Abstract
Asthma is a chronic inflammatory disease of the airways that afflicts over 30 million individuals in the United States and over 300 million individuals worldwide. The inflammatory response in the airways is often characterized by the analysis of sputum which contains multiple types of cells including neutrophils, macrophages, lymphocytes, and rare bronchial epithelial cells. Subtyping patients using microscopy of the sputum has identified both neutrophilic and eosinophilic infiltrates in airway inflammation. However, with the extensive heterogeneity among these cell types, a higher resolution understanding of the inflammatory cell types present in the sputum is needed to dissect the heterogeneity of disease. Improved recognition of the distinct phenotypes and sources of inflammation in asthmatic granulocytes, may identify relevant pathways for clinical management or investigation of novel therapeutic mediators. Here, we employed mass cytometry or CyTOF (Cytometry by Time-Of-Flight) to quantify frequency and define functional status of sputum derived airway cells in asthmatic patients and healthy controls. This in-depth single cell analysis identified multiple distinct subtypes of airway immune cells between healthy controls and asthmatic patients, and within an asthmatic patient cohort, especially in neutrophils. Significance was discovered by statistical analysis as well as a data-driven unbiased clustering approach. Our multidimensional assessment identifies differences in cellular function that may contribute to diverse clinical responses and provide meaningful insights to advance our knowledge of asthmatic inflammation.
Keywords: neutrophil, eosinophil, dendritic cell, mass cytometry, inflammation
Summary Sentence:
An in-depth single cell analysis identifies multiple distinct subtypes of airway immune cells and provides insights of cellular function relevant for pathogenesis of asthma.
Introduction
Asthma is a chronic inflammatory disease of the airways that afflicts over 30 million individuals in the United States and over 300 million individuals worldwide [1]. It is characterized by bronchial hyperreactivity and variable, chronic respiratory symptoms that are non-specific in the clinic--asthma is now recognized to be clinically and biologically heterogenous [2]. While a majority of individuals have mild disease and mild symptoms that are easily controlled, 10–15% of individuals have severe asthma and require higher doses of inhaled corticosteroids (ICS), are more likely to require hospitalization or oral corticosteroids (OCS), and have worse lung function [3]. Biologically, most patients have airway inflammation that is characterized by T2 inflammation, a specific IgE production, mucus production, and infiltration of eosinophils, T cells, and monocytes [4]. A minority of patients have what is termed non-T2 disease and lack allergies. The inflammatory response in these patients is poorly understood but is typically associated with neutrophilic inflammation and no specific IgE response [5, 6].
Heterogeneity is a hallmark of asthma symptomatology and response to therapy [7]. There is increasing appreciation that asthma is driven by both adaptive and innate immune responses to varying degrees in every individual. This underscores the need for a higher resolution understanding of the inflammatory responses associated with the disease. Neutrophils are generally the most abundant immune cells recovered from sputum, however, the heterogeneity of these cells and their function in the airway in asthma remains incompletely understood [8]. While the cytokines and effector cells associated with a T2 inflammatory response have been extensively studied, our understanding of granulocyte populations including eosinophils and neutrophils remains limited. A more in-depth understanding of granulocyte subsets present in the airway and their function in both T2 and non-T2 disease will improve our understanding of asthma heterogeneity and move us towards more precise approaches to treating asthmatic patients [9].
Powerful technical advances now allow phenotypic profiling of protein and gene expression from primary airway cells with only a few thousand cells. These single cell technologies have provided techniques that can resolve disease in humans at an unprecedented level of detail, capturing the clinical and biological heterogeneity of disease. In addition, definition of the microbiome—from airway and gut--may provide a mechanistic link to disease severity [10]. Thus, we now have the potential to identify inflammatory pathways within a single patient, paving the way for more targeted therapies to treat asthmatics who remain refractory to medication even with current biologics [11].
We have previously taken advantage of the novel cell profiling platform, CyTOF, to detect cells in induced sputum and numerous studies have validated this platform for multiparameter profiling of single cells from heterogeneous populations [12–15]. Here we describe the methods that are currently in use to non-invasively collect and analyze cells from sputum samples from patients enrolled in the Yale Center for Asthma and Airway Diseases (YCAAD) asthma phenotyping protocol [16, 17]. We focus on the profiling of neutrophils and eosinophils using standard gating approaches and computational profiling and a hybrid of both approaches. This in-depth profiling can define functional cell subsets relevant both for investigation of disease mechanisms and for the potential integration of single-cell technology into clinical trial research.
Methods
Human Subjects
Asthmatic and healthy control participants were enrolled with written informed consent under the guidelines of the Institutional Ethics Committee of Yale University which approved this study. Samples were obtained as part of the Yale Center for Asthma and Airway Diseases (YCAAD) phenotyping protocol which collects clinical characteristics and induced sputum samples according to established protocols [18–20]. Asthma diagnosis was adopted from the Global Initiative for Asthma (GINA) guidelines including historical evidence of variable airflow obstruction determined by an improvement in FEV1 > 12% and 200 ml compared to baseline after a short-acting bronchodilator, or treatment with corticosteroids, 20% diurnal variation of peak expiratory flow rates on 2 days over a 2–3 week period, or methacholine reactivity causing a 20% decrease in FEV1 (PC20) of <8 mg/ml [18, 21]. Healthy controls were non-smokers (former smokers had not smoked in 1 year or more and smoked ≤ 10 pack years) without fever or antibiotics at the time of sputum induction and confirmed to have no history of lung disease per patient report and/or medical record review.
Sample collection and processing
Airway cell samples were acquired by sputum induction with hypertonic saline as described previously with minimal processing and gentle treatment of cells to optimize viability [7, 12]. Mucus plugs were isolated from sputum sample on the day of collection, washed, dissolved in DTT (Calbiochem/EMD Millipore, Billerica, MA), and centrifuged to generate cell pellet and supernatant as described. Cell viability was assessed microscopically using trypan blue staining. Airway cells were resuspended in RPMI (0.5 – 2.0 × 106 cells/ml) and treated with 25 U/mL DNAse (Sigma-Aldrich, St. Louis, MO) and 150U/mL collagenase IV (Worthington Chemical, Lakewood, NJ) for 15 minutes at 37°C as described previously [7, 12, 18–20].
