body cleaners and those with unclear identities) accounted for 39.64% (44/111), 17.12% (19/111) and 43.24% (48/111) of all the secondary cases. Univariate logistic regression analysis showed that blood relatives had greater risk of infection (OR = 6.35) while the medical personal had lower risk of infection (OR = 0.47). With regard to types of contact, direct blood/bloody secretion contact, bloody droplet contact, airborne contact and urine/feces/sweat contact accounted for 84.76% (89/105), 32.38% (34/105), 66.67% (70/105) and 21.90% (23/105) of the secondary cases. The optimum multivariate logistic regression model (adjusted R2 = 0.49) showed direct blood/bloody secretion and bloody droplet contacts were related to the greater risk of infection (OR = 33.81 and 2.27, respectively) (Table 4) (S3 Text).
Table 4. Risk factors of SFTS human-to-human transmission in China and South Korea, 1996–2019.
| Factors | Contacts (No.) | Attack rate | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|---|
| Overall | Secondary case | P | OR (95%CI) | P | OR (95%CI) | ||
| Relationship | |||||||
| Blood relatives of index case | 59 | 44 | 74.58% | <0.01 | 6.35(3.26–12.37) | N/A | N/A |
| 174 | 55 | 31.61% | |||||
| Medical personnel | 85 | 19 | 22.35% | <0.01 | 0.47(0.26–0.83) | N/A | N/A |
| 225 | 86 | 38.22% | |||||
| Types of contact | |||||||
| Direct blood/bloody secretion contact | 119 | 89 | 74.79% | <0.01 | 38.01(19.73–73.23) | <0.01 | 33.81(17.44–65.53) |
| 221 | 16 | 7.24% | |||||
| Bloody droplet contact | 57 | 34 | 59.65% | <0.01 | 4.41(2.44–7.99) | 0.048 | 2.27(1.01–5.19) |
| 283 | 71 | 25.09% | |||||
| Airborne contact | 288 | 70 | 24.31% | <0.01 | 0.16(0.08–0.30) | N/A | N/A |
| 52 | 35 | 67.31% | |||||
| Urine /Feces/Sweat contact | 33 | 23 | 69.70% | <0.01 | 6.31(2.88–13.82) | N/A | N/A |
| 307 | 82 | 26.71% | |||||
Discussion
To better understand the epidemiological and clinical characteristic of SFTS human-to-human transmission, this study made an analysis of 27 clusters of SFTS human-to-human transmission in China and South Korea during 1996–2019. This study revealed that the clusters of SFTS human-to-human transmission had obvious spatio-temporal distinction. It occurred among elderly people between March and October in the central and eastern China, where SFTS was endemic [3]. The incubation period of SFTS human-to-human transmission was 3–15 days (median of 10.0 days), which is almost consistent with SFTS tick-bite transmission (generally 7–14 days, with average of 9 days) [3].
Farmers are a larger driver in SFTS cases, which may due to farmland and hills being a particularly well-suited habitat for the ticks and farmers had more opportunities to contact with the ticks, though only 11 of them had the definite history of tick bite. Compare to the index cases, the secondary cases developed milder clinical manifestations and better outcomes. It may be explained by the following reasons. Firstly, the diagnosis and treatment of 27 index cases were often delayed, while the secondary cases were relatively timely. In this study, the average time from onset to admission in the index cases was 2.3 days longer than that in the secondary cases. Meanwhile, except for 4 retrospectively confirmed clusters, only 43.48% (10/23) of the index cases were confirmed before death and the misdiagnosis existed, whereas all secondary cases be clearly diagnosed. Early diagnosis and early treatment are important to the clinical outcome of disease, thus the SFTS diagnostic awareness and diagnostic level of medical personnel should be improved. Secondly, the index cases were older than the secondary cases, with poor physical function. Age has been considered significantly related to the disease progression and clinical outcome of SFTS [51].
The risk of SFTS human-to-human transmission continues to be low. Two main reasons may account for this. For one, there was similarities in number (1–3 clusters) and scale (2–12 cases) of the clusters every year, which suggest no change in the risk of SFTS human-to-human transmission. For another, the SAR was 1.72%-55.0% and the average R0 of SFTS human-to-human transmission was 0.13 (95%CI:0.11–0.16), which means this transmission was not self-sustaining and is unable to generate a major epidemic. It much less than the R0 of Ebola virus disease in multiple outbreaks, though there were differences in model structure and underlying assumptions [52], for example, Chowell et.al estimated R0 at 1.33 to 1.35 for the Ebola outbreak in Uganda using SEIR model and employing a Bayesian estimation method. Legrand et.al estimated R0 at 2.7 in Uganda using a different modeling approach, which took into account three different transmission settings (community, hospital settings and during funerals).
