Abstract
Many phytochemicals can affect the growth and development of plants and insects which can be used as biological control agents. In this study, different concentrations of crude, hexane, chloroform, butanol, and aqueous extracts of Euphorbia nivulia Buch.-Ham., an endemic plant of the Cholistan desert in South Punjab of Pakistan, were analysed for their chemical constituents. Their various concentrations were also tested for their phytotoxic and insecticidal potential against duckweed, Lemna minor L., and the dusky cotton bug, Oxycarenus hyalinipennis Costa. various polyphenols, i.e., quercetin, gallic acid, caffeic acid, syringic acid, coumaric acid, ferulic acid, and cinnamic acid were detected in different concentrations with different solvents during the phytochemical screening of E. nivulia. In the phytotoxicity test, except for 100 μg/mL of the butanol extract gave 4.5% growth regulation, no phytotoxic lethality could be found at 10 and 100 μg/mL of all the extracts. The highest concentration, 1000 μg/mL, of the chloroform, crude, and butanol extracts showed 100, 63.1, and 27.1% of growth inhibition in duckweed, respectively. In the insecticidal bioassay, the highest O. hyalinipennis mortalities (87 and 75%) were recorded at 15% concentration of the chloroform and butanol extracts of E. nivulia. In contrast, the lower concentrations of the E. nivulia extracts caused the lower mortalities. Altogether, these findings revealed that E. nivulia chloroform extracts showed significant phytotoxicity while all the extracts showed insecticidal potential. This potential can be, further, refined to be developed for bio-control agents.
1. Introduction
In nature, living organisms compete for food, space, water, and light within their communities. Plants exercise chemical interactions with other plants and biotic components around their vicinity by discharging various secondary metabolites called allelochemicals [1]. These allelochemicals may have positive or negative impacts on other plants and animals. Plants produce a vast array of phytochemicals to ensure plant survival in the stresses caused by hostile plants, animals, insects, and microbes. These phytochemicals can crumble the competing plants by affecting their growth or causing chlorosis and wilting, leading towards the death of hostile plants termed phytotoxicity [1,2].
Many plants, including weeds, have been investigated for their phytotoxic potential against different weeds [3]. These plants inhibit the germination and growth of different crops by releasing certain water-soluble phytotoxins into their adjacent environment [4,5]. Alien species chemicals are allelopathic to the native plant species that invade plants to establish in a new ecosystem and environment [6]. Most of the active allelochemicals include cinnamic acids, flavonoids, and various terpenes are considered good sources of natural herbicides [7]. In addition to have phytotoxicity, allelochemicals are also found to be involved in plant-insect interactions with their insecticidal capacities [8]. In agricultural practices, the insecticidal capacity of plants can be used to manage insect pests, which are a serious problem by decreasing the yield of crops [3].
In agriculture and forest management, weeds and insect pests cause substantial obstructions in the growth and productivity of crops and trees. Hence, tons of pesticides are applied to the agrosystem for better outcomes [9]. Chemical pesticides for crop protection cause environmental contamination due to toxic residues accumulation in addition to pesticide resistance development in pest organisms [10]. For sustainable agriculture, there is a demand to screen potential phytochemicals for phytotoxic and entomotoxic capabilities to strengthen the integrated pest management system [11]. For such purpose, several natural plant compounds were identified as active ingredients of many pesticides. It has been reported that extracts of Azadirachta indica, Calotropis procera, Cassia fistula, Chrysanthemum coronarium, Lantana camara, Murraya koenigii, and Punica granatum showed insecticidal or nematocidal effects against some insects with high safety-index to mammals. Therefore, phytochemicals would be promising bio-control agents in sustainable agriculture in the future [12].
Among angiosperms, Euphorbiaceae family is comprised of about 340 genera and about 8000 species [13]. It has been reported that many phytochemicals of Euphorbiaceae plants showed insecticidal, larvicidal, ovicidal actions against different insects [14,15]. Euphorbia tirucalli, E. pulcherrima, and E. antiquorum are known to have good larvicidal properties against mosquitoes and other insects [16]. Additionally, E. pulcherima extracts also exhibited insecticidal activity against fall armyworm with toxic effect and higher insect growth regulation (IGR) activity [17].
Euphorbia nivulia Buch.-Ham. has gained the researcher’s attention due to its outstanding biological activities, but there is still limited information reported [18]. Northern and central India is the native habitat of this plant, and it is also found in Myanmar and Pakistan [13]. It contains compounds of diterpenes and triterpenes [19]. The latex contains alkaloids, glycosides, phenolic compounds, tannins, and terpenes [18]. In a previous study, the aqueous leaf extract revealed good toxicity and insect growth regulation (IGR) against Plutella xylostella [20].
Oxycarenus hyalinipennis Costa, commonly known as dusky cotton bug, is an emerging insect pest of cotton. Generally, it causes severe damage at the seedling stage. Adults of dusky cotton bugs are 4–4.3 mm long, having black thorax with shining white wings, while nymphs are pinkish with red-orange abdomen [21]. Their heavy infestation causes multiple injuries resulted into reduced seed oil, seed weight, and cotton yield. They inject toxic saliva into bolls leading towards greasy spots appearance [22]. It has been reported that this widespread species can also be found in Southern Europe [23]. There were previous studies that investigated the utilization of plant chemicals to suppress this pest [12,24].
The present study aimed to test the phytotoxic and insecticidal capacity of E. nivulia extracts against Lemna minor and O. hyalinipennis, respectively, to reveal its biological control potentials.
