Abstract
Background
Acute kidney injury (AKI) is frequent during hospitalization and may contribute to adverse short- and long-term consequences. Acute kidney disease (AKD) reflects the continuing pathological processes and adverse events developing after AKI. We aimed to evaluate the association of AKD, long-term adverse renal function and mortality in a cohort of patients with sepsis.
Methods
We performed a retrospective analysis of adult patients with septic AKI admitted to the Division of Intensive Medicine of the Centro Hospitalar Lisboa Norte (Lisbon, Portugal) between January 2008 and December 2014. Patients were categorized according to the development of AKI using the Kidney Disease: Improving Global Outcomes (KDIGO) classification. AKI was defined as an increase in absolute serum creatinine (SCr) ≥0.3 mg/dL or by a percentage increase in SCr ≥50% and/or by a decrease in urine output to <0.5 mL/kg/h for >6 h. AKD was defined as presenting at least KDIGO Stage 1 criteria for >7 days after an AKI initiating event. Adverse renal outcomes (need for long-term dialysis and/or a 25% decrease in estimated glomerular filtration rate after hospital discharge) and mortality after discharge were evaluated.
Results
From 256 selected patients with septic AKI, 53.9% developed AKD. The 30-day mortality rate was 24.5% (n = 55). The mean long-term follow-up was 45.9 ± 43.3 months. The majority of patients experience an adverse renal outcome [n = 158 (61.7%)] and 44.1% (n = 113) of patients died during follow-up. Adverse renal outcomes, 30-day mortality and long-term mortality after hospital discharge were more frequent among AKD patients [77.5 versus 43.2% (P < 0.001), 34.1 versus 6.8% (P < 0.001) and 64.8 versus 49.1% (P = 0.025), respectively]. The 5-year cumulative probability of survival was 23.2% for AKD patients, while it was 47.5% for patients with no AKD (log-rank test, P < 0.0001). In multivariate analysis, AKD was independently associated with adverse renal outcomes {adjusted hazard ratio [HR] 2.87 [95% confidence interval (CI) 2.0–4.1]; P < 0.001} and long-term mortality [adjusted HR 1.51 (95% CI 1.0–2.2); P = 0.040].
Conclusions
AKD after septic AKI was independently associated with the risk of long-term need for dialysis and/or renal function decline and with the risk of death after hospital discharge.
Keywords: AKD, AKI, critical care, long term, outcomes, sepsis
BACKGROUND
Acute kidney injury (AKI) is a complex syndrome that can develop as a consequence of multiple pathologies [1]. AKI is defined as an increase in baseline serum creatinine (SCr) or a decrease in urine output (UO) within 48 h [2].
The incidence of AKI has increased in recent decades and ranges from 2% in the community setting to ~20% in hospitalized patients and up to 60% in critical care units [3–6]. AKI is associated with poor short- and long-term outcomes, namely in-hospital mortality, progression to chronic kidney disease (CKD), cardiovascular disease (CVD) and long-term mortality [7–9].
Sepsis is one of the most common causes of AKI in critically ill patients, accounting for up to 50% of cases [5, 10]. Septic AKI patients have distinct characteristics from patients with AKI not associated with sepsis [11, 12]. Indeed, septic AKI is associated with higher disease severity scores at admission, requirement of vasoactive drugs, need for mechanical ventilation, non-renal organ failure, prolonged lengths of intensive care unit (ICU) and hospital stay, increased in-hospital mortality and a higher probability of recovery of renal function at the time of discharge from hospital [13–17]. The short-term survival of patients with sepsis has improved in recent years; however, the impact of sepsis may affect the long-term prognosis of patients by decreasing functional and cognitive status and quality of life, increasing cardiovascular risk and increasing long-term mortality risk [18–23].
There is a lack of evidence on long-term outcomes of patients with septic AKI. In this study we evaluated long-term adverse renal function and mortality after acute kidney disease (AKD) in critically ill patients with sepsis.
MATERIALS AND METHODS
This is a single-centre retrospective analysis of septic AKI patients admitted to the Division of Intensive Medicine of the Centro Hospitalar Universitário Lisboa Norte between January 2008 and December 2014. Data collection was performed in January 2020.
This study was approved by the Ethical Committee in agreement with institutional guidelines. Due to the retrospective and non-interventional nature of the study, informed consent was waived by the Ethical Committee.
Participants
Eligible patients were adult patients (≥18 years of age) with a diagnosis of sepsis at admission to the Division of Intensive Medicine who developed AKI within the first week of ICU hospitalization.
Exclusion criteria were CKD patients on renal replacement therapy (RRT), patients who underwent RRT 1 week prior to admission to the ICU, patients who were discharged or died <2 days after ICU admission, patients who died in the hospital and patients lost to follow-up.
Variables and outcomes
Patient variables were collected from individual clinical records. The protocol for all patients in this ICU includes daily determination of SCr and hourly UO.
The following variables were analysed: patient demographic characteristics (age, gender, ethnicity, body weight and height), comorbidities [diabetes mellitus, hypertension, chronic obstructive pulmonary disease (COPD), CVD, cirrhosis, CKD and/or malignancy], main diagnosis on admission (medical versus surgical), source of infection, laboratory values at admission (serum haemoglobin, neutrophil, lymphocyte count, platelet count, serum albumin, SCr, arterial blood gas and pH analysis), disease severity according to the Simplified Acute Physiologic Score (SAPS) II [24] as determined by the worst variables documented throughout the first 24 h of ICU admission, fluid balance during ICU admission, mechanical ventilation, vasopressor use and requirement for RRT.
The outcomes measured were mortality within 30 days after discharge, long-term adverse renal outcomes and long-term mortality.
Definitions
The Kidney Disease: Improving Global Outcomes (KDIGO) classification according to both SCr and UO criteria was used to define AKI (Table 1) [2]. Pre-admission SCr (SCr within the previous 3 months) was considered a baseline value. When unavailable, baseline SCr was estimated from the Modification of Diet in Renal Disease equation, accepting the lower limit of a normal baseline glomerular filtration rate (GFR) of 75 mL/min/1.73 m2 [2].