Blood relatives of the index case had more risk to get infection, who provided more bedside care (39.64%). Meanwhile, genetic susceptibility might be related with SFTSV infection. For example, Sun et al. [53] reported that all three sisters infected with SFTSV and eventually died, while only 4.05% (3/74) of individuals living in the same areas were asymptomatic and others were uninfected. While medical personnel had lower risk to get infection. They get more personal protection equipment even incomplete (face shield and goggles gloves), which could comparatively protect contacts from infection [14,16].
The index cases were highly contagious at least within 2–18 days of onset, leading to secondary infection. Among types of contact, direct blood/bloody secretion contact was the major risk factor for SFTS human-to-human transmission. 84.76% of the secondary cases contact blood/bloody secretion. Particularly, part of secondary cases only contacted with blood of corpse were infected, which indicates that blood remains infectious for a long time even after death of the index cases. Bloody droplet contact might be the risk factor for SFTS human-to-human transmission. Jeong et al. [54] had detected SFTSV from tracheal aspirate of SFTS case. In this study, 89.47% of the index cases had respiratory symptoms and some also had mouth hemorrhagic symptoms, which make SFTSV possible to transmit through bloody droplet with close proximity. And 6 secondary cases who exposed to index patient during endotracheal intubation without direct blood contact got infection in Anhui Province [32]. Urine, feces and sweat contact may not be the risk factor for SFTS human-to-human transmission. None of secondary cases was caused by mere exposure to urine or feces in this study, though SFTSV was detected in urine specimens of SFTS patients in previous study [55]. In addition, 2 secondary cases in Shandong Province only contacted with sweat of the index case and got infection [40]. Thus, exposure to non-hemorrhagic secretions cannot be ruled out as a possible transmission mode and should still be avoided. Airborne cannot be confirmed as the risk factor for SFTS human-to-human transmission in this study, however, 2 secondary cases only stayed in mouring hall but no directly contacted with the index case were infected in Zhejiang Province [17]. SFTS human-to-human transmission also might be linked to the frequency and duration of contact with the index case, individual susceptibility and immune status [9].
This study had a few limitations. Firstly, we excluded the clusters without detail and the SFTS cases who could be either human-to-human or co-exposure to tick. It may underestimate the practical level of SFTS human-to-human transmission. Secondly, the data was mainly collected from previous publications, thus part of information were missing.
In summary, the clusters of SFTS human-to-human transmission in China and South Korea during 1996–2019 had spatio-temporal distinction. Targeted tick removal should be carried out in high-incidence seasons and areas. Meanwhile, the risk of SFTS human-to-human transmission continues to be low, however, ongoing assessment of SFTS human-to-human transmission is crucial for public health authorities.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors would like to thank all the scholars in this field for providing basic information support for this study.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
CJB received funding from Jiangsu Provincial Major Science & Technology Demonstration Project (No. BE2017749); WDL received funding from Jiangsu Provincial natural Science Foundation (No. BK20151595) and Jiangsu Provincial Medical Major Talent and Youth Talent (No.ZDRC2016032 & QNRC2016542); CJB and WDL received funding from Key Medical Discipline of Epidemiology (No. ZDXK A2016008). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. January 2017-First Annual review of diseases prioritized under the Research and Development Blueprint. 2017 Jan 24 [Cited 2019 Dec 17]. Available from: https://www.who.int/news-room/events/detail/2017/01/24/default-calendar/january-2017-first-annual-review-of-diseases-prioritized-under-the-research-and-development-blueprint.
