Abstract
Background:
Evaluation of aortic stenosis (AS) requires calculation of the aortic valve area (AVA), which relies on the assumption of a circular-shaped left ventricular outflow tract (LVOT). However, the LVOT is often elliptical, and the circular assumption underestimates the true LVOT area. Biplane imaging using transthoracic echocardiography (TTE) allows for direct planimetry of the LVOT area. We aim to assess the feasibility of obtaining LVOT area by this technique, and its impact on the discordance between AVA and gradient criteria in AS grading.
Methods:
We prospectively studied 134 patients (median age 80 years, interquartile range (IQR) 73–87 years; 39% female) with AS, including 82 (61%) with severe AS and 52 (39%) with mild or moderate AS. LVOT area was traced by direct planimetry (LVOTAbiplane) and compared with LVOT area calculated by circular assumption (LVOTAcirc). In a subset of patients who underwent cardiac computed tomography (CT), direct planimetry of LVOT area (LVOTACT) was used as a reference standard.
Results:
LVOTAbiplane was significantly larger than LVOTAcirc (4.20 cm2 (IQR 3.66–4.90 cm2) vs 3.73 cm2 (IQR 3.14–4.15 cm2), p < 0.001). Among 30 patients who underwent cardiac CT, LVOTAbiplane had better agreement with LVOTACT than LVOTAcirc (mean bias −0.45 ± 0.63 cm2 vs −1.02 ± 0.63 cm2, p < 0.0001). Of 82 patients with severe AS (AVA ≤ 1 cm2 using LVOTAcirc), 40 (49%) had discordant mean gradient (MG < 40 mmHg). By using LVOTAbiplane, patients with discordant AVA and MG decreased from 49% to 27% (p = 0.004), and 29% of patients with severe AS were reclassified as moderate AS, with the highest percentage of reclassification in the low-gradient AS with preserved left ventricular ejection fraction group.
Conclusions:
Direct planimetry by biplane imaging avoids the inherent underestimation of LVOT area using the circular assumption. LVOT area obtained by biplane planimetry can lead to better concordance between AVA and MG, and classification of AS severity.
Keywords: aortic stenosis, left ventricular outflow tract, direct planimetry, simultaneous biplane imaging, 2D transthoracic echocardiography
Introduction
Aortic stenosis (AS) is an increasingly common condition, with a prevalence of 9.8% in adults over 80 years of age [1]. As the population ages, it is projected that the number of patients living with AS will double by 2050 [2]. Accurate assessment of AS severity is essential for clinical management and therapeutic decision-making in this growing patient population. Transthoracic echocardiography (TTE) is the main diagnostic imaging modality to assess AS severity, which is based on quantitative parameters such as the aortic valve area (AVA), transvalvular mean gradient (MG) and peak AS jet velocity [3]. However, in up to 40% of patients with severe AS, the echocardiographic parameters are discordant (e.g. AVA ≤ 1 cm2 and MG < 40 mmHg) [4]. One of the main sources of error in AVA calculation by continuity equation is inaccurate measurement of the left ventricular outflow tract (LVOT) diameter and the assumption of a circular LVOT geometry. Presuming the LVOT to be circular necessitates that an erroneous diameter measurement be mathematically squared.
Multiple studies using three-dimensional (3D) echocardiography, cardiac computed tomography (CT), and cardiac magnetic resonance (CMR) have shown that in the majority of patients, the LVOT shape is elliptical, with a larger mediolateral (ML) than anteroposterior (AP) diameter [5–12]. Current guidelines, however, recommend the assumption of a circular LVOT area using the single-diameter measurement of the LVOT in the parasternal long-axis view [3]. In fact, this LVOT diameter corresponds to the AP or minor diameter of the ellipse, thereby resulting in underestimation of the true LVOT area, and by extension, the calculated stroke volume index (SVi) and AVA [13].
Simultaneous biplane (also known as biplane) imaging using two-dimensional (2D) TTE is widely accessible, easy to perform, and allows for direct planimetry of the LVOT in a cross-sectional view. In this study, we hypothesize that LVOT area by biplane planimetry is more accurate and will lead to improved concordance and classification of AS severity compared to the standard LVOT method using circular assumption. Our objectives are: 1) to examine the feasibility of measuring LVOT area from TTE biplane imaging, 2) to compare LVOT area derived from biplane planimetry with cardiac CT as the reference standard, and 3) to examine the impact of biplane LVOT area on the discordance of AS grading and re-classification in a subset of patients with severe AS.
Methods
Patient population
From April 2018 to April 2019, we prospectively enrolled a random sample of 171 patients, who were referred to the Massachusetts General Hospital Echocardiography Laboratory for evaluation of AS. All patients were at least 18 years of age, had at least mild aortic valve stenosis (defined by peak aortic valve velocity of > 2.5 m/s), and had no prior surgery or intervention on the aortic valve or ascending aorta. Thirty-seven patients were excluded for the following reasons: 17 had inadequate echocardiographic biplane image quality, 17 had moderate or severe aortic or mitral regurgitation, and three had LVOT velocity > 1.5 m/s. Baseline demographics, clinical history, echocardiographic and CT imaging data were collected. The study was approved by our institutional review board.
