Abstract
Objectives
To evaluate and compare the biofilm formation between labial and lingual orthodontic brackets.
Materials and Methods
Twenty patients with a mean age of 24 ± 8.8 who had received labial or lingual orthodontic treatment were enrolled in the study. Biofilm formation on 80 brackets was analyzed quantitatively with the Rutherford backscattering detection method. Five micrographs were obtained per bracket with views from the vestibule/lingual, mesial, distal, gingival, and occlusal aspects. Quantitative analysis was carried out with surface analysis software (ImageJ 1.48). Data were analyzed by Mann-Whitney U and Kruskal-Wallis tests (α = 0.05).
Results
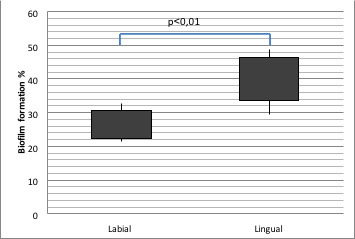
Total biofilm formation was 41.56% (min 29.43% to max 48.76%) on lingual brackets and 26.52% (min 21.61% to max 32.71%) on labial brackets. Differences between the two groups were found to be significant. No difference was observed in intraoral location. The biofilm accumulation was mostly located on gingival, mesial, and distal surfaces for both groups.
Conclusions
The biofilm accumulation on lingual orthodontic therapy was found to be more than labial orthodontic therapy.
Keywords: Biofilm, Lingual orthodontics
INTRODUCTION
To meet the increasing esthetic expectations of orthodontic patients, lingual treatment technique was developed and became a powerful alternative to labial treatment especially with advances in recent years.1,2 After the insertion of the brackets to labial and lingual surfaces, ecological changes occur in the amount, composition, metabolic activity, and pathogenicity of oral microbiota. This may lead to an increased rate of gingivitis and caries lesions.3–9 These side effects can be explained by a higher number of plaque-retentive areas, physiochemical properties of biomaterials, and the inability to remove the plaque mechanically.10 In addition, access of saliva to these areas is restricted and the effectiveness of the tongue in removing food residues from the mouth is reduced. It is important for orthodontists to know the changes that occur in the oral environment after the insertion of lingual brackets, which are quite different from conventional labial techniques in dimension, position, and difficulty in access during hygiene measures.
After the insertion of brackets, the initial biofilm layer that forms on bracket surfaces worsens the periodontal condition but, after a while, the host microorganism balance is restored.11 However, intraoral materials wear out over time due to particles of food, beverages, and corrosion.12,13 In this case, such wear would have a significant effect on the biofilm formation, as it would change the surface roughness and energy.14 It is therefore important to investigate the amount of biofilm on and around the brackets. The amount of biofilm on bracket surfaces is also an indicator of the biofilm around the brackets.
There are conflicting results in the literature regarding which treatment modality is superior in hygiene.15–21 However, no studies have been reported that aimed to evaluate the amount of biofilm formation on lingual brackets and to compare them with that on labial brackets. The aim of this study was to compare the amount of biofilm formation on bracket surfaces between lingual and labial treatments. The null hypothesis of the study was that there would be no difference between lingual and labial brackets in the extent of biofilm formation.
MATERIALS AND METHODS
This study was carried out with the approval of Başkent University Medical and Health Sciences Research Council (project no: D-KA 15/05, accepted: 07.04.2015, number: 94603339 / 18-050.01.08.01-429) and supported by Başkent University Research Fund. Based on a power analysis, it was determined that a sample of 10 patients per group was needed for a 20% effect size change to represent a statistically significant difference in biofilm amount. With a total of 20 patients, 80% power and 95% confidence levels were achieved.