Table 1.
Definition and staging of AKI according to the KDIGO classification and definition of AKD according to the ADQI
| AKI | Stage 1 | ↑ SCr ≥0.3 mg/dL or ↑ SCr ≥1.5–1.9× within any 48-h period |
| UO <0.5 mL/kg/h for >6 h | ||
| Stage 2 | ↑ SCr >2–2.9× | |
| UO <0.5 mL/kg/h for >12 h | ||
| Stage 3 | ↑ SCr ≥3× or ↑SCr to ≥4 mg/dL | |
| or RRT start | ||
| UO <0.3 mL/kg/h for >24 h | ||
| or anuria for >12 h | ||
| AKD | AKI KDIGO Stage ≥1 present ≥7 days after an AKI initiating event | |
Sepsis was diagnosed according to the Third International Consensus Definitions as an acute change in total sequential organ failure assessment score ≥2 points consequent to the infection [25].
Diabetes mellitus was diagnosed according to the American Diabetes Association criteria [26] and hypertension was diagnosed according to the seventh report of the Joint National Committee [27]. COPD comprised emphysema and chronic bronchitis and CVD was considered as present whenever a history of cerebrovascular disease, chronic heart failure of any cause, cardiac ischaemic disease and/or peripheral arterial disease was documented; also, a previous diagnosis on clinical records was considered sufficient for the confirmation of these diagnoses. The presence of CKD was estimated according to the baseline SCr as an estimated GFR (eGFR) <60 mL/min/1.73 m2 [28]. The neutrophil, lymphocyte and platelet (NLP) ratio at admission was calculated as (neutrophil count × 100)/(lymphocyte count × platelet count).
AKD was defined by presenting at least KDIGO Stage 1 criteria for >7 days after an AKI initiating event [29].
Long-term adverse renal outcomes were defined as the need for long-term dialysis and/or a 25% decrease in eGFR calculated from the discharge eGFR, as previously applied [30].
Statistical methods
Categorical variables were described as the total number and percentage for each category, whereas continuous variables were described as the mean ± SD. Normally distributed continuous variables were compared with the Student’s t-test, non-normally distributed continuous variables were compared with the Mann–Whitney U-test and categorical variables were compared with the chi-squared test.
Univariate analysis was used to determine statistically significant factors that may have contributed to long-term adverse renal outcomes and mortality in AKD patients. These factors were then analysed using the Cox regression method for a multivariate analysis.
The Kaplan–Meier method was used to determine cumulative mortality curves, which were compared using the log-rank test. Patients were censored at the last follow-up date (January 2020) if alive. Patients lost to follow-up were excluded from all analyses.
Data were expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). No sensitivity analyses were carried out. Statistical significance was defined as P < 0.05. Analyses were performed with the statistical software package SPSS 21.0 for Windows (IBM, Armonk, NY, USA).
RESULTS
Participants
After analysis of the ICU patient admissions register, 723 critically ill septic patients were selected as potentially eligible. Of these, 57 did not develop AKI during hospitalization, 122 had CKD on RRT, 144 had been hospitalized for <48 h or had less than two SCr determinations, none required RRT in the week preceding ICU admission, 143 died during hospitalization and 1 was lost to follow-up. Consequently, we focused on a final cohort of 256 septic AKI patients (Figure 1). We registered no missing data.
FIGURE 1.
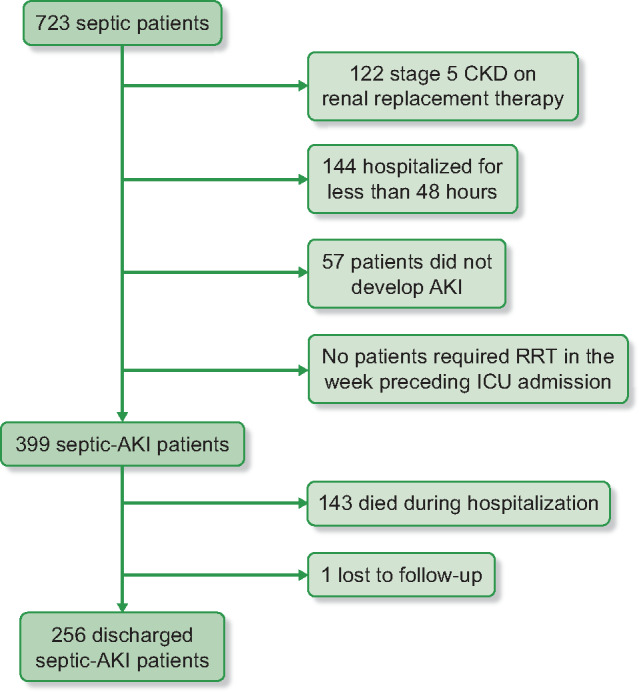
Flow chart of patient selection.
Demographics, clinical patient variables and long-term outcomes, including comparisons between the AKD and no-AKD groups, are described in Table 2.
Table 2.