Transthoracic echocardiography image acquisition and analysis
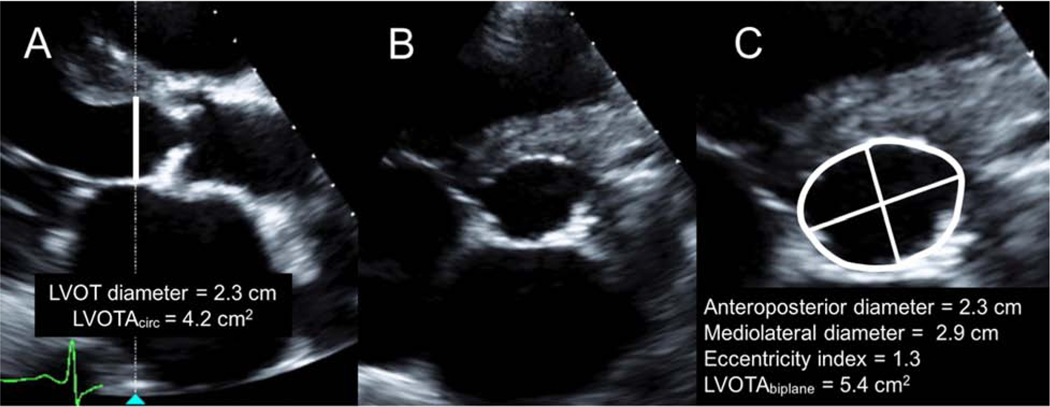
Comprehensive 2D and Doppler TTE was performed using a commercially available echocardiographic system (EPIQ 7, Philips Medical Systems, Andover, MA) with the X5–1 broadband-array transducer. Data were digitally recorded for offline analysis (Syngo Dynamics, Siemens Healthineers, Forchheim, Germany). AVA was calculated using the continuity equation according to published guidelines [3]. Standard LVOT diameter (LVOTd) was measured from the zoomed parasternal long-axis view at mid-systole, approximately 5 mm below the aortic valve annulus, and from inner edge to inner edge of the tissue-blood interface. Special care was taken to maximize LVOT diameter and to optimize LVOT border delineation. Standard LVOT area (LVOTAcirc) was calculated using circular assumption . Simultaneous biplane imaging was used in a zoomed view to visualize the LVOT cross-sectional area in short-axis view (Figure 1). Special care was taken to ensure that the alignment of the biplane cursor was perpendicular to the long-axis of the LVOT in mid-systole. Biplane LVOT area was measured by direct planimetry (LVOTAbiplane). The LVOT AP and ML diameters were also obtained from biplane imaging and the eccentricity index (EI) was calculated using the ratio of ML to AP dimensions, with EI >1.1 indicating an elliptical shape [14]. The LVOT velocity time integral (VTI) was measured from the apical view using pulsed-wave Doppler, with the sample volume located at approximately 5 mm proximal to the aortic valve, taking care to obtain a laminar flow curve. Maximal aortic velocity, aortic valve VTI, and peak and mean aortic gradients were measured using continuous-wave Doppler from the apical, right parasternal or suprasternal views, and data from the view with the highest velocity were recorded. In patients with atrial fibrillation, each data point was averaged over 5 consecutive cardiac cycles.
Figure 1.
Simultaneous biplane imaging of the left ventricular outflow tract (LVOT) demonstrating: (A) the parasternal long-axis view with standard LVOT diameter measurement and LVOT area calculated using circular assumption (LVOTAcirc), (B) the simultaneous parasternal short-axis view using biplane imaging, and (C) the anteroposterior and mediolateral diameters of LVOT, eccentricity index, and direct planimetry of the LVOT area (LVOTAbiplane) measured using the simultaneous parasternal short-axis view.
Cardiac CT image acquisition and analysis
A subset of patients with severe AS underwent cardiac CT for evaluation for transcatheter aortic valve replacement (TAVR). These patients were scanned with a 128-slice or 192-slice dual-source CT scanners (SOMATOM Definition Flash or SOMATOM Definition Force, Siemens Healthineers) with prospective ECG triggering. Intravenous contrast (Isovue 370, Iopamidol, Bracco Diagnostics Inc, NJ) volume and rate were determined by the patient’s weight and scan time using the CertegraP3T algorithm (Bayer, Warrendale, PA), and imaging was performed in a bolus-triggered mode. Images were reconstructed using iterative reconstruction algorithm (strength level 3 out of 5, I131F SAFIRE/ Bv40 ADMIRE, Siemens Healthineers) and 0.75 mm thickness with increment of 0.4 mm at 220–440 ms (20 ms interval). Images were analyzed on a dedicated workstation (Syngo Dynamics, Siemens Healthineers). From the contrast-enhanced dataset, three orthogonal planes in multiplanar reconstruction were aligned to obtain the double-oblique transverse view of the LVOT at 5 mm below the aortic valve annulus. LVOT area (LVOTACT) was measured by direct planimetry during mid-systole, at approximately 260–320 ms depending on the heart rate. LVOT AP and ML diameters were also measured at the same level to calculate the EI. The modified AVA (AVACT) was calculated by inputting the LVOTACT into the continuity equation along with Doppler VTIs from TTE. The LVOT measurements were performed by two independent observers (S.L. and V.B.), who were blinded to each other and to all echocardiographic data.
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) values if normally distributed, and as median with interquartile range (IQR) if non-normally distributed. Categorical variables were presented as counts with percentages. The paired t test and Wilcoxon signed-rank test were used to compare continuous variables for difference within patients. Data were assessed for normal distribution and homogeneity of variances using the Shapiro–Wilk test and the Levene’s test, respectively. Chi-square was used for comparison of categorical variables. Correlation and agreement between the LVOT areas obtained by different methods (standard TTE, biplane TTE, and cardiac CT) were determined by the Pearson correlation or the Spearman’s rank correlation and the Bland-Altman analysis, respectively. Interobserver (S.L., L.H.) and intraobserver (S.L.) reproducibility of biplane LVOT area was performed in a random set of 20 patients by two independent blinded observers and evaluated by use of intraclass correlation coefficient (ICC) for absolute agreement and the Bland-Altman method. A good agreement was defined as > 0.80, and the limits of agreement were defined as the mean difference ± 2 SD. A probability value of < 0.05 was considered significant. All statistical analyses were performed with SPSS version 23 (SPSS Inc, Chicago, IL).
Results
Baseline clinical and echocardiographic characteristics
The study population consisted of 134 patients (median age 80 years (IQR 73–87 years); 39% female). Based on AVA ≤ 1 cm2 calculated by continuity equation using the standard LVOT area, 82 (61%) patients had severe AS and 52 (39%) patients had mild or moderate AS. Table 1 shows the baseline demographic, clinical, and echocardiographic characteristics of all study patients. Overall, 64% of all study subjects had tricuspid aortic valve, and 14% had bicuspid aortic valve; the remainder (22%) had undifferentiated valve morphology due to severe valve calcification. The majority (87%) of patients had normal left ventricular ejection fraction (LVEF ≥ 50%). Normal LV size was observed in 79% of patients. The median peak and mean aortic valve gradients were 54 mmHg (IQR 39–73 mmHg) and 30 mmHg (IQR 21–42 mmHg), respectively. The median AVA calculated using LVOTAcirc was 0.98 cm2 (IQR 0.81–1.17 cm2). The AVA was not indexed, as the majority (94%) of patients had body surface area ≥ 1.5 m2.