Sample Labelling and Mass Cytometry Acquisition
Surface markers on fresh airway cells were labeled prior to fixation for optimal labeling as described previously [12]. Metal-conjugated antibodies (Table S1) for labeling cells were purchased (Fluidigm, Markham, ON), or carrier-free antibodies were conjugated in house using MaxPar X8 labeling kits according to manufacturer’s instructions (Fluidigm). EPX antibody was the generous gift of Elizabeth Jacobsen, PhD from the Mayo Clinic, Scottsdale, AZ [22]. Briefly, cells were washed with CyFACS buffer (PBS with 0.1% BSA, 2 nM EDTA, 0.05% Na azide; Maxpar® Cell Staining Buffer; Fluidigm, South San Francisco, CA), resuspended in 5 μM cisplatin (Enzo Life Sciences) for 5 minutes, and quenched with 100% FBS. Samples were incubated with 11% Fc blocking buffer (Biolegend, Dedham, MA), surface labeled for 30 min on ice, fixed (BD FACS Lyse), and frozen at -80°C. Intracellular labeling was conducted on batches of samples (10–20/day). Fixed cells were permeabilized (BD FACS Perm II) for labeling with intracellular antibodies for 45 min on ice. Cells were suspended overnight in iridium interchelator (125 nM; Fluidigm) in 2% paraformaldehyde in PBS and washed 1X in PBS and 2X in H2O immediately before acquisition. Samples were run on Helios 2 CyTOF instrument at a flow rate of 30 μL/min and a minimum of 100,000 events was collected.
CyTOF data processing and analysis
Raw FCS files were bead normalized [23] and imported to Cytobank for gating. Live cells were determined following exclusion of debris (DNAlo), dead cells (cisplatinhi), and doublets (event count >40) using the default data transformation of a hyperbolic arcsine with cofactor 5 (Figure S1). Individual subsets from the data were furthered compared using CyTOFKit, an R package for analyzing mass cytometry data (github.com/JinmiaoChenLab/cytofkit). Cells were visualized using a tSNE plot and clustered using the FlowSOM algorithm [24] on functional markers HLA-DR, IL-5, CD69, IL-5R, eotaxin, MIP-1β, IL-6, Siglec-8, IL-8, CD62L, CCR3, and CD16 with k = 9 clusters and random seed 42. Statistical analysis was performed using GraphPad Prism 8.
Results
Profile of airway cell phenotypes
To provide more in-depth definition of neutrophils in asthma and heterogeneity of functional status that may be relevant clinically or therapeutically, we quantified immune cell subsets of untreated sputum samples in a cohort of well-characterized adult asthmatic patients compared to healthy volunteers (Table 1). We employed a 42-marker antibody panel focused on defining neutrophil phenotypes and functional status (Table S1). Cell counts and frequencies of immune cell subsets were determined after manual gating according to a standard strategy (Fig. S1) with exclusion of debris (DNAlo), doublets (event count >40), and dead cells (cisplatinhi). When we compared samples of sputum from asthmatic subjects and healthy controls, we found that the total number of live cells recovered from a sputum sample was not different between asthmatic (AS) and healthy control (HC) subjects (Fig 1A; AS 29,217 ± 5,108 vs HC 62,144 ± 17,509, mean ± sem; Mann Whitney NS). The viability of cells from both groups was high (71.4%) and was not different between asthmatic patients and healthy controls (AS 70.6 ± 2.7%; HC 73.3 ± 6.2%, NS). Similarly, the percentage of live singlet cells detected was equal for both groups (28% of total events). Cytokeratin+ squamous epithelial cells were present at <5% of total and were not different between groups (AS 4.1 ± 1.3; HC 2.0 ± 0.6; NS). This demonstrates that the total yield of viable cells is sufficient for in depth characterization of sputum as we have previously shown statistically equivalent detection of major cell subjects in peripheral blood mononuclear cells from as few as 10,000 cells [25].
Table 1.
Study Subject Demographics and Clinical Status
| Severe Asthmatics (n = 7) | Moderate/Mild Asthmatics (n = 11) | Healthy Controls (n = 9) | |
|---|---|---|---|
| Demographics | |||
| Age mean ± SD, | 48.14 ± 14.22 | 50.36 ± 15.70 | 43.89 ± 16.82 |
| Sex Female, (n), % | (5/7) 71.43% | (7/11) 63.64% | (8/9) 88.89% |
| Race/Ethnicity (n) | |||
| Caucasian | 3 | 6 | 5 |
| African American/Black | 4 | 5 | 1 |
| Asian | 0 | 0 | 2 |
| Other | 0 | 0 | 1 |
| Hispanic/Latino | 1 | 1 | 0 |
| BMI mean ± SD | 38.38 ± 5.31 | 28.44 ± 6.33 | 25.39 ± 7.10 |
| Clinical Characteristics | |||
| FEV1 % (mean ± SD) | 65.5 ± 18.22 | 74.36 ± 16.43 | |
| L (mean ± SD) | 1.98 ± 1.09 | 2.11 ± 0.68 | |
| BD Reversibility % (mean ± SD) | 4.5 ± 6.3 | 8.2 ± 5.5 | |
| ICS total/day mcg (mean ± SD) | 934 ± 206 | 268 ± 203 | |
| Biologic use | (2/7) 28.57% | (1/7) 14.29% | |
Figure 1. Frequency of cells from sputum.
