Abstract
Background
Endothelial dysfunction is associated with cardiovascular events and mortality in various disease states, including end-stage renal disease (ESRD). Novel technological approaches have emerged for real-time assessment of endothelial reactivity. This study examined skin microcirculation using laser speckle contrast imaging (LSCI) before and after arterial occlusion in ESRD patients undergoing haemodialysis (HD) or peritoneal dialysis (PD).
Methods
The 38 HD patients were matched in a 1:1 ratio with 38 PD patients (for age, sex and dialysis vintage) and 38 controls (for age and sex). Skin microvascular reactivity parameters assessed with LSCI included baseline perfusion, occlusion perfusion and peak perfusion during post-occlusive reactive hyperaemia (PORH); time to peak perfusion; proportional change from baseline to peak perfusion; baseline and peak cutaneous vascular conductance (CVC); proportional change from baseline to peak CVC and amplitude of the PORH response (i.e. the difference between peak and baseline CVC).
Results
Baseline perfusion [HD: 46.97 ± 14.6; PD: 49.32 ± 18.07; controls: 42.02 ± 11.94 laser specle perfusion units (LSPU), P = 0.097] and peak post-occlusion perfusion (104.77 ± 28.68 versus 109.04 ± 40.77 versus 116.96 ± 30.96 LSPU, P = 0.238) did not differ significantly between groups. However, the post-occlusive vascular response was completely different since the proportional increase from baseline to peak perfusion (HD: 133 ± 66; PD: 149 ± 125; controls: 187 ± 61%, P = 0.001) was significantly lower in ESRD patients and time to peak response was lower in HD but similar in PD patients compared with controls (HD: 7.24 ± 6.99; PD: 10.68 ± 9.45; controls: 11.11 ± 5.1 s, Kruskal–Wallis P = 0.003; pairwise comparisons: HD versus controls, P = 0.002; HD versus PD, P = 0.154; PD versus controls, P = 0.406). ESRD patients also had lower levels of peak CVC, indicating the maximum capillary recruitment (HD: 1.05 ± 0.3; PD: 1.07 ± 0.44; controls: 1.57 ± 0.52 LSPU/mmHg, P < 0.001), lower proportional increase of CVC at peak (P < 0.001) and lower amplitude of the PORH response, a measure of the difference between baseline and maximum capillary recruitment (P = 0.001).
Conclusions
Using this novel non-invasive technology, endothelial post-occlusive forearm skin vasodilatory response was found to be similar between HD and PD patients and significantly impaired compared with controls. Future studies are needed to assess the prognostic implications of this microcirculatory functional defect.
Keywords: endothelial dysfunction, end-stage renal disease, haemodialysis, laser speckle contrast imaging (LSCI), peritoneal dialysis
INTRODUCTION
Atherosclerosis is a natural process following increasing age; it can begin even in childhood and after a long period of silent progression becomes clinically evident usually at middle age [1]. Atherosclerotic changes begin with various types of injury to the vascular endothelium [2]. The term endothelial dysfunction refers to alterations in the functional endothelial phenotype from a quiescent to an active state with expression of inflammatory molecules (cytokines, chemokines and adhesion molecules), deregulation of redox balance and vascular tone control [1, 3]. Over the years, several methods for clinical assessment of endothelial function have emerged and the central role of endothelial dysfunction in the pathogenesis of cardiovascular disease has been established. Invasive and non-invasive measures of endothelial function have an additional prognostic value in the assessment of cardiovascular risk in various populations [4, 5]. Markers of endothelial dysfunction have been associated with greater albuminuria and renal function decline in patients with chronic kidney disease (CKD) as well as left and right ventricular dysfunction, cardiovascular events and all-cause mortality in patients with end-stage renal disease (ERSD) [6–8].
Among the different methodological approaches to assess endothelial function, starting from the most invasive intracoronary infusion of acetylcholine in the mid-1980s, less invasive and non-invasive techniques, including venous occlusion plethysmography (measuring changes in forearm blood flow before and after infusion of vasoactive substances, i.e. acetylcholine) or Doppler ultrasound assessment of flow-mediated dilation (FMD) in the forearm arteries (measuring changes in brachial artery diameter before and after a 5-min ischaemia period), both reflecting endothelium-dependent vasodilation as a result of nitric oxide (NO) release, gained popularity because of their confirmed validity and reproducibility [9]. Although much simpler, these techniques require relevant infrastructure, careful assessments and experienced operators [1, 9]. Laser speckle contrast imaging (LSCI) is a novel technique that when coupled with reactivity tests such as post-occlusive reactive hyperaemia (PORH) and local thermal hyperaemia (LTH) showed improved spatial and temporal reproducibility compared with laser Doppler flowmetry (LDF) [10, 11]. It has been used for evaluation of different circulatory beds, from measurement of retinal blood flow and intraoperative monitoring of cerebral, gastric and hepatic microcirculation to evaluation of perfusion of ulcers in patients with systemic sclerosis and diabetes mellitus (DM) [12–16]. Recently LSCI was used for assessment of endothelium-dependent vasodilator responses in patients with type 1 DM, coronary artery disease (CAD) and Raynaud’s phenomenon [17–19].
Although the LSCI technique gradually evolved in its present form several years ago [20] and has been previously applied in the fields of ophthalmology, dermatology, rheumatology, neurology and even gastro-intestinal tract surgery [21], as of this writing, no studies have been conducted using this method in patients with CKD. Therefore this study aimed to assess skin microvascular endothelial function with LSCI coupled with a PORH protocol in patients with end-stage renal disease (ESRD) undergoing haemodialysis (HD) or peritoneal dialysis (PD) compared with controls.