Table 1.
Baseline clinical and echocardiographic characteristics of all patients (n=134).
| Clinical characteristics: | |
|---|---|
| Age (years) | 80 (73–87) |
| Sex (% female) | 39% |
| Height (cm) | 166 ± 11 |
| Weight (kg) | 79 ± 17 |
| Body surface area (m2) | 1.9 ± 0.2 |
| Hypertension | 121 (90%) |
| Hyperlipidemia | 117 (87%) |
| Diabetes mellitus | 40 (30%) |
| Obstructive CAD, prior PCI or CABG | 57 (43%) |
| Atrial fibrillation or flutter | 51 (38%) |
| Stroke or transient ischemic attack | 21 (16%) |
| Echocardiographic parameters: | |
| Left ventricular end-diastolic dimension (mm) | 44 ± 6 |
| Left ventricular end-systolic dimension (mm) | 30 ± 7 |
| Left ventricular ejection fraction (%) | 67 (61–73) |
| Aortic valve morphology | |
| Tricuspid | 86 (64%) |
| Bicuspid | 18 (14%) |
| Unknown | 30 (22%) |
| Aortic valve peak gradient (mmHg) | 54 (39–73) |
| Aortic valve mean gradient (mmHg) | 30 (21–42) |
| Aortic valve area (cm2) | 0.98 (0.81–1.17) |
| Stroke volume index (ml/m2) | 46 ± 12 |
CABG: coronary artery bypass grafting; CAD: coronary artery disease; PCI: percutaneous coronary intervention; SD, standard deviation. Continuous variables are presented as mean ± standard deviation if normally distributed, and as median with interquartile range if non-normally distributed.
Biplane 2D TTE: LVOT geometry and area by direct planimetry
The median AP and ML LVOT diameters measured by biplane TTE were 2.09 cm (IQR 1.96–2.28 cm) and 2.49 cm (IQR 2.35–2.68 cm), respectively, with a mean EI of 1.19 ± 0.11 (Table 2). The majority of patients (81%) had EI > 1.1, indicating an elliptical LVOT geometry. Among all study patients, LVOTAbiplane was significantly larger than LVOTAcirc (4.20 cm2 (IQR 3.66–4.90 cm2) vs 3.73 cm2 (IQR 3.14–4.15 cm2), p < 0.001). The SVi calculated using LVOTAbiplane was also significantly larger than that calculated using LVOTcirc (SVibiplane vs SVicirc: 54 ± 13 vs 46 ± 12 ml/m2, p < 0.001). Similarly, the average AVA calculated by continuity equation using LVOTAbiplane was significantly larger than that using LVOTAcirc (AVAbiplane vs AVAcirc: 1.14 cm2 (IQR 0.94–1.37 cm2) vs 0.98 cm2 (IQR 0.81–1.17 cm2), p < 0.001).
Table 2.
LVOT geometry, stroke volume index, and aortic valve area of all study patients and a subset of patients with cardiac CT available, stratified by different imaging methods.
| All study patients (n=134) | Standard TTE | Biplane TTE | p-value |
|---|---|---|---|
| LVOT AP diameter (cm) | 2.18 (2.00–2.30) | 2.09 (1.96–2.28) | 0.002 |
| LVOT ML diameter (cm) | -- | 2.49 (2.35–2.68) | -- |
| Eccentricity index | -- | 1.19 ± 0.11 | -- |
| LVOT area (cm2) | 3.73 (3.14–4.15) | 4.20 (3.66–4.90) | <0.001 |
| Stroke volume index (ml/m2) | 46 ± 12 | 54 ± 13 | <0.001 |
| Aortic valve area (cm2) | 0.98 (0.81–1.17) | 1.14 (0.94–1.37) | <0.001 |
| Subset of patients with cardiac CT (n=30) | Standard TTE | Biplane TTE | Cardiac CT |
| LVOT AP diameter (cm) | 2.15 (2.00–2.20) ‡ | 2.04 (1.94–2.12) † | 2.11 (1.94–2.28) |
| LVOT ML diameter (cm) | -- | 2.43 (2.30–2.64) * | 2.67 (2.54–2.88) |
| Eccentricity index | -- | 1.21 ± 0.10 † | 1.28 ± 0.10 |
| LVOT area (cm2) | 3.63 (3.14–3.80) * | 3.92 (3.56–4.37) † | 4.38 (3.80–4.98) |
| Stroke volume index (ml/m2) | 44 ± 9 * | 51 ± 12 † | 56 ± 13 |
| Aortic valve area (cm2) | 0.78 (0.66–0.88) * | 0.94 (0.73–1.00) † | 0.95 (0.84–1.15) |
AP: anteroposterior; CT: computed tomography; LVOT: left ventricular outflow tract; ML: mediolateral; TTE: transthoracic echocardiography. Continuous variables are presented as mean ± standard deviation if normally distributed, and as median with interquartile range if non-normally distributed.
p < 0.001 vs CT
p < 0.01 vs CT
p = non-significant vs CT
Cardiac CT: LVOT geometry and area by direct planimetry
Thirty patients with severe AS underwent cardiac CT for TAVR evaluation. The AP and ML LVOT diameters measured on cardiac CT were 2.11 cm (IQR 1.94–2.28 cm) and 2.67 cm (IQR 2.54–2.88 cm), respectively, with a mean EI of 1.28 ± 0.10 (Table 2). All patients (n = 30) who underwent cardiac CT had EI > 1.1, indicating an elliptical LVOT shape. The LVOT area measured by direct planimetry on cardiac CT (LVOTACT) was 4.38 cm2 (IQR 3.80–4.98 cm2). By substituting LVOTACT into the stroke volume equation and the continuity equation, the modified SVi and AVA were calculated as 56 ± 13 ml/m2 and 0.95 cm2 (IQR 0.84–1.15 cm2), respectively.