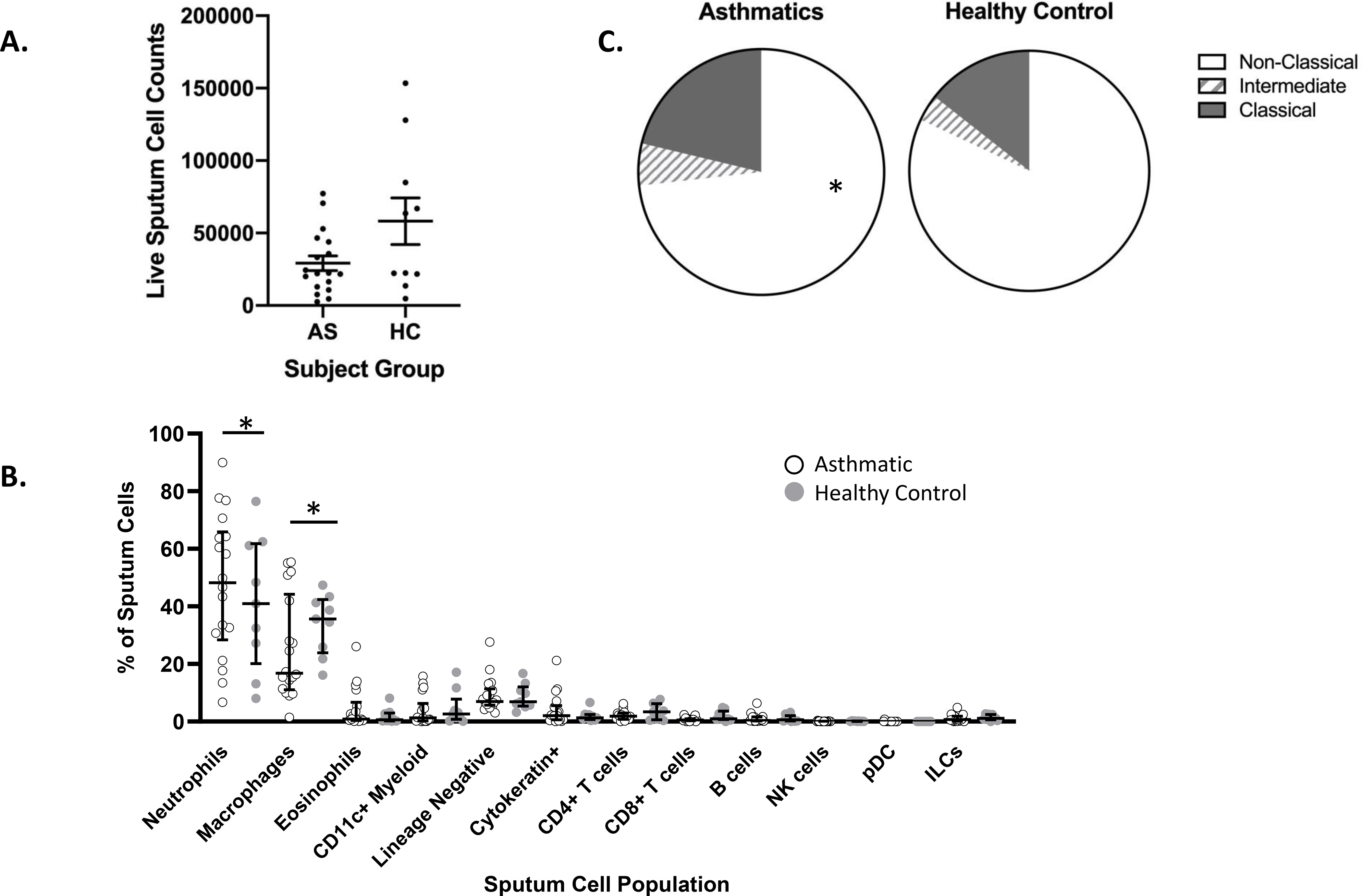
Induced sputum samples from asthmatic (AS, n=18) and healthy control (HC, n= 9) subjects were labeled fresh on the day of collection with metal-conjugated antibodies and analyzed by mass cytometry. (A) Cell yield mean ± sem, NS (B) Frequency of cell subsets (C) Frequency of macrophage subsets. Data shown is median with interquartile range; Wilcoxon signed rank test, * P<0.01.
Altered frequency of immune cell subsets in asthmatic patients
To provide in depth profiles of airway cells in asthma, sputum samples were labeled with antibodies for cell lineage specific surface markers (n=28). We quantified differences in 13 distinct cell subsets simultaneously using a manual gating strategy to define airway cell types (Fig. S1). As expected, the most abundant live cell population in sputum in both asthmatics and healthy controls was neutrophils, which were higher in frequency in asthmatic patients consistent with increased inflammation in asthma (Fig. 1B; AS 48.2; HC 40.9; p< 0.01). Macrophages, the second most abundant cell type in sputum, were lower in asthmatic patients compared to healthy controls (Fig. 1B; AS 16.8%; HC 35.6%; P<0.01). Macrophages in sputum have historically been defined morphologically. Here we used expression levels of macrophage markers to define distinct subsets of “classical” CD14++CD16-, “intermediate” CD14++CD16+ and “non-classical” CD14+/lowCD16+ monocytes [26] which have been shown to have distinct functional roles in tissue [27, 28]. Macrophages from sputum were predominantly CD16+ (non-classical) and were lower in asthmatic patients (Fig. 1C; AS 12.2% vs HC 25.8%). CD16+ cells are defined as more anti-inflammatory [27, 28] which may be relevant to the heterogeneity of asthma. Additional cells types present in lower frequencies are readily detected by CyTOF (Fig. 1B) and defined by expression of surface markers as T lymphocytes (CD4+ and CD8+), B lymphocytes, eosinophils, NK cells, dendritic cells, and cytokine-producing innate lymphoid cells (ILCs) [29]. No significant differences in frequencies between asthmatic patients and healthy controls were detected for these less abundant cell types.
Heterogeneity and functional status of neutrophils in asthmatic patients
Sputum cell composition across asthmatic patients was strikingly heterogenous which is a known characteristic of asthma [30]. While overall the asthmatic patients had more neutrophils compared to the healthy controls, individual asthmatics showed dramatic differences from one another in the proportion of neutrophils (Fig. 2A). Indeed, the proportion of neutrophils ranges from 6.7% to 83.3%. Other cells types were also highly variable including macrophages (1.5%53.9%) and eosinophils (range 0.1–25.9% of total). Lower levels of eosinophils may also be due to being well controlled with medication (i.e. ICS or mepolizumab) at the time of the visit [31]. Differences detected in the frequencies of 13 distinct immune cell subsets highlights significant differences expected between patients and controls (Fig. 2B) and may have implications for pathogenesis and subtyping of asthma [3, 30, 32].
Figure 2. Frequencies of sputum cell sub-populations in asthmatics and healthy controls.