Twenty individuals over the age of 17 years who underwent fixed orthodontic treatment with lingual brackets (STb, Ormco Corporation, Glendora, CA, USA) or labial metal brackets (Victory Series, 3M Unitek, Monrovia, CA, USA) were enrolled in this study. The exclusion criteria were presence of a systemic disease, smoking habit, history of periodontal disease, extensive dental restorations, history of antibiotics or any antibacterial mouth rinse product use during the last 6 weeks before debonding, and the application of professional dental hygiene procedures in the last 3 months. The patients were treated by the same clinician. During treatment, the patients in both groups were informed about brushing with a Modified Bass Technique, and an interdental brush was recommended. During the control appointments, hygiene motivation and education were given whenever levels started to decrease. Brackets were collected from consecutively debonded patients between September 2014 and December 2014 until the required sample size was reached.
The group with labial metal brackets consisted of 10 individuals with a mean age of 25.30 ± 10.69 years (six females, four males). The mean treatment time was 24.4 ± 8.36 months. The group with lingual brackets consisted of 10 individuals with a mean age of 22.60 ± 6.64 years (seven females, three males). The mean treatment time was 25.8 ± 8.92 months (Table 1).
Table 1.
Chronological Ages, Treatment Time, and Sex of the Individuals Involved in the Study
| F |
M |
Age (Year) |
Treatment Time (Month) |
|||||
|
x ± sd |
Min |
Max |
x ± sd |
Min |
Max |
|||
| Labial treatment | 6 | 4 | 25.30 ± 10.69 | 17 | 51 | 24.4 ± 8.36 | 13 | 42 |
| Lingual treatment | 7 | 3 | 22.60 ± 6.64 | 17 | 34 | 25.8 ± 8.92 | 13 | 37 |
| Total | 13 | 7 | 24 ± 8.8 | 17 | 51 | 25.1 ± 8.45 | 13 | 42 |
The brackets of these 20 patients were investigated after the completion of orthodontic treatment. The same experienced clinician removed the brackets and prepared them for evaluation and investigated the photomicrographs. The brackets were removed using the same debonding plier (Lingual debonding plier, ETM 431 with Modified Hook). Extra care was taken to avoid the iatrogenic removal of the biofilm layer. After debonding, archwires were carefully removed, mildly rinsed, and air-dried with an air-water spray to remove debris, and stored in separate boxes at room temperature. Central incisor and second premolar brackets were equally and consecutively selected from either first and third quadrants or second and fourth quadrants for both the labial and lingual groups. Four brackets were collected from each patient; a total of 80 brackets (40 labial, 40 lingual) were obtained for investigation.
Biofilm formation was screened using the Rutherford backscattering detection (RBSD) method on a scanning electron microscope (SEM) (JSM-6400, JEOL, Tokyo, Japan).22–24 This technique measures backscattering of high energy electrons impinging on a sample. Due to the different atomic weights, lighter elements such as carbon atoms in the biofilm appear as dark areas, and the heavier elements such as iron in stainless steel appear as bright areas. (Figure 1) Biofilm coverage was verified with SEM at high magnification and elemental analysis of the surfaces were made using energy-dispersive x-ray spectroscopy (EDS / EDX). Elemental analysis was performed because, on the SEM images, relatively unusual brighter areas with clear borders were observed in non-biofilm-covered areas (Figure 2).
Figure 1.
A secondary electron image at × 100 magnification.
Figure 2.
Areas seen relatively brighter in the lingual brackets.
Photomicrographs from the mesial, distal, occlusal, gingival, and vestibular-lingual aspects (five aspects) of each bracket were obtained by RBSD method at 20 kV and × 20 magnification. (Figures 3 and 4) A total of 400 photomicrographs were analyzed for quantitative biofilm formation analysis using the ImageJ 1.48 software program (for Windows, National Institutes of Health, Bethesda, MD). Biofilm-covered areas appeared to be in different gray values. A binary image was obtained by determining the threshold value in the gray scale. The dark areas of the biofilm, the entire bracket surface area, and their ratio were calculated. Then, the ratio of the total biofilm areas on all five surfaces of the bracket to the total surface area of the bracket was calculated.
