Abstract
Background
The use of dialysis fluids (DFs) during haemodialysis has been associated with increased oxidative stress and reduced serum magnesium (Mg) levels, contributing to chronic inflammation. Since the role of Mg in modulating immune function and reducing oxidative stress has been demonstrated, the aim of this study was to characterize in vitro whether increasing the Mg concentration in DFs could protect immune cells from oxidative stress and damage.
Methods
The effect of citrate [citrate dialysis fluid (CDF), 1 mM] or acetate [acetate dialysis fluid (ADF), 3 mM] dialysates with low (0.5 mM; routinely used) or high (1 mM, 1.25 mM and 2 mM) Mg concentrations was assessed in THP-1 human monocytes. The levels of reactive oxygen species (ROS), malondialdehyde (MDA) and oxidized/reduced (GSSG/GSH) glutathione were quantified under basal and inflammatory conditions (stimulation with lipopolysaccharide, LPS).
Results
The increase of Mg in CDF resulted in a significant reduction of ROS production under basal and inflammatory conditions (extremely marked in 2 mM Mg; P < 0.001). These effects were not observed in ADF. Interestingly, in a dose-dependent manner, high Mg doses in CDF reduced oxidative stress in monocytes under both basal and inflammatory conditions. In fact, 2 mM Mg significantly decreased the levels of GSH, GSSG and MDA and the GSSG/GSH ratio in relation to 0.5 mM Mg.
Conclusions
CDF produces lower oxidative stress than ADF. The increase of Mg content in DFs, especially in CDF, could have a positive and protective effect in reducing oxidative stress and damage in immune cells, especially under inflammatory conditions.
Keywords: acetate, chronic kidney disease, citrate, dialysate, haemodialysis, inflammation, magnesium, monocytes, oxidative stress
INTRODUCTION
Cardiovascular diseases (CVDs) remain the main cause of morbidity and mortality among chronic kidney disease (CKD) patients, especially in those undergoing haemodialysis (HD) [1–3]. Several risk factors inherent to the dialysis procedure itself could contribute to this high incidence/prevalence of CVDs [4, 5]. Several sources of evidence support the theory that the pathophysiological mechanism by which these risk factors induce CVDs is closely related to their ability to generate systemic inflammation, which is characterized by excessive production and release of pro-inflammatory compounds [4–7]. These mediators also lead to the activation of signalling cascades that trigger the overproduction of oxidant compounds, such as reactive oxygen species (ROS), contributing to oxidative stress and amplifying cellular damage [7]. Since a relationship between oxidative stress, vascular cell damage and the systemic inflammation has been extensively reported in HD patients with CVDs, preventing oxidative stress in these subjects has become a priority therapeutic objective [6, 8].
Immune cells continuously generate oxidants and inflammatory compounds to perform their defensive functions. However, when the overproduction of oxidant compounds is not well controlled by the antioxidant defences, an alteration occurs in the redox balance, leading to oxidative stress; thus, certain biomolecules are oxidized, promoting remarkable detrimental consequences on cellular functioning [9]. Several sources of evidence indicate the strong involvement of oxidative–inflammatory stress in the progression of renal injury and atherosclerosis in CKD patients [6, 8, 10–12]. Moreover, uraemic- and dialysis-associated factors contribute to oxidative–inflammatory stress in HD patients, including the use of bioincompatible dialyser membranes and dialysates [12].
Dialysis fluid (DF) composition is critical to optimize uraemic toxin removal while preserving fluid, electrolytes and acid–base balance [13]. DFs are produced by treated purified water, bicarbonate concentrate and an acid concentrate, which is needed to prevent calcium/magnesium carbonate precipitation occurring by the addition of bicarbonate. The most commonly used acid is acetic acid (3–10 mM) [14]. However, acetate dialysis fluid (ADF) is associated with several side effects and induces leukocyte activation, resulting in increased ROS production and the release of inflammatory compounds [15–17]. Thus, citrate dialysis fluid (CDF) was developed to improve HD biocompatibility by replacing the small amount of acetic acid with more physiological citric acid [18]. At the concentrations routinely used in clinical practice, ADF increases oxidative stress and activates other pro-inflammatory stimuli typical of HD patients, whereas CDF does not induce this activation, which could make it a suitable alternative in clinical practice [17].
Magnesium (Mg) plays a key role in numerous biological processes [19, 20]. Low Mg levels are associated with CVDs and all-cause mortality in HD patients [20, 21]. Moreover, hypomagnesaemia is associated with increased oxidative–inflammatory stress in CKD, suggesting that an increase in Mg levels is a useful therapy [20, 21]. Routine HD with the standard 0.5 mM Mg dialysate reduces Mg in most patients [22, 23], who often need an increase in dialysate Mg concentration [19]. The maintenance of serum Mg levels in HD patients depends on intestinal absorption, uptake and release by bone, and residual renal function and the composition of the DFs in relation to both the Mg concentration and the type of acid [19]. A recent multicentre, randomized, prospective study has described Mg reduction in relation to the use of CDF [24]. Several investigators suggested a CDF formulation with an Mg concentration higher than usual (0.5 mM), because higher Mg levels might be beneficial or indeed needed when using 1 mM citrate concentrates [18, 25]. Furthermore, the increase of Mg concentration in DFs such as in ADF is reportedly associated with a decrease of intra- and post-dialysis arrhythmias [26], the serum’s propensity to inhibit calcification [27] and cardiovascular mortality in HD patients [28, 29]. Therefore, higher Mg concentrations in DFs could be a potential mechanism to modify oxidative–inflammatory stress in HD patients [25]. However, more studies are necessary to reinforce clinical translation in this field.
This study aimed to evaluate, in vitro, whether the increase of Mg concentrations in different DFs could have a beneficial/protective effect on oxidative stress in immune cells. Several oxidative stress and damage parameters were assessed in human monocytes culture in two DFs generally used in clinical practice: citrate (CDF, 1 mM) or acetate (ADF, 3 mM) dialysates with standard (0.5 mM) or high (1 mM, 1.25 mM and 2 mM) Mg concentrations.