2. Material and methods
2.1. Preparation of plant extracts
The aerial parts (leave, stems and flowers) of E. nivulia were collected from desert areas (29.3892848° N, 71.7878353° E) of Bahawalpur, Pakistan in a local field farm of the university and do not need a permit. The plants were identified and authenticated by the taxonomist of the Department of Botany, The Islamia University of Bahawalpur, Pakistan with voucher specimen no. EN-AP-05-12-041. The collected plant material was segmented into pieces and dried under shade at room temperature for 40 days. Later, it was ground into powder using an electric grinder and sieved through a mesh (No. 60). Then, 100 g plant powder was dissolved in 1000 ml of ethanol (70%) at room temperature for 15 days with stirring (once a day). Then, the mixture was filtered three times with muslin cloth separately. Further, filtration was performed by filter paper (Whatman Grade-1). This filtrate was then evaporated under low pressure (-760mm Hg) and temperature on a rotary evaporator (Heidholph Laborota, 4000-efficient, Germany). It resulted in a thick semi-solid brownish gummy mass that was placed in the oven for drying (Memmert Beschichung Loading, Model 100–800, Germany). Then, dried material was weighed, labeled, and stored at 4°C after the percent yield calculation for further experimentation. The same procedure was repeated using other solvents (hexane, chloroform, butanol, water).
2.2. Phytochemical screening
To identify different phytoconstituents (alkaloids, flavonoids, glycosides, phenols, saponins, tannins, etc.), qualitative phytochemical screening of the plant crude extract and its different solvent-based fractions was performed using standard procedures described by World Health Organization (WHO). Total phenolic content (TPC) was quantified, using Folin-Ciocalteu’s technique, as mg gallic acid equivalent (GAE) per gram of the extract [25]. Total flavonoid content (TFC) was assessed by the modified colorimetric method [26]. High-Performance Liquid Chromatography (HPLC) of the extracts was also performed at Central Hi-Tech Laboratory, University of Agriculture, Faisalabad, Pakistan as described previously by Pak-Dek et al., in 2020. The test samples were hydrolyzed by mixing and homogenizing 50 mg of plant extracts in 24 mL methanol. Then, 16 ml of distilled water was added followed by 10 ml of HCl (6M). This mixture was, then, heated for 2h at 95°C. The resultant solution was filtered through a 0.45 μm nylon filter before HPLC analysis. The HPLC analysis was carried out using Waters HPLC system furnished with Waters-2487 dual wavelength absorbance detector, Waters-600 Pump and controlled by Waters empower 2-software (Waters, Milford, MA). The separations were done using Waters reverse-phase (RP) Symmetry C-18 column (150*3.9 mm, 5 μm) at room temperature. The mobile phase comprised of de-ionized water with TFA (pH 2.5) as solvent-A and 99.99% methanol as solvent-B. The following gradient was used: 100–50% solvent-A (0 to 20 min), 50–40% solvent-A (20 to 30 min) and 40–100% solvent-A (30 to 40 min). The mobile-phase flow rate was held at 1 ml/ min and the detector was kept at 280 nm. Identification of phenolic compounds was established by comparing the retention time and UV-Visible spectra of the peaks with those previously obtained by injection of standards. Results were compared with the internal library of analytes maintained at Hi-Tech Lab. Quantification was performed by external calibration. The peak identifications and quantifications were done based on the judgment of retention time and area of standards, correspondingly [27].
2.3. Lemna minor culture and phytotoxicity bioassay
L. minor plants with 2–3 fronds were collected from the local university pond for this experiment. The pond water was filtered and autoclaved to use in growth media for L. minor. A phytotoxic bioassay was performed using an E-medium, prepared by mixing 0.68 g KH2PO4, 1.5 g KNO3, 1.18 g Ca(NO2)2.4H2O, 0.49 g 0.0028 g H3BO3, 0.0036 g MnCl2.4H2O, 0.0054 g FeCl2.4H2O, 0.0002 g ZnSO4.5H2O, 0.0002 g CuSO4.5H2O, 0.00012 g Na2MO4.2H2O and 0.0112 g EDTA in 1000 ml distilled water and adjusting pH between 6–7 by adding KOH (Stock solution). 100 ml stock solution was added to 900 ml distilled water to make a working E-medium. Similarly, 30 mg crude extract was also dissolved in 1.5 ml Ethanol solvent serving as stock solute. Three flasks were inoculated with 10, 100, and 1000 μl of solution from the stock solution for 10, 100 and 1000μg/ml. 20 ml working E-medium and L. minor plants (with a rosette of two to three fronds) were added to each flask (a total of 20 fronds). Other flasks were supplemented with E-medium and reference (standard-drug) plant growth inhibitors and promoters as -ve and +ve control treatments. These flasks were positioned in growth chambers for seven days. The treated plants were observed daily for plant growth, and the number of fronds in each flask was recorded on day 7. Results were analysed as percent growth regulation regarding -ve control using the following equation:
Following criterion was used for assessing phytotoxicity: 0–39% growth inhibition was low activity, 40–59% inhibition was moderate activity, 60–69% inhibition was an excellent activity, and above 70% inhibition was significant activity [28].
2.4. Oxycarenus hyalinipennis culture and insecticidal bioassay
O. hyalinipennis adults were collected from university research fields (The Islamia University of Bahawalpur, Pakistan) during 2019 and reared under laboratory conditions (25±2°C, and 65±5% R.H.). The insect population was maintained on their natural food (water-soaked fuzzy seeds of cotton) in plastic chambers (36×60×60 cm) provided with the aerial flow till next generation and third instar nymphs from this generation were used in experiments. Twenty O. hyalinipennis nymphs (3rd instar), to have a uniform population, were positioned in Petri-dish (15 cm diameter) containing cotton seedlings with roots enclosed by wet cotton to keep them alive. Afterwards, they were sprayed with 5, 10, and 15% solutions of crude, aqueous, butanol, chloroform, and hexane extracts of E. nivulia and insecticide Oberon (spiromesifen), using a fine hand-sprayer machine (Flip & Spray™ Bottles, Thomas Scientific, USA). A control treatment was maintained by applying water spray. The treatments were triplicated under laboratory conditions (25±1°C, 65±5% RH) [29].