Patients’ baseline characteristics and comparison according to the development of AKD
| Characteristic | All (N = 256) | AKD patients (n = 138) | Renal recovery (n = 118) | P-value |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age (years) | 62.6 ± 22.6 | 63.9 ± 15.9 | 61.2 ± 15.4 | 0.172 |
| Gender (male), n (%) | 144 (56.3) | 78 (56.5) | 66 (55.9) | 0.924 |
| Race (Caucasian), n (%) | 245 (95.7) | 131 (94.4) | 114 (96.6) | 0.508 |
| Comorbidities, n (%) | ||||
| Hypertension | 118 (46.1) | 65 (47.1) | 53 (44.9) | 0.726 |
| Diabetes | 57 (22.3) | 36 (26.1) | 21 (17.8) | 0.112 |
| CVD | 75 (29.3) | 47 (34.1) | 28 (23.7) | 0.070 |
| COPD | 18 (7.0) | 8 (5.8) | 10 (8.5) | 0.404 |
| Cirrhosis | 10 (3.9) | 6 (4.3) | 4 (3.4) | 0.693 |
| Neoplasia | 50 (19.5) | 33 (23.9) | 17 (14.4) | 0.056 |
| CKD | 142 (55.5) | 94 (68.1) | 48 (40.7) | <0.001 |
| Baseline SCr (mg/dL) | 1.27 ± 0.6 | 1.5 ± 0.7 | 1.0 ± 0.5 | <0.001 |
| Baseline eGFR (mL/min/1.73 m2) | 64.1 ± 32.3 | 53.8 ± 28.0 | 76.2 ± 32.7 | <0.001 |
| ICU admission | ||||
| Medical admission, n (%) | 140 (54.7) | 69 (50.0) | 71 (60.2) | 0.103 |
| Infection source, n (%) | ||||
| Abdominal | 106 (41.4) | 60 (43.5) | 46 (39.0) | |
| Respiratory | 76 (29.7) | 38 (27.5) | 38 (32.2) | |
| Kidney | 35 (13.7) | 21 (15.2) | 14 (11.9) | 0.853 |
| Skin | 20 (7.8) | 10 (7.2) | 10 (8.5) | |
| Others | 6 (2.3) | 3 (2.2) | 3 (2.5) | |
| Unknown | 8 (3.1) | 4 (2.9) | 4 (3.4) | |
| Nephrotoxins | 89 (34.8) | 47 (34.1) | 42 (35.6) | 0.797 |
| SAPS II | 46.1 ± 15.6 | 45.2 ± 16.2 | 47.1 ± 14.9 | 0.343 |
| Admission SCr (mg/dL) | 2.46 ± 1.5 | 3.0 ± 1.7 | 1.8 ± 1.1 | <0.001 |
| Haemoglobin (g/dL) | 10.8 ± 2.0 | 10.7 ± 2.1 | 10.8 ± 1.9 | 0.781 |
| Serum albumin (g/dL) | 1.9 ± 0.6 | 2.0 ± 0.5 | 1.9 ± 0.6 | 0.084 |
| Acidaemia (pH <7.5), n (%) | 80 (31.3) | 48 (34.8) | 32 (27.1) | 0.187 |
| NLP ratio | 13.6 ± 22.6 | 12.78 ± 20.13 | 14.7 ± 25.3 | 0.504 |
| Mechanical ventilation, n (%) | 182 (71.1) | 97 (70.3) | 85 (72.0) | 0.759 |
| Vasopressors, n (%) | 173 (67.6) | 90 (62.2) | 83 (70.3) | 0.383 |
| Fluid balance (L) | 3.4 ± 4.7 | 2.9 ± 4.3 | 3.9 ± 5.1 | 0.081 |
| AKI characteristics | ||||
| KDIGO Stage 1, n (%) | 70 (27.3) | 34 (24.6) | 36 (30.5) | 0.293 |
| KDIGO Stage 2, n (%) | 79 (30.9) | 38 (27.5) | 41 (34.7) | 0.213 |
| KDIGO Stage 3, n (%) | 107 (41.8) | 66 (47.8) | 41 (34.7) | 0.034 |
| RRT, n (%) | 43 (16.8) | 24 (17.4) | 19 (16.1) | 0.783 |
| Length of stay in hospital (days) | 37.7 ± 36.1 | |||
| At discharge | ||||
| Discharge SCr (mg/dL) | 1.42 ± 1.2 | 2.01 ± 1.3 | 0.68 ± 0.21 | <0.001 |
| Discharge eGFR (mL/min/1.73 m2) | 68.0 ± 39.1 | 39.2 ± 19.7 | 101.7 ± 27.5 | <0.001 |
| AKD, n (%) | 138 (53.9) | |||
| Outcomes | ||||
| Follow-up duration (months) | 45.9 ± 43.3 | |||
| 30-day mortality, n (%) | 55 (24.5) | 47 (34.1) | 8 (6.8) | <0.001 |
| Adverse renal outcomes, n (%) | 158 (61.7) | 107 (77.5) | 51 (43.2) | <0.001 |
| eGFR last follow-up )mL/min/1.73 m2) | 59.3 ± 37.6 | 36.2 ± 22.4 | 84.2 ± 35.4 | <0.001 |
| Need for long-term dialysis, n (%) | 26 (10.2) | 23 (16.7) | 3 (2.5) | <0.001 |
| Decrease of at least 25% of eGFR, n (%) | 132 (51.6) | 84 (60.9) | 48 (40.7) | <0.001 |
| Long-term mortality, n (%) | 113 (44.1) | 59 (64.8) | 54 (49.1) | 0.025 |
Values presented as mean ± SD unless stated otherwise.
The mean age of patients was 62.6 ± 22.6 years. Most patients were Caucasian [n = 245 (95.7%)] and predominantly male [n = 144 (56.3%)]. Regarding comorbidities, 22.3% (n = 57) had diabetes mellitus, 46.1% (n = 118) had hypertension, 29.3% (n = 75) had CVD, 7.0% (n = 18) had COPD, 3.9% (n = 10) had cirrhosis, 19.5% (n = 50) had a previous diagnosis of malignancy and 55.5% (n = 142) had CKD. Baseline SCr was unknown and estimated in 37.5% of patients. The mean baseline eGFR was 64.1 ± 32.3 mL/min/1.73 m2. Most admissions were medical [n = 140 (54.7%)]. Concerning infection source, most were abdominal [n = 106 (42.4%)], respiratory [n = 76 (29.7%)], urinary tract [n = 35 (13.7%)] and skin [n = 20 (7.8%)].
At ICU admission, the mean SAPS II was 46.1 ± 15.6, mean SCr was 2.46 ± 1.5 mg/dL, mean haemoglobin was 10.8 ± 2.0g/dL, mean serum albumin was 1.9 ± 0.6 mg/dL, mean NLP ratio was 13.6 ± 22.6 and 31.3% of patients were acidotic (n = 80). During ICU admission, 71.1% (n = 182) required mechanical ventilation, 67.6% (n = 173) required vasopressor support, 34.8% (n = 89) were exposed to nephrotoxins and the mean fluid balance was 3.4 ± 4.7 L.