MATERIALS AND METHODS
HD fluids
Two types of DFs were used:
ADF: containing acetate (3 mM)
CDF: containing citrate (1 mM)
The composition of the DFs is shown in Table 1. Samples were taken from the Hansen connector to the dialysate input, once the machine had been stabilized (conductivity and temperature). Hank’s medium with similar standard Mg concentration (0.5 mM) to DFs was used as control solution. A vial of magnesium sulphate (150 mg/mL solution of MgSO4 heptahydrate; Genfarma®, Altan Pharma, Madrid, Spain) was used for supplementing ADF, CDF and control solution with 1, 1.25 and 2 mM Mg concentrations.
Table 1.
Composition of DFs used
| DFs-Reference | AcetateSoftPac® Baxter | CitrateSelectBag Citrate® Baxter |
|---|---|---|
| Na, mM | 140 | 140 |
| K, mM | 2 | 2 |
| Ca, mM | 1.50 | 1.65 |
| Mg, mM | 0.50 | 0.50 |
| Cl, mM | 109.5 | 106.5 |
| Citrate, mM | 0 | 1 |
| Acetate, mM | 3 | 0 |
| Bicarbonate, mM | 34 | 34 |
| Glucose, mg/dL | 100 | 100 |
Na, sodium; K, potassium; Ca, calcium; Mg, magnesium; Cl, chlorine.
Human monocytes cultures
Human monocytes (THP-1 cell line, Cat#88081201; Sigma-Aldrich, St Louis, MO, USA) were cultured in RPMI-1640 medium with 1% penicillin–streptomycin (Basel, Switzerland) and 10% fetal bovine serum (FBS, Sigma-Aldrich).
All different assays were performed under basal and stimulating pro-inflammatory conditions, such as those induced by bacterial lipopolysaccharide (LPS), to assess whether the antioxidant capacity of Mg could protect patients from potential high-risk situations.
Measurement of ROS
Intracellular ROS production was measured in THP-1 monocytes using a method [30] based on the fluorescence emitted by 2′,7′-dichlorodihydrofluorescein (DCF-DA, Sigma-Aldrich) which is oxidized in the cytoplasm by ROS to 2′,7′-dichlorofluorescein, with several modifications [25]. Briefly, THP-1 cell suspension adjusted to 0.2 × 106 cells/well in the different DFs or control solution were loaded into 96-well plates (Nunclon Delta, Thermo Fisher Scientific, Waltham, MA, USA) and incubated for 30 min (37°C) with or without LPS (1 μg/mL, Escherichia coli serotype 0127: B8 endotoxin, Sigma-Aldrich). Previously, monocytes were pre-treated 30 min with different Mg concentrations (1, 1.25 and 2 mM). After LPS incubation, monocytes were incubated with DCF-DA (1 mM; 30 min; 37°C). Fluorescence of samples was measured with excitation/emission = 485–535 nm. Results were expressed as units ‘relative of fluorescence’ (URF). The percentage (%) of ROS production in response to LPS stimulation (related to the basal value without LPS) was also calculated.
To simulate the chronic inflammatory state that CKD patients suffer in HD, we chose LPS (1 μg/mL) to induce cell activation, because bacterial cell wall components mimic the infectious state and promote inflammatory conditions [31, 32].
Glutathione content assay
Both reduced (GSH) and oxidized (GSSG) glutathione levels were quantified using a fluorimetric method [33, 34], with several modifications [25]. This assay was carried out on frozen THP-1 monocytes (3 × 106 cells) aliquots, which were previously cultivated in CDF or control solution with different Mg concentrations (0.5, 1, 1.25 and 2 mM) and incubated (4 h; 37°C; 5% CO2) with or without LPS (1 μg/mL). Monocytes were lysed with phosphatebuffered saline (0.1 M, pH 8, Sigma-Aldrich), sonicated and centrifuged (16 000g; 30 min). Supernatants were used for glutathione quantification. Fluorescence was measured at excitation/emission = 350–420 nm. Protein contents of different samples were assessed using the ‘Biocinchoninic Acid (BCA) protein assay’ kit (Sigma-Aldrich). Serum albumin (BSA, Sigma-Aldrich) was used as standard. Results are expressed as GSH or GSSG nmol/mg protein. Moreover, the GSSG/GSH ratio was calculated for each sample.
Lipid peroxidation assay
Malondialdehyde (MDA) content was assessed using the ‘Lipid peroxidation MDA Assay’ kit (Biovision, Milpitas, CA, USA), which quantified the adduct formation of MDA with thiobarbituric acid (TBA; Sigma-Aldrich). The assay was carried out on frozen THP-1 monocytes (3 × 106 cells) aliquots, which were previously cultivated in CDF or control solution with several Mg concentrations (0.5, 1, 1.25 and 2 mM) and incubated (4 h; 37°C; 5% CO2) with or without LPS (1 μg/mL). Monocytes were lysed (lysis buffer with butylated hydroxytoluene 0.1 mM), sonicated and centrifuged (13 000g; 10 min). Later, supernatants were mixed with TBA, incubated in a water bath (1 h; 95°C) and ice-cold (10 min) to stop the reaction. Finally, samples were mixed with n-butanol (Sigma-Aldrich) and centrifuged (13 000g; 10 min) to collect the upper organic phase for spectrophotometric measurement at 532 nm. The protein levels of the samples were quantified as mentioned above. Results were expressed as nmol MDA/mg protein.
Cell viability assay
In all experiments, the viability of THP-1 monocytes was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kit (Sigma-Aldrich) [25]. THP-1 cells (0.2 × 106 cells/well) in 96-well, flat-bottomed plates were incubated in ADF, CDF or control solution with different Mg concentrations (0.5, 1, 1.25 and 2 mM) and then stimulated or not with LPS (1 μg/mL) for 4 h. Later, the solutions were replaced with MTT solution (0.5 mg/mL) for 4 h (37°C; 5% CO2). Then, the MTT solution was removed and the formazan crystals were solubilized (1 h; 37°C) using a lysis buffer (DMSO, Sigma-Aldrich). Absorbance was measured at 570 nm. Results are expressed as percentages (%) relative to results obtained with control cells.