2.5. Data analysis
Evaluation of the toxicity of different treatments was based on the corrected mortality percentage calculated through Abbot’s formula [30]:
Where n = insect population, T = treated, Co = control.
The corrected percent mortality data were analysed using factorial analysis of variance (ANOVA) by Minitab 16.1 software application. The means were separated via Tukey’s HSD test at a 5% significance level to quantify the treatment’s impact.
3. Results
3.1. Phytochemical screening
The E. nivulia extracts contain many phytochemicals like glycosides, alkaloids, saponins, flavonoids, phenols, tannins, carbohydrates, and their types were highly affected by the extracting solvents (Table 1) [31,32].
Table 1. Phytochemical evaluation of various extracts of Euphorbia nivulia Buch.-Ham.
| Test | Crude Aq. EtOH Extract | Hexane Extract | Chloroform Extract | Butanol Extract | Aqueous Extract |
|---|---|---|---|---|---|
| Carbohydrates | |||||
| Fehling’s Test | + | - | + | + | + |
| Alkaloids | |||||
| Hager’s Test | + | - | - | - | - |
| Mayer’s Test | + | - | - | - | - |
| Wagner’s Test | + | - | - | - | - |
| Glycosides | |||||
| Keller Kiliani Test | + | - | - | - | - |
| Phenolic Compounds | |||||
| FeCl3 Test | + | - | + | + | + |
| Flavonoids | |||||
| Alkali Test | + | - | + | + | + |
| Tannins | |||||
| FeCl3 Test | + | - | + | + | + |
| Saponins | |||||
| Froth Test | + | - | - | + | + |
3.2. Total flavonoid and phenolic content (TFC and TPC)
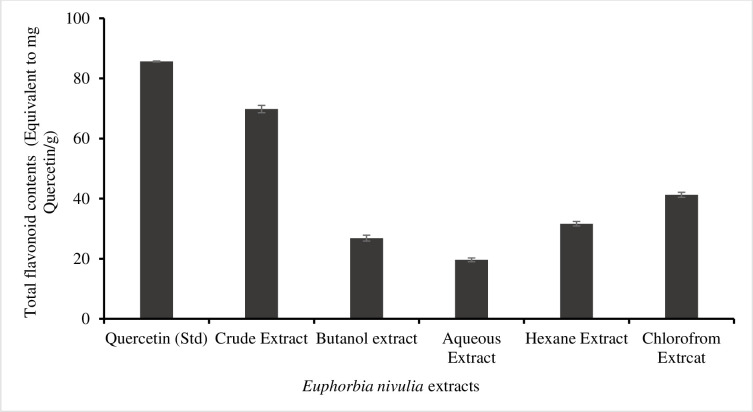
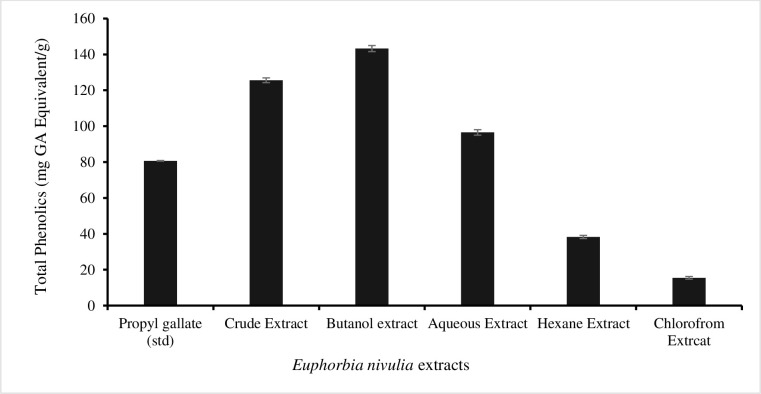
The highest flavonoid content of 69.80±1.21 mg/g was found in the E. nivulia crude extract (Fig 1). Followed by 41.26±1.23, 31.65±1.22, 26.85±0.93, and 19.63±1.14 mg/g were found in crude, hexane, butanol and aqueous extracts, respectively. In the analysis of total phenolic contents, the highest was 143.26±2.65 mg/g in the butanol extract, followed by the crude extract of 125.6±1.32 mg/g, the aqueous extracts of 96.53±2.01 mg/g, the chloroform extract of 38.27±3.21 mg/g, and the hexane extract of 15.49±1.92 mg/g (Fig 2).
Fig 1. Total flavonoid contents in Euphorbia nivulia Buch.-Ham. crude extract and various fractions.
Fig 2. Total phenolic contents in Euphorbia nivulia Buch.-Ham. crude extract and various fractions.