Regarding AKI stage, 27.3% (n = 70) were KDIGO Stage 1, 30.9% (n = 79) were Stage 2, 41.8% (n = 79) were Stage 3 and 16.8% (n = 43) required RRT. The mean length of hospital stay was 37.7 ± 36.1 days. At discharge, the mean SCr was 1.42 ± 1.2 mg/dL, mean eGFR was 68.0 ± 39.1 mL/min/1.73 m2 and 53.9% (n = 138) of patients had criteria for AKD.
The 30-day mortality rate post-discharge was 24.5% (n = 55). The mean long-term follow-up was 45.9 ± 43.3 months. The mean eGFR at the last follow-up was 59.3 ± 37.6 mL/min/1.73 m2. The majority of patients experienced adverse renal outcomes [n = 158 (61.7%)], such as a decrease of at least 25% of discharge GFR [n = 132 (83.5%)] and the need for long-term dialysis [n = 26 (16.5%)]. During follow-up, 44.1% (n = 113) of patients died (Table 3).
Table 3.
Patient characteristics according to adverse renal outcomes and mortality
| Characteristics | No adverse renal outcomes(n = 98) | Adverse renal outcomes (n = 158) | P-value | Long-term survival (n = 88) | Long-term mortality (n = 113) | P-value |
|---|---|---|---|---|---|---|
| Patient characteristics | ||||||
| Age (years) | 61.2 ± 15.8 | 63.5 ± 15.6 | 0.248 | 62.1 ± 14.9 | 61.6 ± 16.9 | 0.125 |
| Gender (male), n (%) | 62 (63.3) | 82 (51.7) | 0.075 | 45 (51.1) | 71 (62.8) | 0.096 |
| Race (Caucasian), n (%) | 96 (98.0) | 149 (94.3) | 0.161 | 85 (96.6) | 106 (93.8) | 0.368 |
| Comorbidities, n (%) | ||||||
| Hypertension | 43 (43.0) | 75 (47.5) | 0.575 | 45 (51.1) | 48 (42.5) | 0.222 |
| Diabetes | 18 (18.4) | 39 (24.7) | 0.238 | 21 (23.9) | 23 (20.4) | 0.551 |
| CVD | 24 (24.5) | 51 (32.3) | 0.183 | 29 (33.0) | 30 (26.5) | 0.322 |
| COPD | 8 (8.2) | 10 (6.3) | 0.577 | 5 (5.7) | 8 (7.1) | 0.689 |
| Cirrhosis | 5 (5.1) | 5 (3.2) | 0.437 | 3 (3.4) | 6 (5.3) | 0.518 |
| Neoplasia | 19 (19.4) | 31 (19.6) | 0.964 | 19 (21.6) | 21 (18.6) | 0.596 |
| CKD | 47 (48.0) | 95 (60.1) | 0.057 | 40 (45.5) | 67 (56.8) | 0.051 |
| Baseline SCr (mg/dL) | 1.2 ± 0.5 | 1.3 ± 0.7 | 0.166 | 1.1 ± 0.5 | 1.4 ± 0.7 | 0.009 |
| ICU admission, n (%) | ||||||
| Medical admission | 58 (59.2) | 82 (51.9) | 0.255 | 42 (47.7) | 63 (53.4) | 0.258 |
| Infection source, n (%) | ||||||
| Abdominal | 38 (38.8) | 68 (43.0) | 0.142 | 40 (45.5) | 48 (40.7) | 0.431 |
| Respiratory | 36 (36.7) | 40 (25.3) | 17 (19.3) | 37 (31.4) | ||
| Kidney | 7 (7.1) | 28 (17.7) | 11 (12.5) | 11 (9.3) | ||
| Skin | 8 (8.2) | 12 (7.6) | 11 (12.5) | 9 (7.6) | ||
| Others | 4 (4.1) | 2 (1.3) | 3 (3.4) | 2 (1.7) | ||
| Unknown | 3 (3.1) | 5 (3.2) | 3 (3.4) | 4 (3.4) | ||
| Nephrotoxins | 34 (34.7) | 55 (34.8) | 0.985 | 30 (34.1) | 35 (29.7) | 0.639 |
| SAPS II | 45.5 ± 13.8 | 46.5 ± 16.6 | 0.615 | 48.7 ± 14.0 | 46.0 ± 15.9 | 0.206 |
| Admission SCr (mg/dL) | 2.1 ± 1.1 | 2.7 ± 1.7 | 0.002 | 2.1 ± 1.3 | 2.6 ± 1.6 | 0.022 |
| Haemoglobin (g/dL) | 10.9 ± 1.9 | 10.7 ± 2.1 | 0.377 | 10.8 ± 1.8 | 10.8 ± 2.1 | 0.900 |
| Serum albumin (g/dL) | 2.0 ± 0.6 | 1.9 ± 0.5 | 0.846 | 1.9 ± 0.6 | 1.9 ± 0.6 | 0.683 |
| Acidaemia (pH < 7.5), n (%) | 27 (27.6) | 53 (33.5) | 0.315 | 22 (25.0) | 41 (34.7) | 0.087 |
| NLP ratio | 14.7±24.7 | 13.0±21.3 | 0.566 | 15.7±26.2 | 14.7±24.2 | 0.790 |
| Mechanical ventilation, n (%) | 71 (72.4) | 111 (70.3) | 0.706 | 66 (75.0) | 80 (67.8) | 0.507 |
| Vasopressors, n (%) | 66 (67.3) | 107 (67.7) | 0.950 | 67 (76.1) | 74 (62.7) | 0.102 |
| Fluid balance, L | 3.9 ± 5.0 | 3.1 ± 4.1 | 0.160 | 3.2 ± 4.0 | 3.7 ± 5.5 | 0.435 |
| AKI characteristics | ||||||
| KDIGO Stage 1, n (%) | 30 (30.6) | 40 (25.3) | 0.114 | 30 (34.1) | 31 (26.3) | 0.460 |
| KDIGO Stage 2, n (%) | 35 (35.7) | 44 (27.8) | 24 (27.3) | 29 (24.6) | ||
| KDIGO Stage 3, n (%) | 33 (33.7) | 74 (46.8) | 34 (38.6) | 53 (44.9) | ||
| RRT, n (%) | 17 (17.3) | 26 (16.5) | 0.853 | 14 (15.9) | 21 (17.8) | 0.620 |
| Outcomes | ||||||
| Length of stay in hospital (days) | 42.4 ± 40.3 | 34.7 ± 33.0 | 0.097 | 37.0 ± 36.1 | 37.0 ± 35.4 | 0.990 |
| AKD, n (%) | 31 (31.6) | 107 (67.7) | <0.001 | 32 (36.4) | 59 (50.0) | 0.025 |
| Adverse renal outcomes, n (%) | 42 (47.7) | 74 (65.5) | 0.011 | |||
| 30-day mortality, n (%) | 13 (113.3) | 42 (26.6) | 0.012 | |||
| Long-term mortality, n (%) | 52 (53.1) | 116 (73.4) | 0.001 | |||
Values presented as mean ± SD unless stated otherwise.