MATERIALS AND METHODS
Study participants
Patients undergoing HD or PD followed up in two tertiary nephrology departments of northern Greece were recruited from March 2017 until October 2019. We included as cases adult patients (>18 years) with ESRD who had been treated with HD on a standard thrice-weekly schedule for at least 3 months. A blinded member of our team matched HD patients with potential controls that were under PD treatment for at least 3 months in our departments, as well as a group of controls comprised of healthy individuals or subjects with mild newly diagnosed hypertension or hypercholesterolaemia. Patients with non-functioning old arteriovenous fistulae on both arms or on the opposite arm from the one used as a vascular access for dialysis that could interfere with the protocol measurements were excluded. HD and PD patients were matched for age (±5 years), gender and dialysis vintage (±2 years) and with controls for age and sex. The protocol was approved by the ethical committee of the School of Medicine, Aristotle University of Thessaloniki and all participants provided informed written consent to participate in the study.
Study protocol
Demographics, anthropometric characteristics, comorbidities, concomitant medications and dialysis-related parameters were recorded for every participant. ESRD patients and controls were asked to visit the Hypertension Unit after having refrained from consuming food, coffee, alcohol and tobacco for ~12 h. Patients on HD were evaluated 1 h before the second or third dialysis session of the week, while patients on PD arrived 1 h before their programmed follow-up visit. All participants had received their morning drugs.
All subjects underwent a physical examination and venous blood sampling for routine laboratory tests. Office blood pressure (BP) measurements were recorded by a doctor on the basis of the average of three office measurements (obtained after the participant had stayed in a sitting position for at least 5 min), according to current guidelines [22], and mean blood pressure (MBP) was subsequently calculated as diastolic BP (DBP) + 1/3[systolic BP (SBP) − DBP]. Thereafter, skin microvascular reactivity was evaluated with LSCI, as described below.
LSCI assessment
The PeriCam PSI system (Perimed, Järfälla, Sweden) was used to assess LSCI, in combination with a PORH protocol, in a quiet room, framed by dark curtains, the temperature set at 23 ± 1°C and after at least 20 min of bed rest. The penetration depth of the laser beam with a wavelength of 785 nm was estimated at ~300 μm, providing a visual representation of more superficial skin microvascular structures (i.e. capillary loops and the upper microcirculatory plexus). The laser head was fixed at a distance of 15 ± 1 cm from the ventral surface of the forearm opposite the arm with the vascular access after it had been immobilized by a vacuum cushion and a sphygmomanometer cuff had been placed above the elbow (Figure 1). The selected distance was in accordance with the manufacturer’s recommendations and inside the 10–30 cm range, which reportedly has no influence on skin blood flow values [23]. Two circular sites >10 mm2 were selected for evaluation [i.e. regions of interest (ROIs)], avoiding visible veins, hair and areas of increased pigmentation or broken skin. Images were recorded at a frame rate of 21 images/s and a resolution of 0.41–0.46 mm.
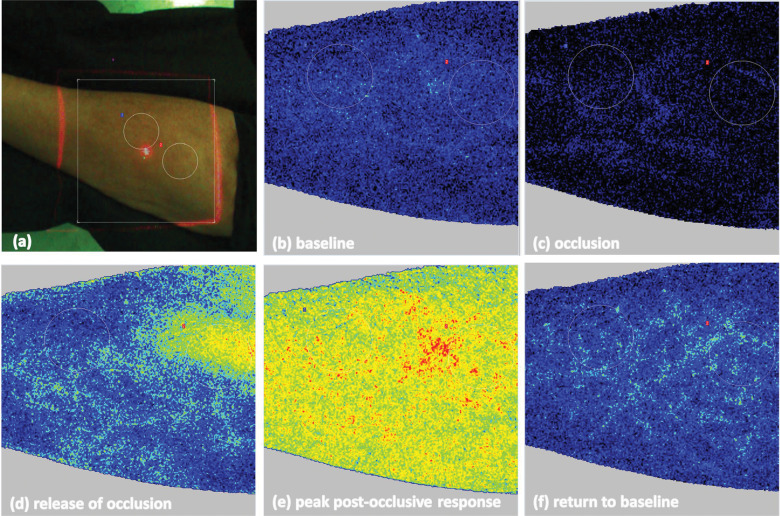
FIGURE 1:

Evaluation of skin endothelial function with LSCI (PeriCam PSI system) depicting the position of the forearm under the laser light beam and the two skin sites used for measurement of microvascular responses.
Baseline perfusion was recorded for 2 min, followed by a 5-min occlusion period after inflation of the cuff at least 50 mmHg above the SBP. Abrupt deflation of the cuff at the end of the occlusion period was followed by a maximum response of blood flow and a 5-min recording period of post-occlusion reactive hyperaemia, including return to basal status. Analysis was performed using the manufacturer’s software (PIMSoft, Perimed). Estimated variables of interest included the following: (i) baseline perfusion, (ii) occlusion perfusion and (iii) post-occlusive hyperaemic peak perfusion, expressed in LSPU and calculated from values obtained from each study period and averaged over appropriate time frames (times of interest). These time frames were 60 s for baseline and occlusion and 3 s for peak hyperaemia, in accordance with existing evidence for reduction of the variability of cutaneous blood flow measurements [24]; (iv) time to peak (s) was measured from the moment of the sphygmomanometer cuff deflation to the moment of maximal post-occlusion perfusion; (v) proportional (%) change of LSPU calculated as (peak LSPU – baseline LSPU) × 100; (vi) baseline cutaneous vascular conductance (CVC) and (vii) peak CVC were calculated during baseline and maximum post-occlusive hyperaemic response (peak CVC) by dividing respective perfusion units with MBP (LSPU/mmHg); (viii) amplitude of the PORH response calculated as the difference between peak CVC and baseline CVC (in LSPU/mmHg); and (iv) proportional (%) change of CVC calculated as (peak CVC – baseline CVC) × 100.