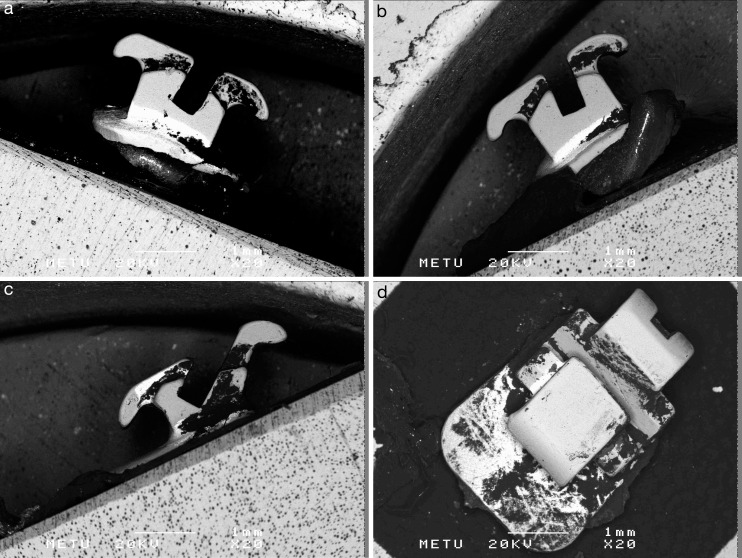
Figure 3.
RBSD method photomicrographs of a labial mandibular central bracket with perspective from the mesial (a), gingival (b), distal (c), occlusal (d), and vestibular (e) aspects. RBSD indicates Rutherford backscattering detection.
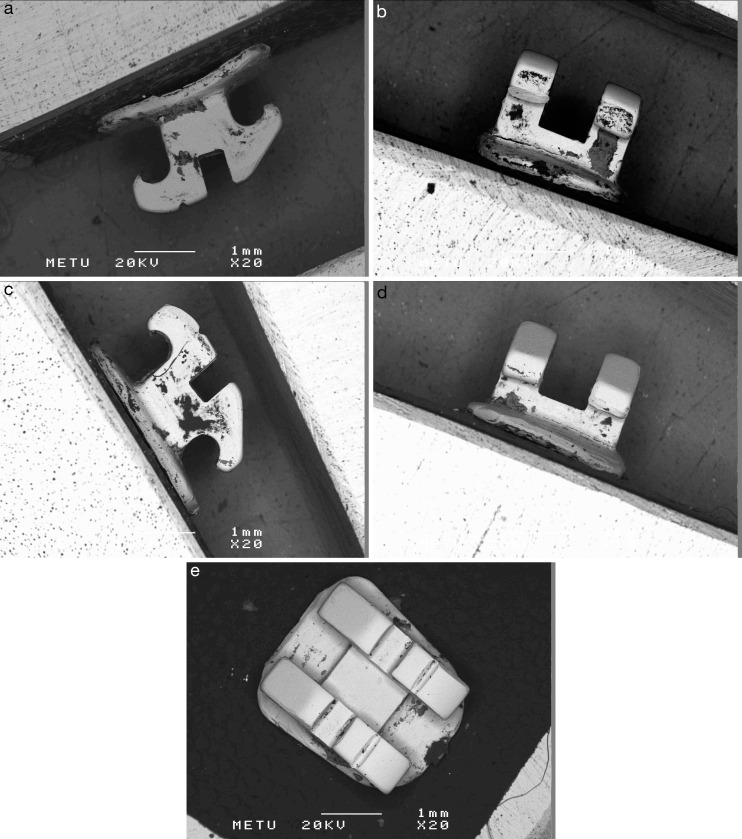
Figure 4.
RBSD method photomicrographs of a lingual mandibular central incisor bracket viewed from the mesial (a), gingival (b), distal (c), occlusal (d), and vestibular (e) aspects.
Statistical Analysis
A statistical analysis was performed using SPSS / PC-version 20.0 for Windows (IBM, Armonk, NY, USA) program. The Kolmogorov-Smirnov test and Shapiro-Wilk test were applied to determine normal distribution. Data were compared by use of the Mann-Whitney U‐test. Kruskal-Wallis one-way variance analysis was used for multiple tests. The level of significance was set as 0.05.