Correlation and agreement of LVOT area by echocardiography and CT
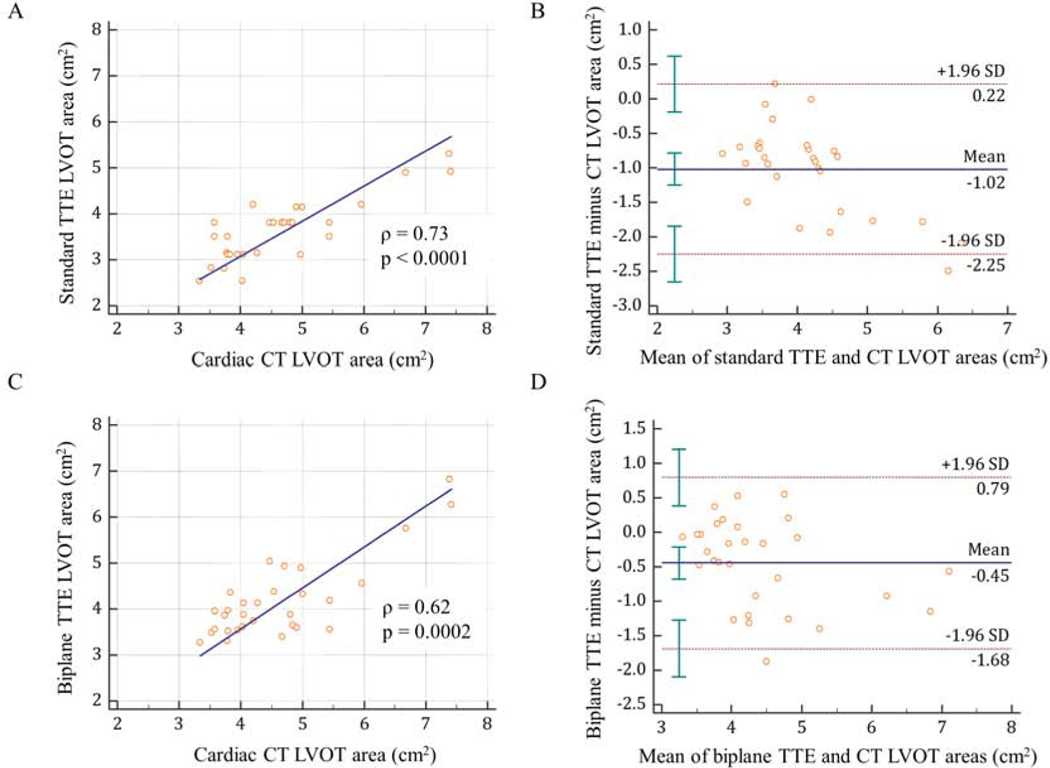
Among the 30 patients with cardiac CT available, we compared LVOTAcirc and LVOTAbiplane with LVOTACT as a reference standard (Figure 2). The median LVOTACT was 4.38 cm2 (IQR 3.80–4.98 cm2), which was significantly larger than both the LVOTAcirc (3.63 cm2 (IQR 3.14–3.80 cm2), p < 0.001) and LVOTAbiplane (3.92 cm2 (IQR 3.56–4.37 cm2), p < 0.01). Compared to CT, both the standard and biplane TTE methods underestimated LVOT area by 24 ± 13%, and 10 ± 14%, respectively. Although both the LVOTAcirc and LVOTAbiplane showed good correlation with LVOTACT, with a Spearman’s rank correlation coefficient (ρ) of 0.73 and 0.62, respectively, the biplane method had better agreement with CT than the standard method. The mean bias between LVOTAcirc vs LVOTACT was −1.02 ± 0.63 cm2 (95% CI, −1.25 to −0.78 cm2), and between LVOTAbiplane vs LVOTACT was −0.45 ± 0.63 cm2 (95% CI, −0.68 to −0.21 cm2).
Figure 2.
Correlation and Bland-Altman analysis of LVOT area between standard TTE and cardiac CT (A, B), and between biplane TTE and cardiac CT (C, D).
CT: computed tomography; LVOT: left ventricular outflow tract; TTE: transthoracic echocardiography.
Impact on concordance of severe AS grading and classification
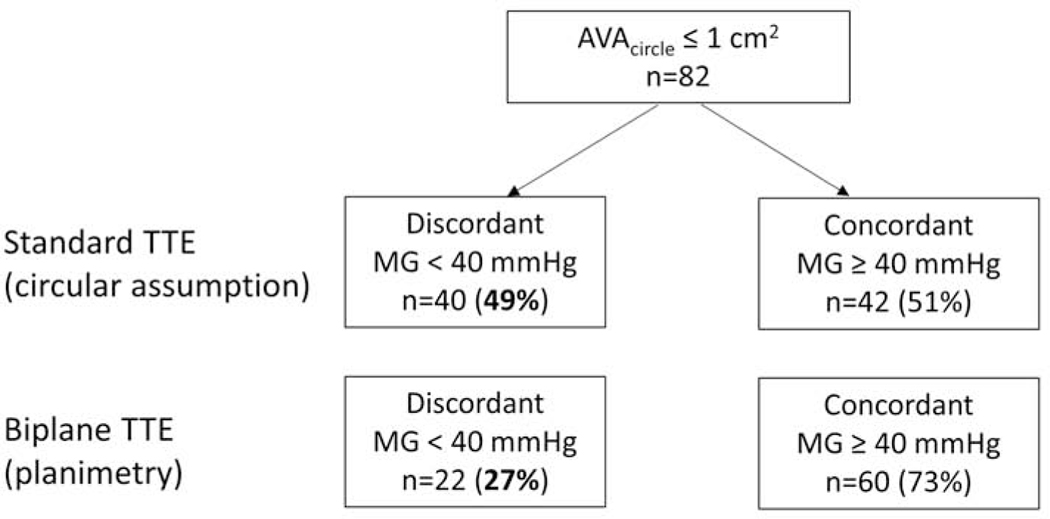
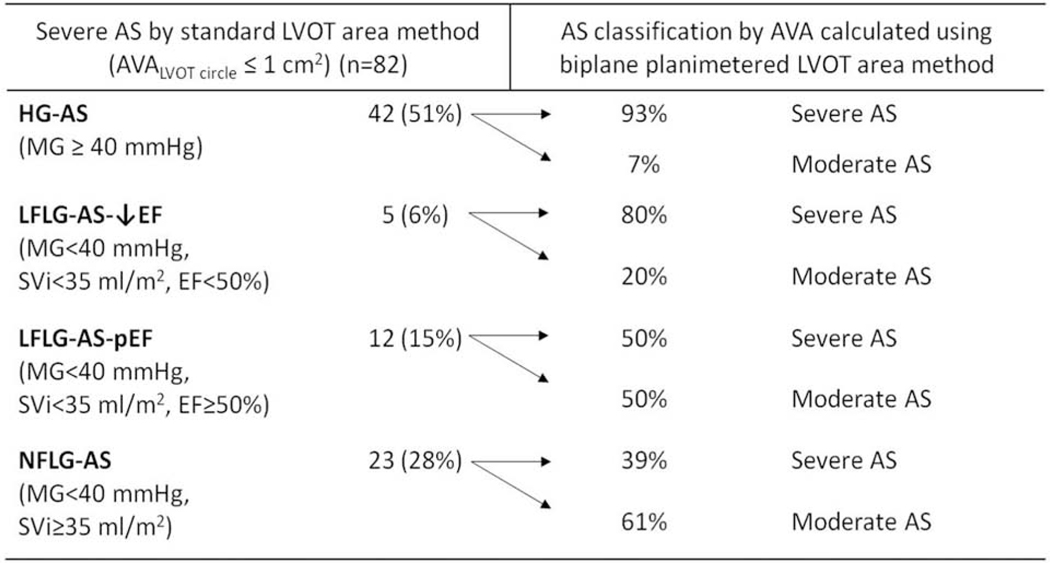
In the subset of patients with severe AS (n = 82, defined by AVAcirc ≤ 1 cm2), 42 (51%) patients had concordant mean gradient (i.e. MG ≥ 40 mmHg) and 40 (49%) patients had discordant mean gradient (i.e. MG < 40 mmHg) (Figure 3). In those patients with discordant mean gradient, 5 (6%) had classic low-flow low-gradient (LF-LG) AS with abnormal LVEF (MG < 40 mmHg, SVi < 35 ml/m2, LVEF < 50%), 12 (15%) had LF-LG AS with preserved LVEF (MG < 40 mmHg, SVi < 35 ml/m2, LVEF ≥ 50%), and 23 (28%) had normal-flow low-gradient (NF-LG) AS (MG < 40 mmHg, SVi ≥ 35 ml/m2, LVEF ≥ 50%) (Figure 4). By substituting LVOTAbiplane into the continuity equation to calculate AVA, the proportion of patients with discordant MG decreased from 49% to 27% (p = 0.004) (Figure 3). Since low stroke volume could lead to a low transvalvular mean gradient, we subsequently analyzed only patients (n = 61) with AVAcirc ≤ 1 cm2 and SVi ≥ 35 ml/m2. After this adjustment, the biplane LVOT method still led to a significant reduction in the proportion of patients