- 2.Yu X, Liang M, Zhang S, Liu Y, Li JD, Sun Y, et al. Fever with thrombocytopenia associated with a novel Bunyavirus in China. N Engl J Med, 2011; 364:1523–32. 10.1056/NEJMoa1010095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhan J, Wang Q, Cheng J, Hu B, Li J, Zhan F, et al. Current status of severe fever with thrombocytopenia syndrome in China. Virol Sin, 2017; 32:51–62. 10.1007/s12250-016-3931-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi T, Maeda K, Suzuki T, Ishido A, Shigeoka T, Kamei T, et al. The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J Infect Dis, 2014; 209:816–27. 10.1093/infdis/jit603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park S, Han M, Yun S, Park C, Lee W, Ryou J. Severe fever with thrombocytopenia syndrome virus, South Korea, 2013. Emerg Infect Dis, 2014; 20:1880–2. 10.3201/eid2011.140888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tran XC, Yun Y, Van An L, Kin SH, Thao NTP, Man PKC, et al. Endemic Severe Fever with Thrombocytopenia Syndrome, Vietnam. Emerg Infect Dis, 2019; 25:1029–1031. 10.3201/eid2505.181463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gai Z, Zhang Y, Liang M, Jin C, Zhang S, Zhu C, et al. Clinical progress and risk factors for death in severe fever with thrombocytopenia syndrome patients. J Infect Dis, 2012; 206:1095–102. 10.1093/infdis/jis472 [DOI] [PubMed] [Google Scholar]
- 8.Luo L, Zhao L, Wen H, Zhang Z, Liu J, Fang L, et al. Haemaphysalis longicornis Ticks as Reservoir and Vector of Severe Fever with Thrombocytopenia Syndrome Virus in China, Emerg Infect Dis, 2015; 21:1770–1776. 10.3201/eid2110.150126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao C, Guo X, Qi X, Hu J, Zhou M, Varma JK, et al. A family cluster of infections by a newly recognized bunyavirus in eastern China, 2007: further evidence of person-to-person transmission. Clin Infect Dis, 2011; 53:1208–14. 10.1093/cid/cir732 [DOI] [PubMed] [Google Scholar]
- 10.Gai Z, Liang M, Zhang Y, Zhang S, Jin C, Wang S, et al. Person-to-person transmission of severe fever with thrombocytopenia syndrome bunyavirus through blood contact. Clin Infect Dis, 2012; 54:249–52. 10.1093/cid/cir776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Li Q, Hu W, Wu J, Wang Y, Mei L, et al. Person-to-person transmission of severe fever with thrombocytopenia syndrome virus. Vector Borne Zoonotic Dis, 2012; 12:156–60. 10.1089/vbz.2011.0758 [DOI] [PubMed] [Google Scholar]
- 12.Bao C, Qi X, Wang H. A Novel Bunyavirus in China. N Engl J Med, 2011; 365:864. 10.1056/NEJMc1106000 [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Hu K, Zou J, Xiao J. A cluster of cases of human-to-human transmission caused by severe fever with thrombocytopenia syndrome bunyavirus. Int J Infect Dis, 2013; 17:e206–8. 10.1016/j.ijid.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 14.Tang X, Wu W, Wang H, Du Y, Liu L, Kang K, et al. Human-to-human transmission of severe fever with thrombocytopenia syndrome bunyavirus through contact with infectious blood. J Infect Dis, 2013; 207:736–9. 10.1093/infdis/jis748 [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Deng B, Zhang J, Cui W, Yao W, Liu P. Person-to-person asymptomatic infection of severe fever with thrombocytopenia syndrome virus through blood contact. Intern Med, 2014; 53:903–6. 10.2169/internalmedicine.53.1164 [DOI] [PubMed] [Google Scholar]
- 16.Jiang X, Zhang S, Jiang M, Bi Z, Liang M, Ding S, et al. A cluster of person-to-person transmission cases caused by SFTS virus in Penglai, China. Clin Microbiol Infect, 2015; 21:274–9. 10.1016/j.cmi.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 17.Gong Z, Gu S, Zhang Y, Sun J, Wu X, Ling F, et al. Probable aerosol transmission of severe fever with thrombocytopenia syndrome virus in southeastern China. Clin Microbiol Infect, 2015; 21:1115–20. 10.1016/j.cmi.2015.07.024 [DOI] [PubMed] [Google Scholar]
