Abstract
Hypoxia-inducible factor prolyl-hydroxylase inhibitors belong to a new class of orally administered drugs for treating anemia in patients with chronic kidney disease (CKD). The prevalence of hypothyroidism is disproportionately high in patients with CKD on hemodialysis. We report a rapid suppression of thyroid-stimulating hormone (TSH) and decrease in free triiodothyronine (T3) and free tetraiodothyronine levels after switching from darbepoetin alfa to roxadustat in a hemodialysis patient with hypothyroidism on levothyroxine therapy. This was reversed after stopping roxadustat. Roxadustat has structural similarity with T3 and is a selective activating ligand for thyroid hormone receptor-β possibly suppressing TSH release.
Keywords: end-stage renal disease, hemodialysis, hypothyroidism, hypoxia-inducible factor prolyl-hydroxylase inhibitor, levothyroxine sodium hydrate, roxadustat
BACKGROUND
Renal anemia is a common complication among patients with chronic kidney disease (CKD). Anemia is associated with increased morbidity and mortality related to cardiovascular disease, as well as increased risk of hospitalization [1]. Therefore, treatment of anemia is essential for CKD patients. Development of hypoxia-inducible factor prolyl-hydroxylase (HIF-PH) inhibitors could be the next step in the quest for safe and effective ways to increase hemoglobin levels in patients with end-stage renal disease (ESRD).
Roxadustat is an oral HIF-PH inhibitor that promotes erythropoiesis by increasing endogenous erythropoietin, improving iron regulation and reducing hepcidin [2]. Hence, roxadustat has the potential to be a new therapeutic option for treating renal anemia in patients with ESRD.
On the other hand, ESRD is associated with perturbations in thyroid hormone concentrations and increased prevalence of hypothyroidism. Therefore, it is anticipated that there will be an increase in the use of roxadustat in patients with ESRD complicated by hypothyroidism after the launch of this drug. Here, we present a case of rapid and reversible suppression of thyroid-stimulating hormone (TSH) and thyroid hormone levels after switching from darbepoetin alfa to roxadustat in a hypothyroid hemodialysis patient treated with levothyroxine.
CASE REPORT
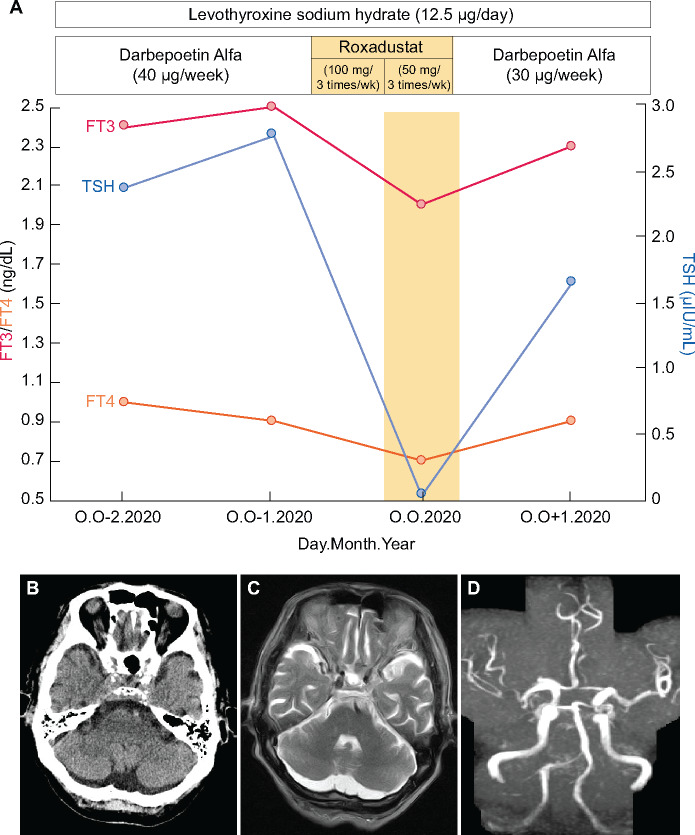
We present a case of an 85-year-old male with benign nephrosclerosis on hemodialysis and history of hypothyroidism due to chronic thyroiditis, who was taking a low dose of levothyroxine sodium hydrate. Thyroid hormone levels were well controlled. We switched darbepoetin alpha (40 μg/week) to roxadustat (100 mg, 3 times/week). At 1 month after switching to roxadustat, TSH, free triiodothyronine (FT3) and free tetraiodothyronine (FT4) had markedly decreased (TSH: 2.781 to 0.038 μIU/mL; FT3: 2.5 to 2.0 ng/dL and FT4: 1.0 to 0.7 ng/dL) (Figure 1A). Clinical symptoms such as general malaise and loss of appetite were observed after switching to roxadustat. There was no increase in low-density lipoprotein (LDL) cholesterol or creatinine kinase levels. Computed tomography and magnetic resonance imaging of the head did not disclose any abnormal finding (Figure 1B–D). We suspected an adverse effect of roxadustat and discontinued it immediately. After stopping the roxadustat, the TSH and thyroid hormone levels returned to the normal range.