AKD and long-term outcomes
Patients with AKD were more likely to have higher baseline SCr [1.5 ± 0.7 versus 1.0 ± 0.5 mg/dL, P < 0.001; unadjusted odds ratio (OR) 4.5 (95% CI 2.70–8.33), P < 0.001], higher admission SCr [3.0 ± 1.7 versus 1.8 ± 1.1 mg/dL, P < 0.001; unadjusted OR 2.00 (95% CI 1.57–2.52), P < 0.001] and KDIGO Stage 3 AKI [47.8 versus 34.7%, P = 0.034; unadjusted OR 1.72 (95% CI 1.04–2.85), P = 0.035]. In a multivariate analysis, only baseline SCr [adjusted OR 3.06 (95% CI 1.66–5.63), P < 0.001] and SCr at admission [adjusted OR 1.62 (95% CI 1.25–2.10), P < 0.001] were associated with AKD development (Table 4).
Table 4.
Univariate and multivariate analyses of factors predictive of long-term renal outcomes and mortality in septic AKI patients
| Long-term renal outcomes |
Mortality |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Unadjusted HR (95% CI) | P-value | Adjusted HR (95% CI) | P-value | Unadjusted HR (95% CI) | P-value | Adjusted HR (95% CI) | P-value |
| Demographics | ||||||||
| Age | 1.00 (0.9–1.0) | 0.772 | 1.00 (1.0–1.1) | 0.992 | ||||
| Male | 0.96 (0.7–1.3) | 0.776 | 1.37 (0.9–2.0) | 0.108 | ||||
| Caucasian | 1.03 (0.5–2.0) | 0.943 | 0.96 (0.4) | 0.906 | ||||
| Comorbidities | ||||||||
| Hypertension | 0.89 (0.7–1.2) | 0.469 | 0.72 (0.5–1.1) | 0.088 | ||||
| Diabetes | 1.02 (0.7–1.5) | 0.924 | 0.80 (0.5–1.3) | 0.325 | ||||
| CVD | 1.14 (0.8–1.6) | 0.456 | 0.81 (0.5–1.2) | 0.326 | ||||
| COPD | 0.86 (0.5–1.6) | 0.643 | 0.96 (0.5–2.0) | 0.899 | ||||
| Cirrhosis | 0.82 (0.3–2.0) | 0.663 | 1.50 (0.7–3.4) | 0.339 | ||||
| Neoplasia | 1.01 (0.8–1.2) | 0.942 | 0.92 (0.7–1.2) | 0.499 | ||||
| CKD | 1.64 (1.0–2.7) | 0.058 | 1.75 (1.0–3.1) | 0.052 | ||||
| Baseline SCr | 1.39 (1.1–1.7) | 0.001 | 1.07 (0.8–1.5) | 0.595 | 1.39 (1.1–1.7) | 0.002 | 1.28 (1.0–1.6) | 0.055 |
| Medical admission | 1.11 (0.8–1.5) | 0.520 | 1.25 (0.9–1.8) | 0.232 | ||||
| Infection source | ||||||||
| Abdominal | 0.92 (0.67–1.27) | 0.622 | 0.93 (0.6–1.3) | 0.682 | ||||
| Respiratory | 1.00 (0.69–1.43) | 0.980 | 1.51 (1.0–2.2) | 0.139 | ||||
| Kidney | 1.81 (1.2–2.7) | 0.050 | 0.89 (0.5–1.7) | 0.712 | ||||
| Skin | 0.64 (0.4–1.1) | 0.133 | 0.65 (0.3–1.3) | 0.223 | ||||
| Others | 1.18 (0.5–2.9) | 0.713 | 0.58 (0.2–1.6) | 0.288 | ||||
| At UCI admission | ||||||||
| SAPS II | 1.00 (0.9–1.0) | 0.581 | 0.99 (0.9–1.0) | 0.191 | ||||
| Admission SCr | 1.18 (1.1–1.3) | <0.001 | 1.03 (0.8–1.1) | 0.587 | 1.15 (1.0–1.3) | 0.008 | 1.10 (1.0–1.2) | 0.132 |
| Haemoglobin | 1.00 (0.9–1.1) | 0.969 | 1.03 (0.9–1.1) | 0.525 | ||||
| Serum albumin | 1.14 (0.9–1.5) | 0.368 | 1.11 (0.8–1.6) | 0.532 | ||||
| pH <7.35 | 1.29 (0.9–1.8) | 0.133 | 1.42 (1.0–2.1) | 0.075 | ||||
| NLP ratio | 1.00 (0.9–1.0) | 0.851 | 1.00 (1.0–1.1) | 0.824 | ||||
| Nephrotoxins | 0.92 (0.7–1.3) | 0.606 | 0.85 (0.6–1.3) | 0.439 | ||||
| During ICU admission | ||||||||
| Mechanical ventilation | 0.91 (0.6–1.3) | 0.571 | 0.91 (0.6–1.4) | 0.639 | ||||
| Vasopressors | 0.91 (0.6–1.3) | 0.570 | 0.83 (0.6–1.2) | 0.359 | ||||
| Fluid balance | 1.00 (1.0–1.1) | 0.746 | 1.00 (1.0–1.1) | 0.319 | ||||
| KDIGO Stage 1 | 0.67 (0.5–1.0) | 0.133 | 0.78 (0.5–1.2) | 0.233 | ||||
| KDIGO Stage 2 | 1.17 (0.8–1.7) | 0.389 | 1.01 (0.7–1.5) | 0.974 | ||||
| KDIGO Stage 3 | 1.23 (0.9) | 0.204 | 1.23 (0.9–1.8) | 0.268 | ||||
| RRT | 1.12 (0.7–1.7) | 0.591 | 1.19 (0.7–1.9) | 0.475 | ||||
| At discharge | ||||||||
| AKD | 3.07 (2.2–4.3) | <0.001 | 2.87 (2.0–4.1) | <0.001 | 1.70 (1.2–2.5) | 0.005 | 1.51 (1.0–2.2) | 0.040 |
After discharge, the 30-day mortality was higher in AKD patients (34.1 versus 6.8%, P < 0.001).