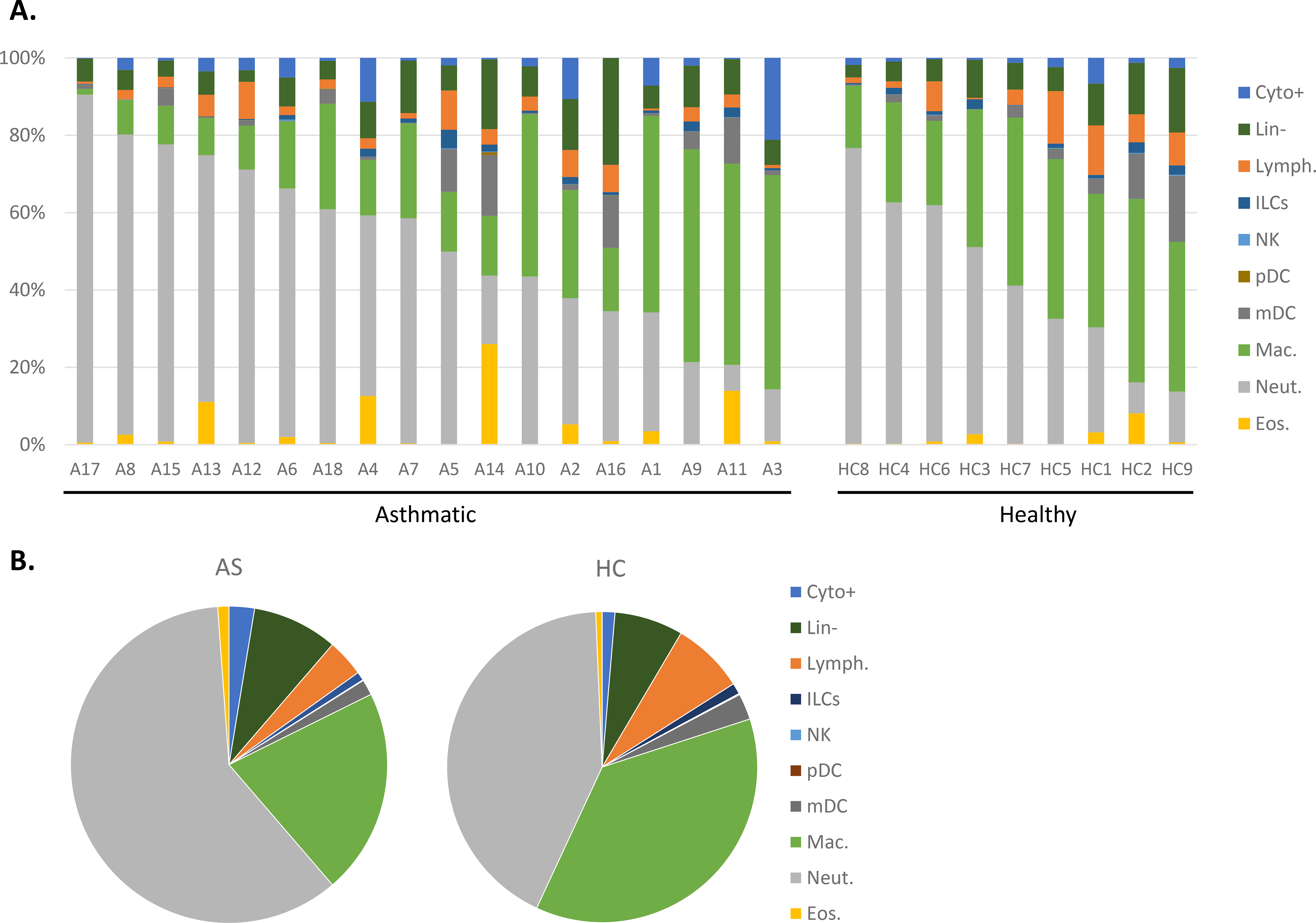
Induced sputum samples from asthmatic (AS, n=18) and healthy control (HC, n= 9) subjects were labeled fresh on the day of collection with metal-conjugated antibodies and analyzed by mass cytometry. Frequency of immune cell subsets displayed using Boolean analysis (A) and median values in each population by group (B).
To assess the implications of these differences in cell frequencies for pathogenesis, we assessed the functional status of the airway cells. Differences in cellular activation status between asthmatic patients and healthy controls can be readily detected ex vivo by quantifying levels of key immune markers in untreated sputum. CyTOF supports analysis of 18 functional markers (Table S1) in each of the 13 distinct subsets simultaneously. While this in-depth multidimensional assessment provides valuable insights for cellular function, the scale and complexity of CyTOF data present challenges for routine analytic approaches. To accelerate analysis and identify additional structure in the CyTOF data, we focused on granulocyte subsets (CD66b+, CD15+; Fig S1) as these cells are the most abundant in sputum and have a known role in asthma pathogenesis. To gain functional information about subpopulations, we employed a data-driven technique for clustering and dimensionality reduction, FlowSOM [33], to generate an unsupervised self-organizing map. The FlowSOM heatmap analysis revealed 9 distinct clusters of granulocytes with different levels of expression of functional markers which can be interpreted to define functional cell subsets (Fig. 3). IL-8, a key marker and an important neutrophil chemotactic factor, is present at very high levels in cluster 1 and 9 and very low levels in cluster 3, 6 and 8. Similarly, pro-inflammatory cytokines IL-6 and MIP-1β, which identify more activated phenotypes, are very highly expressed in cluster 3 and absent in clusters 5 and 6.
Figure 3. Functional analysis of asthmatic and healthy control granulocyte populations.