RESULTS
The difference between the amount of biofilm formation of the different bracket surfaces was statistically significant in both groups (P < .001) (Table 2). In both groups, the highest values of biofilm were found at the gingival, mesial, and distal surfaces. The lowest amount of biofilm was present on the vestibular surface of labial brackets and on the occlusal surface of lingual brackets. The amount of biofilm was significantly higher in the lingual brackets than in the labial brackets for all bracket surfaces except the occlusal (P < .01).
Table 2.
Comparative Evaluation of Biofilm Formation With Respect to Bracket Surfaces
| Surface, % |
Labial |
Lingual |
|||||||
| n |
Median |
Min |
Max |
n |
Median |
Min |
Max |
P Valuea |
|
| Mesial | 40 | 31.68 | 16.54 | 49.98 | 40 | 51.77 | 34.8 | 71.99 | .001** |
| Distal | 40 | 34.47 | 21.19 | 37.28 | 40 | 55.63 | 44.85 | 65.18 | .001** |
| Occlusal | 40 | 24.29 | 14.78 | 43.85 | 40 | 23.27 | 7.96 | 36.18 | .853 |
| Gingival | 40 | 41.84 | 32.37 | 66.42 | 40 | 77.52 | 59.03 | 88.64 | .001** |
| Vestibule-lingual | 40 | 18.17 | 10.85 | 26.15 | 40 | 30.7 | 21.36 | 40.41 | .001** |
| P valueb | 0.000*** | 0.000*** | |||||||
Mann-Whitney U.
Kruskal-Wallis.
P < .01; *** P < .001.
The amount of biofilm formation was 41.56% (min 29.43% to max 48.76%) of the total bracket surface on lingual brackets and 26.52% (min 21.61% to max 32.71%) on the labial brackets. The difference between the relative biofilm formation in the labial and lingual groups was statistically significant. (P < .01) (Table 3)
Table 3.
Comparative Evaluation of Relative Biofilm Formation on Labial Brackets and Lingual Bracketsa
| % |
N |
Median |
Min |
Max |
P Value |
| Labial brackets | 10 | 26.52 | 21.61 | 32.71 | .001* |
| Lingual brackets | 10 | 41.56 | 29.43 | 48.76 |
Mann-Whitney U‐Test; *P < .01.
When the results of biofilm accumulation with respect to location (maxillary central, maxillary second premolar, mandibular central, mandibular second premolar) (P > .05) were investigated, at all locations except maxillary central brackets, significantly higher biofilm accumulation was found on lingual brackets than on labial brackets (P < .05) (Table 4).
Table 4.
Comparative Evaluation of the Biofilm Formation With Respect to the Intraoral Locations of the Brackets
| Location (Bracket), % |
Treatment Group |
||||||||
| Labial |
Lingual |
||||||||
| n |
Median |
Min |
Max |
n |
Median |
Min |
Max |
P Valuea |
|
| Max5 | 10 | 24.53 | 15.92 | 44.35 | 10 | 37.68 | 27.17 | 48.05 | .011* |
| Max1 | 10 | 23.5 | 13.18 | 47.77 | 10 | 34.36 | 13.09 | 63.58 | .075 |
| Mand1 | 10 | 30.27 | 12.56 | 46.39 | 10 | 39.92 | 27.43 | 61.87 | .043* |
| Mand5 | 10 | 18.24 | 14.7 | 40.19 | 10 | 39.6 | 29.04 | 61.18 | .001** |
| P value++ | 0.18 | 0.803 | |||||||
Mann-Whitney U.
Kruskal-Wallis.
P < .05.
P < .01.
EDS Analysis
While the labial bracket material was detected to be made up only of stainless steel alloy (72.67% Fe, 17.28% Cr, 4.28% Ni, 2.64% Cu, 0.72% Mn, 2.39% Si), lingual bracket material was detected to contain a high rate of gold element (70.16% Au, 14.45% Fe, 10.44% Ni, 4.94% Cr) in the brighter uncovered areas and stainless steel alloy in the darker uncovered areas. Thus, the material properties of the labial and lingual brackets were not identical (Figure 5).