Adverse renal outcomes (77.5 versus 43.2%, P < 0.001) and long-term mortality (64.8 versus 49.1%, P = 0.025) were more frequent among AKD patients (Table 3). In multivariate analysis, AKD was independently associated with adverse renal outcomes [adjusted HR 2.87 (95% CI 2.0–4.1), P < 0.001] and long-term mortality [adjusted HR 1.51 (95% CI 1.0–2.2), P = 0.040]. Additionally, mortality during follow-up was also higher in patients who experienced adverse renal outcomes (65.5 versus 47.7%, P = 0.011) (Table 4).
The 5-year cumulative probability of survival was 23.2% for AKD patients, while it was 47.5% for patients with no AKD (log-rank test, P < 0.0001) (Figure 2).
FIGURE 2.
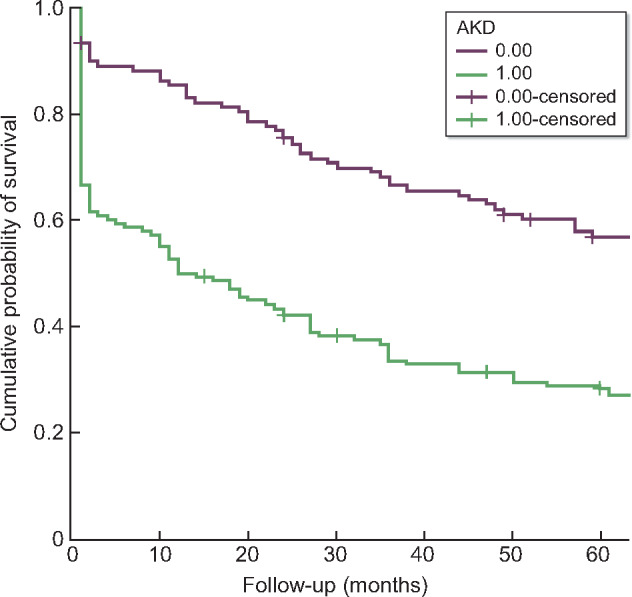
Comparison of cumulative mortality curves according to the development of post-operative AKD. Log-rank test P < 0.0001.
DISCUSSION
In this retrospective study of a cohort of 256 critically ill septic patients who developed AKI, AKD was independently associated with the risk of developing long-term adverse renal outcomes and of death after hospital discharge.
We found that AKD after septic AKI was associated with poor long-term renal function and long-term mortality: patients with AKD had a 2.8-fold higher risk of long-term dialysis or a 25% decrease in eGFR and a 1.5-fold higher risk of death than patients with no AKD.
In a previous study we analysed 457 critically ill septic patients hospitalized between January 2008 and December 2014 and compared the diagnostic and prognostic ability of the Risk, Injury, Failure, Loss of kidney function, End-stage kidney disease (RIFLE), Acute Kidney Injury Network and KDIGO classifications [31]. The incidence of AKI was 87.5% using the KDIGO classification, and AKI was independently associated with in-hospital mortality [adjusted OR 2.7 (95% CI 1.2–6.2), P = 0.021] [31, 32]. In the current analysis, we investigated the occurrence of AKD (presenting at least KDIGO Stage 1 criteria for >7 days after an AKI initiating event) after septic AKI and its association with adverse renal outcomes (need of long-term dialysis and/or a 25% decrease in eGFR after hospital discharge) and mortality of the patients of the same cohort who were discharged alive.
The impact of AKI on long-term renal function decline and mortality has been previously reported [30, 33–37]. In this study we demonstrated that AKD patients were a subpopulation of AKI patients with increased risk of renal function decline and mortality.
Indeed, renal recovery after AKI and its impact on patient outcomes has increasingly become the focus of research [38, 39].
Pannu et al. [40] reported a lower risk of adverse renal outcomes or mortality in patients with renal function recovery after AKI, defined as a return to within 25% above baseline SCr, in a population of community and hospital patients. Renal recovery was also significantly associated with lower risk for cardiovascular events in a study of hospitalized AKI patients by Omotoso et al. [41]. AKI has also been associated with increased risk of 30-day post-discharge mortality [42].
Considering that several different definitions of renal recovery and its impact in long-term outcomes have been used in the literature, the Acute Disease Quality Initiative (ADQI) workgroup proposed a standard definition of AKD as a condition in which AKI KDIGO Stage ≥1 is present >7 days after AKI start [29]. AKD persisting >90 days is considered CKD [29]. Thus AKD represents a period in which therapeutic interventions might be critical to alter the progression of kidney disease.