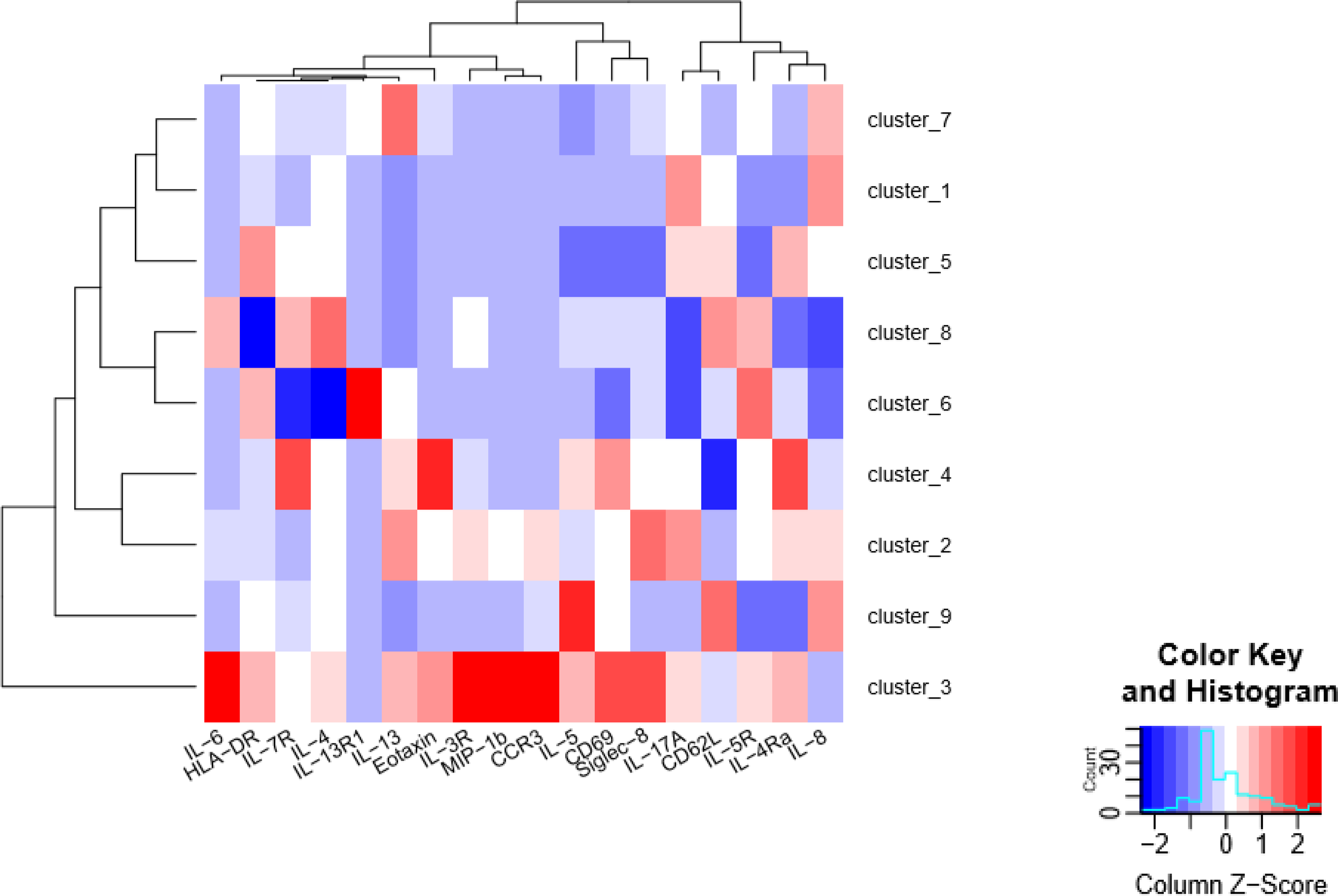
Induced sputum samples were labeled fresh on the day of collection with metal-conjugated antibodies and analyzed by mass cytometry. Analysis by FlowSOM clustering heatmap of the median expression levels of functional markers of granulocyte subsets for AS (n=4) and HC (n=3).
The functional differences in the clusters were more readily visualized in a two-dimensional tSNE plot of all samples with the FlowSOM clusters overlaid (Fig. 4A). The largest number of cells are found in clusters 1 and 4 and the fewest in clusters 6 and 8. The majority of the neutrophils were in cluster 1, which includes expression of IL-17A and IL-8, both pro-inflammatory cytokines associated with neutrophil recruitment and activation. Further, striking differences in neutrophil status are apparent when the clusters are separated between the asthmatic patients (Fig. 4B) and healthy controls (Fig. 4C). In-depth examination of clusters being expressed in one but not the other group shows clusters 2, 3, and 4 were highly expressed in asthmatics and absent in the healthy controls. Markers in these clusters reflect high expression of IL-6, MIP-1β, CCR3, Siglec-8, IL-13, IL-17 which are characteristic of an inflammatory activity associated with cell activation [34]. In contrast, clusters 5 and 6 are largely present only in healthy controls and express little to no cytokines, and are negative for CD69, a marker of cell activation, and would be expected to contribute less to inflammatory processes. Both clusters 5 and 6 contain cells expressing HLA-DR, while cluster 5 was positive for the IL-4Rα and cluster 6 had increased IL-5R and IL-13R1 expression. This clustering suggests two specialized subsets of neutrophils in the airway of healthy donors which, in an individual with asthma, could become activated when there is T2 inflammation and increased levels of IL-4, -5, and -13 being generated by activated bronchial epithelial cells and T cells. Cluster 4, which is also highly expressed in asthma and largely absent in healthy controls, includes neutrophils which are positive for eotaxin expression, a recruitment factor for eosinophils, and IL-7R, which plays an important role in the activation of Type 2 innate lymphoid cells [35]. Neutrophil subsets in cluster 4 also express low levels of CD62L (CD62Ldim), a marker of an activated neutrophil subset reported to play a role in inflammation and airway cell responses [36, 37]. Expression of CCR3 and Siglec-8 in clusters 2 and 3 suggest these clusters include eosinophils, which are critical mediators of allergic asthma. Indeed, detailed examination of cluster 3, with high expression of IL-3R, CD69, IL-5, combined with low levels of CD62L, suggests that this population may represent inflammatory eosinophils, a recently described subset in asthmatic sputum [38]. Future studies will benefit from further definition of eosinophil populations with use of the eosinophil peroxidase marker (EPX) (Fig S2).
Figure 4. Functional clustering of asthmatic and healthy control granulocyte populations.
