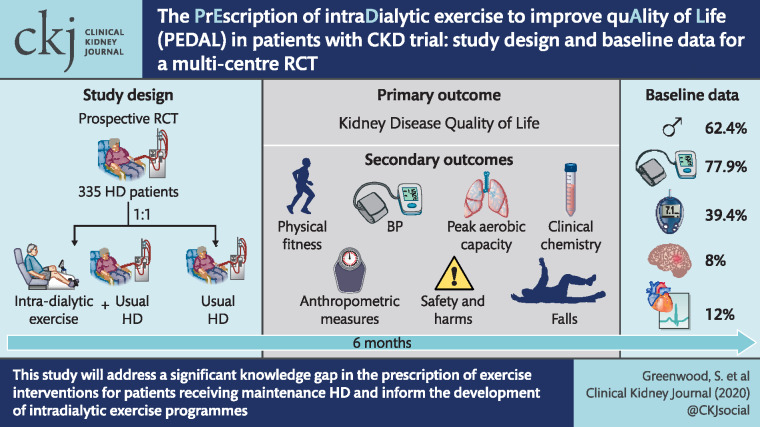
Abstract
Background
Exercise interventions designed to improve physical function and reduce sedentary behaviour in haemodialysis (HD) patients might improve exercise capacity, reduce fatigue and lead to improved quality of life (QOL). The PrEscription of intraDialytic exercise to improve quAlity of Life study aimed to evaluate the effectiveness of a 6-month intradialytic exercise programme on QOL and physical function, compared with usual care for patients on HD in the UK.
Methods
We conducted a prospective, pragmatic multicentre randomized controlled trial in 335 HD patients and randomly (1:1) assigned them to either (i) intradialytic exercise training plus usual care maintenance HD or (ii) usual care maintenance HD. The primary outcome of the study was the change in Kidney Disease Quality of Life Short Form (KDQOL-SF 1.3) Physical Component Score between baseline and 6 months. Additional secondary outcomes included changes in peak aerobic capacity, physical fitness, habitual physical activity levels and falls (International Physical Activity Questionnaire, Duke’s Activity Status Index and Tinetti Falls Efficacy Scale), QOL and symptom burden assessments (EQ5D), arterial stiffness (pulse wave velocity), anthropometric measures, resting blood pressure, clinical chemistry, safety and harms associated with the intervention, hospitalizations and cost-effectiveness. A nested qualitative study investigated the experience and acceptability of the intervention for both participants and members of the renal health care team.
Results
At baseline assessment, 62.4% of the randomized cohort were male, the median age was 59.3 years and 50.4% were white. Prior cerebrovascular events and myocardial infarction were present in 8 and 12% of the cohort, respectively, 77.9% of patients had hypertension and 39.4% had diabetes. Baseline clinical characteristics and laboratory data for the randomized cohort were generally concordant with data from the UK Renal Registry.
Conclusion
The results from this study will address a significant knowledge gap in the prescription of exercise interventions for patients receiving maintenance HD therapy and inform the development of intradialytic exercise programmes both nationally and internationally.
Trial Registration
ISRCTN N83508514; registered on 17 December 2014.
Keywords: chronic kidney disease, end-stage renal failure, exercise, haemodialysis, physical activity, quality of life
Graphical Abstract
INTRODUCTION
Haemodialysis (HD) is a major treatment option for patients with end-stage kidney failure. Over 27 000 patients receive dialysis for chronic kidney disease (CKD) in the UK and 80% of these are treated with HD [1]. Improved dialysis techniques and management of co-existing disease have made HD more tolerable and many new patients can anticipate a longer life expectancy [2], although not always with a good quality of life (QOL).
Both physical inactivity and impaired physical function are strongly associated with increased morbidity, mortality and reduced QOL in patients on HD [2, 3]. Reduced QOL is also independently associated with mortality in patients on HD. Reports indicate that 36-item Short Form Health Survey (SF-36) QOL-Physical Component Scores (PCSs) of <25 were associated with a 93% increased risk of death and a 56% increased risk of hospitalization in HD patients, while a 10-point decrease in the PCS translated into a 25% increased risk of death within 2 years [4]. Conversely, a 1-point increase in the PCS was associated with a 3.5% improvement in the odds of death [5]. Interventions designed to increase physical function and reduce sedentary behaviour in HD patients may mitigate CVD risk, improve physical functioning, improve fitness for potential future kidney transplantation, lower levels of fatigue and in turn lead to improved QOL. A study by Painter et al. [6] reported an average 4-point increase in the PCS in the intervention group (in-centre intradialytic cycling or individualized home exercise programme), and an average 6-point decrease in the score in the non-intervention group. DeOreo [7] reported that for every increase in PCS of 5 points, there is an approximately 10% increase in the probability of survival.
Evidence from several systematic reviews [8–21] indicates that a range of exercise training interventions show potential to improve exercise capacity and physical function in patients with dialysis-dependent CKD. The greatest effects were reported after 6 months of exercise and were associated with both supervised and higher intensities of exercise. However, most of the studies reviewed were small trials, many of which were not methodologically robust, and non-intradialytic interventions were occasionally included in the evidence synthesis [15]. Relatively few of the reviewed studies on intradialytic exercise training were appropriately powered to detect QOL outcomes, and none included a cost-effectiveness analysis. Moreover, adverse events in the published literature were only recorded in a minority of studies [16] or were poorly reported [15]. A recent systematic review [21] on intradialytic exercise training suggests that aerobic and resistance exercise programmes, delivered alone, can improve aerobic capacity but that a combination of both can improve a greater range of outcomes, including exercise capacity, depression and some elements of QOL. In contrast, a recent systematic review by Young et al. [17] indicated that there was insufficient evidence demonstrating whether cycling exercise during HD improves patient outcomes. The recommendations emerging from these systematic reviews indicated the need for (i) high-quality, adequately powered randomized controlled trials (RCTs) of intradialytic exercise and (ii) routine collection and reporting of adverse event data associated with participation in these trials. The generation of this information may help to more fully identify recommendations for an exercise delivery pathway for HD patients and ultimately its clinical implementation [20].