Figure 2 depicts the speckle pattern obtained after analysis of the scattered laser light during a full LSCI examination coupled with a PORH challenge.
FIGURE 2:
(A) ROIs for LSCI measurements in the patient’s forearm marked with white circles. Images (speckle pattern) obtained during different phases of the exam: (B) baseline, (C) occlusion, (D) release of occlusion, (E) peak post-occlusive hyperaemic response and (F) return to baseline perfusion after peak response.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences version 22.0 (IBM, Armonk, NY, USA). Continuous variables are presented as mean ± standard deviation (SD) or as median [interquartile range (IQR)] according to the normality of the distribution, examined with the Kolmogorov–Smirnov test for samples ≥50 and Shapiro–Wilk test for samples <50. Categorical variables are presented as absolute frequencies and percentages [n (%)]. For continuous variables, one-way analysis of variance (ANOVA) or Kruskal–Wallis test was used for between-group comparisons of more than three groups and Bonferroni or the respective non-parametric post hoc test, where applicable, was used for pairwise comparisons according to the normality of the distribution. Student’s t-test and Mann–Whitney test, where applicable, were used for comparisons between two groups. Chi-square tests were used for comparisons of categorical variables. P-values <0.05 (two-tailed) were considered statistically significant for all comparisons.
RESULTS
Table 1 presents demographic characteristics, dialysis vintage, comorbidities, antihypertensive medication and laboratory data for all study participants. As expected, no differences were observed between the 38 HD and 38 PD patients for age, sex and dialysis vintage or between the 38 HD patients and 38 controls for age and sex. Body mass index was not different between PD patients, HD patients and controls (P = 0.603). As expected from the design of the study, since we included as controls either healthy individuals or subjects with mild hypertension or hypercholesterolaemia, their renal function was preserved (estimated glomerular filtration rate 93.37 ± 12.32 mL/min/1.73 m2), whereas the prevalence of hypertension, diabetes and cardiovascular disease was much lower in controls. Of the 76 patients undergoing HD, 90.8% were on high-flux HD and 9.2% were on online haemodiafiltration. Of the 38 patients undergoing PD, 31.6% were on continuous ambulatory PD and 68.4% were on automated PD.
Table 1.
Baseline characteristics of the study participants
| Parameters | HD patients | PD patients | Controls | P-value |
|---|---|---|---|---|
| (n = 38) | (n = 38) | (n = 38) | ||
| Female, n (%) | 15 (39.5) | 15 (39.5) | 15 (39.5) | 1.00 |
| Age (years) | 62.99 ± 12.58 | 62.74 ± 14.45 | 59.03 ± 7.47 | 0.269 |
| Dialysis vintage (months) | 24.47 (14.29–40.81) | 18.53 (10.94–35.59) | 0.391 | |
| BMI (kg/m2) | 27.49 ± 4.41 | 28.52 ± 5.68 | 28.33 ± 4.06 | 0.603 |
| Hypertension , n (%) | 29 (76.3) | 33 (86.8) | 8 (21.1) | <0.001 |
| Diabetes, n (%) | 11 (28.9) | 9 (23.7) | 0 (0) | <0.001 |
| Hypercholesterolaemia, n (%) | 23 (60.5) | 22 (57.9) | 16 (42.1) | 0.25 |
| Cardiovascular disease, n (%) | 18 (47.4) | 17 (44.8) | 0 (0) | <0.001 |
| Smoking, n (%) | 8 (21.1) | 7 (18.4) | 14 (38.9) | 0.094 |
| eGFR (CKD-EPI) (mL/min/1.73 m2) | 93.37 ± 12.32 | |||
| Total cholesterol (mg/dL) | 158 ± 43.76 | 167.05 ± 38.9 | 203.24 ± 37.48 | <0.001 |
| LDL cholesterol (mg/dL) | 86.9 ± 32.68 | 90.09 ± 38.42 | 132.07 ± 32.22 | <0.001 |
| Hb (g/dL) | 11.43 ± 1.55 | 11.47 ± 1.43 | 14.43 ± 1.49 | <0.001 |
| Number of antihypertensive drugs, n (range) | 1 (1–2) | 2 (1–3) | 0 (0–0) | <0.001 |
| Office SBP (mmHg) | 141.63 ± 17.73 | 141.37 ± 20.29 | 124.89 ± 13.15 | <0.001 |
| Office DBP (mmHg) | 79.18 ± 10.18 | 83.34 ± 13.88 | 79.01 ± 7.52 | 0.151 |
| ACEi/ARB, n (%) | 5 (13.2) | 20 (52.6) | 4 (10.5) | <0.001 |
| CCBs, n (%) | 7 (18.4) | 15 (39.5) | 4 (10.5) | 0.008 |
| β-blockers, n (%) | 24 (63.2) | 27 (71.1) | 1 (2.6) | <0.001 |
| Centrally acting agents, n (%) | 1 (2.6) | 5 (13.2) | 0 (0) | 0.046 |
| Diuretics, n (%) | 6 (15.8) | 22 (57.9) | 0 (0) | <0.001 |
| Nitroderivatives, n (%) | 4 (10.5) | 2 (5.3) | 0 (0) | 0.163 |
| Statins, n (%) | 21 (55.3) | 26 (68.4) | 1 (2.6) | <0.001 |
Values are presneted as mean ± SD unless stated otherwise.
For continuous variables the P-value is generated from ANOVA or Kruskal–Wallis test depending on the normality of the distribution for each variable with the exception of dialysis vintage, where the P-value is generated by Mann–Whitney test. For categorical variables, the P-value is generated by chi-square test.
BMI: body mass index; eGFR: estimated glomerular filtration rate; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration equation; CCB: calcium channel blockers; Hb: haemoglobin.