Statistical analysis
Statistical analyses were performed with the SPSS 21.0 statistical package (SPSS Inc., Chicago, IL, USA). Data are expressed as mean ± standard deviation (SD). All tests were two-tailed, with a significance level of 0.05. Both normality and homogeneity of the variances were checked by the Kolmogorov–Smirnov and Levene’s tests, respectively. Differences between groups were studied by one-way analysis of variance followed by post hoc test analysis. The Tukey test was used for post hoc comparisons when variances were homogeneous, whereas its counterpart Games-Howell was used when variances were not homogeneous.
RESULTS
Effect of the addition of high Mg concentrations to the DFs on ROS production under basal and inflammatory conditions
First, THP-1 monocytes were cultured in ADF, CDF or control solution, all with similar standard Mg concentration (0.5 mM). ROS generation was quantified under basal and LPS-stimulated conditions (Figure 1). Monocytes cultured in both ADF and CDF (P < 0.001 and P < 0.01, respectively) produced a significantly higher basal ROS production than the control solution (Figure 1A). Similar results were observed in LPS-stimulated conditions (P < 0.001; Figure 1A). However, CDF induced lower ROS production (P < 0.05) than ADF. In fact, a significant decrease in ROS production (% LPS stimulation) was found in THP-1 cells cultured with CDF (P < 0.05) in comparison with ADF (Figure 1B).
FIGURE 1:
Intracellular ROS production in human monocyte (THP-1) cultivated with acetate (ADF, 3 mM) or citrate (CDF, 1 mM) dialysates with 0.5 mM Mg concentration. (A) Level of ROS (URF) under basal or inflammatory conditions (stimulation with LPS, 1 µg/mL). (B) Percentage of stimulation of ROS in response to LPS. Each column represents the mean ± SD of 5–10 values corresponding to that number of experiments, and each value being the mean of duplicate assays. **P < 0.01 and ***P < 0.01 versus control solution. ○P < 0.05 versus ADF.
A similar experiment was performed using higher Mg concentrations (1, 1.25 and 2 mM) in DFs and control solution to test the beneficial/detrimental effect of this element. Under basal conditions, ROS generation varies depending on the DFs used (Figure 2). Mg significantly decreased basal ROS production by monocytes cultured in CDF (Figure 2C), which was extremely marked with 2 mM Mg (P < 0.01). Similar results were found in the control solution (Figure 2A), where reduced ROS content was detected with 1 mM and 1.25 mM Mg (P < 0.01) in comparison with standard Mg. By contrast, high Mg in ADF induced a marked increase in basal ROS generation (1 and 2 mM Mg, P < 0.05; Figure 2B).
FIGURE 2:
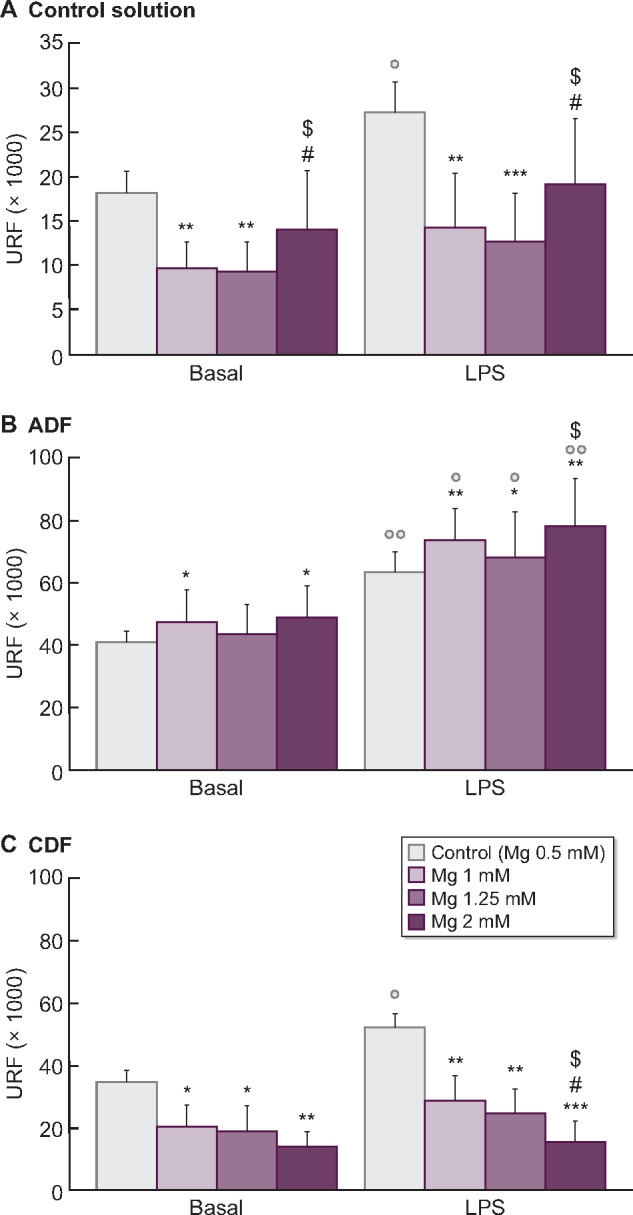
ROS (URF) levels under basal and inflammatory conditions (stimulation with LPS, 1 µg/mL) in human THP-1 monocyte cell line cultured with (A) control solution or with (B) acetate (ADF, 3 mM) and (C) citrate (CDF, 1 mM) dialysates with standard 0.5 mM Mg (control) or with high Mg concentrations (1, 1.25 and 2 mM). Each column represents the mean ± SD of 5–10 values corresponding to that number of experiments, with each value being the mean of duplicate assays. *P < 0.05, **P < 0.01 and ***P < 0.01 versus Mg 0.5 mM (control). #P < 0.05 versus Mg 1 mM. $P < 0.05 versus Mg 1.25 mM. ○P < 0.05 and ○○P < 0.01 versus basal conditions.