The PrEscription of intraDialytic exercise to improve quAlity of Life (PEDAL) study was designed in response to a National Institute for Health Research (NIHR) commissioned call to evaluate the clinical benefit and cost-effectiveness of exercise during HD (intradialytic exercise) in patients with end-stage kidney disease. At the time of planning and developing the PEDAL study (2012), there was extremely limited evidence on the effectiveness of intradialytic cycling on patient-centred outcomes such as QOL indices. In CKD Stages 2–5, overall quality of life indices was improved with exercise training. Improved scores in the self-reported physical function sub-scores of the questionnaires used appeared to be the main drivers for the overall improvement. Other elements of QOL such as vitality, social function and general health did not show a systematic change [8]. Short-term (2–6 months) structured and supervised moderate-intensity aerobic training programmes (mainly cycling) have been reported to induce a systematic and large improvement in cardiorespiratory fitness [volume of oxygen consumption (VO2peak)] of 17–50%, with an overall mean difference between treatment and control groups of 5.22 mL/kg/min. Such improvements exceed the clinically important criterion of 1 MET (3.5 mL/kg/min). Thus, we utilized the evidence base promoting intradialytic cycling for improved cardiorespiratory fitness to investigate whether the physiological benefits derived from cycling, could extend to perceived enhanced QOL. Furthermore, as inconsistent improvements have been noted for objectively measured functional capacity indices (walking speed/distance, sit-to-stand performance) from previous intradialytic cycling studies, we chose to evaluate key secondary outcome measures of objective physical function.
The rationale for intradialytic exercise is both intuitively and pragmatically appealing as the environment of unit-based HD provides a platform for longer term sustainable implementation of exercise rehabilitation programmes, and thus could promote exercise-enhancing behaviours in HD patients. The pre-existing need for patients to attend for standard thrice weekly, 4 h-long HD sessions provides a practical opportunity to deliver a structured and supervised rehabilitation programme with an enhanced potential for participation, associated with a substantially reduced patient burden in terms of time, effort and travel costs.
MATERIALS AND METHODS
Primary objective
The primary objective of this trial was to determine, in comparison with usual care, whether usual care augmented by intradialytic exercise training for a period of 6 months improved The Kidney Disease Quality of Life Short Form (KDQOL-SF 1.3) Physical Component Score (PCS) in CKD Stage 5 patients receiving maintenance HD.
Secondary objective
Secondary objectives were to determine, in comparison with usual care, whether usual care augmented by intradialytic exercise training improved:
Physical function and physical activity (PA) outcomes:
Peak aerobic capacity
Physical fitness indicators
Gait speed
Lower limb strength
Clinical measures:
Resting blood pressure
Haemoglobin, serum phosphate, parathyroid hormone
Arterial stiffness (pulse wave velocity)
Anthropometric measures:
Body mass index (BMI)
Waist and hip circumference
Patient-reported outcomes:
Quality of life and symptom burden assessments (EQ5D)
Habitual PA levels (International Physical Activity Questionnaire)
Duke’s Activity Status Index
Falls confidence (Tinetti Falls Efficacy Scale)
Economic analysis of cost-effectiveness:
Quality-adjusted life years (QALYs) will be derived from EQ5D
Resource use associated with physiotherapy assistant time
Resource use associated with delivery of exercise programme
Equipment costs
Hospital admissions and medication use will be recorded (with consent) from the patient’s clinical records
Qualitative study:
A nested qualitative study investigated the experience and acceptability of the intervention for both participants and members of the renal care team
Design, setting and participants
This was a prospective, pragmatic multicentre RCT with blinded outcome assessment. The flow of participants through the trial is summarized in Table 1. We aimed to recruit prevalent adult patients (aged >18 years), treated as outpatients, undergoing in-centre (hospital unit, satellite unit) maintenance HD. Participants were recruited from 10 sites in five ‘regions’ spread across the UK. The research sites selected were broadly representative of contemporary UK HD units and geographically covered a wide range of the UK. These centres were selected as they also provided access to large numbers of prevalent HD patients. London Fulham Research Ethics Committee approved the protocol (14/LO/1851; NCT02222402), and the study was prospectively registered (ISRCTN N83508514). The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) Checklist is available in Supplementary data, File S1 of this report.
Table 1.
Overview of trial schedule
| Outcome measure/procedure | Screening | Baseline | 6 months |
|---|---|---|---|
| Informed consent | X | ||
| Inclusion/exclusion criteria | X | ||
| Medical history/demographics | X | ||
| Adverse events recorded | X | X | |
| Review/record medication | X | X | |
| Anthropometric measurements | |||
| Height | X | X | |
| Weight | X | X | |
| BMI | X | X | |
| Waist-to-hip ratio | X | X | |
| QOL (and symptom burden) assessments | |||
| KDQOL-SF 1.3 questionnaire | X | X | |
| EQ5D questionnaire | X | X | |
| Cardiovascular assessments | |||
| Resting blood pressure | X | X | |
| Pulse wave velocity | X | X | |
| Functional capacity measurements | |||
| Peak aerobic capacity (VO2 peak and/or peak power output) | X | X | |
| Sit-to-stand 60 | X | X | |
| Functional mobility (10mTUG) | X | X | |
| DASI Questionnaire | X | X | |
| Tinetti Falls Efficacy Scale | X | X | |
| Habitual PA | |||
| IPAQ questionnaire | X | X | |
| Clinical chemistry | |||
| Hb | X | X | |
| Phosphate | X | X | |
| Parathyroid hormone | X | X | |
| Medication | |||
| ESAs | X | X |
10mTUG, 10 m Timed Up and Go test; ESAs, erythropoiesis-stimulating agents.