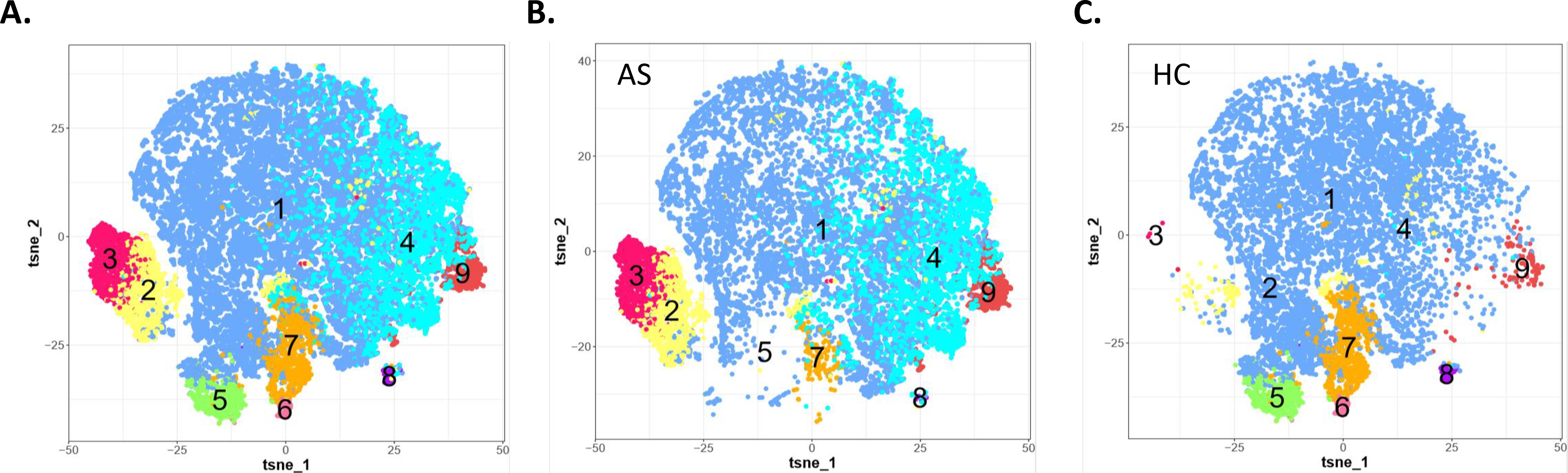
Induced sputum samples were labeled fresh on the day of collection with metal-conjugated antibodies and analyzed by mass cytometry. Visualization of FlowSOM clusters by Tsne for neutrophil clusters from all samples (A) and separated for AS (B) andHC (C).
Discussion
Here we have employed the high parameter cellular data obtained using CyTOF to provide detailed functional status of airway cells. We have identified multiple subtypes of airway immune cells which show distinct profiles both between healthy controls and asthmatic patients, and within an asthmatic patient cohort. This demonstrates the power that single cell profiling technologies bring to understanding phenotypes of asthma that have broad therapeutic and scientific implications. CyTOF can overcome the historical obstacles of tremendous heterogeneity in a relatively small cell sample size of sputum. The enhanced marker detection (>45 markers) combined with advanced clustering techniques dramatically improves our ability to analyze live heterogenous cell subsets. Improved recognition of distinct cellular phenotypes and functions provide insights into cell functions such as recently described low density neutrophils which correlate with disease progression in lung cancer patients [39]. In concert with single cell RNASeq, these advances have recently identified a new cell type, the ionophore, that contributes to the pathogenesis of cystic fibrosis [40], and changes in alveolar macrophages and epithelial cells during the progression of pulmonary fibrosis [41]. These approaches may identify relevant pathways that significantly advance the personalization of clinical asthma management and identification of novel therapeutic mediators.
As these in-depth methods gain a foothold in translational studies, it is a natural progression to expand the use of CyTOF into clinical trials. Use of single-cell technologies in clinical trials can augment findings with important mechanistic insights derived from collected samples. High dimensional datasets can give investigators a comprehensive view of disease pathogenesis within individual patients and across cohorts, shed light on heterogeneous cellular responses, and may help reveal mechanisms relevant to understanding responders and non-responders. To this end, our group has established relevant parameters to support integrating single cell technology into a multi-site clinical trial. In collaboration with the Inner-City Asthma Consortium (ICAC), we have introduced an immune sub-study to the ongoing MUPPITS-2 trial, a phase III study evaluating the efficacy of mepolizumab in pediatric asthmatics (ClinicalTrials.gov Identifier: NCT03292588) [42, 43]. We have adapted our methods to assess sputum samples collected as part of the trial and hope that the addition of CyTOF to this study will identify meaningful differences that contribute to diverse clinical responses and advance our knowledge of asthmatic inflammation.
Supplementary Material
Acknowledgements
This work was supported in part by the National Institutes of Health (ICAC-3; R01 HL118346, R01 HL-095390, 1K01HL125514–01). None of the authors have any commercial or other association that may pose a conflict of interest for this work. This work was developed with the CyTOF working group of the Inner-City Asthma Consortium (ICAC) with PI William W. Busse, University of Wisconsin School of Medicine, Madison WI. The authors gratefully acknowledge the valuable assistance of the Yale Center for Asthma and Airway Diseases, the Yale CyTOF facility, and Ms. Nicole Grant and Ms. Jean Estrom.