Figure 5.
Graph showing the EDS results of (a) regular bracket and (b) brackets showing brighter areas. EDS indicates energy-dispersive x-ray spectroscopy.
DISCUSSION
Brackets are the most important plaque deposit areas as soon as they are placed in the mouth. There are studies in the literature examining biofilm formation on labial bracket surfaces. However, there is no study examining the amount of biofilm accumulation on lingual bracket surfaces.
The RBSD method was used to analyze the quantitative extent of the biofilm-covered area. This technique was previously used in some studies to detect biofilm on the surfaces of dental materials.22–25 With this SEM technique, a quantitative analysis is carried out on photomicrographs of biofilm-covered and nonbiofilm-covered areas by surface analysis software, since they appear in different gray values. This method allowed only 2D evaluation of biofilm covered areas. It was not possible to determine the biofilm thickness and bacterial diversity.
In both groups, biofilm accumulation was mostly observed on the gingival, mesial, and distal surfaces of the brackets. These areas are the most common sites of white spot lesion formation around the brackets.26 In the literature, similar studies that were carried out on brackets with labial placement also supported these findings.22,25,27 These findings can be explained by the resistance to shearing forces in areas that were protected from mechanical cleaning and the effect of salivary flow.14,28
The null hypothesis was rejected because the amount of relative total biofilm on the lingual brackets was found to be significantly higher at all locations. Similarly, Lombardo et al.20 revealed that gingival inflammation and the amount of Streptococcus mutans were higher in lingual treatment both clinically and microbiologically. It has also been shown in survey studies that lingual patients were more distressed than labial bracket patients in food residues left around braces and were having more discomfort while brushing18,21 and they were doing it blindly.
Lombardo et al.20 also noted that the gingival bleeding index of the labial group was significantly increased compared with the lingual group in the first month of treatment, while the plaque index was not related to the type of treatment.19 In their in vivo study, Sfondrini et al.29 eliminated the effect of archwire and ligature by bonding the same brackets to the labial and lingual surfaces. They reported that the bracket position did not have any effect on periodontal and microbial parameters. Van der Veen et al.30 found that white spot lesions formed on labial surfaces and developed 4.8 times more than on lingual surfaces. The contradictory findings could be due to the fact that the dental plaque also contains other bacteria than the streptococcus species and that the amount of plaque may not be directly related to the streptococcal prevalence.5 Since all of these previous studies examined the short-term impact, it is possible that there could be a difference compared with the current study, which examined biofilm accumulation at the end of treatment. Significant differences were observed in short-, medium-, and long-term biofilm formation in oral materials.12,13,31 Long-term outcomes may be affected by changes in cooperation in brushing, oral hygiene motivation, and dietary habits.14 Therefore, according to the current results, oral hygiene of lingual patients might have reduced in the long term due to limited visibility of the lingual side. Although the cleaning effect of the tongue is mentioned for the palatal region,26 there are bracket surfaces where the tongue is not effective in cleaning.14,28 For the lingual technique, more biofilm accumulation may have occurred due to features such as shorter interbracket distance16 and closer position of the brackets to the gingiva.
There was no significant difference in biofilm formation with respect to the intraoral locations of the brackets (anterior, posterior, mandible, maxilla) in either group. Most studies that evaluated the development of caries reported that more lesions developed in the maxillary anterior than in the mandibular anterior region.5,30 Also, there were studies that supported that plaque formation and gingival enlargement were more frequent on posterior teeth than on anterior teeth.32–34 These conflicting findings may be due to differences in the brackets used, the parameters assessed, the methodology applied, and the difference in oral hygiene.
With the detection of “Au” element in lingual brackets, EDS analysis revealed that the material properties of the labial and lingual brackets were not the same. In the literature, gold possesses the highest antibacterial activity, and has biofilm adhesion inhibitory effect.35–37 Nevertheless, in their in vivo study, Dittmer et al. found that, in the short term, the initial biofilm formation on stainless steel surfaces was less compared to gold.38 This study was unable to find an answer to this elemental composition. It may be a coincidence or due to the use of same manufacturer's band with another appliance.