Under inflammatory conditions, the increase of Mg in CDF resulted in a significant reduction of ROS production (1 and 1.25 mM Mg, P < 0.01; 2 mM Mg, P < 0.001; Figure 2C). Interestingly, in a dose-dependent manner, high Mg concentrations in CDF reduced the percentage of ROS produced in LPS response (Figure 3). These effects were not observed in ADF, where high Mg rendered monocytes more sensitive to the LPS action, inducing ROS production (1 and 2 mM Mg, P < 0.01; Figure 2B). Moreover, a significant increase ROS production (%) in LPS response was observed with the highest Mg concentration (2 mM) in comparison with standard Mg (P < 0.05; Figure 3).
FIGURE 3:

ROS production (%) in response to LPS stimulation (1 µg/mL) in human THP-1 monocyte cell line cultured with acetate (ADF, 3 mM) or citrate (CDF, 1 mM) with standard 0.5 mM Mg (control) or with high Mg concentrations (1, 1.25 and 2 mM). Each column represents the mean ± SD of 5–10 values corresponding to that number of experiments, and each value being the mean of duplicate assays. *P < 0.05, **P < 0.01 and ***P < 0.01 versus Mg 0.5 mM (control). #P < 0.05 and ##P < 0.01 versus Mg 1 mM. $$P < 0.01 versus Mg 1.25 mM. ○P < 0.05, ○○P < 0.01 and ○○○P < 0.001 versus ADF.
High Mg concentrations reduce oxidative stress and damage induced by CDF under basal and inflammatory conditions
Since the increase of Mg concentrations in CDF had a beneficial effect by decreasing ROS production, we also evaluated if this effect could occur in other biomarkers associated with redox balance and lipid damage. Therefore, intracellular GSH and GSSG levels, the GSSG/GSH ratios (markers of redox balance) and MDA content (lipid peroxidation marker) were assessed in monocytes cultured in CDF with 0.5 mM Mg or with high Mg concentrations (1, 1.25 and 2 mM) under basal or inflammatory conditions (LPS, 1 µg/mL). In general, when monocytes were cultivated with standard Mg, LPS induced higher oxidative stress (decreased GSH, P < 0.05; increased GSSG and GSSG/GSH ratio, P < 0.05; Figure 4) and damage (increased MDA, P < 0.05; Figure 5) than in basal conditions.
FIGURE 4:
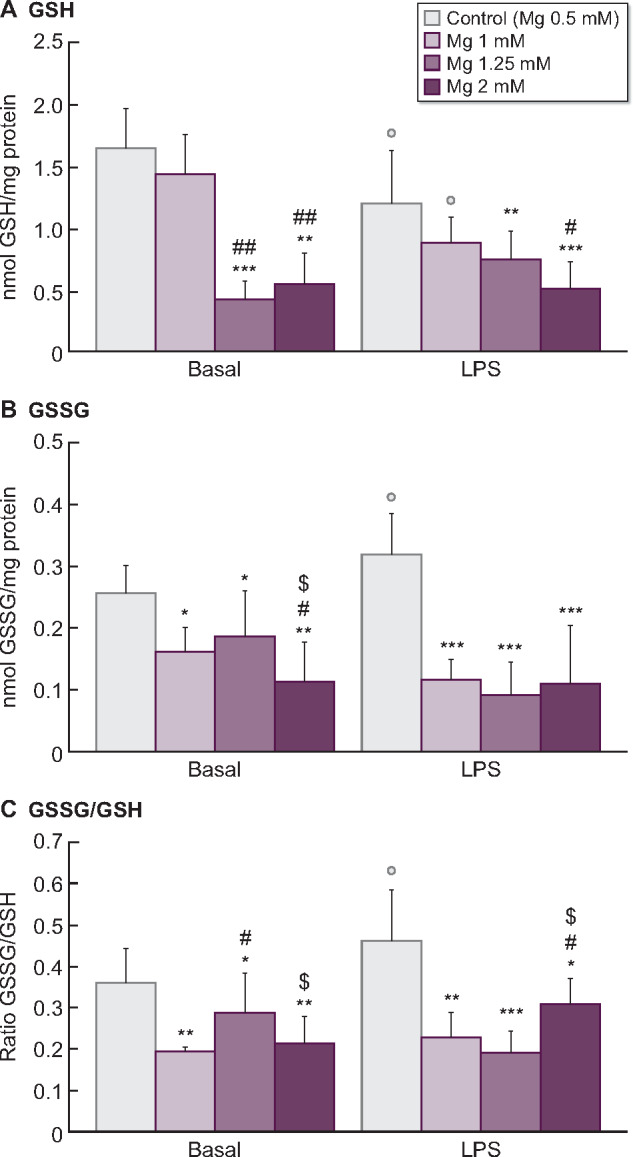
Effect of citrate (CDF, 1 mM) dialysate with standard 0.5 mM Mg (control) or with high Mg concentrations (1, 1.25 and 2 mM) on the intracellular levels of (A) reduced (GSH) and (B) oxidized (GSSG) glutathione (nmol/mg protein), as well as on (C) the redox balance GSSG/GSH ratio assessed in human monocyte cultures (THP-1) cultivated under basal or inflammatory conditions (stimulation with LPS, 1 µg/mL). Each column represents the mean ± SD of 5–10 values corresponding to that number of experiments, and each value being the mean of duplicate assays. *P < 0.05, **P < 0.01 and ***P < 0.01 versus Mg 0.5 mM (control). #P < 0.05 and ##P < 0.01 versus Mg 1 mM. $P < 0.05 versus Mg 1.25 mM. ○P < 0.05 versus basal conditions.