Office SBP levels were found to be significantly higher in HD and PD patients compared with controls (HD: 141.63 ± 17.73; PD: 141.37 ± 20.29; controls: 124.89 ± 13.15 mmHg, P < 0.001), whereas DBP was similar between groups. As expected, differences between groups were also noted in the total number of prescribed antihypertensive drugs [HD: 1 (IQR 1–2); PD: 2 (1–3); controls: 0 (0–0), P < 0.001] and the use of angiotensin-converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs) (13.2% versus 52.6% versus 10.5%, P < 0.001) and statins (55.3% versus 68.4% versus 2.6%, P < 0.001).
Table 2 presents levels of skin microvascular parameters in patients undergoing HD and PD and their controls assessed with LSCI coupled with PORH. Figure 3 depicts characteristic examples of different skin microcirculation flow diagrams obtained in subjects from the groups undergoing HD or PD and controls using LSCI. As shown in the Table 2, baseline perfusion (HD: 46.97 ± 14.6; PD: 49.32 ± 18.07; controls: 42.02 ± 11.94 LSPU, P = 0.097) did not differ between the three groups. During occlusion, significantly lower perfusion was found in controls compared with patients under HD and PD (HD: 12.5 ± 6.1 ; PD: 15.25 ± 8.95 ; controls: 6.53 ± 4.16 LSPU, P < 0.001; pairwise comparisons: HD versus controls: P < 0.001; PD versus controls: P < 0.001; HD versus PD: 0.898).
Table 2.
Skin microvascular reactivity parameters assessed with LSCI coupled with PORH
| Parameters | HD patients (n = 38) | PD patients (n = 38) | Controls (n = 38) | P-value Kruskal– Wallis | P-value for pairwise comparisons |
||
|---|---|---|---|---|---|---|---|
| HD versus PD | HD versus controls | PD versus controls | |||||
| Baseline perfusion (LSPU) | 46.97 ± 14.6 | 49.32 ± 18.07 | 42.02 ± 11.94 | 0.097 | |||
| Occlusion perfusion (LSPU) | 12.5 ± 6.1 | 15.25 ± 8.95 | 6.53 ± 4.16 | <0.001 | 0.898 | <0.001 | <0.001 |
| Peak perfusion (LSPU) | 104.77 ± 28.68 | 109.04 ± 40.77 | 116.96 ± 30.96 | 0.238 | |||
| Increase of perfusion from baseline to peak response (%) | 133 ± 66 | 149 ± 125 | 187 ± 61 | 0.001 | 1.000 | 0.002 | 0.004 |
| Time to peak (s) | 7.24 ± 6.99 | 10.68 ± 9.45 | 11.11 ± 5.10 | 0.003 | 0.154 | 0.002 | 0.406 |
| Baseline CVC (LSPU/mmHg) | 0.48 ± 0.16 | 0.48 ± 0.2 | 0.59 ± 0.26 | 0.139 | |||
| Peak CVC (LSPU/mmHg) | 1.05 ± 0.30 | 1.07 ± 0.44 | 1.57 ± 0.52 | <0.001 | 1.000 | <0.001 | <0.001 |
| Increase of CVC from baseline to peak response (%) | 133 ± 66 | 146 ± 126 | 186 ± 63 | <0.001 | 1.000 | 0.003 | 0.005 |
| Amplitude of the PORH response (LSPU/mmHg) | 0.58 ± 0.24 | 0.58 ± 0.41 | 0.98 ± 0.32 | 0.001 | 1.000 | <0.001 | <0.001 |
Values presneted as mean ± SD unless stated otherwise.
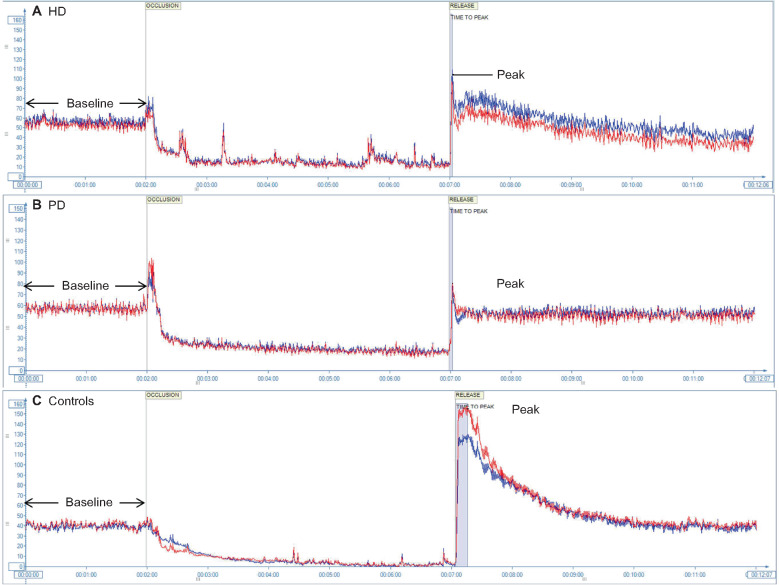
FIGURE 3:
Depiction of changes in perfusion units across time during a typical exam performed using LSCI coupled with a PORH challenge in (A) an HD patient, (B) a PD patient and (C) a control. The blue and red lines represent the two circular sites (ROIs) that were evaluated in each patient. Characteristic findings are the lower time to peak and decreased post-occlusion peak perfusion in ESRD patients.