The high standard deviations of biofilm amounts indicated the difference in the amount of biofilm-covered surfaces between individuals. This can be explained by the effects of factors such as nutrition, oral hygiene, and tongue activity.39,40 Therefore, the effects of individual factors on oral hygiene should not be overlooked while interpreting the results. Individuals undergoing lingual treatment should be reinstructed and remotivated regarding oral hygiene at every appointment. In addition to routine brushing techniques, additional methods such as floss and interdental brushes should be recommended for both labial and lingual treatments. The results from this study should be considered when deciding on the type of treatment for individuals with poor oral hygiene.
The limitation of this study was that periodontal parameters, bacterial composition, and carious lesions were not evaluated. In future studies, long-term effects of biofilm accumulation on clinical and microbiological parameters of lingual and labial treatment can be evaluated related to the different sizes of brackets and bracket materials.
CONCLUSIONS
In this study comparing the amount of biofilm formation on lingual and labial orthodontic bracket surfaces, the following results were obtained:
The null hypothesis was rejected. At all locations, biofilm formation was found significantly higher on lingual brackets than it was on labial brackets.
The gingival, mesial, and distal surfaces are the areas where biofilm accumulation is highest for labial and lingual brackets.
REFERENCES
- 1.Gorman JC. Treatment of adults with lingual orthodontic appliances. Dent Clin North Am. 1988;32(3):589–620. [PubMed] [Google Scholar]
- 2.Gorman JC, Smith RJ. Comparison of treatment effects with labial and lingual fixed appliances. Am J Orthod Dentofacial Orthop. 1991;99(3):202–209. doi: 10.1016/0889-5406(91)70002-E. [DOI] [PubMed] [Google Scholar]
- 3.Atack NE, Sandy JR, Periodontal Addy M. and microbiological changes associated with the placement of orthodontic appliances. A review. J Periodontol. 1996;67:78–85. doi: 10.1902/jop.1996.67.2.78. [DOI] [PubMed] [Google Scholar]
- 4.Naranjo AA, Trivino LM, Jaramillo A, Betancourth M, Botero JE. Changes in the subgingival microbiota and periodontal parameters before and 3 months after bracket placement. Am J Orthod Dentofac Orthop. 2006;130:275.e17–e22. doi: 10.1016/j.ajodo.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Ahn SJ, Lim BS, Lee SJ. Prevalence of cariogenic streptococci on incisor brackets detected by polymerase chain reaction. Am J Orthod Dentofac Orthop. 2007;131:736–741. doi: 10.1016/j.ajodo.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 6.van Gastel J, Quirynen M, Teughels W, Coucke W, Carels C. Longitudinal changes in microbiology and clinical periodontal variables after placement of fixed orthodontic appliances. J Periodontol. 2008;79:2078–2086. doi: 10.1902/jop.2008.080153. [DOI] [PubMed] [Google Scholar]
- 7.Artun J. A post treatment evaluation of multibonded lingual appliances in orthodontics. Eur J Orthod. 1987;9(3):204–210. doi: 10.1093/ejo/9.3.204. [DOI] [PubMed] [Google Scholar]
- 8.Demling A, Demling C, Schwestka-Polly R, Stiesch M, Heuer W. Short-term influence of lingual orthodontic therapy on microbial parameters and periodontal status. A preliminary study. Angle Orthod. 2010;80(3):480–484. doi: 10.2319/061109-330.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demling A, Demling C, Schwestka-Polly R, Stiesch M, Heuer W. Influence of lingual orthodontic therapy on microbial parameters and periodontal status in adults. Eur J Orthod. 2009;31(6):638–642. doi: 10.1093/ejo/cjp064. [DOI] [PubMed] [Google Scholar]