FIGURE 5:
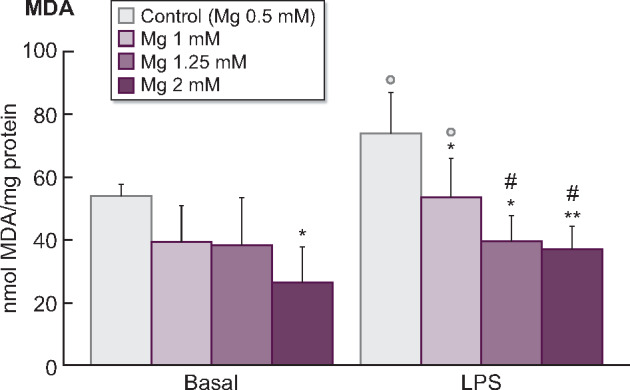
Effect of citrate (CDF, 1 mM) dialysate with standard 0.5 mM Mg (control) or with high Mg concentrations (1, 1.25 and 2 mM) on MDA content assessed in human monocyte cultures (THP-1) cultivated under basal or inflammatory conditions (stimulation with LPS, 1 µg/mL). Each column represents the mean ± SD of 5–10 values corresponding to that number of experiments, and each value being the mean of duplicate assays. *P < 0.05 and **P < 0.01 versus Mg 0.5 mM (control). #P < 0.05 versus Mg 1 mM. ○P < 0.05 versus basal conditions.
Interestingly, in a dose-dependent manner, high Mg concentrations reduced the oxidative stress and damage observed in monocytes cultured with 0.5 mM Mg. Regarding glutathione, monocytes cultured with high Mg showed significantly lower GSH levels (1.25 mM Mg, P < 0.001; 2 mM Mg, P < 0.01; Figure 4A) and lower GSSG amounts (1 and 1.25 mM Mg, P < 0.05; 2 mM Mg, P < 0.01; Figure 4B) than those observed with standard 0.5 mM Mg. Similar results were observed under LPS stimulation, where high Mg also potentiated a marked reduction of GSH (1.25 mM Mg, P < 0.01; 2 mM Mg, P < 0.001; Figure 4A) and GSSG (P < 0.001; Figure 4B) levels. Furthermore, high Mg in CDF promoted a decreased GSSG/GSH ratio under basal and LPS-stimulated conditions in comparison with standard Mg (Figure 4C). Regarding lipid oxidative damage (Figure 5), treatment of monocytes with 1, 1.25 and 2 mM Mg prior to LPS stimulation significantly decreased MDA levels in a dose-dependent manner (1 and 1.25 mM Mg, P < 0.05; 2 mM Mg, P < 0.01) in comparison with standard Mg.
The viability of monocytes was evaluated in all the assays performed. The results of cell viability (MTT assay) showed that ADF and CDF had no cytotoxic effects (all values >90%) and the values of cell viability ranged from 92 ± 5% to 96 ± 4% (ADF and CDF 0.5 mM Mg, respectively). The mean percentage of viability after exposure to high Mg concentrations (1 mM, 1.25 mM and 2 mM Mg) was 93 ± 6%, 90 ± 4% and 89 ± 5% in ADF, and 95 ± 7%, 96 ± 5% and 92 ± 7% in CDF, respectively. No statistically significant difference was observed between the experimental and control solutions.
DISCUSSION
The use of DFs during HD has been associated with increased oxidative stress and reduced serum Mg levels, contributing to oxidative damage and chronic inflammation in HD patients [10, 17]. Herein, we analysed in vitro the effect of increasing Mg concentration (1, 1.25 and 2 mM) in different DFs on oxidative stress and damage in human monocytes, especially under inflammatory conditions. In culture, THP-1 cells can be activated by LPS, serving as an effective model to investigate the mechanisms involved in vascular damage associated with inflammatory activity [17, 35]. Given that previous studies had shown that dialysates themselves differentially induce ROS production [17, 25], we included the most common DFs used in clinical practice, ADF (3 mM) and CDF (1 mM). Our study clearly showed that the increase in Mg concentrations in ADF or CDF significantly modulates changes in oxidative stress in monocytes. CDF produces lower ROS production than ADF. Interestingly, in a dose-dependent manner, high Mg concentrations in CDF resulted in a marked reduction of oxidative stress (decreased ROS, GSSG and GSSG/GSH) and lipid damage (decreased MDA) in monocytes, especially under LPS stimulation.
HD promotes excessive oxidative stress, due to the loss of antioxidants and the accumulation of oxidative products during HD procedures [10, 11]. Our results confirmed that ADF and CDF induced an increased ROS basal production in monocytes. Since oxidation and inflammation processes are interlinked processes [7], we hypothesized that ROS production in monocytes could also be triggered by an increase in basal inflammation. Thus, under LPS stimulation, a marked ROS production was observed in the monocytes cultured with ADF and CDF. However, substantial differences between ADF and CDF were observed despite their identical Mg concentration (0.5 mM). Interestingly, CDF induced significantly lower ROS production than ADF. These results confirm previous studies, which revealed that CDF does not induce oxidative stress or inflammation both in vitro and in vivo, as compared with ADF [18, 25, 36, 37]. In fact, fluids with acetate induced greater ROS activity in immunocompetent cells of both healthy subjects and HD patients than citrate-containing fluids [18]. ADF reportedly induces leucocyte activation and cytokine production [38], whereas both ROS production and inflammatory cytokine release were minimal during acetate-free biofiltration [15, 39]. Until recently, acetate has been used as a buffer in conventional dialysates. However, ADF leads to immune cell activation, promoting inflammation and arterial hypotension [15]. Because CDF does not induce cell activation [18] and has a positive impact on haemodynamic tolerance [24], CDF could be used as an alternative in clinical practice.