AKI can contribute to the development of CKD by acute endothelial injury, nephron loss, glomerular hypertrophy and fibrosis [43, 44]. Additionally, AKI associated with sepsis has particular detrimental characteristics due to the inflammatory milieu [45]. In septic shock patients, renal recovery has been demonstrated to have survival impact in the short and long term [46].
Lopes et al. [47] described the long-term impact of AKI in 234 septic patients. In this study, AKI, defined according to the RIFLE criteria, was an independent predictor of 2-year mortality [HR 3.2 (95% CI 1.6–6.5), P = 0.001]. Rubin et al. [48] also demonstrated an increased risk of CKD development in 232 critically ill patients.
In a retrospective study by Kim et al. [49] of 2208 patients with septic shock, AKI was associated with mortality; however, it did not correlate with the development of CKD in a 1-year follow-up. Interestingly, in this study, higher SCr at discharge was independently associated with CKD development [adjusted OR 2.686 (95% CI 1.499–4.812), P < 0.001] [49].
In contrast, in the Finnish Acute Kidney Injury (FINNAKI) study, AKI was not an independent predictor of 3-year mortality among 2336 30-day survivors of critical illness [50]. Nevertheless, this study reports the association of CKD and long-term outcomes and cannot exclude the possible increase in post-3-year mortality associated with progression to CKD [50].
This highlights the importance of the findings of our study. Our study is the first to describe the association of AKD, as defined by the ADQI definition, and long-term renal function decline and mortality in critically ill septic AKI patients. AKD after AKI is therefore an important diagnosis to be properly managed to prevent negative long-term outcomes.
Renal recovery was also evaluated in 1742 patients with AKI KDIGO Stages 2 and 3 by Fiorentino et al. [51]. In this study, renal recovery at discharge was defined as a return of SCr to within 150% of baseline without dialysis and was associated with better long-term survival [51], whereas non-recovery of renal function was associated with increased mortality in a 3-year follow-up [51]. Interestingly, they developed a model for prediction of renal recovery at discharge, including baseline SCr, AKI on Day 1, use of in-hospital RRT, Apache III score and CKD, which showed an area under the ROC curve of 0.79 [51]. The modest number of patients in our cohort has not allowed us to develop a model to identify patients at risk for AKD among AKI patients.
Further studies focusing on AKD are required to improve early recognition of these high-risk patients, in whom to employ preventive measures and therapeutic interventions to decrease CKD progression and mortality.
Certain limitations have to be noted. First, the single-centre and retrospective nature with a small cohort of patients restricts the generalization of our results. Second, we did not evaluate patients’ rehospitalizations, which could exacerbate renal function deterioration and increase mortality. Third, the development of proteinuria or CVD during follow-up was not accounted for; both are factors that influence renal function and long-term mortality. Fourth, we did not analyse causes of mortality. Fifth, the use of SCr to estimate AKD may overestimate renal recovery in septic patients due to loss of muscle mass, change in volume distribution, changes in renal reserve and hyperfiltration. Finally, we were unable to determine differences in long-term outcomes according to AKI severity, which can largely be related to the limited size of our cohort.
Despite these limitations, our study has numerous strengths. To the best of our knowledge, this is the first study comparing the incidence of AKD as defined by the ADQI and long-term outcomes in critically ill septic patients. Also, both SCr and UO criteria were used to define and categorize AKI. Only one patient was lost to follow-up. Finally, most of the studied variables were routinely registered during daily clinical practice.
CONCLUSION
In this retrospective study, we demonstrated that AKD after septic AKI is independently associated with the risk of long-term need of dialysis and/or renal function decline and with the risk of death after hospital discharge. Taking preventive measures to minimize the occurrence of AKD after AKI could potentially contribute to improved long-term outcomes.
FUNDING
There was no funding for this study.
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest. The results presented in this article have not been published previously in whole or part.
REFERENCES
- 1. Ronco C, Bellomo R, Kellum JA.. Acute kidney injury. Lancet 2019; 394: 1949–1964 [DOI] [PubMed] [Google Scholar]
- 2. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron 2012; 120: c179–c184 [DOI] [PubMed] [Google Scholar]
- 3. Hoste EA, Clermont G, Kersten A. et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care 2006; 10: R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bagshaw SM, George C, Bellomo R.. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care 2007; 11: R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoste EAJ, Bagshaw SM, Bellomo R. et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015; 41: 1411–1423 [DOI] [PubMed] [Google Scholar]
- 6. Ali T, Khan I, Simpson W. et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol 2007; 18: 1292–1298 [DOI] [PubMed] [Google Scholar]
- 7. Hoste EAJ, Kellum JA, Selby NM. et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol 2018; 14: 607–625 [DOI] [PubMed] [Google Scholar]
- 8. Odutayo A, Wong CX, Farkouh M. et al. AKI and long-term risk for cardiovascular events and mortality. J Am Soc Nephrol 2017; 28: 377–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bihorac A, Yavas S, Subbiah S. et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg 2009; 249: 851–858 [DOI] [PubMed] [Google Scholar]
- 10. Uchino S. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005; 294: 813. [DOI] [PubMed] [Google Scholar]
- 11. Bellomo R, Kellum JA, Ronco C. et al. Acute kidney injury in sepsis. Intensive Care Med 2017; 43: 816–828 [DOI] [PubMed] [Google Scholar]
- 12. Zarbock A, Gomez H, Kellum JA.. Sepsis-induced acute kidney injury revisited. Curr Opin Crit Care 2014; 20: 588–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoste E. Acute renal failure in patients with sepsis in a surgical ICU: predictive factors, incidence, comorbidity, and outcome. J Am Soc Nephrol 2003; 14: 1022–1030 [DOI] [PubMed] [Google Scholar]