FIGURE 1:
(A) Clinical course of thyroid hormones during the switch from darbepoetin alpha to roxadustat. (B) Noncontrast head CT shows no abnormal finding. (C) MRI of the head shows no infarction finding in the pituitary grand. (D) MR angiography shows no occlusion of the major cerebral arteries. CT, computed tomography; MRI, magnetic resonance imaging. Normal ranges: TSH: 0.34–3.88 μIU/mL; FT3: 2.1–4.1 ng/dL; FT4: 0.9–1.7 ng/dL.
DISCUSSION
This is the first report to suggest that roxadustat rapidly suppresses TSH, FT3 and FT4 in a patient with ESRD. HIF-PH inhibitors have the potential to increase endogenous erythropoietin production and enhance iron availability to the bone marrow in patients with CKD. Thus, the number of patients using this drug is expected to increase in the future. In particular, roxadustat is orally administered three times per week, and compliance can be ensured if the drug is delivered at every hemodialysis session. In this case, hypothyroidism was very well controlled with a low dosage of levothyroxine. The rapid change in TSH and thyroid hormone levels after switching from darbepoetin alfa to roxadustat and the reversibility after switching back to darbepoetin alfa suggest that roxadustat affected thyroid hormone levels.
This finding raises the question of why TSH was rapidly suppressed after switching to roxadustat. One of the serious adverse effects of HIF-PH inhibitors is thrombosis. However, there were no findings of infarction due to thrombosis in the pituitary gland and hypothalamus. In Japan, three types of HIF-PH inhibitors (daprodustat, roxadustat and vadadustat) are widely used, but hypothyroidism has been confirmed as an adverse event in clinical trials only in roxadustat. Thus, the structural features of roxadustat might affect thyroid hormone dynamics. A previous report suggested that some drugs, such as the retinoid X receptor-selective ligands, suppress the activity of the TSH gene promoter, thereby causing central hypothyroidism [3]. Roxadustat has structural similarity with T3 and is a selective activating ligand for thyroid hormone receptor-β, possibly suppressing TSH release [4]. Moreover, roxadustat can partially cross the blood–brain barrier in mice [5]. Thus, roxadustat possibly has an affinity for thyrotropin-releasing hormone receptor, producing an allosteric effect in the pituitary gland and/or hypothalamus. Although there were symptoms suggestive of hypothyroidism after switching to roxadustat, there was no increase in LDL cholesterol or creatinine kinase levels. Thus, roxadustat may have partially induced hypothyroidism via T3-like allosteric effects.
In conclusion, this report calls attention to the fact that roxadustat could possibly suppress TSH in patients on hemodialysis.
PATIENT CONSENT
We obtained written informed consent from the patient for publication of this case report. Ethical approval for publication was not required by Kawasaki Medical School.
ACKNOWLEDGEMENTS
The authors would like to thank the patient and his family for their contribution to this report. The authors also thank ThinkSCIENCE, Inc. for English language editing.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Horl WH. Anaemia management and mortality risk in chronic kidney disease. Nat Rev Nephrol 2013; 9: 291–301 [DOI] [PubMed] [Google Scholar]
- 2. Besarab A, Provenzano R, Hertel J. et al. Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol Dial Transplant 2015; 30: 1665–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sherman SI, Gopal J, Haugen BR. et al. Central hypothyroidism associated with retinoid X receptor-selective ligands. N Engl J Med 1999; 340: 1075–1079 [DOI] [PubMed] [Google Scholar]
- 4. Yao B, Wei Y, Zhang S. et al. Revealing a mutant-induced receptor allosteric mechanism for the thyroid hormone resistance. iScience 2019; 20: 489–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoppe G, Yoon S, Gopalan B. et al. Comparative systems pharmacology of HIF stabilization in the prevention of retinopathy of prematurity. Proc Natl Acad Sci USA 2016; 113: E2516–E2525 [DOI] [PMC free article] [PubMed] [Google Scholar]