Dialysis patients are particularly vulnerable to the effect of Mg deficiency, which may explain the increased risk of CVDs in these subjects [40]. The use of low Mg dialysates (0.25–0.5 mM; routinely prescribed) is a risk factor for hypomagnesaemia in HD patients [40]. However, there is still no consensus regarding the optimal Mg concentration for dialysate. Although the use of a low Mg dialysate may have harmful haemodynamic effects [40, 41], the impact of Mg in HD patients still remains unclear in clinical routine practice. Chronic hypomagnesaemia could result in excessive ROS production, supporting a role for Mg in altering the threshold antioxidant capacity [40]. Thus, increased ROS production is associated with low-intracellular Mg levels [42]. Our study found that the increase in Mg concentration (1.25 and 2 mM) in dialysates had a dual effect on ROS production by monocytes, depending on the type of DFs used. Thus, high Mg concentrations decreased ROS production in monocytes cultured with CDF but not with ADF. Interestingly, this effect was more exacerbated under inflammatory conditions. In fact, the highest Mg concentration (2 mM) in CDF produced a marked reduction of ROS generated in response to LPS stimulation. By contrast, this highest Mg concentration in ADF promoted an increase in ROS production. Our results are in consonance with a previous in vitro study that supports a different modulation of oxidative stress in monocytes depending on the Mg concentration in several DFs [25]. Moreover, low serum Mg and immune system activation seem to co-operate to promote oxidative stress associated with endothelial dysfunction [43]. Increased ROS production and DNA damage were found in Mg-deprived endothelial cultured cells [43]. Therefore, increasing Mg concentrations in DFs, especially in CDF, could have a positive effect in decreasing ROS associated with inflammation, thereby improving the redox balance in immune cells.
Low serum Mg levels are common in many patients on HD and 5% are overtly hypomagnesaemic in those dialysed against a concentration of 0.5 mM [44]. This low dialysate Mg concentration inevitably induces a post-dialysis hypomagnesaemia [45], promoting acute haemodynamic effects [40, 41]. In relation to our data, it is important to note that the use of CDF might affect Mg concentration, because citrate may form a complex with Mg, which is dialysable, and increase the intradialytic removal of Mg. Therefore, some authors propose the use of CDF with higher Mg levels than usual (0.5 mM) to solve the problem of the reduction in Mg described in relation with the use of CDF [24], which could also have a beneficial effect on cardiovascular mortality in CKD patients [29].
An increase in oxidative lipid damage and reduced plasma concentrations of antioxidants are closely associated with the HD procedure [10–12, 45, 46]. Our study also showed that LPS stimulation remarkably decreased the GSH content and increased both GSSG and MDA levels and the GSSG/GSH ratio in monocytes cultured in CDF with 0.5 mM Mg. Interestingly, in a dose-dependent manner, high Mg concentrations in CDF decreased GSH, GSSG and MDA content in monocytes, especially with 2 mM Mg under LPS stimulation. A disturbance of the glutathione antioxidant system, including overall compromised blood levels of GSH and increased GSSG, has been reported in CKD and HD patients [46]. Moreover, the GSSG/GSH ratio has been proposed as a good maker of redox balance in dialysed and not yet dialysed patients [47]. Although reduced GSH could have detrimental effects on promoting oxidative damage, the observed and marked GSSG/GHS reduction indicates a good maintenance of redox balance. Although we have not yet elucidated the molecular mechanisms underlying the potential action of high Mg levels in CDF in decreasing GSH/GSSG, several studies demonstrate a direct relationship between cellular Mg status and circulating GSSG/GSH [42, 47]. Thus, a lower GSSG/GSH ratio was associated with higher intracellular levels of Mg in red blood cells of hypertensive patients [42]. Regarding lipid peroxidation, several studies revealed that CDF decreases glycoxidation and lipid peroxidation products compared with conventional ADF [45], as well as decreasing the degranulation of polymorphonuclear leukocytes and platelets, and contributing to reduce oxidative damage during HD [47]. Mg deficiency is also associated with increased lipid oxidative tissue damage [42]. Mg deficiency is reportedly related to a marked increase in plasma and kidney MDA levels [48], whereas increased Mg intake reduced lipid peroxidation and improved hyperlipidaemia in rats [49]. Therefore, our results support the notion that the use of CDF containing high Mg concentrations has possible beneficial effects through reducing lipid damage during HD. This could be because the citrate would exert direct or indirect antioxidant effects due to its iron chelation activity and interaction with redox signalling pathways (regulation of the cellular antioxidant response) [50].
Our study provides useful information about the beneficial effects of the increase of Mg concentration in CDF on reducing oxidative stress and damage in monocytes. However, there are two major limitations in this study that could be addressed in future research. First, our results are based on in vitro experiments using a human monocyte cell line. Thus, experimental results must be viewed with caution and conclusions must not be inappropriately extrapolated. Secondly, our results might have been affected by other electrolytes present in the DFs. Therefore, further pre-clinical and clinical studies, especially randomized trials, will be required to validate our findings and to confirm the positive effects of the increase of Mg in DFs on improving clinical outcomes in HD patients.
CONCLUSION
This is the first study to reveal that the increase of Mg content in DFs has different effects on oxidative stress in human monocytes. Interestingly, high Mg concentrations (1.25 mM and 2 mM) in CDF have a positive effect, reducing oxidative stress and lipid damage, especially under inflammatory conditions. By contrast, high Mg in ADF enhances ROS production. Although further studies in clinical practice are needed, the increase of Mg concentration in CDF may provide a potential therapeutic tool to prevent excessive oxidative stress and damage and subsequent CVDs in HD patients.
ACKNOWLEDGEMENTS
We are grateful to Ignacio Hernández-Agramonte and Carlos Álvarez-Martín for their critical review of this manuscript.
FUNDING
This study was funded by Instituto de Salud Carlos III through the projects ‘PI17/01029’ and ‘PI19/00240’ (co-funded by European Regional Development Fund ‘A way to make Europe’), Santander/UCM PR41/17-20964, Sociedad Española de Nefrología and Universidad de Alcalá grants (UAHGP2018-4 and CCG2018/BIO-010). C.V. was funded by a fellowship programme ‘Contratos Asociados a Proyectos de Investigación’, Universidad de Alcalá, Madrid, Spain.