- 18.Huang D, Jiang Y, Liu X, Wang B, Shi J, Su Z, et al. A cluster of symptomatic and asymptomatic infections of severe fever with thrombocytopenia syndrome caused by person-to-person transmission. Am J Trop Med Hyg, 2017; 97:396–402. 10.4269/ajtmh.17-0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Y, Wu H, Gao J, Zhou X, Zhu R, Zhang C, et al. Two confirmed cases of severe fever with thrombocytopenia syndrome with pneumonia: implication for a family cluster in East China. BMC Infect Dis, 2017; 17:537. 10.1186/s12879-017-2645-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu J, Shi C, Li Z, Guo X, Qian Y, Tan W, et al. A cluster of cases of severe fever with thrombocytopenia syndrome bunyavirus infection in China, 1996: A retrospective serological study. PLoS Negl Trop Dis, 2018; 12:e00066036. 10.1371/journal.pntd.0006603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon J, Lee H, Jeon JH, Kwon Y, Kim H, Wang EB, et al. Aerosol transmission of severe fever with thrombocytopenia syndrome virus during resuscitation. Infect Control Hosp Epidemiol, 2018; 19:1–4. 10.1017/ice.2018.330 [DOI] [PubMed] [Google Scholar]
- 22.Jung IY, Choi W, Kim J, Wang E, Park SW, Lee WJ, et al. Nosocomial person-to-person transmission of severe fever with thrombocytopenia syndrome. Clin Microbiol Infect, 2019; 25:631–3. 10.1016/j.cmi.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 23.Hu J, Li Z, Cai J, Liu D, Zhang X, Jiang R, et al. A cluster of Bunyavirus-associated severe fever with thrombocytopenia syndrome cases in a coastal plain area in China, 2015: identification of a previously unidentified endemic region for severe fever with thrombocytopenia Bunyavirus. Open Forum Infect Dis, 2019; 6:z209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tao W, Tao X. A cluster of 8 cases of severe fever with thrombocytopenia syndrome caused by bunyavirus. J Jiangsu Univ, 2011; 21:91–2. (in Chinese) [Google Scholar]
- 25.Ye L, Ren Y, Mao S, Liu D. Report of a household clustering of novel bunyavirus infection. Dis Surveill, 2012; 27:987–9. (in Chinese) [Google Scholar]
- 26.Tang X, Cui N, Kang K, Wang H, You A, Zhao G,et al. Analysis on the diagnosis and treatment of a cluster of cases infected by new bunyavirus. Chin J Pre Med, 2012; 46:110–3. (in Chinese) [PubMed] [Google Scholar]
- 27.Lin G, Yang X, Yu M. A cluster of 4 cases of severe fever with thrombocytopenia syndrome. Seek Med Ask Med, 2012; 10:741. (in Chinese) [Google Scholar]
- 28.Teng H, Li A, Xu N, Liu A. Novel bunyavirus infection in a family: report of four cases. Chin Gen Pract, 2012; 15:1529–30. (in Chinese) [Google Scholar]
- 29.Qu R, Guo Z, Qin X. Epidemiological investigation on cluster outbreak of fever with thrombocytopenia syndrome in Rongcheng city. J Trop Med, 2013; 13:1289–91. (in Chinese) [Google Scholar]
- 30.Zhang Y, Cui R, Yu D, Liu Y, Meng X, Miao C, et al. Clinical characteristics and epidemiology of novel bunyavirus human infection cases in Dandong city, 2010–2012. Chin J Public Health, 2013; 29:1495–8. (in Chinese) [Google Scholar]
- 31.Yue A, Qu R, Sun L. Investigation of family aggregation with fever and thrombocytopenia syndrome. Prev Med Trib Vol, 2014; 20:134–5. (in Chinese) [Google Scholar]
- 32.Lv Y, Wu J, Xu P, Xie S, Li K, Hu J, et al. Human-to-human transmission epidemic of sever fever with thrombocytopenia syndrome in western Anhui Province. Chin J Public health, 2014; 30:1129–32. (in Chinese) [Google Scholar]
- 33.Cheng Z, Wang F, Qian B, Chen E, Wu J. Investigation and disposal on the first cluster outbreak of person to person transmission of severe fever with thrombocytopenia syndrome in southern Anhui Province. Chin J Dis Control Pre, 2014; 18:1055–8. (in Chinese) [Google Scholar]
- 34.Xu Z, Xu G. A cluster of severe fever with thrombocytopenia caused by bunyavirus. World Health Digest, 2014; 16:39–40. (in Chinese) [Google Scholar]
- 35.Huang W, Xu X, Wu H, Zhang L, Miao Z, Lv H. Investigation and analysis on the transmission of severe fever with thrombocytopenia syndrome via contact with patient. Chin J Vector Biol &Control, 2015; 26:172–5. (in Chinese) 10.1088/0031-9155/50/15/N02 [DOI] [PubMed] [Google Scholar]
- 36.Cong L. Clinical analysis of 53 patients with severe fever with thrombocytopenia syndrome in People ‘ s Hospital of Penglai city. Infect Dis Info, 2015; 1:32–5. (in Chinese) [Google Scholar]