References
- 1.Kuruvilla ME, Vanijcharoenkarn K, Shih JA, Lee FE. Epidemiology and risk factors for asthma. Respir Med 2019; 149:16–22. [DOI] [PubMed] [Google Scholar]
- 2.Kaur R, Chupp G. Phenotypes and endotypes of adult asthma: Moving toward precision medicine. J Allergy Clin Immunol 2019; 144:1–12. [DOI] [PubMed] [Google Scholar]
- 3.Yan X, Chu JH, Gomez J, Koenigs M, Holm C, He X, Perez MF, Zhao H, Mane S, Martinez FD, Ober C, Nicolae DL, Barnes KC, London SJ, Gilliland F, Weiss ST, Raby BA, Cohn L, Chupp GL. Noninvasive analysis of the sputum transcriptome discriminates clinical phenotypes of asthma. Am J Respir Crit Care Med 2015; 191:1116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chupp GL, Kaur R, Mainardi A. New Therapies for Emerging Endotypes of Asthma. Annu Rev Med 2019. [DOI] [PubMed]
- 5.Fahy JV. Type 2 inflammation in asthma - present in most, absent in many (vol 15, pg 57, 2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; Nature Reviews Immunology 2015; 15:130 -. [Google Scholar]
- 6.Lambrecht BN, Hammad H, Fahy JV. The Cytokines of Asthma. Immunity 2019; 50:975–91. [DOI] [PubMed] [Google Scholar]
- 7.Yan X, Chu J, Gomez J, Koenigs M, Holm C, He X, Perez MF, Zhao H, Mane S, Martinez FD, Ober C, Nicolae DL, Barnes KC, London SJ, Gilliland F, Weiss ST, Raby BA, Cohn L, Chupp GL. Non-invasive analysis of the sputum transcriptome discriminates clinical phenotypes of asthma. Amer J Respir Crit Care Med 2015; 191:1116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy BD, Noel PJ, Freemer MM, Cloutier MM, Georas SN, Jarjour NN, Ober C, Woodruff PG, Barnes KC, Bender BG, Camargo CA Jr., Chupp GL, Denlinger LC, Fahy JV, Fitzpatrick AM, Fuhlbrigge A, Gaston BM, Hartert TV, Kolls JK, Lynch SV, Moore WC, Morgan WJ, Nadeau KC, Ownby DR, Solway J, Szefler SJ, Wenzel SE, Wright RJ, Smith RA, Erzurum SC. Future Research Directions in Asthma: An NHLBI Working Group Report. Am J Respir Crit Care Med 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodruff PG, Modrek B, Choy DF, Jia GQ, Abbas AR, Ellwanger A, Koth LL, Arron JR, Fahy JV. T-helper Type 2-driven Inflammation Defines Major Subphenotypes of Asthma (vol 180, pg 388, 2009). [DOI] [PMC free article] [PubMed] [Google Scholar]; American Journal of Respiratory and Critical Care Medicine 2009; 180:796-. [Google Scholar]
- 10.Levan SR, Stamnes KA, Lin DL, Panzer AR, Fukui E, McCauley K, Fujimura KE, McKean M, Ownby DR, Zoratti EM, Boushey HA, Cabana MD, Johnson CC, Lynch SV. Elevated faecal 12,13-diHOME concentration in neonates at high risk for asthma is produced by gut bacteria and impedes immune tolerance. Nat Microbiol 2019; 4:1851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters MC, Wenzel SE. Intersection of biology and therapeutics: type 2 targeted therapeutics for adult asthma. Lancet 2020; 395:371–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao Y, Welp T, Liu Q, Niu N, Wang X, Britto CJ, Krishnaswamy S, Chupp GL, Montgomery RR. Multiparameter single cell profiling of airway inflammatory cells. Cytometry B Clin Cytom 2017; 92:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bendall SC, Simonds EF, Qiu P, Amir el AD, Krutzik PO, Finck R, Bruggner RV, Melamed R, Trejo A, Ornatsky OI, Balderas RS, Plevritis SK, Sachs K, Pe'er D, Tanner SD, Nolan GP. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 2011; 332:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michlmayr D, Pak TR, Rahman AH, Amir ED, Kim EY, Kim-Schulze S, Suprun M, Stewart MG, Thomas GP, Balmaseda A, Wang L, Zhu J, Suarez-Farinas M, Wolinsky SM, Kasarskis A, Harris E. Comprehensive innate immune profiling of chikungunya virus infection in pediatric cases. Mol Syst Biol 2018; 14:e7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dutertre CA, Becht E, Irac SE, Khalilnezhad A, Narang V, Khalilnezhad S, Ng PY, van den Hoogen LL, Leong JY, Lee B, Chevrier M, Zhang XM, Yong PJA, Koh G, Lum J, Howland SW, Mok E, Chen J, Larbi A, Tan HKK, Lim TKH, Karagianni P, Tzioufas AG, Malleret B, Brody J, Albani S, van Roon J, Radstake T, Newell EW, Ginhoux F. Single-Cell Analysis of Human Mononuclear Phagocytes Reveals Subset-Defining Markers and Identifies Circulating Inflammatory Dendritic Cells. Immunity 2019; 51:573–89 e8. [DOI] [PubMed] [Google Scholar]
- 16.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, Dziura JD, Reed J, Coyle AJ, Kiener P, Cullen M, Grandsaigne M, Dombret M, Aubier M, Pretolani M, Elias JA. A chitinase-like protein in the lung and circulation of patients with severe asthma. New England Journal of Medicine 2007; 357:2016–27. [DOI] [PubMed] [Google Scholar]
- 17.Gomez JL, Crisafi GM, Holm CT, Meyers DA, Hawkins GA, Bleecker ER, Jarjour N, Severe Asthma Research Program I, Cohn L, Chupp GL. Genetic variation in chitinase 3-like 1 (CHI3L1) contributes to asthma severity and airway expression of YKL-40. J Allergy Clin Immunol 2015; 136:51–8 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, Dziura JD, Reed J, Coyle AJ, Kiener P, Cullen M, Grandsaigne M, Dombret MC, Aubier M, Pretolani M, Elias JA. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med 2007; 357:2016–27. [DOI] [PubMed] [Google Scholar]
- 19.Ober C, Tan Z, Sun Y, Possick JD, Pan L, Nicolae R, Radford S, Parry RR, Heinzmann A, Deichmann KA, Lester LA, Gern JE, Lemanske RF Jr., Nicolae DL, Elias JA, Chupp GL. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med 2008; 358:1682–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin JC, Gagnon L, He X, Baum ED, Karas DE, Chupp GL. Improvement in asthma control and inflammation in children undergoing adenotonsillectomy. Pediatr Res 2014; 75:403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.GINA. Global Initiative for Asthma. Asthma management and prevention for adults and children older than 5 years. A pocket guide for health professionals. 2019. https://ginasthmaorg/wp-content/uploads/2019/04/GINA-2019-main-Pocket-Guide-wmspd.