3.3. HPLC analysis
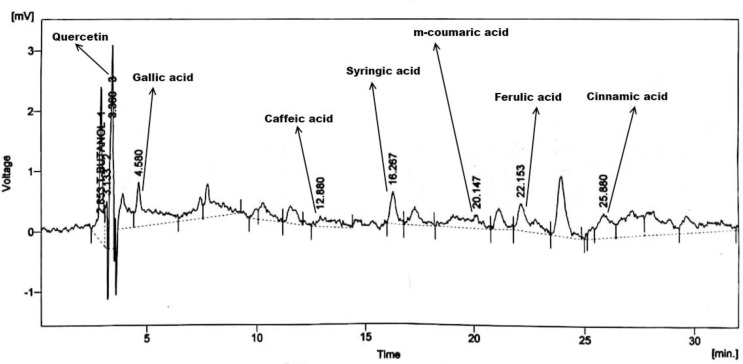
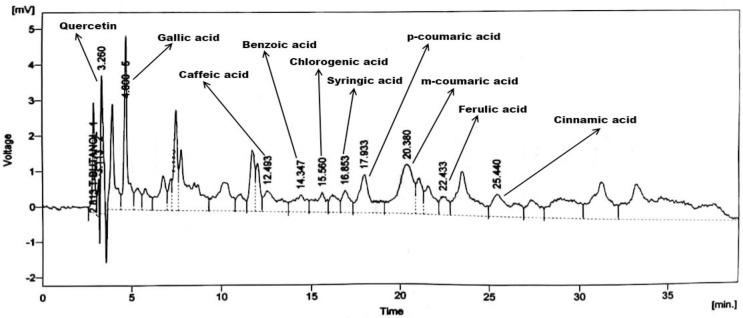
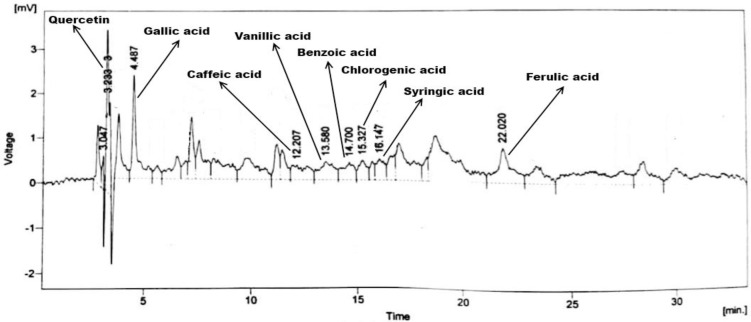
The chromatographic fingerprinting and quantitative sketching of E. nivulia extracts were done by HPLC analysis (Figs 3–5). The results confirmed the presence of polyphenols include quercetin, gallic acid, caffeic acid, vanillic acid, benzoic acid, chlorogenic acid, syringic acid, and ferulic acid in E. nivulia crude, butanol, and aqueous extracts using the library of external standards of polyphenols for calculation of polyphenol quantities in the same extracts. The presence of various polyphenols, i.e., quercetin, gallic acid, caffeic acid, syringic acid, coumaric acid, ferulic acid, and cinnamic acid in E. nivulia crude extract has already been reported (Table 2) [32]. These polyphenols were recorded as 2.13, 2.19, 1.55, 2.59, 1.85, 0.47, 0.68, 1.58, 1.22 and 1.63 (ppm/mg) in the butanol extract (Table 3). While the same polyphenols were quantified in aqueous extract of E. nivulia as 1.87, 1.24, 0.81, 1.22, 1.57, 0.92, 0.32, 2.48 ppm/mg, respectively (Table 4).
Fig 3. HPLC chromatogram of Euphorbia nivulia Buch.-Ham. crude extract.
Fig 5. HPLC chromatogram of the Euphorbia nivulia Buch.-Ham. butanol extract.
Table 2. The qualitative and quantitative analysis of phenolic compounds in the Euphorbia nivulia Buch.-Ham. crude extract using HPLC.
| Compounds | Retention time (min) | Area (mV s) | Phenolic content quantity (ppm/mg) |
|---|---|---|---|
| 1. Quercitin | 3.36 | 27.85 | 1.47 |
| 2. Gallic acid | 4.58 | 27.59 | 0.99 |
| 3. Caffeic acid | 12.85 | 9.55 | 0.43 |
| 4. Syringic acid | 16.25 | 9.78 | 0.24 |
| 5. m-coumaric acid | 20.14 | 16.67 | 0.19 |
| 6. Ferulic acid | 22.15 | 17.43 | 1.25 |
| 7. Cinnamic acid | 25.38 | 2.97 | 0.11 |
Table 3. The qualitative and quantitative analysis of phenolic compounds in the Euphorbia nivulia Buch.-Ham. butanol extract using HPLC.
| Compounds | Retention time (min) | Area (mV s) | Phenolic content quantity (ppm/mg) |
|---|---|---|---|
| 1. Quercitin | 3.26 | 37.86 | 2.13 |
| 2. Gallic acid | 4.60 | 61.19 | 2.19 |
| 3. Caffeic acid | 12.49 | 33.71 | 1.55 |
| 4. Benzoic acid | 14.34 | 24.51 | 2.59 |
| 5. Chlorogenic acid | 15.56 | 23.83 | 1.85 |
| 6. Syringic acid | 16.85 | 18.90 | 0.47 |
| 7. p-coumaric acid | 17.93 | 52.81 | 0.68 |
| 8. m-coumaric acid | 20.38 | 83.84 | 1.58 |
| 9. Ferulic acid | 22.43 | 17.18 | 1.22 |
| 10. Cinnamic acid | 25.44 | 46.69 | 1.63 |
Table 4. The qualitative and quantitative analysis of phenolic compounds in the Euphorbia nivulia Buch.-Ham. aqueous extract using HPLC.
| Compounds | Retention time (min) | Area (mV s) | Phenolic content quantity (ppm/mg) |
|---|---|---|---|
| 1. Quercitin | 3.23 | 35.47 | 1.87 |
| 2. Gallic acid | 4.48 | 34.60 | 1.24 |
| 3. Caffeic acid | 12.20 | 17.74 | 0.81 |
| 4. Vanillic aid | 13.58 | 19.62 | 1.22 |
| 5. Benzoic acid | 14.70 | 14.97 | 1.57 |
| 6. Chlorogenic acid | 15.32 | 11.82 | 0.92 |
| 7. Syringic acid | 16.14 | 13.04 | 0.32 |
| 8. Ferulic acid | 22.02 | 34.57 | 2.48 |
Fig 4. HPLC chromatogram of Euphorbia nivulia Buch.-Ham. aqueous extract.