Recruitment and eligibility
The majority of potential participants for the PEDAL study were identified during routine HD clinical consultations and concurrent evaluation of clinical records to confirm eligibility for participation. Patients already established on HD for >3 months were eligible and easily identified from hospital databases and dialysis logs. If considered eligible for the study, they were approached by a member of the renal health care team who discussed the study and provided them with a Participant Information Sheet (PIS) with further details to read. After allowing the patient a minimum of 24 h to read and consider the information in the PIS and to consult with family members, the research team approached the patient (usually during the next dialysis session) to answer any questions. If the patient was agreeable to proceed, an appointment was made for familiarization and baseline outcome assessment sessions. Written informed consent was obtained by a member of the research team prior to any study assessments.
Inclusion criteria
Prevalent CKD Stage 5 patients receiving maintenance HD therapy for >3 months, who were male or female, aged >18 years and who were able to provide written informed consent.
Exclusion criteria
Patients with expected survival on dialysis of <6 months [i.e. those with severe heart failure (New York Heart Association ≥3)], patients for whom dialysis withdrawal was being considered, patients likely to receive a live-donor transplant or transfer to peritoneal dialysis in the period of time, all patients within 3 months of initiation of HD (patients in this time frame are generally less clinically stable, many having vascular access procedures performed and with much higher rates of intercurrent events, including death and hospitalization), patients deemed to be clinically unstable by their treating physician, bilateral lower limb amputations, patients with dementia or severe cognitive impairment and other patients unable to give informed consent, patients with psychiatric disorders (who are not treated and stable) and patients who were pregnant.
Randomization and blinding
Randomization was conducted via a centrally controlled web-based randomization system, run by the Glasgow Clinical Trials Unit (GCTU). To ensure balanced assignment across critical variables, a minimization algorithm was employed, taking into account baseline age, gender and diabetes status. It was impossible to blind the ‘treating’ physiotherapy assistants or the participants, and thus the study implemented a blinded outcome assessment and analysis.
Intervention arms
Intradialytic exercise prescription and training programme
The intradialytic exercise prescription was based on current PA guidelines for the elderly [22], and for people with diabetes [23] and cardiovascular disease [24]. These recommend a minimum target amount of 1000 kcal per week be expended in PA for health benefits, with optimal benefits associated with weekly target PA accumulation of 1500–2000 kcal or at least 150 min/week at moderate-intensity exercise and at least 2 days a week of resistance-based training for muscular endurance and strength gains. As opportunity for structured prescribed exercise was largely restricted to patients’ three HD days, the training aim was for the patients to accumulate as great a proportion of this minimum threshold level of 1500 kcal per week via intradialytic exercise, as possible. A target overall volume of exercise was calculated in EE units (kcal/week) using the FIIT principle (Frequency, Intensity, Time, Type) and provided for all patients, with an individualized progression plan towards this goal.
The following progression plan was devised as a general guide:
Introductory/adoption phase
A minimum cycling duration of 21 min/dialysis session was used as a starting point target (150 min of moderate-intensity exercise/7 days). By the end of the first 4 weeks all patients were expected to cycle for 21 min either in 2 × 10 min bouts or continuously at the low end of the moderate exercise intensity range.
The progression requirements were that, by Week 8, all participants should be able to cycle for at least 21 min continuously on the cycle ergometer (63 min/week) at the low end of the moderate exercise intensity range. Minimum target overall volume of exercise prescription was set at 140 kcal/cycling session.
Progression/adoption phase
This phase is expected to last up to 12–14 weeks. During these weeks, emphasis was given on progressing exercise stimulus via duration mainly. A reasonable and achievable exercise target for this group would be a minimum cycling duration of ∼21–30 min/dialysis session at moderate intensity. Minimum target overall volume of exercise prescription was set at ∼1000–1200 kcal/week or ∼170 kcal/dialysis exercise session.
Behaviour development phase
This phase is expected to last up to 24 weeks. All patients should be able to achieve a target duration ranging from 30 to 40 min/dialysis session (90–120 min/week) at moderate to vigorous exercise intensity (55–70% VO2 reserve). Minimum target overall volume of exercise was set at ∼1500 kcal/week (or ∼214 kcal/dialysis session) and this would be achieved via adjustments to duration and intensity.
The prescribed individualized training intensity was derived from a peak aerobic capacity (VO2peak) assessment, using a 1-min ramp incremental protocol on a cycle ergometer. New exercise intensity ranges were established at the 3-month follow-up assessment point. Exercise prescription was set at a workload corresponding to 40–75% of VO2 reserve. We also made a note of rate of perceived exertion (RPE), heart rate (HR) and BP responses corresponding to these ranges of exercise intensity during the incremental cycle testing protocol and used these indices to guide and monitor progression until the next planned assessment point (3 months) at which time the exercise prescription was renewed.
Using a modified and custom-made cycle ergometer (MONARK), aerobic exercise was performed in a semi-recumbent position, three times per week during the first 2 h of HD. Exercise duration and intensity were recorded and monitored for each exercise session via exercise diaries. Also, RPE, BP and HR were recorded during the training sessions. The energy expenditure goals were deliverable via a progressive increase in intradialytic cycling from short bouts of 8–10 min in duration to start with, to 21–40 min or longer, at the prescribed exercise intensity, resulting in 55, 69 and 75%, respectively, of the 1000 kcal target weekly minimum PA volume being achieved. Twice per week participants also completed lower extremity muscular conditioning exercise, using ankle weights, after the aerobic cycling exercise. Physiotherapy assistants (PTA, band 4 Technical Instructors) were employed in each region to deliver the intradialytic intervention. This role, supervised and quality assured by a regional coordinator, involved the technical implementation of the exercise prescription produced by the regional Research Assistant who was blinded to treatment allocation.