with discordant AS grading, from 38% to 20% (p = 0.03), compared to the circular LVOT method. Overall, 24 (29%) patients with severe AS by the standard AVA calculation were reclassified into the moderate category after substituting the biplane LVOT area into the continuity equation. Specifically, 7% of patients (n = 3/42) in the high-gradient (HG) AS group, 20% (n = 1/5) in the classic LF-LG AS with abnormal LVEF group, 50% (n = 6/12) in the LF-LG AS with normal LVEF group, and 61% (n = 14/23) in the NF-LG group were reclassified as moderate AS (Figure 4).
Figure 3.
Proportion of patients with severe AS and discordant or concordant aortic valve mean gradient, stratified by the standard- and biplane-TTE methods of measuring left ventricular outflow tract area.
AVA: aortic valve area; MG: mean gradient; TTE: transthoracic echocardiography.
Figure 4.
Reclassification of aortic stenosis severity using left ventricular outflow tract area obtained by simultaneous biplane transthoracic echocardiography, among patients with severe aortic stenosis as defined by the conventional aortic valve area calculation.
AS: aortic stenosis; AVA: aortic valve area; EF: ejection fraction; HG: high-gradient; LFLG: low-flow low-gradient; LVOT: left ventricular outflow tract; MG: mean gradient; NFLG: normal-flow low-gradient.
Reproducibility of LVOT area
LVOT area by biplane TTE had an intraobserver variability of 0.88 (95% CI: 0.72–0.95) with mean bias of 0.14 ± 0.46 cm2, and an interobserver variability of 0.95 (95% CI: 0.85–0.98) with mean bias of 0.15 ± 0.32 cm2. LVOT area by cardiac CT had an intraobserver variability of 0.95 (95% CI: 0.83–0.99) with mean bias of 0.09 ± 0.33 cm2, and an interobserver variability of 0.94 (95% CI: 0.79–0.98) with mean bias of 0.09 ± 0.37 cm2.
Discussion
Our study is the first to systematically examine the use of 2D simultaneous biplane TTE imaging in the assessment of LVOT area by direct planimetry. The main findings of our study are: 1) measurement of LVOT area using biplane TTE is feasible in 90% of patients with AS; 2) LVOT area obtained by biplane TTE is significantly larger and has better agreement with cardiac CT than the standard TTE method; and 3) among patients with severe AS, biplane-derived LVOT area can lead to improved concordance between AVA and MG, and classification of AS type, particularly in the subgroup of patients with low-gradient AS and preserved LVEF.