- 10.Boyd RL. Longitudinal evaluation of a system for self-monitoring plaque control effectiveness in orthodontic patients. J Clin Periodontol. 1983;10:380–388. doi: 10.1111/j.1600-051x.1983.tb01287.x. [DOI] [PubMed] [Google Scholar]
- 11.Ristic M, Svabic MV, Sasic M, Zelic O. Clinical and microbiological effects of fixed orthodontic appliances on periodontal tissues in adolescents. Orthod Craniofac Res. 2007;10:187–195. doi: 10.1111/j.1601-6343.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 12.Matasa CG. Pros and cons of the reuse of direct-bonded appliances. Am J Orthod Dentofacial Orthop. 1989;96:72–76. doi: 10.1016/0889-5406(89)90232-1. [DOI] [PubMed] [Google Scholar]
- 13.Lin MC, Lin SC, Lee TH, Huang HH. Surface analysis and corrosion resistance of different stainless steel orthodontic brackets in artificial saliva. Angle Orthod. 2006;76:322–329. doi: 10.1043/0003-3219(2006)076[0322:SAACRO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Quirynen M, Bollen CML. The influence of surface roughness and surface free energy on supra- and subgingival plaque formation in man. A review of the literature. J Clin Periodontol. 1995;22:1–14. doi: 10.1111/j.1600-051x.1995.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 15.Hohoff A, Stamm T, Kühne N, et al. Effects of a mechanical interdental cleaning device on oral hygiene in patients with lingual brackets. Angle Orthod. 2003;73(5):579–587. doi: 10.1043/0003-3219(2003)073<0579:EOAMIC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Raige SF. A lingual light-wire technique. J Clin Orthod. 1982;16(8):534–544. [PubMed] [Google Scholar]
- 17.Fujita K. Multilingual-bracket and mushroom arch wire technique. A clinical report. Am J Orthod. 1982;82(2):120–140. doi: 10.1016/0002-9416(82)90491-2. [DOI] [PubMed] [Google Scholar]
- 18.Caniklioglu C, Ozturk Y. Patient discomfort: a comparison between lingual and labial fixed appliances. Angle Orthod. 2005;75(1):86–91. doi: 10.1043/0003-3219(2005)075<0086:PDACBL>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Sinclair PM, Cannito MF, Goates LJ, Solomos LF, Alexander CM. Patient responses to lingual appliances. J Clin Orthod. 1986;20(6):396–404. [PubMed] [Google Scholar]
- 20.Lombardo L, Gorgun O, Panza C, Scuzzo G, Siciliani G. Changes in oral environment after placement of lingual and labial orthodontic appliances. Prog Orthod. 2013;14(1):28. doi: 10.1186/2196-1042-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyawaki S, Yasuhara M, Koh Y. Discomfort caused by bonded lingual orthodontic appliances in adult patients as examined by retrospective questionnaire. Am J Orthod Dentofacial Orthop. 1999;115(1):83–88. doi: 10.1016/s0889-5406(99)70320-3. [DOI] [PubMed] [Google Scholar]
- 22.Demling A, Elter C, Heidenblut T, et al. Reduction of biofilm on orthodontic brackets with the use of a polytetrafluoroethylene coating. Eur J Orthod. 2010;32(4):414–418. doi: 10.1093/ejo/cjp142. [DOI] [PubMed] [Google Scholar]
- 23.Elter C, Heuer W, Demling A, et al. Supra- and subgingival biofilm formation on implant abutments with different surface characteristics. Int J Oral Maxillofac Implants. 2008;23(2):327–334. [PubMed] [Google Scholar]
- 24.Demling A, Heuer W, Elter C, et al. Analysis of supra- and subgingival long-term biofilm formation on orthodontic bands. Eur J Orthod. 2009;31(2):202–206. doi: 10.1093/ejo/cjn090. [DOI] [PubMed] [Google Scholar]