Usual care: HD therapy
Usual care was based on UK Renal Association Guidelines for haemodialysis [1] and included management of blood pressure (BP), treatment of anaemia, phosphate control and cardiovascular risk mitigation strategies. For the purposes of the trial, we specified that usual care, in both arms of the trial, should allow all of these treatments to continue unchanged so that we were investigating any additional benefit of the intradialytic exercise training intervention to usual care.
Adherence
We attempted to minimize the loss to follow-up in this study by (i) emphasizing to participants the importance of their attendance at follow-up assessments even if they were no longer compliant with the intervention, (ii) reducing outcome assessment appointments to a maximum of two non-dialysis day visits, (iii) using a reminder protocol for non-dialysis day assessment appointments that utilized prompts via the dialysis unit staff, letters and telephone contact, (iii) providing travel remuneration (including, where necessary, taxi costs) and (iv) provision of training in issues related to compliance for all study staff who came into contact with the participants.
Assessment outcomes and their measurement
Baseline clinical information and study visits
A summary of the study schedule is shown in Table 1. Clinical data including cause of CKD, comorbidities and medications were collected via participant interviews and reviews of clinical databases and records at the baseline study visit. Follow-up data collection was scheduled at 6 months post-randomization. The 6-month follow-up was chosen as the primary outcome time point as this has been reported as the optimum duration for the largest effect sizes following exercise rehabilitation research trials.
Primary outcome
The primary endpoint for this study was the change in KDQOL-SF 1.3 PCS between baseline and 6 months. The KDQOL-SF 1.3 is a disease-specific QOL measure. The KDQOL-SF 1.3 questionnaire includes the SF-36 as the generic core plus symptoms/problems of kidney disease scales. The SF-36 questionnaire has 36 items compiled into eight scales: physical functioning (PF), role functioning/physical (RP), bodily pain, general health (GH), vitality (VT), social functioning (SF), role functioning/emotional (RE) and mental health (MH). These scales are scored from 0 to 100; a higher score is more positive (i.e. less pain or less limitation). Normalized scores representing overall physical functioning and mental functioning are calculated from the individual scales and are presented as the Physical Component Score (PCS) and the mental component scale. The PCS includes the dimensions of PF, RP, bodily pain, GH, VT and SF.
Key secondary outcomes
QOL
A documented change in the EQ5D from baseline to 6 months was a key QOL secondary outcome.
Peak aerobic capacity
VO2peak was determined by an incremental cycling exercise tolerance protocol. Breath-by-breath gas exchange was measured using cardiopulmonary exercise testing equipment calibrated prior to each patient assessment. The exercise testing protocol started from a 3-min unloaded cycle, followed by ramp increases in resistance of 15 W/min until one of the following occurred: (i) a plateau in oxygen uptake, (ii) attainment of respiratory exchange ratio ≥1.15 or (iii) patient requested to stop. Average oxygen uptake of the final 20 s of the test was recorded as the VO2peak (L/min). Electrocardiogram and HR were continuously monitored, and BP was recorded every 1 or 2 min throughout the ramp incremental test. RPE, using the CR100 RPE scale and angina scale was recorded every minute for safety.
Physical performance tests
Patient physical function was assessed by the sit-to-stand-60 (STS60) test [25] and the 10-metre timed-up-go (10mTUG) test [26], both of which have been used as accurate and valid measures of lower leg strength, balance, coordination, gait speed and physical function in HD patients.
Anthropometric measures
Measures of height, body mass, BMI and waist circumference were performed.
Cardiovascular risk
Carotid–femoral pulse wave velocity (PWV) is considered the gold standard for non-invasive arterial stiffness assessment in clinical practice [27] and has been suggested as a surrogate cardiovascular endpoint. This was assessed with the Vicorder system (Skidmore Industries, UK). The Vicorder system is small, portable, non-invasive and non-operator dependant. In addition, it was available in all centres to ensure comparability of the data. Conditions for assessment, as stated by the expert consensus statement by Laurent et al. [27], were adhered to for all measurements. The measurement protocol by Hickson et al. [28] was used, mathematically removing the additional femoral segment from the Vicorder standard protocol to correct for any inherent bias at high arterial PWV. The average of three measurements (of 20 consecutive signals) was recorded at each time point. Resting predialysis BP was assessed.
Physical function questionnaires, PA and fear of falling
Patients completed the following questionnaires to capture data about physical activity, activities of daily living and falls: The International Physical Activity Questionnaire Long Form (IPAQ-LF) [29], The Duke Activity Status Index (DASI)—a self-reported 12-item questionnaire that assesses activities of daily living [30]. The Tinetti Falls Efficacy Scale measured fear of falling [31].
Blood tests
Clinical blood tests were collected pre-dialysis and included haemoglobin (Hb), serum phosphate and parathyroid hormone.
Medication
Dosages of erythropoiesis-stimulating agents were recorded.
Safety and monitoring of the intervention
Safety and monitoring data included discontinuation from the exercise intervention and permanent study withdrawals with reasons, compliance with the exercise programme and adherence to the exercise prescription and serious adverse events.
All key secondary outcome measures will be reported in the publication of the primary results.