Continuing to use a circular LVOT assumption in routine TTE is problematic in the assessment of aortic stenosis. Prior studies using 3D imaging have demonstrated that the LVOT geometry is elliptical in 70–100% of patients with aortic stenosis [5–9, 15, 16]. Our study is consistent with these findings, demonstrating that 81% of patients with AS had an elliptical LVOT by biplane TTE. Furthermore, the LVOT undergoes remodeling in patients with AS, and has increased ellipticity at peak systole, thereby resulting in an even greater underestimation of the true LVOT area compared to control subjects [17]. 2D TTE has been shown to consistently underestimate LVOT area by 17–33% compared to cardiac CT or CMR [9, 14, 18–20]. Our study used CT as a reference standard, and demonstrated that 2D TTE underestimated LVOT area by 24 ± 13% (mean bias of 1.02 ± 0.63 cm2). Biplane-derived LVOT area had better agreement with cardiac CT than the standard TTE method, and underestimated LVOT area by 10 ± 14% (mean bias of −0.45 ± 0.63 cm2). Despite this improvement, there remained a small but significant difference between LVOTAbiplane and LVOTACT. We believe the main reason is the superior spatial resolution of CT compared to TTE, particularly in the imaging of the lateral aspects of the LVOT. Nonetheless, our degree of underestimation was similar to that reported by 3D transesophageal echocardiography (TEE). In 53 patients with severe AS undergoing TAVR evaluation, direct planimetry of LVOT area using 3D TEE underestimated CT-derived LVOT area by 7.7% (or mean bias of −0.37 ± 0.37 cm2) [21]. In 35 patients with and without AS who underwent both 3D TEE and cardiac CT, the mean bias between these two methods in assessing LVOT area was −0.46 ± 0.56 cm2, which was similar to our results using biplane TTE [22]. However, unlike TEE which is invasive and associated with procedural complications, biplane imaging is non-invasive, easy to perform, and can be readily incorporated into a patient’s routine TTE.
Direct planimetry by biplane TTE also helps to resolve discrepancies between AVA and MG in classifying AS severity. The percentage of patients with severe AS by AVA and concordant MG increased from 51% to 73% after substituting the biplane-derived LVOT area into the continuity equation. This finding is in keeping with prior studies, which showed a similar improvement in the degree of concordance by using the elliptical LVOT area. In 51 patients with suspected severe AS, CT-derived LVOT area improved concordance between AVA < 0.8 cm2 and dimensionless index (DI) ≤ 0.25 from 73% to 92% compared to 2D TTE [5]. In another study comparing CT to 2D TTE among 52 patients with severe AS, concordance between AVAi < 0.6 cm2/m2 and DI < 0.25 improved from 79% to 98% when CT-derived LVOT area was substituted into the continuity equation [18]. Finally, planimetry of LVOT area by 3D TEE in 33 patients with severe AS improved the concordance between AVA and mean gradient from 61% to 82%, compared to 2D TTE [8]. Thus, the biplane method has a similar impact on the concordance of AS grading parameters compared to other more advanced or invasive imaging modalities.
The biplane technique also yields similar results on AS classification as compared to studies performed on 3D imaging techniques. In our study, 29% of patients with severe AS were reclassified into the moderate category using the LVOT area by biplane planimetry, amongst which the highest proportion of reclassification was in the low-gradient AS with preserved LVEF group. Specifically, 50% of patients with paradoxical LF-LG AS and 61% of patients with NF-LG AS were reclassified as moderate AS. Our reclassification rate is comparable to other studies, which have reported an overall rate of 15–30% using 3D TEE, cardiac CT or CMR [8, 18–21, 23, 24]. In a study using CMR, 25% of patients with severe AS by 2D TTE were reclassified into moderate AS when using CMR-derived LVOT area, with the highest proportion of reclassification (55%) in the NF-LG group [20]. Similarly, in another study using cardiac CT, 16% of patients were reclassified into moderate AS, again with the highest reclassification rate (52%) in the NF-LG group [23].
Accurate assessment of LVOT area is critical in determining the AVA, which in combination with other imaging and clinical characteristics, affect therapeutic decisions in patients with AS. The results of our study are likely to have the greatest impact on patients with low-gradient AS and preserved LVEF, which is also the category with the greatest clinical dilemma. AVA measurements in this particular group of patients have been shown to be most likely underestimated by standard TTE when compared to the modified AVA using CT-derived LVOT area, or the heart team final diagnosis [25]. Furthermore, studies have shown that patients with paradoxical LF-LG severe AS tend to have worse survival than patients with moderate AS, and benefit from aortic valve replacement; in contrast, patients with NF-LG severe AS have similar outcomes as those with moderate AS, and the effect of aortic valve replacement on survival was neutral. [26, 27]. Therefore, it is crucial to accurately distinguish between low-gradient true severe AS and moderate AS.
Several clinical tools exist to help reconcile the discordant measures of AS severity, though each has its limitations. Dobutamine stress echocardiography is routinely performed in patients with classic LF-LG AS and abnormal LVEF; however, it is not recommended in patients with low-gradient AS and preserved LVEF. Quantitative aortic valve calcium scoring by non-contrast cardiac CT has been shown to be a predictor of stenosis severity, but its added value is unclear and it does not account for valvular fibrosis [28]. Direct planimetry of the anatomic AVA using cardiac CT or other imaging methods is often limited by artifacts from severe calcification, and lacks longitudinal outcome data. The use of a hybrid imaging approach, which combines the elliptical LVOT area obtained by 3D imaging with the Doppler flow velocities by echocardiography to yield a modified AVA, has been proposed [29]. However, the optimal cut-off value to define severe AS using the hybrid method remains controversial [30]. Clavel et al. have shown that a modified AVA of ≤ 1.2 cm2 using the hybrid CT approach predicts mortality, but similar cut-off values have not been well studied for other imaging methods [6]. Furthermore, each 3D imaging technique is subject to limitations. For instance, contrast-enhanced cardiac CT has superior spatial resolution, but is associated with risks of contrast administration and radiation, and does not provide hemodynamic data. CMR also has excellent spatial resolution, but it is time-consuming and less available. 3D TTE has been used to measure LVOT area in limited studies [20, 31, 32]; however, in a study by Gaspar et al., its image quality was found to be inadequate, and the reproducibility of LVOT area measurements by 3D TTE was inferior to 2D TTE [14]. 3D TEE is invasive and has procedural complications associated with sedation and esophageal intubation. The biplane planimetry method using TTE avoids procedure-related risks, and the need for off-line image processing and cropping. It can also be done at the time of initial image acquisition, obviating the need for supplemental imaging.
The circular LVOT assumption inherently underestimates the true LVOT area, and affects the accuracy of assessing AS severity. Our study describes a method using the same readily available technology to directly measure LVOT area in a way that is reproducible, time-efficient, and makes no geometric assumptions. In addition, the planimetry method of determining area is already the recommended standard for mitral stenosis and thus is not a novel concept in echocardiography laboratories. Direct planimetry using simultaneous biplane TTE imaging offers a simple and more accurate alternative to the conventional LVOT diameter method, and would not be difficult to implement in daily practice.