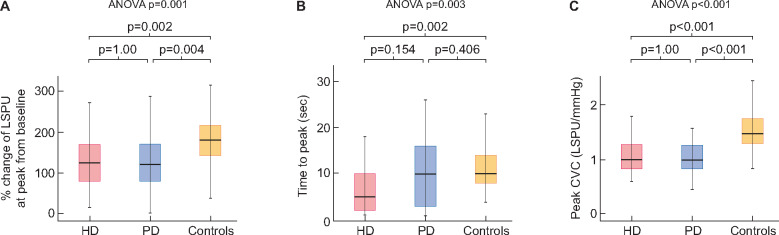
After release of occlusion, peak perfusion was also not different between groups (HD: 104.77 ± 28.68 ; PD: 109.04 ± 40.77 ; controls: 116.96 ± 30.96 LSPU, P = 0.238). However, the post-occlusive vascular response differed significantly between the three groups. In particular, time to peak hyperaemic response was numerically lower in patients under HD than patients under PD and controls (HD: 7.24 ± 6.99 ; PD: 10.68 ± 9.45 ; controls: 11.11 ± 5.1 s, P = 0.003) (Figure 4). In pairwise comparisons, the difference between HD and PD patients did not reach statistical significance but that between HD and controls did (P = 0.154 and P = 0.002, respectively) (Figure 4B). In addition, significant differences were observed in the proportional increase of perfusion from baseline to peak post-occlusive response (HD: 133 ± 66; PD: 149 ± 125; controls: 187 ± 61%, P = 0.001). Pairwise comparisons revealed a significantly lower maximum proportional increase of post-occlusive perfusion in HD patients versus controls and in PD patients versus controls (P = 0.002 and P = 0.004, respectively) (Figure 4A).
FIGURE 4:
(A) Proportional change of perfusion from baseline at peak post-occlusive hyperaemic response in patients on HD, PD and controls. (B) Time to peak post-occlusive hyperaemic response in patients on HD, PD and controls. (C) Cutaneous vascular conductance at peak post-occlusive hyperaemic response in patients on HD, PD and controls. P-values on the right of each diagram correspond to Kruskal–Wallis test; P-values on the top of each diagram indicate pairwise comparisons.
Of note, significant differences between the three groups were noted in peak CVC, indicating the maximum capillary recruitment (HD: 1.05 ± 0.3 ; PD: 1.07 ± 0.44 ; controls: 1.57 ± 0.52 LSPU/mmHg, P < 0.001). Pairwise comparisons confirmed significantly lower peak CVC in patients on HD or PD than controls (P < 0.001 for both) (Figure 4C). Analysis of the proportional increase of CVC at peak response from baseline showed similar results (HD: 133 ± 66; PD: 146 ± 126; controls: 186 ± 63%, P < 0.001; pairwise comparisons: HD versus controls, P = 0.003; PD versus controls, P = 0.005). Similarly, patients under both dialysis modalities showed lower amplitude of the PORH response (a measure of the difference between baseline and maximum capillary recruitment) compared with controls (HD: 0.58 ± 0.24 ; PD: 0.58 ± 0.41 ; controls: 0.98 ± 0.32 LSPU/mmHg, P = 0.001; pairwise comparisons to controls, P < 0.001 for both). As depicted in Table 2, there were no significant differences in any of these parameters between PD and HD patients.
DISCUSSION
This is the first study to examine endothelial function with LSCI at the forearm skin microcirculation in patients undergoing HD and PD versus matched controls. We observed that patients under HD and PD exhibit similar perfusion and CVC during all periods studied as well as similar proportional changes of perfusion and CVC from baseline to peak; we only noted a numerically but not significantly shorter time to peak in HD patients compared with their PD counterparts. Most importantly, we found that ESRD patients under both dialysis modalities show an impaired post-occlusive vascular response, as time to peak, peak CVC, proportional changes of perfusion and CVC and the amplitude of PORH response (a measure of the difference between baseline and maximum capillary recruitment) were much lower than that of controls. These data suggest severely disturbed skin capillary recruitment and endothelial function in response to occlusion in ESRD patients.
Endothelium dysfunction represents a disturbed balance between vasoactive molecules that constrict or relax the vessel, inappropriately favouring the former under conditions of increased blood flow [25]. Increased intracellular oxidative stress, subclinical inflammation, insulin resistance and other factors interfere with the vasodilation process, normally mediated by a number of agents such as NO, prostaglandins (PGI2 and PGE2) and a family of endothelial-derived hyperpolarizing factors (EDHFs), which in turn promote the development of hypertension and atherosclerosis [26, 27]. Markers of endothelial dysfunction, such as asymmetric dimethylarginine (ADMA), the endogenous inhibitor of NO synthase, oxidant stress and inflammation have been shown to increase with renal function decline [28], whereas increased ADMA and C-reactive protein levels are associated with incident cardiovascular disease (CVD) and mortality in ESRD patients [29, 30].
Although important, the above studies on endothelial dysfunction in ESRD use surrogate markers, and works with direct assessment of microcirculation and endothelium-dependent vasodilation in this population, including comparative data between different dialysis modalities, are scarce. A previous study of living donor transplant recipients on PD (n = 12), HD (n = 14) or before dialysis (n = 15) showed a trend towards worse in vitro acetylcholine-induced vasorelaxation of harvested inferior epigastric arteries in HD compared with PD patients (P = 0.11) [31]. FMD is the gold-standard technique for in vivo evaluation of conduit artery endothelial function, as it is non-invasive and there is sufficient evidence supporting its firm link to biology and association with cardiovascular outcomes [1]. However, its use is limited by high cost, the need for expert operators and results are influenced by conditions such as temperature, exercise and food consumption [32]. Verbeke et al. [33] studied variations of vasomotor tone in response to hand-warming by comparing shear stress (SS)-mediated changes in brachial artery diameter obtained by FMD between ESRD patients with and without known CVD [33]. Patients with ESRD and CVD exhibited no response to low and mild SS increases and a ‘paradoxical’ vasoconstriction during high SS; a negative association between changes in brachial artery diameters and the presence of ESRD or CVD was noted [33]. Measurement of skin blood flow offers the advantage of easy accessibility to a representative vascular bed. A previous study of 63 patients under dialysis with LDF showed that abnormal flow parameters during post-occlusive and thermal interrogation of the microvasculature are clustered in patients with CVD or diabetes, but also in 50% of those without clinically evident CVD or diabetes [34]. A subsequent study showed that LDF thermal hyperaemic challenge variables offer an additive to the Framingham score predictive value for future cardiovascular events and death in ESRD [35].