3.4. Phytotoxicity bioassay
Table 5 showed the phytotoxic effects of E. nivulia extracts against L. minor. All concentrations of hexane and water extracts showed no phytotoxic effects. Except for 100 μg/mL of the butanol extract, all other extracts at 10 and 100 μg/mL resulted in no effects on the growth regulation of L. minor. By contrast, crude and chloroform extract treatments at 1000 μg/mL have higher effects with 63% and 100% growth regulation using, respectively.
Table 5. In vitro phytotoxic effects of different Euphorbia nivulia Buch.-Ham. extracts against Lemna minor L.
| Concentration (μg/mL) | Crude extract | Chloroform extract | Butanol extract | Hexane extract | Aqueous extract | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of fronds | % Growth regulation | No. of fronds | % Growth regulation | No. of fronds | % Growth regulation | No. of fronds | % Growth regulation | No. of fronds | % Growth regulation | |
| 0 | 57 | 0 | 41 | 0 | 44 | 0 | 41 | 0 | 41 | 0 |
| 10 | 57 | 0 | 41 | 0 | 44 | 0 | 41 | 0 | 41 | 0 |
| 100 | 57 | 0 | 41 | 0 | 42 | 4.5 | 41 | 0 | 41 | 0 |
| 1000 | 21 | 63.15 | 00 | 100 | 32 | 27.1 | 41 | 0 | 41 | 0 |
| Result | Good activity at highest concentration | Significant activity at highest concentration | Low activity at highest concentration | No activity | No activity | |||||
Standard drug: Paraquat (Mc. Laughlin et al. 1991) under incubation condition as 28 ± 1 oC.
3.5. Insecticidal bioassay
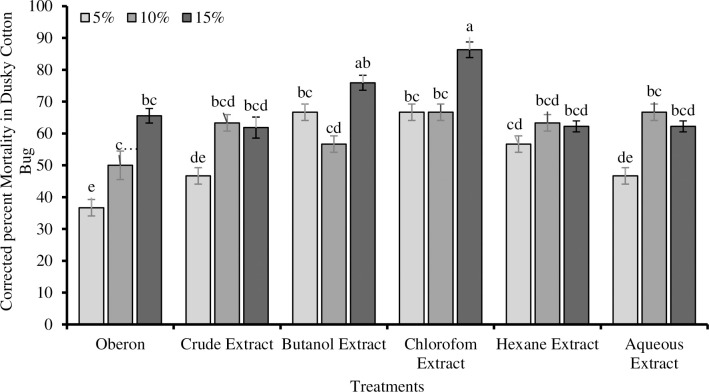
Fig 6 showed the effects of E. nivulia extracts on the mortality of O. hyalinipennis. It was found that the crude extract at 15, 10, and 5% concentrations resulted in 61, 62, and 48% mortality, respectively. Two of the highest mortalities were found as 87 and 75%, when O. hyalinipennis were treated with 15% concentrations of E. nivulia chloroform and butanol extracts, respectively. While, 15, 10, and 5% solutions of hexane and aqueous extracts showed 61, 62, 58, 61, 68, and 47% mortalities in O. hyalinipennis, respectively. The standard insecticide solution, Oberon, at concentrations of 15, 10, and 5%, resulted in 65, 50, and 36% mortalities in O. hyalinipennis, respectively.
Fig 6. Corrected % mortality in Oxycarenus hyalinipennis (dusky cotton bug) after exposure to different solvent-based extracts of Euphorbia nivulia Buch.-Ham. and an insecticide Oberon, at different concentrations.
4. Discussion
In E. nivulia, the investigation of phenolic compounds was limited to only a few compounds such as gallic acid, caffeic acid, and syringic acid [32]. In the present study, the phenolic profile of E. nivulia extracts was done by HPLC qualitative and quantitative analysis. It expanded the knowledge regarding various polyphenols, including quercetin, gallic acid, caffeic acid, vanillic acid, benzoic acid, chlorogenic acid, syringic acid, m-coumaric acid, p-coumaric acid, cinnamic acid, vanillic acid, and ferulic acid. The current research was further extended to investigate their phytotoxic and insecticidal potentials of crude extract and other solvent fractions of E. nivulia. It has been reported that phenolics, particularly water-soluble allelochemicals are responsible for the most allelopathic activities and thus, could cause growth-inhibitory effects to individual plants [33]. A few polyphenols, such as cinnamic acid derivatives (caffeic and p-coumaric acids), benzoic acid derivatives (vanillic and syringic acids), could impose deleterious impact (due to their water solubility) on their neighboring vicinity that present in adequate quantities [34].
Furthermore, phytochemicals, including phenolic acids, are germination and plant growth inhibitors [5]; this harmful effect of these phenolics might be due to their additive or synergistic actions [33]. Gibberellic acid controls α-amylase production and is also inhibited by phenolics [35]. These allelopathic phytotoxins affect the target species’ structural and physiological cell functions, leading to weakened germination and reduced growth [36].
Phytotoxic bioassay showed the depressive impact of E. nivulia crude and chloroform extracts on germination, growth, and some biochemical aspects of L. minor. The allelopathic potential of crude extract and its fractions in various concentrations was demonstrated in weeds’ germination. Significant (P ≤ 0.05) delay in the time to start germination and E50 over the control was provoked by the plant samples. The study’s findings confirmed the significant inhibitory effects against the tested species for seedling germination and growth. These significant phytotoxic effects are due to allelochemicals existing in the plant species affecting the different physiological processes, possibly through their effects on enzymes responsible for phytohormone synthesis and the inhibition of nutrients movement ion absorption by affecting plasma membrane penetrability [37].