AUTHORS’ CONTRIBUTIONS
C.V., M.A., P.d.S., J.C. and R.R. were involved in the concept and design. C.V. was involved in the experiment and the acquisition of data. C.V., M.A., P.d.S., G.B., J.C., R.P. and R.R. were involved in the analysis and/or interpretation of data and contributed to manuscript drafting and critical revision. Final approval of the version to be published was provided by all authors.
CONFLICT OF INTEREST STATEMENT
P.d.S. holds a grant from Baxter and received honoraria for participation as a speaker at meetings of Baxter and sponsorships of scientific congresses by Nipro and Baxter. The authors declare no other conflicts of interest.
REFERENCES
- 1. Go AS, Chertow GM, Fan D. et al. Chronic kidney disease and the risks of death, cardio-vascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305 [DOI] [PubMed] [Google Scholar]
- 2. Di Angelantonio E, Chowdhury R, Sarwar N. et al. Chronic kidney disease and risk of major cardiovascular disease and non-vascular mortality: prospective population based cohort study. BMJ 2010; 341: c4986–c4986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chien KL, Lin HJ, Lee BC. et al. A prediction model for the risk of incident chronic kidney disease. Am J Med 2010; 123: 836–846 [DOI] [PubMed] [Google Scholar]
- 4. Spittle MA, Hoenich NA, Handelman GJ. et al. Oxidative stress and inflammation in hemodialysis patients. Am J Kidney Dis 2001; 38: 1408–1413 [DOI] [PubMed] [Google Scholar]
- 5. Pawlak K, Naumnik B, Brzósko S. et al. Oxidative stress – a link between endothelial injury, coagulation activation, and atherosclerosis in haemodialysis patients. Am J Nephrol 2004; 24: 154–161 [DOI] [PubMed] [Google Scholar]
- 6. Filiopoulos V, Hadjiyannakos D, Metaxaki P. et al. Inflammation and oxidative stress in patients on hemodiafiltration. Am J Nephrol 2008; 28: 949–957 [DOI] [PubMed] [Google Scholar]
- 7. Vida C, Gonzalez E, Fuente M.. Increase of oxidation and inflammation in nervous and immune systems with aging and anxiety. Curr Pharm Des 2014; 20: 4656–4678 [DOI] [PubMed] [Google Scholar]
- 8. Panth N, Paudel KR, Parajuli K.. Reactive oxygen species: a key hallmark of cardiovascular disease. Adv Med 2016; 2016: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fuente M, Miquel J.. An update of the oxidation-inflammation theory of aging: the involvement of the immune system in oxi-inflamm-aging. Curr Pharm Des 2009; 15: 3003–3026 [DOI] [PubMed] [Google Scholar]
- 10. Liakopoulos V, Roumeliotis S, Zarogiannis S. et al. Oxidative stress in hemodialysis: causative mechanisms, clinical implications, and possible therapeutic interventions. Semin Dial 2019; 32: 58–71 [DOI] [PubMed] [Google Scholar]
- 11. Daenen K, Andries A, Mekahli D. et al. Oxidative stress in chronic kidney disease. Pediatr Nephrol 2019; 34: 975–991 [DOI] [PubMed] [Google Scholar]
- 12. Ling XC, Kuo KL.. Oxidative stress in chronic kidney disease. Ren Replace Ther 2018; 4: 53 [Google Scholar]
- 13. Misra M. Basic mechanisms governing solute and fluid transport in hemodialysis. Hemodial Int 2008; 12: S25–S28 [DOI] [PubMed] [Google Scholar]
- 14. Pizzarelli F, Cerrai T, Dattolo P. et al. On-line hemodiafiltration with and without acetate. Nephrol Dial Trasplant 2006; 21: 1648–1651 [DOI] [PubMed] [Google Scholar]
- 15. Masuda A, Hagiwara S, Tanimoto M. et al. Effects of acetate-free citrate dialysate on glycoxidation and lipid peroxidation products in hemodialysis patients. Nephron Extra 2012; 2: 256–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Todeschini M, Macconi D, Fernández NG. et al. Effect of acetate-free biofiltration and bicarbonate hemodialysis on neutrophil activation. Am J Kidney Dis 2002; 40: 783–793 [DOI] [PubMed] [Google Scholar]
- 17. Pérez-García R, Ramírez R, de Sequera P. et al. Citrate dialysate does not induce oxidative stress or inflammation in vitro as compared to acetate dialysate. Nefrologia 2017; 37: 630–637 [DOI] [PubMed] [Google Scholar]
- 18. Ahmad S, Callan R, Cole JJ. et al. Dialysate made from dry chemicals using citric acid increases dialysis dose. Am J Kidney Dis 2000; 35: 493–499 [DOI] [PubMed] [Google Scholar]
- 19. Alhosaini M, Leehey DJ.. Magnesium and dialysis: the neglected cation. Am J Kidney Dis 2015; 66: 523–531 [DOI] [PubMed] [Google Scholar]
- 20. Ikee R. Cardiovascular disease, mortality, and magnesium in chronic kidney disease: growing interest in magnesium-related interventions. Ren Replace Ther 2018; 4: 1–9 [Google Scholar]
- 21. Nielsen FH. Magnesium deficiency and increased inflammation: current perspectives. J Inflamm Res 2018; 11: 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leenders NHJ, Ittersum FJ, Hoekstra T. et al. Routine hemodialysis induces a decline in plasma magnesium concentration in most patients: a prospective observational cohort study. Sci Rep 2018; 8: 10256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sakaguchi Y, Fujii N, Shoji T. et al. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int 2014; 85: 174–181 [DOI] [PubMed] [Google Scholar]
- 24. De Sequera P, Pérez-García R, Molina-Nuñez M. et al. Prospective randomised multicentre study to demonstrate the benefits of haemodialysis without acetate (with citrate): ABC-treat Study. Acute effect of citrate. Nefrología 2019; 39: 424–433 [DOI] [PubMed] [Google Scholar]