Limitations
While we highlight an important finding relevant to AS diagnosis, our findings should be interpreted in context of the following limitations. First, any 2D technique involves rotational errors in plane alignment and imprecision in plane positioning due to translation of the heart during the cardiac cycle [33]. Nonetheless, proper alignment of the biplane cursor is feasible in 90% of patients in our study. In our experience, alignment of the biplane cursor with the LVOT can be optimized by moving the probe to a lower parasternal window, typically one rib space inferior to the standard view. Acquiring multiple cardiac cycles also facilitate attaining a mid-systolic frame with optimal alignment. Second, not all patients in our study had cardiac CT for comparison. However, in the subset of patients with severe AS undergoing TAVR evaluation, we were able to demonstrate good correlation and better agreement between biplane TTE and CT-planimetered LVOT area compared to standard TTE method. Third, most outcomes data in AS are based on AVA calculated using standard LVOT area with circular assumption, and in this study, we did not examine the clinical implications of the biplane technique. Long-term follow-up with a larger number of patients is needed to assess the impact of the biplane-derived LVOT area method on clinical decision-making and outcomes.
Conclusions
Direct planimetry of the LVOT area by biplane imaging is a feasible, non-invasive, and widely available method that avoids inherent underestimation of LVOT area using circular assumption. It has better agreement with cardiac CT than the conventional single-diameter method, and can lead to better concordance between AVA and MG in AS grading. This technique has the potential to be the preferred TTE method for assessing LVOT area and AS severity, particularly in the subgroup of patients with low-gradient ‘severe’ AS by AVA and preserved LVEF.
Acknowledgments
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of interest: Dr. Ghoshhajra has received institutional research support from Siemens Healthcare.
Abbreviations:
- AP
Anteroposterior
- AS
Aortic stenosis
- AVA
Aortic valve area
- CMR
Cardiac magnetic resonance
- CT
Computed tomography
- EI
Eccentricity index
- HG
High gradient
- LF-LG
Low-flow low-gradient
- LVEF
Left ventricular ejection fraction
- LVOT
Left ventricular outflow tract
- LVOTA
Left ventricular outflow tract area
- LVOTd
Left ventricular outflow tract diameter
- MG
Mean gradient
- ML
Mediolateral
- NF-LG
Normal-flow low-gradient
- SVi
Stroke volume index
- TAVR
Transcatheter aortic valve replacement
- TEE
Transesophageal echocardiography
- TTE
Transthoracic echocardiography
- VTI
Velocity-time integral
- 2D
Two-dimensional
- 3D
Three-dimensional
Footnotes
All other authors have no relationships relevant to the contents of this paper to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Eveborn GW, Schirmer H, Heggelund G, Lunde P, Rasmussen K. The evolving epidemiology of valvular aortic stenosis. the Tromso study. Heart. 2013;99:396–400. [DOI] [PubMed] [Google Scholar]
- [2].Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- [3].Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, et al. Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30:372–92. [DOI] [PubMed] [Google Scholar]
- [4].Clavel MA, Burwash IG, Pibarot P. Cardiac Imaging for Assessing Low-Gradient Severe Aortic Stenosis. JACC Cardiovasc Imaging. 2017;10:185–202. [DOI] [PubMed] [Google Scholar]
- [5].O’Brien B, Schoenhagen P, Kapadia SR, Svensson LG, Rodriguez L, Griffin BP, et al. Integration of 3D imaging data in the assessment of aortic stenosis: impact on classification of disease severity. Circ Cardiovasc Imaging. 2011;4:566–73. [DOI] [PubMed] [Google Scholar]
- [6].Clavel MA, Malouf J, Messika-Zeitoun D, Araoz PA, Michelena HI, Enriquez-Sarano M. Aortic valve area calculation in aortic stenosis by CT and Doppler echocardiography. JACC Cardiovasc Imaging. 2015;8:248–57. [DOI] [PubMed] [Google Scholar]
- [7].Caballero L, Saura D, Oliva-Sandoval MJ, Gonzalez-Carrillo J, Espinosa MD, Garcia-Navarro M, et al. Three-Dimensional Morphology of the Left Ventricular Outflow Tract: Impact on Grading Aortic Stenosis Severity. J Am Soc Echocardiogr. 2017;30:28–35. [DOI] [PubMed] [Google Scholar]
- [8].Saitoh T, Shiota M, Izumo M, Gurudevan SV, Tolstrup K, Siegel RJ, et al. Comparison of left ventricular outflow geometry and aortic valve area in patients with aortic stenosis by 2-dimensional versus 3-dimensional echocardiography. Am J Cardiol. 2012;109:1626–31. [DOI] [PubMed] [Google Scholar]
- [9].Garcia J, Kadem L, Larose E, Clavel MA, Pibarot P. Comparison between cardiovascular magnetic resonance and transthoracic Doppler echocardiography for the estimation of effective orifice area in aortic stenosis. J Cardiovasc Magn Reson. 2011;13:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sagmeister F, Weininger M, Herrmann S, Bernhardt P, Rasche V, Bauernschmitt R, et al. Extent of size, shape and systolic variability of the left ventricular outflow tract in aortic stenosis determined by phase-contrast MRI. Magn Reson Imaging. 2018;45:58–65. [DOI] [PubMed] [Google Scholar]
- [11].Doddamani S, Grushko MJ, Makaryus AN, Jain VR, Bello R, Friedman MA, et al. Demonstration of left ventricular outflow tract eccentricity by 64-slice multi-detector CT. Int J Cardiovasc Imaging. 2009;25:175–81. [DOI] [PubMed] [Google Scholar]
- [12].Bhatia N, Dawn B, Siddiqui TS, Stoddard MF. Impact and predictors of noncircular left ventricular outflow tract shapes on estimating aortic stenosis severity by means of continuity equations. Tex Heart Inst J. 2015;42:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Alskaf E, Kardos A. The mystery of defining aortic valve area: what have we learnt from three-dimensional imaging modalities? J Echocardiogr. 2018;16:130–8. [DOI] [PubMed] [Google Scholar]