Similarly, significant inhibition was observed at the higher concentrations of the plant extracts. The decreased plant germination may result from the allelochemical stress due to inhibition of water uptake [38] and altered activity of gibberellic acid, which is known for regulation of amylase production during the germination course. Cell elongation and division may also be inhibited by allelochemical [39]. The release of phytotoxins from incorporated crop residues by leaching or decomposition leads to reduced growth and development [40]. Allelopathic compounds are rapidly solubilized and imbibed by the germinating seeds, retard or delay emergence, and adversely affect the subsequent seedling growth. Phenolics, glycosides, terpenoids, and other secondary metabolites found in the crude extract and various plant fractions can exhibit phytotoxicity in plants [41–43]. Several workers have reported the allelopathic property of saponins [44,45] and amino acids against plants [20]. Phenolic compounds with their derivatives play an inhibitory role in stressing germination and seedling growth. They have allelopathic applications as herbicides in agriculture and forestry [34]. Phytotoxins may affect the membrane absorbency, ion-uptake, photosynthetic electron transport and respiratory chains, enzyme activity, and cell division [46]. Hence, it can be inferred that these active ingredients detected in this species may be responsible for Lemna plant growth inhibition.
E. nivulia extracts showed strong insecticidal activity because euphorbiaceous plants contain chemical constituents like triterpenoids and related compounds (alcohols, sterols, and hydrocarbons), phenolic compounds, alkaloids, cyanogenic glucosides, and glucosinolates [47]. Previously, several studies had shown pesticidal and insecticidal effects of Euphorbia plants [48]. Euphorbia plants are rich in latex, and latex toxicity is well established and reported. For example, the latex of E. tirucalli is composed of a range of toxic substances, including phenolics, ellagic acid and tannins [49], triterpenes [50,51], and diterpene esters [52]. These are the most relevant compounds to entomotoxicity and numerous biological activities [53,54]. Similarly, insecticidal properties of the latex of E. antiquorum have been reported previously De. Silva et al [16]. This is probably the first report on the phytotoxic and insecticidal potentials of E. nivulia crude extract and its various fractions. In our study, the mortality increased with increased concentration at all the doses at 96h exposure to the extracts showing remarkable insecticidal activity against the dusky cotton bug. Previously, N. tobacum and C. procera exhibited significant mortalities in O. hyalinipennis during various phytochemicals screening [24]. It is an indication to promote green chemistries for the effective management of agricultural pests. Indeed, phytochemical analyses and screening for active biological compounds can help greatly in this regard.
5. Conclusion
The findings of the present study suggested that chloroform and crude extracts of E. nivulia have good phytotoxic potential at their higher concentration. In contrast, all solvent-based extracts showed variable insecticidal potential against dusky cotton bugs. This might be due to different chemical constituents are extracted with different solvents with different actions. So, further studies of phytochemicals extracted with different solvents and conditions from E. nivulia could be focused on the selective action against harmful weeds and insect pests of field crops to avail opportunities to benefit sustainable agriculture in the future.
Acknowledgments
The authors acknowledge the Department of Entomology, Faculty of Agriculture and Environment Science, The Islamia University of Bahawalpur, Pakistan, and Department of Pharmacognosy, Faculty of Pharmacy & Pharmaceutical Sciences, University of Karachi, Karachi, Pakistanfor facilitating to carry out this study. The authors would like to extend their sincere appreciation to the Taif University Researchers Supporting Project number (TURSP—2020/141) from the Deanship of Scientific Research at Taif University, Taif, Saudi Arabia.
Data Availability
All relevant data are within the manuscript.
Funding Statement
The research is supported by the Endowment Fund Secretariate (EFS), University of Agriculture, Faislabad, Pakistan. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Cheng F.; Cheng Z. Research Progress on the use of Plant Allelopathy in Agriculture and the Physiological and Ecological Mechanisms of Allelopathy. Front Plant Sci. 2015, 6: 1020. 10.3389/fpls.2015.01020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lungu L.; Popa C.V.; Morris J; Savoiu M., Evaluation of phytotoxic activity of Melia azedarach L. extracts on Lactuca sativa L. Rom. Biotechnol. Lett. 2011, 16(2): 6089–6095. [Google Scholar]
- 3.Jabran K.; Mahajan G.; Sardana V.; Chauhan B.S. Allelopathy for weed control in agricultural systems. Crop. Prot. 2015, 72: 57–65. [Google Scholar]
- 4.Singh H.P.; Batish D.R.; Setia N.; Kohli R.K. Herbicidal activity of volatile oils from Eucalyptus citriodora against Parthenium hysterophorus. Annu. Appl. Biol. 2005, 146(1): 89–94. [Google Scholar]