Other outcomes
Qualitative study
A constructivist phenomenological approach was used to learn about views and perceptions of participants and build greater understanding of varied perspectives of both service users and providers [32, 33]. Focus groups and individual semi-structured interviews were used as most appropriate to the stage of the study and location of data collection. Purposive sampling continued until data saturation was reached and was used to ensure varied experiences and viewpoints were represented, for example, including service users and providers from different study regions, including people in both study arms and people with different participation rates and response to the intervention.
Health economic analysis
QALYs were derived from utility scores generated using a standard UK algorithm from the EQ5D. Resource use associated with physiotherapy assistant time and training (in use of equipment and delivery of personal exercise programmes per patient) time was recorded prospectively using timesheets for the intervention group. Equipment costs were calculated using the annuity method with discounting rates set as per National Institute for Health and Care Excellence (NICE) base case. Hospital admissions and medication use were recorded (with consent) from the patient’s clinical records.
Sample size
Primary outcome: KDQOL-SF 1.3 PCS
A sample size calculation, based on an assumed difference of 4 points in the PCS score and a standard deviation (SD) of 10 points as seen in the study by Painter et al. [6], and conservatively comparing 6-month KDQOL-SF 1.3 PCS between groups by two-sample t-test (two-sided 5% significance level), resulted in a sample size of 133 completers per group with 83% power to detect a mean difference of four points in KDQOL-SF 1.3 PCS. To allow for a 30% loss to follow-up over 6 months as seen in Phase II trials [23], 190 participants per group were suggested to be randomized. This sample size calculation did not take into account adjustment for baseline PCSs. Subsequent analysis of within-trial change in the PCS from baseline, adjusted for baseline levels and randomization minimization variables, suggests that the study will have 80% power to detect a four-point difference with only 87 participants per group with complete data at baseline and 6-month follow-up. Likewise, 115 participants per group would be needed if a zero score is imputed for deaths prior to 6 months.
Key secondary outcome
Analysis of blinded within-trial data adjusting for baseline levels and randomization minimization variables suggest that a study of 44 participants per group would have 90% power to detect the minimum clinically important difference in peak aerobic capacity of 3.5 mL/kg/min (1 MET).
Statistical analyses
A full statistical analysis plan will be signed off prior to database lock and study unblinding.
Descriptive statistics of clinical and socio-demographic variables at baseline are presented split by treatment group.
Primary outcome
The primary outcome measure (change from baseline to 6 months in KDQOL-SF 1.3 PCS) will be compared between the control and intervention groups using a normal linear model adjusting for baseline KDQOL-SF 1.3 PCS and the randomization minimization variables. The findings will be presented as the (adjusted) mean difference (95% confidence interval) between the treatment groups. The main analysis will be carried out on research participants with PCS assessments from baseline to 6 months. Two sensitivity analyses will also be carried out, first imputing a score of zero for those who die prior to 6 months and secondly, based on all participants with a baseline PCS using the method of multiple imputation.
Secondary outcomes
Other continuous outcomes will be analysed as for the primary outcome. The Bonferroni correction will be applied for multiple comparisons between groups.
Binary outcomes will be compared between treatment groups using logistic regression models adjusting for baseline value. The results will be reported as the adjusted odds ratio, with a 95% confidence interval.
Time-to-event outcomes will be calculated as time from randomization and will be compared between treatment groups using Cox proportional hazards regression models. The results will be reported as the adjusted hazard ratio for intervention versus control, with a 95% confidence interval.
Data involving counts of events will be compared between treatment groups using negative binomial regression models adjusting for length of follow-up. The results will be reported as the adjusted treatment effect, with a 95% confidence interval.
Subgroup analyses
The effect of treatment on the primary outcome will be compared between those who have an exercise prescription at baseline (those who have VO2peak at baseline) and those who do not. This will be done using a linear regression model predicting the primary outcome from treatment, whether or not exercise prescription at baseline is available, and the interaction of both variables, adjusting for minimization variables.
Within the intervention group, the primary outcome will be compared between those who have completed <30% of the expected exercise sessions, those who have completed ≥30% but <50%, those who have completed ≥50% but <70%, and those who have completed >70% of the expected exercise sessions. This will be done using a linear regression model predicting the primary outcome from the above categories for the expected exercise sessions completed, adjusting for minimization variables.
The primary outcome will also be assessed comparing the control group with the three completer groups defined above.
In addition, the relation of percentage of sessions completed at 6 months follow-up to the primary outcome will be analysed in two ways:
As a linear regression predicting the primary outcome from the percentage of sessions completed at 6 months follow-up, adjusting for baseline PCS and minimization variables, in the intervention group only.
As linear regression predicting primary outcome from the percentage of sessions completed at 6 months follow-up, adjusting baseline PCS for minimization variables, in all patients, setting the percentage of sessions completed to 0 in the control group.
Safety analyses
Discontinuations from the intervention and permanent study withdrawals and their reasons will be tabulated as will adherence to the exercise prescription. Serious adverse events will be tabulated by system organ class and body system. The safety analysis is based on collection of and description of all hospitalizations for any cause whether related, possibly related or unrelated, and all other reported Serious Adverse Events (SAEs).
Data monitoring and quality assurance
The trial was coordinated by a Trial Management Group (TMG), consisting of the Chief Investigator, GCTU Assistant Director and Senior Clinical Trial Manager and a statistician. The Trial Manager coordinated the study and was accountable to the Chief Investigator. The Central Trial Office (King’s College Hospital & Glasgow Clinical Trials Unit) provided support to each site. The Trial Office was responsible for randomization, collection of data in collaboration with the research coordinator, data processing and analysis. Publication and dissemination of the study results will be coordinated by the Trial Office in collaboration with the Chief Investigator and Investigators. A Trial Steering Committee (TSC) was established to oversee the conduct and progress of the trial. The study’s funder, National Institute for Health Research (NIHR) HTA, formally appointed the chair and members after the nominations from the TMG. A charter was drawn up to describe membership, roles and responsibilities of the TSC. The Independent Data Monitoring Committee was an independent group of experts, consisting of a nephrologist, physiotherapist, lay member and a statistician, who monitored patient safety and treatment efficacy data while the clinical trial was ongoing; the primary mandate of this committee was to protect patient safety.