- 22.Ochkur SI, Kim JD, Protheroe CA, Colbert D, Moqbel R, Lacy P, Lee JJ, Lee NA. The development of a sensitive and specific ELISA for mouse eosinophil peroxidase: assessment of eosinophil degranulation ex vivo and in models of human disease. J Immunol Methods 2012; 375:138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finck R, Simonds EF, Jager A, Krishnaswamy S, Sachs K, Fantl W, Pe'er D, Nolan GP, Bendall SC. Normalization of mass cytometry data with bead standards. Cytometry A 2013; 83:483–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nowicka M, Krieg C, Crowell HL, Weber LM, Hartmann FJ, Guglietta S, Becher B, Levesque MP, Robinson MD. CyTOF workflow: differential discovery in high-throughput high-dimensional cytometry datasets. F1000Res 2017; 6:748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao Y, Liu R, Shin MS, Trentalange M, Allore H, Nassar A, Kang I, Pober J, Montgomery RR. CyTOF supports efficient detection of immune cell subsets from small samples. J Immunol Methods 2014; 415:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziegler-Heitbrock L, Hofer TP. Toward a refined definition of monocyte subsets. Front Immunol 2013; 4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cignarella A, Tedesco S, Cappellari R, Fadini GP. The continuum of monocyte phenotypes: Experimental evidence and prognostic utility in assessing cardiovascular risk. J Leukoc Biol 2018. [DOI] [PubMed] [Google Scholar]
- 28.Ruiz-Alcaraz AJ, Carmona-Martinez V, Tristan-Manzano M, Machado-Linde F, Sanchez-Ferrer ML, Garcia-Penarrubia P, Martinez-Esparza M. Characterization of human peritoneal monocyte/macrophage subsets in homeostasis: Phenotype, GATA6, phagocytic/oxidative activities and cytokines expression. Sci Rep 2018; 8:12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barlow JL, McKenzie ANJ. Innate Lymphoid Cells of the Lung. Annu Rev Physiol 2019; 81:429–52. [DOI] [PubMed] [Google Scholar]
- 30.Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology 2006; 11:54–61. [DOI] [PubMed] [Google Scholar]
- 31.Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, Wardlaw AJ, Pavord ID. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet 2002; 360:1715–21. [DOI] [PubMed] [Google Scholar]
- 32.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D'Agostino R Jr., Castro M, Curran-Everett D, Fitzpatrick AM, Gaston B, Jarjour NN, Sorkness R, Calhoun WJ, Chung KF, Comhair SA, Dweik RA, Israel E, Peters SP, Busse WW, Erzurum SC, Bleecker ER, National Heart L, Blood Institute's Severe Asthma Research P. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med 2010; 181:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Gassen S, Callebaut B, Van Helden MJ, Lambrecht BN, Demeester P, Dhaene T, Saeys Y. FlowSOM: Using self-organizing maps for visualization and interpretation of cytometry data. Cytometry A 2015; 87:636–45. [DOI] [PubMed] [Google Scholar]
- 34.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nature Medicine 2012; 18:716–25. [DOI] [PubMed] [Google Scholar]
- 35.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol 2015; 16:45–56. [DOI] [PubMed] [Google Scholar]
- 36.Tak T, Wijten P, Heeres M, Pickkers P, Scholten A, Heck AJR, Vrisekoop N, Leenen LP, Borghans JAM, Tesselaar K, Koenderman L. Human CD62L(dim) neutrophils identified as a separate subset by proteome profiling and in vivo pulse-chase labeling. Blood 2017; 129:3476–85. [DOI] [PubMed] [Google Scholar]
- 37.Ekstedt S, Safholm J, Georen SK, Cardell LO. Dividing neutrophils in subsets reveals a significant role for activated neutrophils in the development of airway hyperreactivity. Clin Exp Allergy 2019; 49:285–91. [DOI] [PubMed] [Google Scholar]
- 38.Mesnil C, Raulier S, Paulissen G, Xiao X, Birrell MA, Pirottin D, Janss T, Starkl P, Ramery E, Henket M, Schleich FN, Radermecker M, Thielemans K, Gillet L, Thiry M, Belvisi MG, Louis R, Desmet C, Marichal T, Bureau F. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest 2016; 126:3279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaul ME, Eyal O, Guglietta S, Aloni P, Zlotnik A, Forkosh E, Levy L, Weber LM, Levin Y, Pomerantz A, Nechushtan H, Eruslanov E, Singhal S, Robinson MD, Krieg C, Fridlender ZG. Circulating neutrophil subsets in advanced lung cancer patients exhibit unique immune signature and relate to prognosis. FASEB J 2020. [DOI] [PubMed] [Google Scholar]
- 40.Montoro DT, Haber AL, Biton M, Vinarsky V, Lin B, Birket SE, Yuan F, Chen S, Leung HM, Villoria J, Rogel N, Burgin G, Tsankov AM, Waghray A, Slyper M, Waldman J, Nguyen L, Dionne D, Rozenblatt-Rosen O, Tata PR, Mou H, Shivaraju M, Bihler H, Mense M, Tearney GJ, Rowe SM, Engelhardt JF, Regev A, Rajagopal J. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 2018; 560:319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reyfman PA, Walter JM, Joshi N, Anekalla KR, McQuattie-Pimentel AC, Chiu S, Fernandez R, Akbarpour M, Chen CI, Ren Z, Verma R, Abdala-Valencia H, Nam K, Chi M, Han S, Gonzalez-Gonzalez FJ, Soberanes S, Watanabe S, Williams KJN, Flozak AS, Nicholson TT, Morgan VK, Winter DR, Hinchcliff M, Hrusch CL, Guzy RD, Bonham CA, Sperling AI, Bag R, Hamanaka RB, Mutlu GM, Yeldandi AV, Marshall SA, Shilatifard A, Amaral LAN, Perlman H, Sznajder JI, Argento AC, Gillespie CT, Dematte J, Jain M, Singer BD, Ridge KM, Lam AP, Bharat A, Bhorade SM, Gottardi CJ, Budinger GRS, Misharin AV. Single-Cell Transcriptomic Analysis of Human Lung Provides Insights into the Pathobiology of Pulmonary Fibrosis. Am J Respir Crit Care Med 2019; 199:1517–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gergen PJ, Teach SJ, Togias A, Busse WW. Reducing Exacerbations in the Inner City: Lessons from the Inner-City Asthma Consortium (ICAC). J Allergy Clin Immunol Pract 2016; 4:22–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wood RA, Togias A, Wildfire J, Visness CM, Matsui EC, Gruchalla R, Hershey G, Liu AH, O'Connor GT, Pongracic JA, Zoratti E, Little F, Granada M, Kennedy S, Durham SR, Shamji MH, Busse WW. Development of cockroach immunotherapy by the Inner-City Asthma Consortium. J Allergy Clin Immunol 2014; 133:846–52 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