- 5.Batish D.R.; Singh H.P.; Kaur S.; Kohli R.K.; Yadav S.S. Caffeic acid affects early growth, and morphogenetic response of hypocotyl cuttings of mung bean (Phaseolus aureus). J. Plant Physiol. 2008, 165(3): 297–305. 10.1016/j.jplph.2007.05.003 [DOI] [PubMed] [Google Scholar]
- 6.Callaway R.M.; Ridenour W.M. Novel weapons: invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2(8): 436–443. [Google Scholar]
- 7.Singh H.P.; Batish D.R.; Pandher J.K.; Kohli R.K. Assessment of allelopathic properties of Parthenium hysterophorus residues. Agr. Ecosyst. Environ. 2003, 95(2–3): 537–541. [Google Scholar]
- 8.Weir T.L.; Park S.W.; Vivanco J.M. Biochemical and physiological mechanisms mediated by allelochemicals. Curr. Opin. Plant. Biol. 2004, 7(4): 472–479. 10.1016/j.pbi.2004.05.007 [DOI] [PubMed] [Google Scholar]
- 9.Akkari H.; Ezzine O.; Dhahri S.; Bchir F.; Rekik M.; Hajaji, Aziz, M.; et al. Chemical composition, insecticidal and in vitro anthelmintic activities of Ruta chalepensis (Rutaceae) essential oil. Ind. Crop. Prod. 2015, 74: 745–751. [Google Scholar]
- 10.Nenaah G.E.; Ibrahim S.I.; Al-Assiuty B.A. Chemical composition, insecticidal activity and persistence of three Asteraceae essential oils and their nanoemulsions against Callosobruchus maculatus (F.). J. Stored Prod. Res. 2015, 61: 9–16. [Google Scholar]
- 11.Ahmad H.; Ali N.; Ahmad B.; Khan I. Screening of solanum surrattense for antibacterial, antifungal, phytotoxic and haemagglutination. J. Tradit. Chin. Med. 2012, 32(4): 616–620. 10.1016/s0254-6272(13)60080-1 [DOI] [PubMed] [Google Scholar]
- 12.Khan M.F.; Ahmed S.M. Toxicity of neem fruit extract and seed oil against Oxycarenus (Heteroptera) of cotton crop. Acta Biol. Cracov. Zool. 2000, 42: 14–21. [Google Scholar]
- 13.Radcliffe-Smith A. Euphorbiaceae Flora of Pakistan (Last Modified On 6/3/2011), Tropicos.org. Missouri Botanical Garden, web site:< http://www.tropicos.org/Name/12800166. 2011. [Google Scholar]
- 14.Rahuman A.A.; Gopalakrishnan G.; Venkatesan P.; Geetha K. Larvicidal activity of some Euphorbiaceae plant extracts against Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitol. Res. Parasitology Res. 2008, 102(5): 867–73. [DOI] [PubMed] [Google Scholar]
- 15.Yusuf Y.; Efendi K.; Diantasari S. Larvicidal Activity Test of Ethanolic Extract of (Euphorbia tirucalli Linn) Stem on Aedes aegypti Larvae. Sys. Rev. Pharm. 2020, 11(3): 388 392. 10.1016/j.jcot.2020.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Silva W.P.; Manuweera G.K.; Karunaratne S.H.P. Insecticidal activity of Euphorbia antiquorum L. latex and its preliminary chemical analysis. J. Natl. Sci. Found. 2008, 36(1): 15–23. [Google Scholar]
- 17.Almeida V.T.; Ramos V.M.; Saqueti M.B.; Gorni P.H.; Pacheco A.C.; de Leão R.M. Bioactivity of ethanolic extracts of Euphorbia pulcherrima on Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Afr. J. Biotechnol. 2017, 16(13): 615–622. [Google Scholar]
- 18.Mahajan R.T.; Badgujar S.B. Bioprospecting of Euphorbia nivulia Buch .-ham. Int. J. Phytopharm. 2011, 2(2): 37–42. [Google Scholar]
- 19.Ravikanth V.; Reddy V.L.N.; Reddy A.V.; Ravinder K.; Rao T.P.; Ram T.S.; et al. Three new ingol diterpenes from Euphorbia nivulia: evaluation of cytotoxic activity. Chem. Pharm. Bull. 2003, 51(4): 431–434. 10.1248/cpb.51.431 [DOI] [PubMed] [Google Scholar]
- 20.Uma M.S.; Prasanna P.M.; Reddy G.V.M.; Kumar A.R.V. Efficacy of some Euphorbiaceae plant extracts against cabbage diamondback moth, Plutella xylostella L. Karnataka J. Agr. Sci. 2009, 22(3): 688–689. [Google Scholar]
- 21.Smith T.R.; Brambila J. A major pest of cotton, Oxycarenus hyalinipennis (Heteroptera: Oxycarenidae) in the Bahamas. Flor. Entomol. 2008, 91: 479–482. [Google Scholar]
- 22.Schaefer C.W.; Panizzi A.R. (Eds.). Heteroptera of economic importance. 2000, CRC press. [Google Scholar]
- 23.De Jong Y. Fauna Europaea—all European animal species on the web. Biodivers. Data J. 2014, 2: e4034. 10.3897/BDJ.2.e4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbas M.; Hafeez F.; Farooq M.; Ali A. Dusky cotton bug Oxycarenus spp. (Hemiptera: Lygaeidae); Hibernating sites and management by using plant extracts under laboratory conditions. Polish J. Ent. 2015, 84: 127–136. [Google Scholar]
- 25.Chlopicka J.; Pasko P.; Gorinstein S.; Jedryas A.; Zagrodzki P. Total phenolic and total flavonoid content, antioxidant activity and sensory evaluation of pseudocereal breads. Lwt-Food. Sci. Technol. 2012, 46(2): 548–555. [Google Scholar]
- 26.Wolfe K.; Wu X.; Liu R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51(3): 609–614. 10.1021/jf020782a [DOI] [PubMed] [Google Scholar]
- 27.Pak-Dek MS.; Osman A.; Sahib NG. Effects of extraction techniques on phenolic components and antioxidant activity of Mengkudu (Morinda citrifolia L.) leaf extracts. J. Med. Plants Res. 2011, 5(20): 5050–5057. [Google Scholar]
- 28.Acosta-Motos J.R.; Ortuño M.F.; Bernal-Vicente A.; Diaz-Vivancos P.; Sanchez-Blanco M.J.; Hernandez J.A. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy, 2017, 7(1): 18. [Google Scholar]