Baseline characteristics of randomized participants
Of the 335 randomized patients, 62.4% were male (Table 2). The median [interquartile range (IQR)] age was 59.3 (48.8–70.5) years. Half of the randomized population was white and 34.3% was Black African/Black Caribbean. The mean (SD) weight was 80.5 (20.1) kg and most participants were classified as overweight or obese [median (IQR) BMI of 27.8 (24.0–32.2) kg/m2]. Thirteen percent reported a musculoskeletal or orthopaedic condition, and patients had an Hb between 100 and 120 g/dL at baseline.
Table 2.
Baseline characteristics in all randomized participants (intention to treat population)
| Characteristic | Value | N | Summary |
|---|---|---|---|
| Age (years) | Mean (SD) | 335 | 59.4 (14.7) |
| Median (Q1, Q3) | 59.3 (48.8–70.5) | ||
| Gender | n (%) Female | 335 | 126 (37.6) |
| Ethnicity | n (%) White | 169 (50.4) | |
| n (%) Black Caribbean | 47 (14.0) | ||
| n (%) Black African | 335 | 68 (20.3) | |
| n (%) South Asian (Indian, Pakistani, Bangladeshi) | 39 (11.6) | ||
| n (%) Chinese | 2 (0.6) | ||
| n (%) Other | 10 (3.0) | ||
| Weight (kg) | Mean (SD) | 333 | 80.5 (20.1) |
| Median (Q1, Q3) | 77.5 (66.0–91.7) | ||
| BMI (kg/m2) | Mean (SD) | 333 | 28.7 (6.7) |
| Median (Q1, Q3) | 27.8 (24.0–32.2) | ||
| Smoking | n (%) Current | 335 | 42 (12.5) |
| n (%) Former | 98 (29.3) | ||
| n (%) Never | 195 (58.2) | ||
| SBP (mmHg) | Mean (SD) | 332 | 136.1 (21.9) |
| Median (Q1, Q3) | 135.0 (121.3–150.8) | ||
| DBP (mmHg) | Mean (SD) | 332 | 73.6 (14.1) |
| Median (Q1, Q3) | 73.0 (63.3–82.0) | ||
| Peripheral vascular disease | n (%) Yes | 335 | 11 (3.3) |
| Diabetes | n (%) Yes | 335 | 132 (39.4) |
| Hypertension | n (%) Yes | 335 | 261 (77.9) |
| Hyperlipidaemia | n (%) Yes | 335 | 71 (21.2) |
| Previous MI | n (%) Yes | 335 | 41 (12.2) |
| Heart failure | n (%) Yes | 335 | 32 (9.6) |
| Cerebrovascular events | n (%) Yes | 335 | 26 (7.8) |
| Cardiovascular conditions | n (%) Yes | 335 | 69 (20.6) |
| Musculoskeletal and orthopaedic condition | n (%) Yes | 335 | 43 (12.8) |
| Hb (g/L) | Mean (SD) | 320 | 110.3 (13.5) |
| Median (Q1, Q3) | 110.0 (102.0–119.0) | ||
| CRP (mg/L) | Mean (SD) | 315 | 14.5 (19.9) |
| Median (Q1, Q3) | 7.0 (3.0–17.1) | ||
| URR (%) | Mean (SD) | 318 | 71.5 (8.1) |
| Median (Q1, Q3) | 72.7 (67.0–77.0) |
Continuous variables are shown as mean (SD) or median (Q1, Q3).
SBP, systolic blood pressure; DBP: diastolic blood pressure; CRP, C-reactive protein; URR, urea reduction ratio; MI, myocardial infarction.
Prior cardiovascular events and risk factors
A history of major adverse cardiovascular events affected the minority of participants, reflecting the incident nature of the population; prior stroke and myocardial infarction were present in 8 and 12% of the cohort, respectively. A history of heart failure or peripheral vascular disease was recorded for 4 and 10% of participants, respectively. At baseline, 78% had hypertension, 39% had diabetes, 21% had hyperlipidaemia and 58% had never smoked.
Ethics approval and consent to participate
London Fulham Research Ethics Committee approved this protocol (14/LO/1851). The study was prospectively registered (ISRCTN N83508514).
Availability of data and materials
Findings from the study will be disseminated at national and international conferences. All data published from this study will be available if requested in writing from the chief investigator.
Trial status
The current version is Version 5, 20 June 2019. Recruitment finished on 31 April 2019. The study completed on 28 February 2020. Trial sponsor: King’s College Hospital, Denmark Hill, London SE5 9RS, UK.
DISCUSSION
Physical inactivity and impaired physical function are strongly associated with increased morbidity, mortality and reduced health-related QOL in HD patients [2, 3]. However, these patients do not usually receive formal exercise-based rehabilitation as part of routine care. Physical inactivity is a modifiable risk factor and exercise interventions designed to improve physical function and reduce sedentary behaviours may improve health-related outcomes and be cost-effective in the longer term. However, despite the evidence from a number of systematic reviews on the potential efficacy of exercise training interventions for patients with dialysis-dependent CKD [8–21], there remains a pressing need for high-quality evidence from RCTs in order to evaluate the clinical benefit and cost-effectiveness of intradialytic exercise. Without this evidence, there is unlikely to be any traction in the commissioning of routine exercise-based interventions for patients with dialysis-dependent CKD, including intradialytic exercise training. In addition, it is essential that cost-effectiveness information is generated to aid decision making around the potential value of the clinical implementation of an exercise delivery pathway for patients on HD [20].