LSCI represents a novel non-invasive technique for real-time assessment of microcirculation, which is based on analysis of laser light backscattered from the skin forearm to the detector and is provided in the form of a random interference (speckle) pattern [36]. This speckle pattern is generated from fluctuations in the movement of small-scale particles (i.e. red blood cells) inside the tissue, and thus blurring of the speckle image can be related to blood flow [36]. In contrast to LDF, LSCI provides the clinical advantage of simplicity and faster acquisition time, along with higher resolution and reproducibility [10, 11, 21], and has been used in the study of several vascular beds [14–16]. Endothelium-dependent vasodilation with LSCI coupled with a PORH challenge was studied by de Matheus et al. [17] in 50 type 1 diabetic patients; the study showed an impaired response, with lower peak perfusion, peak CVC and amplitude of the PORH response in diabetic patients compared with 30 healthy controls. Similarly, Borges et al. [18] showed impaired reactive hyperaemic forearm vasodilation in males with CAD with LSCI, while impaired dorsum vasodilation in patients with Raynaud syndrome secondary to systemic sclerosis [19] was also reported.
In this study, all PORH parameters were lower in patients under HD and PD compared with controls, reflecting an impaired endothelial response. In agreement with findings in diabetic [17] and CAD patients [18] discussed above, we noted a similar pattern in the two most commonly studied LSCI indexes, which is lower CVC at maximum response and smaller amplitude of PORH response in ESRD patients compared with controls. However, in our study, perfusion at maximum response did not differ between the two ESRD groups and controls, in contrast to the findings of de Matheus et al. [17] in diabetic individuals. Moreover, as we evaluated per protocol a larger set of LSCI parameters, we also noted differences between ESRD patients and controls regarding perfusion at occlusion and time to peak response indexes. Whether these observations are relevant to a more detailed protocol than that used in previous studies [17, 18] or reflect true underlying differences between ESRD and other patient populations remains to be clarified by future research.
Of note, as of this writing, the effects of antihypertensive agents that are commonly used in CKD on microvascular reactivity parameters assessed with LSCI have not been evaluated in human studies. In a recent animal study, angiotensin II–treated rats exhibited significantly lower PORH response compared with controls [37]. Initiation of antihypertensive treatment in newly diagnosed hypertensive patients was associated with an increase of the post-occlusive hyperaemic response evaluated with LDF [38], which is another technique assessing microvascular responses with laser methodology, however, with lower reproducibility than LSCI, as discussed above. In general, renin–angiotensin–system blockers, calcium antagonists and vasodilating β-blockers have been shown to improve endothelial function assessed with a variety of methods [39]; the effects of these agents on LSCI parameters warrant investigation in future studies.
Mechanisms leading to endothelial dysfunction in ESRD are multifactorial and associated with a high prevalence of both traditional risk factors (i.e hypertension, diabetes, hypercholesterolaemia) and non-traditional ones, such as increased oxidative stress, hyperhomocysteinaemia, insulin resistance and inflammation [40]. An increase of oxidative stress, with enhanced generation of reactive oxygen species, and homocysteine can lead to accumulation of ADMA, reduced bioavailability of NO and endothelial dysfunction in animal models and human studies in CKD [40]. On the other hand, the vasoconstrictor endothelin-1 (ET-1) was found to be elevated in dialysis patients, while evidence supports ET-1 can be a mediator of the effects of insulin resistance in the vasculature [27]. Finally, chronic inflammation, a prominent feature in pre-dialysis and dialysis patients, has long been associated with release of cell-surface adhesion molecules and impaired endothelial-dependent vasorelaxation [41].
Notably, patients on HD in our study exhibited a numerically shorter time to post-occlusive hyperaemic peak perfusion than PD patients and a significantly shorter time than controls. This may suggest a slightly worse endothelial response in HD compared with PD patients, exactly as noted in the aforementioned study comparing ex vivo arterial endothelial function (P = 0.11) [31]. This is a hypothesis to be tested in future studies.
Another interesting finding was that of lower perfusion observed during occlusion in controls compared with ESRD patients. One plausible explanation for this could be the effect of normal sympathetic neurogenic control of vasomotor tone characterized by rapid adaptations to different physiological conditions in healthy subjects [42]. In contrast, sympathetic overdrive is present in CKD even from the early stages [43]. A reciprocal relationship between endothelial function and sympathetic activity under physiological states has been previously reported [44], while sympathetic nerve traffic values were positively associated with ADMA levels in patients with CKD Stages 2–4 [45]. Thus sympathetic overdrive of ESRD could be involved in decreased suppression of perfusion during occlusion, as well as decreased endothelial response during post-reactive hyperaemia.
To our knowledge this is the first study using LSCI in ESRD and the first examining the comparison with the functional integrity of forearm cutaneous microcirculation in HD and PD patients using a non-invasive method. Among the strengths of this study is the careful design, including blinded matching of HD and PD patients for a set of crucial parameters, resulting in the absence of differences for several characteristics. We also included a carefully matched group of controls that were healthy or had new-onset hypertension or dyslipidaemia. Our strict protocol tried to minimize the influence of external factors (noise, temperature, light) on the results. One unavoidable limitation of our study is the different prevalence of comorbid conditions and intake of medications that could interfere with an endothelial response (ACEis/ARBs, statins) between ESRD patients and controls.