- 29.Da Silva Mesquita R.; Kyrylchuk A.; Grafova I.; Kliukovskyi D.; Bezdudnyy A.; Rozhenko A. Synthesis, molecular docking studies, and larvicidal activity evaluation of new fluorinated neonicotinoids against Anopheles darlingi larvae. PLoS ONE. 2020, 15(2): e0227811. 10.1371/journal.pone.0227811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abbott W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18(2): 265–267. [Google Scholar]
- 31.Younus M.; Hasan M.M.; Abbas K.; Sarwar G. Pharmacognostic and physicochemical screening of Euphorbia nivulia Buch.-Ham. Pak. J. Pharm. Sci. 2019, 32(3): 1111–1119. [PubMed] [Google Scholar]
- 32.Younus M.; Hasan M.M. α-Glucosidase Inhibitory, Anti-Oxidant, and Anti-Hyperglycemic Effects of Euphorbia nivulia–Ham. in STZ-Induced Diabetic Rats. Dose-Response: An Inter. J. 2020, 2020: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blum U. Allelopathic interactions involving phenolic acids. J. Nematol. 1996, 8(3): 259. [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z.H.; Wang Q.; Ruan X.; Pan C.D.; Jiang D.A. Phenolics and plant allelopathy. Molecules, 2010, 15(12): 8933–52. 10.3390/molecules15128933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Einhellig F.A. Interactions involving allelopathy in cropping systems. J. Agron. 1996, 88(6): 886–893. [Google Scholar]
- 36.Duke S.O.; Dayan F.E.; Romagni J.G.; Rimando A.M.; Rosén P.; Dåbakk E.; et al. Natural products as sources of herbicides: current status and future trends. Weed Res. 2000, 40(1): 99–111. [Google Scholar]
- 37.Daizy R.; Manpreet B.K.; Harminder P.S.; Ravinder K.K. Phytotoxicity of a medicinal plant, Anisomeles indica, against Phalaris minor and its potential use as natural herbicide in wheat fields. Crop Protec. 2007, 26(7): 948–952. [Google Scholar]
- 38.Tawaha A.M.; Turk M.A. Allelopathic effects of black mustard (Brassica nigra) on germination and growth of wild barley (Hordeum spontaneum). J. Agron. Crop Sci. 2003, 189(5): 298–303. [Google Scholar]
- 39.Oudhia P.; Pandey N.; Tripathi R.S. Allelopathic effects of weeds on germination and seedling vigor of hybrid rice. Int. Rice Res. Notes. 1999, 24(2): 1–1. [Google Scholar]
- 40.Birkett M.A.; Chamberlain K.; Hooper A.M.; Pickett J.A. Does allelopathy offer real promise for practical weed management and for explaining rhizosphere interactions involving higher plants? Plant and Soil, 2001, 232(1–2): 31–39. [Google Scholar]
- 41.Abrosca B.D.; DellaGreca M.; Fiorentino A.; Monaco P.; Natale A.; Oriano P.; et al. Structural characterization of phytotoxic terpenoids from Cestrum parqui. Phytochemistry, 2005, 66(22): 2681–2688. 10.1016/j.phytochem.2005.09.011 [DOI] [PubMed] [Google Scholar]
- 42.Jha P.; Norsworthy J.K.; Riley M.B.; Bridges W. Annual changes in temperature and light requirements for germination of Palmer amaranth (Amaranthus palmeri) seeds retrieved from soil. Weed Sci. 2010, 58(4): 426–432. [Google Scholar]
- 43.Mancini E.; Camele I.; Elshafie H.S.; De Martino L.; Pellegrino C.; Grulova D.; et al. Chemical composition and biological activity of the essential oil of Origanum vulgare ssp. hirtum from different areas in the Southern Apennines (Italy). Chem. Biochem. 2014, 11(4): 639–651. [DOI] [PubMed] [Google Scholar]
- 44.Lee D.L.; Prisbylla M.P.; Cromartie T.H.; Dagarin D.P. Howard S.W.; Provan W.M.; et al. The discovery and structural requirements of inhibitors of p-hydroxyphenylpyruvate dioxygenase. Weed Sci. 1997, 45(5): 601–609. [Google Scholar]
- 45.Chaieb I.; Mediouni J. Insecticidal Efficacy of Dust/Saponins Association. Tunis. J. Plant Prot. 2012, 7: 90–95. [Google Scholar]
- 46.Romero-Romero T.; Anaya A.L.; Cruz-Ortega R. Screening for effects of phytochemical variability on cytoplasmic protein synthesis pattern of crop plants. J. Chem. Ecol. 2002, 28(3): 617–629. 10.1023/a:1014504531418 [DOI] [PubMed] [Google Scholar]
- 47.Rizk A.F.M. The chemical constituents and economic plants of the Euphorbiaceae. Bot. J. Linn. Soc. 1987, 94(1–2): 293–326. [Google Scholar]
- 48.Ayatollahi A.M.; Ghanadian M.; Afsharypuor S.; Siddiq S.; Pour-Hosseini S.M. Biological screening of Euphorbia aellenii. Iran. J. Pharm. Res. 2010, 9(4): 429. [PMC free article] [PubMed] [Google Scholar]
- 49.Lin W.; Huaqin H.E.; Yichun G.U.O.; Fangyu C.H.E.N. Rice allelopathy and its physiobiochemical characteristics. J. Appl. Ecol. 2001, 12(6): 871–875. [Google Scholar]
- 50.Khan A.Q.; Kazmi S.N.U.H.; Ahmed Z.; Malik A. Euphorcinol: A new pentacyclic triterpene from Euphorbia tirucalli. Planta Med. 1989, 55(3): 290–291. 10.1055/s-2006-962008 [DOI] [PubMed] [Google Scholar]
- 51.Rasool N.; Khan A.Q. A taraxerane type triterpene from Euphorbia tirucalli. Phytochemistry. 1989, 28: 1193–1196. [Google Scholar]
- 52.Khan A.Q.; Malik A. A new macrocyclic diterpene ester from the Latex of Euphorbia tirucalli. J. Nat. Prod. 1990, 53: 728–731. [Google Scholar]
- 53.Sandhyarani G.; Kumar K.P. Insecticidal activity of ethanolic extract of leaves of Euphorbia nivulia. Eur. J. Pharmacol. Toxicol. 2014, 4(2): 102–104. [Google Scholar]
- 54.Vimal J.B.; Das S.S.M. Euphorbia antiquorum latex and its mosquitocidal potency against Aedes aegypti. J. Entomol. Zool. Stud. 2014, 2: 267–269. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.