The primary aim of the PEDAL study was to determine whether intradialytic exercise training, in comparison with usual care, improves health-related QOL in CKD Stage 5 patients receiving maintenance HD therapy. This will be the first large multicentre RCT that has been appropriately powered to investigate the effect of intradialytic exercise rehabilitation, delivered pragmatically by physiotherapy assistants, on health-related QOL in patients receiving HD therapy.
The baseline demographic data of the PEDAL trial cohort broadly aligns with the ‘real-world’ dialysis population in the UK as detailed by the latest data from the UK Renal Registry [34] and also the Proactive IV irOn Therapy in hemodiALysis patients (PIVOTAL) trial, a recent large interventional study conducted in UK renal units [35]. Baseline age, sex, BMI, BP, diabetes prevalence and the proportion of current smokers are all similar to UK Renal Registry data [34]. However, the current study included a larger proportion of Black African and Black Caribbean participants, and a lesser proportion of White participants, than what is representative of the broader prevalent dialysis population in the UK. The baseline data are similar to most dialysis populations worldwide [36–39]. There are some differences in rates of diabetes prevalence, with lower rates in our patient cohort compared with dialysis populations in the USA and Asia, which may limit application of the results to all other dialysis populations.
The results of the PEDAL trial will address a significant knowledge gap in the promotion and prescription of exercise for patients receiving HD therapy. If the study demonstrates that the exercise intervention is beneficial, there will be a strong case for implementation of intradialytic exercise training for patients with similar inclusion/exclusion criteria who are receiving HD therapy. The study was ‘open-label’ and that does increase the risk of bias in favour of the intervention. We did however attempt to mitigate this with blinded assessments. The PEDAL study was deliberately designed to be a pragmatic intervention that could be implemented with relative ease. There is potential for the results of the PEDAL study to be realized by patients in participating trial centres who have equipment and personnel immediately, and for the results to be used to aid national and international commissioning of intradialytic exercise training programmes.
PATIENT CONSENT
Not applicable.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
FUNDING
This study was funded by a grant from The National Institute for Health Research (grant number: NIHR-HTA 12/23/09). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research or the Department of Health. The funders had no role in the design, collection, analysis, interpretation of the data or writing of this protocol.
AUTHORS’ CONTRIBUTIONS
Authorship followed ICMJE guidelines. S.A.G., J.M., P.A.K., I.C.M., I.F., A.C.S. and T.H.M. were responsible for the inception and design of the project and prepared the manuscript. S.A.G., S.B., J.B., I.D., K.F., I.F., P.A.K., M.K., J.M., I.C.M., C.-M.M., C.R., M.W.T., P.C.T., D.C.W., M.Y., S.M., C.W., S.K. and T.H.M. contributed to the design of the study, provided methodological input and contributed to manuscript development. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
Supplementary Material
REFERENCES
- 1. Ashby D, Borman N, Burton J. et al. Renal Association Clinical Practice Guideline on Haemodialysis. BMC Nephrol 2019; 20: 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stack AG, Molony DA, Rives T. et al. Association of physical activity with mortality in the US dialysis population. Am J Kidney Dis 2005; 45: 690–701 [DOI] [PubMed] [Google Scholar]
- 3. Sietsema KE, Amato A, Adler SG. et al. Exercise capacity as a predictor of survival among ambulatory patients with end-stage renal disease. Kidney Int 2004; 65: 719–724 [DOI] [PubMed] [Google Scholar]
- 4. Knight EL, Ofsthun N, Teng M. et al. The association between mental health, physical function, and hemodialysis mortality. Kidney Int 2003; 63: 1843–1851 [DOI] [PubMed] [Google Scholar]
- 5. Lowrie EG, Curtin RB, LePain N. et al. Medical outcomes study short form-36: a consistent and powerful predictor of morbidity and mortality in dialysis patients. Am J Kidney Dis 2003; 41: 1286–1292 [DOI] [PubMed] [Google Scholar]
- 6. Painter P, Carlson L, Carey S. et al. Physical functioning and health-related quality-of-life changes with exercise training in hemodialysis patients. Am J Kidney Dis 2000; 35: 482–492 [DOI] [PubMed] [Google Scholar]
- 7. DeOreo PB. Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am J Kidney Dis 1997; 30: 204–212 [DOI] [PubMed] [Google Scholar]
- 8. Heiwe S, Jacobson SH.. Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev 2011; 5: CD003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heiwe S, Jacobson SH.. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis 2014; 64: 383–393 [DOI] [PubMed] [Google Scholar]
- 10. Cheema BS, Chan D, Fahey P. et al. Effect of progressive resistance training on measures of skeletal muscle hypertrophy, muscular strength and health related quality of life in patients with chronic kidney disease: a systematic review and meta-analysis. Sports Med 2014; 44: 1125–1138 [DOI] [PubMed] [Google Scholar]
- 11. Segura-Orti E. Exercise in hemodialysis patients: a literature systematic review. Nefrologia 2010; 30: 236–224 [DOI] [PubMed] [Google Scholar]
- 12. Smart N, Steele M.. Exercise training in haemodialysis patients: a systematic review and meta-analysis. Nephrology (Carlton) 2011; 16: 626–632 [DOI] [PubMed] [Google Scholar]
- 13. Salhab N, Karavetian M, Kooman J. et al. Effects of intradialytic aerobic exercise on hemodialysis patients: a systematic review and meta-analysis. J Nephrol 2019; 32: 549–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sheng K, Zhang P, Chen L. et al. Intradialytic exercise in hemodialysis patients: a systematic review and meta-analysis. Am J Nephrol 2014; 40: 478–490 [DOI] [PubMed] [Google Scholar]