- 37.Gu S, Wu X, Zhou B, Ling F, Zhang H, Huang Y, et al. Epidemiological investigation on an outbreak of severe fever with thrombocytopenia syndrome in northwest Zhejiang province. Chin J Epidemic, 2015; 36:364–7. (in Chinese) [PubMed] [Google Scholar]
- 38.Ma Y, Zhao W. Analysis of clinical features of severe fever with thrombocytopenia syndrome in 65 cases. Chin J Med Pharm, 2015; 21:3325–7. (in Chinese) [Google Scholar]
- 39.Liang Y, Qi S, Wu H, Zhou L. Investigation and analysis of the first cluster of severe fever with thrombocytopenia syndrome in Dalian city. Chin Primary Health Care, 2015; 29:58–9. (in Chinese) [Google Scholar]
- 40.Wang Y, Qu X. Investigation on a family clustering occurrence of severe fever with thrombocytopenia syndrome. Inter J Epidemiol Infect Dis, 2016; 43:62–4. (in Chinese) [Google Scholar]
- 41.Wu H, Yan G. An epidemiological investigation of an epidemic of aggregation severe fever with thrombocytopenia syndrome. J Med Pest Control, 2016; 32:1056–7. (in Chinese) [Google Scholar]
- 42.Ma T, Xu Q, Li C, Zhang Z, Feng L, Li W, et al. Epidemiological characteristics of severe fever with thrombocytopenia syndrome in Nanjing, China from 2010 to 2016. Modern Preventive Medicine, 2017; 44:2890–4. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 43.Cui R, Yu D, Miao C. Analysis of the epidemic characteristics of severe fever with thrombocytopenia syndrome in Dandong city of Liaoning province, 2010–2015. Chin J Vector Biol &Control, 2017; 28:60–3. (in Chinese) 10.1088/0031-9155/50/15/N02 [DOI] [PubMed] [Google Scholar]
- 44.Lv Y, Xu P, Sun J, Hu J, Gong T, Xie S, et al. Surveillance of severe fever with thrombocytopenia syndrome in Lu’an, 2011–2015. Chin Trop Med, 2017; 17:809–12. (in Chinese) [Google Scholar]
- 45.He Y, Huang Y, He S, Qi B, Zhao W, Wu F. Epidemiological investigation of a cluster of severe fever with thrombocytopenia syndrome in Tongling city. Chin Prev Med, 2018; 19:553–6. (in Chinese) [Google Scholar]
- 46.Cai H, Zhang H, Dai Z. Nursing care of 2 patients with new bunyavirus family cluster disease. Shanghai Nurs, 2018; 18:87–8. (in Chinese) [Google Scholar]
- 47.Sang D, Liu D, Liu Z, Zhou T, Shang D. One case of a nurse infected with the novel bunyavirus. Chin J Emeg Med, 2019; 28:901–3. (in Chinese) [Google Scholar]
- 48.Ge R, Chi Q, Chen Y. Investigation on the first cluster of severe fever with thrombocytopenia syndrome in Suzhou city. Jiangsu J Prev Med, 2019; 30:195–6. (in Chinese) [Google Scholar]
- 49.Chinese Ministry of Health. Prevention guide severe fever with thrombocytopenia (syndrome 2010 ed). Chin J Clin Infect Dis, 2011; 4:193–4. (in Chinese) [Google Scholar]
- 50.Bjornstad, Ottar N. Epidemics: Models and Data using R. University Park, USA: Springer International Publishing, 2018. [Google Scholar]
- 51.Jia B, Wu W, Huang R, Wang G, Song P, Li Y, et al. Characterization of clinical features and outcome for human-to-human transmitted severe fever with thrombocytopenia syndrome. Infect Dis (Lond), 2018; 50:601–8. 10.1080/23744235.2018.1449962 [DOI] [PubMed] [Google Scholar]
- 52.Chowell G, Nishiura H. Transmission dynamics and control of Ebola virus disease (EVD): a review. BMC Med, 2014; 12:196. 10.1186/s12916-014-0196-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun J, Tang Y, Ling F, Chang Y, Ye X, Shi W, et al. Genetic susceptibility is one of the determinants for severe fever with thrombocytopenia syndrome virus infection and fatal outcome: An Epidemiological Investigation. PLoS One, 2015; 10:e132968. 10.1371/journal.pone.0132968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jeong EJ, Song JY, Lim CS, Lee I, Park MS, Choi MJ, et al. Viral shedding from diverse body fluids in a patient with severe fever with thrombocytopenia syndrome. J Clin Virol, 2016; 80:33–5. 10.1016/j.jcv.2016.04.018 [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, He Y, Dai Y, Xiong Y, Zheng H, Zhou D, et al. Hemorrhagic fever caused by a novel Bunyavirus in China: pathogenesis and correlates of fatal outcome. Clin Infect Dis, 2012; 54:527–33. 10.1093/cid/cir804 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.