In conclusion, using a novel non-invasive technology, this study observed that endothelial post-occlusive forearm cutaneous vasodilatory response was significantly impaired in ESRD patients compared with controls. Overall, the hyperaemic proportional change in peak perfusion and the amplitude of PORH response did not differ between HD and PD patients, whereas a trend towards even more impaired time to peak in HD patients was observed. These findings support that HD and PD patients exhibit similarly impaired endothelial response; any subtle potential differences should be examined by larger studies. LSCI is a non-invasive, non-contact, real-time technique that provides reproducible and high-resolution images without physically demanding or time-consuming procedures, something that is important for patients on dialysis. The cost of the equipment is within the range of a credible ultrasound device, while the operator’s learning curve and the short demand of operator time (<15 min per test) may add to its cost-effectiveness for assessing endothelial function. Thus it could be used in future longitudinal studies aiming to evaluate the associations of microcirculation dysfunction with future cardiovascular events in this high-risk population.
ACKNOWLEDGEMENTS
The authors would like to express their gratitude to the doctors and nurses of the two departments of nephrology where the study was undertaken for their help.
AUTHORS’ CONTRIBUTIONS
S.D., A.P. and P.A.S. contributed to the conception and design of this study. M.A., A.L., P.D. and B.N. performed the data acquisition. M.A., E.G. and E.M. performed the data interpretation and analysis. M.A. and C.L. performed the statistical analysis. S.D. and P.A.S. contributed to the mentorship. Each author contributed important intellectual content during manuscript drafting and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
FUNDING
This article was not supported by any source and represents an original effort of the authors.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Deanfield JE, Halcox JP, Rabelink TJ.. Endothelial function and dysfunction: testing and clinical relevance. Circulation 2007; 115: 1285–1295 [DOI] [PubMed] [Google Scholar]
- 2. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993; 362: 801–809 [DOI] [PubMed] [Google Scholar]
- 3. Gimbrone MA, García-Cardeña G.. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 2016; 118: 620–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brevetti G, Silvestro A, Schiano V et al.. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation 2003; 108: 2093–2098 [DOI] [PubMed] [Google Scholar]
- 5. Halcox JPJ, Schenke WH, Zalos G. et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation 2002; 106: 653–658 [DOI] [PubMed] [Google Scholar]
- 6. Seliger SL, Salimi S, Pierre V. et al. Microvascular endothelial dysfunction is associated with albuminuria and CKD in older adults. BMC Nephrol 2016; 17: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dubin RF, Guajardo I, Ayer A. et al. Associations of macro- and microvascular endothelial dysfunction with subclinical ventricular dysfunction in end-stage renal disease. Hypertension 2016; 68: 913–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. London GM, Pannier B, Agharazii M. et al. Forearm reactive hyperemia and mortality in end-stage renal disease. Kidney Int 2004; 65: 700–704 [DOI] [PubMed] [Google Scholar]
- 9. Flammer AJ, Anderson T, Celermajer DS. et al. The assessment of endothelial function: from research into clinical practice. Circulation 2012; 126: 753–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tew GA, Klonizakis M, Crank H. et al. Comparison of laser speckle contrast imaging with laser Doppler for assessing microvascular function. Microvasc Res 2011; 82: 326–332 [DOI] [PubMed] [Google Scholar]
- 11. Roustit M, Millet C, Blaise S. et al. Excellent reproducibility of laser speckle contrast imaging to assess skin microvascular reactivity. Microvasc Res 2010; 80: 505–511 [DOI] [PubMed] [Google Scholar]
- 12. Srienc AI, Kurth-Nelson ZL, Newman EA.. Imaging retinal blood flow with laser speckle flowmetry. Front Neuroenergetics 2010; 2: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hecht N, Woitzik J, Dreier JP. et al. Intraoperative monitoring of cerebral blood flow by laser speckle contrast analysis. Neurosurg Focus 2009; 27: E11. [DOI] [PubMed] [Google Scholar]
- 14. Ambrus R, Achiam MP, Secher NH. et al. Evaluation of gastric microcirculation by laser speckle contrast imaging during esophagectomy. J Am Coll Surg 2017; 225: 395–402 [DOI] [PubMed] [Google Scholar]
- 15. Ruaro B, Sulli A, Smith V. et al. Short-term follow-up of digital ulcers by laser speckle contrast analysis in systemic sclerosis patients. Microvasc Res 2015; 101: 82–85 [DOI] [PubMed] [Google Scholar]
- 16. Mennes OA, van Netten JJ, van Baal JG. et al. Assessment of microcirculation in the diabetic foot with laser speckle contrast imaging. Physiol Meas 2019; 40:065002. [DOI] [PubMed] [Google Scholar]
- 17. de M Matheus AS, Clemente ELS, de Lourdes Guimarães Rodrigues M. et al. Assessment of microvascular endothelial function in type 1 diabetes using laser speckle contrast imaging. J Diabetes Complicat 2017; 31: 753–757 [DOI] [PubMed] [Google Scholar]
- 18. Borges JP, Lopes GO, Verri V. et al. A novel effective method for the assessment of microvascular function in male patients with coronary artery disease: a pilot study using laser speckle contrast imaging. Braz J Med Biol Res 2016; 49: e5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Della Rossa A, Cazzato M, d’Ascanio A. et al. Alteration of microcirculation is a hallmark of very early systemic sclerosis patients: a laser speckle contrast analysis. Clin Exp Rheumatol 2013; 31: 109–114 [PubMed] [Google Scholar]