- 15. Chung YC, Yeh ML, Liu YM.. Effects of intradialytic exercise on the physical function, depression and quality of life for haemodialysis patients: a systematic review and meta-analysis of randomised controlled trials. J Clin Nurs 2017; 26: 1801–1813 [DOI] [PubMed] [Google Scholar]
- 16. Pu J, Jiang Z, Wu W. et al. Efficacy and safety of intradialytic exercise in haemodialysis patients: a systematic review and meta-analysis. BMJ Open 2019; 9: e020633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Young HML, March DS, Graham-Brown MPM. et al. Effects of intradialytic cycling exercise on exercise capacity, quality of life, physical function and cardiovascular measures in adult haemodialysis patients: a systematic review and meta-analysis. Nephrol Dial Transplant 2018; 33: 1436–1445 [DOI] [PubMed] [Google Scholar]
- 18. Huang M, Lv A, Wang J. et al. Exercise Training and Outcomes in Hemodialysis Patients: Systematic Review and Meta-Analysis. Am J Nephrol 2019; 50: 240–254 [DOI] [PubMed] [Google Scholar]
- 19. Zhao QG, Zhang HR, Wen X. et al. Exercise interventions on patients with end-stage renal disease: a systematic review. Clin Rehabil 2019; 33: 147–156 [DOI] [PubMed] [Google Scholar]
- 20. Clarkson MJ, Bennett PN, Fraser SF. et al. Exercise interventions for improving objective physical function in patients with end-stage kidney disease on dialysis: a systematic review and meta-analysis. Am J Physiol Renal Physiol 2019; 316: F856–F872 [DOI] [PubMed] [Google Scholar]
- 21. Gomes Neto M, de Lacerda FFR, Lopes AA. et al. Intradialytic exercise training modalities on physical functioning and health-related quality of life in patients undergoing maintenance hemodialysis: systematic review and meta-analysis. Clin Rehabil 2018; 32: 1189–1202 [DOI] [PubMed] [Google Scholar]
- 22.Department of Health, Social Services and Public Safety, Scottish Government, Welsh Government, Department of Health. Start Active, Stay Active: A Report on Physical Activity from the Four Home Countries’ Chief Medical Officers2011. (online). http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_128209 (11 February 2020, date last accessed)
- 23. Colberg SR, Albright AL, Blissmer BJ. et al. Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc 2010; 42: 2282–2303 [DOI] [PubMed] [Google Scholar]
- 24. Fleg JL, Forman DE, Berra K. et al. Secondary prevention of atherosclerotic cardiovascular disease in older adults: a scientific statement from the American Heart Association. Circulation 2013; 128: 2422–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Segura-Orti E, Martinez-Olmos FJ.. Test-retest reliability and minimal detectable change scores for sit-to-stand-to-sit tests, the six-minute walk test, the one-leg heel-rise test, and handgrip strength in people undergoing hemodialysis. Phys Ther 2011; 91: 1244–1252 [DOI] [PubMed] [Google Scholar]
- 26. Abe Y, Matsunaga A, Matsuzawa R. et al. Determinants of slow walking speed in ambulatory patients undergoing maintenance hemodialysis. PLoS One 2016; 11: e0151037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laurent S, Cockcroft J, Van Bortel L. et al.; on behalf of the European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27: 2588–2605 [DOI] [PubMed] [Google Scholar]
- 28. Hickson SS, Butlin M, Broad J. et al. Validity and repeatability of the Vicorder apparatus: a comparison with the SphygmoCor device. Hypertens Res 2009; 32: 1079–1085 [DOI] [PubMed] [Google Scholar]
- 29. Lee PH, Macfarlane DJ, Lam TH. et al. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act 2011; 8: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coutinho-Myrrha MA, Dias RC, Fernandes AA. et al. Duke Activity Status Index for cardiovascular diseases: validation of the Portuguese translation. Arq Bras Cardiol 2014; 102: 383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tinetti ME, Richman D, Powell L.. Falls efficacy as a measure of fear of falling. J Gerontol 1990; 45: P239–243 [DOI] [PubMed] [Google Scholar]
- 32. Crotty M. The foundations of social research: Meaning and perspective in the research process. London, Thousand Oaks, CA: Sage Publications, 1998 [Google Scholar]
- 33. Grbich C. Qualitative research in health. An introduction. London: Sage, 1999 [Google Scholar]
- 34. Evans K, Pyart R, Steenkamp R. et al. UK renal registry 20th annual report of the renal association. Nephron 2018; 139: 1–12 [DOI] [PubMed] [Google Scholar]
- 35. Macdougall IC, White C, Anker SD. et al. , on behalf of the PIVOTAL Trial investigators. Randomized trial comparing proactive, high-dose versus reactive, low-dose intravenous iron supplementation in hemodialysis (PIVOTAL): study design and baseline data. Am J Nephrol 2018; 48: 260–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saran R, Robinson B, Abbott KC. et al. US Renal Data System 2018 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2019; 73: A7–A8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goodkin DA, Bragg-Gresham JL, Koenig KG. et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol 2003; 14: 3270–3277 [DOI] [PubMed] [Google Scholar]
- 38. Duranton F, Kramer A, Szwarc I. et al. Geographical variations in blood pressure level and seasonality in hemodialysis patients. Hypertension 2018; 71: 289–296 [DOI] [PubMed] [Google Scholar]
- 39. Lopes AA, Bragg-Gresham JL, Satayathum S. et al. Health-related quality of life and associated outcomes among hemodialysis patients of different ethnicities in the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2003; 41: 605–615 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Findings from the study will be disseminated at national and international conferences. All data published from this study will be available if requested in writing from the chief investigator.