- 25. Vida C, Carracedo J, Sequera P. et al. Increasing the magnesium concentration in various dialysate solutions differentially modulates oxidative stress in a human monocyte cell line. Antioxidant (Basel) 2020; 9: 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tumlin JA, Roy-Chaudhury P, Koplan BA. et al. ; on behalf of the MiD investigators and Committees. Relationship between dialytic parameters and reviewer confirmed arrhythmias in hemodialysis patients in the monitoring in dialysis study. BMC Nephrol 2019; 20: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Floege J. Magnesium concentration in dialysate is higher better? Clin J Am Soc Nephrol 2018; 13: 1309–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu L, Li H, Wang SX.. Serum magnesium and mortality in maintenance hemodialysis patients. Blood Purif 2017; 43: 31–36 [DOI] [PubMed] [Google Scholar]
- 29. Schmaderer C, Braunisch MC, Suttmann Y. et al. Reduced mortality in maintenance haemodialysis patients on high versus low dialysate magnesium: a pilot study. Nutrients 2017; 9: 926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang H, Joseph JA.. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 1999; 27: 612–616 [DOI] [PubMed] [Google Scholar]
- 31. Tucureanu MM, Rebleanu D, Constantinescu CA. et al. Lipopolysaccharide-induced inflammation in monocytes/macrophages is blocked by liposomal delivery of Gi-protein inhibitor. Int J Nanomedicine 2017; 13: 63–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prestigiacomo V, Weston A, Messner S. et al. Pro-fibrotic compounds induce stellate cell activation, ECM-remodelling and Nrf2 activation in a human 3D-multicellular model of liver fibrosis. PLoS One 2017; 12: e0179995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hissin PJ, Hilf R.. Effects of estrogen to alter amino acid transport in R3230AC mammary carcinomas and its relationship to insulin action. Cancer Res 1979; 39: 3381–3387 [PubMed] [Google Scholar]
- 34. Vida C, Martinez de Toda I, Garrido A. et al. Impairment of several immune functions and redox state in blood cells of Alzheimer’s Disease patients. Relevant role of neutrophils in oxidative stress. Front Immunol 2018; 8: 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ronald JA, Ionescu CV, Rogers KA. et al. Differential regulation of transendothelial migration of THP-1 cells by ICAM-1/LFA-1 and VCAM-1/VLA-4. J Leukoc Biol 2001; 70: 601–609 [PubMed] [Google Scholar]
- 36. Bryland A, Wieslander A, Carlsson O. et al. Citrate treatment reduces endothelial death and inflammation under hyperglycaemic conditions. Diab Vasc Dis Res 2012; 9: 42–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Molina Nuñez M, de Alarcón R, Roca S. et al. Citrate versus acetate-based dialysate in on-line hemodiafiltration. A prospective cross-over study. Blood Purif 2015; 39: 181–187 [DOI] [PubMed] [Google Scholar]
- 38. Carozzi S, Nasini MG, Caviglia PM. et al. Acetate free biofiltration. Effects on peripheral blood monocyte activation and cytokine release. Asaio J 1992; 38: 52–54 [PubMed] [Google Scholar]
- 39. Higuchi T, Yamamoto C, Kuno T. et al. A comparison of bicarbonate hemodialysis, hemodiafiltration, and acetate-free biofiltration on cytokine production. Ther Apher Dial 2004; 8: 460–467 [DOI] [PubMed] [Google Scholar]
- 40. van de Wal-Visscher ER, Kooman JP, van der Sande FM.. Magnesium in chronic kidney disease: should we care? Blood Purif 2018; 45: 173–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Afshinnia F, Doshi H, Rao PS.. The effect of different dialysate magnesium concentrations on QTc dispersion in hemodialysis patients. Ren Fail 2012; 34: 408–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barbagallo M, Dominguez LJ, Tagliamonte MR. et al. Effects of glutathione on red blood cell intracellular magnesium: relation to glucose metabolism. Hypertension 1999; 34: 76–82 [DOI] [PubMed] [Google Scholar]
- 43. Wolf FI, Trapani V, Simonacci M. et al. Magnesium deficiency and endothelial dysfunction: is oxidative stress involved? Magnes Res 2008; 21: 58–64 [PubMed] [Google Scholar]
- 44. Maier JA, Bernardini D, Rayssiguier Y. et al. High concentrations of magnesium modulate vascular endothelial cell behaviour in vitro. Biochim Biophys Acta 2004; 1689: 6–12 [DOI] [PubMed] [Google Scholar]
- 45. Pun PH, Middleton JP.. Dialysate potassium, dialysate magnesium, and hemodialysis risk. J Am Soc Nephrol 2017; 28: 3441–3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Poulianiti KP, Kaltsatou A, Mitrou GI. et al. Systemic redox imbalance in chronic kidney disease: a systematic review. Oxid Med Cell Longev 2016; 2016: 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Santangelo F, Witko-Sarsat V, Drueke T. et al. Restoring glutathione as a therapeutic strategy in chronic kidney disease. Nephrol Dial Transplant 2004; 19: 1951–1955 [DOI] [PubMed] [Google Scholar]
- 48. Hans CP, Chaudhary DP, Bansal DD.. Magnesium deficiency increases oxidative stress in rats. Indian J Exp Biol 2002; 40: 1275–1279 [PubMed] [Google Scholar]
- 49. Olatunji LA, Soladoye AO.. Increased magnesium intake prevents hyperlipidemia and insulin resistance and reduces lipid peroxidation in fructose-fed rats. Pathophysiology 2007; 14: 11–15 [DOI] [PubMed] [Google Scholar]
- 50. Wu X, Dai H, Liu L. et al. Citrate reduced oxidative damage in stem cells by regulating cellular redox signaling pathways and represent a potential treatment for oxidative stress-induced diseases. Redox Biol 2019; 21: 101057. [DOI] [PMC free article] [PubMed] [Google Scholar]