- 20. Boas DA, Dunn AK.. Laser speckle contrast imaging in biomedical optics. J Biomed Opt 2010; 15:011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heeman W, Steenbergen W, van Dam G et al. Clinical applications of laser speckle contrast imaging: a review. J Biomed Opt 2019; 24: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sarafidis PA, Persu A, Agarwal R. et al. Hypertension in dialysis patients: a consensus document by the European Renal and Cardiovascular Medicine (EURECA-m) working group of the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) and the Hypertension and the Kidney working group of the European Society of Hypertension (ESH). Nephrol Dial Transplant 2017; 32: 620–640 [DOI] [PubMed] [Google Scholar]
- 23. Mahé G, Haj-Yassin F, Rousseau P. et al. Distance between laser head and skin does not influence skin blood flow values recorded by laser speckle imaging. Microvasc Res 2011; 82: 439–442 [DOI] [PubMed] [Google Scholar]
- 24. Rousseau P, Mahé G, Haj-Yassin F. et al. Increasing the “region of interest” and “time of interest”, both reduce the variability of blood flow measurements using laser speckle contrast imaging. Microvasc Res 2011; 82: 88–91 [DOI] [PubMed] [Google Scholar]
- 25. Brunner H, Cockcroft JR, Deanfield J. et al. Endothelial function and dysfunction. Part II: association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens 2005; 23: 233–246 [DOI] [PubMed] [Google Scholar]
- 26. Giles TD, Sander GE, Nossaman BD. et al. Impaired vasodilation in the pathogenesis of hypertension: focus on nitric oxide, endothelial-derived hyperpolarizing factors, and prostaglandins. J Clin Hypertens (Greenwich) 2012; 14: 198–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sarafidis PA, Bakris GL.. Review: insulin and endothelin: an interplay contributing to hypertension development? J Clin Endocrinol Metab 2007; 92: 379–385 [DOI] [PubMed] [Google Scholar]
- 28. Raptis V, Georgianos PI, Sarafdis PA. et al. Elevated asymmetric dimethylarginine is associated with oxidant stress aggravation in patients with early stage autosomal dominant polycystic kidney disease. Kidney Blood Press Res 2013; 38: 72–82 [DOI] [PubMed] [Google Scholar]
- 29. Zoccali C, Bode-Böger S, Mallamaci F. et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet 2001; 358: 2113–2117 [DOI] [PubMed] [Google Scholar]
- 30. Tripepi G, Mallamaci F, Zoccali C.. Inflammation markers, adhesion molecules, and all-cause and cardiovascular mortality in patients with ESRD: searching for the best risk marker by multivariate modeling. J Am Soc Nephrol 2005; 16(Suppl 1): S83–S88 [DOI] [PubMed] [Google Scholar]
- 31. Chung A, Yang H, Kim JM. et al. Arterial stiffness and functional properties in chronic kidney disease patients on different dialysis modalities: an exploratory study. Nephrol Dial Transplant 2010; 25: 4031–4041 [DOI] [PubMed] [Google Scholar]
- 32. Corretti MC, Anderson TJ, Benjamin EJ. et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002; 39: 257–265 [DOI] [PubMed] [Google Scholar]
- 33. Verbeke FH, Pannier B, Guérin AP. et al. Flow-mediated vasodilation in end-stage renal disease. Clin J Am Soc Nephrol 2011; 6: 2009–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stewart J, Kohen A, Brouder D. et al. Noninvasive interrogation of microvasculature for signs of endothelial dysfunction in patients with chronic renal failure. Am J Physiol Heart Circ Physiol 2004; 287: H2687–H2696 [DOI] [PubMed] [Google Scholar]
- 35. Kruger A, Stewart J, Sahityani R. et al. Laser Doppler flowmetry detection of endothelial dysfunction in end-stage renal disease patients: correlation with cardiovascular risk. Kidney Int 2006; 70: 157–164 [DOI] [PubMed] [Google Scholar]
- 36. Briers JD, Webster S.. Laser speckle contrast analysis (LASCA): a nonscanning, full-field technique for monitoring capillary blood flow. J Biomed Opt 1996; 1: 174–179 [DOI] [PubMed] [Google Scholar]
- 37. Mottard N, Berkowitz DE, Santhanam L.. Assessing renal microvascular reactivity by laser speckle-contrast imaging in angiotensin-II-treated mice. Int J Nephrol Renovasc Dis 2020; 13: 45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rossi M, Bradbury A, Magagna A. et al. Investigation of skin vasoreactivity and blood flow oscillations in hypertensive patients: effect of short-term antihypertensive treatment. J Hypertens 2011; 29: 1569–1576 [DOI] [PubMed] [Google Scholar]
- 39. Silva IVG, de Figueiredo RC, Rios D.. Effect of different classes of antihypertensive drugs on endothelial function and inflammation. Int J Mol Sci 2019; 20:3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Endemann DH, Schiffrin EL.. Endothelial dysfunction. J Am Soc Nephrol 2004; 15: 1983–1992 [DOI] [PubMed] [Google Scholar]
- 41. Stenvinkel P. Endothelial dysfunction and inflammation-is there a link? Nephrol Dial Transplant 2001; 16: 1968–1971 [DOI] [PubMed] [Google Scholar]
- 42. Wallin BG, Charkoudian N.. Sympathetic neural control of integrated cardiovascular function: insights from measurement of human sympathetic nerve activity. Muscle Nerve 2007; 36: 595–614 [DOI] [PubMed] [Google Scholar]
- 43. Grassi G, Quarti-Trevano F, Seravalle G. et al. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension 2011; 57: 846–851 [DOI] [PubMed] [Google Scholar]
- 44. Otto ME, Svatikova A, Barretto Rb de M. et al. Early morning attenuation of endothelial function in healthy humans. Circulation 2004; 109: 2507–2510 [DOI] [PubMed] [Google Scholar]
- 45. Grassi G, Seravalle G, Ghiadoni L. et al. Sympathetic nerve traffic and asymmetric dimethylarginine in chronic kidney disease. Clin J Am Soc Nephrol 2011; 6: 2620–2627 [DOI] [PMC free article] [PubMed] [Google Scholar]