- 14. Bagshaw SM, George C, Bellomo R, ANZICS Database Management Committee. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care 2008; 12: R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Angus DC, Linde-Zwirble WT, Lidicker J. et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29: 1303–1310 [DOI] [PubMed] [Google Scholar]
- 16. Bagshaw SM, Uchino S, Bellomo R. et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol 2007; 2: 431–439 [DOI] [PubMed] [Google Scholar]
- 17. Bagshaw SM, Lapinsky S, Dial S et al.. Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med 2009; 35: 871–881 [DOI] [PubMed] [Google Scholar]
- 18. Linder A, Guh D, Boyd JH. et al. Long-term (10-year) mortality of younger previously healthy patients with severe sepsis/septic shock is worse than that of patients with nonseptic critical illness and of the general population. Crit Care Med 2014; 42: 2211–2218 [DOI] [PubMed] [Google Scholar]
- 19. Prescott HC, Costa DK.. Improving long-term outcomes after sepsis. Crit Care Clin 2018; 34: 175–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prescott HC, Kepreos KM, Wiitala WL. et al. Temporal changes in the influence of hospitals and regional healthcare networks on severe sepsis mortality. Crit Care Med 2015; 43: 1368–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stevenson EK, Rubenstein AR, Radin GT. et al. Two decades of mortality trends among patients with severe sepsis. Crit Care Med 2014; 42: 625–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ou S-M, Chu H, Chao P-W. et al. Long-term mortality and major adverse cardiovascular events in sepsis survivors. A nationwide population-based study. Am J Respir Crit Care Med 2016; 194: 209–217 [DOI] [PubMed] [Google Scholar]
- 23. Shankar-Hari M, Rubenfeld GD.. Understanding long-term outcomes following sepsis: implications and challenges. Curr Infect Dis Rep 2016; 18: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Le Gall JR. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993; 270: 2957–2963 [DOI] [PubMed] [Google Scholar]
- 25. Singer M, Deutschman CS, Seymour CW. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315: 801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care 2009; 32(Suppl 1): S13–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chobanian AV. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289: 2560–2572 [DOI] [PubMed] [Google Scholar]
- 28.Kidney Disease: Improving Global Outcomes CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 29. Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 workgroup. Nat Rev Nephrol 2017; 13: 241–257 [DOI] [PubMed] [Google Scholar]
- 30. Chawla LS, Amdur RL, Shaw AD. et al. Association between AKI and long-term renal and cardiovascular outcomes in United States Veterans. Clin J Am Soc Nephrol 2014; 9: 448–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pereira M, Rodrigues N, Godinho I. et al. Acute kidney injury in patients with severe sepsis or septic shock: a comparison between the ‘Risk, Injury, Failure, Loss of kidney function, End-stage kidney disease’ (RIFLE), Acute Kidney Injury Network (AKIN) and Kidney Disease: Improving Global Outcomes (KDIGO) classifications. Clin Kidney J 2017; 10: 332–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gameiro J, Fonseca JA, Jorge S. et al. Neutrophil, lymphocyte and platelet ratio as a predictor of mortality in septic-acute kidney injury patients. Nefrologia 2020; doi: 10.1016/j.nefro.2019.11.006 [DOI] [PubMed] [Google Scholar]
- 33. Sawhney S, Mitchell M, Marks A. et al. Long-term prognosis after acute kidney injury (AKI): what is the role of baseline kidney function and recovery? A systematic review. BMJ Open 2015; 5: e006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gameiro J, Neves JB, Rodrigues N. et al. Acute kidney injury, long-term renal function and mortality in patients undergoing major abdominal surgery: a cohort analysis. Clin Kidney J 2016; 9: 192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lafrance J-P, Miller DR.. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol 2010; 21: 345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thakar CV, Christianson A, Himmelfarb J. et al. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol 2011; 6: 2567–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chawla LS, Amdur RL, Amodeo S. et al. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 2011; 79: 1361–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xu J, Xu X, Shen B. et al. Evaluation of five different renal recovery definitions for estimation of long-term outcomes of cardiac surgery associated acute kidney injury. BMC Nephrol 2019; 20: 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Göcze I, Wiesner C, Schlitt HJ, et al. Renal recovery. Best Pract Res Clin Anaesthesiol 2017; 31: 403–414 [DOI] [PubMed] [Google Scholar]
- 40. Pannu N, James M, Hemmelgarn B et al.. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol 2013; 8: 194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Omotoso BA, Abdel-Rahman EM, Xin W. et al. Acute kidney injury (AKI) outcome, a predictor of long-term major adverse cardiovascular events (MACE). Clin Nephrol 2016; 85: 1–11 [DOI] [PubMed] [Google Scholar]
- 42. Horkan CM, Purtle SW, Mendu ML. et al. The association of acute kidney injury in the critically ill and postdischarge outcomes. Crit Care Med 2015; 43: 354–364 [DOI] [PubMed] [Google Scholar]
- 43. Wald R, Quinn RR, Li P et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 2009; 302: 1179–1185 [DOI] [PubMed] [Google Scholar]
- 44. Ferenbach DA, Bonventre JV.. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol 2015; 11: 264–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Godin M, Murray P, Mehta RL.. Clinical approach to the patient with AKI and sepsis. Semin Nephrol 2015; 35: 12–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sood MM, Shafer LA, Ho J. et al. Early reversible acute kidney injury is associated with improved survival in septic shock. J Crit Care 2014; 29: 711–717 [DOI] [PubMed] [Google Scholar]
- 47. Kim J, Kim Y-J, Ryoo S. et al. One-year progression and risk factors for the development of chronic kidney disease in septic shock patients with acute kidney injury: a single-centre retrospective cohort study. J Clin Med 2018; 7: 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mildh H, Pettilä V, Korhonen A-M et al.. Three-year mortality in 30-day survivors of critical care with acute kidney injury: data from the prospective observational FINNAKI study. Ann Intensive Care 2016; 6: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fiorentino M, Tohme FA, Wang S. et al. Long-term survival in patients with septic acute kidney injury is strongly influenced by renal recovery. PLoS One 2018; 13: e0198269. [DOI] [PMC free article] [PubMed] [Google Scholar]