- [14].Gaspar T, Adawi S, Sachner R, Asmer I, Ganaeem M, Rubinshtein R, et al. Three-dimensional imaging of the left ventricular outflow tract: impact on aortic valve area estimation by the continuity equation. J Am Soc Echocardiogr. 2012;25:749–57. [DOI] [PubMed] [Google Scholar]
- [15].Burgstahler C, Kunze M, Loffler C, Gawaz MP, Hombach V, Merkle N. Assessment of left ventricular outflow tract geometry in non-stenotic and stenotic aortic valves by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2006;8:825–9. [DOI] [PubMed] [Google Scholar]
- [16].De Vecchi C, Caudron J, Dubourg B, Pirot N, Lefebvre V, Bauer F, et al. Effect of the ellipsoid shape of the left ventricular outflow tract on the echocardiographic assessment of aortic valve area in aortic stenosis. J Cardiovasc Comput Tomogr. 2014;8:52–7. [DOI] [PubMed] [Google Scholar]
- [17].Mehrotra P, Flynn AW, Jansen K, Tan TC, Mak G, Julien HM, et al. Differential left ventricular outflow tract remodeling and dynamics in aortic stenosis. J Am Soc Echocardiogr. 2015;28:1259–66. [DOI] [PubMed] [Google Scholar]
- [18].Pinto Teixeira P, Ramos R, Rio P, Moura Branco L, Portugal G, Abreu A, et al. Modified continuity equation using left ventricular outflow tract three-dimensional imaging for aortic valve area estimation. Echocardiography. 2017;34:978–85. [DOI] [PubMed] [Google Scholar]
- [19].Utsunomiya H, Yamamoto H, Horiguchi J, Kunita E, Okada T, Yamazato R, et al. Underestimation of aortic valve area in calcified aortic valve disease: effects of left ventricular outflow tract ellipticity. Int J Cardiol. 2012;157:347–53. [DOI] [PubMed] [Google Scholar]
- [20].Maes F, Pierard S, de Meester C, Boulif J, Amzulescu M, Vancraeynest D, et al. Impact of left ventricular outflow tract ellipticity on the grading of aortic stenosis in patients with normal ejection fraction. J Cardiovasc Magn Reson. 2017;19:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ng AC, Delgado V, van der Kley F, Shanks M, van de Veire NR, Bertini M, et al. Comparison of aortic root dimensions and geometries before and after transcatheter aortic valve implantation by 2- and 3-dimensional transesophageal echocardiography and multislice computed tomography. Circ Cardiovasc Imaging. 2010;3:94–102. [DOI] [PubMed] [Google Scholar]
- [22].Otani K, Takeuchi M, Kaku K, Sugeng L, Yoshitani H, Haruki N, et al. Assessment of the aortic root using real-time 3D transesophageal echocardiography. Circ J. 2010;74:2649–57. [DOI] [PubMed] [Google Scholar]
- [23].Kamperidis V, van Rosendael PJ, Katsanos S, van der Kley F, Regeer M, Al Amri I, et al. Low gradient severe aortic stenosis with preserved ejection fraction: reclassification of severity by fusion of Doppler and computed tomographic data. Eur Heart J. 2015;36:2087–96. [DOI] [PubMed] [Google Scholar]
- [24].Jainandunsing JS, Mahmood F, Matyal R, Shakil O, Hess PE, Lee J, et al. Impact of three-dimensional echocardiography on classification of the severity of aortic stenosis. Ann Thorac Surg. 2013;96:1343–8. [DOI] [PubMed] [Google Scholar]
- [25].Arsalan M, Squiers JJ, Filardo G, Pollock B, DiMaio JM, Parva B, et al. Effect of Elliptical LV Outflow Tract Geometry on Classification of Aortic Stenosis in a Multidisciplinary Heart Team Setting. JACC Cardiovasc Imaging. 2017;10:1401–2. [DOI] [PubMed] [Google Scholar]
- [26].Maes F, Boulif J, Pierard S, de Meester C, Melchior J, Gerber B, et al. Natural history of paradoxical low-gradient severe aortic stenosis. Circ Cardiovasc Imaging. 2014;7:714–22. [DOI] [PubMed] [Google Scholar]
- [27].Eleid MF, Sorajja P, Michelena HI, Malouf JF, Scott CG, Pellikka PA. Flow-gradient patterns in severe aortic stenosis with preserved ejection fraction: clinical characteristics and predictors of survival. Circulation. 2013;128:1781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Simard L, Cote N, Dagenais F, Mathieu P, Couture C, Trahan S, et al. Sex-Related Discordance Between Aortic Valve Calcification and Hemodynamic Severity of Aortic Stenosis: Is Valvular Fibrosis the Explanation? Circ Res. 2017;120:681–91. [DOI] [PubMed] [Google Scholar]
- [29].Delgado V, Clavel MA, Hahn RT, Gillam L, Bax J, Sengupta PP, et al. How Do We Reconcile Echocardiography, Computed Tomography, and Hybrid Imaging in Assessing Discordant Grading of Aortic Stenosis Severity? JACC Cardiovasc Imaging. 2019;12:267–82. [DOI] [PubMed] [Google Scholar]
- [30].Pibarot P, Clavel MA. Left ventricular outflow tract geometry and dynamics in aortic stenosis: implications for the echocardiographic assessment of aortic valve area. J Am Soc Echocardiogr. 2015;28:1267–9. [DOI] [PubMed] [Google Scholar]
- [31].Doddamani S, Bello R, Friedman MA, Banerjee A, Bowers JH Jr., Kim B, et al. Demonstration of left ventricular outflow tract eccentricity by real time 3D echocardiography: implications for the determination of aortic valve area. Echocardiography. 2007;24:860–6. [DOI] [PubMed] [Google Scholar]
- [32].Khaw AV, von Bardeleben RS, Strasser C, Mohr-Kahaly S, Blankenberg S, Espinola-Klein C, et al. Direct measurement of left ventricular outflow tract by transthoracic real-time 3D-echocardiography increases accuracy in assessment of aortic valve stenosis. Int J Cardiol. 2009;136:64–71. [DOI] [PubMed] [Google Scholar]
- [33].Menzel T, Mohr-Kahaly S, Wagner S, Fischer T, Bruckner A, Meyer J. Calculation of left ventricular outflow tract area using three-dimensional echocardiography. Influence on quantification of aortic valve stenosis. Int J Card Imaging. 1998;14:373–9. [DOI] [PubMed] [Google Scholar]