- 25.Lindel ID, Elter C, Heuer W, et al. Comparative analysis of long-term biofilm formation on metal and ceramic brackets. Angle Orthod. 2011;81(5):907–914. doi: 10.2319/102210-616.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stratemann MW, Shannon IL. Control of decalcification in orthodontic patients by daily self-administered application of a water-free 0.4 per cent stannous fluoride gel. Am J Orthod. 1974;66(3):273–279. doi: 10.1016/0002-9416(74)90291-7. [DOI] [PubMed] [Google Scholar]
- 27.Sukontapatipark W, El-Agroudi MA, Selliseth NJ, Thunold K, Selvig KA. Bacterial colonization associated with fixed orthodontic appliances. A scanning electron microscopy study. Eur J Orthod. 2001;23(5):475–484. doi: 10.1093/ejo/23.5.475. [DOI] [PubMed] [Google Scholar]
- 28.Hannig M. Transmission electron microscopy of early plaque formation on dental materials in vivo. Eur J Oral Sci. 1999;107(1):55–64. doi: 10.1046/j.0909-8836.1999.eos107109.x. [DOI] [PubMed] [Google Scholar]
- 29.Sfondrini MF, Debaggi M, Zara F, et al. Influence of lingual bracket position on microbial and periodontal parameters in vivo. J Appl Oral Sci. 2012;20(3):357–361. doi: 10.1590/S1678-77572012000300011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Veen MH, Attin R, Schwestka-Polly R, Wiechmann D. Caries outcomes after orthodontic treatment with fixed appliances: do lingual brackets make a difference? Eur J Oral Sci. 2010;118(3):298–303. doi: 10.1111/j.1600-0722.2010.00733.x. [DOI] [PubMed] [Google Scholar]
- 31.Schade CT, Schaberl JW, Lawley A. Stainless steel AISI grades for PM applications. Int J Powder Metall. 2008;44(3):57–67. [Google Scholar]
- 32.Auschill TM, Hellwig E, Sculean A, Hein N, Arweiler NB. Impact of the intraoral location on the rate of biofilm growth. Clin Oral Investig. 2004;8(2):97–101. doi: 10.1007/s00784-004-0255-6. [DOI] [PubMed] [Google Scholar]
- 33.Zachrisson BU, Alnaes L. Periodontal condition in orthodontically treated and untreated individuals. I. Loss of attachment, gingival pocket depth and clinical crown height. Angle Orthod. 1973;43(4):402–411. doi: 10.1043/0003-3219(1973)043<0402:PCIOTA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 34.Kloehn JS, Pfeifer JS. The effect of orthodontic treatment on the periodontium. Angle Orthod. 1974;44(2):127–134. doi: 10.1043/0003-3219(1974)044<0127:TEOOTO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 35.Berry CW, Moore TJ, Safar JA, Henry CA, Wagner MJ. Antibacterial activity of dental implant metals. Implant Dent. 1992;1(1):59–65. doi: 10.1097/00008505-199200110-00006. [DOI] [PubMed] [Google Scholar]
- 36.Auschill TM, Arweiler NB, Brecx M, Reich E, Sculean A, Netuschil L. The effect of dental restorative materials on dental biofilm. Eur J Oral Sci. 2002;110(1):48–53. doi: 10.1046/j.0909-8836.2001.101160.x. [DOI] [PubMed] [Google Scholar]
- 37.Passariello C, Gigola P. Adhesion and biofilm formation by periodontopathogenic bacteria on different commercial brackets. Eur J Paediatr Dent. 2013;14(3):199–203. [PubMed] [Google Scholar]
- 38.Dittmer MP, Helleman CF, Grade S, et al. Comparative three-dimensional analysis of initial biofilm formation on three orthodontic bracket materials. Head Face Med. 2015;11:10. doi: 10.1186/s13005-015-0062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lundstrom F, Krasse B. Streptococcus mutans and lactobacilli frequency in orthodontic patients; the effect of chlorhexidine treatments. Eur J Orthod. 1987;9(2):109–116. doi: 10.1093/ejo/9.2.109. [DOI] [PubMed] [Google Scholar]
- 40.Eliades T, Eliades G, Brantley WA. Microbial attachment on orthodontic appliances: I. Wettability and early pellicle formation on bracket materials. Am J Orthod Dentofacial Orthop. 1995;108(4):351–360. doi: 10.1016/s0889-5406(95)70032-3. [DOI] [PubMed] [Google Scholar]